Abstract
Escherichia coli is a major player in foodborne illnesses, capable of forming biofilms on dairy facilities, leading to milk contamination. Thus, we examined the capacity of E. coli strains from raw milk bulk tanks to form biofilms on surfaces made of polystyrene, stainless steel, and silicone; the potential links between biofilm formation with genes responsible for fimbriae and virulence factors of extra-intestinal E. coli (ExPEC); and the susceptibility of biofilm-forming isolates to iodine and chlorhexidine digluconate. Out of 149 E. coli strains, 42.28% (63/149) formed biofilm on polystyrene, 56.38% (84/149) on silicone, and 21.48% (32/149) on stainless steel. The frequency of genes was: fimH (100%), hlyA (5.4%), irp2 (2.7%), sitA (10.7%), ompT (43.6%), and traT (98%). No biofilm developed when disinfectants were used prior to biofilm formation. However, iodine and chlorhexidine digluconate allowed 25.40% (16/63) of isolates displaying growth after a mature biofilm was formed. The presence of biofilm on different surfaces emphasizes the vital need for thorough equipment cleaning, both in farms and in industrial dairy settings. Rapid disinfection is crucial, given the reduced susceptibility of potentially pathogenic E. coli after biofilm maturity.
Keywords:
dairy; disinfectants; enterobacteria; ExPEC; milk; pathogen; polystyrene; silicone; stainless steel 1. Introduction
There is a global concern surrounding the quality of milk intended for consumption. Nevertheless, instances of pasteurization failure or the consumption of unpasteurized milk can expose humans to risks, such as Escherichia coli infection [1]. Raw milk from bulk tanks has the potential to harbor pathogenic microorganisms, which can originate from diverse sources. These sources encompass farm animals, primarily through mastitis, as well as potential contamination from milking equipment or inadequately sanitized milk cooling tanks, along with the broader dairy environment [2]. Notably, a deficiency in comprehensive data exists concerning the molecular identification of E. coli within these bulk tanks and its persistence and control.
E. coli has the capacity to create biofilms within dairy environments. Despite the considerable issues biofilms pose in the food industry, their formation in these settings remains not fully understood, making effective biofilm control an ongoing challenge. Biofilm formation is contingent upon an array of factors, including the microorganisms’ ability to adhere to surfaces, the progression through the stages of mature biofilm development, the characteristics of the adhered surface, and the prevailing environmental conditions, encompassing temperature, pH, and nutrient availability [3,4].
An additional crucial aspect concerning biofilm formation involves the sensitivity of strains to disinfectants routinely utilized in dairy chain, such as iodine and chlorhexidine digluconate. The selection of the optimal disinfectant for surface sanitation hinges on a multitude of factors, including the surface’s composition, temperature, duration of exposure to residues, pH levels, and the inherent resistance of bacteria that form biofilms to disinfectants [3,4].
Recognizing the significance of biofilms within the milk production process and their implications for public health, this current study delved into the capability of E. coli strains, isolated from milk bulk tanks, to generate biofilms on various surfaces including polystyrene, stainless steel, and silicone. Additionally, we examined the relationship between biofilm development and the presence of genes responsible for fimbriae, as well as genes associated with extra-intestinal E. coli virulence factors (ExPEC). Furthermore, we explored the susceptibility of biofilm-forming isolates to iodine and chlorhexidine digluconate. Thus, our study provides new insights to the international scientific community, as it is one of the few comprehensive studies conducted in Brazil that encompasses the characterization of virulence potential (fimbriae and ExPEC) and biofilm formation of E. coli on different materials used in the dairy production chain, along with the evaluation of the effectiveness of disinfectants widely employed in sanitation processes.
2. Materials and Methods
This study adhered to the guidelines established by the Ethics Committee on Animal Use (CEUA/FMVZ—UNESP/Botucatu, Sao Paulo, Brazil), under protocol number 136/2017.
2.1. Farms Description and Isolation of Escherichia coli
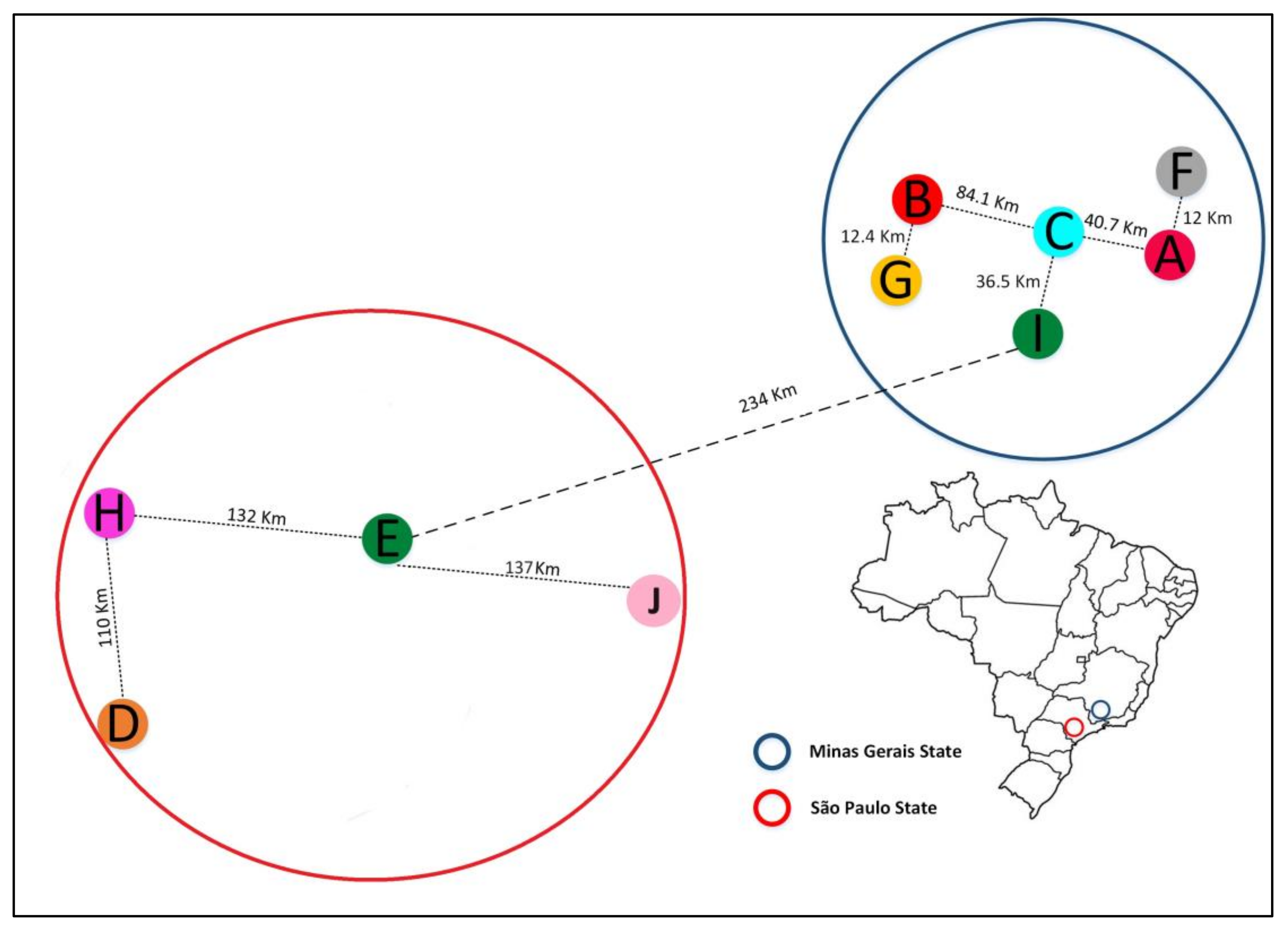
A total of 149 E. coli isolates were collected from bulk tank milk samples, selected using a convenience sampling approach, originating from ten farms situated in the states of Minas Gerais and São Paulo, located in southeast Brazil (Figure 1). These milk samples were collected over the span of 2017 to 2019.
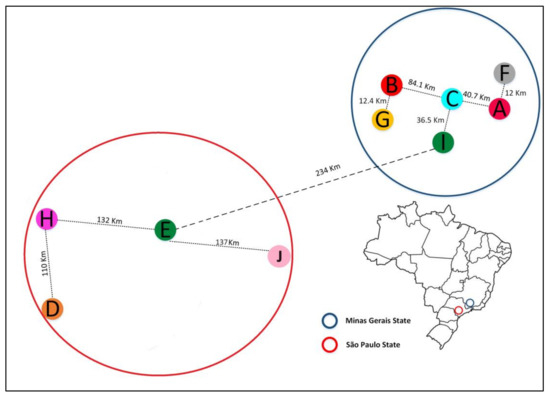
Figure 1.
Location of the 10 (ten) dairy farms sampled in the study, which are located in the states of São Paulo and Minas Gerais, Brazil.
Eligibility for participation in the study was based on specific criteria:
- Cows of Holstein breed or Holstein crossbreeds.
- Farms housing a minimum of 200 lactating cows.
- Milk yield exceeding 20 kg per cow per day.
- Bulk tank milk somatic cell count (SCC) below 400,000 cells/mL.
- Utilization of milk machines on the farms.
- Availability of electronic health and production records.
Milk samples were aseptically collected and placed into sterile bottles, then promptly refrigerated to maintain their quality until the initiation of the microbiological analysis. The assessment involved incubating serial dilutions (ranging from 100 to 10−3) of the milk samples in Brain Heart Infusion (BHI) broth, at a 1:10 ratio, and allowing them to incubate at 37 °C for 4 h. Subsequently, 0.1 mL from each dilution was plated onto chromogenic medium (Chromocult® Agar for coliforms, Merck Millipore, Burlington, MA, USA)) and incubated at 37 °C for 24 h. The identification of colonies presumptive of E. coli was accomplished using conventional phenotypic tests [5].
2.2. Biofilm Formation and Efficacy of Antiseptics for Its Control
We assessed biofilm production following the protocol outlined by Vasudevan and colleagues [6]. In essence, following a 24 h incubation period at 35 °C, the cultures cultivated in Luria Bertani (LB) medium were appropriately diluted (to a 0.5 MacFarland scale) utilizing a turbidimeter. Subsequently, 200 µL aliquots were dispensed in triplicate within 96-well polystyrene microplates (Kasvi, São José dos Pinhais, Paraná, Brazil), simulating an abiotic surface, and incubated once again at 35 °C for 48 h. Upon completion of the incubation, the wells were meticulously washed with PBS (pH 7.4). A subsequent step involved staining the wells with 1% crystal violet solution, allowing the stain to act for 15 min, followed by three thorough washes with the same buffer. Post-washing, the microplates were left to dry, subsequently undergoing spectrophotometric analysis at a wavelength of 540 nm. As a corrective measure for absorbance values, uninoculated LB medium was utilized as a blank [6]. Each instance of phenotypic biofilm formation expression was determined by conducting three OD540 readings for each sample. Within every analyzed plate, both a positive and a negative sample were present. The outcomes were then classified into two groups: biofilm formers and non-biofilm formers. This classification was achieved by computing the cutoff point as follows: the mean OD540 value of the negative control strain, augmented by three times the standard deviation, was calculated across the three replicates per plate to establish the cutoff OD (ODc) specific to each plate [7].
For the tests involving stainless steel and silicone, we utilized coupons that were meticulously washed, dried, and sterilized via autoclaving. These coupons had a 1 cm diameter and were fabricated from 304 stainless steel or silicone material. Using sterile forceps, the plugs were placed within individual wells of a 24-well plate, which was germ-free and fitted with a lid. This arrangement was established in triplicate, and each well received a 300 µL aliquot from a 108 CFU dilution. Subsequently, the plate was incubated at 35 °C for 48 h, as per the method outlined by Oosterik and colleagues [7]. After the incubation period, the coupons were transferred to a new plate. This transfer was a precautionary step to prevent the inadvertent quantification of biofilm that might potentially form on the surrounding polystyrene areas of coupons composed of different materials. Once on the new plate, the procedures occurred as described for polystyrene tests.
Strains exhibiting the ability to generate biofilm on polystyrene surfaces underwent susceptibility testing with disinfectants routinely employed in milking lines, namely iodine and chlorhexidine digluconate. To perform the test, the Minimum Bactericidal Concentration (MBC) was determined within a three-hour incubation period in Luria Bertani broth (LB). The bacterial inoculum, approximately 108 CFU/mL, was prepared within LB broth using MacFarland scale. The procedure employed a sterile 96-well microplate. Starting from the initial well, a concentration of 2% disinfectant solution (200 µL) was added. Subsequent wells underwent serial dilutions, using a base-two scale. Each well was supplemented with a 100 µL aliquot of the bacterial suspension, generating dilutions spanning from 1% to 0.5%. After thorough mixing, the microplate was incubated at 35 °C for 3 h. For MBC determination, aliquots from each well were cultivated on TSA Agar at 35 °C for 24 h. The MBC value represented the lowest dilution of the disinfectant capable of completely inhibiting growth on the agar plate.
Once the Minimum Bactericidal Concentration (MBC) was determined, the subsequent steps aimed to validate the obtained results. The process transitioned to cultivating a mature biofilm within a 96-well microplate over a 72 h period. After this incubation period, the wells underwent four rounds of washing with sterile PBS. Each wash cycle involved the introduction of 200 µL of the disinfectant solution (ranging from 0.5% to 2%). The microplate was then incubated at 35 °C for 3 h to mimic the industrial-scale sanitation process. In the next step, the efficacy of the antimicrobial treatment was confirmed by plating the contents of each well on TSA agar to observe the absence of growth. A control was established by inoculating a well solely with LB medium.
2.3. Genes Detection through Polymerase Chain Reaction (PCR)
Two different gene panels were used in this study. The first one contemplated gene encoded different types of fimbriae and related to the formation of biofilms: fimA, fimE, ecpRAB, ecpA, elfA [8] csgD [9], and hcpA [10].
The second panel contained genes related to extra-intestinal E. coli virulence factors (ExPEC):
- Adhesins: fimH [11], iha [12], papA [13], papC, afaBC, and sfaDE [14]
- Toxins: hlyA [15], sat, vat [16], cnf [17], and cdt [11]
- Siderophores: iroN [18], irp2 [19], iucD [20], ireA [16], and sitA [21]
- Invasion: ibe10 [22]
- Serum resistance: traT [13] kpsMTll [18], and ompT [23]
Briefly, all bacterial colonies underwent the following procedure: they were suspended in 200 µL of sterile water, boiled for 10 min, and then rapidly frozen for 10 min. Subsequently, these suspensions were subjected to centrifugation at 10,000× g for 1 min, and the resulting supernatants were employed as templates for the polymerase chain reaction (PCR) to amplify DNA. Each PCR reaction mixture comprised 2.0 µL of DNA template, 7.0 µL of Go Taq Green Master Mix (Promega, Madison, WI, USA), 4.0 µL of nuclease-free H2O, and 0.34 µmol l−1 of each pair of primers, constituting a total volume of 15 µL. The amplification process was conducted within a thermocycler, and the resulting reaction fragments were subjected to 1.5% agarose gel electrophoresis. The images of these reactions were captured using an image capture system.
2.4. Statistical Analysis
Initially, frequency distributions were produced to describe the prevalence of the studied genes and the prevalence of biofilm-forming strains in the materials used. Then, we used a logistic regression model (PROC LOGISTIC, SAS Institute, Cary, NC, USA) to estimate the chances of biofilm formation among the studied materials. For each material studied, a chi-square or Fisher test (PROC FREQ, SAS Institute) was used to compare the proportion of biofilm formation between the positive and negative isolates for each researched gene. All analyses were performed at a significance level of 5%.
3. Results
Among a collection of 149 E. coli strains extracted from bulk tank milk samples, it was found that 42.28% (63/149) exhibited the capacity to generate biofilm when placed on polystyrene surfaces. This ability increased to 56.38% (84/149) when silicone was employed as the substrate, and in the case of stainless steel, 21.48% (32/149) demonstrated biofilm-forming potential.
Comparing these outcomes against the benchmark of stainless steel, the odds ratio for biofilm development on polystyrene was calculated to be 2.68 (with a 95% confidence interval ranging from 1.61 to 4.46), while for silicone, the odds ratio stood at 4.72 (with a confidence interval of 2.84 to 7.87).
We have detailed the presence of genes linked to fimbriae and ExPEC in Table 1. Specifically, for ExPEC genes, the observed prevalence was as follows: fimH 100%, hlyA 5.4%, irp 2 2.7%, sitA 10.7%, ompT 43.6%, and traT 98%.

Table 1.
Absolute and relative prevalence of biofilm formation associated genes and ExPEC genes detection in 149 E. coli isolated from bulk tank milk, Brazil.
The correlation between genes associated with biofilm formation and ExPEC and the ability to form biofilm in vitro on polystyrene, stainless steel, and silicone substrates for E. coli isolates sourced from bulk tank milk is outlined in Table 2 and Table 3.

Table 2.
Association of genes related to biofilm production with in vitro biofilm production in polystyrene, stainless steel, and silicone of E. coli isolates obtained from bulk tank milk, Brazil.

Table 3.
Association of genes related to the production of virulence factors for extra-intestinal E. coli (ExPEC) with the in vitro production of polystyrene, stainless steel, and silicone biofilms from E. coli isolates obtained from bulk tank milk, Brazil.
Sixty-three samples with the ability to produce biofilm in polystyrene were submitted to the determination of MBC for disinfectant susceptibility evaluation. None of the isolates formed biofilm when both disinfectants were added to the bacterial inoculum and subjected to incubation for 3 h. However, when iodine and chlorhexidine digluconate were added after the formation of mature biofilm (72 h of incubation) and incubated for 3 h, 25.40% (16/63) of the isolates demonstrated growth in the TSA medium for at least one of the agents tested.
4. Discussion
Inadequate equipment hygiene can lead to microorganism buildup on surfaces, promoting biofilm formation in dairy environments and industry. This study enhances the understanding of biofilm development on various materials. This is concerning for potential milk recontamination, leading to product deterioration and shorter shelf life [24]. Additionally, it poses public health risks through disease transmission. The materials chosen for this study, including polystyrene, stainless steel, and silicone, allow for comparisons with in vitro investigations on biofilm formation. The use of 304 stainless steel, together with 316, is known for its physical and mechanical properties, thermal stability, cleaning capacity, and corrosion resistance, aligns with its widespread use in industrial settings.
The study showed clear trends in biofilm development on different materials. Specifically, biofilms were found in 42.28% of tested isolates on polystyrene, 56.38% on silicone, and 21.48% on stainless steel surfaces. When comparing these materials, a significant difference in biofilm formation emerged. Biofilm formation on polystyrene was 2.68 times more likely than on stainless steel, and a substantial 4.72 times more likely than on silicone. This supports the choice of stainless steel for industrial use, as it demonstrated the lowest tendency for biofilm formation, aligning with its inherent characteristics.
The choice of polystyrene for this study was made to facilitate comparison with existing research on E. coli biofilm formation. It is important to note that literature on this topic is limited, particularly regarding isolates from milk sources. A study by Milanov et al. [25] investigated biofilm production in LB and tryptone soy broth (TSB) mediums at two temperatures (20 °C and 37 °C) using 25 E. coli isolates from cases of bovine mastitis. Their results showed biofilm production rates of 64% at 20 °C and 56% at 37 °C in LB medium, and 68% and 24% at 20 °C and 37 °C, respectively, in TSB medium.
Biofilm formation potential is not limited to specific strains or species; various microorganisms can establish biofilms in dairy settings. In Brazil, a comprehensive study examined multiple sources (mastitic milk, milk from bulk tanks, milking equipment, and the hands of milkers), revealing biofilm formation by Staphylococcus aureus on surfaces such as polystyrene (45.2%), stainless steel (41.9%), and rubber (38.7%) [26]. Another study explored the biofilm-forming ability of S. aureus on both biotic surfaces, such as bovine mammary epithelium cells and HeLa cells, as well as abiotic surfaces [27]. Recent advancements have highlighted the significant pathogenicity factors inherent to coagulase-negative Staphylococcus species. These factors have been identified as influential in shaping the biofilm-forming capacity, even extending their impact to other species within the same genus [28].
This study used stainless steel to mimic the milking equipment, the milk bulk tank, and the dairy equipment. The E. coli survival under stress conditions and the ability to form biofilms depends on the isolate’s serotype and characteristics. Pathogenic strains are more resistant to temperature, pressure, and disinfectants routinely used in the industry [29]. Another major problem associated with E. coli biofilms is related to pathogenic serotypes once they require fewer individuals to establish themselves as an infectious dose. Thus, even a weak and low-quality biofilm can promote the contamination or recontamination of food in industrial facilities [30]. A notable prevalence of biofilm was observed on stainless steel surfaces. This observation brings to the forefront a significant concern within the context of milk processing plants. These findings diverge from the study conducted by Cherif-Antar and colleagues [31], in which none of the 25 E. coli isolates gathered from various points within the dairy displayed the capability to produce biofilms.
While limited data exists regarding the propensity of bacterial isolates from milk to form biofilms on silicone surfaces, this material is intrinsically conducive to biofilm formation due to its surface attributes that facilitate adherence. Silicone holds merit due to its outstanding biocompatibility, durability, and versatility in various applications [32]. In the context of our study, the rationale was to emulate the liners utilized within milking environments. Notably, the extensive use of silicone in the medical domain, such as in catheters and prostheses, underscores its pivotal role and the consequential importance it carries for combating nosocomial infections [32]. In this investigation, we have successfully demonstrated a notable frequency of biofilm development on silicone surfaces, reaching 56.38%. This represents the highest rate among the three materials examined. This result has important implications, especially given the difficulty in using common disinfectants for silicone cleaning. Chlorhexidine, acetic acid, and mandelic acid were found to be ineffective in eradicating biofilm cells [33]. Aside from surface properties, it is crucial to highlight microbial factors that contribute to biofilm formation. The complex molecular pathways governing this phenomenon are not fully understood, indicating gaps in our knowledge.
Numerous studies have linked various genes in E. coli isolates to biofilm production. One key example is the role of fimbriae, adhesive protein structures that play essential roles in biofilm formation [4]. Fimbriae encompass various types, each contributing uniquely to the process. The cluster of fim genes encodes structural components of type I fimbrial organelles, commonly found in the Enterobacteriaceae family [34]. Mutants in these genes showed reduced initial binding during biofilm formation analysis on polyvinyl chloride (PVC) [35]. In our study, fimA and fimE genes were highly prevalent (100% and 99.3%), but as there were no negative samples for these genes, we could not establish a correlation with biofilm production on the three materials.
A recently characterized form of adhesion is the ECP (E. coli common pilus), produced by most E. coli pathogroups, whether intestinal or extraintestinal [36]. Initially identified as a temperature-dependent fimbria [37], studies have shown that strains lacking ECP have significantly reduced adherence to epithelial cells [36]. Our in vitro tests support these findings, as we found no correlation between the ecpRAB gene and the presence of biofilm-forming isolates on surfaces such as polystyrene, stainless steel, and silicone. Additionally, there was no apparent link between the ecpA gene and biofilm occurrence on stainless steel surfaces.
Regarding curli fimbriae, the csgD gene regulates key components crucial for biofilm development [38]. Ryu and Beuchat [39] demonstrated that curli production significantly influences biofilm formation on stainless steel surfaces. Despite a high occurrence (96%) of the csgD gene in our study, we did not find a conclusive association with biofilm formation on any of the materials tested (p > 0.05). This suggests a more intricate relationship, as the csgD can both up-regulate and down-regulate factors in curli biosynthesis [38]. In this study, we also examined laminin-binding fimbriae governed by the elfA gene. This flexible fiber structure can bind to laminin, an extracellular matrix protein. It is speculated to contribute to E. coli adhesion [40]. In a study involving human samples, the elfA gene was found in 72% of samples, but the corresponding protein structure was not identified in an immunofluorescence assay [8]. Interestingly, when analyzing samples from E. coli isolates from milk in bulk tanks, the elfA was present in all samples (100%). While this widespread presence made it challenging to establish a direct link with biofilm production on the three materials, the universal presence of the elfA in all biofilm-forming samples suggests its involvement in E. coli biofilm formation from food sources.
We also assessed the presence of the hcpA gene, which corresponds to type IV pili. This structure holds implications beyond mere adherence, as it is associated with triggering inflammation in host cells due to Shiga-toxin-producing E. coli (STEC) [40]. Notably, individuals affected by hemolytic uremic syndrome (SUH) exhibited the presence of type IV pili [10]. Moreover, a link between this form of fimbria and E. coli adhesion to cells was demonstrated by introducing mutations in the hcpA gene, leading to a reduction in adherence among mutant strains [10]. While the hcpA gene was detected in only 6.7% (10/149) of the samples in our study, a significant positive correlation was established between its presence and biofilm formation on polystyrene surfaces (p = 0.0182). This finding underscores the potential contribution of type IV pili to the process of biofilm formation.
Our study emphasizes the critical role of surface exposure duration in enabling mature biofilm development in E. coli. This time factor can lead to robust biofilm formation, even in the presence of disinfectants like iodine and chlorhexidine digluconate. Interestingly, our results show that simultaneous application of these disinfectants inhibits biofilm formation. However, as the biofilm matures over a 72 h incubation period, a distinct trend emerges. Specifically, among E. coli biofilm-forming isolates on polystyrene surfaces, 25.40% (16/63) exhibit resistance to at least one of the disinfectants used. This highlights the potential for certain E. coli biofilm strains to develop resistance to disinfectants over time, underscoring the dynamic nature of biofilm formation and its interaction with disinfection practices. Therefore, implementing an appropriate disinfection regimen is crucial to prevent biofilm maturation and the accumulation of substances that could serve as nutrients for their growth [41]. It is important to note that the effectiveness of disinfectants significantly decreases when surfaces are contaminated with debris or food particles [42].
Recently, the pathotype of E. coli implicated in infections beyond the intestinal tract has been labeled as ExPEC (Extraintestinal Pathogenic E. coli). ExPEC’s virulence factors can cause disease in both animals and humans, influenced by factors such as adhesins, toxins, siderophores, and serum resistance mechanisms [43]. The hlyA gene encodes E. coli enterohemorrhagic hemolysin and is widespread among various pathotypes, playing a significant role in adhesion, inducing inflammatory responses, and damaging epithelial cells [44,45]. ExPEC faces iron scarcity in animal hosts, prompting the use of siderophores to acquire chelated iron from host proteins. Siderophores also contribute to ExPEC’s virulence, with each siderophore having specific characteristics that enhance its adaptation to environmental conditions and immune evasion [46]. Notably, our molecular analysis detected the presence of genes encoding irp2 and sitA among the five siderophore genes studied.
Virulence factors in ExPEC strains serve as a defense against the host’s immune system. Most strains possess a protective layer of lipopolysaccharides (LPS) that guards against opsonization and lysis by the complement system [47]. They can also express membrane proteins (traT), increasing resistance against serum and, consequently, the complement system [48], and ompT, capable of cleaving immunoglobulins and other host defense proteins [49]. Although our detection covered only six of the 21 analyzed genes categorized as virulence factors in ExPEC, it is crucial to recognize their multifaceted roles in infection and disease development within the host. This underscores the importance of investigating these factors in animal-derived products, which can potentially act as carriers for disease transmission. Such research has implications for safeguarding public health and preventing the spread of infections.
5. Conclusions
The occurrence of biofilm formation on various surfaces underscores the critical importance of thorough equipment cleaning procedures, both in farm settings and industrial dairy environments. Swift disinfection using iodine and chlorhexidine digluconate is essential due to the diminished susceptibility of potentially pathogenic E. coli to these disinfectants once a mature biofilm has developed.
The challenge of effectively disinfecting these surfaces persists, requiring ongoing research and innovation to address this complex issue. Ultimately, maintaining hygienic conditions and preventing persistent biofilm formation is pivotal in ensuring the safety and quality of dairy products and minimizing potential risks to public health.
Author Contributions
Conceptualization: S.J.A.S., H.L., M.G.R., J.C.d.F.P., V.L.M.R., R.T.H. and D.d.S.L.; Methodology, formal analysis, investigation and data curation: S.J.A.S., F.d.F.G., G.A.M.R., S.T.G., F.M.D., B.C.L., M.d.S.R.M., A.C.Y., E.C.d.S., G.N.d.M., A.B.B. and S.B.L.; Resources, funding acquisition and project administration: S.J.A.S., H.L., M.G.R., J.C.d.F.P., V.L.M.R., R.T.H. and D.d.S.L.; Writing—original draft preparation and review and editing: S.J.A.S., H.L., G.A.M.R. and A.B.B.; Supervision: H.L. All authors have read and agreed to the published version of the manuscript.
Funding
This study was funded by The São Paulo Research Foundation (FAPESP) support research grant number 2015/19688-8 and scholarship grant number 2017/07246-6.
Institutional Review Board Statement
This study adhered to the guidelines established by the Ethics Committee on Animal Use (CEUA/FMVZ—UNESP/Botucatu, Sao Paulo state), Brazil, under protocol number 136/2017.
Informed Consent Statement
Not applicable.
Data Availability Statement
Raw data will be available upon request.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Guh, A.; Phan, Q.; Nelson, R.; Purviance, K.; Milardo, E.; Kinney, S.; Mshar, P.; Kasacek, W.; Cartter, M. Outbreak of Escherichia coli O157 associated with raw milk, Connecticut, 2009. Clin. Infec. Dis. 2010, 12, 1411–1417. [Google Scholar] [CrossRef] [PubMed]
- LeJeune, J.T.; Besser, T.E.; Rice, D.H.; Ber, J.L.; Stilborn, R.P.; Hancock, D.D. Longitudinal Study of Fecal Shedding of Escherichia coli O157:H7 in Feedlot Cattle: Predominance and Persistence of Specific Clonal Types despite Massive Cattle Population Turnover. Appl. Environm. Microbiol. 2004, 70, 377–384. [Google Scholar] [CrossRef] [PubMed]
- Van Houdt, R.; Michiels, C.W. Role of bacterial cell surface structures in Escherichia coli biofilm formation. Res. Microbiol. 2005, 156, 626–633. [Google Scholar] [CrossRef] [PubMed]
- Van Houdt, R.; Michiels, C.W. Biofilm formation and the food industry, a focus on the bacterial outer surface. J. Appl. Microbiol. 2010, 109, 1117–1131. [Google Scholar] [CrossRef] [PubMed]
- Trabulsi, L.R.; Ordoñez, J.; Martinez, M. Enterobacteriaceae. In Microbiologia, 4th ed.; Trabulsi, L.R., Alterthum, F., Eds.; Editora Atheneu: Sao Paulo, SP, Brazil, 2005; pp. 269–276. (In Portuguese) [Google Scholar]
- Vasudevan, P.; Nair, M.K.M.; Annamalai, T.; Venkitanarayanan, K.S. Phenotypic and genotypic characterization of bovine mastitis isolates of Staphylococcus aureus for biofilm formation. Vet. Microbiol. 2003, 92, 179–185. [Google Scholar] [CrossRef] [PubMed]
- Oosterik, L.H.; Tuntufye, H.N.; Butaye, P.; Goddeeris, B.M. Effect of serogroup, surface material and disinfectant on biofilm formation by avian pathogenic Escherichia coli. Vet. J. 2014, 202, 561–565. [Google Scholar] [CrossRef] [PubMed]
- Hernandes, R.T.; Velsko, I.; Sampaio, S.C.F.; Elias, W.P.; Robins-Browne, R.M.; Gomes, T.A.T.; Girón, J.A. Fimbrial adhesins produced by Atypical Enteropathogenic Escherichia coli strains. Appl. Environ. Microbiol. 2011, 77, 8391–8399. [Google Scholar] [CrossRef] [PubMed]
- Saldaña, Z.; Xicohtencatl-Cortes, J.; Avelino, F.; Phillips, A.D.; Kaper, J.B.; Puente, J.L.; Girón, J.A. Synergistic role of curli and cellulose in cell adherence and biofilm formation of attaching and effacing Escherichia coli and identification of Fis as a negative regulator of curli. Environm. Microbiol. 2009, 11, 992–1006. [Google Scholar] [CrossRef]
- Xicohtencatl-Cortes, J.; Monteiro-Neto, V.; Ledesma, M.A.; Jordan, D.M.; Francetic, O.; Kaper, J.B.; Puente, J.L.; Girón, J.A. Intestinal adherence associated with type IV pili of enterohemorrhagic Escherichia coli O157: H7. J. Clin. Invest. 2007, 117, 3519–3529. [Google Scholar] [CrossRef]
- Tennant, S.M.; Tauschek, M.; Azzopardi, K.; Bigham, A.; Bennett-Wood, V.; Hartland, E.L.; Qi, W.; Whittam, T.; Robins-Browne, R.M. Characterisation of atypical enteropathogenic E. coli strains of clinical origin. BMC Microbiol. 2009, 9, 1–11. [Google Scholar] [CrossRef]
- Scaletsky, I.C.A.; Aranda, K.R.S.; Souza, T.B.; Silva, N.P.; Morais, M.B. Evidence of pathogenic subgroups among atypical enteropathogenic Escherichia coli strains. J. Clinic. Microbiol. 2009, 47, 3756–3759. [Google Scholar] [CrossRef] [PubMed]
- Johnson, J.R.; Stell, A.L. Extended Virulence Genotypes of Escherichia coli Strains from Patients with Urosepsis in Relation to Phylogeny and Host Compromise. J. Infect. Dis. 2000, 181, 261–272. [Google Scholar] [CrossRef] [PubMed]
- Le Bouguenec, C.; Archambaud, M.; Labigne, A. Rapid and specific detection of the pap, afa, and sfa adhesin-encoding operons in uropathogenic Escherichia coli strains by polymerase chain reaction. J. Clinic. Microbiol. 1992, 30, 1189–1193. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, S.; Terai, A.; Yuri, K.; Kurazono, H.; Takeda, Y.; Yoshida, O. Detection of urovirulence factors in Escherichia coli by multiplex polymerase chain reaction. FEMS Immunol. Med. Microbiol. 1995, 12, 85–90. [Google Scholar] [CrossRef] [PubMed]
- Ewers, C.; Li, G.; Wilking, H.; Kiebling, S.; Alt, K.; Antáo, E.M.; Laturnus, C.; Diehl, I.; Glodde, S.; Homeier, T.; et al. Avian pathogenic, uropathogenic, and newborn meningitis-causing Escherichia coli: How closely related are they? Int. J. Medic. Microbiol. 2007, 297, 163–176. [Google Scholar] [CrossRef] [PubMed]
- Tóth, I.; Hérault, F.; Beutin, L.; Oswald, E. Production of cytolethal distending toxins by pathogenic Escherichia coli strains isolated from human and animal sources: Establishment of the existence of a new cdt variant (type IV). J. Clin. Microbiol. 2003, 41, 4285–4291. [Google Scholar] [CrossRef] [PubMed]
- Johnson, J.R.; Russo, T.A.; Tarr, P.I.; Carlino, U.; Bilge, S.S.; Vary JR, J.C.; Stell, A.L. Molecular epidemiological and phylogenetic associations of two novel putative virulence genes, iha and iroN (E. coli), among Escherichia coli isolates from patients with urosepsis. Infect. Immun. 2000, 68, 3040–3047. [Google Scholar] [CrossRef] [PubMed]
- Czeczulin, J.R.; Whittam, T.S.; Henderson, I.R.; Navarro-Garcia, F.; Nataro, J.P. Phylogenetic analysis of enteroaggregative and diffusely adherent Escherichia coli. Infect. Immun 1999, 67, 2692–2699. [Google Scholar] [CrossRef]
- Herrero, M.; De Lorenzo, V.; Neilands, J.B. Nucleotide sequence of the iucD gene of the pCoIV-K30 aerobactin operon and topology of its product studied with phoA and lacZ gene fusions. J. Bacteriol. 1988, 170, 56–64. [Google Scholar] [CrossRef]
- Rodriguez-Siek, K.E.; Giddings, C.W.; Doetkott, C.; Johnson, T.J.; Nolan, L.K. Characterizing the APEC pathotype. Vet. Res. 2005, 36, 241–256. [Google Scholar] [CrossRef]
- Huang, S.H.; Wass, C.; Fu, Q.; Prasadarao, N.V.; Stins, M.; Kim, K.S. Escherichia coli invasion of brain microvascular endothelial cells in vitro and in vivo: Molecular cloning and characterization of invasion gene ibe10. Infect. Immun. 1995, 63, 4470–4475. [Google Scholar] [CrossRef] [PubMed]
- Johnson, J.R.; O’Bryan, T.T.; Low, D.A.; Ling, G.; Delavari, P.; Fasching, C.; Russo, T.A.; Carlino, U.; Stell, A.L. Evidence of commonality between canine and human extraintestinal pathogenic Escherichia coli strains that express papG allele III. Infect. Immun. 2000, 68, 3327–3336. [Google Scholar] [CrossRef] [PubMed]
- Kasnowski, M.C.; Mantilla, S.P.S.; Oliveira, L.A.T.; Francoo, R.M. Biofilm formation in the food industry and surface validation methods. Rev. Cient. Eletr. Med. Vet. 2010, 15, 1–23. [Google Scholar]
- Milanov, D.; Prunic, B.; Velhner, M.; Todorovic, D.; Polacek, V. Investigation of biofilm formation and phylogenetic typing of Escherichia coli strains isolated from milk of cows with mastitis. Acta Sci. Vet. 2015, 65, 202–216. [Google Scholar] [CrossRef]
- Lee, S.H.; Mangolin, B.L.; Gonçalves, J.L.; Neeff, D.V.; Silva, M.P.; Cruz, A.G.; Oliveira, C.A. Biofilm-producing ability of Staphylococcus aureus isolates from Brazilian dairy farms. J. Dairy Sci. 2014, 97, 1812–1816. [Google Scholar] [CrossRef] [PubMed]
- Castilho, I.G.; Dantas, S.T.A.; Langoni, H.; Araújo, J.P., Jr.; Fernandes, A., Jr.; Alvarenga, F.C.L.; Maia, L.; Cagnini, D.Q.; Rall, V.L.M. Host-pathogen interactions in bovine mammary epithelial cells and HeLa cells by Staphylococcus aureus isolated from subclinical bovine mastitis. J. Dairy Sci. 2017, 100, 6414–6421. [Google Scholar] [CrossRef] [PubMed]
- Goetz, C.; Tremblay, Y.D.N.; Lamarche, D.; Blondeau, A.; Gaudreau, A.M.; Labrie, J.; Malouin, F.; Jacques, M. Coagulase-negative staphylococci species affect biofilm formation of other coagulase-negative and coagulase-positive staphylococci. J. Dairy Sci. 2017, 8, 6454–6464. [Google Scholar] [CrossRef] [PubMed]
- Chagnot, C.; Caccia, N.; Loukiadis, E.; Ganet, S.; Durand, A.; Bertin, Y.; Talon, R.; Astruc, T.; Desvaux, M. Colonization of the meat extracellular matrix proteins by O157 and non-O157 enterohemorrhagic Escherichia coli. Int. J. Food Microbiol. 2014, 188, 92–98. [Google Scholar] [CrossRef]
- Marouani-Gadri, N.; Firmesse, O.; Chassaing, D.; Sandris-Nielsen, D.; Arneborg, N.; Carpentier, B. Potential of Escherichia coli O157:H7 to persist and form viable but non-culturable cells on a food-contact surface subjected to cycles of soiling and chemical treatment. Int. J. Food Microbiol. 2010, 144, 96–103. [Google Scholar] [CrossRef]
- Cherif-Antar, A.; Moussa–Boudjemâa, B.; Didouh, N.; Medjahdi, K.; Mayo, B.; Flórez, A.B. Diversity and biofilm-forming capability of bacteria recovered from stainless steel pipes of a milk-processing dairy plant. Dairy Sci. Technol. 2016, 96, 27–38. [Google Scholar] [CrossRef]
- Wang, R.; Neoh, K.G.; Shi, Z.; Kang, E.T.; Tambyah, P.A.; Chiong, E. Inhibition of Escherichia coli and Proteus mirabilis adhesion and biofilm formation on medical grade silicone surface. Biotechnol. Bioeng. 2012, 109, 336–345. [Google Scholar] [CrossRef] [PubMed]
- Stickler, D.; Hewett, P. Activity of antiseptics against Escherichia coli growing as biofilms on silicone surfaces. Eur. J. Clin. Microbiol. Infect. Dis. 1989, 8, 974–978. [Google Scholar] [CrossRef] [PubMed]
- Fernández, L.A.; Berenguer, J. Secretion and assembly of regular surface structures in Gram-negative bacteria. FEMS Microbiol. Rev. 2000, 24, 21–44. [Google Scholar] [CrossRef] [PubMed]
- Pratt, L.A.; Kolter, R. Genetic analysis of Escherichia coli biofilm formation: Roles of flagella, motility, chemotaxis and type I pili. Mol. Microbiol. 1998, 30, 285–293. [Google Scholar] [CrossRef] [PubMed]
- Rendón, M.A.; Saldaña, Z.; Erdem, A.L.; Monteiro-Neto, V.; Vázquez, A.; Kaper, J.B.; Puente, J.l.; Girón, J.A. Commensal and pathogenic Escherichia coli use a common pilus adherence factor for epithelial cell colonization. Proc. Natl. Acad. Sci. USA 2007, 104, 10637–10642. [Google Scholar] [CrossRef] [PubMed]
- Pouttu, R.; Westerlund-Wikström, B.; Lång, H.; Alsti, K.; Virkola, R.; Saarela, U.; Siitonen, A.; Kalkkinen, N.; Korhonen, T.K. MatB, a common fimbrillin gene of Escherichia coli, expressed in a genetically conserved, virulent clonal group. J. Bact. Res. 2001, 183, 4727–4736. [Google Scholar] [CrossRef] [PubMed]
- Brombacher, E.; Dorel, C.; Zehnder, A.J.B.; Landini, P. The curli biosynthesis regulator CsgD coordinates the expression of both positive and negative determinants for biofilm formation in Escherichia coli. Microbiology 2003, 149, 2847–2857. [Google Scholar] [CrossRef] [PubMed]
- Ryu, J.H.; Beuchat, L.R. Biofilm formation by Escherichia coli O157:H7 on stainless steel: Effect of exopolysaccharide and Curli production on its resistance to chlorine. Appl. Environm. Microbiol. 2005, 71, 247–254. [Google Scholar] [CrossRef]
- Farfan, M.J.; Torres, A.G. Molecular mechanisms that mediate colonization of Shiga toxin-producing Escherichia coli strains. Infect. Immun. 2012, 80, 903–913. [Google Scholar] [CrossRef]
- Sharma, M.; Anand, S.K.; Prasad, D.N. In vitro propagation of mixed species biofilms using online consortia for dairy processing lines. Milchwissenschaft 2003, 58, 270–274. [Google Scholar]
- Sinde, E.; Carballo, J. Attachment of Salmonella spp. and Listeria monocytogenes to stainless steel, rubber and polytetrafluoroethylene: The influence of free energy and the effect of commercial sanitizers. Food Microbiol. 2000, 17, 439–447. [Google Scholar] [CrossRef]
- Kaper, J.B.; Nataro, J.P.; Mobley, H.L. Pathogenic Escherichia coli. Nat. Rev. Microbiol. 2004, 2, 123–140. [Google Scholar] [CrossRef] [PubMed]
- Badouei, M.A.; Morabito, S.; Najafifar, A.; Mazandarani, E. Molecular characterization of enterohemorrhagic Escherichia coli hemolysin gene (EHEC-hlyA)-harboring isolates from cattle reveals a diverse origin and hybrid diarrheagenic strains. Infect. Genet. Evol. 2016, 39, 342–348. [Google Scholar] [CrossRef]
- Bielaszewska, M.; Mellmann, A.; Zhang, W.; Köck, R.; Fruth, A.; Bauwens, A.; Peters, G.; Karch, H. Characterisation of the Escherichia coli strain associated with an outbreak of haemolytic uraemic syndrome in Germany, 2011: A microbiological study. Lancet Infect. Dis. 2011, 11, 671–676. [Google Scholar] [CrossRef] [PubMed]
- Garénaux, A.; Caza, M.; Dozois, C.M. The Ins and Outs of siderophore mediated iron uptake by extra-intestinal pathogenic Escherichia coli. Vet. Microbiol. 2011, 153, 89–98. [Google Scholar] [CrossRef] [PubMed]
- Johnson, J.R. Microbial virulence determinants and the pathogenesis of urinary tract infection. Infect. Dis. Clin. North Am. 2003, 17, 261–278. [Google Scholar] [CrossRef] [PubMed]
- Johnson, J.R. Virulence factors in Escherichia coli urinary tract infection. Clin. Microbiol. Rev. 1991, 4, 80–128. [Google Scholar] [CrossRef] [PubMed]
- Stathopoulos, C. Structural features, physiological roles, and biotechnological applications of the membrane proteases of the OmpT bacterial endopeptidase family: A micro-review. Membr. Cell Biol. 1998, 12, 1–8. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).