Abstract
Traditional organic antimicrobials mainly act on specific biochemical processes such as replication, transcription and translation. However, the emergence and wide spread of microbial resistance is a growing threat for human beings. Therefore, it is highly necessary to design strategies for the development of new drugs in order to target multiple cellular processes that should improve their efficiency against several microorganisms, including bacteria, viruses or fungi. The present review is focused on recent advances and findings of new antimicrobial strategies based on metal complexes. Recent studies indicate that some metal ions cause different types of damages to microbial cells as a result of membrane degradation, protein dysfunction and oxidative stress. These unique modes of action, combined with the wide range of three-dimensional geometries that metal complexes can adopt, make them suitable for the development of new antimicrobial drugs.
Keywords:
metal complexes; new antimicrobial strategies; metallo-drugs; silver; copper; zinc; iron; ruthenium; gallium; bismuth; vanadium; synergic effects 1. Introduction
The search for new active antimicrobial compounds is of growing interest since the current clinical pipeline remains insufficient to tackle the challenge of increasing emergence and spread of antimicrobial resistance. In the United States, each year more than 2.8 million people suffer from an antibiotic-resistant infection, resulting in more than 35,000 deaths [1]. In Europe, antibiotic resistance is responsible for an estimated 33,000 deaths annually [2]. According to the World Health Organization (WHO), the newly approved products have limited clinical benefits over existing treatments and almost 75% of the antimicrobials under clinical development are simply derivatives of already known and used molecules existing on the market, and for which multiple resistance mechanisms are well established [3]. Therefore, there is an urgent need for the development of new antimicrobial agents.
Since the discovery of antibiotics by Alexander Fleming in the 1920s, most of the current compounds developed by medicinal chemists around the world are almost exclusively purely organic. Although metals and their complexes have been employed since ancient times, they were generally used for their applications as catalysts or materials, and their properties were often associated with toxicity. However, the use of structurally defined metal complexes in medicine mostly appeared at the beginning of the 20th century with the discovery of the arsenic-containing organometallic complex as the first effective treatment of syphilis (Salvarsan) [4]. Since then, many other metal complexes have been found to be useful in medicinal chemistry, like the development of a famous mercuric-based antiseptic agent (Mercurochrome) or the treatment of rheumatoid arthritis by a gold complex agent (Auranofin) [5]. Nevertheless, the most relevant examples in the field of medicinal chemistry are undoubtedly the platinum-based anticancer drugs cisplatin, oxaliplatin and carboplatin [6]. These complexes are still currently used in nearly 50% of all cancer treatments as chemotherapeutic agents, often in combination with other drugs. Over the past two decades, several metal-based complexes (based on silver, copper, iron gold, bismuth, gallium, etc.) have been designed and have reached human clinical trials for the treatment of cancer, malaria and neurodegenerative diseases [7,8,9].
Despite their high potential in such diseases, little attention has been paid to their application as antimicrobial compounds. The vast diversity of metals, types of ligands, and geometries makes metal-based coordination complexes very useful in accessing a highly underexplored chemical space for drug development, and especially for the design of new antimicrobials [10]. Unlike the majority of organic molecules, which possess only one- or two- dimensional topologies, metal complexes can adopt three-dimensional structures available through metal coordination chemistry, the latter offering the possibility to create a wide variety of antimicrobials. Furthermore, metal-based complexes may provide unique modes of action: exchange or release of ligands, redox activation and catalytic generation of toxic species (reactive oxygen species, ROS), as well as depletion of essential substrates [11,12], making them able to abolish enzyme activities, disrupt membrane function or damage DNA.
2. Metal Complex-Based Antimicrobial Compounds
Metal ions or metal ion binding components play important roles in biological processes, and their rational design could be used to develop new therapeutic drugs or diagnostic probes. The fact that metal atoms easily lose electrons and form positively charged ions makes them soluble in biological fluids. Due to their electron deficiency, they can readily interact with electron-rich biomolecules such as DNA or proteins, and therefore participate either in a catalytic mechanism or in the stabilization/determination of their tertiary or quaternary structures.
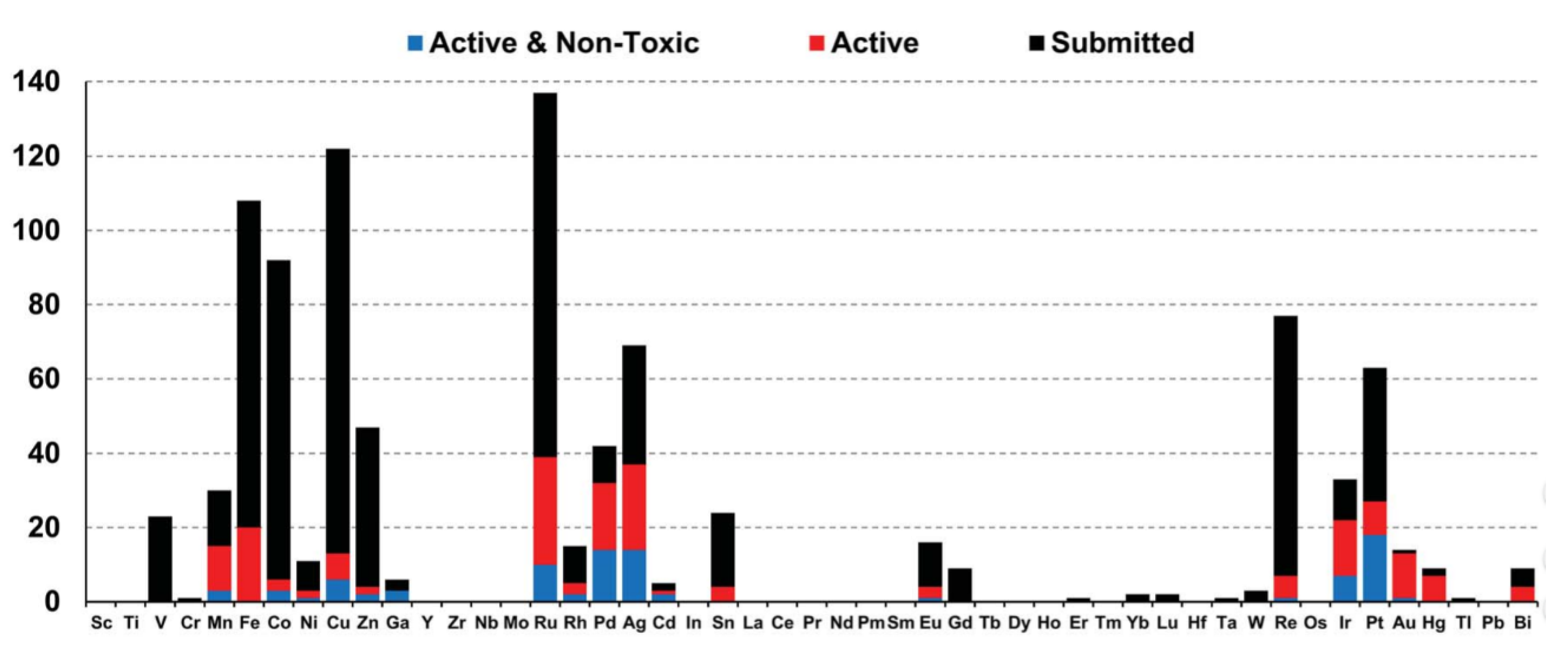
Depending on the type of metal ion coordination complexes and organometallics, they offer a wide range of oxidation states, coordination numbers and geometries, leading to a virtually unlimited number of structures and conformations. With the improvement of knowledge and understanding of biological processes, judicious metal–ligand combinations can be designed with appropriate geometry for specific interactions. For example, some of them have already been used to inhibit enzymes, label proteins, image cells, probe biomacromolecules, alter bioavailability or provide contrast as MRI agents [13]. In addition, the broad range of metal–ligand combinations makes it possible to design new entities with various physical properties and chemical reactivities including charge, solubility, rates of ligand exchange, strengths of metal–ligand bonds, Lewis acidity, metal- and ligand-based redox potentials, outer-sphere interactions, and ligand conformations [14]. Therefore, compared to organic drugs, the structural and electronic properties of such complexes offer biological and chemical diversity, making them very attractive in the field of medicinal chemistry, especially as antimicrobial agents with novel modes of action to treat drug-resistant diseases. From this point of view, the transition metals accompanied by some other metals are the most promising for disease treatments, while the heavy lanthanides are more investigated for their radioactive and photoluminescent properties. This is illustrated by the growing amount of this kind of organometallic species deposited on open databases specialized in bioactive compounds. Hence, a study of Blaskovich and co-workers, who investigated the compounds submitted on the Community for Open Antimicrobial Drug Discovery, classified the organometallic compounds according to the nature of their metal element and their activity and toxicity [15]. These graphs (Figure 1) therefore reflect the most interesting ions for future work.
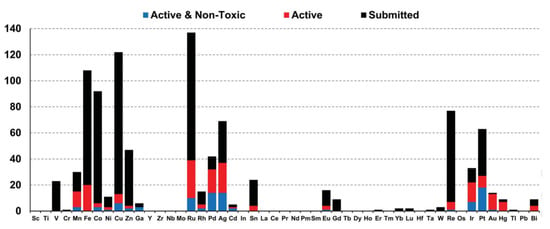
Figure 1.
Distribution of organometallic compounds submitted to Co-ADD in function of their metal [15].
In this review, we will discuss the major discoveries in the non-traditional field of metal complex-based antibiotic compounds, focusing on the last decade and the most promising elements. In particular, we will focus on silver, copper, zinc, iron, ruthenium, gallium, bismuth and vanadium.
2.1. Silver
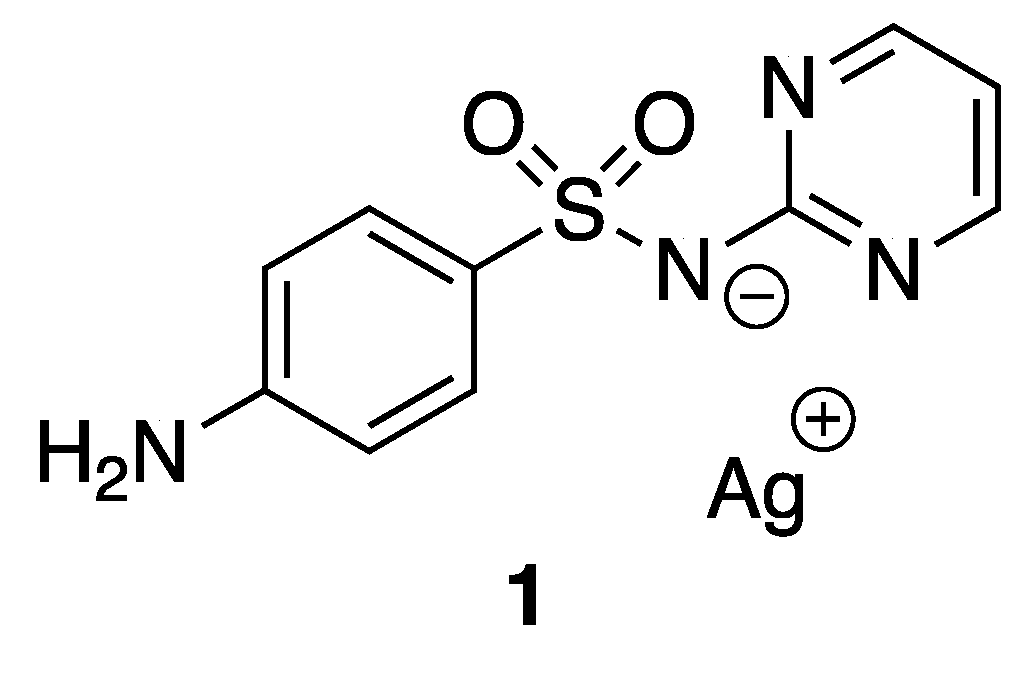
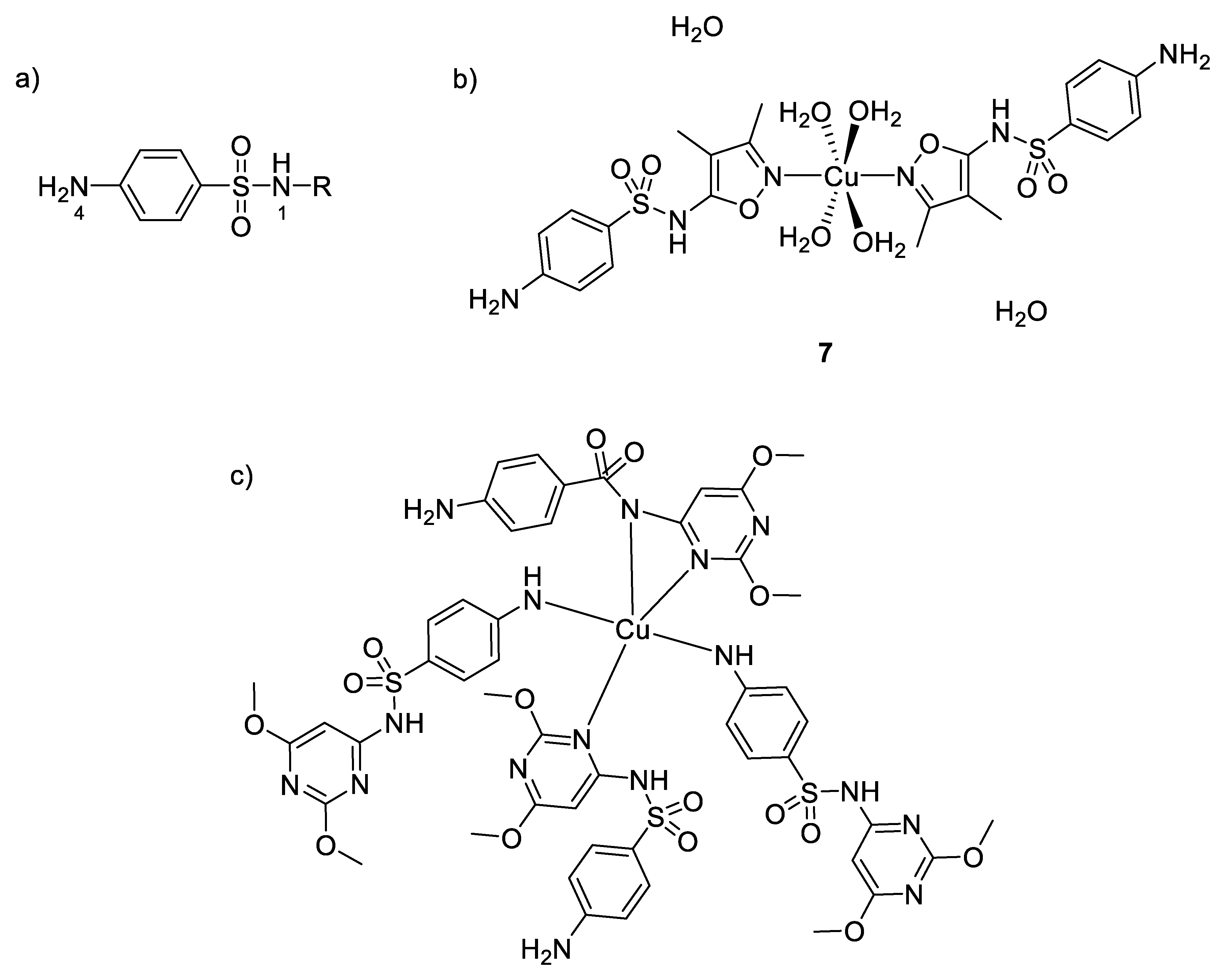
Silver and its compounds have long been used as antimicrobial agents. In the 18th and 19th centuries, silver compounds have found a range of applications in medicine, especially with respect to infectious diseases. Colloidal silver was used, for example, for wound antisepsis and silver nitrate for the treatment of burn wounds [16]. Even though the discovery of antibiotics in the early part of the 20th century drastically reduced the use of silver, numerous silver-based compounds are still employed for their medicinal and low toxicity properties. Indeed, around 300 clinical trials involving silver-based compounds and formulations for diverse applications are currently ongoing [17]. One example to illustrate the efficiency and the potential activity of silver compounds is silver sulfadiazine 1 (Figure 2), known as Silvadene®, which was approved in 1968 by the Food and Drug Administration (FDA) for use as a broad-spectrum antibiotic for burn wounds. This compound acts as a reservoir of silver(I) in the wound, liberating these ions slowly. The interest in silver-based new materials as antimicrobial agents is exponentially increasing and has been reviewed previously [18].
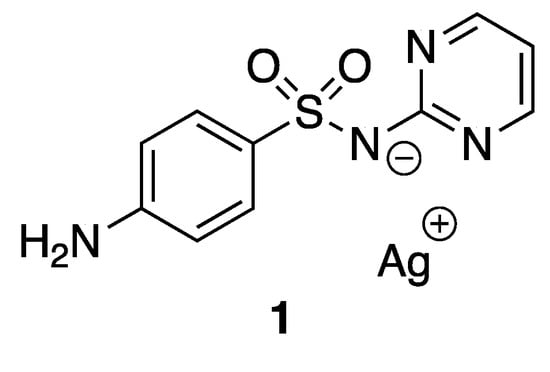
Figure 2.
Silver sulfadiazine.
Even though silver and its complexes have shown cytotoxic effects against Gram-positive/Gram-negative bacteria and fungi, their mechanisms of action are not well understood. However, the most common one described in the literature is related to a slow release of the active silver(I) ion, which reacts with the thiol groups of proteins or with key functional groups of enzymes [19,20], coordinating ligands just acting as a carrier for the silver(I) ion. These interactions lead to the denaturation of proteins and the impairing of the membrane function [21,22]. The magnitude of antimicrobial properties of silver complexes is then related to the ease with which they participate to ligand exchange reactions. Additionally, in some cases, silver ions can produce ROS, known to target mainly lipids, DNA, RNA and proteins, causing severe consequences such as malfunction of membranes, proteins, and the DNA replication machinery [23,24]. Furthermore, upon treatment with silver, condensed DNA molecules have been observed in the bacterial cytoplasm, thereby leading to a loss of its ability to replicate, and thus resulting in the death of the bacteria [21].
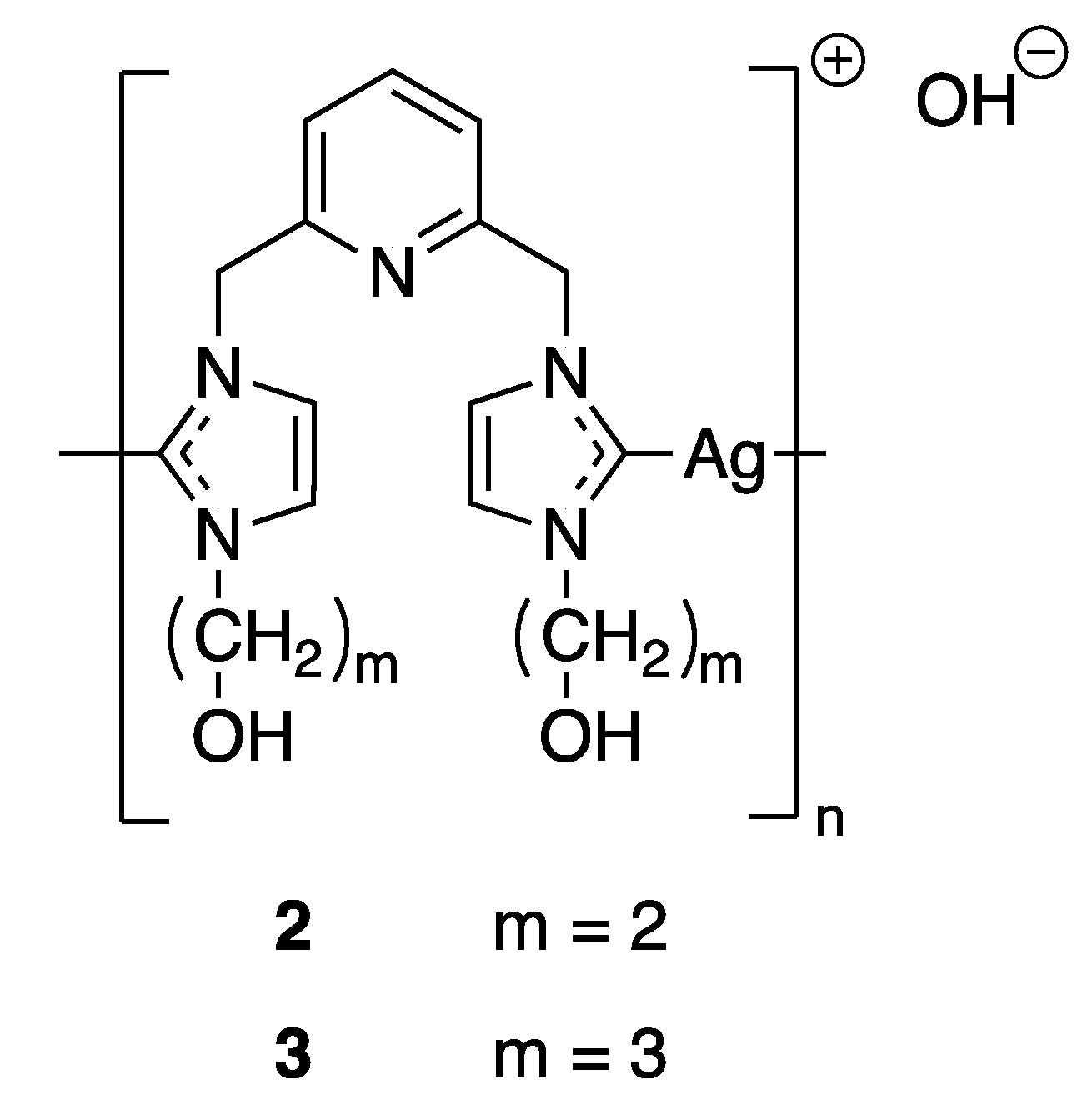
Even though a complete understanding of the mechanism of silver ions fighting bacteria has not been reached until present days, it has been suggested that the activity of silver-based complexes is strictly connected to their water solubility and stability, lipophilicity, redox ability and rate of release silver ions. These properties are governed by the choice of suitable ligands and by slight modulations in their electronic and steric effects. All of these factors are fundamental for maintaining their bioavailability over an extended period of time and preventing reinfection or resistance. Therefore, taking into account these features, a wide variety of new classes of silver complexes have garnered attention for their antimicrobial properties, in particular, N-heterocyclic carbene (NHC) complexes, phosphine complexes or N-heterocyclic complexes of silver(I) [25]. Because of their electronic properties, NHC ligands form very stable bonds with the majority of metal ions and thus improve the stability of the complexes. In terms of the function of substituents that are directed towards the metallic center, NHC ligands are able to protect metal ions and have a strong impact on steric accessibility. Thus, silver NHC complexes are mainly used to modulate the silver release and to control the systemic delivery of silver. For example, Youngs and coworkers synthesized in 2004 several pincer Ag(I)-carbene complexes (Figure 3) and tested their antimicrobial properties against Escherichia coli (E. coli), Staphylococcus aureus (S. aureus), and Pseudomonas aeruginosa (P. aeruginosa). The minimum inhibitory concentrations (MICs) of compounds 2 and 3 were evaluated and showed better bacteriostatic activity than AgNO3, even at much lower concentration [26]. The success of this first Ag(I)-NHC antimicrobial was followed by the development of other derivatives in order to improve their biological activities [27]. Moreover, phosphines and N-heterocyclic ligands have also been extensively screened in association with silver and some of them have shown promising applications against both bacteria [28] and fungi [29]. The authors proposed different modes of action behind this non-proliferation of bacteria and cells as the Ag(I) complexes could possibly interfere with DNA through intercalation or disrupt the cell membrane.
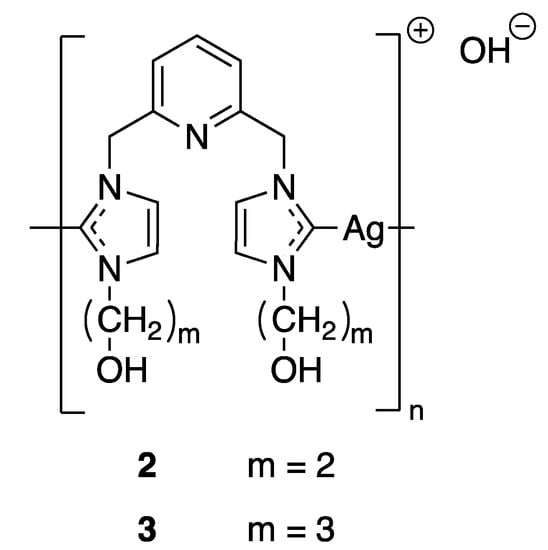
Figure 3.
Structures of Ag(I)-NHC complexes synthesized by Youngs and coworkers [26].
However, despite the great amount of research undertaken and the number of new silver complexes synthesized, most of them have limited efficacy in vivo because of their rapid clearance, which is typical for small molecule-based drugs inside the body. A possible solution to overcome this drawback could be to encapsulate those active species into biodegradable nanoparticles for transportation and delivery [30,31].
Several silver complexes displaying antibacterial properties have been published during these last decades. Without giving an exhaustive list, which could be very long, the most recent results should be mentioned, which showed the best results for a series of metal–antibiotic complexes against different bacterial strains. Thus, while the silver–ampicillin complex shows only a weak inhibition effect against E. coli and S. aureus compared to ampicillin alone (2- to 4-fold decrease of MIC), the inhibition is strongly enhanced against K. pneumonia, A. baumannii and P. aeruginosa with a 20- to 114-fold drop of MIC. It is nevertheless noticed that the MICs for the silver–ampicillin complex are similar to those of silver alone, except for a slight decrease against S. aureus, but the authors noted that one has to consider the silver concentration, which is twice as low for the 1:1 complex compared to the silver alone. Moreover, the non-linear evolution of the MIC with respect to the silver ratio leads the authors to conclude to a synergy between the silver(I) ion and its ligand. This is nicely illustrated by measuring the zones of inhibition of a silver/antibiotic mixture (antibiotic = ampicillin or penicillin G, see Table 1), showing an enhancement of the activity with the increase of the silver concentration, whereas silver nitrate alone displays the lowest activity [32]. The authors explain this synergetic effect by a decrease of the kinetic rate, although the Michaelis constant increased due to the presence of silver ions. Overall, the ratio kcat/KM decreases, resulting in a slowdown of the antibiotic hydrolysis by the β-lactamase enzymes expressed by the resistant bacteria. Thus, the more silver ions there are in the mixture, the more the enzymes are downturned, allowing the majority of the antibiotic molecules to cross the bacterial cell wall and to reach their target (the peptidoglycan membrane) before being hydrolyzed.

Table 1.
Zone of inhibition measured by disk-diffusion assays for silver–ampicillin and silver–penicillin G complexes with different ratios [32].
Other studies aiming to rehabilitate “old” antibiotics that encounter a lot of resistance by themselves have been carried out in recent years by associating them with silver. This is illustrated for instance in a publication of Morones-Ramirez et al., which showed the growth over 3 h of Escherichia coli in the presence of some antibiotics with and without silver. Whereas the concentrations of ofloxacin and ampicillin were adjusted to be bacteriostatic (no growth, no death) alone, the addition of 15 µM of silver nitrate resulted in 10- to 50-fold bacterial death. Even more, although vancomycin is normally inactive against Gram-negative bacteria, 30 µg/mL associated with 30 µM of silver nitrate divided the bacterial concentration by a factor of 10,000 [33]. Finally, rather than associating antibiotics with silver (I) ions, one can also consider association with silver nanoparticles, numerous examples of which are given in a review by Ziora et al. [34]. Nanoparticles have the advantage of releasing silver ions slowly, making it possible to distribute a continuous dose in the organism. Another way to release silver ions progressively is to embed them with or without antibiotics in particles such as zeolites [35] or into sol–gel coatings. Hence, McHale et al. showed that 0.7% w/w of silver ions in a sol–gel coating was enough to avoid any bacterial growth on surfaces, while 0.3% w/w of silver ions with coumarin led to only a slight increase of Enterobacter cloacea (but the coatings with silver ions alone were more efficient against S. aureus MRSA). Nevertheless, sol–gel coatings doped with silver ions and coumarin displayed silver release on a longer period than sol–gel coatings doped with only silver nitrate [36].
2.2. Copper
Many transition metals are associated with numerous biological processes that are indispensable to life processes. They are the most abundantly found trace elements present in biological systems. They can coordinate with C- or N- terminals from proteins in a variety of models, and thereby play a vital role in the conformation and utility of living macromolecules. Thus, these metal ions are nowadays present in several inorganic pharmaceuticals used as drugs against different kinds of diseases, ranging from antibacterial and antifungal to anticancer applications [37,38].
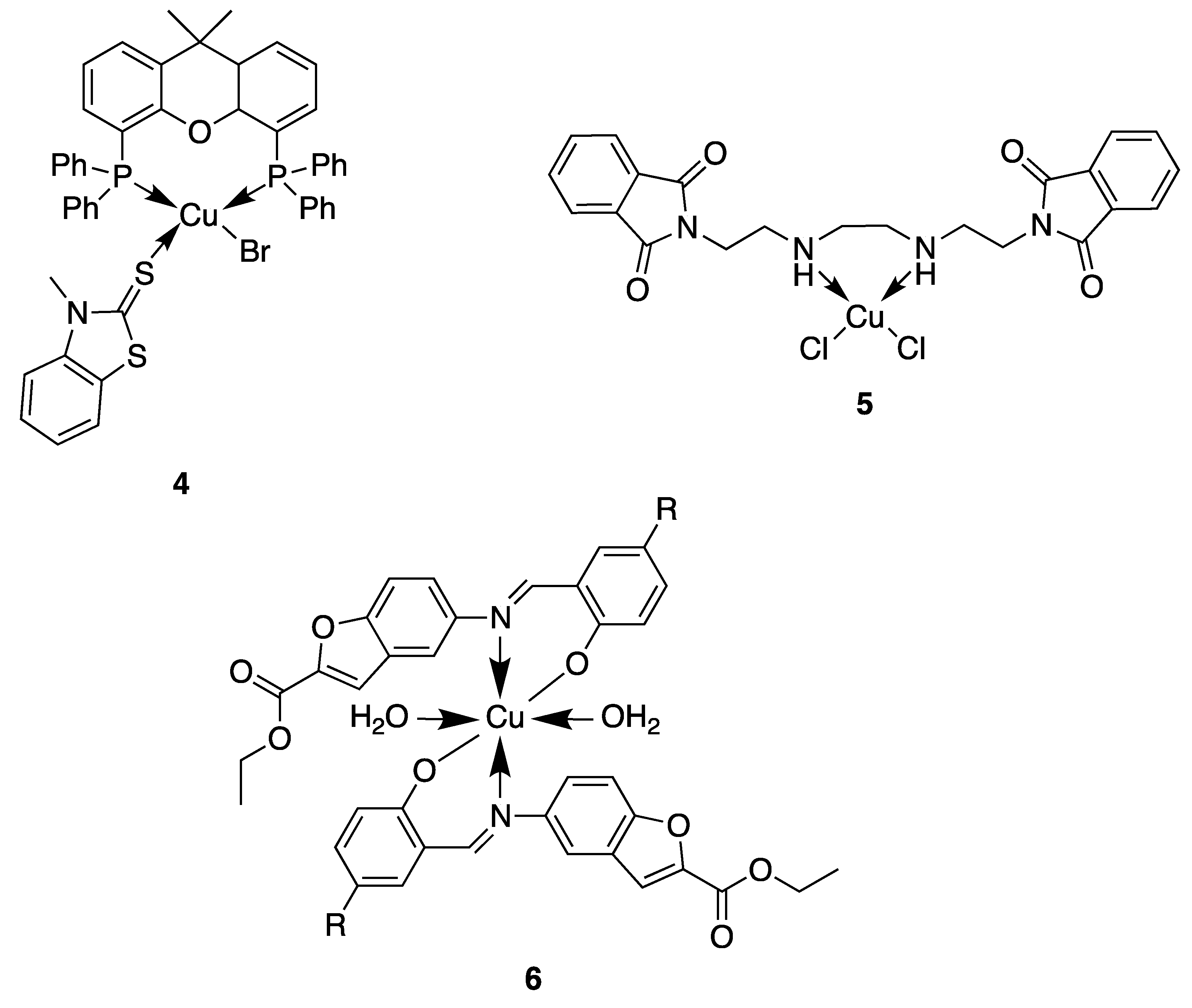
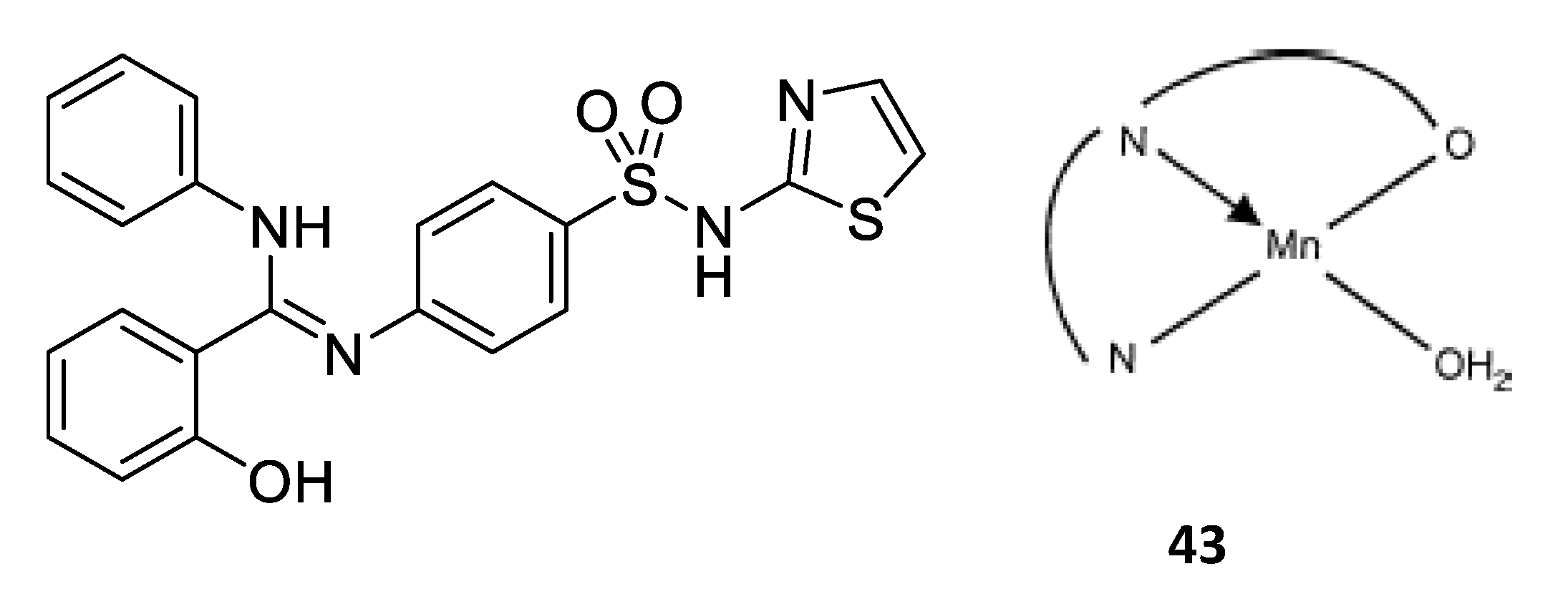
In this context, copper is an essential trace element present as a cofactor in many enzymes as, for example, in superoxide dismutase. In this enzyme and many other metabolic pathways, copper acts as a redox agent. Furthermore, free copper ions are reported to have toxic effects against both bacteria and fungi [39]. Based on this behavior, numerous researchers used the coordination of organic molecules to copper in order to improve the antimicrobial activity. They showed different mechanisms of action depending on the geometry of the complexes and the nature of the ligand (Figure 4) [40,41]. Here, we will discuss some of the most relevant examples described in the literature. For instance, compound 4, based on a tetrahedral mixed-ligand copper(I) bromide complex, was 100-fold more active against both Gram-negative and Gram-positive bacteria (Escherichia coli, Xanthomonas campestris, Bacillus subtilis and Bacillus cereus) compared to the clinical antibiotic ampicillin. The authors showed that the mechanism of action of 4 was based on damage of the bacterial membrane through the generation of reactive oxygen species [42]. Another example showing the effectiveness of copper complexes in order to inhibit the bacterial growth is related to the synthesis of a phthalimide-based copper(II) complex 5 [43]. Due to their planar aromatic rings, phthalimide moieties and derivatives possess different biologically active targets and have been studied for their potent anticancer [44], antimicrobial [45], anti-inflammatory [46] and antimalarial activities [47]. The efficacy of these drugs can be credited to their DNA-interacting capabilities. Then, by coordinating this phthalimide ligand to a copper(II) ion, a complex with square-planar geometry is obtained, which exhibited excellent antibacterial activity against different bacterial strains, especially against Salmonella enterica (IC50 = 0.0019 μg/mL), compared to the ligand itself and the clinical antibiotic ciprofloxacin. This higher antibacterial activity could be attributed to an enhanced interaction of the complex with DNA. Furthermore, a multitude of Schiff base–copper(II) complexes have been synthesized for their antimicrobial properties [48,49,50,51]. This class of ligands is very interesting in the sense that they are excellent coordinating molecules with strong donor groups (azomethine or imine group) and can exhibit variety in the structure of their metal complexes. They have also found application in a broad range of biological activities like antibacterial, antifungal, anti-tuberculosis, antimalarial and antiviral properties [52]. Based on these facts, Nazirkar et al. synthesized complexes of Cu(II) coordinated by new Schiff base derivatives with a benzofuran core (compound 6) [53]. They observed that some of their complexes had excellent antibacterial activity against Mycobacteria Tuberculosis (MIC = 1.6 μg/mL) which is almost two-fold higher than that of pyrazinamide and ciprofloxacin (MIC = 3.125 μg/mL), and almost four-fold higher than that of streptomycin (MIC = 6.25 μg/mL). This could be explained for the first time by the presence of active pharmacophores in the molecular structures of the newly synthesized ligands (benzofuran moiety, imine group and well-positioned halogen), which might interfere in the mitosis cell mechanism and lead to a stop of the bacteria growth. However, Cu(II) complexes showed improved antibacterial activities as compared with their parent Schiff base ligands. The authors explained this by an increase of the lipophilicity of the complexes, which occurs after the complexation of the organic residue around the copper ion which favors their transfer across the lipid membrane of the bacterial cell wall. Indeed, according to the Ligand Field Theory (LFT), overlapping of metal orbitals with orbitals from ligands induce a minimization of the positive charge on the metal by gaining the electrons from donor groups of the Schiff base ligands. The delocalization of the electrons from the ligands to the central metal atom enhances the lipophilic nature of the complex. Thus, the degree of lipophilicity of a molecule is a key factor governing the magnitude of antimicrobial activity.
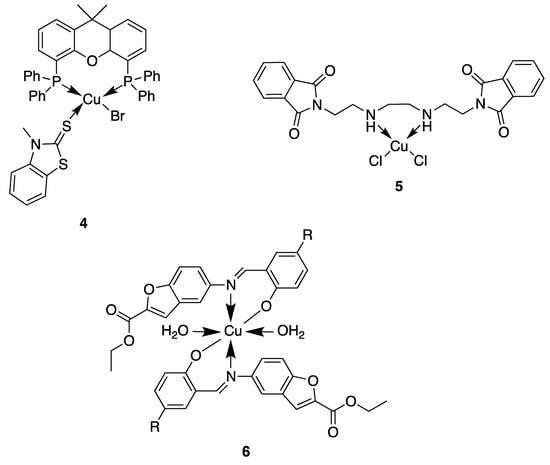
Figure 4.
Structure of antimicrobial copper complexes [42,43,53].
Moreover, a series of copper complexes with sulfonamide ligands have also been synthesized for their antimicrobial properties [54]. Figure 5 shows the general structure of sulfonamides and the coordination versatility of these compounds, which make them very useful in inorganic chemistry. They act as monodentate ligands through the 4N atom or Nh (nitrogen from heterocycle R), as bidentate through the 1N and Nh or bridging two metal ions through 4N and Nh, as bidentate to one Cu(II) through Nh and 1N and bridging to an adjacent Cu(II) through the 4N. It is also well known that sulfonamides are competitive antagonisms of PABA (p-aminobenzoic acid) and then interfere in the biosynthesis of tetrahydrofolic acid, which is quite essential to the bacterial metabolism. Nonetheless, some studies have shown different toxicological and pharmacological properties between sulfonamides and their metal complex counterparts [55]. For example, Kremer et al. evaluated the antimicrobial activity of their synthesized complexes and ligands against both Gram-positive and Gram-negative bacteria (S. aureus and E. coli) [54]. They showed that their copper(II) complexes with five-membered heterocyclic ring substituents (sulfisoxazole 7, sulfamethoxazole, sulfamethizole) were more active than the free sulfonamides (in opposition to the copper(II) complexes with six-membered heterocyclic ring substituents, showing no improvement of activity compared to their title ligand). To understand the microbiological behaviors of these complexes, one has to consider knowledge about the activity of free sulfonamides, where only the ionic form is the active antibacterial species [56]. However, due to its low lipophilicity, the penetration efficiency across the lipoidal bacterial membrane is very low for this anionic form. In addition, therefore, as mentioned above, the complexation of this kind of ligands with metal ions could be one possibility to increase their lipophilicity leading to an enhanced permeation of the drug inside the cell. In the case of copper(II) complexes, the most efficient ones coordinate through the heterocyclic N atom, thus maintaining the anionic form of sulfonamides. Furthermore, intracellular Cu(II) can also act as a redox center and undergoes reduction to Cu(I). This latter ion may catalyze the reduction of O2 to O2−, while the remaining copper(II) ions could participate to the dismutation mechanism of O2− to H2O2 [57]. Hence, the reactive oxygen species produced by such redox reactions cause severe damages to cell and participate synergistically to the high toxicity observed and the increased MIC values obtained with these complexes compared to the free sulfonamides.
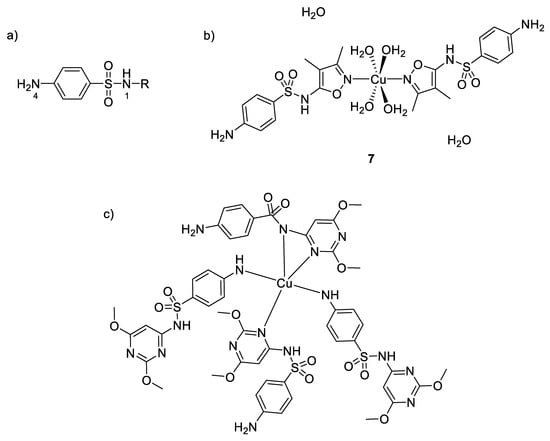
Figure 5.
(a) General structure of sulfonamides showing the labelling of the atoms; (b) structure of [Cu(sulfisoxazole)2(H2O)4]∙2H2O 7- the two oxazole rings and the copper ion are in the same plane (c) structure of a six-membered heterocycle substituted sulfonamide with its environment [54].
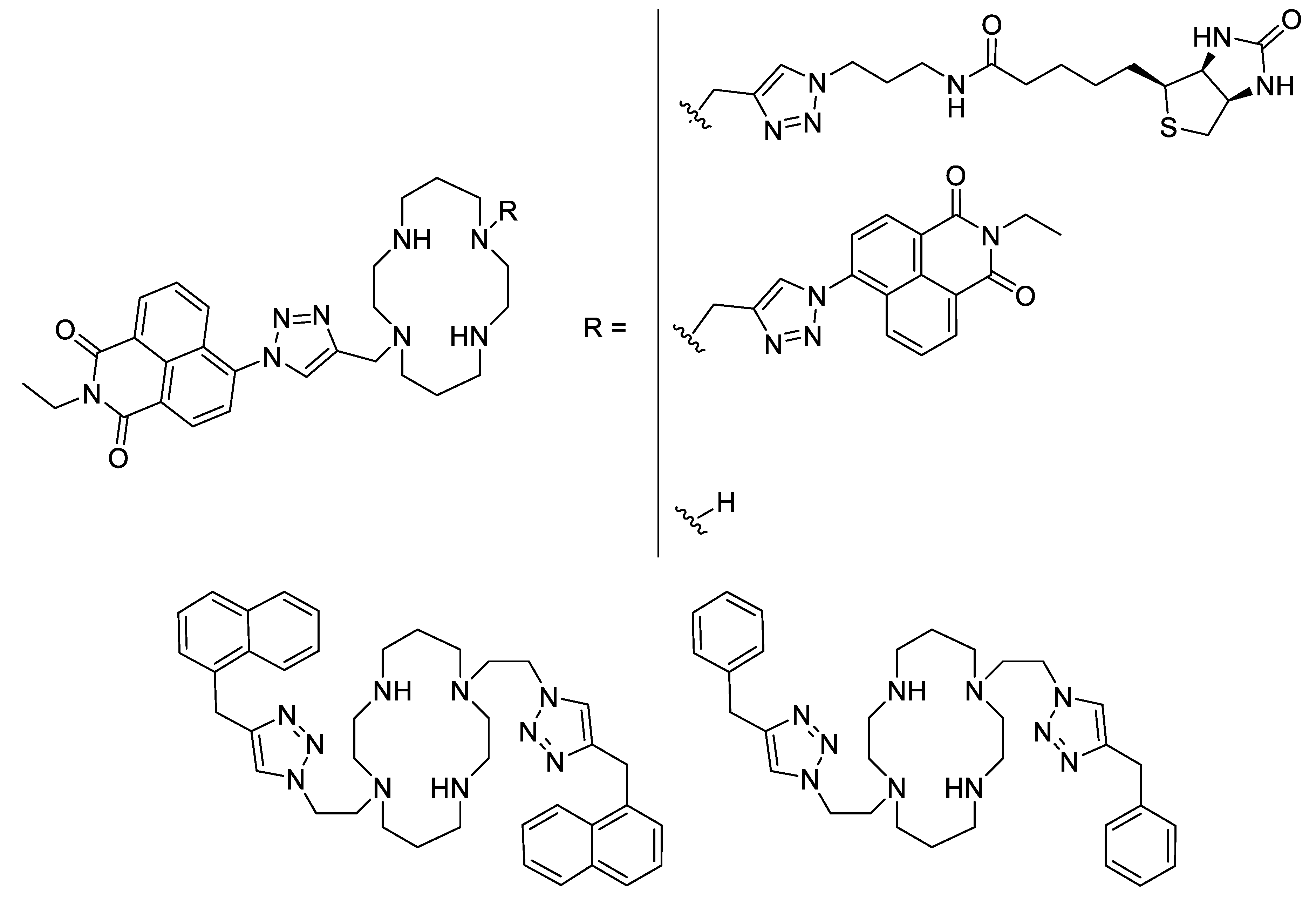
Besides Schiff bases and sulfonamides, another interesting ligand type could be the cyclams. Cyclam derivatives and their zinc- and particularly copper complexes were demonstrated to be active against the Mycobacterium tuberculosis bacteria. Thus, a series of cyclam ligands bearing one or two triazole moieties substituted by a naphthalimide showed a good potency against some Mycobacterium strains with MICs in the low micromolar range (Figure 6). It was shown that a disubstituted cyclam displayed a higher activity than the monosubstituted one [58]. Further, by investigating the influence of the substituent, it was observed that a simple naphthalene is better than a naphthalimide (25 µM vs 6.25 µM against M. tuberculosis). For most of these ligands, their complexation with zinc (II) ions does not improve their antimycobacterial activity, but their copper analogues display 2- to 4-fold decrease of their MIC. Finally, the highest difference between ligand alone and copper-complexes are for two substituted cyclams where the naphthalimide was changed for a smaller phenyl or benzyl part. In both cases, whereas the ligand alone and its zinc-complex have very high MICs (50 µM for the phenyl-substitution and more than 100 µM for the benzyl-substitution), their copper complexes showed a strong enhancement with a 8- to more than 16-fold decrease of the MICs (6.25 µM in both cases) [59].
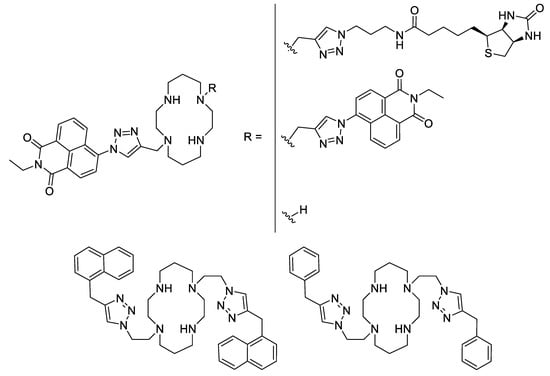
Figure 6.
Some of the triazole-based substituted cyclam ligands [58,59].
As shown in these few examples and according to the literature, many copper-based complexes have been designed over the past few decades, possessing different kinds of ligands, substituents and geometries that influence their antimicrobial activities. Regarding their structural and electronic properties, those complexes showed a promising action on microorganisms and are relevant candidates for further pharmaceutical studies.
2.3. Zinc
The average human body contains 2–3 g of zinc, which makes it the second most abundant d-block natural metal ion in humans after iron. As it is involved in many vital cellular reactions at its low endogenous concentrations, it is an essential element for most living species [60,61]. Zn2+ ions have been the topic of several studies, demonstrating their key role in metalloenzymes and/or metal-based pharmaceuticals [62,63,64], especially as a recognized antiseptic [65]. Their antimicrobial activities are explained by two different mechanisms: (i) a direct interaction with microbial membranes leading to membrane destabilization and enhanced permeability [66]; (ii) an interaction with nucleic acids and deactivation of enzymes of the respiratory system [67]. Taken together, these two modes of action lead to cell death.
As Zn2+ ions are very close to Cu2+ ions in terms of size and charge density, their interactions with O-, N- or S- donor ligands are quite similar. For example, novel Schiff base Zn(II) metal coordination complexes can be designed for their interesting antimicrobial properties. Yamgar et al. synthesized Zn(II) complexes possessing significant antifungal activities compared with standard Fluconazole, that are 4 and 10 times higher against Candida albicans and Aspergillus niger, respectively [68]. In their comparative studies, almost all metal complexes showed increased activity compared with Schiff base ligand. They attributed these enhancements to the greater lipophilic nature of the complexes, which facilitates the penetration through the lipid membrane as discussed above. Additionally, Sheikhshoaie et al. showed promising antimicrobial activities, as their square pyramidal Zn(II) complexes had both bacteriostatic and bactericidal effects against a wide range of bacteria and fungi [69].
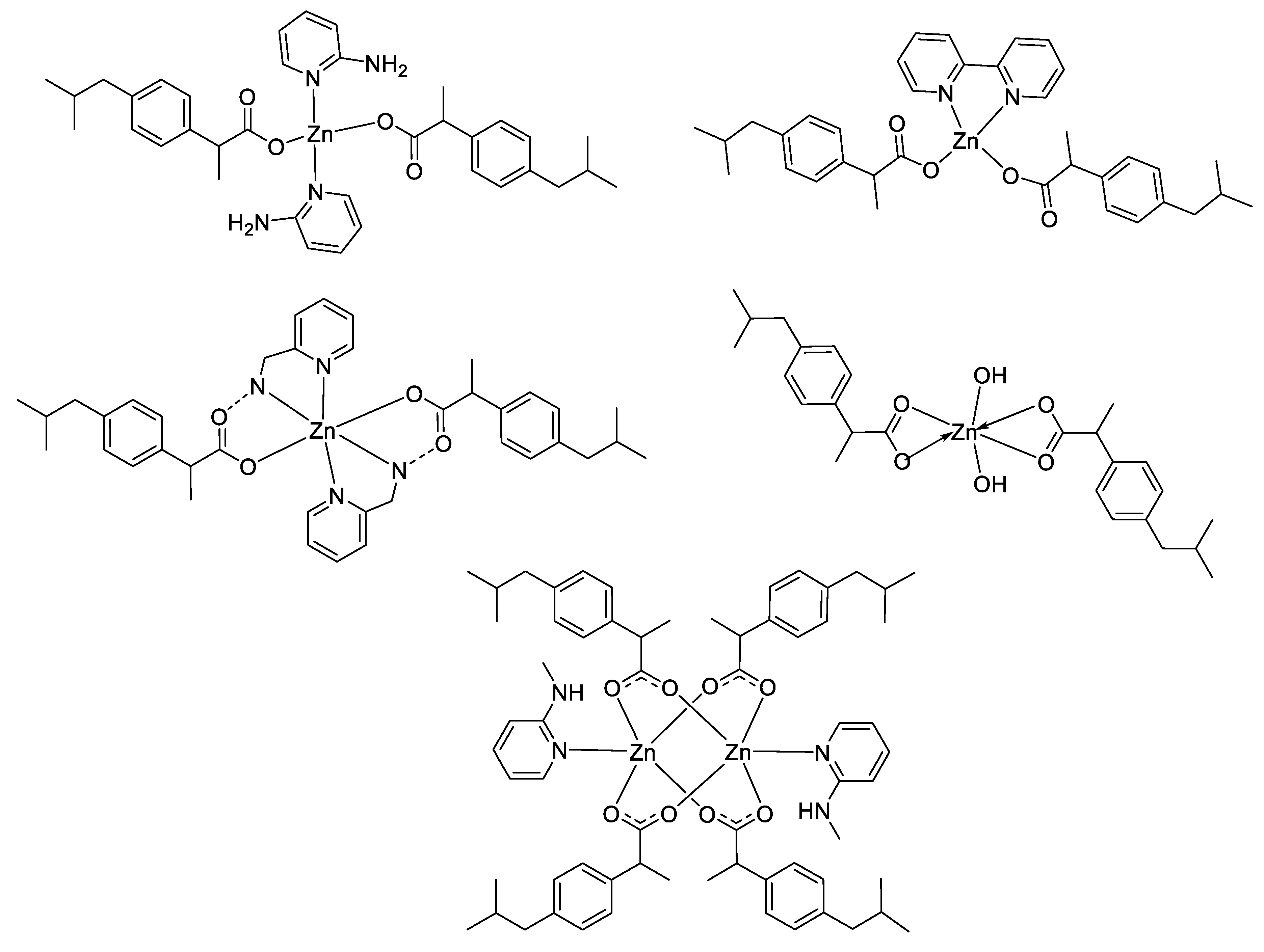
However, according to the Irving–Williams series and the Ligand Field Stabilization Energy (LFSE), the stability of copper(II) complexes is higher than that of zinc(II) complexes. In general, complex stability increases as the ionic radius decreases across the series. Nevertheless, unlike Cu2+, showing a high stability that can be attributed to LFSE obtained through the Jahn–Teller distortion, Zn2+ has low stability due to a lack of LFSE for its d10 electronic configuration, and thus has no preference for any specific ligand field geometry. These principles may be an explanation of the differences obtained between these two latter metal complexes in some studies in terms of antimicrobial activities [51,53,58,59,69], where the stronger affinity of Cu(II) for biomolecules could enhance the permeability of the Cu(II) complexes through cell membrane [70]. For instance, considering the large panel of complexes synthesized by Nazirkar et al., Cu(II) complexes possessing higher antibacterial activity against Mycobacteria Tuberculosis showed stronger efficacy compared to their Zn(II) counterparts by a factor of 62.5 [53]. Nonetheless, the electronic configuration and the ability of Zn2+ ions to have no geometry preferences could also be an advantage in some cases in order to design unique structural complexes that are not suitable with other metals. For instance, Abu Ali et al. synthesized five Zn(II) complexes with the nonsteroidal anti-inflammatory drug Ibuprofen in the presence of N-donor heterocyclic ligands, and having different carboxylate coordination modes (monodentate, bidentate and bridging bidentate, see Figure 7) [71]. They determined their crystal structures by single-crystal X-ray diffraction and have shown that their compounds have completely different structures and shapes, ranging from distorted tetrahedral to hexagonal to square planar coordination geometries. They also investigated in vitro antibacterial activities of their complexes against both Gram-positive (Micrococcus luteus, Staphylococcus aureus and Bacillus subtilis) and Gram-negative (Escherichia coli, Klebsiella pneumoniae and Proteus mirabilis) bacteria, and the results obtained exhibited a strong influence of the geometry of the complexes on their antimicrobial activities.
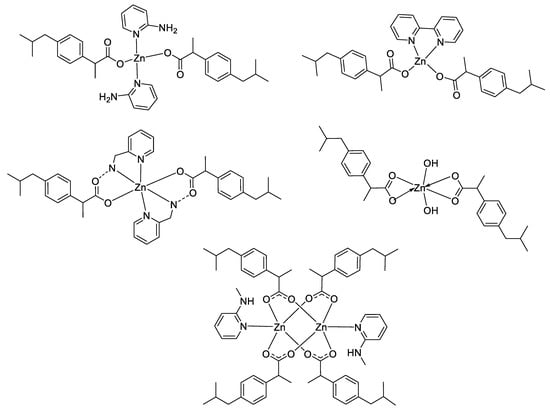
Figure 7.
Structure of zinc–Ibuprofen complexes [71].
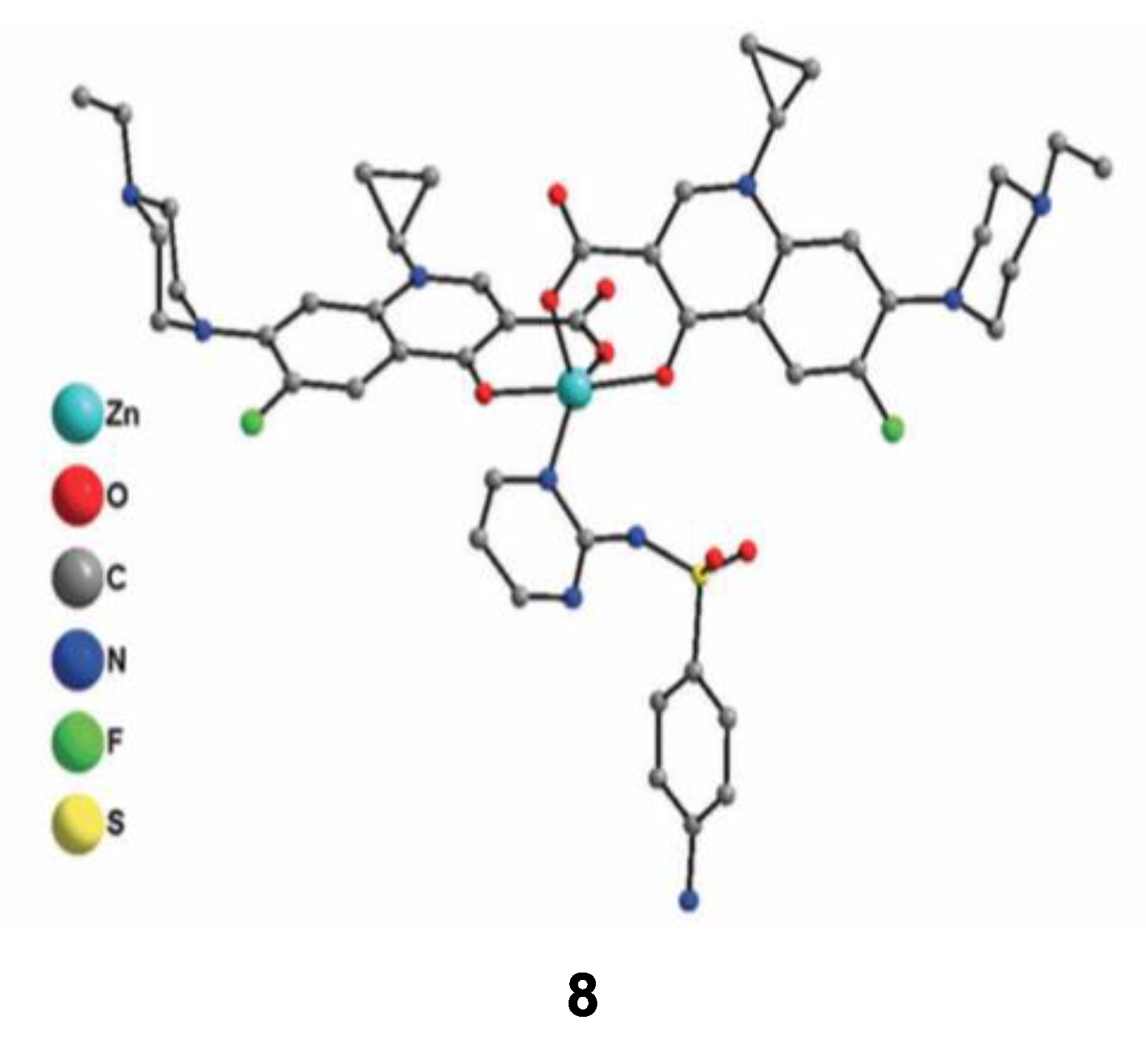
According to the previously described properties, some new strategies have been developed in order to enhance the antimicrobial properties of such complexes. One of them is focusing on the development of new metallo-antibiotics that associate, all in one molecule, metal ions and organic antibiotics. Due to their multicomponent structure, these molecules can interact with several targets in the bacteria. The weak interactions between Zn2+ ions and ligands should offer the possibility to release easily active molecules inside the target. In this context, Boughougal et al. synthesized a new model of a Zn-based complex (compound 8) based on the association of two complementary antibiotics as ligands (sulfadiazine and enrofloxacin), and an antiseptic central Zn(II) cation (Figure 8) [72]. Structural determination of this complex was carried out using X-ray diffraction on single crystals and showed a cationic metal heart, where Zn2+ was located in an almost perfect square-base pyramidal geometry. The charge balance is ensured by ClO4− as counter-ions. Antimicrobial experiments were also performed, and the results obtained showed MIC values of this latter complex lower than 0.5 μg/mL against different bacteria strains (E. coli, S. aureus and E. faecalis), which was much better than those integrating only one type of antibiotic (sulfadiazine or enrofloxacin). Combining the cationic charge of the compound that facilitates the interaction with the lipoidal bacterial membrane leading to an enhanced penetration ability inside the cell, the synergetic effect of each chemical entity appears to strongly increase the antimicrobial activity of this model. Further optimization concerning the choice of antibiotics binding metal ions in order to provide an optimal association could be promising in the future for the development of new bioactive molecules to treat multi-drug resistant diseases.
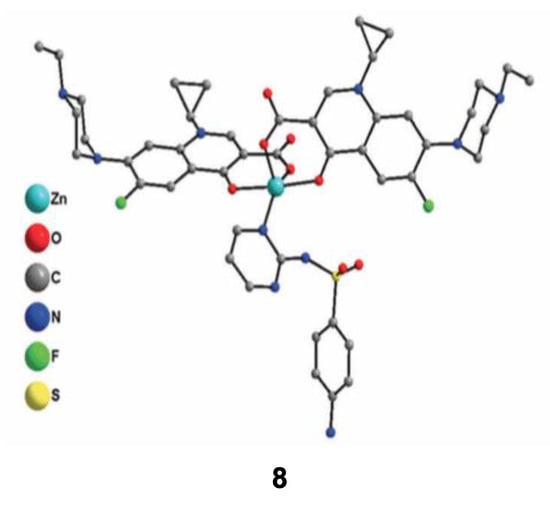
Figure 8.
Crystal structure of the cationic Zn(II) complex 8 with sulfadiazine and enrofloxacin antibiotics used as ligands. Reprinted with permission from ref. [72]. Copyright 2020, Royal Chemical Society.
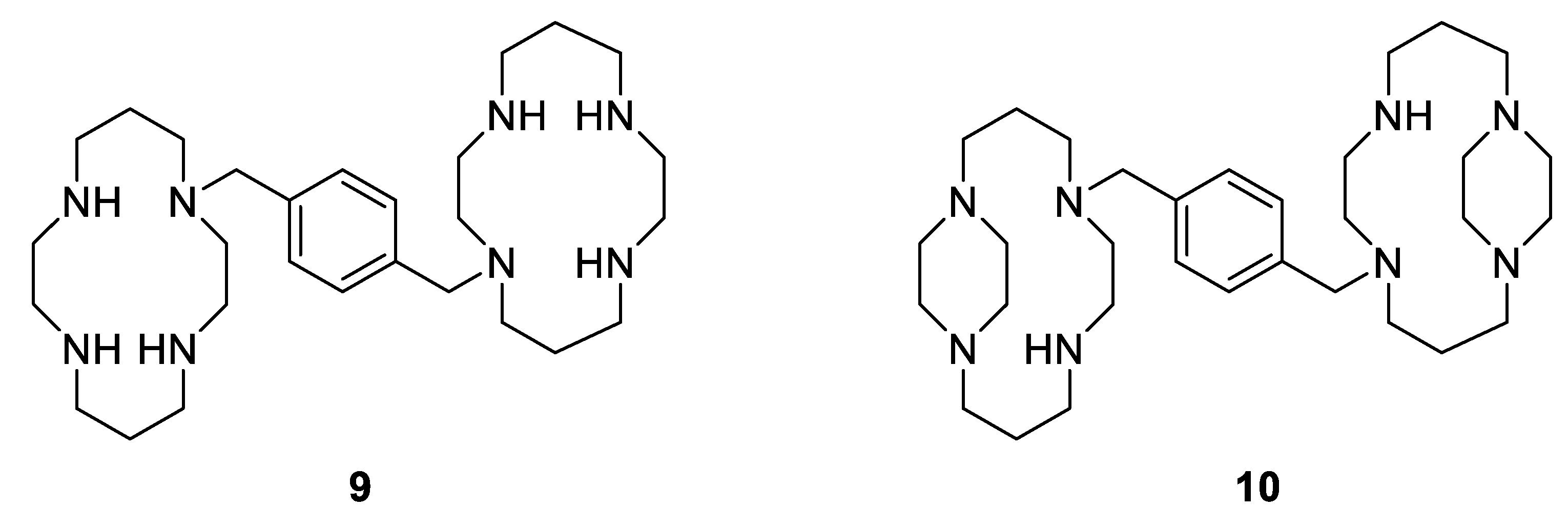
Moreover, another example showing the great importance of the geometry and the structural configuration of a metallodrug is the complexation of a specific cyclam to Zn(II). Xylylbicyclam is a potent anti-HIV agent and is in clinical use as a stem-cell mobilizing drug (AMD3100, Figure 9, Compound 9). Its target is the co-receptor CXCR4, which assists the entry of HIV into cells and anchors stem cells in the bone marrow [73]. Additionally, it has been reported that there is a close correlation between the antiviral activity and the binding to the CXCR4 [74]. Therefore, as cyclams are known to be strong metal-chelating agents, some studies have shown that the complexation of AMD3100 to several metal ions, especially Zn2+, into the cyclam rings enhances the co-receptor binding strength (by a factor of 36 in the case of Zn2+), resulting in good anti-HIV activity [75]. Further studies have also reported that there are only specific metallo-macrocycle configurations that can be recognized by the active site of the proteins to specific amino acid sidechains, H-bonding and hydrophobic interactions [76], allowing optimization of drug design. Archibald et al. synthesized, for example, a configurationally restricted analogue of bismacrocyclic cyclam CXCR4 receptor antagonist (Figure 9 compound 10) and its Zn(II) complex [77]. They showed that this latter complex adopts only one configuration in solution leading to an enhancement of interactions with the protein receptor and an improvement of its anti-HIV activity, which is three times more potent than [Zn2(AMD3100]4+.
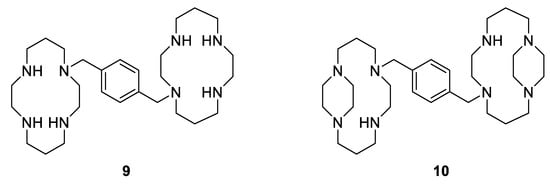
Figure 9.
Antiviral macrocyclic bicyclams AMD3100 9; constrained analogue of AMD3100 10 [77].
Rather than using antimicrobial compounds as ligands to obtain metal complexes, some assays were carried out to associate metal complexes with antimicrobials as cofactors. This is the case, for instance, for Starzac and her group, who were interested in the antimicrobial properties of thiadiazole complexes of zinc and copper. Over a four members series of metal complexes (two copper complexes coordinating two thiadiazole ligands, and two zinc complexes coordinating one thiadiazole and one acetate), only the two zinc complexes displayed a definite antibacterial effect, with a MIC of 1.36 mM and 1.06 mM against S. aureus, and of 2.71 mM and 2.12 mM against E. coli, respectively. This is better than the ligand alone (2.39 mM and 4.78 mM), but much higher than current antibacterials like kanamycin, which demonstrates MICs of 8.05 and 16.10 µM with respect to the two bacterial strains. Only one of these two zinc complexes was tested together with kanamycin against S. aureus; this induced the fall of their respective MICs, which became 0.34 mM for the thiadiazole–zinc complex, and 1.03 µM for the kanamycin [78]. Thus, rather than perform antimicrobial assays directly on new compounds, it is sometimes interesting to use them as cofactors of known antibiotics to determine their synergistic effects. This will be discussed in more depth in the last chapter of our review (Section 3).
Therefore, the electronic properties of Zn2+ offer the possibility to design new antimicrobial drugs that may have a wide range of structural geometries and interesting multicomponent arrangements for interacting with several targets in the bacteria. These features could be further investigated in order to enhance the activity and the selectivity as well as the bioavailability of the Zn(II) complexes.
2.4. Iron
As the most abundant transition metal in the human body, iron performs many important functions. It is primarily involved in the transfer of oxygen from lungs to tissues by forming a complex, known as heme, between its ferrous form (Fe2+) and protoporphyrin IX in hemoglobin and myoglobin. A large number of enzymes also require iron as a cofactor for their functions, especially for their electron transfer properties (cytochromes, iron-sulfur proteins) or for their ability to transport and store iron (transferrin, ferritin).
Furthermore, it is well known that iron is an essential transition metal ion for the growth of pathogenic bacteria, which have different processes for iron acquisition [79]. Taking into account that iron can be coordinated by organic molecules presenting antimicrobial activity, the development of new metallo-antibiotics based on the process of iron acquisition could be a possible strategy in order to enhance the antimicrobial activity of active drugs inside the cell by using iron as a carrier. Numerous examples were reported in the literature about iron complexes with antimicrobial activity involving this strategy. For example, Tarallo et al. synthesized new iron–quinoxaline derivative compounds to obtain new and more potent therapeutic tools against tuberculosis [80]. Previous studies have already displayed the antibacterial activity of quinoxaline derivatives [81], and thus by coordinating those ligands to iron, the authors expected to increase their bioactivity. The results showed that the newly developed iron complexes have a significantly higher activity than the free ligands against Mycobacteria Tuberculosis, with MIC values of 0.78 μg/mL and 3.9–6.2 μg/mL respectively. These results are also comparable to or better than those obtained for clinical antibiotics such as streptomycin (MIC = 1.00 μg/mL), ciprofloxacin (MIC = 2.00 μg/mL) or gentamicin (MIC = 2.00–4.00 μg/mL). The high potential activity of these iron complexes could be explained by the fact that iron(III) acts as a carrier of bioactive ligands and thereby producing an enhanced concentration of these molecules inside the mycobacterial cells. Additionally, interesting results have attracted attention towards triazole derivatives, which are associated with various biological activities including antimicrobial properties, especially when they are functionalized with amino groups in order to obtain various Schiff bases [82,83]. Kharadi synthesized for instance Fe(III) complexes of 1,2,4-triazole Schiff bases that possess better antimicrobial activity against different Gram-positive and Gram-negative bacteria strains than that of the respective free ligands under identical experimental conditions [84]. The author attributed this bioactivity to the presence of quinolones in the complexes that interfere with enzyme production. Furthermore, as already mentioned above, chelation increases the lipophilic nature of the central metal atom, which in turn favors the permeation of the complex through the membrane of the microorganism and, hence, enhancing its activity.
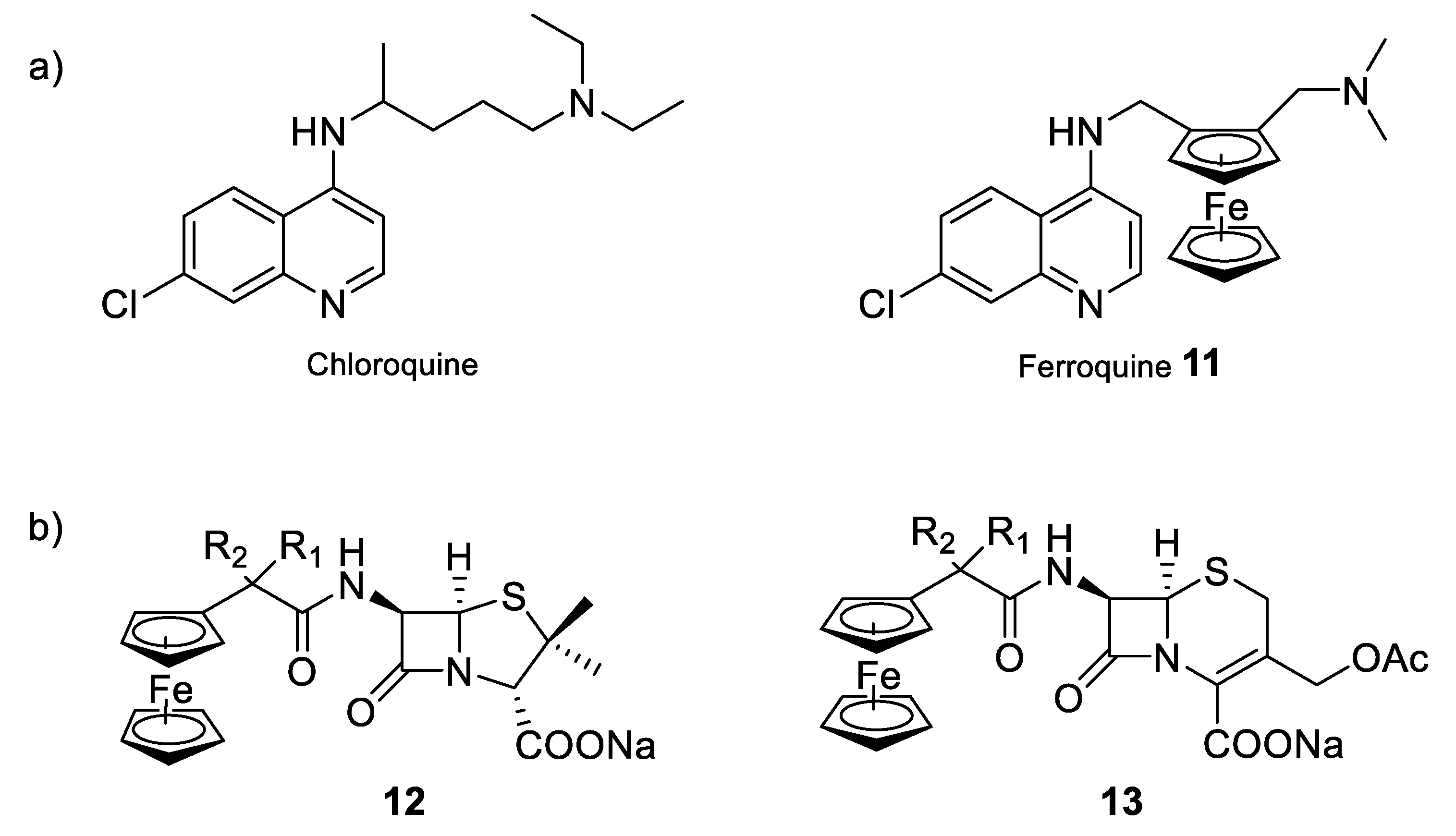
Moreover, another strategy that could be an option to enhance the antimicrobial properties of currently used organic drugs is the design of bio-organometallic derivatives containing either the antimicrobial drug, the active moiety of the drug, or using the metal to mimic a part of the drug [85]. Biot et al. demonstrated, for example, that the addition of a ferrocenyl moiety into the structure of the antimalarial chloroquine, to give the so-called organometallic complex ferroquine (compound 11) allowed additional modes of action compared to the parent organic drug (Figure 10a) [86]. Due to the presence of the ferrocene moiety, ferroquine is active against chloroquine-resistant parasitic strains. This higher efficiency can be explained by the redox properties of iron. Indeed, in addition to having a similar mechanism of action to chloroquine, ferroquine can also produce reactive oxygen species that are able to kill the parasites resistant to chloroquine. Ferroquine is thus one of the most advanced organometallic drug candidates, soon to enter in clinical phase III trials. Other examples have been reported for the preparation of bio-organometallic derivatives having antimicrobial properties and incorporating iron in the molecular structure. Edwards et al. prepared for the first time, in 1975, semi-synthetic derivatives of penicillin and cephalosporin (compounds 12 and 13) in which the conventional phenyl or heteroaromatic group has been replaced by a ferrocene moiety (Figure 10b) [87]. Since then, other ferrocenylamide derivatives of these two β-lactam antibiotics were synthesized and the resulting compounds showed variable degrees of antibacterial activity depending on the proximity of the metal atom and the β-lactam ring.
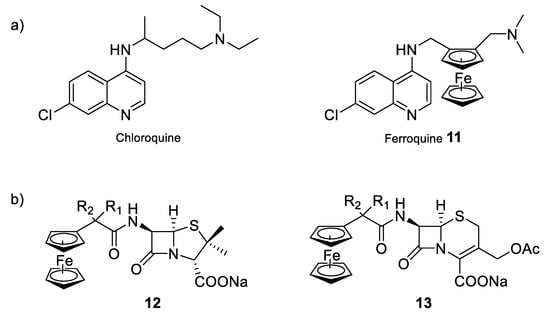
Figure 10.
Bio-organometallic compounds of conventional antibacterial drugs. (a) Schematic structures of chloroquine and ferroquine, two of the most commonly used antimalarial compounds [86]; (b) semi-synthetic derivatives of penicillin and cephalosporin [87].
By using the nutrient assimilation machinery of the bacteria, the development of new metallo-antibiotics based on the ability of active organic molecules to easily coordinate iron ions could be a promising approach in order to enhance the antimicrobial activity. Further strategies including the design of new organometallic derivatives of conventional antimicrobial drugs based on structure-relationship methods is still underdeveloped and due to their modes of action that are different from the current antimicrobial agents, they may be applied to tackle multi-drug resistant diseases.
2.5. Ruthenium
In general, transition metals of the second and third row, with closed electron shells have shown interesting applications in medicinal inorganic chemistry when structurally rigid compounds are required. The replacement of a metal center with its heavier homologs of the same group leads to isostructural complexes with mild changes of the geometrical parameters but that differ significantly in the redox properties or in kinetics. The replacement of the ferrocenyl moiety by a ruthenocene unit to give ruthenoquine, a structural analog of ferroquine presented in the previous section, led for example to a decreased potency due to the absence of redox chemistry [88]. Other studies revealed that the chemical inertness of ruthenium complexes could also be used to define a portion of space with a precise geometry yielding in a structure that has stereo-electronic complementarity with the active site of a target. This concept was employed by the group of Meggers, wherein, mimicking the natural product staurosporin, Ru(II) complexes were designed in order to act as inhibitors of protein kinases [89].
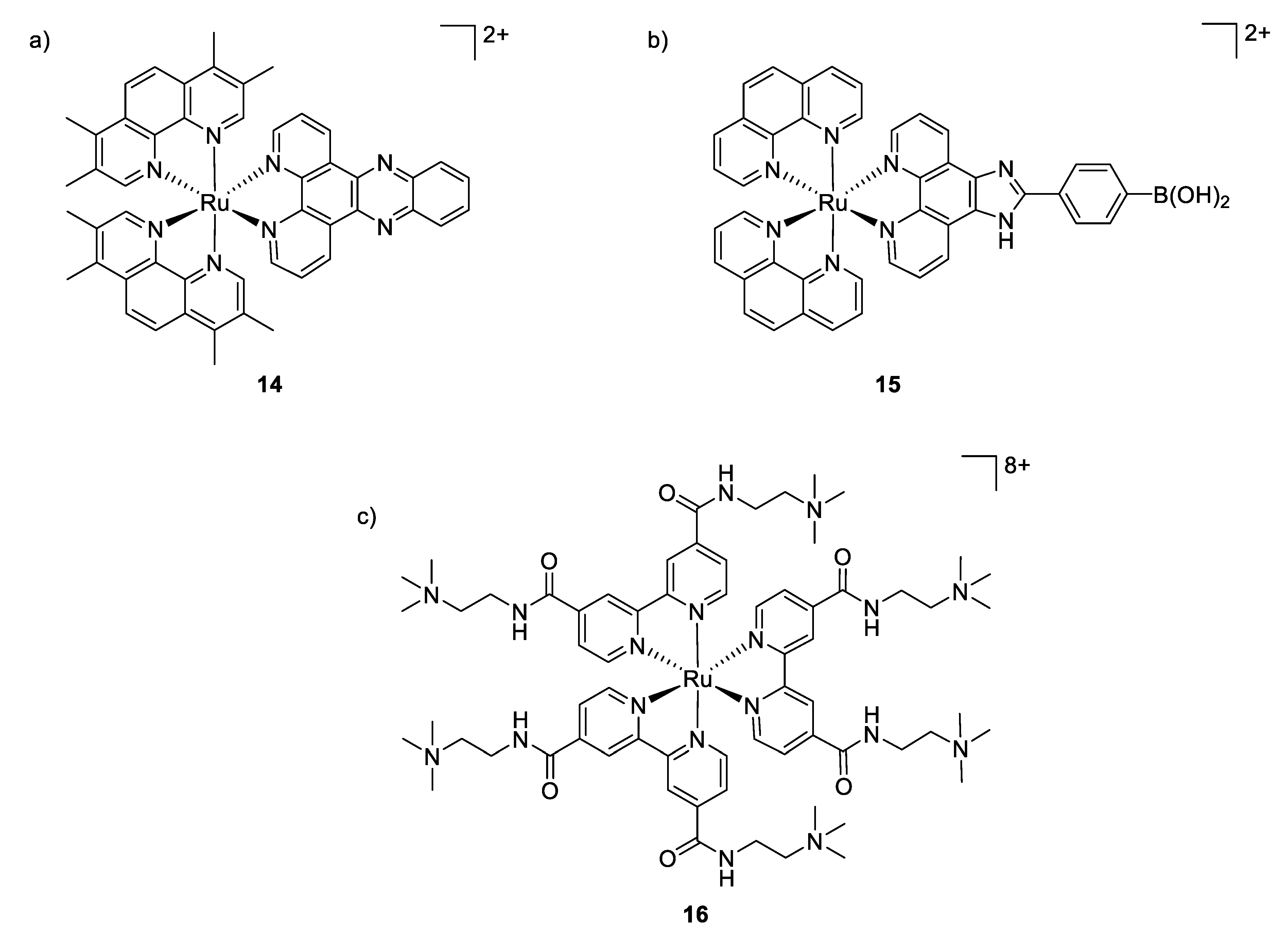
Although ruthenium complexes have been widely studied for their anticancer activity, some interesting antimicrobial properties were also described since the mid-twentieth century by Dwyer [90,91]. Among all the tested transition metals, it was shown that the inert polypyridylruthenium(II) complex [Ru(Me4phen)3]2+ exhibited remarkable antimicrobial activity in vitro, particularly against Gram-positive strains [92]. The activity of these octahedral complexes is probably due to their ability to interact with nucleic acids through intercalation with aromatic bases, aided by electrostatic attraction between the positive charged-metal complex and the negative charged phosphate groups. Aldrich-Wright and co-workers also reported that their mononuclear Ru(II) complexes [Ru(2,9-Me2phen)2(dppz)]2+ (compound 14, Figure 11) incorporating DNA intercalating ligands exhibited significant antibacterial activity against Gram-positive bacteria (B. subtilis and S. aureus, MIC = 2–8 μg/mL) [93]. However, these complexes were not active against the Gram-negative bacterium E. coli. The mechanism of action remained unclear but the hypothesis mentioned is that the Ru(II) complexes were not able to cross the outer membrane of E. coli.
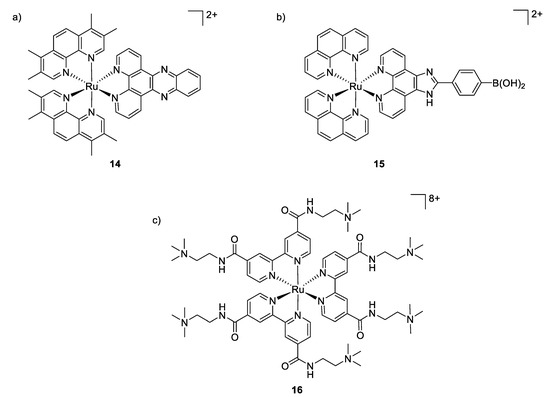
Figure 11.
Structures of antimicrobial ruthenium complexes presenting different modes of action. (a,b) Interaction with DNA through intercalation; (c) aPDT-active Ru(II) complex [92,94,95].
The same was observed by Sun et al., who tested a [Ru(phen2)(p-BPIP)]2+ against E. coli, M. tetragenus, S. aureus, and some moistures. Antibacterial activity was found only against both Gram-positive bacteria. Further scanning electron microscopy studies showed non-smooth cell walls, and parts of the cytoplasm outside of the bacteria cell. Finally, it was demonstrated that the complex causes the fragmentation of the DNA. Therefore, it was concluded that the treatment of Gram-positive bacteria by the ruthenium complex resulted in a perforated membrane due to its interaction with the bacterial cell wall, increasing the permeability, even if the main mechanism of action of [Ru(phen2)(p-BPIP)]2+ (Figure 11, compound 15) is the damage of the DNA and RNA [94].
Furthermore, ruthenium polypyridyl complexes can also act as photosensitizers, generating ROS upon light irradiation for use in antibacterial photodynamic therapy (aPDT). The multi-target feature of ROS not only renders aPDT highly potent in killing even multidrug-resistant bacteria, but also makes bacteria difficult to develop any resistance against these multiple attacks. However, it is a great challenge to inactivate selectively bacterial cells while leaving mammalian cells unaffected. In this context, ruthenium polypyridyl complexes are promising candidates as aPDT agents due to their overall positive charge, which may promote interactions with the negatively charged bacterial membrane, their high 1O2 yields, and excellent chemical stability and photostability. In 2007, Donnelly et al. were the first to describe these features by analyzing photophysical and microbiological behaviors of their Ru(II) complexes. They showed that upon white light irradiation, their compounds presented MIC values of 12.5, 50 and ≤12.5 μg/mL against S. aureus, P. aeruginosa and C. albicans, respectively. Unfortunately, no toxicological studies against human cell lines were reported [96]. More recently, Feng et al. depicted a series of charged ruthenium complexes by using quaternary ammonium-modified bipyridine as ligand [95]. Their results indicated that the most highly charged complex (Figure 11, compound 16), bearing eight positive charges, exhibited the most potent aPDT activity against S. aureus, displaying 6–7 log reduction in bacterial viability (comparable to the traditional antibiotic vancomycin at equal concentrations) when irradiated with 470 nm light, while only minor activity was observed against Gram-negative bacterium E. coli, probably due to its dense and compact outer membrane which may hamper the photodegradation. Damages and deformations of cell walls in S. aureus have also been observed by scanning electron microscopy (SEM) for the aPDT-treated cells, pointing to the highly negatively charged bacterial surfaces as the target of this class of compounds. Co-culture experiments revealed the selective photoinactivation of compound 16 toward bacterial cells over mammalian cells. The spherical octahedral coordination structure and hydrophilic cationic character of the Ru(II) core may lack the interaction with cytoplasmic membranes and therefore could be responsible for their lower affinity toward mammalian cells.
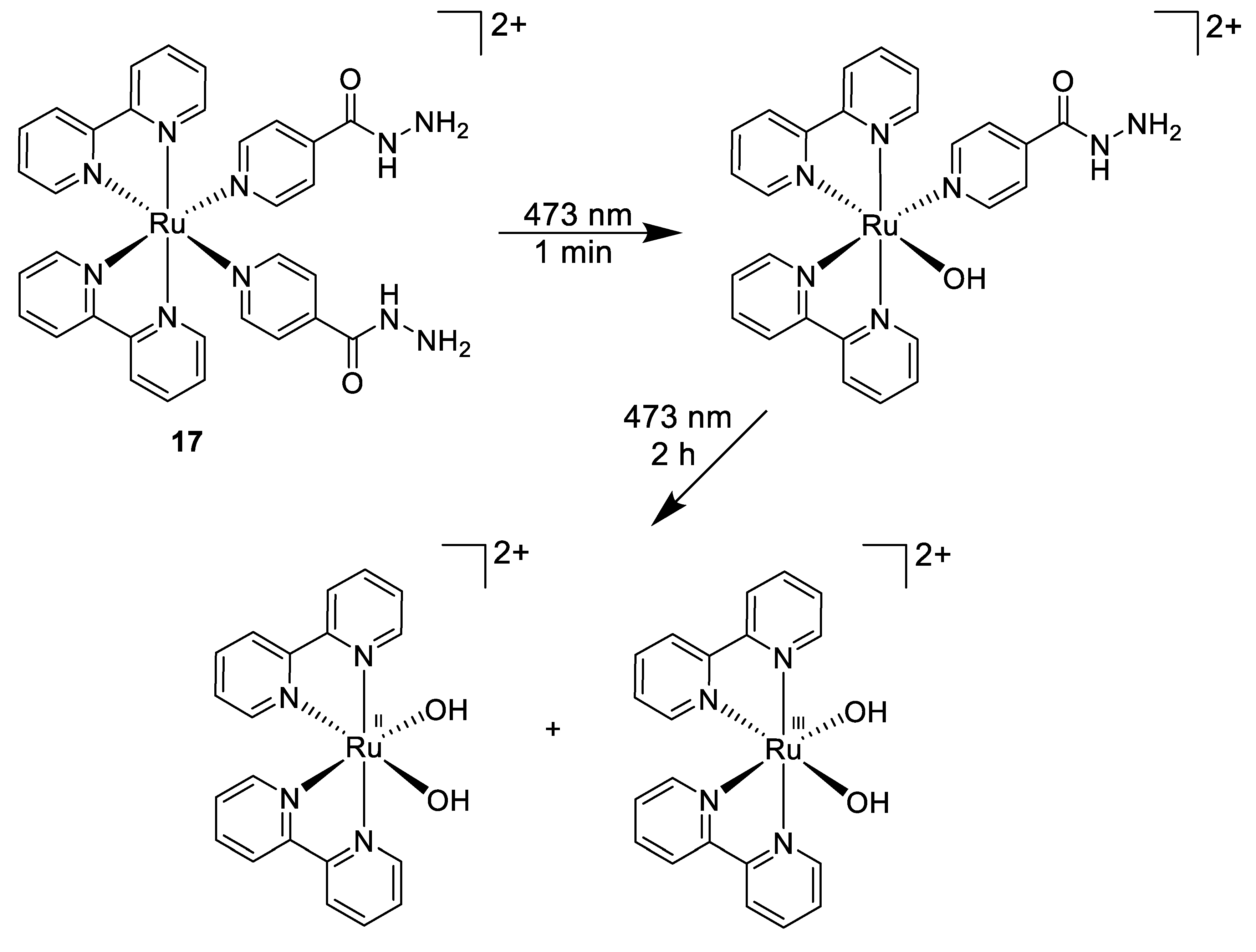
Another light-mediated strategy was pursued by Smith et al., who designed a photoactive ruthenium(II) complex incorporating the anti-tuberculosis drug isoniazid (Figure 12, compound 17) [97] that could be further released from the Ru(II) core upon 463 nm light irradiation. This results in a selective high potency towards Mycobacterium smegmatis (MIC = 4 μM) over both Gram-positive (B. subtilis) and Gram-negative (E. coli) bacteria within no activity was observed. This was a 5.5-fold increase in potency compared to the title compound isoniazid. Moreover, this Ru(II) compound was found to be non-toxic to human lung cell line.
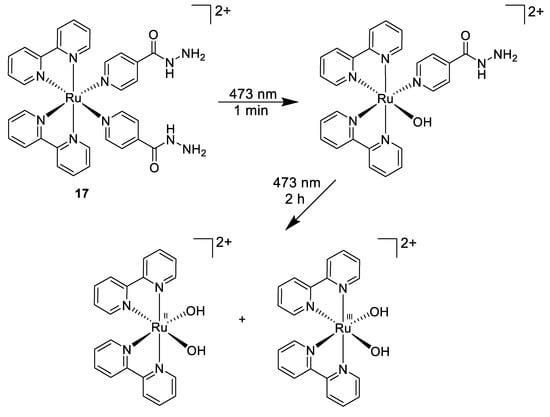
Figure 12.
Stepwise photoactivation of the antibacterial prodrug cis-[Ru(bpy)2(INH)2]2+ 17 [97].
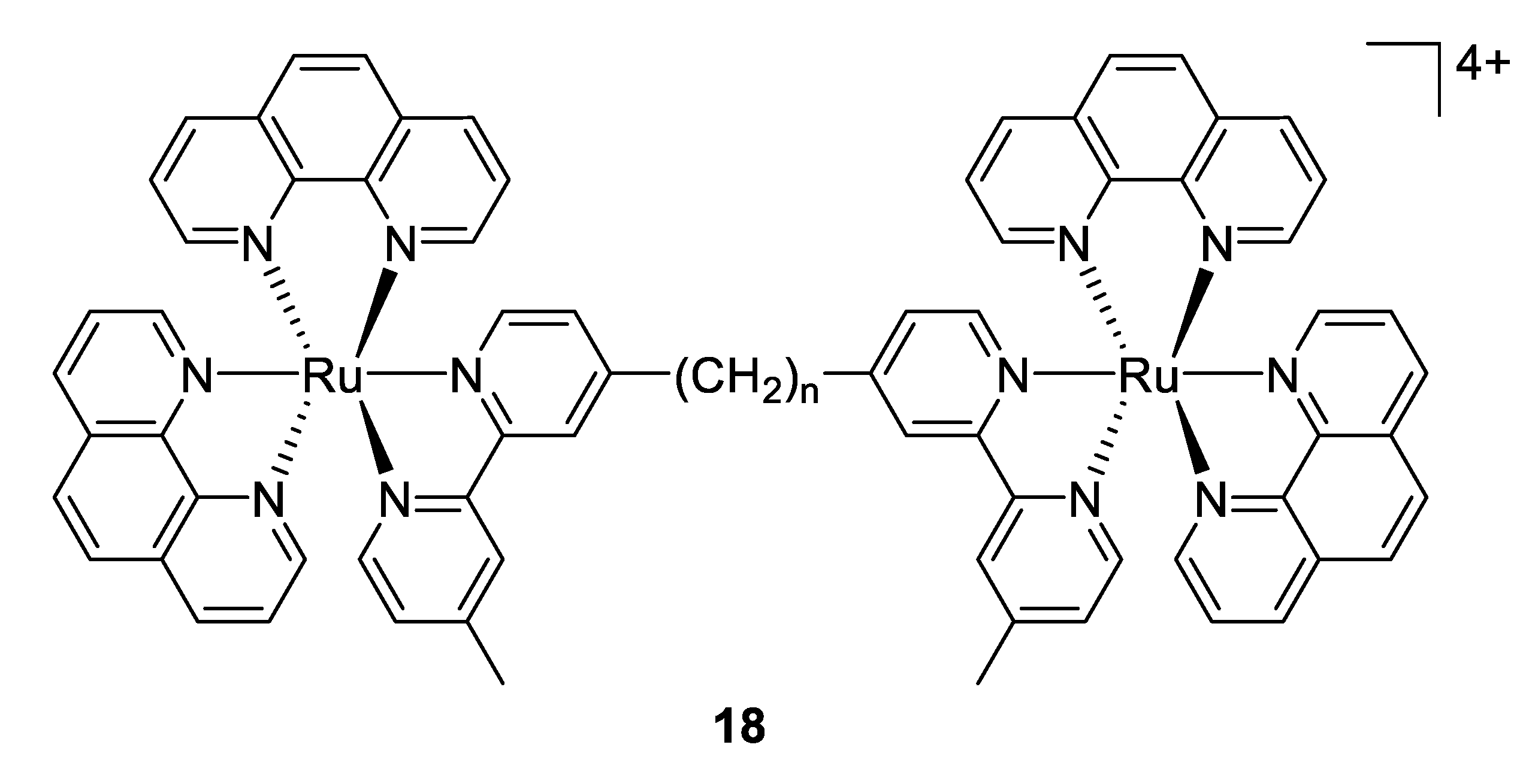
Another approach reported firstly by Keene and Collins in 2011 was to investigate the antimicrobial activity of an extensive range of mono-, di- and oligonuclear inert polypyridylruthenium(II) complexes [98]. The results demonstrated that for the dinuclear Ru(II) complexes linked by long flexible alkane chains (compounds 18, Figure 13) are highly active against both Gram-positive and Gram-negative bacteria (with MIC values that are comparable or better than the well-known antibiotic gentamicin), but are considerably less toxic to human eukaryotic cells. In subsequent studies, the same group exhibited that the antimicrobial activity of these complexes is correlated with the level of cellular uptake. As the longer alkyl chain length leads to more lipophilic compounds, the authors concluded that the increasing lipophilicity is responsible for the higher uptake [99]. Later, they showed that compound 18 with n = 16 condensed ribosomes when they existed as polysomes, leading to stop the protein production, and thereby inhibit the bacterial growth [100].
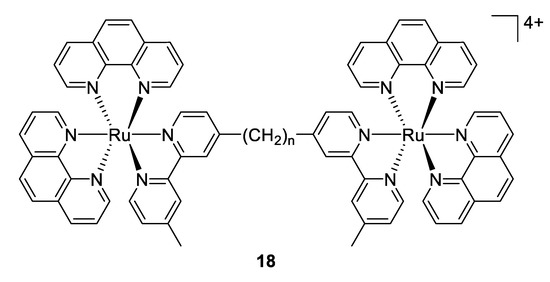
Figure 13.
Structure of dinuclear ruthenium(II) complexes [98].
Although the mode of action of polypyridylruthenium(II) complexes is not completely understood, DNA binding is normally considered as the major interaction leading to the antimicrobial activity. However, some recent interesting studies have shown that these complexes could behave as efficient photoactivable prodrug delivery systems, and both photodynamic antimicrobial therapy and photorelease antimicrobial therapy are promising strategies for overcoming bacterial infections. Taking into account these recent investigations, the design of the next generation of ruthenium-based antimicrobial agents are on the way. Nevertheless, there are only limited data available at this stage concerning in vivo efficiency of these compounds, and future research will have to focus on this area.
2.6. Gallium
Depriving bacteria of essential nutrients, such as iron, is a viable strategy for the development of new antimicrobials. As mentioned earlier, iron plays a vital role for all forms of life, including bacteria, as a cofactor of many vital enzymes. With the emergence of multi drug resistant pathogens, gallium has gained interest due to its ability to interact with proteins involved in iron metabolism of a wide range of bacteria based on an intriguing “Trojan horse” strategy [101,102]. The ionic radius and charge density of Ga3+ (r = 62 pm, ρ = 3.01) is effectively almost identical to Fe3+ (r = 55 pm, ρ = 4.30), and many biological systems involving in microorganisms are not able to distinguish easily these two metal ions. Therefore, it has been shown in different studies that Ga3+ ions can inhibit the growth of many bacterial and fungal species by interfering with iron-dependent metabolic pathways [103,104]. As the insertion of Ga3+ into the active site of Fe-dependent proteins and enzymes renders them inactive, it is worth mentioning that unlike Fe3+, Ga3+ cannot be reduced under physiological conditions in biological systems [105]. It is also important to note that gallium is resistant to known bacterial efflux pumps, preventing the development of bacterial resistance.
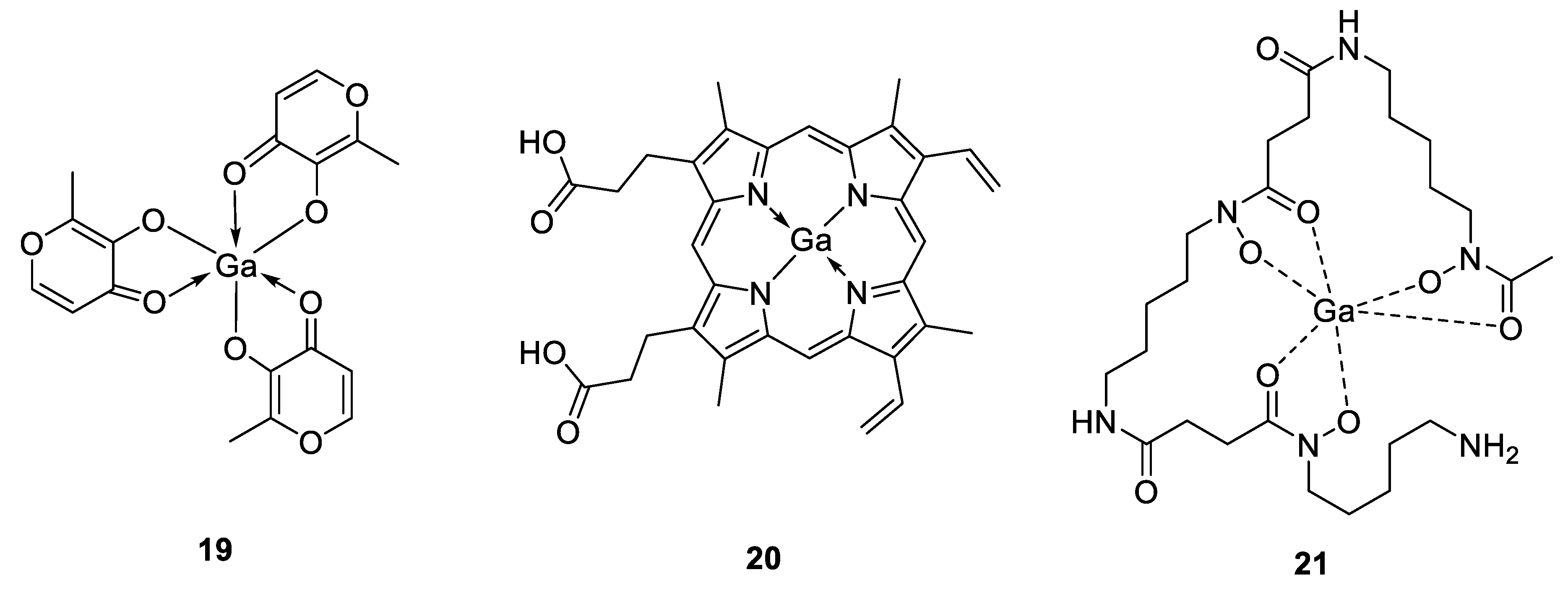
The antimicrobial properties of gallium were discovered in 1931 by Levaditi et al. who reported that gallium tartrate eradicated syphilis in rabbits and Trypanosoma evansi in mice [106]. However, with the introduction and use of antibiotics, gallium has been overlooked until recently. Since then, gallium compounds, ranging from simple gallium salts to more complex structures have advanced in preclinical and clinical investigations [107]. They can be grouped as first-, second- and third-generation gallium compounds, which become increasingly diverse with the binding ability of gallium to different ligands. In this context, gallium nitrate represents the first generation of gallium compounds, as it was the first drug to enter in clinical trials, approved by the Food and Drug Administration (FDA) for the treatment of calcium-associated hypercalcemia and commercialized since 2012 with the brand name Ganite®. This latter gallium formulation has been shown to possess promising activity against different bacterial strains such as P. aeruginosa, A. baumanii and M. tuberculosis [107]. Other gallium(III) salts such as GaCl3 or Ga(III)-citrate, Ga(NO3)3 also exhibited potent suppressive activity on P. aeruginosa biofilm formation in vitro and in murine lung infection models [108]. The second generation of gallium compounds led from simple gallium salts to gallium complexes with organic anions, like gallium citrate or gallium maltolate (compound 19, Figure 14). This latter compound consists of Ga3+ bound to three maltol ligands in a stable coordination geometry with increased solubility and reduced the probability of forming toxic precipitates, allowing better bacterial cell growth inhibition and apoptosis than the first generation of gallium-based compounds, which could be explained by a greater cellular uptake [109]. DeLeon et al. demonstrated that gallium maltolate promoted the survival of all P. aeruginosa-infected, thermally injured mice at significantly lower Ga concentrations than Ga(NO3)3, while it appeared to be well tolerated at all of the doses delivered, as no gross clinical signs of toxicity were observed [110]. The authors also showed a considerable inhibition of the growth of both S. aureus and A. baumannii pathogens, exhibiting the high potential of gallium maltolate to treat both Gram-positive and Gram-negative bacteria in wounds. They hypothesized that the increased lipophilicity of the gallium maltolate formulation and its reduced tendency to form insoluble gallate precipitates could be the reason for the observed greater efficacy over Ga(NO3)3. In addition, by relying on the antimicrobial activity of gallium compounds against a variety of microorganisms, Aridis Pharmaceuticals developed a novel formulation based on gallium citrate (Panaecin™) that recently entered clinical trials for evaluation of its efficacy in certain infections. The third generation of gallium complexes in preclinical evaluation includes gallium bound to ligands such as hydroxamic acid, protoporphyrins, pyridine, hydrazones and others [111,112,113,114,115]. Different studies revealed for example that the heme-mimetic gallium protoporphyrin IX (GaPPIX, compound 20, Figure 14) is likely to exploit heme-uptake routes to enter bacterial cells, where it could inhibit the iron metabolism, resulting in the perturbation of vital cellular functions [116]. According to this, it has been shown that GaPPIX possesses a good antibacterial activity against several bacterial species, including S. aureus, A. baumannii, M. smegmatis or P. aeruginosa [114,116,117,118]. Because Ga(NO3)3 and Ga porphyrin disrupt different pathways of bacterial ion acquisition and use, a combination of these two types of gallium compounds would result in enhanced antimicrobial activity. This concept was used by Choi et al. who described the in vitro synergistic effect against both Gram-positive (methicillin-resistant S. aureus, MRSA) and Gram-negative bacteria (K. pneumoniae and P. aeruginosa) by combining these two latter components, while a significant reduction of the bacterial populations in K. pneumoniae and P. aeruginosa biofilms have been observed [119]. The same synergistic strategy was employed by Banin et al., who used a combination of a gallium(III) complex (GaDFO, compound 21, Figure 14), where the ligand desferrioxamine is a strong siderophore involved in P. aeruginosa iron metabolism with the anti-Pseudomonas antibiotic gentamicin [120]. Their in vivo studies showed that such combination was able to reduce the bacterial infiltration and final scar size by about 50% in a rabbit keratitis model compared to topical application of gentamicin alone.
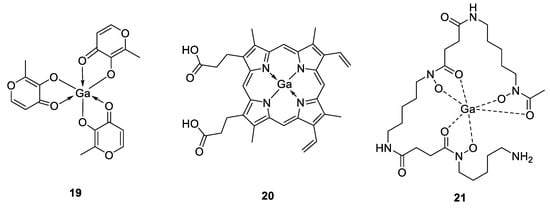
Figure 14.
Structures of antimicrobial gallium(III) complexes [109,116,120].
Moreover, it is also important to note that most studies exhibited the antimicrobial activity of gallium compounds employed iron-poor media, usually through the addition of an iron chelator. Indeed, high concentrations of iron have shown to reduce gallium activity. This was shown by Hijazi et al. who provided a comparative study of Ga(NO3)3, gallium maltolate and gallium protoporphyrin IX, belonging to the first, second and third generation of Ga(III) formulations, respectively [104]. They investigated the antimicrobial activity of these three compounds against ESKAPE species (E. faecium, S. aureus, K. pneumoniae, A. baumannii, P. aeruginosa and Enterobacter species) under different growth conditions (standard culture medium Mueller Hinton broth, MHB; iron-depleted MHB, DMHB and RPMI-1640 supplemented with 10% human serum, RPMI-HS) containing different iron proportions. The authors showed that the antibacterial properties of the gallium compounds depend strongly on the media. All ESKAPE species were resistant to the more labile compounds Ga(NO3)3 and gallium maltolate in MHB and DMHB (MIC > 32 μM), while GaPPIX showed some bactericidal activity under these conditions against S. aureus (MIC = 0.06–0.12 μM) and A. baumannii (MIC = 16–32 μM) strains. Conversely, in RPMI-HS, the presence of serum albumin, which interferes with GaPPIX but not with Ga(NO3)3 or gallium maltolate indicates that among the three Ga(III) compounds tested, the FDA-approved gallium nitrate and the orally active gallium maltolate were the most effective under conditions that better simulate the low iron content in in vivo environment.
Recently, Pandey et al. introduced and assessed the gallium(III) complex of ciprofloxacin-functionalized siderophore desferrichrome as a potential theranostic conjugate [121]. They demonstrated the ability of their compound to act both as a potential therapeutic system for bacterial infection using an in vitro assay and a tracer-based approach employing the radioactive 67Ga. Their results highlighted first the good antibacterial activity of their theranostic gallium conjugate against both Gram-negative (E. coli, MIC = 0.23 μM) and Gram-positive (S. aureus, P. aeruginosa and K. pneumoniae with MIC = 1.9, 3.8 and 12.5 μM respectively) strains in iron-deficient media. Secondly, the authors showed that their radiolabeled-67Ga complexes were able to quantify time-dependent in vitro uptake in bacteria, and in vivo pharmacokinetics in mice.
In conclusion, several studies have demonstrated the antimicrobial activity of Ga(III) both in vitro and in vivo. These promising results raise the hope that gallium will confirm its efficacy in clinical trials and will become a valuable therapeutic option in order to cure untreatable bacterial infections. The recent development of gallium conjugates as new theranostic platforms also opens the door for future investigations in bacterial infections by combining both diagnostic and therapeutic tools.
2.7. Bismuth
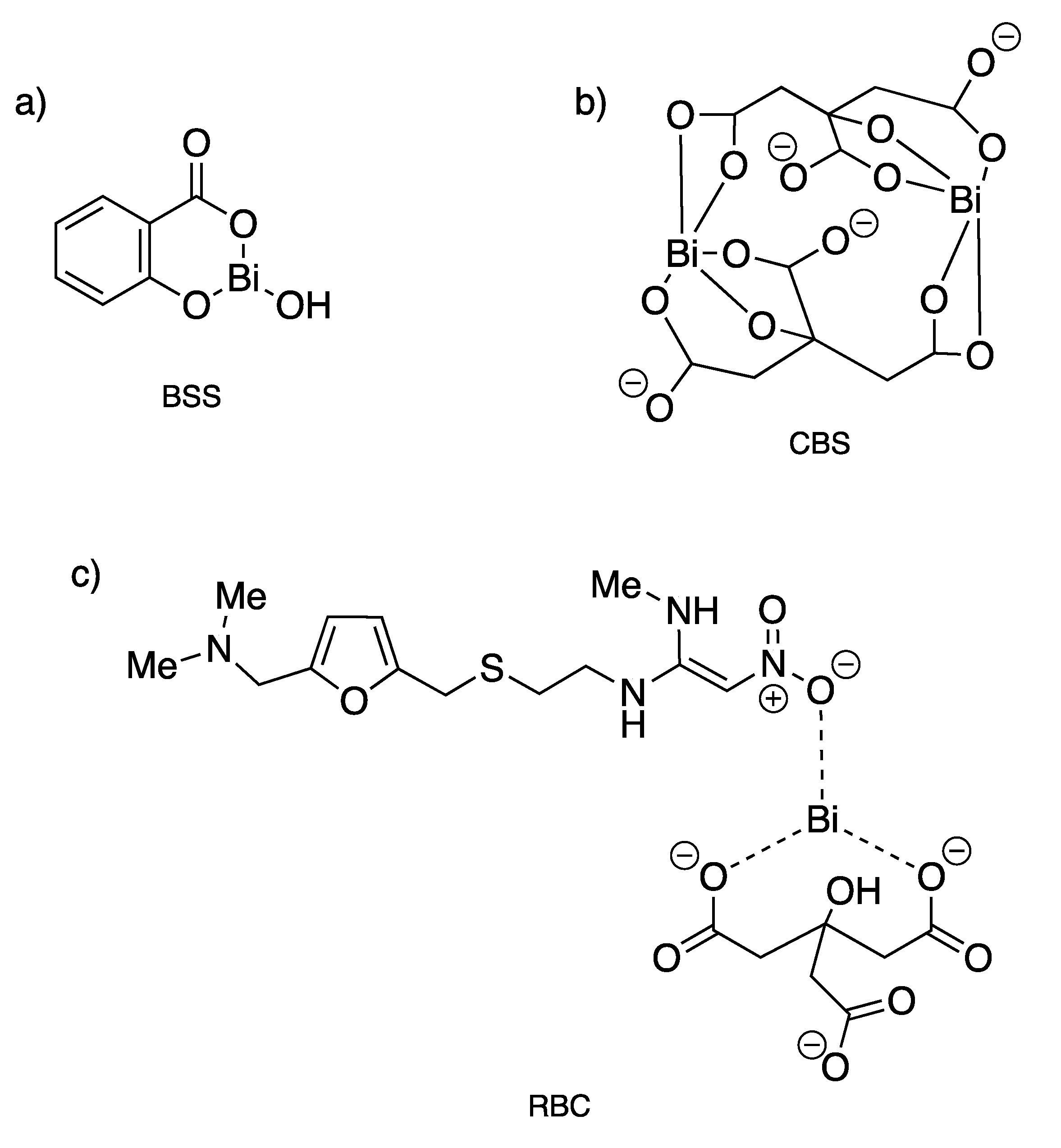
Since the 18th century, bismuth compounds have been used in medicine for the treatment of syphilis, colitis, wound infection and quartan malaria, but mostly for gastrointestinal disorders [122]. Indeed, Bi(III) is well known to exhibit remarkably low toxicity against humans as it is well tolerated at high doses, while being potently toxic against bacteria. Three bismuth-based drugs, namely bismuth subsalicylate (BSS, Pepto-Bismol®), colloidal bismuth subcitrate (CBS, De-Nol®) and ranitidine bismuth citrate (RBC, Pylorid®), have been used clinically in combination with antibiotics to treat infection associated with Helicobacter pylori (Figure 15), a bacterium that leads to the generation of gastritis, ulcers in the gastrointestinal tract, and gastric cancers [123]. Clarithromycin or metronidazole used as the antibiotics of choice to kill this bacterium induce actually a decrease in bactericidal efficiency over time with an increase bacterial resistance. This acquired resistance can then be partially overcome through coadministration of some antibiotics (metronidazole, tetracycline hydrochloride, amoxicillin, clarithromycin) with CBS or RBC, so-called bismuth-based triple or quadruple therapy [124,125]. Generally, the antiulcer activity of bismuth-containing medicines is explained by the precipitation of bismuth, probably as BiOCl and bismuth citrate complexes, within the ulcer crater resulting in the formation of a protective coating, which contributes to the healing of the lesion [122].
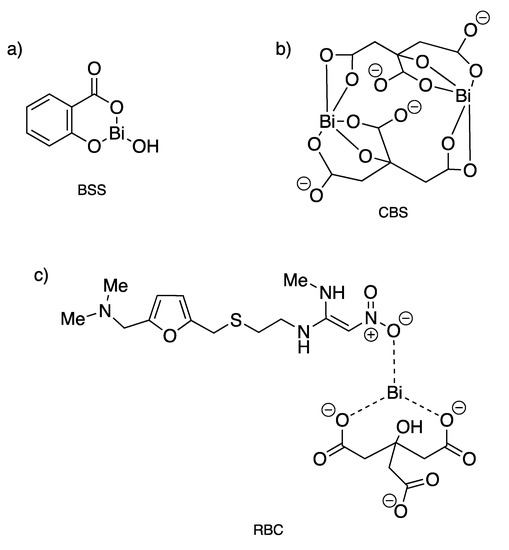
Figure 15.
Structures of clinically used bismuth(III) compounds to treat infection associated with H. pylori. (a) Bismuth subsalicylate; (b) colloidal bismuth subcitrate; (c) ranitidine bismuth citrate [123].
Even if the exact molecular mechanisms describing the anti-H. pylori activity of Bi-based drugs have not been fully understood, the biological targets of such compounds are clearly related to their binding with specific proteins and enzymes [126]. Recent structural studies of bismuth complexes have indicated that Bi(III) has a variable coordination number (from 3 to 10), an irregular coordination chemistry and an acidic behavior, properties which render this metal ion highly effective to interact with a wide range of proteins. Among all of them, transferrin and lactoferrin proteins used by H. pylori for iron acquisition have been shown to be the specific targets of different Bi-based compounds. The binding of bismuth to these proteins can prevent the acquisition of iron into the pathogens, resulting in the perturbation of their biological pathways and their death [126]. It is also well known that Bi(III) has a high affinity with thiolate ligands, and thiolation of Bi(III) is thought to be one of the major biochemical fates of bismuth in biological fluids and cells. Bi(III) readily binds to the cysteine residues in amino acids and peptides, making Bi-based drugs particularly effective for inhibiting several enzymes from H. pylori, for example, ureases or cytosolic alcohol dehydrogenase (ADH) [127,128]. Urease, a dinuclear Ni(II) enzyme that converts urea into ammonia and carbonic acid, is crucial for the bacteria to grow in the highly acidic stomach environment. Zhang et al. demonstrated, for instance, that their bismuth complexes, Bi(EDTA), Bi(Cys)3 and RBC, inhibited urease activity effectively through both competitive and non-competitive inhibition modes. The authors suggest that Bi(III) binds to a cysteine residue of the enzyme located at the entrance of the urease active site [127]. Additionally, CBS has also been shown to inhibit alcohol dehydrogenase (ADH) in H. pylori. ADH is a zinc-containing enzyme responsible for the oxidation of alcohols to acetaldehydes, which are toxic to gastric cells and causing mucosal damage. Jin et al. proposed that the inhibition of ADH by Bi(III) is probably due to its direct interference with the zinc(II) binding sites through a non-competitive process involving interactions with thiol groups [128]. There are an increasing number of novel Bi(III) complexes that have been developed recently as potential agents for the treatment of H. pylori. The group of Andrews designed, for example, numerous bismuth-based complexes with excellent anti-H. pylori activity based on aminoarenesulfonate ligands possessing MIC values (MIC = 0.049 μg/mL) much lower than those obtained for BSS (MIC = 12.5 μg/mL), CBS (MIC = 12.5 μg/mL) and RBC (MIC = 8 μg/mL) for the strains 251 and B128 [129].
Furthermore, several bismuth complexes developed over the years have been accessed for other antibacterial and antifungal activities. For instance, Lessa et al. designed different Bi(III) thiosemicarbazone complexes, as this class of thiol/thione compound is reported to have wide pharmacological applications like antiparasitic, antibacterial, anticancer and antiviral properties [130]. The authors demonstrated that upon coordination to Bi(III), the antibacterial activities of both thiosemicarbazones and bis(thiosemicarbazones) increased against the Gram-positive bacteria, especially against S. aureus, where the activity of some of their complexes (MIC = 5.5–6.1 μM) was 15 to 64 times more potent than their corresponding free ligands. The same group also reported the antimicrobial activity of hydrazone derivatives Bi(III) complexes against different bacterial (S. aureus, E. faecalis, S. epidermidis, P. aeruginosa) and fungal (C. albicans) strains [131]. In this study, the ligands investigated were found to be inactive against the panel of microorganisms previously mentioned. However, upon coordination to Bi(III), the antimicrobial activity increased significantly against all Gram-positive bacteria and to a lesser extent against Gram-negative P. aeruginosa. Among all of the bismuth-based complexes developed, two of them were shown to be more active against S. aureus (MIC = 0.2 and 0.3 μM) than tetracycline (MIC = 7.2 μM), a clinical antibiotic used as positive control. Additionally, one of their Bi(III) hydrazone complexes exhibited better activity (MIC = 44 μM) against C. albicans than fluconazole (MIC = 59 μM), a well-known antifungal agent. Unfortunately, despite these very promising results, no further experiments were performed by the authors in order to explain the mechanism of action of such bismuth compounds on their antimicrobial activities.
Recently, Sun and coworkers demonstrated that CBS and related bismuth compounds could be novel and potent inhibitors of metallo-β-lactamases (MBLs), especially NDM-1 (New Delhi MBLs), VIM-2 (Verona integron-encoded MBLs) and IMP-4 (imipenemases) [132]. MBLs are zinc(II)-containing enzymes that activate a nucleophilic water molecule to cleave the β-lactam ring, conferring bacterial resistance to currently used organic antibiotics. In contrast, Bi(III) compounds inhibit at micromolar levels the enzyme through an irreversible replacement of two Zn(II) ions by one Bi(III) ion in the active site, leading to the abolition of MBL activity both in vitro and in vivo. The authors explained this unique mechanism by the intrinsic properties of Bi(III), which are the relatively large size of the metal ion and its high coordination numbers, and its thiol-philic feature, which makes interactions with the cysteine residues in the active site easy. Thus, the inhibition of MBLs by Bi(III) compounds restores the antimicrobial activity of β-lactam antibiotics (meropenem, MER), whereas CBS itself showed no or minor growth inhibition toward either NDM-1-positive or -negative bacteria. Then, the combined use of CBS with MER led to a synergistic effect that may represent a new approach for the discovery of MBLs therapies [132].
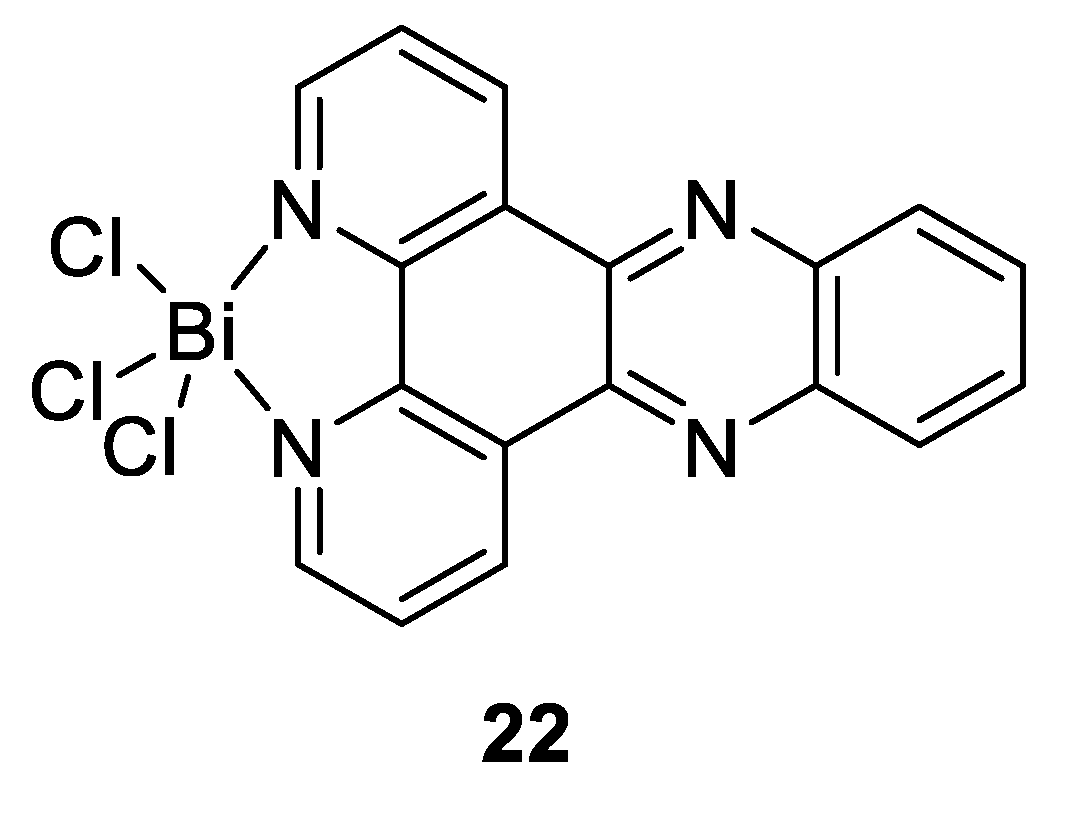
Other studies have also revealed that bismuth drugs could have unexpected medicinal applications due to the ability of Bi(III) to exert its action through binding to the key enzymes, and thereby disrupt key pathological pathways in the pathogen. As mentioned above, Bi(III) has a high affinity with thiolate ligands, that makes enzymes possessing cysteine residues in their active sites potential targets for this metal ion. This feature was used by Yang et al., who tested different bismuth compounds in order to evaluate their antiviral activities against severe acute respiratory syndrome coronavirus (SARS-CoV) [133]. They showed that RBC (Figure 15c) was particularly efficient as a strong inhibitor of the SARS-CoV helicase, an enzyme containing a cysteine-rich Zn(II)-binding domain, which blocks virus replication. There have also been recent reports on the development of new Bi compounds that can successfully be used to treat leishmaniasis, a disease caused by some parasites [134]. Those compounds were effectively found to be potential alternatives to current antimony(V)-based antileishmanial drugs given by their close proximity in the periodic table, their similar biological chemistry in terms of their effects and their modes of action, while providing lower mammalian cell toxicities and opportunities of oral delivery. Andrews and Demicheli are the two main working groups investigating the use of bismuth complexes on Leishmania. For example, Lizarazo-Jaimes et al. reported the synthesis of the [Bi(dppz)Cl3] complex (compound 22, Figure 16) and tested its antileishmanial activity against wild-type (WT) and Sb-resistant (SbR) strains of L. infantum chagasi and L. amazonensis, which are associated with visceral and cutaneous leishmaniasis, respectively [135]. The authors showed that this Bi(III) complex was slightly more active than dppz alone, and at least 77 and 2400 times more active than potassium antimonyl tartrate used as reference in WT and Sb(III)-resistant strains, respectively. Because the starting reagent BiCl3 had very little activity against Leishmania, it was suggested that the metal ion alone was not sufficient to have anti-leishmaniasis activity, but rather improved the ability of dppz to inhibit the growth of promastigotes through complexation. The authors further proposed that the leishmanicidal activity of the dppz complex may occur via its interaction with parasite DNA through intercalation and/or a modulation of the hydrophilicity profile of the compound.
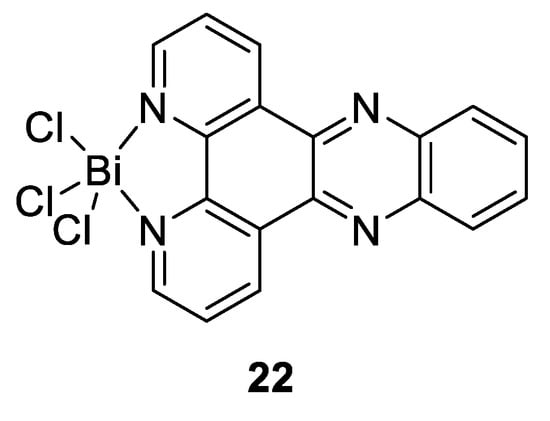
Figure 16.
Structure of antileishmanial-Bi(III) complex [Bi(dppz)Cl3] [135].
Overall, bismuth-based compounds are routinely used for the treatment of gastrointestinal disorders and were recently found to possess potential antimicrobial, antiviral and antileishmanial properties. It is likely that proteins are the key biomolecular targets of Bi(III), particularly proteins possessing thiol-rich domains or metal binding sites with attractive coordination environments for Bi. Due to the low toxicity of Bi(III) salts and the development of novel bismuth-based compounds with considerable and varied biological activity, there is no doubt that Bi has the potential to play new and important roles in medicinal chemistry in the upcoming years.
2.8. Vanadium
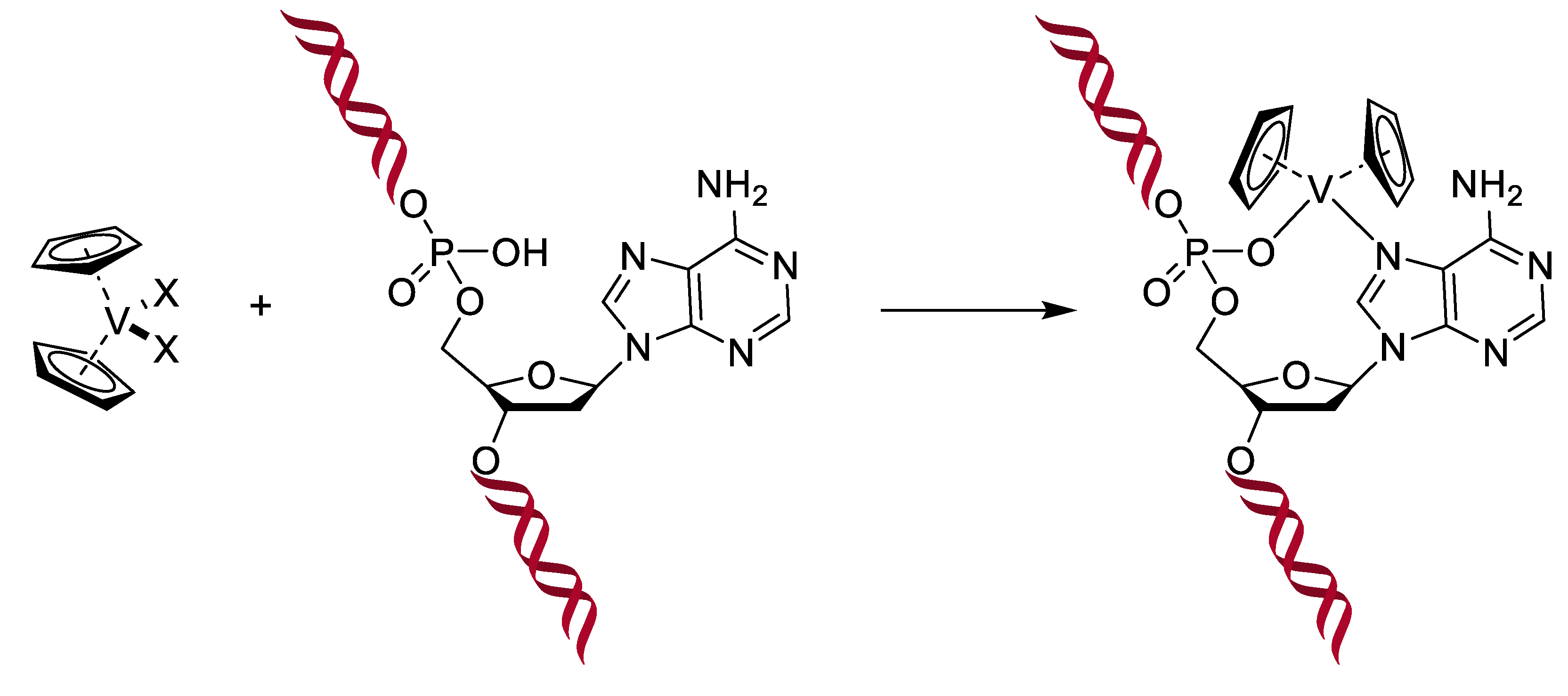
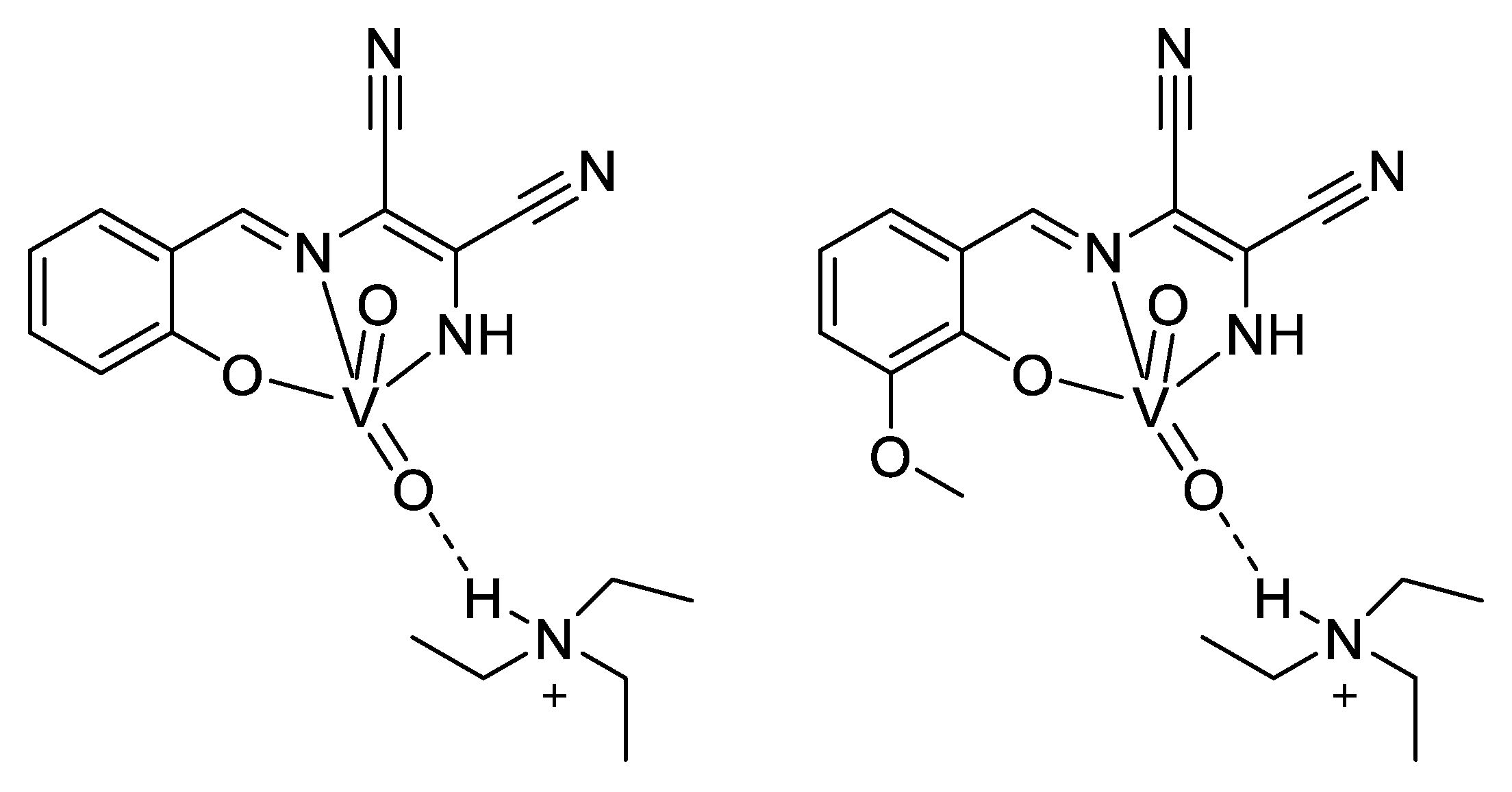
Vanadium is present as cofactors in certain enzymes, such as haloperoxidases and in some kinds of nitrogenases expressed by nitrogen metabolizing bacteria. It is even found at high concentrations (0.15 M) in the blood of some marine animals like sea squirts [136]. Moreover, the structure of the vanadate VO43− is very close to that of phosphate PO43−. In addition, there are other similarities, such as in esterification-type reactions; vanadate can therefore be considered a competitor of phosphate. Knowing the great importance of phosphate in several very different biochemical pathways (kinases, ATP, etc.), vanadate is becoming an interesting target to study, and that is even more true when looking at their pKa (7.2 for phosphate means a similar ratio for H2PO4− and HPO42−, while it is 8.2 for vanadate, meaning that H2VO4− is the main species at the physiological pH) and at the coordination (phosphorous can typically not be coordinated by more than five ligands, while vanadium can accept six). Altogether, it was demonstrated that if vanadate easily replaces the phosphate in an enzyme, it can form stable complexes with the enzyme’s target, inhibiting the enzyme [135]. As a small aside about our antimicrobial topic, two consequences of this similarity with phosphate are in the treatment of diabetes and cancers: Vanadium is able to interfere in the metabolism of glucose, making it possible to reduce the acute as well as the secondary consequences of diabetes by mimicking insulin. Indeed, diabetes appears when the insulin-receptors of cells no longer recognize insulin. Yet, the linking of insulin to its receptors normally results in the phosphorylation of a tyrosine residue, which starts a chain reaction. In the absence of insulin, or in case of non-response, the tyrosine is dephosphorylated by another enzyme. Thus, when diabetics are treated with vanadate, it can bind to the active site of this last enzyme and inhibit the dephosphorylation, leading to an activation of the insulin signaling pathway even when the insulin receptor does not react to insulin [137]. Moreover, intervening in the phosphorylation reactions, vanadate therefore causes some issues for cells that have a high need for phosphate. This is the case for tumor cells, which present an uncontrolled development. Hence, uptake of vanadium complexes has been shown to induce cellular death in different kinds of tumors. Another proposed mechanism of action is the generation of ROS in tumoral cells, and it was also shown that vanadocene-derived compounds (by analogy with ferrocene) are able to interact with the DNA, forming a stable complex leading to apoptosis (see Figure 17). Other metallocenes show similar activity, particularly titanocene, molybdenocene, and niobocene [136,137].
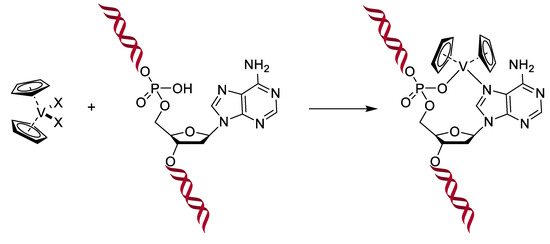
Figure 17.
Interaction of metallocenes within the DNA, X represents halogen atoms [137].
To summarize, vanadium participates in the regulation of the phosphate pathways, and is considered to be an essential oligo element, but may become toxic in too large quantities, explaining why these compounds stay aside for the moment [136,138] Nevertheless, to overcome the low biodisponibility of orally taken mineral salts, as only a very low amount of salt successes usually to pass the gastrointestinal tract into the blood system due to low lipophilicity, the hope is on vanadium complexes, which are currently a main part of the research about this element. The goal is therefore to maximize the biodisponibility with a non-toxic ligand, forming stable complexes in the gastrointestinal tract that are able to dissociate once in the bloodstream [136,139].
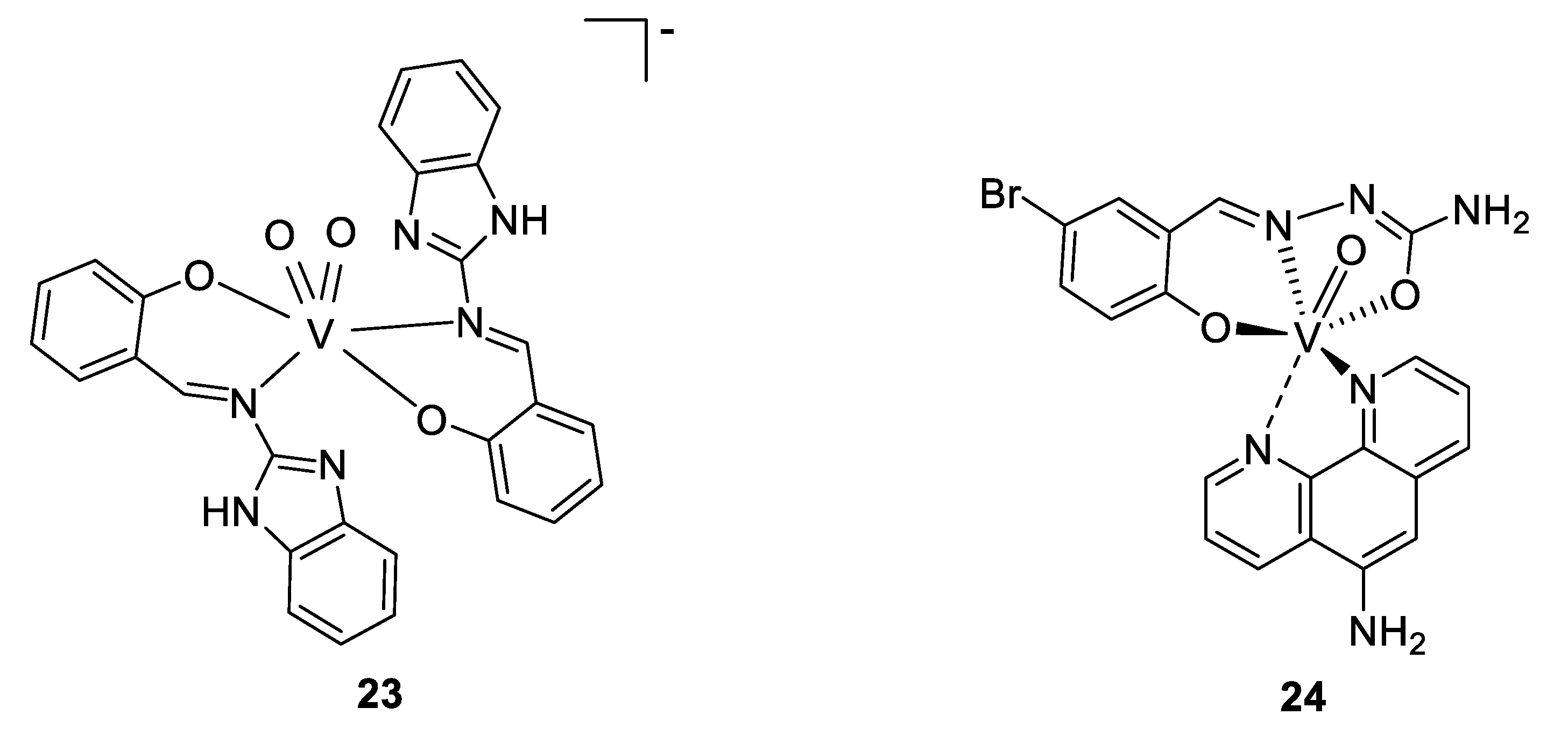
To come back to our antimicrobial focus, there is ongoing research surrounding the use of vanadium complexes against some parasites. One of the first studies reporting results in this domain was published in the early 2000s, where Barti et al. tested some protozoicidal organic molecules as ligands for different transition metals, like molybdenum, vanadium, and tungsten. All the complexes showed a lower IC50 against an amoeba (Entamoeba histolytica) than their respective ligands alone, and the best results were found for a vanadium complex of 2-(salicylideneimine)benzimidazole (Figure 18, compound 23), exhibiting an IC50 value of 2.35 µM (compared to 9.20 µM for the title ligand and 2.99 µM for the molybdenum complex; tungsten complex n.d.) [140].
The research continued (and is continuing) around some other parasites, mainly against tropical parasites, and particularly against Trypanosoma cruzi. This protozoan parasite causes Chagas disease, resulting in 10,000 deaths per year and 8 million infected people. Several publications dealing with vanadium complexes for potential antitrypanosomal treatment are available, and some of this work was done by the group of Dinorah Gambino. They proposed several vanadium complexes based on salicylaldehyde- and polypyridyl-derivatives ligands [141,142,143]. Another example is a vanadium complex of aminophen and bromosalycilaldehyde (Figure 18, compound 24), whose IC50 was shown to be in the low micromolar range (0.27–3.8 µM) [144,145] against T. cruzi, similar to the standard drug nifurtimox, but a live/dead assay only resulted in a trypanostatic effect, with the parasites recovering to normal growth after the cutting off treatment. Moreover, the compound did not present toxicity against murine macrophages serving as a mammalian model. Indeed, its IC50 is around 50 µM, almost 200 times higher than against T. cruzi, reflecting a high selectivity. Investigation around the mechanism of action showed that whereas only 2.4% of the dissolved complex was taken up by the parasites (which is a similar amount to what was observed for other metallodrugs such as cisplatin), a high concentration on vanadium was observed within the DNA and the RNA (0.089 ng of vanadium/µg of DNA, and 0.006 ng of vanadium/µg of RNA), suggesting a strong interaction with DNA. A deficiency of the mitochondria is hypothesized as well, due to the high level in the cytoplasm of some organites used in the mitochondria. Moreover, the analysis of the protein expression concluded to overexpression of transporters and drug efflux, and of some proteins involved in the transcription. This is coherent with the presence of vanadium in DNA, the deficiency of the mitochondria, and overexpression of some proteins involved in reduction/oxidation pathways and hydrolysis, suggesting the vanadium complex to cause some redox disorders [144,145].
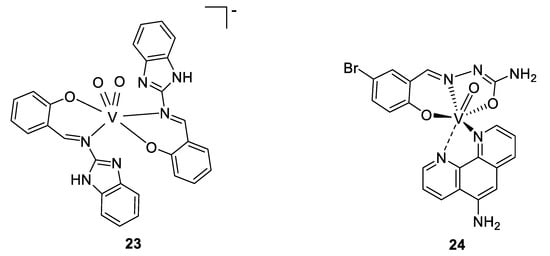
Figure 18.
Proposed structures for compound 23, and V(IV)-O(Brsal)(aminophen) 24 [140,144,145].
Figure 18.
Proposed structures for compound 23, and V(IV)-O(Brsal)(aminophen) 24 [140,144,145].
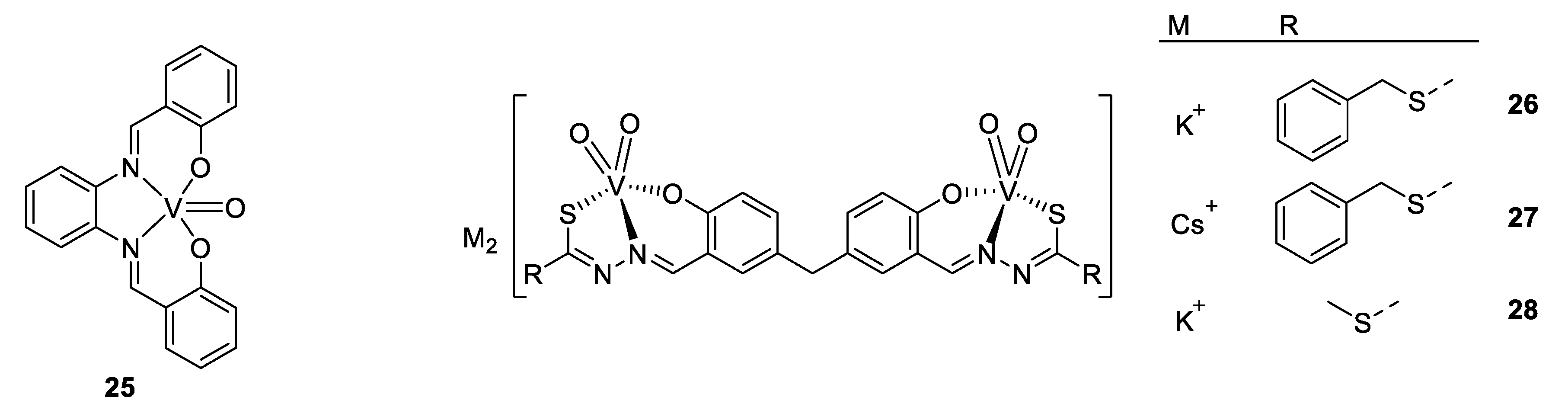
Vanadium complexes were also developed against Leishmania species, another parasite responsible for leishmaniosis, and close to T. cruzi. The next example deals with a vanadium–stilbene complex (Figure 19, compound 25), which is again based on a salicylic acid moiety like the previous examples. These “salen-derivative” ligands have some advantages, e.g., the ability to form very stable complexes. X-ray analysis of the complex showed a slightly distorted square pyramidal structure around the vanadium cation with oxygen atoms being slightly closer to vanadium than the nitrogen atoms, which can be explained by the strong Lewis acid properties of V(IV), and the anionic character of phenol compared to the imine [146]. The complex displays an IC50 of 3.51 µM against L. amazonensis, which is slightly higher than for other vanadyl polypyridyl complexes. The mechanism of action was proposed to involve the mitochondria, and authors underline the great interest of this target, as the mitochondria of parasite work differently from those of mammalian, resulting in a higher selectivity, and moreover, the parasite being unicellular species, they have only one mitochondrion [147].
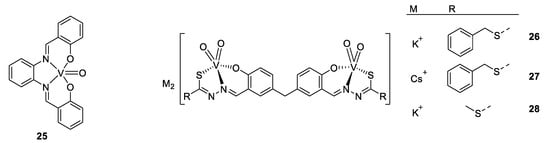
Figure 19.
Structures of 25 V(IV)O(sal-HBPD) and compounds 26–28 [146,147,148].
As V(IV) was demonstrated to be subject to oxidation, our last, but not least, example is V(V) complexes, active against an amoeba, Entamoeba histolytica (see Figure 19 compounds 26–28). Its activity is similar to those seen before, with an IC50 value of around 0.09–0.85 µM, while the standard drug for amoebas, Metronizadol, has 1.68 µM [148].
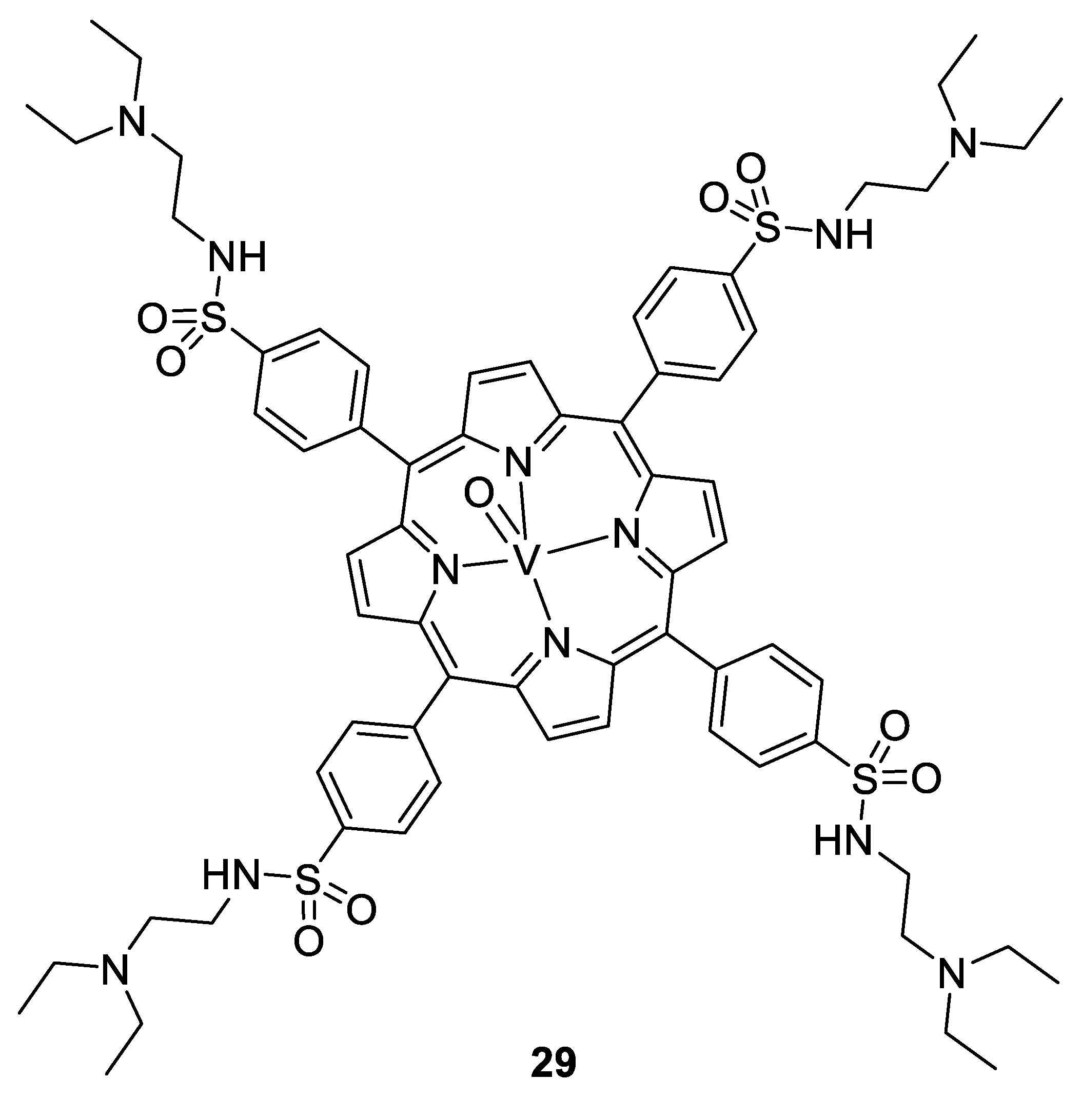
Vanadium complexes can be efficient against viruses and bacteria too. In particular, oxovanadium complexes of thiourea, [149] polyoxovanadates, [150], and oxovanadium porphyrins showed efficiency against HIV, with more than 97% of inhibition at 5 µM for the compound 29 (Figure 20). They act by linking to the HIV-1 reverse transcriptase, and a complementary computational study proposed a binding to the CD4 protein, blocking the entry of the virus to the cells [151]. Some polyoxovanadates incorporating silicon, tungsten, and/or boron show activity even at less than 1 µM (K5[SiVW11O40] and K7[BVW11O40]) [150].
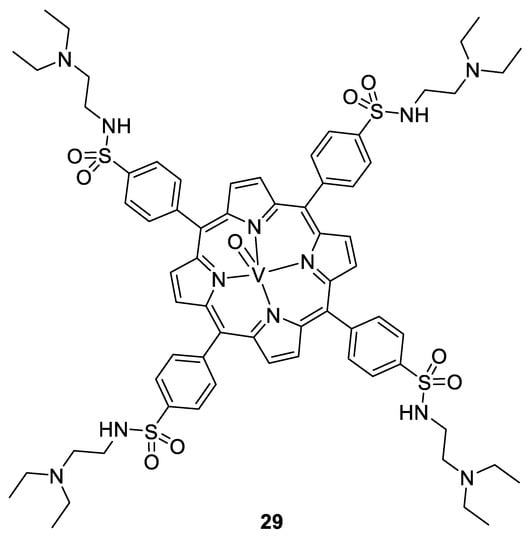
Figure 20.
V(IV)O-porphyrin complex 29 [151].
Hydrazone complexes of vanadium(V) 30–32 (see Figure 21) with an octahedral geometry were obtained by Wu and co-workers. These three V(V) complexes show antibacterial activity against B.subtilis, S. aureus, and E. coli, with MICs between 1.2 and 37.5 µg/mL, which is much better than their respective title ligands (9.4 to >150 µg/mL, Table 2). It may be noted that complex 30 shows the lowest potency, illustrating the key importance of the substitution of the aromatic ring [152].
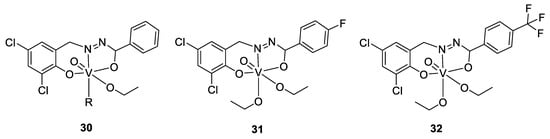
Figure 21.
Structures of complexes 30–32. R represents an ethanol or a methanol ligand [152].

Table 2.
MIC (µg/mL) of the complexes 30–32, and of their title ligands [152].
Schiff base complexes of vanadium also display antibacterial activity, as it was experimented by Khaleghi et al. Results in measuring the inhibition zone are, however, dependent on the tested bacteria, as the two vanadium complexes (see Figure 22) were not active at all against Gram-negative bacteria P. aeruginosa, E. coli and K. pneumonia. They nevertheless showed higher activity than the vanadium sulfate and the ciprofloxacin standard against the Gram-positive bacteria Listeria monocytigenes, E. faecalis and against the fungus C. albicans (respective MIC: 0.62, 1.25 and 2.5 mg/mL). Finally, the vanadium complexes impaired also the formation of biofilms of some bacteria [153].
Figure 22.
Schiff-base complex of vanadium(V) [153].
2.9. Other Metal-Based Complexes as Antimicrobial Agents
In recent decades, many other metal-based complexes have been reported in the literature due to their very promising antimicrobial properties. Here, we will discuss the most relevant examples.
As mentioned in the early stage of this review, gold has garnered attention for its medicinal properties since the 19th century in treating syphilis, tuberculosis and inflammatory rheumatoid diseases. Auranofin (compound 33, Figure 23), for example, is a gold-based FDA-approved arthritis drug and is currently used in clinical trials for its anticancer applications [18]. Recently, it has been repurposed as a potential antimicrobial agent and was found to be effective against many Gram-positive bacteria, including multidrug resistant strains, as well as M. tuberculosis [154]. However, almost no activity was observed toward Gram-negative species. The exact molecular mechanism of auranofin is still unclear but it is postulated that its activity is provided by the inhibition of the bacterial thioredoxin reductase (Trx), an essential enzyme for maintaining the thiol–redox balance and protecting against reactive oxidative species, through the interaction with the redox-active selenocysteine or cysteine residues that are present in the active site [155]. The ineffectiveness of auranofin toward Gram-negative bacteria was suggested to be the result of their glutathione system that is able to compensate for the loss of the reducing ability of Trx [154]. An alternative hypothesis was also proposed, suggesting that the outer membranes of Gram-negative bacteria were able to prevent auranofin accumulation as an effective barrier [156]. In this context, some groups have decided to design auranofin analogs in order to improve the antimicrobial properties of this gold-based drug. For example, Wu et al. prepared 40 auranofin analogs by modulating the structures of the thiol and phosphine ligands, and tested their activities against ESKAPE pathogens, establishing a structure–activity relationship [157]. They demonstrated that the Gram-negative activity of auranofin could be effectively modulated by altering the thiol and phosphine ligands, while some compounds exhibited bacterial inhibition (MIC) and killing (MBC) activities up to 65-fold higher than that of auranofin (compounds 34–36 Figure 23). Moreover, preliminary in vitro cytotoxicity experiments performed on A549 cells (human lung epithelial cells) revealed that the active analogues had mammalian cell toxicities either similar to or lower than that of auranofin. These findings suggest that the properties of auranofin can be optimized for additional antimicrobial applications. Furthermore, other studies have shown that auranofin is efficacious in a mouse model of MRSA systemic infection [156], demonstrating its ability in in vivo experiments. It was also demonstrated that auranofin can be used in combination with traditional antibiotics (ciprofloxacin, linezolid, gentamicin) as an alternative strategy in order to provide a significant protection from mortality associated with MRSA infections and to reduce the potential emergence of drug resistance [156]. Taken together, those studies strongly suggest that auranofin has significant promise to be repurposed as an effective antimicrobial agent for the treatment of systemic bacterial infections.
Figure 23.
Structure of auranofin (33) and its antimicrobial analogues [157].
Only limited reports on the antimicrobial properties of organometallic iridium(III)-based complexes have been published in the literature. In 2013, Keene and Collins compared the bacteriostatic and bactericidal activities of chloride-containing ruthenium(II) and iridium(III) complexes against four strains of bacteria [158]. They have shown that unlike Ru(II)-polypyridyl complexes, the Ir(III) counterparts were only bacteriostatic, rather than bactericidal. The authors suggested that the origin of the difference in activities probably comes from the higher overall charge of iridium compounds (+4 before aquation of the chloride ligands compared to the +2 for the ruthenium counterparts), which probably prevents their crossing through the membrane. Additionally, the chloride anions of the iridium complexes were found to be more labile than for the corresponding ruthenium complexes, which might also affect their accumulation within the bacteria, and thereby their bactericidal activity. Nevertheless, further studies based on cyclometallated polypyridyl iridium complexes revealed promising antibacterial activities against both Gram-positive and Gram-negative bacteria and demonstrated that their mechanisms of action are mainly attributed to their ability to interact with DNA through intercalation or to act as photosensitizers, generating ROS upon light irradiation [158,159,160,161,162]. Furthermore, the third-row transition metal ion Ir(III) is also supposed to be relatively kinetically inert and therefore is likely to reach its drug target sites with its initial ligands still bound. This could offer the opportunity to design novel metallo-antibiotics associating, all in one molecule, metal ions and active organic molecules. For instance, Chen et al. introduced biguanide ligands, including the antidiabetic drug metformin, into organoiridium cyclopentadienyl complexes (compound 37, Figure 24) [162]. Several of these complexes exhibited potent activity against both Gram-negative and Gram-positive bacteria, including MRSA with MICs as low as 0.125 μg/mL, which is four times more potent than the clinically used antibiotic vancomycin. However, all biguanide complexes have little activity towards the P. aeruginosa strain (MIC > 32 μg/mL). The authors hypothesized that this inefficiency was probably due to the membrane permeability and the presence of a very effective efflux system, making this bacterial strain particularly resistant to many antibiotics. Additionally, some compounds also displayed high activity against the fungal strains C. albicans and C. neoformans, with MICs values as low as 0.25 μg/mL, which is 32 times more active than the reference compound fluconazole. Moreover, when co-administered with vancomycin, three complexes showed synergistic activity and can restore the activity of the antibiotic against vancomycin-resistant Enterococci. It was also shown that some of these compounds could disrupt S. aureus biofilm formation. Another important point is that these novel Ir(III)-biguanide complexes exhibit low toxicity toward mammalian cells, indicating high selectivity. Furthermore, the authors investigated the potential targets and the mechanism of action of their complexes. They suggested that their organometallic complexes could enter the bacteria (without disrupting the cell walls) and then undergo ligand exchange reaction with thiol-containing biomolecules, displacing the biguanide ligand, which might interfere with important cell enzymes. As the biguanide ligand on its own had no antimicrobial effect, it is supposed that the overall structure of the complex is required in order to induce a biological action.
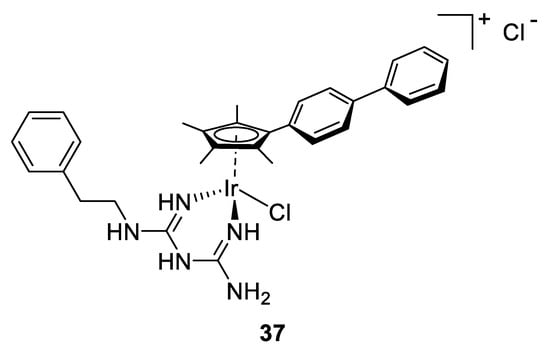
Figure 24.
Structure of antimicrobial biguanide Ir(III) complex [162].
Cobalt is an essential metal that is found in very low abundance in the body and is well known to possess biochemical roles, especially as an integral part of vitamin B12, a cofactor involved in different human key processes such as in fatty acids metabolism, in the synthesis and regulation of DNA, and in energy production. Cobalt in biological systems exists almost exclusively as Co(II) or Co(III), though Co(I) and Co(IV) are known as well [163]. In coordination complexes, these two latter predominant oxidation states make them particularly suitable for therapeutic applications. Co(III) ions form predominantly octahedral inert complexes, while Co(II) ions can induce the complexation of four, five or six ligands and their d7 electronic configuration makes Co(II)-based complexes particularly labile. The biological activity of cobalt complexes was first reported by Dwyer et al. in 1952, who exhibited in vitro micromolar bactericidal activity of their compounds, while a low systemic toxicity was observed in mice [89]. Since then, different studies have shown that cobalt(III) complexes could be efficiently used for antibacterial, antifungal and antiviral applications [164]. Most of the time, their antimicrobial properties were attributed to the greater lipophilic nature of the complexes as compared to the parent ligands, which improves their penetration across the cell membrane and thereby their activity. Furthermore, it has been shown that a platform based on both Co(II) and Co(III) metal ions can be designed with labile ligands that can undergo exchange with biomolecule moieties such as amino acid side chains. To date, the only cobalt-based complex that has reached phase II clinical trials is Doxovir (CTC-96, compound 38, Figure 25), a Co(III) Schiff base complex used for topical treatment of herpes simplex virus type 1, and it was demonstrated that this compound could also be used to treat viral eye infections [165]. Although the entire mechanism of action of this drug is not fully understood, its promising antiviral activity involved the inhibition of membrane fusion events required for viral entry into cells, through ligand exchange with proteins [166]. It was then suggested that the Co(III) Schiff base complex undergoes a dissociative exchange of its labile 2-methylimidazole axial ligand and interacts with proteins by coordinating histidine residues [167]. The large difference in reactivity between the Co(III) and Co(II) allows specific activation mechanisms and makes cobalt an ideal candidate for use in redox-activated prodrugs [168,169]. It is well known that the ligand exchange rates of Co(III) complexes are several orders of magnitude lower than those of the Co(II) complexes [170]. An appropriately designed Co(III) complex can then undergo bio-reductive activation in order to produce a cobalt species that can acts as an effective redox-responsive drug carrier. This principle can be applied to the selective release of bioactive ligands, which means that a complex can be administered as an inert and biologically inactive Co(III) complex, and then be converted to a labile and active Co(II) complex. Such a strategy has been widely studied in the past decades for anticancer applications [171,172]. However, to the best of our knowledge, no reports have been published in the literature concerning the use of cobalt complexes for the delivery of antimicrobial drugs through a bio-reductive activation. This way could be further explored by researchers for the development of new Co-based complexes that are able to induce selective release of active ligands in specific chemical environments, such as in bacteria.
Figure 25.
Structure of cobalt-based complex Doxovir used for topical treatment of HSV-1 [166].
Despite nickel being considered in very low quantities as an oligo-element, essential for animals and vegetable growth, it is classified in high quantities as carcinogenic for humans, explaining maybe why nickel complexes have not been investigated as much for their antibacterial properties as other metal ion complexes. Indeed, there is currently no nickel-based drug available, and only a few publications have investigated the antibacterial effects of nickel complexes further than fast in vitro assays giving the inhibition zone or the MIC. Nevertheless, some examples of the use of Schiff bases as a ligand for potential antibacterial nickel complexes can be found in the literature. Chalabian F. et al. studied for instance the activity of two nickel–Schiff base complexes 39–40 (Figure 26). Compared to the antibiotic standard gentamycin, the metal complexes showed higher inhibition area against Streptococcus pyogenes, but less good results against Bacillus anthracis and Staphylococcus aureus. It was also noticed that if the title ligand and the metal complexes had identical MICs against the two first bacteria strains, the ligand alone displayed the highest MIC against S. aureus (25 mg/mL), while the complexes showed lowest (6.25 mg/mL) [173].
Figure 26.
Structures of Schiff base complexes of nickel(II) [173].
Another kind of nickel ligand is the thiocarbamide. However, although respectable, the activity of the hexakis thiocarbamide nickel(II) nitrate [Ni[CS(NH2)2]6](NO3)2 is lower than other known antibiotics, with inhibition zones, for instance, only 0.4 to 0.7 times those of chloramphenicol [174]. It would nevertheless be interesting to test these compounds against resistant bacteria, as they probably do not target the same essential metabolic pathways.
Rather than developing completely new therapeutic agents, other scientists have tried to improve the efficiency of current antibiotics by using them as ligands. Hence, by comparing the activity of Ceclor with its Co- and Ni- complexes against both Gram-negative and Gram-positive bacteria strains with the disk-diffusion method, it was observed that both metal derivatives (Figure 27) showed higher inhibition areas that the title Ceclor, and the Ni complex had even better results than the Co complex against Streptococcus pyogenes and Escherichia coli [175]. If the role of cobalt and nickel is not described in the paper, it is known that Ceclor, as a β-lactam antibiotic, acts by impairing the cross-linking of the peptidoglycans forming the bacterial cell wall. A second example is the nickel complexes of the kefzol, another β-lactam antibiotic. They displayed a higher inhibition zone than the antibiotic alone against Staphyloccocus aureus, Escherichia coli, Klebsiella pneumonia, and Proteus mirabilis. It was also noticed that the NiL2 complex had better efficiency than the NiL complex, which was in accordance with the antibiotic nature of the ligand [176].
Figure 27.
Proposed structures of nickel complexes with kefzol [176].
Moreover, nickel is present as cofactors in a half-dozen of bacterial enzymes, most of them involved in the production or use of gases. A non-exhaustive list of enzymes using nickel ions includes the urease which catalyzes the hydrolysis of urea, a kind of superoxide dismutase, which uses nickel ions against reactive oxidative species, the glyoxylase I of Leishmania major, which needs nickel ion to act as Lewis acid in the conversion of methylglyoxal to lactate (in other eukaryotic organisms, the nickel ion is replaced by zinc), and the NiFe hydrogenase, which catalyzes the reversible redox reaction between protons and dihydrogen. Originally, one subunit of the Methyl-CoM reductase used a nickel ion in its +1 oxidation state, and one of its discussed mechanisms includes an intermediate stage with a Ni(III) ion [177,178]. Thus, bacteria need nickel ions for normal growth, and it has also been shown that the calprotectin, a chelator secreted by the human immune system, can confine nickel ions. Due to these higher nickel requirements for bacteria compared to humans, some studies have therefore focused on the chelation of the nickel ions naturally present in the human body to impair bacterial growth, rather than investigating the antibacterial properties of this element. From this point of view, and knowing the great importance of metal ions in general for the human proteins, the need to have a very selective chelator lead to the testing of dimethylglyoxime as a potential antibacterial compound. Dimethylglyoxime is a bidentate ligand, and two molecules coordinate Ni(II) with a square planar geometry (Figure 28, compound 41). In vitro assays on one Klebsiella pneumonia strain and two Salmonella enterica serovar Typhimurium strains resulted in MICs in the low millimolar range, and the conclusion that the dimethylglyoxime has a bacteriostatic effect rather being bactericidal [179]. As expected, the presence of dimethylglyoxime inhibits the hydrogenase activity of the two Salmonella and impairs the urease enzyme of a Klebsiella species. Furthermore, while some other toxicology tests are required before considering dimethylglyoxime as a drug candidate, preliminary assays showed that high quantities of the chelators are not toxic for mice and larvae, but better reduce mortality for mice and insects in case of Salmonella infection, even if some traces are measurable in the liver of the mice [179].
Figure 28.
Structure of dimethylglyoxime and nickel-dimethylglyoxime complex [179].
Nickel chelators are also used in the case of allergies. Indeed, this element is the most common cause of metal allergy by skin contact and accounts for more cases than all other metals together. Thus, in the case of eczema caused by nickel, the use of chelators can help to treat dermatitis by sequestering the responsible metal ions [180].
In opposition to nickel, manganese ions are present in several enzymes as cofactors, both in humans and pathogens. Then, chelating the cellular manganese could be tricky without disturbing the metal cation homeostasis of the host organism. Therefore, the research rather tried to look at the antibacterial effect of manganese complexes. Although no drug based on a manganese complex is currently under commercialization, preliminary evaluations of antibacterial properties of some complexes have been published. Thus, Chaudhary et al. synthesized a series of manganese(II) complexes 42. The ligands are constituted of two diaminopyridine or diethylenetriamine molecules linked on each side by an amide bond to a malonic, succinic, glutaric, or adipic acid linker. They result in macrocycles containing six nitrogen atoms able to coordinate the manganese ion, even if further analysis shows that only those belonging to the amide function coordinate the cation in a plane, accompanied by two chlorides on both sides to reach an octahedral geometry [181]. Antibacterial assays showed only a slightly improved activity of the macrocycles against Pseudomonas cepacicola, Staphylococcus aureus, and Xanthomonas compestris compared to the activity of the ligand moieties alone. For instance, by considering the ligand constituted of two diaminopyridine and two adipic acid molecules (compound series 42, model structure on left, n = 4, Figure 29), 3.5 and 4.1 mM of respectively adipic acid and DMAP are needed to get a 3 mm inhibition zone against P. cepacicola, while the manganese complex displays a 3-fold larger inhibition zone for only 0.9 mM. By comparison, at 0.9 mM the standard streptomycin results in a 3 mm inhibition zone. Moreover, there is a greater enhancement of some of the complexes (mainly those containing a DMAP moiety) over their building blocks at higher concentration. By multiplying the concentration by a factor of 2, the respective inhibition zones of DMAP, adipic acid and the Mn complex are 4 mm, 4 mm and 16 mm. However, the improvements are slighter against S. aureus (4 mm, 6 mm, 15 mm, whereas it is 17 mm for streptomycin) and X. compestris (4 mm, 6 mm, 12 mm; 5 mm for the streptomycin). Another issue is the results of the toxicity tests were nevertheless performed in vivo on rats, showing a lack of fertility [181].
Figure 29.
Structures of the manganese complexes series 42 [181].
Manganese is known for its antifertility properties, and other kinds of manganese complexes encountered the same issue. Bansal et al. developed ligands based on an imine obtained by the condensation of a salicylanilide with a sulphathiazole. One can notice the sulfonamide moiety, which has already been discussed in the copper part (Section 2.2). In addition to the slight antifungal activity of 43 (see Figure 30) by inhibiting the growth of Aspergillus niger, the manganese complex synthesized showed good inhibition results against Gram-negative bacteria, with a 2- to 9-fold bigger inhibition zone compared to the streptomycin standard, and compared to the ligand alone. This is explained by the authors by improved lipophilicity due to the chelation of the cation. Indeed, chelation causes a decrease in the polarity around the cation. Then, the complex can interact more efficiently with the bacteria cell wall and pass through it more easily. The diameter of the inhibition area was however similar to the standard for Staphylococcus aureus, a Gram-positive bacteria. Finally, despite these data, it was observed that the manganese complex has the lowest results compared to complexes between the same ligands and tin or silicon [182].
Figure 30.
Structure of the ligand and proposed structure for the manganese complex [182].
Anacona and Bastardo also developed a manganese(II) complex with a hexaaza macrocycle Schiff base ligand (Figure 31). Based on a phenanthroline, two diaminonaphthoquinones, and dibromoethane, they obtained no crystals suitable for diffraction; therefore, they assumed, based on the similarity with the literature, that the ligand formed a planar and tetradentate base around the cation, completed by two water molecules or two bromine anions to obtain an octahedral configuration. Antimicrobial assays show no effect on Pseudomonas aeruginosa and E. coli, a small effect on S. aureus, but with a less good inhibition than sodium penicillinate except at 2 µg/mL, and higher inhibition of Bacillus cereus than sodium penicillinate [183].
Figure 31.
Schiff base ligand for manganese [183].
2.10. Mixing Metal into Complexes
To further improve the antimicrobial activity of metal complexes, one solution could be to mix different metals. In addition, this way has the frequent advantage of decreasing the toxicity of the metal ions by reducing their respective MICs; thus, the synthesis and antimicrobial analysis of a series of metal and dimetal complexes based on a salen ligand Cu, Ag, Zn and Bi were performed (Figure 32). Indeed, thanks to their N2O2 site and their numerous possible derivatives, salen-derivatives are versatile and can eventually form several compartments able to accommodate different metal ions. Here, the complexes demonstrated large inhibition zones for the bimetallic complexes, comparable to, even if slightly smaller than, those caused by the pure salts, whereas the monometallic complexes did not display any inhibition. According to the authors, this lack of activity was due to the solubility of the compounds. Indeed, the bimetallic complexes are all charged, displaying higher solubility across agar and therefore better activity using the agar diffusion technique. In contrast, monometallic complexes are all neutral, making their dissolution in the agar difficult. Another reason could be the strength of the salen ligand, drastically limiting the progressive release of its metal ion, while the second one is less strongly coordinated. This is confirmed by the biological analysis: the antimicrobial activities of the bimetallic compounds are similar to those of the second metal ion, suggesting that the coordination hides the first one to its environment, reducing its biodisponibility [184].
Figure 32.
Monometallic and bimetallic complexes based on salen ligand [184].
As a last but not least example in this review about metal complexes, it is possible to gather several different metal ions. By combining manganese with rhenium and iron or ruthenium, Bandow et al. designed hetero triorganometallic compounds FcPNA and RcPNA (Figure 33, compounds 44 and 45) containing a cymantrene residue where the manganese possess a tetrahedral symmetry, a ferrocene or ruthenocene, and a (dipicolyl)Re(CO)3 group. With a peptide-mimic moiety, a positively charged moiety, and three organometallic residues constituted of rather lipophilic ligands, the compounds shared similarities with the lipidic membrane of bacteria, allowing good permeability. Hence, by determining the MICs of the two complexes and the small organometallic residues by themselves, it was shown that FcPNA had better efficiency than RcPNA, and that the building blocks did not show any activity at high concentrations (512 µg/mL). Thus, FcPNA was demonstrated to have 4–6 times lower MICs than amoxicillin, similar MICs to norfloxacin, and 5–6 times higher MICs than vancomycin against Bacillus subtilis and Staphylococcus aureus. The best results were against resistant S. aureus MRSA, where the MIC for FcPNA was divided by 100 compared to amoxicillin. The authors report that it also has a good activity against vancomycin-intermediate S. aureus. Moreover, it seems that FcPNA is bactericidal rather than bacteriostatic. Finally, no lysis of the bacteria membrane (leading to the dissolution of the bacteria) was observed, suggesting that the compound’s target is into the bacteria, and that the complex is able to pass through the membrane. Nevertheless, the drawback is that it is also able to interact with proteins, as was demonstrated by measuring the MIC of FcPNA in the presence of bovine serum albumin. The 5-fold increase indicates a strong binding of the FcPNA with the serum. Thus, cytotoxicity studies give dispersed results, with more than 90% survival for CAco-2, L6.C11, or HEpG2 cells, when MCF7 cells have only a 10% viability in the presence of FcPNA. Finally, neither complex demonstrated any activity against E. coli, A. baumannii, or P. aeruginosa, even at the limit of solubility (25 µg/mL) [185]. This is in keeping with the interactions of the complexes with the bacteria membrane, as the three last bacteria are Gram-negative strains, and thus harbor two membranes surrounding a peptidoglycan cell wall in opposition with B. subtilis and S. aureus, which are Gram-positive bacteria and have only a single cytoplasmic membrane constituted of phospholipids.
Figure 33.
Structure of the heterotriorganometallic complexes, M = Fe (FcPNA) 44 or M = Ru (RcPNA) 45 [185].
By comparing the protein expression of B. subtilis in the presence of the complexes to its proteomic library under normal conditions, Bandow et al. observed which proteins were increasingly expressed. These were linked to the cell envelope stress (among others PspA, stabilizing the cell membrane), the energy metabolism (proteins involved in the fatty acid biosynthesis, often observed as a response to the use of antibiotics targeting the membrane), and the general stress response. All these elements led the authors to suggest that the complexes target the cell membrane by embedding in it. Finally, no major difference was observed for bacteria cultivating with FcPNA compared to those cultivated with RcPNA, except for an oxidative stress due to FcPNA. That only FcPNA can be responsible for oxidation can be linked to the different redox properties of the ferrocene and the ruthenocene [185].
3. Synergy of Metal Complexes with Antimicrobials
In this part, we will not present many literature examples, some of which have been previously discussed in their related metal chapter. The aim of this chapter is rather to develop the idea of associating antimicrobials and metal complexes against pathogen bacteria.
The ability of metal complexes to open new routes in therapy may be exploited against resistant bacteria. A second manner to associate metal complexes with antimicrobials is to exploit existing antimicrobial compounds as ligands for metal cations. Indeed, as shown in a lot of the previous examples [32,72,73,77,78,80,84,86,87,97,175,176], the metal complexes displayed higher activity than their ligands or their building-blocks themselves. It is therefore suggested that metal–antibiotic combinations could lead to synergistic effects in terms of their respective antimicrobial properties. A reason for this is found by focusing on the chemical properties of both partners. Among other things, a great issue for the design of new antibiotics is balancing their lipophilic and hydrophilic properties. Indeed, for a drug to be effective in vivo, one important property is to be sufficiently soluble to be brought through the blood to its targeted bacteria, but at the same time to be sufficiently lipophilic to pass through the membrane; those of bacteria as well as the gastrointestinal wall for oral drugs. Thus, some potential antibiotics having a promising activity in vitro do not pass the in vivo step due to having a biodisponibility that is too low. Metal salts are often too polar to interact with the lipid membranes, as well as some organic molecules, designed in this way, they are able to link to their target, generally through H-bonds or electrostatic interactions, and therefore with a lot of polar functions. On the face of it, it would seem there is no reason to combine two “too polar” moieties to decrease the overall hydrophilicity. Nevertheless, as explained previously with the ligand field theory, the complexation of the metal ion with a ligand tends to attenuate the positive charge of the cation by sharing it on a larger area through the orbitals and to decrease the overall electron density of the ligand [55,56,68,84,99,110,164,182]. This is why orienting the research to the synthesis of metal ion-antibiotic complexes could lead to an enhancement of their usual efficiency through an improvement of the biodisponibility and antimicrobial activity [176].
Finally, it is possible to resort to metal complexes as cofactors of antibiotics. As discussed in some examples of this review, metal complexes sometimes display higher antimicrobial activity than the standard antimicrobials, particularly against resistant bacteria such as S. aureus MRSA [32,119,156,161,185]. Therefore, it could be interesting to administer the metal complex together with an antimicrobial to improve both of their effects [32,78]. When they do not have the same targets, this multi-therapy approach leads to reaching the bacteria independently on both ends. The metal complex can act for instance by increasing the porosity of the bacterial cell wall, which facilitates the entry of the antibiotic into the bacteria (see, for instance, the [Ru(phen2)(p-BPIP)]2+ [94]), as well as by protecting the antibiotic against degradation by inactivating the involved enzymes (see for instance some bismuth complexes, where the Bi(III) ion may replace the zinc ions of the metallo-β-lactamase enzymes [133]). Therefore, co-administration of different compounds could be useful either against resistant organisms, or to avoid the emergence of resistance by non-resistant organisms, as it is more complicated to develop two different resistance pathways simultaneously [156,186].
Inducing synergy between the effects of the metal ion and its ligands themselves, resulting in not only the addition of their antimicrobial properties but even their multiplication, may be a good therapeutic option. The presence (or the absence) of synergy is caused by the fractional inhibitory concentration index (FICI): below 0.5, the FICI indicates synergy; between 0.5 and 4.0, it indicates an absence of synergy; and over 4.0, it indicates an antagonism between both parts of the complex. The FICI for combination therapy of n compounds is calculated by the addition over n of the ratio MIC of compound x in combination/MIC of compound x alone [32] Thus, the combination of metal ions with known antibiotics, more than just adding their antimicrobial properties, may improve both of them, as illustrated by the example of silver–ampicillin complexes described previously [32,33]. To improve the synergy and reduce the possibility of resistance emergence, it could be interesting to look on the resistance pathway developed by the bacteria against metal ions and antibiotics. Hence, to resist rifampicin or zinc, bacteria protect themselves by a slight modification of their targets, develop efflux pumps against chloramphenicol, cadmium, or nickel, or decrease the permeability against silver, manganese, or tetracyclines [146]. Therefore, it would be worthwhile studying the main resistance pathways of an antibiotic or a metal cation, and associating it with a cation or an antibiotic with a different resistance system to limit their inhibition. However, this could lead to the development of common resistance mechanisms [146].
4. Conclusions and Future Perspectives
The field of metallodrugs in medicinal inorganic chemistry has grown constantly for around 50 years. However, to date, the proportion of metallodrugs that have reached clinical trials is still very low as compared to the traditional small organic or biological drug molecules. It seems that there is also a lack of public acceptance of the use of metals in the clinic and the “toxicity associated with metals” may hamper the development of metallodrugs for further pharmaceutical applications. Nevertheless, the wide range of metals and their combination with many types of ligands provide a great structural variety (ranging from linear to octahedral and even beyond) and a far more diverse stereochemistry than organic compounds. The rational design of ligands offers medicinal chemists proper control of the organometallic kinetic properties, such as the rate of ligand exchange, which is particularly useful for their interaction with biomolecule moieties such as DNA, enzymes or proteins that are present in the cell. By tuning the ligands, they can also adjust the lipophilicity of the overall metal complex, providing it with the ability to cross the cell membrane, and thus having a considerable impact on its biological activity and its pharmacokinetics. By using the nutrient assimilation machinery of the cell, they were able to design new metallodrugs possessing very promising activities through interaction with growing cell processes, while other strategies include the development of new organometallic derivatives of conventional organic or biological drug molecules based on structure-relationship methods. Additionally, more and more toxicological studies have been performed alongside, and the results point out that metals or metal-based drug molecules are not necessarily less biocompatible than conventional small organic molecules.
In this review article, we focused our attention on the most prominent metal-based complexes developed in the last couple of years for their antimicrobial applications (see an overview in Table 3). We exposed different examples encountered in the literature in order to attract the attention of the readers on the fact that every metal complex displayed different biological properties and mechanisms of action against bacteria, fungi and viruses. The treatment of bacterial infections is still challenging because of a decline in the current arsenal of useful antibiotics and the slow rate of new drug development. Therefore, research and development in the area of antimicrobial metallodrugs is a potential strategy to overcome the global rise of antibiotic resistance. Nevertheless, in many instances, the mechanisms of antimicrobial metal toxicity remain uncertain, and the identification of bacterial targets and uptake pathways are key issues for microbiologists and toxicologists. There are only limited in vivo data currently available for metal complexes, hindering further development of such promising compounds. Hopefully, future studies will be focused on the design of novel strategies for targeting toxic metals in order to have a better understanding of the metal complexes behavior in living organisms. An alternative approach could be the development of nanostructured antimicrobials, which possess new chemical, physical and biological properties due to their small size and large surface area. Even though this new class of compounds has already shown very promising results in terms of their higher bioactive entities released, easier interaction with biomolecules and enhanced selectivity, their development is still at the early stage and further toxicological and mechanistic studies are also required.

Table 3.
Overview of metal complexes discussed in this review article.
To conclude, the future is bright for this field of research, and we believe that in the upcoming years, more metal-based antimicrobial compounds will be able to reach clinical trials and the market.
Author Contributions
K.M.F. conceptualized the manuscript and contributed to the correction and final shaping of it, while M.C. searched literature and wrote the first version part of the manuscript and J.V.S. searched literature and contributed to the writing and the corrections of the second version (in particular, chapters on vanadium and synergic effects). All authors have read and agreed to the published version of the manuscript.
Funding
This research acknowledges funding from an Innosuisse project “GACAPS”.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
References
- Atlanta, GA: US Centers for Disease Control and Prevention. Antibiotic Resistance Threats in the United States. 2019. Available online: https://www.cdc.gov/drugresistance/pdf/threats-report/2019-ar-threats-report-508.pdf (accessed on 29 September 2020).
- European Centre for Disease Prevention and Control. Antibiotic Resistance: An Increasing Threat to Human Health. 2018. Available online: https://antibiotic.ecdc.europa.eu/en/publications-data/antibiotic-resistance-increasing-threat-human-health (accessed on 29 September 2020).
- Geneva: World Health Organization. 2019 Antibacterial Agents in Clinical Development: An Analysis of the Antibacterial Clinical Development Pipeline. Licence: CC BY-NC-SA 3.0 IGO 2019. Available online: https://apps.who.int/iris/handle/10665/330420 (accessed on 29 September 2020).
- Lloyd, N.C.; Morgan, H.W.; Nicholson, B.K.; Ronimus, R.S. The Composition of Ehrlich’s Salvarsan: Resolution of a Century-Old Debate. Angew. Chem. Int. Ed. 2005, 44, 941–944. [Google Scholar] [CrossRef] [PubMed]
- Gasser, G. Metal Complexes and Medicine: A Successful Combination. Chimia (Aarau) 2015, 69, 442–446. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, S. Cisplatin: The First Metal Based Anticancer Drug. Bioorg. Chem. 2019, 88, 102925. [Google Scholar] [CrossRef] [PubMed]
- Biot, C.; Nosten, F.; Fraisse, L.; Ter-Minassian, D.; Khalife, J.; Dive, D. The Antimalarial Ferroquine: From Bench to Clinic. Parasite 2011, 18, 207–214. [Google Scholar] [CrossRef]
- Monro, S.; Colón, K.L.; Yin, H.; Roque, J.; Konda, P.; Gujar, S.; Thummel, R.P.; Lilge, L.; Cameron, C.G.; McFarland, S.A. Transition Metal Complexes and Photodynamic Therapy from a Tumor-Centered Approach: Challenges, Opportunities, and Highlights from the Development of TLD1433. Chem. Rev. 2019, 119, 797–828. [Google Scholar] [CrossRef]
- Kenny, R.G.; Marmion, C.J. Toward Multi-Targeted Platinum and Ruthenium Drugs—A New Paradigm in Cancer Drug Treatment Regimens? Chem. Rev. 2019, 119, 1058–1137. [Google Scholar] [CrossRef] [PubMed]
- Morrison, C.N.; Prosser, K.E.; Stokes, R.W.; Cordes, A.; Metzler-Nolte, N.; Cohen, S.M. Expanding Medicinal Chemistry into 3D Space: Metallofragments as 3D Scaffolds for Fragment-Based Drug Discovery. Chem. Sci. 2020, 11, 1216–1225. [Google Scholar] [CrossRef]
- Gianferrara, T.; Bratsos, I.; Alessio, E. A Categorization of Metal Anticancer Compounds Based on Their Mode of Action. Dalton Trans. 2009, 37, 7588. [Google Scholar] [CrossRef]
- Gasser, G.; Metzler-Nolte, N. The Potential of Organometallic Complexes in Medicinal Chemistry. Curr. Opin. Chem. Biol. 2012, 16, 84–91. [Google Scholar] [CrossRef]
- Mjos, K.D.; Orvig, C. Metallodrugs in Medicinal Inorganic Chemistry. Chem. Rev. 2014, 114, 4540–4563. [Google Scholar] [CrossRef]
- Zhang, P.; Sadler, P.J. Redox-Active Metal Complexes for Anticancer Therapy: Redox-Active Metal Complexes for Anticancer Therapy. Eur. J. Inorg. Chem. 2017, 2017, 1541–1548. [Google Scholar] [CrossRef]
- Frei, A.; Zuegg, J.; Elliott, A.G.; Baker, M.; Braese, S.; Brown, C.; Chen, F.; Dowson, C.G.; Dujardin, G.; Jung, N.; et al. Metal complexes as a promising source for new antibiotics. Chem. Sci. 2020, 11, 2627. [Google Scholar] [CrossRef] [PubMed]
- Alexander, J.W. History of the Medical Use of Silver. Surg. Infect. 2009, 10, 289–292. [Google Scholar] [CrossRef] [PubMed]
- ClinicalTrials.gov is a Database of Privately and Publicly Funded Clinical Studies Conducted around the World. Available online: www.clinicaltrials.gov (accessed on 5 May 2020).
- Eckhardt, S.; Brunetto, P.S.; Gagnon, J.; Priebe, M.; Giese, B.; Fromm, K.M. Nanobio Silver: Its Interactions with Peptides and Bacteria, and Its Uses in Medicine. Chem. Rev. 2013, 113, 4708–4754. [Google Scholar] [CrossRef]
- Liau, S.Y.; Read, D.C.; Pugh, W.J.; Furr, J.R.; Russell, A.D. Interaction of Silver Nitrate with Readily Identifiable Groups: Relationship to the Antibacterial Action of Silver Ions. Lett. Appl. Microbiol. 1997, 25, 279–283. [Google Scholar] [CrossRef]
- Chiericatti, C.; Basílico, J.C.; Basílico, M.L.Z.; Zamaro, J.M. Antifungal Activity of Silver Ions Exchanged in Mordenite. Microporous Mesoporous Mater. 2014, 188, 118–125. [Google Scholar] [CrossRef]
- Feng, Q.; Wu, J.; Chen, G.; Cui, F.; Kim, T.; Kim, J. A Mechanistic Study of the Antibacterial Effect of Silver Ions on Escherichia Coli and Staphylococcus Aureus. J. Biomed. Mater. Res. 2000, 52, 662–668. [Google Scholar] [CrossRef]
- Jung, W.K.; Koo, H.C.; Kim, K.W.; Shin, S.; Kim, S.H.; Park, Y.H. Antibacterial Activity and Mechanism of Action of the Silver Ion in Staphylococcus Aureus and Escherichia Coli. Appl. Environ. Microbiol. 2008, 74, 2171–2178. [Google Scholar] [CrossRef]
- Park, H.-J.; Kim, J.Y.; Kim, J.; Lee, J.-H.; Hahn, J.-S.; Gu, M.B.; Yoon, J. Silver-Ion-Mediated Reactive Oxygen Species Generation Affecting Bactericidal Activity. Water Res. 2009, 43, 1027–1032. [Google Scholar] [CrossRef]
- Tartanson, M.-A.; Soussan, L.; Rivallin, M.; Pecastaings, S.; Chis, C.V.; Penaranda, D.; Roques, C.; Faur, C. Dynamic Mechanisms of the Bactericidal Action of an Al2O3-TiO2-Ag Granular Material on an Escherichia Coli Strain. Appl. Environ. Microbiol. 2015, 81, 7135–7142. [Google Scholar] [CrossRef]
- Medici, S.; Peana, M.; Crisponi, G.; Nurchi, V.M.; Lachowicz, J.I.; Remelli, M.; Zoroddu, M.A. Silver Coordination Compounds: A New Horizon in Medicine. Coord. Chem. Rev. 2016, 327–328, 349–359. [Google Scholar] [CrossRef]
- Melaiye, A.; Simons, R.S.; Milsted, A.; Pingitore, F.; Wesdemiotis, C.; Tessier, C.A.; Youngs, W.J. Formation of Water-Soluble Pincer Silver(I)−Carbene Complexes: A Novel Antimicrobial Agent. J. Med. Chem. 2004, 47, 973–977. [Google Scholar] [CrossRef] [PubMed]
- Johnson, N.A.; Southerland, M.R.; Youngs, W.J. Recent Developments in the Medicinal Applications of Silver-NHC Complexes and Imidazolium Salts. Molecules 2017, 22, 1263. [Google Scholar] [CrossRef] [PubMed]
- Yilmaz, V.T.; Gocmen, E.; Icsel, C.; Cengiz, M.; Susluer, S.Y.; Buyukgungor, O. Di- and Polynuclear Silver(I) Saccharinate Complexes of Tertiary Diphosphane Ligands: Synthesis, Structures, in Vitro DNA Binding, and Antibacterial and Anticancer Properties. J. Biol. Inorg. Chem. 2014, 19, 29–44. [Google Scholar] [CrossRef]
- Kalinowska-Lis, U.; Szewczyk, E.M.; Chęcińska, L.; Wojciechowski, J.M.; Wolf, W.M.; Ochocki, J. Synthesis, Characterization, and Antimicrobial Activity of Silver(I) and Copper(II) Complexes of Phosphate Derivatives of Pyridine And Benzimidazole. ChemMedChem 2014, 9, 169–176. [Google Scholar] [CrossRef]
- Youngs, W.J.; Knapp, A.R.; Wagers, P.O.; Tessier, C.A. Nanoparticle Encapsulated Silvercarbene Complexes and Their Antimicrobial and Anticancer Properties: A Perspective. Dalton Trans. 2012, 41, 327–336. [Google Scholar] [CrossRef]
- Leid, J.G.; Ditto, A.J.; Knapp, A.; Shah, P.N.; Wright, B.D.; Blust, R.; Christensen, L.; Clemons, C.B.; Wilber, J.P.; Young, G.W.; et al. In Vitro Antimicrobial Studies of Silver Carbene Complexes: Activity of Free and Nanoparticle Carbene Formulations against Clinical Isolates of Pathogenic Bacteria. J. Antimicrob. Chemother. 2012, 67, 138–148. [Google Scholar] [CrossRef]
- Möhler, J.S.; Kolmar, T.; Synnatschke, K.; Hergert, M.; Wilson, L.A.; Ramu, S.; Elliott, A.G.; Blaskovich, M.A.T.; Sidjabat, H.E.; Paterson, D.L.; et al. Enhancement of antibiotic-activity through complexation with metal ions - Combined ITC, NMR, enzymatic and biological studies. J. Int. Biochem. 2017, 167, 134–141. [Google Scholar] [CrossRef]
- Morones-Ramirez, J.R.; Winkler, J.A.; Spina, C.S.; Collins, J.J. Silver Enhances Antibiotic Activity against Gram-Negative Bacteria. Sci. Trans. Med. 2013, 5, 190. [Google Scholar] [CrossRef]
- Möhler, J.S.; Sim, W.; Blaskovich, M.A.T.; Cooper, M.A.; Ziora, Z.M. Silver bullets: A new lustre on an old antimicrobial agent. Biotechnol. Adv. 2018, 36, 1391–1411. [Google Scholar] [CrossRef]
- Nagy, A.; Harrison, A.; Sabbani, S.; Munson, R.S., Jr.; Dutta, P.K.; Waldman, W.J. Silver nanoparticles embedded in zeolite membranes: Release of silver ions and mechanism of antibacterial action. Int. J. Nanomed. 2011, 6, 1833–1852. [Google Scholar]
- Jaiswal, S.; Bhattacharya, K.; Sullivan, M.; Walsh, M.; Creaven, B.S.; Laffir, F.; Duffy, B.; McHale, P. Non-cytotoxic antibacterial silver—Coumarin complex doped sol—Gel coatings. Coll. Surf. B Biointerfaces 2013, 102, 412–419. [Google Scholar] [CrossRef] [PubMed]
- Ronconi, L.; Sadler, P.J. Using coordination chemistry to design new medicines. Coord. Chem. Rev. 2007, 251, 1633–1648. [Google Scholar] [CrossRef]
- Hossain, M.S.; Zakaria, C.M.; Kudrat-E-Zahan, M. Metal Complexes as Potential Antimicrobial Agent: A Review. Am. J. Heterocycl. Chem. 2018, 4, 1. [Google Scholar] [CrossRef]
- Sweetman, S.; Blake, P.; Mc Glashan, J.; Neatherco, G. Martindale: The Complete Drug Reference, 35th ed.; Pharmaceutical Press: London, UK, 2006. [Google Scholar]
- Borthagaray, G. Essential Transition Metal Ion Complexation as a Strategy to Improve the Antimicrobial Activity of Organic Drugs. J. Infect. Dis. Epidemiol. 2016, 2, 1–8. [Google Scholar] [CrossRef]
- Nandanwar, S.K.; Kim, H.J. Anticancer and Antibacterial Activity of Transition Metal Complexes. Chem. Select 2019, 4, 1706–1721. [Google Scholar] [CrossRef]
- Evangelinou, O.; Hatzidimitriou, A.G.; Velali, E.; Pantazaki, A.A.; Voulgarakis, N.; Aslanidis, P. Mixed-ligand copper(I) halide complexes bearing 4,5-bis(diphenylphosphano)-9,9-dimethyl-xanthene and N-methylbenzothiazole-2-thione: Synthesis, structures, luminescence and antibacterial activity mediated by DNA and membrane damage. Polyhedron 2014, 72, 122–129. [Google Scholar] [CrossRef]
- Arif, R.; Nayab, P.S.; Ansari, I.A.; Shahid, M.; Irfan, M.; Alam, S.; Abid, M. Synthesis, Molecular Docking and DNA Binding Studies of Phthalimide-Based Copper(II) Complex: In Vitro Antibacterial, Hemolytic and Antioxidant Assessment. J. Mol. Struct. 2018, 1160, 142–153. [Google Scholar] [CrossRef]
- Saravanan, K.; Elancheran, R.; Divakar, S.; Anand, S.A.A.; Ramanathan, M.; Kotoky, J.; Lokanath, N.K.; Kabilan, S. Design, Synthesis and Biological Evaluation of 2-(4-Phenylthiazol-2-Yl) Isoindoline-1,3-Dione Derivatives as Anti-Prostate Cancer Agents. Bioorg. Med. Chem. Lett. 2017, 27, 1199–1204. [Google Scholar] [CrossRef]
- Pan, L.; Li, X.; Gong, C.; Jin, H.; Qin, B. Synthesis of N-Substituted Phthalimides and Their Antifungal Activity against Alternaria Solani and Botrytis Cinerea. Microb. Pathog. 2016, 95, 186–192. [Google Scholar] [CrossRef]
- Bach, D.-H.; Liu, J.-Y.; Kim, W.K.; Hong, J.-Y.; Park, S.H.; Kim, D.; Qin, S.-N.; Luu, T.-T.-T.; Park, H.J.; Xu, Y.-N.; et al. Synthesis and biological activity of new phthalimides as potential anti-inflammatory agents. Bioorg. Med. Chem. 2017, 25, 3396–3405. [Google Scholar] [CrossRef] [PubMed]
- Housman, T.S.; Jorizzo, J.L.; McCarty, M.A.; Grummer, S.E.; Fleischer, A.B.; Sutej, P.G. Low-Dose Thalidomide Therapy for Refractory Cutaneous Lesions of LupusErythematosus. Arch. Dermatol. 2003, 139, 50. [Google Scholar] [CrossRef]
- Nair, M.S.; Arish, D.; Joseyphus, R.S. Synthesis, characterization, antifungal, antibacterial and DNA cleavage studies of some heterocyclic Schiff base metal complexes. J. Saudi Chem. Soc. 2012, 16, 83–88. [Google Scholar] [CrossRef]
- Ejidike, I. Cu(II) Complexes of 4-[(1E)-N-{2-[(Z)-Benzylidene-amino]ethyl}ethanimidoyl]benzene-1,3-diol Schiff Base: Synthesis, Spectroscopic, In-Vitro Antioxidant, Antifungal and Antibacterial Studies. Molecules 2018, 23, 1581. [Google Scholar] [CrossRef]
- Jawoor, S.S.; Patil, S.A.; Toragalmath, S.S. Synthesis and characterization of heteroleptic Schiff base transition metal complexes: A study of anticancer, antimicrobial, DNA cleavage and anti-TB activity. J. Coord. Chem. 2018, 71, 271–283. [Google Scholar] [CrossRef]
- Cao, W.; Liu, Y.; Zhang, T.; Jia, J. Synthesis, characterization, theoretical and antimicrobial studies of tridentate hydrazone metal complexes of Zn(II), Cd(II), Cu(II) and Co(III). Polyhedron 2018, 147, 62–68. [Google Scholar] [CrossRef]
- Przybylski, P.; Huczynski, A.; Pyta, K.; Brzezinski, B.; Bartl, F. Biological Properties of Schiff Bases and Azo Derivatives of Phenols. COC 2009, 13, 124–148. [Google Scholar] [CrossRef]
- Nazirkar, B.; Mandewale, M.; Yamgar, R. Synthesis, characterization and antibacterial activity of Cu(II) and Zn(II) complexes of 5-aminobenzofuran-2-carboxylate Schiff base ligands. J. Taibah Univ. Sci. 2019, 13, 440–449. [Google Scholar] [CrossRef]
- Kremer, E.; Facchin, G.; Estévez, E.; Alborés, P.; Baran, E.J.; Ellena, J.; Torre, M.H. Copper Complexes with Heterocyclic Sulfonamides: Synthesis, Spectroscopic Characterization, Microbiological and SOD-like Activities: Crystal Structure of [Cu(Sulfisoxazole)2(H2O)4]·2H2O. J. Inorg. Biochem. 2006, 100, 1167–1175. [Google Scholar] [CrossRef]
- Pervaiz, M.; Riaz, A.; Munir, A.; Saeed, Z.; Hussain, S.; Rashid, A.; Younas, U.; Adnan, A. Synthesis and Characterization of Sulfonamide Metal Complexes as Antimicrobial Agents. J. Mol. Struct. 2020, 1202, 127284. [Google Scholar] [CrossRef]
- Foye, W.O.; Lemke, T.L.; Williams, D.A. Principles of Medicinal Chemistry; Lea & Febiger: Philadelphia, PA, USA, 1981; pp. 767–768. [Google Scholar]
- Ramadan, A.M. Structural and biological aspects of copper (II) complexes with 2-methyl-3-amino-(3 H)-quinazolin-4-one. J. Inorg. Biochem. 1997, 65, 183–189. [Google Scholar] [CrossRef]
- Mingfeng Yu, M.; Nagalingam, G.; Ellis, S.; Martinez, E.; Sintchenko, V.; Spain, M.; Rutledge, P.J.; Todd, M.H.; Triccas, J.A. Nontoxic Metal–Cyclam Complexes, a New Class of Compounds with Potency against Drug-Resistant Mycobacterium tuberculosis. J. Med. Chem. 2016, 59, 5917–5921. [Google Scholar]
- Spain, M.; Wong, J.K.-H.; Nagalingam, G.; Batten, J.M.; Hortle, E.; Oehlers, S.H.; Jiang, X.F.; Murage, H.E.; Orford, J.T.; Crisologo, P.; et al. Antitubercular Bis-Substituted Cyclam Derivatives: Structure−Activity Relationships and in Vivo Studies. J. Med. Chem. 2018, 61, 3595–3608. [Google Scholar] [CrossRef]
- Cunnane, S.C. Zinc: Clinical and Biochemical Significance; CRC Press: Boca Raton, FL, USA, 2018. [Google Scholar]
- Bertini, I.; Luchinat, C. The reaction pathways of zinc enzymes and related biological catalysts. In Bioinorganic Chemistry; IVANO BERTINI: Mill Valley, CA, USA, 1994; pp. 37–106. [Google Scholar]
- Haas, K.L.; Franz, K.J. Application of Metal Coordination Chemistry To Explore and Manipulate Cell Biology. Chem. Rev. 2009, 109, 4921–4960. [Google Scholar] [CrossRef] [PubMed]
- Yoshikawa, Y.; Yasui, H. Zinc Complexes Developed as Metallopharmaceutics for Treating Diabetes Mellitus based on the Bio-Medicinal Inorganic Chemistry. Curr. Top. Med. Chem. 2012, 12, 210–218. [Google Scholar] [CrossRef]
- Zastrow, M.L.; Pecoraro, V.L. Designing Hydrolytic Zinc Metalloenzymes. Biochemistry 2014, 53, 957–978. [Google Scholar] [CrossRef]
- Pasquet, J.; Chevalier, Y.; Pelletier, J.; Couval, E.; Bouvier, D.; Bolzinger, M.-A. The Contribution of Zinc Ions to the Antimicrobial Activity of Zinc Oxide. Coll. Surf. A Physicochem. Eng. Asp. 2014, 457, 263–274. [Google Scholar] [CrossRef]
- Stanić, V.; Dimitrijević, S.; Antić-Stanković, J.; Mitrić, M.; Jokić, B.; Plećaš, I.B.; Raičević, S. Synthesis, Characterization and Antimicrobial Activity of Copper and Zinc-Doped Hydroxyapatite Nanopowders. Appl. Surf. Sci. 2010, 256, 6083–6089. [Google Scholar] [CrossRef]
- Fang, M.; Chen, J.; Xu, X.; Yang, P.; Hildebrand, H. Antibacterial Activities of Inorganic Agents on Six Bacteria Associated with Oral Infections by Two Susceptibility Tests. Int. J. Antimicrob. Agents 2006, 27, 513–517. [Google Scholar] [CrossRef]
- Yamgar, R.S.; Nivid, Y.; Nalawade, S.; Mandewale, M.; Atram, R.G.; Sawant, S.S. Novel Zinc(II) Complexes of Heterocyclic Ligands as Antimicrobial Agents: Synthesis, Characterisation, and Antimicrobial Studies. Bioinorg. Chem. Appl. 2014, 2014, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Sheikhshoaie, I.; Lotfi, N.; Sieler, J.; Krautscheid, H.; Khaleghi, M. Synthesis, Structures and Antimicrobial Activities of Nickel(II) and Zinc(II) Diaminomaleonitrile-Based Complexes. Transit. Met. Chem. 2018, 43, 555–562. [Google Scholar] [CrossRef]
- Selimović, E.; Jeremić, S.; Ličina, B.; Soldatović, T. Kinetics, DFT Study and Antibacterial Activity of Zinc(II) and Copper(II) Terpyridine Complexes. J. Mex. Chem. Soc. 2018, 62, 1–21. [Google Scholar] [CrossRef]
- Abu Ali, H.; Omar, S.N.; Darawsheh, M.D.; Fares, H. Synthesis, Characterization and Antimicrobial Activity of Zinc(II) Ibuprofen Complexes with Nitrogen-Based Ligands. J. Coord. Chem. 2016, 69, 1110–1122. [Google Scholar] [CrossRef]
- Boughougal, A.; Cherchali, F.Z.; Messai, A.; Attik, N.; Decoret, D.; Hologne, M.; Sanglar, C.; Pilet, G.; Tommasino, J.B.; Luneau, D. New Model of Metalloantibiotic: Synthesis, Structure and Biological Activity of a Zinc(II) Mononuclear Complex Carrying Two Enrofloxacin and Sulfadiazine Antibiotics. N. J. Chem. 2018, 42, 15346–15352. [Google Scholar] [CrossRef]
- De Clercq, E. Inhibition of HIV Infection by Bicyclams, Highly Potent and Specific CXCR4 Antagonists. Mol. Pharmacol. 2000, 57, 833–839. [Google Scholar]
- Esté, J.A.; Cabrera, C.; De Clercq, E.; Struyf, S.; Van Damme, J.; Bridger, G.; SkerlJ, R.T.; Abrams, M.J.; Henson, G.; Gutierrez, A.; et al. Activity of Different Bicyclam Derivatives against Human Immunodeficiency Virus Depends on Their Interaction with the CXCR4 Chemokine Receptor. Mol. Pharmacol. 1999, 55, 67–73. [Google Scholar] [CrossRef]
- Gerlach, L.O.; Jakobsen, J.S.; Jensen, K.P.; Rosenkilde, M.R.; Skerlj, R.T.; Ryde, U.; Bridger, G.J.; Schwartz, T.W. Metal Ion Enhanced Binding of AMD3100 to Asp 262 in the CXCR4 Receptor. Biochemistry 2003, 42, 710–717. [Google Scholar] [CrossRef]
- Liang, X.; Parkinson, J.A.; Weishäupl, M.; Gould, R.O.; Paisey, S.J.; Park, H.; Hunter, T.M.; Blindauer, C.A.; Parsons, S.; Sadler, P.J. Structure and Dynamics of Metallomacrocycles: Recognition of Zinc Xylyl-Bicyclam by an HIV Coreceptor. J. Am. Chem. Soc. 2002, 124, 9105–9112. [Google Scholar] [CrossRef]
- Valks, G.C.; McRobbie, G.; Lewis, E.A.; Hubin, T.J.; Hunter, T.M.; Sadler, P.J.; Pannecouque, C.; De Clercq, E.; Archibald, S.J. Configurationally Restricted Bismacrocyclic CXCR4 Receptor Antagonists. J. Med. Chem. 2006, 49, 6162–6165. [Google Scholar] [CrossRef]
- Karcz, D.; Matwijczuk, A.; Kaminski, D.; Creaven, B.; Ciszkowicz, E.; Lecka-Szlachta, K.; Starzak, K. Structural Features of 1,3,4-Thiadiazole-Derived Ligands and Their Zn(II) and Cu(II) Complexes Which Demonstrate Synergistic Antibacterial Effects with Kanamycin. Int. J. Mol. Sci. 2020, 21, 5735. [Google Scholar] [CrossRef]
- Sheldon, J.R.; Laakso, H.A.; Heinrichs, D.E. Iron Acquisition Strategies of Bacterial Pathogens. Microbiol. Spectr. 2016, 4, 43–85. [Google Scholar] [CrossRef] [PubMed]
- Tarallo, M.B.; Urquiola, C.; Monge, A.; Costa, B.P.; Ribeiro, R.R.; Costa-Filho, A.J.; Mercader, R.C.; Pavan, F.R.; Leite, C.Q.F.; Torre, M.H.; et al. Design of novel iron compounds as potential therapeutic agents against tuberculosis. J. Inorg. Biochem. 2010, 104, 1164–1170. [Google Scholar] [CrossRef] [PubMed]
- Khan, S.A.; Saleem, K.; Khan, Z. Synthesis, characterization and in vitro antibacterial activity of new steroidal thiazolo quinoxalines. Eur. J. Med. Chem. 2007, 42, 103–108. [Google Scholar] [CrossRef] [PubMed]
- Pandeya, S.N.; Sriram, D.; Nath, G.; De Clercq, E. Synthesis, Antibacterial, Antifungal and Anti-HIV Activities of Norfloxacin Mannich Bases. Eur. J. Med. Chem. 2000, 35, 249–255. [Google Scholar] [CrossRef]
- Karegoudar, P.; Prasad, D.J.; Ashok, M.; Mahalinga, M.; Poojary, B.; Holla, B.S. Synthesis, Antimicrobial and Anti-Inflammatory Activities of Some 1,2,4-Triazolo[3,4-b][1,3,4]Thiadiazoles and 1,2,4-Triazolo[3,4-b][1,3,4]Thiadiazines Bearing Trichlorophenyl Moiety. Eur. J. Med. Chem. 2008, 43, 808–815. [Google Scholar] [CrossRef]
- Kharadi, G.J. Antioxidant, Tautomerism and Antibacterial Studies of Fe(III)-1,2,4-Triazole Based Complexes. Spectrochim. Acta Part. A Mol. Biomol. Spectrosc. 2013, 110, 311–316. [Google Scholar] [CrossRef]
- Sierra, M.A.; Casarrubios, L.; de la Torre, M.C. Bio-Organometallic Derivatives of Antibacterial Drugs. Chem. Eur. J. 2019, 25, 7232–7242. [Google Scholar] [CrossRef]
- Biot, C.; Glorian, G.; Maciejewski, L.A.; Brocard, J.S.; Domarle, O.; Blampain, G.; Millet, P.; Georges, A.J.; Abessolo, H.; Dive, D.; et al. Synthesis and Antimalarial Activity in Vitro and in Vivo of a New Ferrocene−Chloroquine Analogue. J. Med. Chem. 1997, 40, 3715–3718. [Google Scholar] [CrossRef]
- Edwards, E.I.; Epton, R.; Marr, G. Organometallic Derivatives of Penicillins and Cephalosporins a New Class of Semi-Synthetic Antibiotics. J. Organomet. Chem. 1975, 85, C23–C25. [Google Scholar] [CrossRef]
- Dubar, F.; Egan, T.J.; Pradines, B.; Kuter, D.; Ncokazi, K.K.; Forge, D.; Paul, J.-F.; Pierrot, C.; Kalamou, H.; Khalife, J.; et al. The Antimalarial Ferroquine: Role of the Metal and Intramolecular Hydrogen Bond in Activity and Resistance. ACS Chem. Biol. 2011, 6, 275–287. [Google Scholar] [CrossRef]
- Bregman, H.; Williams, D.S.; Atilla, G.E.; Carroll, P.J.; Meggers, E. An Organometallic Inhibitor for Glycogen Synthase Kinase 3. J. Am. Chem. Soc. 2004, 126, 13594–13595. [Google Scholar] [CrossRef] [PubMed]
- Dwyer, F.P.; Gyarfas, E.C.; Rogers, W.P.; Koch, J.H. Biological Activity of Complex Ions. Nature 1952, 170, 190–191. [Google Scholar] [CrossRef]
- Li, F.; Collins, J.G.; Keene, F.R. Ruthenium complexes as antimicrobial agents. Chem. Soc. Rev. 2015, 44, 2529–2542. [Google Scholar] [CrossRef] [PubMed]
- Dwyer, F.; Reid, I.; Shulman, A.; Laycock, G.M.; Dixson, S. The Biological Actions of 1,10-Phenanthroline and 2,2′-Bipyridine Hydrochlorides, Quaternary Salts and Metal Chelates and Related Compounds: 1. Bacteriostatic Action on Selected Gram-Positive, Gram-Negative and Acid-Fast Bacteria. Aust. J. Exp. Biol. Med. 1969, 47, 203–218. [Google Scholar] [CrossRef] [PubMed]
- Bolhuis, A.; Hand, L.; Marshall, J.E.; Richards, A.D.; Rodger, A.; Aldrich-Wright, J. Antimicrobial Activity of Ruthenium-Based Intercalators. Eur. J. Pharm. Sci. 2011, 42, 313–317. [Google Scholar] [CrossRef]
- Sun, D.; Zhang, W.; Lv, M.; Yang, E.; Zhao, Q.; Wang, W. Antibacterial activity of ruthenium(II) polypyridyl complex manipulated by membrane permeability and cell morphology. Bioorg. Med. Chem. Lett. 2015, 25, 2068–2073. [Google Scholar] [CrossRef]
- Feng, Y.; Sun, W.; Wang, X.; Zhou, Q. Selective Photoinactivation of Methicillin-Resistant Staphylococcus aureus by Highly Positively Charged Ru(II) Complexes. Chem. Eur. J. 2019, 25, 13879–13884. [Google Scholar] [CrossRef]
- Donnelly, R.; Fletcher, N.; McCague, P.; Donnelly, J.; McCarron, P.; Tunney, M. Design, Synthesis and Photodynamic Antimicrobial Activity of Ruthenium Trischelate Diimine Complexes. LDDD 2007, 4, 175–179. [Google Scholar] [CrossRef]
- Smith, N.A.; Zhang, P.; Greenough, S.E.; Horbury, M.D.; Clarkson, G.J.; McFeely, D.; Habtemariam, A.; Salassa, L.; Stavros, V.G.; Dowson, C.G.; et al. Combatting AMR: Photoactivatable Ruthenium(II)-Isoniazid Complex Exhibits Rapid Selective Antimycobacterial Activity. Chem. Sci. 2017, 8, 395–404. [Google Scholar] [CrossRef]
- Li, F.; Mulyana, Y.; Feterl, M.; Warner, J.M.; Collins, J.G.; Keene, F.R. The Antimicrobial Activity of Inert Oligonuclear Polypyridylruthenium(II) Complexes against Pathogenic Bacteria, Including MRSA. Dalton Trans. 2011, 40, 5032. [Google Scholar] [CrossRef]
- Li, F.; Feterl, M.; Mulyana, Y.; Warner, J.M.; Collins, J.G.; Keene, F.R. In Vitro Susceptibility and Cellular Uptake for a New Class of Antimicrobial Agents: Dinuclear Ruthenium(II) Complexes. J. Antimicrob. Chemother. 2012, 67, 2686–2695. [Google Scholar] [CrossRef]
- Li, F.; Harry, E.J.; Bottomley, A.L.; Edstein, M.D.; Birrell, G.W.; Woodward, C.E.; Keene, F.R.; Collins, J.G. Dinuclear ruthenium(II) antimicrobial agents that selectively target polysomes in vivo. Chem. Sci. 2014, 5, 685–693. [Google Scholar] [CrossRef]
- Bernstein, L. Mechanisms of Therapeutic Activity for Gallium. Pharmacol. Rev. 1998, 50, 665–682. [Google Scholar] [PubMed]
- Chitambar, C.R. The Therapeutic Potential of Iron-Targeting Gallium Compounds in Human Disease: From Basic Research to Clinical Application. Pharmacol. Res. 2017, 115, 56–64. [Google Scholar] [CrossRef] [PubMed]
- de Bastos, T.O.; Maria Soares, B.; Silva Cisalpino, P.; Castro Mendes, I.; dos Santos, R.G.; Beraldo, H. Coordination to Gallium(III) Strongly Enhances the Potency of 2-Pyridineformamide Thiosemicarbazones against Cryptococcus Opportunistic Fungi. Microbiol. Res. 2010, 165, 573–577. [Google Scholar] [CrossRef]
- Hijazi, S.; Visaggio, D.; Pirolo, M.; Frangipani, E.; Bernstein, L.; Visca, P. Antimicrobial Activity of Gallium Compounds on ESKAPE Pathogens. Front. Cell. Infect. Microbiol. 2018, 8, 316. [Google Scholar] [CrossRef]
- Chitambar, C.R. Medical Applications and Toxicities of Gallium Compounds. IJERPH 2010, 7, 2337–2361. [Google Scholar] [CrossRef]
- Levaditi, C.; Bardet, J.; Tchakirian, A.; Vaisman, A. Le gallium, propriétés thérapeutiques dans la syphiliset les trypanosomiases expérimentales. CR Hebd Seances Acad. Sci. Ser. Sci. Nat. 1931, 192, 1142–1143. [Google Scholar]
- Bonchi, C.; Imperi, F.; Minandri, F.; Visca, P.; Frangipani, E. Repurposing of gallium-based drugs for antibacterial therapy: Gallium-Based Antibacterials. BioFactors 2014, 40, 303–312. [Google Scholar] [CrossRef]
- Kaneko, Y.; Thoendel, M.; Olakanmi, O.; Britigan, B.E.; Singh, P.K. The transition metal gallium disrupts Pseudomonas aeruginosa iron metabolism and has antimicrobial and antibiofilm activity. J. Clin. Investig. 2007, 117, 877–888. [Google Scholar] [CrossRef]
- Chitambar, C.R.; Purpi, D.P.; Woodliff, J.; Yang, M.; Wereley, J.P. Development of Gallium Compounds for Treatment of Lymphoma: Gallium Maltolate, a Novel Hydroxypyrone Gallium Compound, Induces Apoptosis and Circumvents Lymphoma Cell Resistance to Gallium Nitrate. J. Pharmacol. Exp. Ther. 2007, 322, 1228–1236. [Google Scholar] [CrossRef] [PubMed]
- DeLeon, K.; Balldin, F.; Watters, C.; Hamood, A.; Griswold, J.; Sreedharan, S.; Rumbaugh, K.P. Gallium Maltolate Treatment Eradicates Pseudomonas aeruginosa Infection in Thermally Injured Mice. Antimicrob. Agents Chemother. 2009, 53, 1331–1337. [Google Scholar] [CrossRef] [PubMed]
- Mendes, I.C.; Soares, M.A.; dos Santos, R.G.; Pinheiro, C.; Beraldo, H. Gallium(III) complexes of 2-pyridineformamide thiosemicarbazones: Cytotoxic activity against malignant glioblastoma. Eur. J. Med. Chem. 2009, 44, 1870–1877. [Google Scholar] [CrossRef]
- Gómez-Ruiz, S.; Gallego, B.; Kaluđerović, M.R.; Kommera, H.; Hey-Hawkins, E.; Paschke, R.; Kaluđerović, G.N. Novel gallium(III) complexes containing phthaloyl derivatives of neutral aminoacids with apoptotic activity in cancer cells. J. Organomet. Chem. 2009, 694, 2191–2197. [Google Scholar] [CrossRef]
- Zanias, S.; Papaefstathiou, G.S.; Raptopoulou, C.P.; Papazisis, K.T.; Vala, V.; Zambouli, D.; Kortsaris, A.H.; Kyriakidis, D.A.; Zafiropoulos, T.F. Synthesis, Structure, and Antiproliferative Activity of Three Gallium(III) Azole Complexes. Bioinorg. Chem. Appl. 2010, 2010, 1–10. [Google Scholar] [CrossRef]
- Arivett, B.A.; Fiester, S.E.; Ohneck, E.J.; Penwell, W.F.; Kaufman, C.M.; Relich, R.F.; Actis, L.A. Antimicrobial Activity of Gallium Protoporphyrin IX against Acinetobacter baumannii Strains Displaying Different Antibiotic Resistance Phenotypes. Antimicrob. Agents Chemother. 2015, 59, 7657–7665. [Google Scholar] [CrossRef]
- Pribisko, M.; Palmer, J.; Grubbs, R.H.; Gray, H.B.; Termini, J.; Lim, P. Cellular uptake and anticancer activity of carboxylated gallium corroles. Proc. Natl. Acad. Sci. USA 2016, 113, E2258–E2266. [Google Scholar] [CrossRef]
- Stojiljkovic, I.; Kumar, V.; Srinivasan, N. Non-iron metalloporphyrins: Potent antibacterial compounds that exploit haem/Hb uptake systems of pathogenic bacteria. Mol. Microbiol. 1999, 31, 429–442. [Google Scholar] [CrossRef]
- Chang, D.; Garcia, R.; Akers, K.; Mende, K.; Murray, C.; Wenke, J.; Sanchez, C. Activity of Gallium Meso- and Protoporphyrin IX against Biofilms of Multidrug-Resistant Acinetobacter baumannii Isolates. Pharmaceuticals 2016, 9, 16. [Google Scholar] [CrossRef] [PubMed]
- Hijazi, S.; Visca, P.; Frangipani, E. Gallium-Protoporphyrin IX Inhibits Pseudomonas aeruginosa Growth by Targeting Cytochromes. Front. Cell. Infect. Microbiol. 2017, 7, 12. [Google Scholar] [CrossRef]
- Choi, S.; Britigan, B.E.; Narayanasamy, P. Dual Inhibition of Klebsiella Pneumoniae and Pseudomonas Aeruginosa Iron Metabolism Using Gallium Porphyrin and Gallium Nitrate. ACS Infect. Dis. 2019, 5, 1559–1569. [Google Scholar] [CrossRef] [PubMed]
- Banin, E.; Lozinski, A.; Brady, K.M.; Berenshtein, E.; Butterfield, P.W.; Moshe, M.; Chevion, M.; Greenberg, E.P.; Banin, E. The potential of desferrioxamine-gallium as an anti-Pseudomonas therapeutic agent. Proc. Natl. Acad. Sci. USA 2008, 105, 16761–16766. [Google Scholar] [CrossRef] [PubMed]
- Pandey, A.; Savino, C.; Ahn, S.H.; Yang, Z.; Van Lanen, S.G.; Boros, E. Theranostic Gallium Siderophore Ciprofloxacin Conjugate with Broad Spectrum Antibiotic Potency. J. Med. Chem. 2019, 62, 9947–9960. [Google Scholar] [CrossRef] [PubMed]
- Sun, H. (Ed.) Biological Chemistr of Arsenic, Antimony and Bismuth; Wiley: Chichester, UK, 2011. [Google Scholar]
- Fock, K.M.; Graham, D.Y.; Malfertheiner, P. Helicobacter pylori research: Historical insights and future directions. Nat. Rev. Gastroenterol. Hepatol. 2013, 10, 495–500. [Google Scholar] [CrossRef] [PubMed]
- Janssen, M.J.R.; Hendrikse, L.; de Boer, S.Y.; Bosboom, R.; de Boer, W.A.; Laheij, R.J.F.; Jansen, J.B.M.J. Helicobacter pylori antibiotic resistance in a Dutch region: Trends over time. Neth. J. Med. 2006, 64, 191–195. [Google Scholar] [PubMed]
- Malfertheiner, P.; Bazzoli, F.; Delchier, J.-C.; Celiñski, K.; Giguère, M.; Rivière, M.; Mégraud, F. Helicobacter pylori eradication with a capsule containing bismuth subcitrate potassium, metronidazole, and tetracycline given with omeprazole versus clarithromycin-based triple therapy: A randomised, open-label, non-inferiority, phase 3 trial. Lancet 2011, 377, 905–913. [Google Scholar] [CrossRef]
- Ge, R.; Sun, H. Bioinorganic Chemistry of Bismuth and Antimony: Target Sites of Metallodrugs. Acc. Chem. Res. 2007, 40, 267–274. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Mulrooney, S.B.; Leung, A.F.K.; Zeng, Y.; Ko, B.B.C.; Hausinger, R.P.; Sun, H. Inhibition of urease by bismuth(III): Implications for the mechanism of action of bismuth drugs. BioMetals 2006, 19, 503–511. [Google Scholar] [CrossRef]
- Jin, L.; Szeto, K.-Y.; Zhang, L.; Du, W.; Sun, H. Inhibition of alcohol dehydrogenase by bismuth. J. Inorg. Biochem. 2004, 98, 1331–1337. [Google Scholar] [CrossRef]
- Busse, M.; Trinh, I.; Junk, P.C.; Ferrero, R.L.; Andrews, P.C. Synthesis and Characterisation of Bismuth(III) Aminoarenesulfonate Complexes and Their Powerful Bactericidal Activity against Helicobacter pylori. Chem. Eur. J. 2013, 19, 5264–5275. [Google Scholar] [CrossRef]
- Lessa, J.A.; Reis, D.C.; Da Silva, J.G.; Paradizzi, L.T.; da Silva, N.F.; de Fátima, A.; Carvalho, M.; Siqueira, S.A.; Beraldo, H. Coordination of Thiosemicarbazones and Bis(thiosemicarbazones) to Bismuth(III) as a Strategy for the Design of Metal-Based Antibacterial Agents. Chem. Biodivers. 2012, 9, 1955–1966. [Google Scholar] [CrossRef]
- Ferraz, K.S.O.; Silva, N.F.; da Silva, J.G.; de Miranda, L.F.; Romeiro, C.F.D.; Souza-Fagundes, E.M.; Mendes, I.C.; Beraldo, H. Investigation on the pharmacological profile of 2,6-diacetylpyridine bis(benzoylhydrazone) derivatives and their antimony(III) and bismuth(III) complexes. Eur. J. Med. Chem. 2012, 53, 98–106. [Google Scholar] [CrossRef]
- Wang, R.; Lai, T.-P.; Gao, P.; Zhang, H.; Ho, P.-L.; Woo, P.C.-Y.; Ma, G.; Kao, R.Y.-T.; Li, H.; Sun, H. Bismuth Antimicrobial Drugs Serve as Broad-Spectrum Metallo-β-Lactamase Inhibitors. Nat. Commun. 2018, 9, 439. [Google Scholar] [CrossRef]
- Yang, N.; Tanner, J.A.; Zheng, B.-J.; Watt, R.M.; He, M.-L.; Lu, L.-Y.; Jiang, J.-Q.; Shum, K.-T.; Lin, Y.-P.; Wong, K.-L.; et al. Bismuth Complexes Inhibit the SARS Coronavirus. Angew. Chem. Int. Ed. 2007, 46, 6464–6468. [Google Scholar] [CrossRef]
- Ong, Y.C.; Kedzierski, L.; Andrews, P.C. Do Bismuth Complexes Hold Promise as Antileishmanial Drugs? Future Med. Chem. 2018, 10, 1721–1733. [Google Scholar] [CrossRef]
- Lizarazo-Jaimes, E.; Monte-Neto, R.; Reis, P.; Fernandes, N.; Speziali, N.; Melo, M.; Frézard, F.; Demicheli, C. Improved Antileishmanial Activity of Dppz through Complexation with Antimony(III) and Bismuth(III): Investigation of the Role of the Metal. Molecules 2012, 17, 12622–12635. [Google Scholar] [CrossRef]
- Akhtiar, R.; Ochiai, E.-I. Pharmacological applications of inorganic complexes. General Pharmacol. 1999, 32, 525–540. [Google Scholar] [CrossRef]
- Rehder, D. Vanadium. Its Role for Humans. In Interrelations between Essential Metal Ions and Human Diseases; Springer: Dordrecht, The Netherlands, 2013; pp. 139–169. [Google Scholar]
- Srivastava, A.K.; Mehdi, M.Z. Insulino-mimetic and anti-diabetic effects of vanadium compounds. Diabet. Med. 2005, 22, 2–13. [Google Scholar] [CrossRef]
- Thompson, K.H.; Liboiron, B.D.; Sun, Y.; Bellman, K.D.D.; Setyawati, I.A.; Patrick, B.O.; Karunaratne, V.; Rawji, G.; Wheeler, J.; Sutton, K.; et al. Preparation and characterization of vanadyl complexes with bidentate maltol-type ligands; in vivo comparisons of anti-diabetic therapeutic potential. J. Biol. Inorg. Chem. 2003, 8, 66. [Google Scholar] [CrossRef]
- Maurya, M.R.; Bharti, N. Synthesis, thermal and spectral studies of oxoperoxo and dioxocomplexes of vanadium(V), molybdenum(VI) and tungsten(VI)with 2-(a-hydroxyalkyl/aryl)benzimidazole. Transit. Met. Chem. 1999, 24, 389–393. [Google Scholar] [CrossRef]
- Benítez, J.; Guggeri, L.; Tomaz, I.; Arrambide, G.; Navarro, M.; Costa Pessoa, J.; Garat, B.; Gambino, D. Design of vanadium mixed-ligand complexes as potential anti-protozoa agents. J. Inorg. Biochem. 2009, 103, 609–616. [Google Scholar] [CrossRef] [PubMed]
- Scalese, G.; Machado, I.; Fontana, C.; Risi, G.; Salinas, G.; Pérez-Díaz, L.; Gambino, D. New heteroleptic oxidovanadium(V) complexes: Synthesis, characterization and biological evaluation as potential agents against Trypanosoma cruzi. J. Biol. Inorg. Chem. 2018, 23, 1265–1281. [Google Scholar] [CrossRef] [PubMed]
- Scalese, G.; Mosquillo, M.F.; Rostán, S.; Castiglioni, J.; Alho, I.; Pérez, L.; Correia, I.; Marques, F.; Costa Pessoa, J.; Gambino, D. Heteroleptic oxidovanadium(IV) complexes of 2-hydroxynaphtylaldimineand polypyridyl ligands against Trypanosoma cruzi and prostate cancer cells. J. Biol. Inorg. Chem. 2017, 175, 154–166. [Google Scholar]
- Fernández, M.; Varela, J.; Correia, I.; Birriel, E.; Castiglioni, J.; Moreno, V.; Costa Pessoa, J.; Cerecetto, H.; González, M.; Gambino, D. A new series of heteroleptic oxidovanadium(iv) compounds with phenanthroline-derived co-ligands: Selective Trypanosoma cruzi growth inhibitors. Dalton Trans. 2013, 33, 11900–11911. [Google Scholar] [CrossRef] [PubMed]
- Mosquillo, M.F.; Smircich, P.; Lima, A.; Gehrke, S.A.; Scalese, G.; Machado, I.; Gambino, D.; Garat, B.; Pérez-Diaz, L. High Throughput Approaches to Unravel the Mechanism of Action of a New Vanadium-Based Compound against Trypanosoma cruzi. Bioinorg. Chem. Appl. 2020, 2020, 1634270. [Google Scholar] [CrossRef] [PubMed]
- Ogunlaja, A.S.; Chidawanyika, W.; Antunes, E.; Fernandes, M.A.; Nyokong, T.; Torto, N.; Tshentu, Z.R. Oxovanadium(IV)-catalyzed oxidation of dibenzothiophene and 4,6-dimethyldibenzothiophene. Dalton Trans. 2012, 41, 13908–13918. [Google Scholar] [CrossRef] [PubMed]
- De Almeida Machado, P.; Zamprogno Mota, V.; de Lima Cavalli, A.C.; Gonçalves de Carvalho, G.S.; Da Silva, A.D.; Gameiro, J.; Cuin, A.; Soares Coimbra, E. High selective antileishmanial activity of vanadium complex with stilbene derivative. Acta Trop. 2015, 148, 120–127. [Google Scholar] [CrossRef]
- Maurya, M.R.; Haldar, C.; Alam Khan, A.; Azam, A.; Salahuddin, A.; Kumar, A.; Costa Pessoa, J. Synthesis, Characterization, Catalytic and Antiamoebic Activity of Vanadium Complexes of Binucleating Bis(dibasic tridentate ONS donor) Ligand Systems. Eur. J. Inorg. Chem. 2012, 15, 2560. [Google Scholar] [CrossRef]
- D’Cruz, O.J.; Dong, Y.; Uckun, F.M. Potent dual anti-HIV and spermicidal activities of novel oxovanadium(V) complexes with thiourea non-nucleoside inhibitors of HIV-1 reverse transcriptase. Biochem. Biophys. Res. Commun. 2003, 302, 253–264. [Google Scholar] [CrossRef]
- Shigeta, S.; Mori, S.; Kodama, E.; Kodama, J.; Takahashi, K.; Yamase, T. Broad spectrum anti-RNA virus activities of titanium and vanadium substituted polyoxotungstates. Antivir. Res. 2003, 58, 265–271. [Google Scholar] [CrossRef]
- Sun, R.W.-Y.; Ma, D.-L.; Wong, E.L.-M.; Che, C.-M. Some uses of transition metal complexes as anti-cancer and anti-HIV agents. Perspect. Dalton Trans. 2007, 43, 4884–4892. [Google Scholar]
- He, L.-H.; Qiu, X.-Y.; Cheng, J.-Y.; Liu, S.-J.; Wu, S.-M. Synthesis, characterization and crystal structures of vanadium(V)complexes derived from halido-substituted tridentate hydrazone compounds with antimicrobial activity. Polyhedron 2018, 156, 105–110. [Google Scholar] [CrossRef]
- Sheikhshoaie, I.; Ebrahimipour, S.Y.; Lotfi, N.; Mague, J.T.; Khaleghi, M. Synthesis, spectral characterization, X-ray crystal structure and antimicrobial activities of two cis dioxido-vanadium(V) complexes incorporating unsymmetrical dimalonitrile-based (NNO) Schiff base ligands. Inorg. Chim. Acta 2016, 442, 151–157. [Google Scholar] [CrossRef]
- Harbut, M.B.; Vilchèze, C.; Luo, X.; Hensler, M.E.; Guo, H.; Yang, B.; Chatterjee, A.K.; Nizet, V.; Jacobs, W.R.; Schultz, P.G.; et al. Auranofin exerts broad-spectrum bactericidal activities by targeting thiol-redox homeostasis. Proc. Natl. Acad. Sci. USA 2015, 112, 4453–4458. [Google Scholar] [CrossRef] [PubMed]
- Angelucci, F.; Sayed, A.A.; Williams, D.L.; Boumis, G.; Brunori, M.; Dimastrogiovanni, D.; Miele, A.E.; Pauly, F.; Bellelli, A. Inhibition of Schistosoma mansoni Thioredoxin-glutathione Reductase by Auranofin: Structural and Kinetic Aspects. J. Biol. Chem. 2009, 284, 28977–28985. [Google Scholar] [CrossRef]
- Thangamani, S.; Mohammad, H.; Abushahba, M.F.N.; Sobreira, T.J.P.; Hedrick, V.E.; Paul, L.N.; Seleem, M.N. Antibacterial activity and mechanism of action of auranofin against multi-drug resistant bacterial pathogens. Sci. Rep. 2016, 6, 22571. [Google Scholar] [CrossRef]
- Wu, B.; Yang, X.; Yan, M. Synthesis and Structure–Activity Relationship Study of Antimicrobial Auranofin against ESKAPE Pathogens. J. Med. Chem. 2019, 62, 7751–7768. [Google Scholar] [CrossRef]
- Pandrala, M.; Li, F.; Feterl, M.; Mulyana, Y.; Warner, J.M.; Wallace, L.; Keene, F.R.; Collins, J.G. Chlorido-containing ruthenium(ii) and iridium(iii) complexes as antimicrobial agents. Dalton Trans. 2013, 42, 4686. [Google Scholar] [CrossRef]
- Lu, L.; Liu, L.-J.; Chao, W.; Zhong, H.-J.; Wang, M.; Chen, X.-P.; Lu, J.-J.; Li, R.; Ma, D.-L.; Leung, C.-H. Identification of an iridium(III) complex with anti-bacterial and anti-cancer activity. Sci. Rep. 2015, 5, 14544. [Google Scholar] [CrossRef]
- Jain, N.; Alam, P.; Laskar, I.R.; Panwar, J. ‘Aggregation induced phosphorescence’ active iridium(III) complexes for integrated sensing and inhibition of bacterial growth in aqueous solution. RSC Adv. 2015, 5, 61983–61988. [Google Scholar] [CrossRef]
- Huang, H.; Banerjee, S.; Sadler, P.J. Recent Advances in the Design of Targeted Iridium(III) Photosensitizers for Photodynamic Therapy. ChemBioChem 2018, 19, 1574–1589. [Google Scholar] [CrossRef] [PubMed]
- Chen, F.; Moat, J.; McFeely, D.; Clarkson, G.; Hands-Portman, I.J.; Furner-Pardoe, J.P.; Harrison, F.; Dowson, C.G.; Sadler, P.J. Biguanide Iridium(III) Complexes with Potent Antimicrobial Activity. J. Med. Chem. 2018, 61, 7330–7344. [Google Scholar] [CrossRef] [PubMed]
- Okamoto, S.; Eltis, L.D. The biological occurrence and trafficking of cobalt. Metallomics 2011, 3, 963. [Google Scholar] [CrossRef]
- Chang, E.L.; Simmers, C.; Knight, D.A. Cobalt Complexes as Antiviral and Antibacterial Agents. Pharmaceuticals 2010, 3, 1711–1728. [Google Scholar] [CrossRef] [PubMed]
- Epstein, S.P.; Wallace, J.A.; Epstein, D.; Stewart, C.C.; Burger, R.M. Efficacy of Cobalt Chelates in the Rabbit Eye Model for Epithelial Herpetic Keratitis. Cornea 1998, 17, 550–557. [Google Scholar] [CrossRef] [PubMed]
- Schwartz, J.A.; Lium, E.K.; Silverstein, S.J. Herpes Simplex Virus Type 1 Entry Is Inhibited by the Cobalt Chelate Complex CTC-96. J. Virol. 2001, 75, 4117–4128. [Google Scholar] [CrossRef]
- Takeuchi, T.; Böttcher, A.; Quezada, C.M.; Meade, T.J.; Gray, H.B. Inhibition of Thermolysin and Human α-Thrombin by Cobalt(III) Schiff Base Complexes. Bioorg. Med. Chem. 1999, 7, 815–819. [Google Scholar] [CrossRef]
- Hall, M.D.; Failes, T.W.; Yamamoto, N.; Hambley, T.W. Bioreductive activation and drug chaperoning in cobalt pharmaceuticals. Dalton Trans. 2007, 36, 3983–3990. [Google Scholar] [CrossRef]
- Heffern, M.C.; Yamamoto, N.; Holbrook, R.J.; Eckermann, A.L.; Meade, T.J. Cobalt derivatives as promising therapeutic agents. Curr. Opin. Chem. Biol. 2013, 17, 189–196. [Google Scholar] [CrossRef]
- Bernhardt, P.V.; Jones, L.A.; Sharpe, P.C. Structural and Electron Self-Exchange Rate Variations in Isomeric (Hexaamine)cobalt(III/II) Complexes. Inorg. Chem. 1997, 36, 2420–2425. [Google Scholar] [CrossRef]
- Renfrew, A.K. Transition Metal Complexes with Bioactive Ligands: Mechanisms for Selective Ligand Release and Applications for Drug Delivery. Metallomics 2014, 6, 1324–1335. [Google Scholar] [CrossRef]
- Renfrew, A.K.; O’Neill, E.S.; Hambley, T.W.; New, E.J. Harnessing the Properties of Cobalt Coordination Complexes for Biological Application. Coord. Chem. Rev. 2018, 375, 221–233. [Google Scholar] [CrossRef]
- Saghatforoush, L.A.; Mehdizadeh, R.; Chalabian, F. Hydrothermal and sono-chemical synthesis of a nano-sized nickel(II) Schiff base complex as a precursor for nano-sized nickel(II) oxide; spectroscopic, catalytic and antibacterial properties. Transit. Met. Chem. 2010, 35, 903–910. [Google Scholar] [CrossRef]
- Revathi, V.; Karthik, K. Physico-chemical properties and antibacterial activity of Hexakis (Thiocarbamide) Nickel(II) nitrate single crystal. Chem. Data Collect. 2019, 21, 100229. [Google Scholar] [CrossRef]
- Chohan, Z.H. Synthesis of cobalt (II) and nickel (II) complexes of Ceclor (Cefaclor) and preliminary experiments on their antibacterial character. Chem. Pharm. Bull. 1991, 39, 1578–1580. [Google Scholar] [CrossRef]
- Chohan, Z.H.; Supuran, C.T.; Scozzafava, A. Metalloantibiotics: Synthesis and Antibacterial Activity of Cobalt(II), Copper(II), Nickel(II) and Zinc(II) Complexes of Kefzol. J. Enzyme Inhib. Med. Chem. 2004, 19, 79–84. [Google Scholar] [CrossRef]
- Ragsdale, S.W. Nickel-based Enzyme Systems. J. Biol. Chem. 2009, 284, 18571–18575. [Google Scholar] [CrossRef]
- Ariza, A.; Vickers, T.J.; Greig, N.; Armour, K.A.; Dixon, M.J.; Eggleston, I.M.; Fairlamb, A.H.; Bond, C.S. Specificity of the trypanothione-dependent Leishmania major glyoxalase I: Structure and biochemical comparison with the human enzyme. Mol. Microbiol. 2006, 59, 1239–1248. [Google Scholar] [CrossRef]
- Benoit, S.L.; Schmalstig, A.A.; Glushka, J.; Maier, S.E.; Edison, A.S.; Maier, R.J. Nickel chelation therapy as an approach to combat multi-drug resistant enteric pathogens. Sci. Rep. 2019, 9, 13851. [Google Scholar] [CrossRef]
- Sharma, A.D. Relationship between nickel allergy and diet. Indian J. Dermatol. Venereol. Leprol. 2007, 73, 307–312. [Google Scholar] [CrossRef]
- Chaudhary, A.; Bansal, N.; Gajraj, A.; Singha, R.V. Antifertility, antibacterial, antifungal and percent disease incidence aspects of macrocyclic complexes of manganese(II). J. Inorg. Biochem. 2003, 96, 393–400. [Google Scholar] [CrossRef]
- Jain, M.; Gaur, S.; Diwedi, S.C.; Joshi, S.C.; Singh, R.V.; Bansal, A. Nematicidal, insecticidal, antifertility, antifungal and antibacterial activities of salicylanilide sulphathiazole and its manganese, silicon and tin complexes. Phosphorus Sulfur Silicon Relat. Elem. 2004, 179, 1517–1537. [Google Scholar] [CrossRef]
- Anacona, J.R.; Bastardo, E. Manganese(II) and palladium(II) complexes containing a new macrocyclic Schiff base ligand: Antibacterial properties. Transit. Chem. 1999, 24, 478–480. [Google Scholar] [CrossRef]
- Finelli, A.; Abram, S.-L.; Hérault, N.; Crochet, A.; Fromm, K.M. Bimetallic Salen-based compounds and tgeir potential applications. Cryt. Growth Des. 2020, 20, 4945–4958. [Google Scholar] [CrossRef]
- Wenzel, M.; Patra, M.; Helmut, C.; Senges, R.; Ott, I.; Stepanek, J.J.; Pinto, A.; Prochnow, P.; Vuong, C.; Langklotz, S.; et al. Analysis of the Mechanism of Action of Potent Antibacterial Hetero-tri-organometallic Compounds: A Structurally New Class of Antibiotics. ACS Chem. Biol. 2013, 8, 1442–1450. [Google Scholar] [CrossRef]
- Kunin, C.M.; Brandt, D.; Wood, H. Bacteriologic studies in rifampin, a new semi-synthetic antibiotic. J. Infect. Dis. 1979, 119, 132–137. [Google Scholar] [CrossRef]
- Marone, P.; Monzillo, V.; Perversi, L.; Carretto, E. Comparative In Vitro Activity of Silver Sulfadiazine, Alone and in Combination with Cerium Nitrate, Against Staphylococci and Gram-Negative Bacteria. J. Chemother. 1998, 10, 17–21. [Google Scholar] [CrossRef]
- Austin, C.B.; Wright, M.S.; Stepanauskas, R.; McArthur, J.V. Co-selection of antibiotic and metal resistance. Trends Microbiol. 2006, 14, 176–182. [Google Scholar] [CrossRef] [PubMed]
- Pitz, A.M.; Woo Park, G.; Lee, D.; Boissy, Y.L.; Vinjé, J. Antimicrobial activity of bismuth subsalicylate on Clostridium difficile, Escherichia coli O157:H7, norovirus, and other common enteric pathogens. Gut Microbes 2015, 6, 93–100. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).