Antimicrobial Activity of Selected Essential Oils against Staphylococcus aureus from Bovine Mastitis
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation of Bacterial Strains
2.2. Essential Oils
2.3. GC/MSD Analysis of EOs
2.4. Determination of Minimum Inhibitory Concentrations (MIC)
2.5. Determination of the Minimum Bactericidal Concentration (MBC)
2.6. Statistical Analysis
3. Results
3.1. Chemical Composition of the Essential Oils
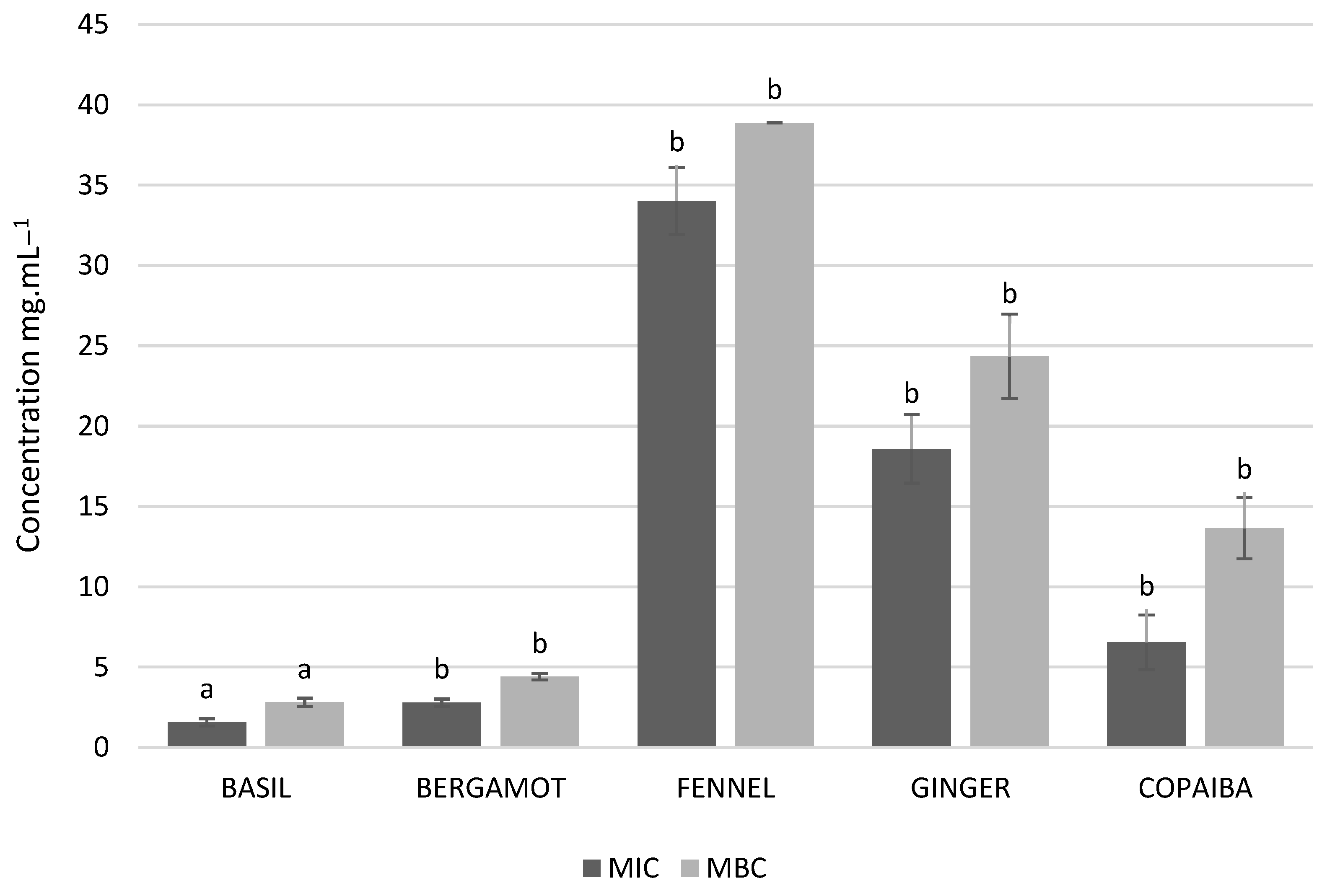
3.2. Antimicrobial Activity of EOs against S. aureus
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| EO | Essential oil |
| MIC | Minimum inhibitory concentration |
| MBC | Minimum bactericidal concentration |
| GC/MSD | Gas chromatography coupled with a mass-selective detector |
| CFU/mL | Colony-forming units per milliliter |
| SM | Subclinical mastitis |
| SCC | Somatic cell count |
| BHI | Brain–heart infusion broth |
| SD | Standard deviation |
| ANOVA | Analysis of variance |
References
- Zaatout, N.; Ayachi, A.; Kecha, M. Staphylococcus Aureus Persistence Properties Associated with Bovine Mastitis and Alternative Therapeutic Modalities. J. Appl. Microbiol. 2020, 129, 1102–1119. [Google Scholar] [CrossRef] [PubMed]
- Bhunia, A.K. Staphylococcus Aureus. In Foodborne Microbial Pathogens; Springer: New York, NY, USA, 2018; pp. 181–192. ISBN 978-0-387-74536-7. [Google Scholar]
- da Silva, A.C.; Rodrigues, M.X.; Silva, N.C.C. Methicillin-Resistant Staphylococcus Aureus in Food and the Prevalence in Brazil: A Review. Braz. J. Microbiol. 2020, 51, 347–356. [Google Scholar] [CrossRef] [PubMed]
- Jenul, C.; Horswill, A.R. Regulation of Staphylococcus Aureus Virulence. Microbiol. Spectr. 2019, 7. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.; Liu, D.; Singh, A.K.; Drolia, R.; Bai, X.; Tenguria, S.; Bhunia, A.K. Tunicamycin Mediated Inhibition of Wall Teichoic Acid Affects Staphylococcus Aureus and Listeria Monocytogenes Cell Morphology, Biofilm Formation and Virulence. Front. Microbiol. 2018, 9, 1352. [Google Scholar] [CrossRef] [PubMed]
- Sharun, K.; Dhama, K.; Tiwari, R.; Gugjoo, M.B.; Iqbal Yatoo, M.; Patel, S.K.; Pathak, M.; Karthik, K.; Khurana, S.K.; Singh, R.; et al. Advances in Therapeutic and Managemental Approaches of Bovine Mastitis: A Comprehensive Review. Vet. Q. 2021, 41, 107–136. [Google Scholar] [CrossRef] [PubMed]
- Gonçalves, J.L.; Kamphuis, C.; Vernooij, H.; Araújo, J.P.; Grenfell, R.C.; Juliano, L.; Anderson, K.L.; Hogeveen, H.; Dos Santos, M.V. Pathogen Effects on Milk Yield and Composition in Chronic Subclinical Mastitis in Dairy Cows. Vet. J. 2020, 262, 105473. [Google Scholar] [CrossRef] [PubMed]
- Tomanić, D.; Samardžija, M.; Kovačević, Z. Alternatives to Antimicrobial Treatment in Bovine Mastitis Therapy: A Review. Antibiotics 2023, 12, 683. [Google Scholar] [CrossRef]
- Gonçalves, J.L.; Kamphuis, C.; Martins, C.M.M.R.; Barreiro, J.R.; Tomazi, T.; Gameiro, A.H.; Hogeveen, H.; dos Santos, M.V. Bovine Subclinical Mastitis Reduces Milk Yield and Economic Return. Livest. Sci. 2018, 210, 25–32. [Google Scholar] [CrossRef]
- Ruegg, P.L. A 100-Year Review: Mastitis Detection, Management, and Prevention. J. Dairy Sci. 2017, 100, 10381–10397. [Google Scholar] [CrossRef]
- da Silva Abreu, A.C.; Matos, L.G.; da Silva Cândido, T.J.; Barboza, G.R.; de Souza, V.V.M.A.; Munive Nuñez, K.V.; Cirone Silva, N.C. Antimicrobial Resistance of Staphylococcus Spp. Isolated from Organic and Conventional Minas Frescal Cheese Producers in São Paulo, Brazil. J. Dairy Sci. 2021, 104, 4012–4022. [Google Scholar] [CrossRef]
- Cheng, W.N.; Han, S.G. Bovine Mastitis: Risk Factors, Therapeutic Strategies, and Alternative Treatments—A Review. Asian-Australas. J. Anim. Sci. 2020, 33, 1699. [Google Scholar] [CrossRef] [PubMed]
- Ni, Z.J.; Wang, X.; Shen, Y.; Thakur, K.; Han, J.; Zhang, J.G.; Hu, F.; Zhao, J.W. Recent Updates on the Chemistry, Bioactivities, Mode of Action, and Industrial Applications of Plant Essential Oils. Trends Food Sci. Technol. 2021, 110, 78–89. [Google Scholar] [CrossRef]
- Tavares, L.; Zapata Noreña, C.P.; Barros, H.L.; Smaoui, S.; Lima, P.S.; Marques de Oliveira, M. Rheological and Structural Trends on Encapsulation of Bioactive Compounds of Essential Oils: A Global Systematic Review of Recent Research. Food Hydrocoll. 2022, 129, 107628. [Google Scholar] [CrossRef]
- Man, A.; Santacroce, L.; Jacob, R.; Mare, A.; Man, L. Antimicrobial Activity of Six Essential Oils against a Group of Human Pathogens: A Comparative Study. Pathogens 2019, 8, 15. [Google Scholar] [CrossRef] [PubMed]
- Álvarez-Martínez, F.J.; Barrajón-Catalán, E.; Herranz-López, M.; Micol, V. Antibacterial Plant Compounds, Extracts and Essential Oils: An Updated Review on Their Effects and Putative Mechanisms of Action. Phytomedicine 2021, 90, 153626. [Google Scholar] [CrossRef] [PubMed]
- Caneschi, A.; Bardhi, A.; Barbarossa, A.; Zaghini, A. Plant Essential Oils as a Tool in the Control of Bovine Mastitis: An Update. Molecules 2023, 28, 3425. [Google Scholar] [CrossRef] [PubMed]
- Lopes, T.S.; Fontoura, P.S.; Oliveira, A.; Rizzo, F.A.; Silveira, S.; Streck, A.F. Use of Plant Extracts and Essential Oils in the Control of Bovine Mastitis. Res. Vet. Sci. 2020, 131, 186–193. [Google Scholar] [CrossRef]
- National Institute of Standards and Technology. NIST Chemistry WebBook SRD 69. Available online: https://webbook.nist.gov/ (accessed on 10 October 2021).
- Adams, R.P. Identification of Essential Oil Components by Gas Chromatography/Mass Spectrometry, 4th ed.; Allured Publ.: Carol Stream, IL, USA, 2007; ISBN 978-1932633214. [Google Scholar]
- Da Silva, A.C.; Iacuzio, R.; Da Silva Cândido, T.J.; Xavier Rodrigues, M.; Cirone Silva, N.C. Resistência Antimicrobiana De Salmonella Spp., Staphylococcus Aureus E Escherichia Coli Isolados De Carcaças De Frangos: Resistência A Antibióticos E Óleos Essenciais. Rev. Bras. Agropecuária Sustentável 2018, 8, 95–103. [Google Scholar] [CrossRef]
- de Souza, V.V.M.A.; Crippa, B.L.; de Almeida, J.M.; Iacuzio, R.; Setzer, W.N.; Sharifi-Rad, J.; Silva, N.C.C. Synergistic Antimicrobial Action and Effect of Active Chitosan-Gelatin Biopolymeric Films Containing Thymus Vulgaris, Ocimum Basilicum and Origanum Majorana Essential Oils against Escherichia Coli and Staphylococcus Aureus. Cell. Mol. Biol. 2020, 66, 214–223. [Google Scholar] [CrossRef]
- Knezevic, P.; Aleksic, V.; Simin, N.; Svircev, E.; Petrovic, A.; Mimica-Dukic, N. Antimicrobial Activity of Eucalyptus Camaldulensis Essential Oils and Their Interactions with Conventional Antimicrobial Agents against Multi-Drug Resistant Acinetobacter Baumannii. J. Ethnopharmacol. 2016, 178, 125–136. [Google Scholar] [CrossRef]
- Xing, C.; Qin, C.; Li, X.; Zhang, F.; Linhardt, R.J.; Sun, P.; Zhang, A. Chemical Composition and Biological Activities of Essential Oil Isolated by HS-SPME and UAHD from Fruits of Bergamot. LWT 2019, 104, 38–44. [Google Scholar] [CrossRef]
- Campos, B.; Pickering, A.C.; Rocha, L.S.; Aguilar, A.P.; Fabres-Klein, M.H.; de Oliveira Mendes, T.A.; Fitzgerald, J.R.; Ribon, A. Diversity and Pathogenesis of Staphylococcus Aureus from Bovine Mastitis: Current Understanding and Future Perspectives. BMC Vet. Res. 2022, 18, 115. [Google Scholar] [CrossRef] [PubMed]
- Silva, V.; Almeida, L.; Gaio, V.; Cerca, N.; Manageiro, V.; Caniça, M.; Capelo, J.L.; Igrejas, G.; Poeta, P. Biofilm Formation of Multidrug-Resistant Mrsa Strains Isolated from Different Types of Human Infections. Pathogens 2021, 10, 970. [Google Scholar] [CrossRef] [PubMed]
- Munive Nuñez, K.V.; da Silva Abreu, A.C.; Gonçalves, J.L.; dos Santos, M.V.; de Oliveira Rocha, L.; Cirone Silva, N.C. Virulence and Antimicrobial Resistance Genes Profiles of Spa Type T605 Methicillin-Susceptible Staphylococcus Aureus Isolated from Subclinical Bovine Mastitis. J. Appl. Microbiol. 2023, 134, lxad057. [Google Scholar]
- Sakkas, H.; Papadopoulou, C. Antimicrobial Activity of Basil, Oregano, and Thyme Essential Oils. J. Microbiol. Biotechnol. 2017, 27, 429–438. [Google Scholar] [CrossRef] [PubMed]
- Falowo, A.B.; Mukumbo, F.E.; Idamokoro, E.M.; Afolayan, A.J.; Muchenje, V. Phytochemical Constituents and Antioxidant Activity of Sweet Basil (Ocimum Basilicum L.) Essential Oil on Ground Beef from Boran and Nguni Cattle. Int. J. Food Sci. 2019, 2019, 2628747. [Google Scholar] [CrossRef]
- Masyita, A.; Mustika Sari, R.; Dwi Astuti, A.; Yasir, B.; Rahma Rumata, N.; Emran, T.B.; Nainu, F.; Simal-Gandara, J. Terpenes and Terpenoids as Main Bioactive Compounds of Essential Oils, Their Roles in Human Health and Potential Application as Natural Food Preservatives. Food Chem. X 2022, 13, 100217. [Google Scholar] [CrossRef] [PubMed]
- Lv, F.; Liang, H.; Yuan, Q.; Li, C. In Vitro Antimicrobial Effects and Mechanism of Action of Selected Plant Essential Oil Combinations against Four Food-Related Microorganisms. Food Res. Int. 2011, 44, 3057–3064. [Google Scholar] [CrossRef]
- Bagheri, L.; Khodaei, N.; Salmieri, S.; Karboune, S.; Lacroix, M. Correlation between Chemical Composition and Antimicrobial Properties of Essential Oils against Most Common Food Pathogens and Spoilers: In-Vitro Efficacy and Predictive Modelling. Microb. Pathog. 2020, 147, 104212. [Google Scholar] [CrossRef]
- de Oliveira, D.F.F.; Nascimento, T.P.; Rodrigues, C.H.; Batista, J.M.S.; Liu, T.P.S.L.; De Medeiros, E.S.; Mota, R.A.; Costa, R.M.P.B.; Porto, T.S.; Porto, C.S.; et al. Antimicrobial Potential of Copaiba Oil (Copaifera Multijuga Hayne-Leguminosae) against Bubaline Mastitis Multiresistant Isolates. An. Acad. Bras. Cienc. 2020, 92, e20200521. [Google Scholar] [CrossRef]
- de Faria, M.J.M.; Braga, C.A.d.S.B.; de Paula, J.R.; André, M.C.D.P.B.; Vaz, B.G.; de Carvalho, T.C.; Romão, W.; Costa, H.B.; Conceição, E.C. Antimicrobial Activity of Copaifera Spp. Against Bacteria Isolated from Milk of Cows with Mastitis. Cienc. Anim. Bras. 2017, 18, e39068. [Google Scholar] [CrossRef]
- Wang, X.; Shen, Y.; Thakur, K.; Han, J.; Zhang, J.G.; Hu, F.; Wei, Z.J. Antibacterial Activity and Mechanism of Ginger Essential Oil against Escherichia Coli and Staphylococcus Aureus. Molecules 2020, 25, 3955. [Google Scholar] [CrossRef] [PubMed]
- Dal Pozzo, M.; Santurio, D.F.; Rossatto, L.; Vargas, A.C.; Alves, S.H.; Loreto, E.S.; Viegas, J. Activity of Essential Oils from Spices against Staphylococcus Spp. Isolated from Bovine Mastitis. Arq. Bras. Med. Veterinária e Zootec. 2011, 63, 1229–1232. [Google Scholar] [CrossRef]
- Ghasemian, A.; Al-Marzoqi, A.H.; Mostafavi, S.K.S.; Alghanimi, Y.K.; Teimouri, M. Chemical Composition and Antimicrobial and Cytotoxic Activities of Foeniculum Vulgare Mill Essential Oils. J. Gastrointest. Cancer 2020, 51, 260–266. [Google Scholar] [CrossRef]
- Moreira Gonçalves, S.; Gomes Motta, J.F.; Ribeiro-Santos, R.; Hidalgo Chávez, D.W.; Ramos de Melo, N. Functional and Antimicrobial Properties of Cellulose Acetate Films Incorporated with Sweet Fennel Essential Oil and Plasticizers. Curr. Res. Food Sci. 2020, 3, 1–8. [Google Scholar] [CrossRef]
- Kwiatkowski, P.; Pruss, A.; Masiuk, H.; Mnichowska-Polanowska, M.; Kaczmarek, M.; Giedrys-Kalemba, S.; Dołęgowska, B.; Zielińska-Bliźniewska, H.; Olszewski, J.; Sienkiewicz, M. The Effect of Fennel Essential Oil and Trans-Anethole on Antibacterial Activity of Mupirocin against Staphylococcus Aureus Isolated from Asymptomatic Carriers. Postep. Dermatologii Alergol. 2019, 36, 308–314. [Google Scholar] [CrossRef]
- Rani, B.; Naz, S.; Saeed, S.; Manan, A.; Chatha, M. Antibacterial Effects of Common Spices against Staphylococcus Aureus under Laboratory Conditions. Biosci. Rev. 2021, 3, 6–16. [Google Scholar] [CrossRef]
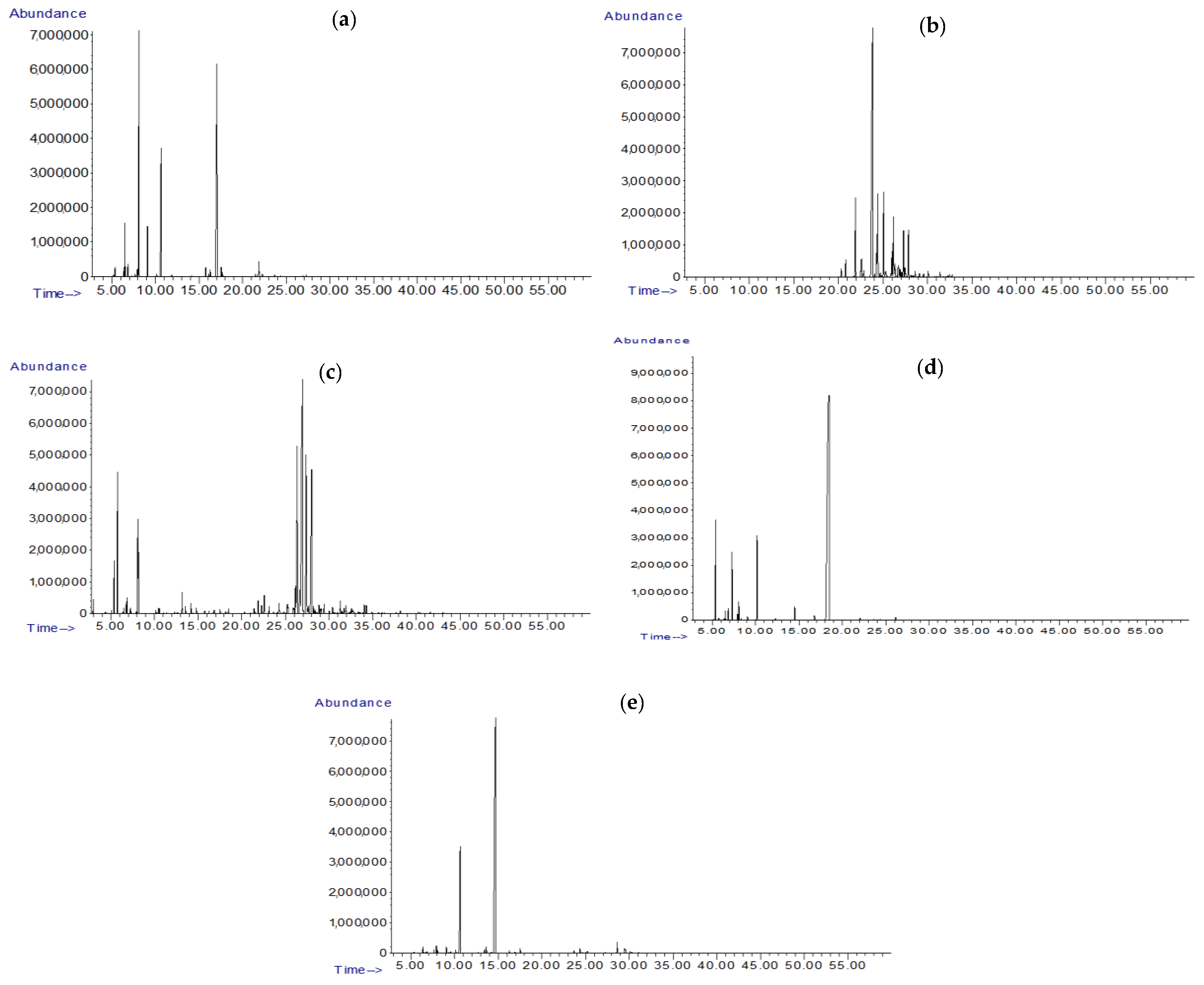
| Botanical Origins | Plant Family | Common Names of EOs | Geographical Origin |
|---|---|---|---|
| Citrus aurantium bergamia | Rutaceae | Bergamot | Brazil |
| Copaifera reticulata | Fabaceae | Copaiba | Brazil |
| Foeniculum vulgare | Apiaceae | Fennel | Brazil |
| Zingiber officinale | Zingiberaceae | Ginger | Brazil |
| Ocimum basilicum | Lamiaceae | Basil | Brazil |
| Essential Oil | RI | RI (Lit.) | Compound | % Rel. |
|---|---|---|---|---|
| Bergamot | 932 | 932 | α-pinene | 0.56 |
| 971 | 969 | sabinene | 0.59 | |
| 975 | 974 | β-pinene | 3.49 | |
| 989 | 988 | β-myrcene | 0.77 | |
| 1023 | 1020 | p-cymene | 0.69 | |
| 1029 | 1029 | Limonene | 29.88 | |
| 1057 | 1054 | γ-terpinene | 3.83 | |
| 1102 | 1095 | Linalool | 16.91 | |
| 1227 | 1227 | nerol (cis-geraniol) | 1.06 | |
| 1239 | 1238 | neral (cis-citral) | 0.69 | |
| 1258 | 1257 | Linalyl acetate | 39.18 | |
| 1269 | 1267 | geranial (trans-citral) | 0.86 | |
| 1372 | 1423 | linalyl butyrate | 1.48 | |
| Copaiba | 1335 | 1335 | δ-elemen | 0.67 |
| 1347 | 1495 | α-cubebene | 1.36 | |
| 1374 | 1374 | α-copaene | 6.81 | |
| 1388 | 1387 | β-cubebene | 0.44 | |
| 1390 | 1389 | β-elemene | 1.75 | |
| 1396 | 1398 | cyperene | 0.54 | |
| 1421 | 1419 | trans-caryophyllene | 47.43 | |
| 1432 | 1434 | γ-elemene | 2.04 | |
| 1435 | 1432 | α-trans-bergamotene | 7.59 | |
| 1452 | 1452 | α-humulene | 7.95 | |
| 1456 | 1454 | trans-β-farnesene | 0.36 | |
| 1458 | 1458 | allo-aromadendrene | 0.43 | |
| 1475 | 1478 | γ-muurolene | 2.43 | |
| 1479 | 1484 | germacrene D | 5.69 | |
| 1483 | - | M = 204 | 1.59 | |
| 1492 | 1493 | epi-cubebol | 1.04 | |
| 1493 | 1500 | bicyclogermacrene | 0.75 | |
| 1497 | 1500 | α-muurolene | 0.66 | |
| 1507 | 1505 | β-bisabolene | 4.11 | |
| 1511 | 1513 | γ-cadinene | 0.6 | |
| 1521 | 1522 | δ-cadinene | 4.11 | |
| 1541 | - | M = 204 | 0.59 | |
| 1579 | 1582 | caryophyllene oxide | 0.59 | |
| 1614 | 1618 | junenol | 0.46 | |
| Fennel | 932 | 932 | α-pinene | 4.56 |
| 975 | 974 | β-pinene | 0.55 | |
| 989 | 988 | β-myrcene | 0.59 | |
| 1004 | 1002 | α-phellandrene | 3.55 | |
| 1023 | 1020 | p-cymene | 0.34 | |
| 1027 | 1029 | limonene | 1.2 | |
| 1056 | 1054 | γ-terpinene | 0.21 | |
| 1088 | 1086 | fenchone | 5.84 | |
| 1197 | 1195 | p-allylanisole (estragole) | 0.97 | |
| 1251 | 1249 | cis-anethole | 0.63 | |
| 1291 | 1282 | trans-anethole | 81.36 | |
| 1478 | 1484 | germacrene D | 0.21 | |
| Ginger | 800 | 801 | hexanal | 0.28 |
| 932 | 932 | α-pinene | 1.66 | |
| 947 | 946 | camphene | 5.01 | |
| 984 | 981 | 6-methyl-5-hepten-2-one | 0.31 | |
| 989 | 988 | β-myrcene | 0.57 | |
| 1028 | 1029 | limonene | 4.88 | |
| 1030 | 1026 | 1,8-cineole (eucalyptol) | 2.27 | |
| 1164 | 1165 | endo-borneol | 1.13 | |
| 1174 | - | M = 166 | 0.24 | |
| 1189 | 1186 | α-terpineol | 0.54 | |
| 1373 | 1374 | α-copaene | 0.74 | |
| 1382 | 1379 | geranyl-acetate | 0.42 | |
| 1390 | 1389 | β-elemene | 1.17 | |
| 1404 | 1405 | sesquitujene | 0.37 | |
| 1431 | 1434 | γ-elemen | 0.45 | |
| 1455 | 1454 | trans-β-farnesene | 0.42 | |
| 1478 | 1478 | γ-muurolene | 1.75 | |
| 1483 | 1479 | ar-curcumene | 14.03 | |
| 1490 | 1496 | valenceno | 1.58 | |
| 1498 | 1493 | α-zingiberene | 32.76 | |
| 1509 | 1505 | β-bisabolene | 11.72 | |
| 1516 | 1520 | 7-epi-α-selenene | 0.62 | |
| 1525 | 1521 | β-sesquiphellandrene | 12.95 | |
| 1531 | 1529 | trans-γ-bisabolene | 0.39 | |
| 1547 | 1548 | elemol | 0.51 | |
| 1562 | 1561 | trans-nerolidol | 0.56 | |
| 1587 | - | M = 222 | 0.34 | |
| 1611 | - | M = 222 | 0.77 | |
| 1628 | - | M = 222 | 0.53 | |
| 1685 | - | M = 222 | 0.56 | |
| 1692 | - | M = 220 | 0.48 | |
| Basil | 971 | 969 | sabinene | 0.57 |
| 1015 | 1014 | α-terpinene | 0.32 | |
| 1023 | 1020 | p-cymene | 0.71 | |
| 1056 | 1054 | γ-terpinene | 0.69 | |
| 1087 | - | n.i. | 0.37 | |
| 1101 | 1095 | linalool | 18.76 | |
| 1171 | 1167 | menthol | 0.61 | |
| 1176 | 1174 | terpin-4-ol | 0.82 | |
| 1202 | 1195 | p-allylanisole (estragole) | 72.86 | |
| 1269 | 1264 | trans-citral (geranial) | 0.64 | |
| 1433 | 1432 | α-trans-bergamotene | 0.7 | |
| 1541 | 1452 | α-humulene | 1.55 | |
| 1563 | 1562 | trans-methoxycinnamaldehyde | 0.76 | |
| 1565 | - | M = 164 | 0.64 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Munive Nuñez, K.V.; Abreu, A.C.d.S.; Almeida, J.M.d.; Gonçalves, J.L.; Bonsaglia, É.C.R.; dos Santos, M.V.; Silva, N.C.C. Antimicrobial Activity of Selected Essential Oils against Staphylococcus aureus from Bovine Mastitis. Dairy 2024, 5, 54-65. https://doi.org/10.3390/dairy5010005
Munive Nuñez KV, Abreu ACdS, Almeida JMd, Gonçalves JL, Bonsaglia ÉCR, dos Santos MV, Silva NCC. Antimicrobial Activity of Selected Essential Oils against Staphylococcus aureus from Bovine Mastitis. Dairy. 2024; 5(1):54-65. https://doi.org/10.3390/dairy5010005
Chicago/Turabian StyleMunive Nuñez, Karen Vanessa, Anderson Clayton da Silva Abreu, Jaqueline Milagres de Almeida, Juliano Leonel Gonçalves, Érika Carolina Romão Bonsaglia, Marcos Veiga dos Santos, and Nathália Cristina Cirone Silva. 2024. "Antimicrobial Activity of Selected Essential Oils against Staphylococcus aureus from Bovine Mastitis" Dairy 5, no. 1: 54-65. https://doi.org/10.3390/dairy5010005
APA StyleMunive Nuñez, K. V., Abreu, A. C. d. S., Almeida, J. M. d., Gonçalves, J. L., Bonsaglia, É. C. R., dos Santos, M. V., & Silva, N. C. C. (2024). Antimicrobial Activity of Selected Essential Oils against Staphylococcus aureus from Bovine Mastitis. Dairy, 5(1), 54-65. https://doi.org/10.3390/dairy5010005







