Monofluorophosphates—New Examples and a Survey of the PO3F2− Anion
Abstract
1. Introduction
2. Materials and Methods
2.1. Syntheses and Single Crystal Growth
2.2. Single Crystal Diffraction and Structure Analysis
2.3. Vibrational Spectroscopy
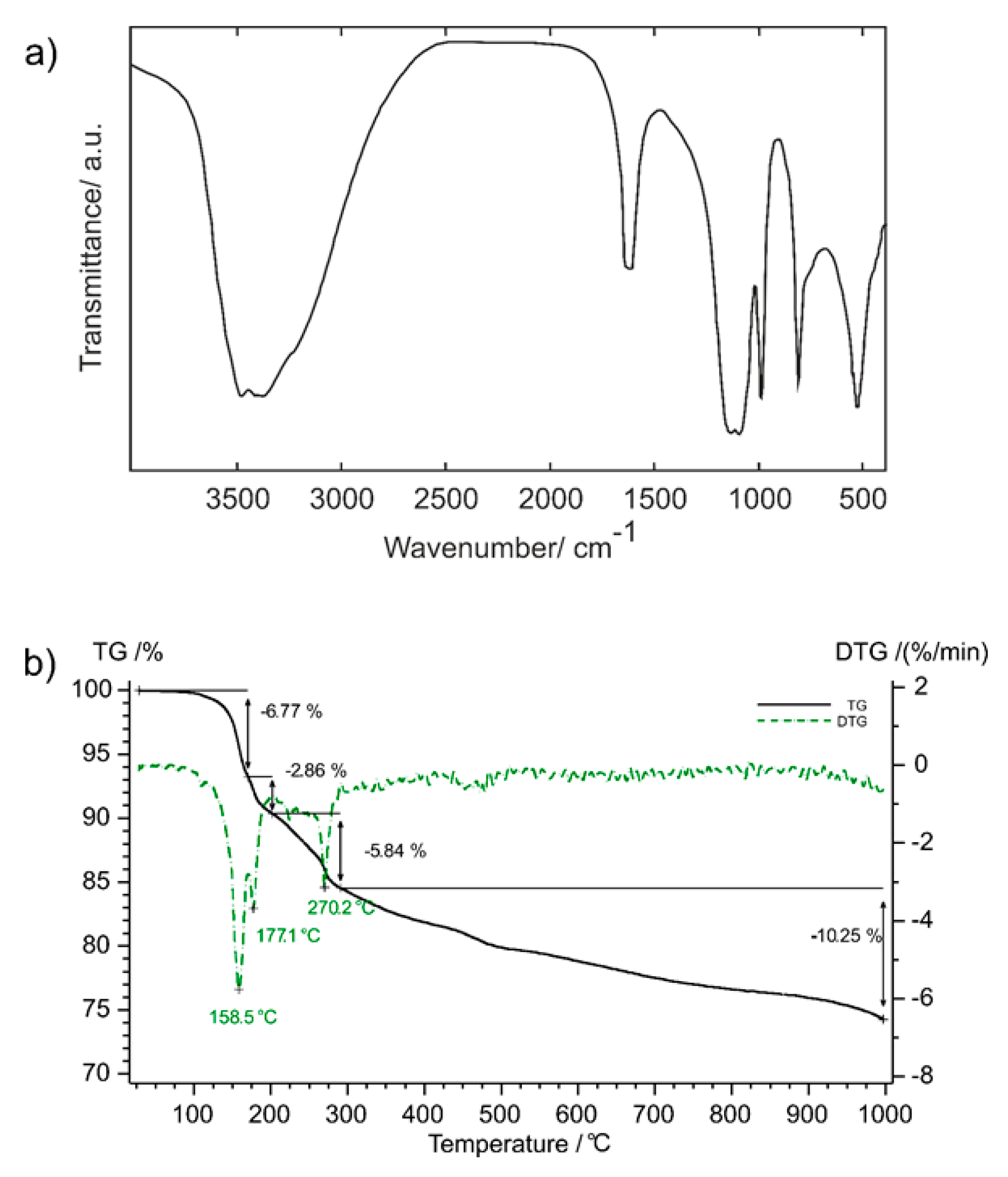
2.4. Thermogravimetry (TG)
3. Results
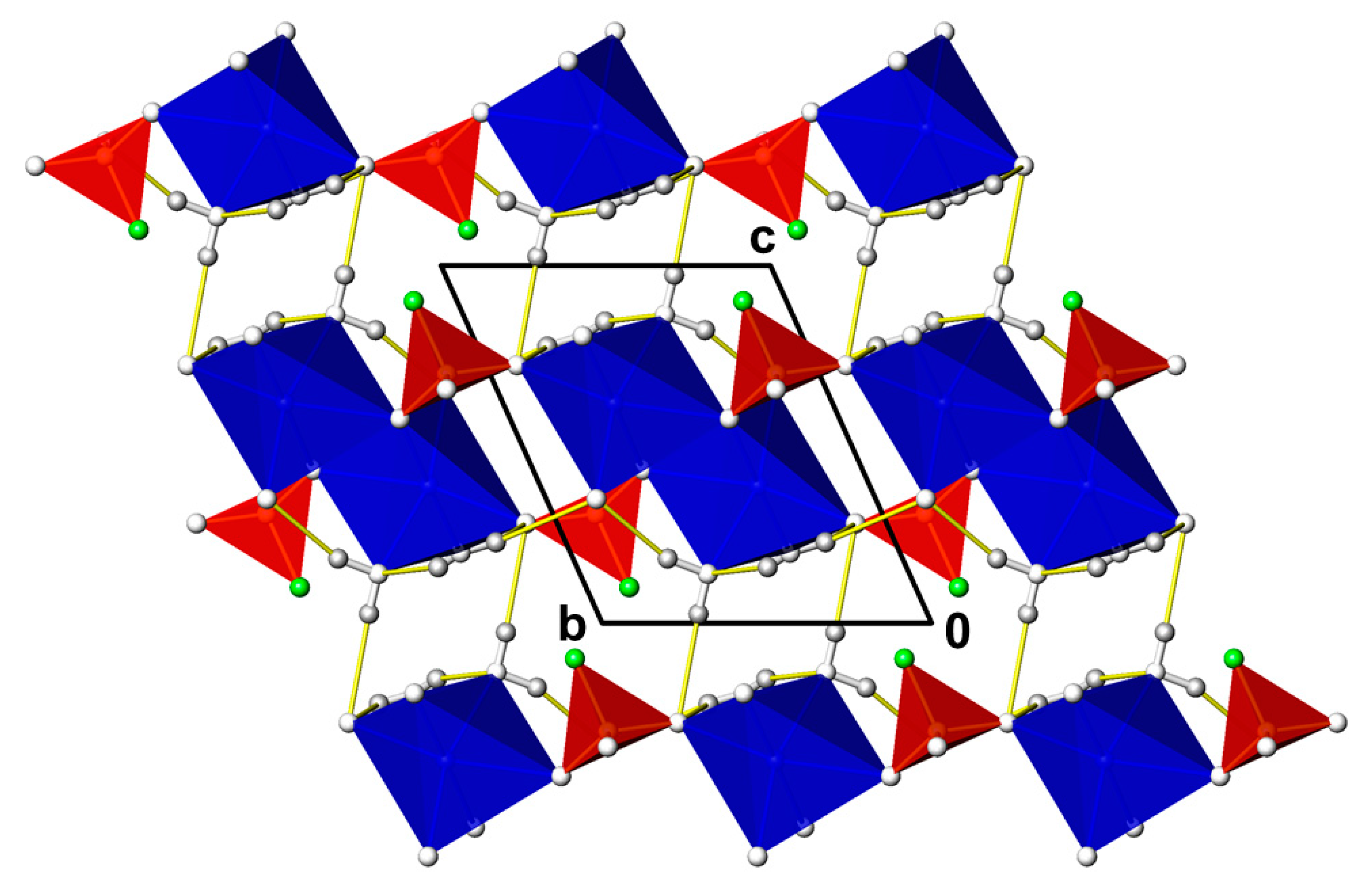
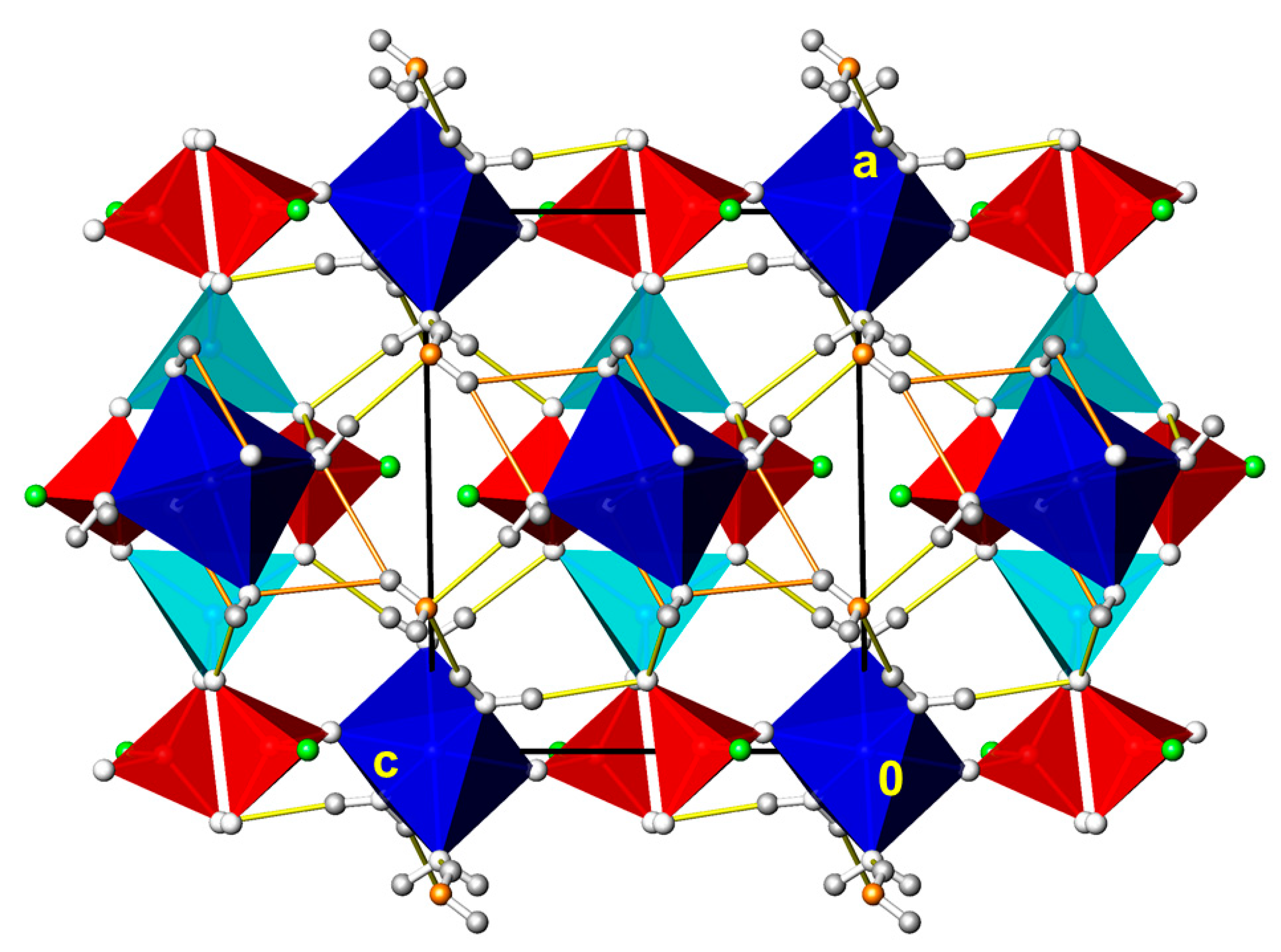
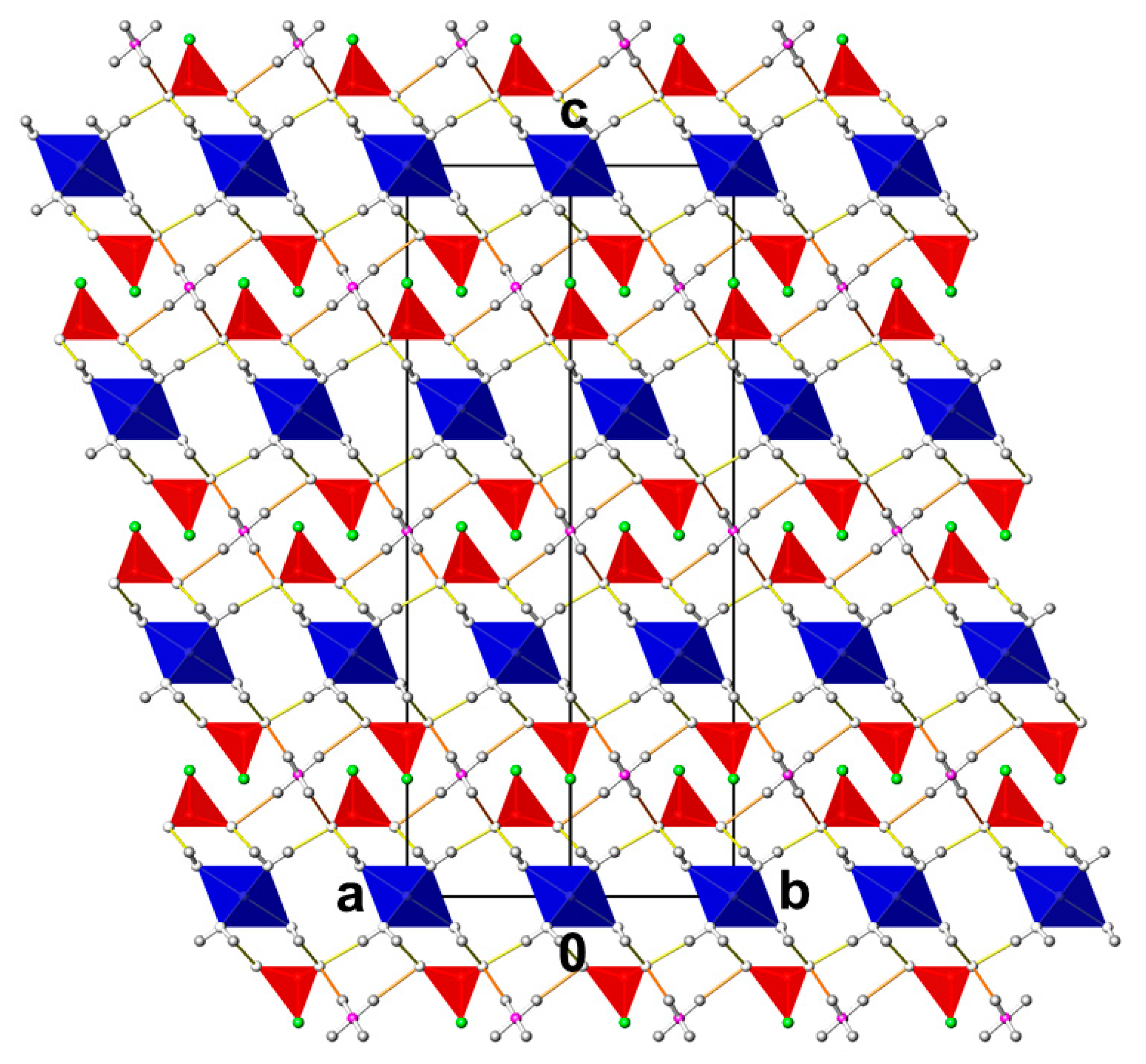
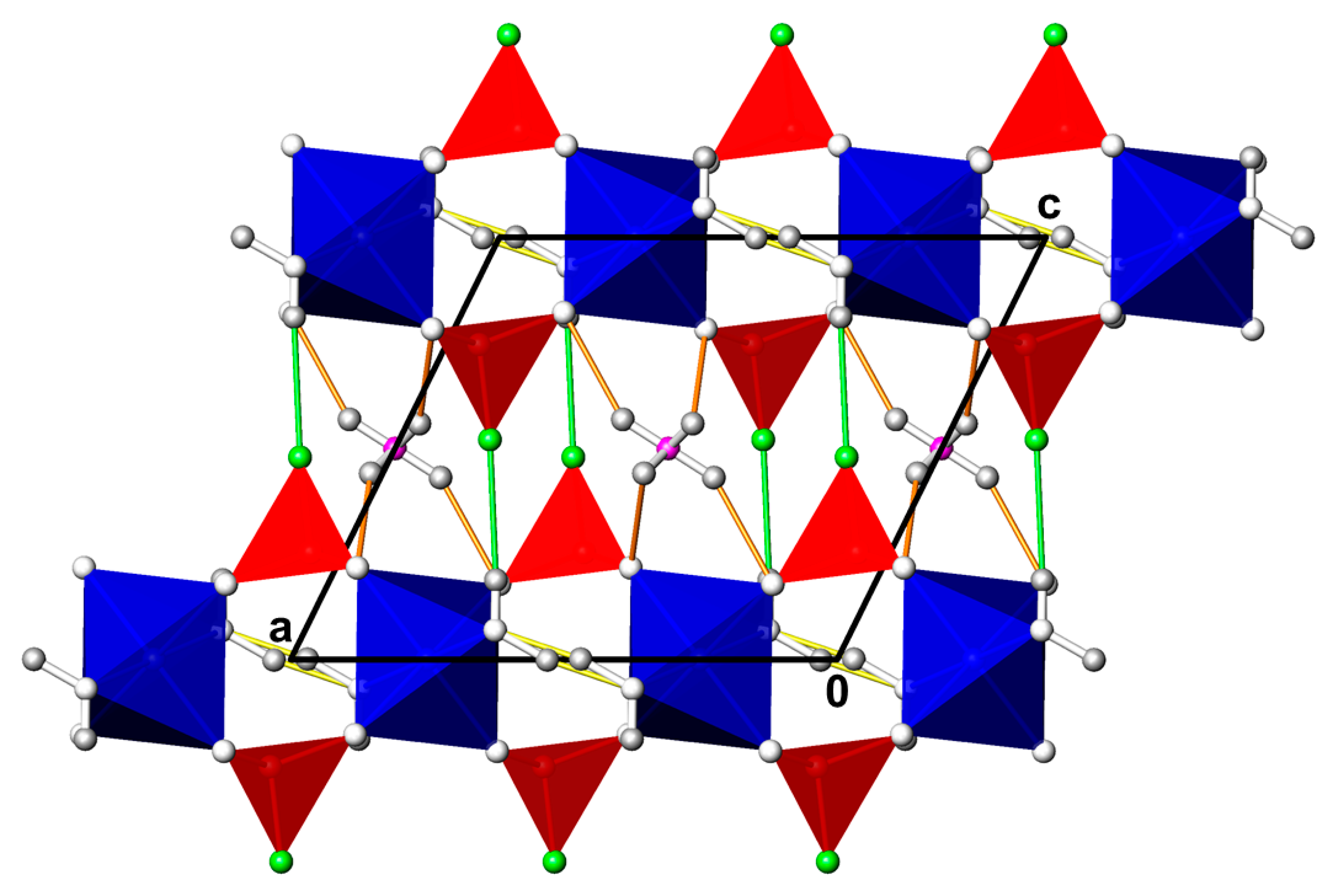
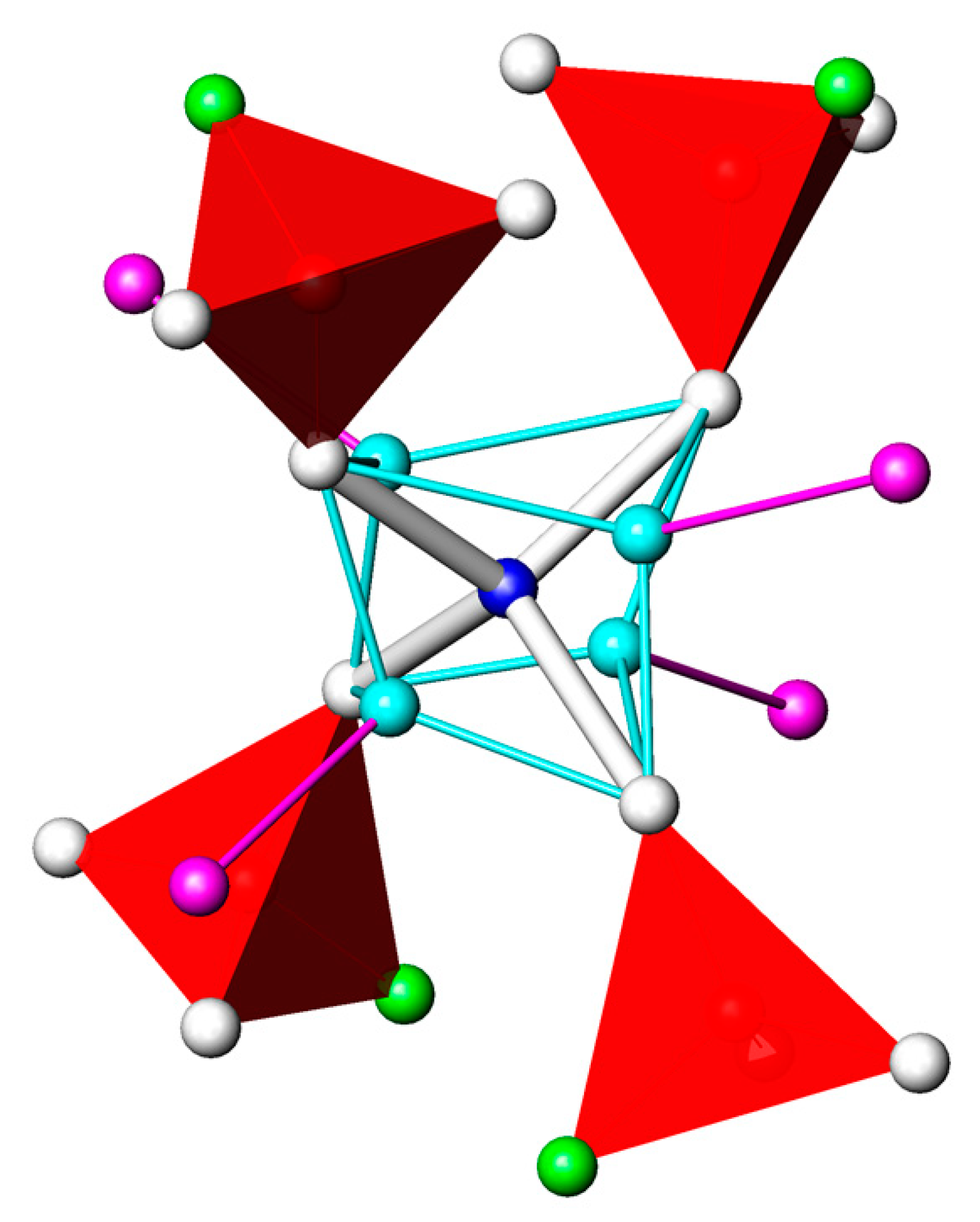
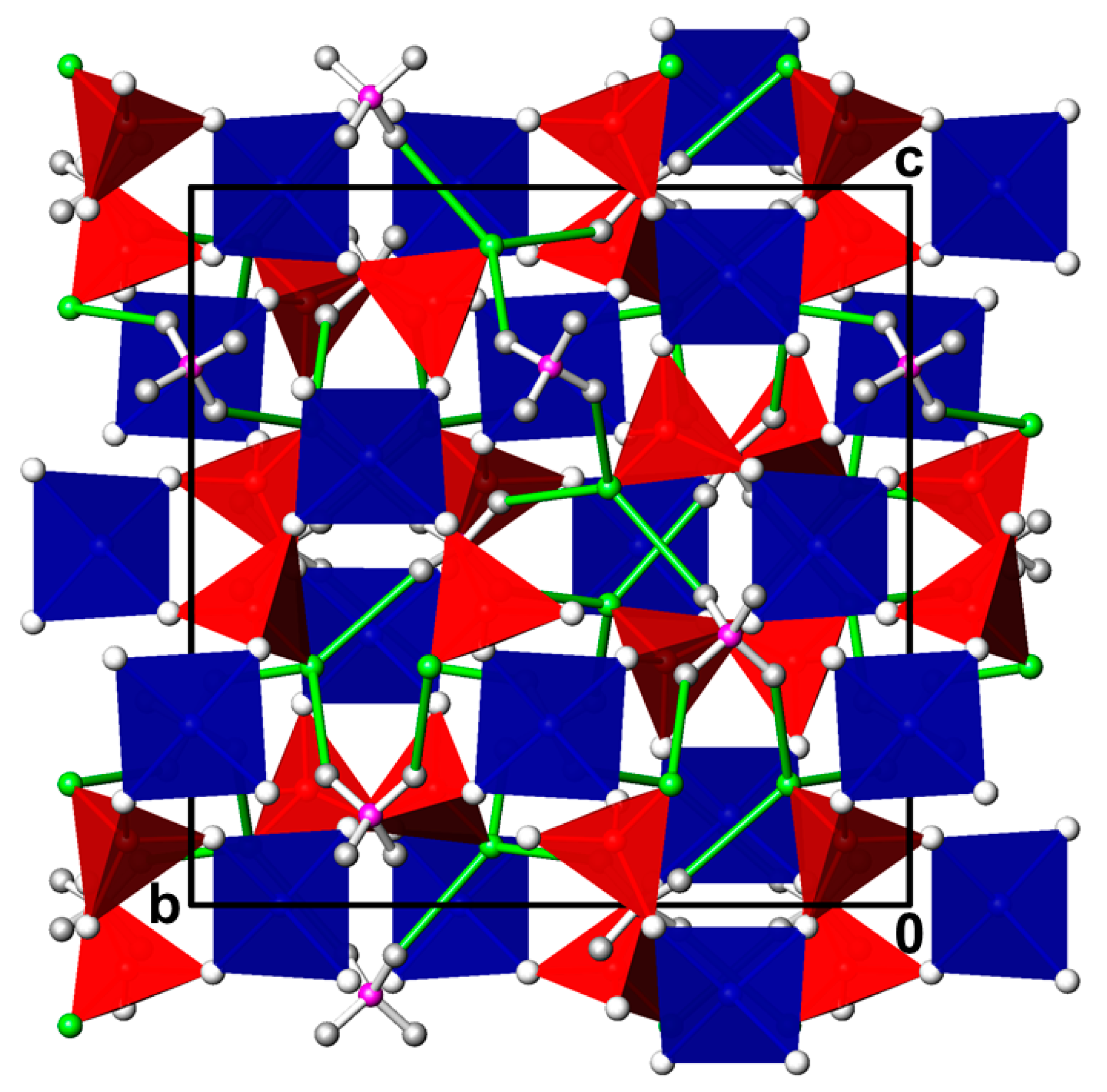
3.1. CdPO3F(H2O)2
- -
- Regarding the vibrations of the water molecules, the O–H stretchings are seen as a relatively broad and clearly splitted band due to the presence of two crystallographically different water molecules. The positions of these bands are characteristic for the presence of hydrogen bridges of medium strength [20], in agreement with the results of the structure analysis. Interestingly, the corresponding deformational mode, δ(H2O), shows also splitting signals.
- -
- The antisymmetric ν(PO3) vibration was not observed in the Raman spectrum, whereas in the IR spectrum it is very strong and broad. In accord with the predictions of the site-symmetry analysis two components can be seen. The corresponding symmetric stretching vibration is the strongest Raman band in both compounds and is also relatively strong in the IR spectrum.
- -
- The ν(P–F) vibration can be clearly identified in the spectra, lying at somewhat higher energy than that observed in the solution Raman spectrum (795 cm−1) [20].
- -
- For the deformational modes only δ(PO3) could be identified, clearly split in the IR spectra as predicted (cf. Table 4), whereas no signals for the δ(FPO3) mode could be found. In the Raman spectrum of a PO3F2− solution, both vibrations are reported at the same energy (520 cm−1) [20], although in the case of crystalline Hg2PO3F, both vibrations were identified at slightly different wavenumbers, with ν5 > ν3 [21].
- -
- The corresponding ν6-PO3-rocking mode was only identified in the Raman spectrum, as a very weak band.
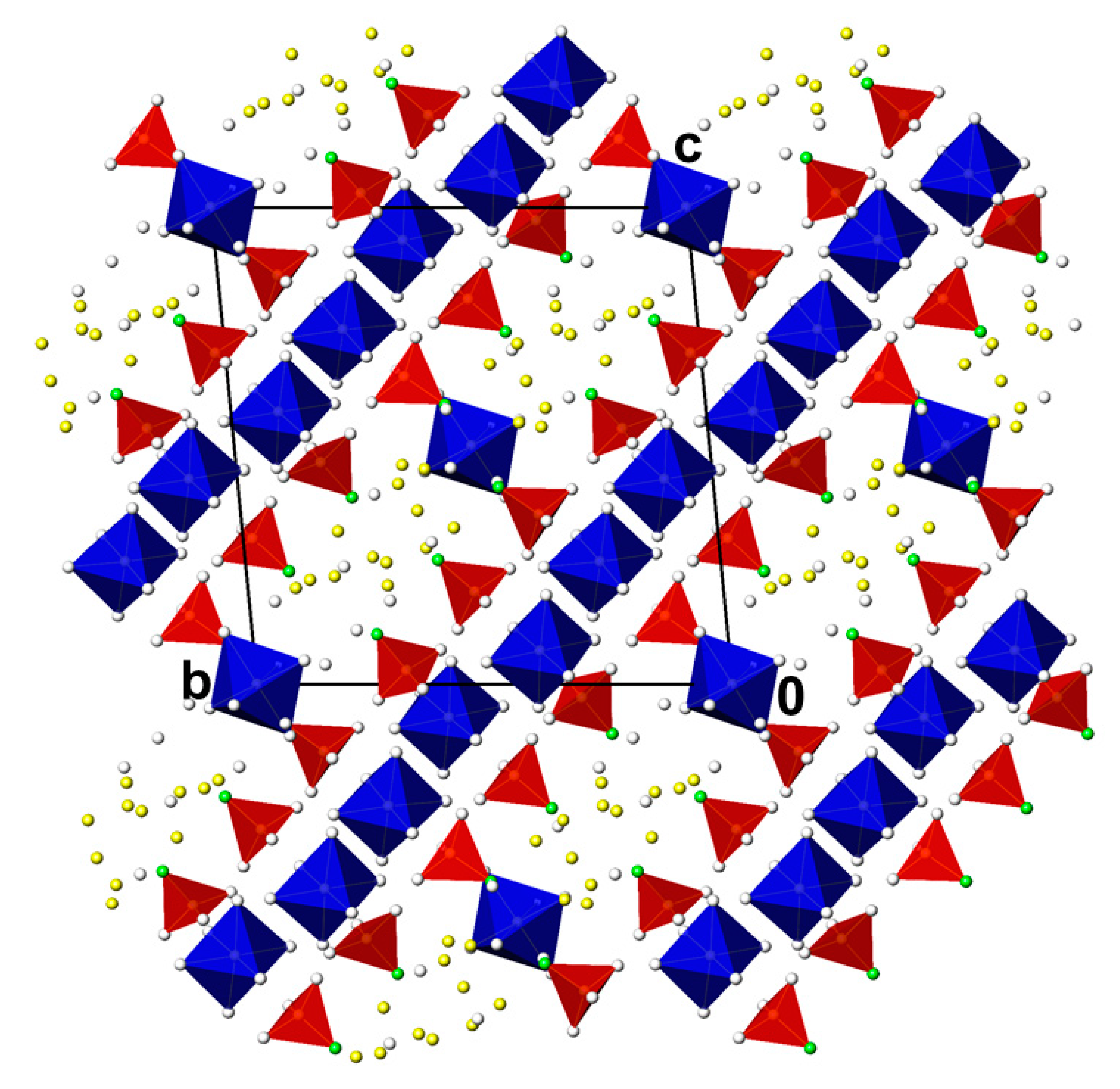
3.2. Cr2(PO3F)3(H2O)18.8
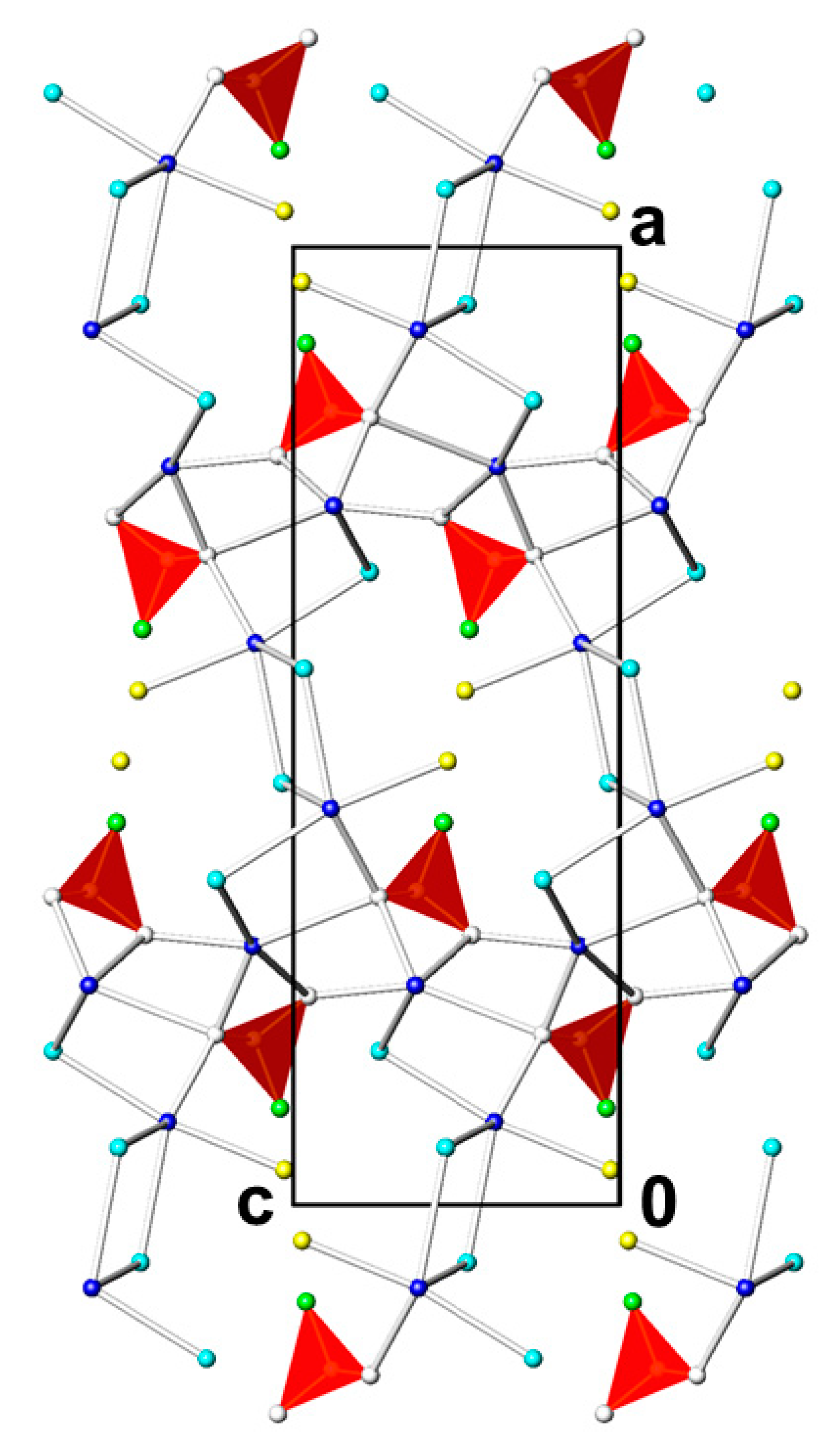
3.3. Pb2(PO3F)Cl2(H2O)
3.4. ZnPO3F(H2O)2.5
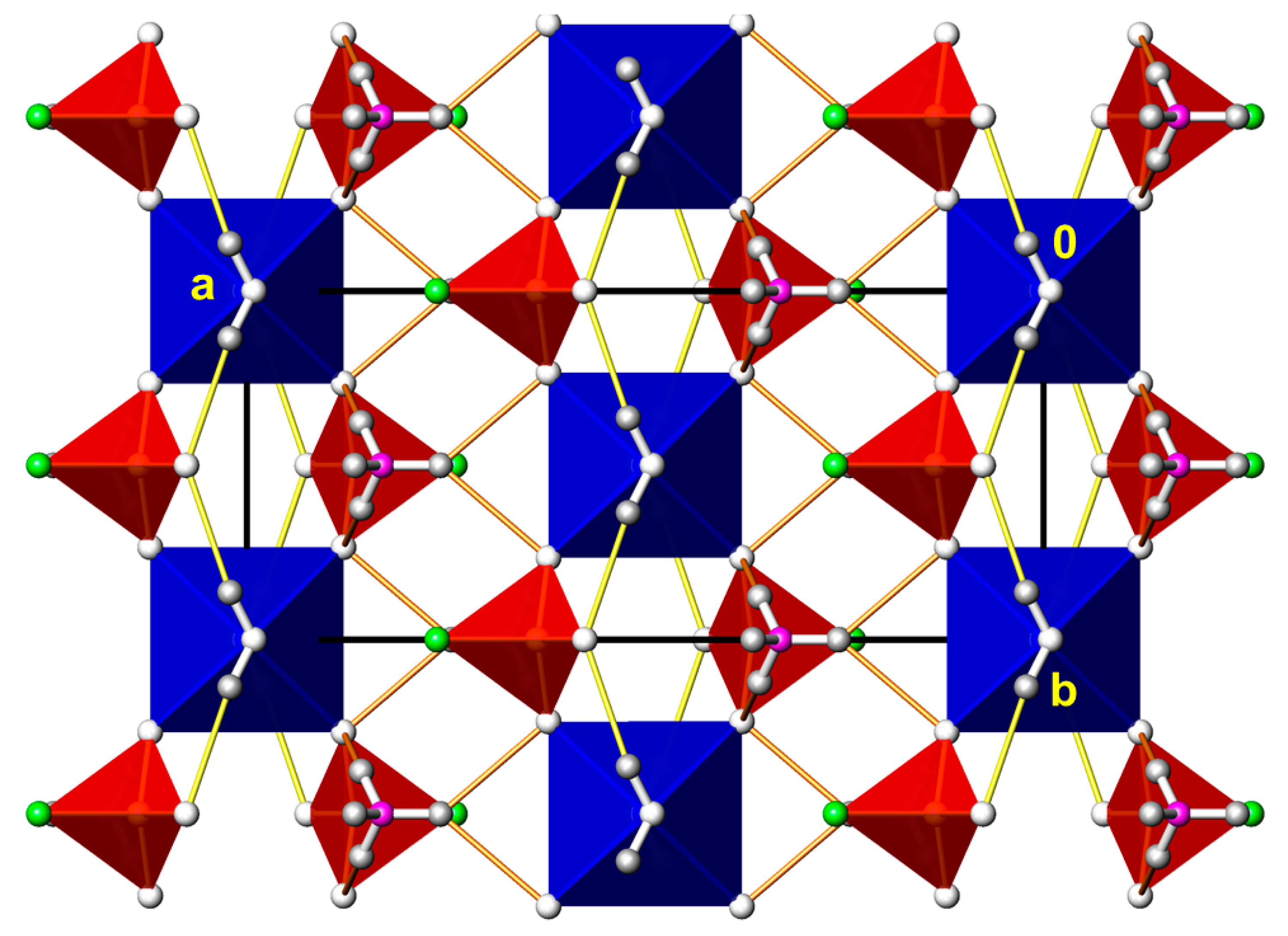
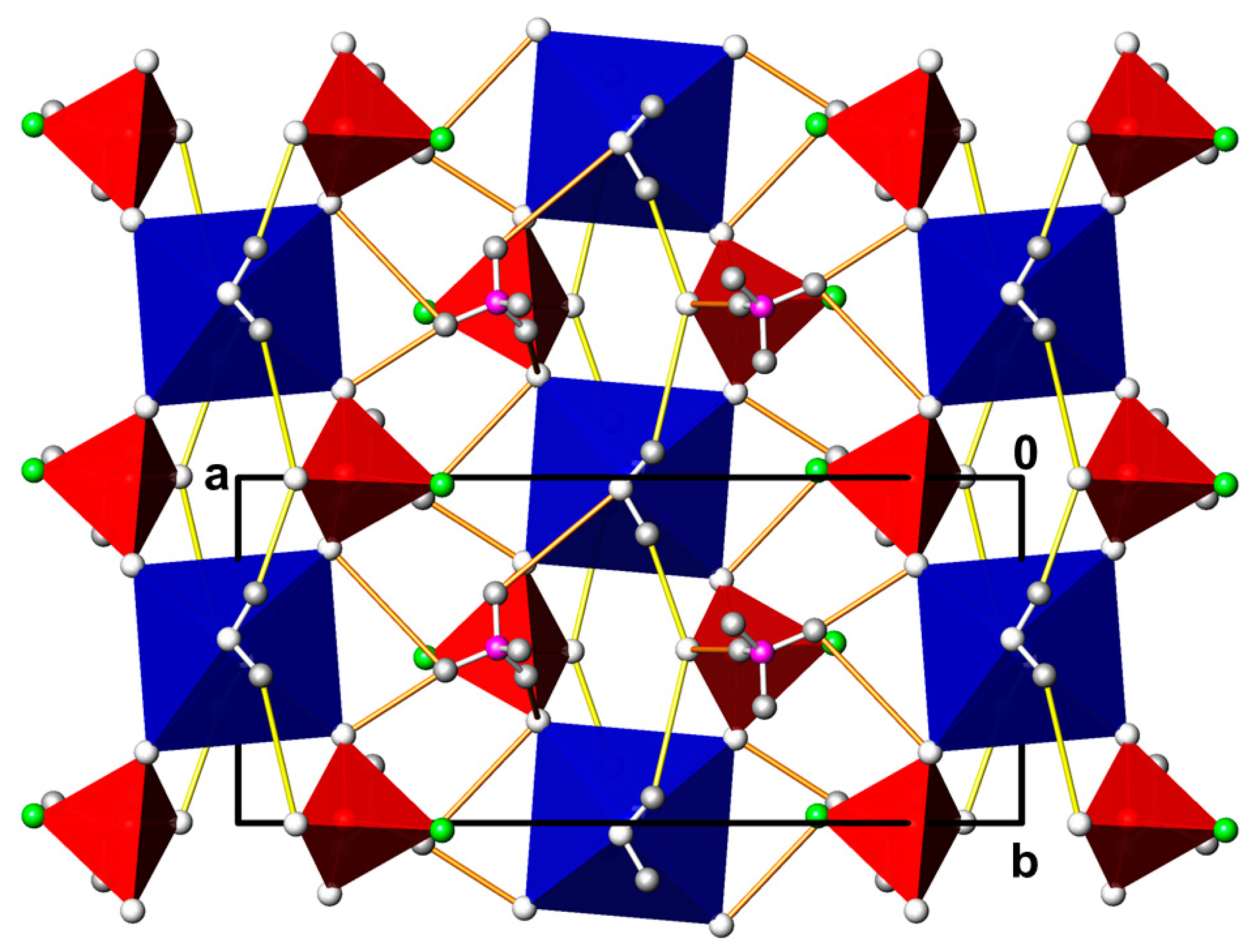
3.5. (NH4)2M(PO3F)2(H2O)2 (M = Co, Mg)
3.6. (NH4)2Mn(PO3F)2(H2O)2
3.7. (NH4)2Ni(PO3F)2(H2O)6
3.8. NH4Cr(PO3F)2(H2O)6
3.9. NH4Cu2(H3O2)(PO3F)2
3.10. (NH4)2Zn(PO3F)2(H2O)0.2
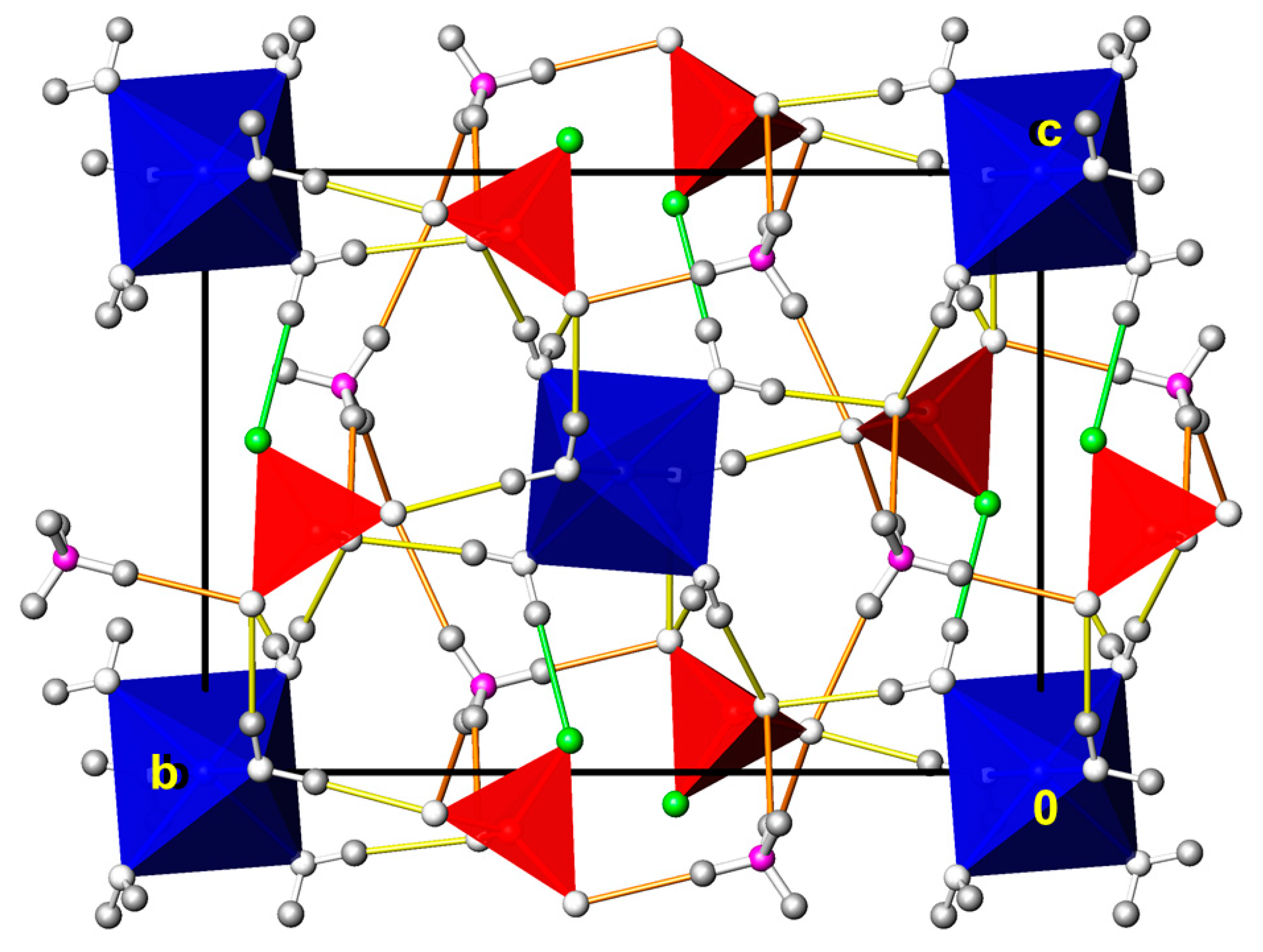
3.11. (NH4)2Zn3(PO3F)4(H2O)
3.12. Survey on the PO3F2− Group
- Mean bond lengths and angles in the PO3F tetrahedron
- Symmetry of the PO3F group in crystal structures
- Isotypism with sulfates
- Hydrogen bonding with the monofluorophosphate F atom as an acceptor
4. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lange, W. Über die Monofluorphosphorsäure und die Ähnlichkeit ihrer Salze mit den Sulfaten. Ber. Dtsch. Chem. Ges. 1929, 62B, 793–801. [Google Scholar] [CrossRef]
- Rea, J.R.; Kostiner, E. The crystal structure of manganese fluorophosphate, Mn2(PO4)F. Acta Crystallogr. 1972, B28, 2525–2529. [Google Scholar] [CrossRef]
- Jin, D.; Qiu, H.; Du, F.; Wei, Y.; Meng, X. Co-doped Na2FePO4F fluorophosphates as a promising cathode material for rechargeable sodium-ion batteries. Solid State Sci. 2019, 93, 62–69. [Google Scholar] [CrossRef]
- Weil, M.; Puchberger, M.; Füglein, E.; Baran, E.J.; Vannahme, J.; Jakobsen, H.J.; Skibsted, J. Single-Crystal Growth and Characterization of Disilver(I) Monofluorophosphate(V), Ag2PO3F: Crystal Structure, Thermal Behavior, Vibrational Spectroscopy, and Solid-State 19F, 31P, and 109Ag MAS NMR Spectroscopy. Inorg. Chem. 2007, 46, 801–808. [Google Scholar] [CrossRef]
- Xiong, L.; Chen, J.; Lu, J.; Pan, C.-Y.; Wu, L.-M. Monofluorophosphates: A New Source of Deep-Ultraviolet Nonlinear Optical Materials. Chem. Mater. 2018, 30, 7823–7830. [Google Scholar] [CrossRef]
- Robinson, M.T. The crystal structures of β-K2SO4 and β-K2PO3F. J. Phys. Chem. 1958, 62, 925–928. [Google Scholar] [CrossRef]
- Rây, R.C. Isomorphism and Chemical Homology. Nature 1930, 126, 310–311. [Google Scholar] [CrossRef]
- Lima-de-Faria, J.; Hellner, E.; Liebau, F.; Makovicky, E.; Parthé, E. Nomenclature of Inorganic Structure Types—Report of the International Union of Crystallography Commission on Crystallographic Nomenclature Subcommittee on the Nomenclature of Inorganic Structure Types. Acta Crystallogr. 1990, A46, 1–11. [Google Scholar] [CrossRef]
- Gates-Rector, S.D.; Blanton, T.N. The Powder Diffraction File: A Quality Materials Characterization Database. Powder Diffr. 2019, 34, 352–360. [Google Scholar] [CrossRef]
- McCubbin, F.M.; Phillips, B.L.; Adcock, C.T.; Tait, K.T.; Steele, A.; Vaughn, J.S.; Fries, M.D.; Atudorei, V.; Vander Kaaden, K.E.; Hausrath, E.M. Discreditation of bobdownsite and the establishment of criteria for the identification of minerals with essential monofluorophosphate (PO3F2−). Am. Mineral. 2018, 103, 1319–1328. [Google Scholar] [CrossRef]
- Schülke, U.; Kayser, R. Herstellung von Fluorophosphaten, Difluorophosphaten, Fluorophosphonaten und Fluorophosphiten in fluoridhaltigen Harnstoffschmelzen. Z. Anorg. Allg. Chem. 1991, 600, 221–226. [Google Scholar] [CrossRef]
- Sheldrick, G.M. A short history of SHELX. Acta Crystallogr. 2008, 64, 112–122. [Google Scholar] [CrossRef]
- Sheldrick, G.M. Crystal structure refinement with SHELXL. Acta Crystallogr. 2015, 71, 3–8. [Google Scholar]
- Durand, J.; Larbot, A.; Cot, L.; Duprat, M.; Dabosi, F. Etude Structurale de ZnPO3F·2.5H2O, Nouvel Inhibiteur de Corrosion. Z. Anorg. Allg. Chem. 1983, 504, 163–172. [Google Scholar] [CrossRef]
- Berraho, M.; R’Kha, C.; Vegas, A.; Rafiq, M. Structure of Ni(H2O)6(NH4)2(PO3F)2. Acta Crystallogr. 1992, C48, 1350–1352. [Google Scholar] [CrossRef]
- Chevier, G.; Giester, G.; Jarosch, D.; Zemann, J. Neutron Diffraction Study of the Hydrogen-Bond System in Cu2K(H3O2)(SO4)2). Acta Crystallogr. 1990, C46, 175–177. [Google Scholar] [CrossRef]
- Ross, S.D. Inorganic Infrared and Raman Spectra; McGraw Hill: London, UK, 1972. [Google Scholar]
- Müller, A.; Baran, E.J.; Carter, R.O. Vibrational spectra of oxo-, thio-, and selenometallates of transition elements in the solid state. Struct. Bond. 1976, 26, 81–139. [Google Scholar]
- Fadini, A.; Schnepel, F.M. Vibrational Spectroscopy: Methods and Applications; Ellis Horwood: Chichester, UK, 1989. [Google Scholar]
- Siebert, H. Anwendungen der Schwingungsspektroskopie in derAnorganischen Chemie; Springer: Berlin, Germany, 1966. [Google Scholar]
- Weil, M.; Puchberger, M.; Baran, E.J. Preparation and characterization of dimercury(I) monofluorophosphate(V), Hg2PO3F: Crystal structure, thermal behavior, vibrational spectra, and solid state 31P and 19F NMR spectra. Inorg. Chem. 2004, 43, 8330–8335. [Google Scholar] [CrossRef]
- Calvo, C.; Au, P.K.L. Crystal structure of Cd2P2O7. Can. J. Chem. 1969, 47, 3409–3416. [Google Scholar] [CrossRef]
- Menz, D.-H.; Kolditz, L. Synthese und thermisches Verhalten von Mg(NH4)2(PO3F)2·2H2O. Z. Chem. 1985, 25, 189–190. [Google Scholar] [CrossRef]
- Menz, D.-H.; Kolditz, L.; Heide, K.; Kunert, C.; Mensing, C. Zur Thermischen Zersetzung von SrPO3F·H2O. Z. Anorg. Allg. Chem. 1986, 540, 191–197. [Google Scholar] [CrossRef]
- Nyburg, S.C.; Steed, J.W.; Aleksovska, S.; Petruševski, V.M. Structure of the alums. I. On the sulfate group disorder in the α-alums. Acta Crystallogr. 2000, B56, 204–209. [Google Scholar] [CrossRef] [PubMed]
- Gmelin Handbook of Inorganic Chemistry. Cr-Chrom (Systemnummer 52); Springer-Verlag: Berlin/Heidelberg, Germany, 1962; Teil B; p. 306ff.
- Berraho, M.; Vegas, A.; Martinez-Ripoll, M.; Rafiq, M. A copper monofluorophosphate, Cu(H2O)2(NH4)2(PO3F)2. Acta Crystallogr. 1994, C50, 666–668. [Google Scholar] [CrossRef]
- Müller, U. Symmetry Relationships between Crystal Structures; IUCr/Oxford Science Publications: Oxford, UK, 2013. [Google Scholar]
- Weil, M. On the (non-)existence of Tutton salts with formula types [M(H2O)6](ClO4)2(H2O)2 and Na2M(SO4)2(H2O)6 (M is a first-row transition metal). Acta Crystallogr. 2013, C69, 990–994. [Google Scholar]
- Moewius, F.; Ziemer, B.; Reck, G.; Meisel, M.; Grunze, H. Darstellung und Kristallstruktur von Kalium-dikupferhydroxid-bis(monofluorophosphat)-Monohydrat Cu2K(OH)(PO3F)2·H2O. Z. Anorg. Allg. Chem. 1987, 547, 75–82. [Google Scholar] [CrossRef]
- Zagorac, D.; Müller, H.; Ruehl, S.; Zagorac, J.; Rehme, S. Recent developments in the Inorganic Crystal Structure Database: Theoretical crystal structure data and related features. J Appl. Crystallogr. 2019, 52, 918–925. [Google Scholar] [CrossRef]
- Weil, M.; Fürst, M. Crystal structure of (1,4-diphenyl-4H-1,2,4-triazol-3-yl)phenylamine difluorophosphate, and a survey of the difluorophosphate anion (PO2F2−). Acta Crystallogr. 2020, E76, 1003–1006. [Google Scholar] [CrossRef]
- Keates, A.C.; Armstrong, J.A.; Weller, M.T. Iron fluorophosphates. Dalton Trans. 2013, 42, 10715–10724. [Google Scholar] [CrossRef]
- Skakle, J.M.S.; Fletcher, J.G.; West, A.R. The crystal structures of potassium oxyfluorides, K3SeO4F and K3PO3F2. An. Quim. Int. Ed. 1996, 92, 358–361. [Google Scholar]
- Steiner, T. The Hydrogen bond in the Solid State. Angew. Chem. Int. Ed. 2002, 41, 48–76. [Google Scholar] [CrossRef]
- Galigne, J.L.; Durand, J.; le Cot, L. Etudes structurales de composes oxyfluores du P(V). I. Structure crystalline de LiKPO3FH2 O. Acta Crystallogr. 1974, B30, 697–701. [Google Scholar] [CrossRef]
- Durand, J.; le Cot, L.; Galigne, J.L. Etudes structurales de composes oxyfluores du P(V). V. Structure crystalline de LiNH4PO3F. Acta Crystallogr. 1978, B34, 388–391. [Google Scholar] [CrossRef]
- Durand, J.; le Cot, L.; Galigne, J.L. Etudes structurales de composes oxyfluores du P(V) II. Structure cristalline de Na2PO3F beta. Acta Crystallogr. 1974, B30, 1565–1569. [Google Scholar] [CrossRef]
- Prescott, H.A.; Troyanov, S.I.; Kemnitz, E. The synthesis and crystal structures of two new hydrated sodium monofluorophosphates: NaHPO3F·2.5H2O (I) and Na2PO3F·10H2O (II). J. Solid State Chem. 2001, 156, 415–421. [Google Scholar] [CrossRef]
- Durand, J.; Granier, W.; le Cot, L.; Galigne, J.L. Etudes structurales de composes oxyfluores du P(V). III. Structure crystalline de NaK3(PO3F)2. Acta Crystallogr. 1975, B31, 1533–1535. [Google Scholar] [CrossRef]
- Fabry, J.; Dušek, M.; Krupkova, R. Ammonium sodium fluorotrioxophosphate monohydrate. Acta Crystallogr. 2007, E63, i92–i94. [Google Scholar] [CrossRef]
- Payen, J.; Durand, J.; le Cot, L.; Galigne, J.L. Etude structurale du monofluorophosphate de potassium K2PO3F. Can. J. Chem. 1979, 57, 886–889. [Google Scholar] [CrossRef]
- Fabry, J.; Dušek, M.; Fejfarova, K.; Krupkova, R.; Vanek, P.; Cisarova, I. Dirubidum fluorotrioxophosphate, Rb2PO3F, at 290 and 130 K, and dicesium fluorotrioxophosphate, Cs2PO3F, at 240 and 100 K. Acta Crystallogr. 2006, C62, i49–i52. [Google Scholar]
- Prescott, H.; Troyanov, S.; Kemnitz, E. The crystal structures of two hydrogen monofluorophosphates: CsHPO3F and Cs3(NH4)2(HPO3F)3(PO3F). Z. Kristallogr. 2000, 215, 240–245. [Google Scholar]
- Krupkova, R.; Fabry, J.; Cisarova, I.; Vanek, P. Bis(ammonium) fluorophosphate at room temperature. Acta Crystallogr. 2002, C58, i66–i68. [Google Scholar] [CrossRef]
- Perloff, A. The crystal structures of hydrated calcium and ammonium monofluorophosphates: CaPO3(H2O)2 and (NH4)2PO3F(H2O). Acta Crystallogr. 1972, B28, 2183–2191. [Google Scholar] [CrossRef]
- Berndt, A.F.; Sylvester, J.M. The crystal structure of ammonium monofluorophosphate: (NH4)2PO3F(H2O). Acta Crystallogr. 1972, B28, 2191–2193. [Google Scholar] [CrossRef]
- Durand, J.; Beys, L.; Hillaire, P.; Aleonard, S.; le Cot, L. Etude structurale de (NH4)2PO3F, H2O a 120 K par diffraction des rayons X et spectroscopie Raman entre 293 et 83 K. Spectrochim. Acta 1978, A34, 123–127. [Google Scholar] [CrossRef]
- Jantz, S.G.; van Wuellen, L.; Fischer, A.; Libowitzky, E.; Baran, E.J.; Weil, M.; Höppe, H.A. Syntheses, crystal structures, NMR spectroscopy, and vibrational spectroscopy of Sr(PO3F).H2O and Sr(PO3F). Eur. J. Inorg. Chem. 2016, 7, 1121–1128. [Google Scholar] [CrossRef]
- Stöger, B.; Weil, M.; Skibsted, J. The crystal structure of BaPO3F revisited—A combined X-ray diffraction and solid-state 19F, 31P MAS NMR study. Dalton Trans. 2013, 42, 11672–11682. [Google Scholar] [CrossRef]
- Marshall, K.L.; Weller, M.T. Synthesis of titanium fluorophosphates and fluorosulfates from hexafluorotitanic acid. Z. Anorg. Allg. Chem. 2014, 640, 2766–2770. [Google Scholar] [CrossRef]
- Weil, M.; Baran, E.J.; Kremer, R.K.; Libowitzky, E. Synthesis, crystal structure, and properties of Mn(PO3F)(H2O)2. Z. Anorg. Allg. Chem. 2015, 641, 184–191. [Google Scholar] [CrossRef]
- Armstrong, J.A.; Williams, E.R.; Weller, M.T. Manganese(III) fluorophosphate frameworks. Dalton Trans. 2013, 42, 2302–2308. [Google Scholar] [CrossRef]
- Wang, G.; Valldor, M.; Dorn, K.V.; Wilk-Kozubek, M.; Smetana, V.; Mudring, A.-V. Ionothermal Synthesis Enables Access to 3D Open Framework Manganese Phosphates Containing Extra-Large 18-Ring Channels. Chem. Mater. 2019, 31, 7329–7339. [Google Scholar] [CrossRef]
- Armstrong, J.A.; Williams, E.R.; Weller, M.T. Fluoride-rich, hydrofluorothermal routes to functional transition metal (Mn, Fe, Co, Cu) fluorophosphates. J. Am. Chem. Soc. 2011, 133, 8252–8263. [Google Scholar] [CrossRef]
- Jiang, J.; Zhu, B.; Zhu, T.; Yang, H.; Jin, Y.; Lü, M. Open-framework ammonium transition metal fluorophosphates with a Kagomé lattice network: Synthesis, structure and magnetic properties. Dalton Trans. 2020, 49, 841–849. [Google Scholar] [CrossRef] [PubMed]
- Zhu, B.; Jiang, J.; Zhu, T.; Yang, H.; Jin, Y.; Choi, K.-Y.; Lü, M. Transition-Metal Monofluorophosphate Ba2M2(PO3F)F6 (M = Mn, Co, and Ni): Varied One-Dimensional Transition-Metal Chains and Antiferromagnetism. Inorg. Chem. 2020, 59, 3794–3804. [Google Scholar] [CrossRef] [PubMed]
- Durand, J.; le Cot, L.; Berraho, M.; Rafiq, M. Structure du monofluorophosphate de cobalt trihydrate. Acta Crystallogr. 1987, C43, 611–613. [Google Scholar]
- Zeibig, M.; Wallis, B.; Moewius, F.; Meisel, M. Darstellung und Kristallstruktur von Kupfer(II)-monofluorophosphat-Dihydrat CuPO3F·2H2O. Z. Anorg. Allg. Chem. 1991, 600, 231–238. [Google Scholar] [CrossRef]
- Berndt, A.F. The crystal structure of SnPO3F. Acta Crystallogr. 1974, B30, 529–530. [Google Scholar] [CrossRef]
- Weil, M. NH4Ag3(PO3F)2, a layered monofluorophosphate(V) with seven different Ag sites. Acta Crystallogr. 2007, C63, i31–i33. [Google Scholar]

| Compound | CdPO3F(H2O)2 | Cr2(PO3F)3(H2O)18.8 | Pb2(PO3F)Cl2(H2O) | ZnPO3F(H2O)2.5 | (NH4)2Co(PO3F)2(H2O)2 | (NH4)2Mg(PO3F)2(H2O)2 | |
|---|---|---|---|---|---|---|---|
| Formula weight | 246.40 | 736.88 | 601.27 | 208.38 | 326.99 | 292.37 | |
| Temperature/°C | 23 | −173 | 23 | −173 | 23 | 23 | |
| Radiation; λ/Å | Mo K; 0.71073 | Mo K; 0.71073 | Mo K; 0.71073 | Mo K; 0.71073 | Mo K; 0.71073 | Mo K; 0.71073 | |
| Diffractometer | SMART CCD | APEX-II CCD | SMART CCD | APEX-II CCD | CAD-4 | SMART CCD | |
| Crystal dimensions / mm | 0.14 × 0.10 × 0.04 | 0.10 × 0.10 × 0.01 | 0.35 × 0.05 × 0.04 | 0.12 × 0.02 × 0.02 | 0.05 × 0.05 × 0.01 | 0.04 × 0.04 × 0.01 | |
| Crystal colour; shape | colourless; fragment | green; plate | colourless; needle | colourless; rod | violet; plate | colourless; plate | |
| Space group, no. | P, 2 | P, 2 | Pnma, 62 | P, 2 | C2/m, 12 | C2/m, 12 | |
| Formula units Z | 2 | 4 | 4 | 4 | 2 | 2 | |
| a/Å | 5.27680(10) | 11.5937(16) | 20.4864(14) | 7.6020(2) | 13.386(3) | 13.374(2) | |
| b/Å | 6.66970(10) | 15.292(2) | 5.3967(4) | 7.6490(2) | 5.3476(9) | 5.3541(8) | |
| c/Å | 7.7037(2) | 15.360(2) | 6.9722(5) | 9.4671(3) | 7.390(2) | 7.3852(11) | |
| α/° | 65.5060(10) | 83.804(6) | 90 | 88.633(2) | 90 | 90 | |
| β/° | 85.9190(10) | 84.203(6) | 90 | 88.888(2) | 114.02(2) | 113.758(3) | |
| γ/° | 75.3940(10) | 82.579(6) | 90 | 87.182(2) | 90 | 90 | |
| V/Å3 | 238.584(9) | 2674.1(6) | 770.84(10) | 549.58(3) | 483.2(2) | 484.01(13) | |
| μ/mm−1 | 4.867 | 1.118 | 44.496 | 4.733 | 2.169 | 0.576 | |
| X-ray density/g·cm–3 | 3.430 | 1.830 | 5.181 | 2.518 | 2.247 | 2.006 | |
| Absorption correction | multi-scan; SADABS | multi-scan; SADABS | multi-scan; SADABS | multi-scan; SADABS | numerical; HABITUS | multi-scan; SADABS | |
| Trans. coef. Tmin; Tmax | 0.549; 0.829 | 0.778; 0.972 | 0.023; 0.269 | 0.545; 0.749 | 0.884; 0.934 | 0.887; 0.922 | |
| Range θmin–θmax | 2.91–35.51 | 1.34–26.00 | 3.09–30.49 | 2.15–43.26 | 3.02–29.96 | 3.01–28.29 | |
| Range | h | −8–7 | −14–14 | −25–29 | −14–14 | −18–18 | −17–17 |
| k | −10–10 | −18–18 | −7–7 | −14–14 | −7–7 | −7–7 | |
| l | −12–12 | −18–18 | −9–9 | −18–18 | −10–10 | −9–9 | |
| Measured reflections | 20950 | 80926 | 8539 | 77904 | 2799 | 3204 | |
| Independent reflections | 2055 | 10504 | 1291 | 8190 | 772 | 660 | |
| Obs.reflections [I > 2σ(I)] | 1927 | 6720 | 1176 | 7431 | 540 | 517 | |
| Ri | 0.0235 | 0.0731 | 0.0390 | 0.0329 | 0.0668 | 0.0525 | |
| Number of parameters | 87 | 681 | 59 | 198 | 55 | 55 | |
| Ext. coef. (SHELXL) | 0.0173(9) | - | 0.00298(15) | 0.0042(4) | - | - | |
| Diff. elec. dens. max; min | 0.51 (0.70, Cd1); | 1.24 (0.94, O8W); | 2.33 (0.80, Pb1); | 1.34 (1.52, O8); | 0.44 (0.49, O1); | 0.57 (0.71, O2); | |
| [e–·Å–3] (dist./Å, atom) | −0.56 (0.65, Cd1) | −0.97 (0.43, O14W) | −1.51 (0.26, Pb2) | −0.48 (0.48, O4) | −0.34 (0.51, H2) | −0.34 (1.23, H2) | |
| R[F2 > 2σ(F2)] | 0.0136 | 0.0605 | 0.0237 | 0.0220 | 0.0280 | 0.0462 | |
| wR2(F2 all) | 0.0297 | 0.2010 | 0.0554 | 0.0523 | 0.0662 | 0.1153 | |
| Goof | 1.081 | 1.036 | 1.062 | 1.225 | 0.989 | 1.031 | |
| CSD number | 2,048,140 | 2,048,141 | 2,048,144 | 2,048,145 | 2,048,134 | 2,048,135 | |
| Compound | (NH4)2Mn(PO3F)2(H2O)2 | (NH4)2Ni(PO3F)2(H2O)6 | NH4Cr(PO3F)2(H2O)6 | NH4Cu2(H3O2)(PO3F)2 | (NH4)2Zn(PO3F)2(H2O)0.2 | (NH4)2Zn3(PO3F)4(H2O) | |
| Formula weight | 323.00 | 398.83 | 374.08 | 376.09 | 301.10 | 642.09 | |
| Temperature/°C | −173 | −173 | −173 | −173 | 23 | −173 | |
| Radiation; λ/Å | Mo K; 0.71073 | Mo K; 0.71073 | Mo K; 0.71073 | Mo K; 0.71073 | Mo K; 0.71073 | Mo K; 0.71073 | |
| Diffractometer | APEX-II CCD | APEX-II CCD | APEX-II CCD | APEX-II CCD | APEX-II CCD | APEX-II CCD | |
| Crystal dimensions/mm | 0.08 × 0.06 × 0.01 | 0.44 × 0.32 × 0.15 | 0.15 × 0.15 × 0.08 | 0.20 × 0.10 × 0.08 | 0.10 × 0.10 × 0.01 | 0.08 × 0.08 × 0.08 | |
| Crystal colour; shape | light-pink; plate | blue; fragment | green; plate | light-blue; parallelepiped | colourless; plate | colourless; octahedron | |
| Space group, no. | P21/n, 14 | P21/c, 14 | Rm, 166 | C2/m, 12 | C2/c, 15 | I3d, 220 | |
| Formula units Z | 2 | 2 | 3 | 2 | 12 | 4 | |
| a/Å | 12.558(3) | 6.2700(3) | 6.5491(2) | 9.1012(8) | 18.9363(17) | 11.3693(4) | |
| b/Å | 5.4559(19) | 12.2845(6) | 6.5491(2) | 6.4121(5) | 7.6955(7) | 11.3693(4) | |
| c/Å | 7.4215(18) | 9.1894(4) | 25.4381(14) | 7.8506(7) | 20.5276(18) | 11.3693(4) | |
| α/° | 90 | 90 | 90 | 90 | 90 | 90 | |
| β/° | 99.918(5) | 106.033(2) | 90 | 116.277(3) | 108.641(2) | 90 | |
| γ/° | 90 | 90 | 120 | 90 | 90 | 90 | |
| V/Å3 | 500.9(2) | 680.27(6) | 944.88(8) | 410.80(6) | 2834.4(4) | 1469.61(16) | |
| μ/mm−1 | 1.697 | 1.745 | 1.245 | 5.631 | 2.976 | 5.415 | |
| X-ray density/g·cm–3 | 2.142 | 1.947 | 1.972 | 3.040 | 2.117 | 2.902 | |
| Absorption correction | multi-scan; SADABS | multi-scan; SADABS | multi-scan; SADABS | multi-scan; SADABS | multi-scan; SADABS | multi-scan; SADABS | |
| Trans. coef. Tmin; Tmax | 0.636; 0.747 | 0.662; 0.749 | 0.661; 0.747 | 0.631; 0.748 | 0.889; 0.995 | 0.656; 0.746 | |
| Range θmin–θmax | 2.98–32.99 | 2.34–45.97 | 2.40–34.71 | 2.89–39.98 | 2.88–31.00 | 4.39–30.00 | |
| Range | h | −18–19 | −12–12 | −10–10 | −16–16 | −27–19 | −15–12 |
| k | −8–8 | −24–24 | −9–10 | −11–11 | −11–11 | −15–15 | |
| l | −11–11 | −18–18 | −40–40 | −14–13 | −23–29 | −16–16 | |
| Measured reflections | 10,452 | 105,648 | 6844 | 7619 | 11,828 | 6077 | |
| Independent reflections | 1886 | 5910 | 557 | 1356 | 4382 | 362 | |
| Obs.reflections [I > 2σ(I)] | 1059 | 5659 | 461 | 1183 | 3079 | 328 | |
| Ri | 0.0881 | 0.0227 | 0.0526 | 0.0336 | 0.0392 | 0.0516 | |
| Number of parameters | 94 | 129 | 30 | 57 | 234 | 33 | |
| Ext. coef. (SHELXL) | - | 0.0022(9) | - | - | - | - | |
| Flack parameter | - | - | - | - | - | 0.026(15) | |
| Diff. elec. dens. max; min | 0.56 (0.63, O1) | 0.53 (0.56, Ni1); | 0.51 (0.77, Cr1); | 1.07 (1.52, H1W); | 0.78 (0.75, O1); | 0.34 (0.78, O1); | |
| [e−·Å−3] (dist./Å, atom) | −0.63 (0.72, P1) | −0.79 (0.55, Ni1) | −0.51 (0.0, Cr1) | −0.83 (0.60, Cu1) | −0.45 (1.36, Zn2) | −0.33 (0.41, Zn1A) | |
| R[F2 > 2σ(F2)] | 0.0404 | 0.0142 | 0.0232 | 0.0239 | 0.0467 | 0.0265 | |
| wR2(F2 all) | 0.1009 | 0.0400 | 0.0621 | 0.0575 | 0.1133 | 0.0533 | |
| Goof | 1.012 | 1.160 | 1.140 | 1.109 | 1.009 | 1.114 | |
| CSD number | 2,048,136 | 2,048,137 | 2,048,142 | 2,048,143 | 2,048,138 | 2,048,139 | |
| CdPO3F(H2O)2 | Cr2(PO3F)3(H2O)18.8 | Pb2(PO3F)Cl2(H2O) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Cd1 | OW1 | 2.2474(10) | Cr1 | O1 | 1.956(3) | 2x | Pb1 | O1 | 2.570(3) | 2x | |
| Cd1 | O2 | 2.2505(9) | Cr1 | O2 | 1.960(3) | 2x | Pb1 | O1W | 2.703(7) | ||
| Cd1 | O1 | 2.2545(9) | Cr1 | O3 | 1.973(3) | 2x | Pb1 | Cl1 | 2.875(2) | ||
| Cd1 | O3 | 2.2867(9) | Cr2 | O4 | 1.949(3) | 2x | Pb1 | Cl2 | 2.9423(10) | 2x | |
| Cd1 | O3 | 2.3310(9) | Cr2 | O5 | 1.959(3) | 2x | Pb1 | Cl2 | 3.048(2) | ||
| Cd1 | OW2 | 2.3865(10) | Cr2 | O6 | 1.971(3) | 2x | Pb2 | O2 | 2.301(5) | ||
| P1 | O1 | 1.5058(9) | Cr3 | O7 | 1.939(3) | Pb2 | O1 | 2.503(3) | 2x | ||
| P1 | O2 | 1.5073(9) | Cr3 | O8 | 1.950(4) | Pb2 | Cl1 | 3.1402(10) | 2x | ||
| P1 | O3 | 1.5155(9) | Cr3 | O9 | 1.952(3) | Pb2 | O2 | 3.142(3) | 2x | ||
| P1 | F1 | 1.5747(8) | Cr3 | O10 | 1.969(4) | Pb2 | O1 | 3.167(3) | 2x | ||
| Cr3 | O11 | 1.975(4) | P1 | O2 | 1.493(6) | ||||||
| O−P1−O | 113.89(5)–114.93(5) | Cr3 | O12 | 1.984(3) | P1 | O1 | 1.517(4) | 2x | |||
| O−P1−F1 | 103.37(5)–104.33(5) | Cr4 | O13 | 1.947(3) | P1 | F1 | 1.564(5) | ||||
| Cr4 | O14 | 1.950(4) | |||||||||
| Cr4 | O15 | 1.955(3) | O−P1−O | 111.92(17)–113.9(2) | |||||||
| ZnPO3F(H2O)2.5 | Cr4 | O16 | 1.972(4) | O−P1−F1 | 105.12(19)–108.2(3) | ||||||
| Zn1 | O10 | 1.9480(7) | Cr4 | O17 | 1.977(3) | ||||||
| Zn1 | O8 | 1.9614(8) | Cr4 | O18 | 1.983(3) | ||||||
| Zn1 | O7 | 1.9647(7) | Cr5 | O19 | 1.942(4) | (NH4)2Co(PO3F)2(H2O)2 | |||||
| Zn1 | O5 | 1.9915(7) | Cr5 | O20 | 1.959(4) | Co1 | O2 | 2.0714(16) | 4x | ||
| Zn1 | P2 | 2.9235(3) | Cr5 | O21 | 1.960(4) | Co1 | O1W | 2.161(3) | 2x | ||
| Zn2 | O9 | 2.0199(8) | Cr5 | O22 | 1.967(4) | P1 | O1 | 1.505(3) | |||
| Zn2 | O9 | 2.0199(8) | 2x | Cr5 | O24 | 1.974(4) | P1 | O2 | 1.5056(17) | 2x | |
| Zn2 | O6 | 2.0941(8) | 2x | Cr5 | O23 | 1.976(4) | P1 | F1 | 1.579(2) | ||
| Zn2 | O4 | 2.1306(8) | 2x | P1 | O11P | 1.505(3) | |||||
| Zn3 | O3 | 1.9979(8) | 2x | P1 | O12P | 1.507(3) | O−P1−O | 112.54(15)–115.25(8) | |||
| Zn3 | O11 | 2.1130(9) | 2x | P1 | O13P | 1.518(3) | O−P1−F1 | 103.27(9)–105.39(14) | |||
| Zn3 | O1 | 2.1308(8) | 2x | P1 | F1 | 1.568(3) | |||||
| P1 | O3 | 1.4866(8) | P2 | O21P | 1.501(4) | ||||||
| P1 | O8 | 1.5123(8) | P2 | O22P | 1.503(4) | (NH4)2Mg(PO3F)2(H2O)2 | |||||
| P1 | O7 | 1.5342(7) | P2 | O23P | 1.521(3) | Mg1 | O2 | 2.0561(18) | 4x | ||
| P1 | F2 | 1.5604(7) | P2 | F2 | 1.564(3) | Mg1 | O1W | 2.124(3) | 2x | ||
| P2 | O9 | 1.4906(8) | P3 | O31P | 1.498(4) | P1 | O1 | 1.501(3) | |||
| P2 | O10 | 1.5167(8) | P3 | O32P | 1.511(3) | P1 | O2 | 1.5045(19) | |||
| P2 | O5 | 1.5302(7) | P3 | O33P | 1.521(3) | P1 | F1 | 1.586(2) | |||
| P2 | F1 | 1.5595(7) | P4 | F3 | 1.563(3) | ||||||
| P4 | O41P | 1.497(4) | O−P1−O | 113.31(16)–114.74(9) | |||||||
| O−P−O | 109.98(5)–115.78(5) | P4 | O42P | 1.503(4) | O−P1−F1 | 103.47(9)–105.35(6) | |||||
| O−P−F | 103.61(4)–108.61(4) | P4 | O43P | 1.523(3) | |||||||
| P5 | F4 | 1.564(3) | |||||||||
| P5 | O51P | 1.494(4) | (NH4)2Ni(PO3F)2(H2O)6 | ||||||||
| NH4Cr(PO3F)2(H2O)6 | P5 | O52P | 1.507(4) | Ni1 | O4 | 2.0295(3) | |||||
| Cr1 | O2W | 1.9622(10) | 6x | P5 | O53P | 1.518(3) | Ni1 | O6 | 2.0624(3) | ||
| P1 | O1 | 1.5106(10) | 3x | P6 | F5 | 1.564(4) | Ni1 | O5 | 2.0747(3) | ||
| P1 | F1 | 1.5676(17) | P6 | O61P | 1.480(4) | P1 | O1 | 1.5082(3) | |||
| P6 | O62P | 1.483(4) | P1 | O2 | 1.5109(3) | ||||||
| O1-P-O1 | 113.44(4) | P6 | O63P | 1.519(3) | P1 | O3 | 1.5113(3) | ||||
| O1-P-F1 | 105.13(4) | P6 | F6B | 1.585(7) | P1 | F1 | 1.6054(3) | ||||
| P6 | F6A | 1.591(9) | |||||||||
| O−P1−O | 113.599(17)–115.710(17) | ||||||||||
| (NH4)2Mn(PO3F)2(H2O)2 | O−P−O | 113.1(2)–114.6(2) | O−P1−F1 | 103.168(16)–104.160(18) | |||||||
| Mn1 | O3 | 2.151(2) | 2x | O−P−F | 90.4(4)–118.9(4) | ||||||
| Mn1 | O1 | 2.1650(19) | 2x | ||||||||
| Mn1 | O1W | 2.240(2) | 2x | (NH4)2Zn3(PO3F)4(H2O) | |||||||
| P1 | O1 | 1.512(2) | (NH4)2Zn(PO3F)2(H2O)0.2 | Zn1A | O1 | 1.934(3) | 4x | ||||
| P1 | O2 | 1.5140(19) | Zn1 | O9 | 1.911(3) | Zn1B | O1 | 1.766(11) | |||
| P1 | O3 | 1.517(2) | Zn1 | O5 | 1.920(3) | Zn1B | O1 | 1.830(11) | |||
| P1 | F1 | 1.5944(19) | Zn1 | O3 | 1.929(2) | Zn1B | O1 | 2.199(10) | |||
| Zn1 | O2 | 1.940(2) | Zn1B | O1W | 2.204(14) | ||||||
| O-P-O | 112.73(12)–114.98(12) | Zn2 | O8B | 1.835(11) | P1 | O1 | 1.497(3) | 3x | |||
| O-P-F1 | 103.15(10)–105.12(10) | Zn2 | O8B | 1.835(11) | P1 | F1 | 1.554(5) | ||||
| Zn2 | O4A | 1.872(5) | |||||||||
| NH4Cu2(H3O2)(PO3F)2 | Zn2 | O4A | 1.872(5) | O−P1−O | 113.24(13) | ||||||
| Cu1 | O2 | 1.9493(10) | 2x | Zn2 | O8A | 1.958(6) | O−P1−F1 | 105.37(16) | |||
| Cu1 | O1H | 2.0217(8) | 2x | Zn2 | O8A | 1.958(6) | |||||
| Cu1 | O1 | 2.3642(10) | 2x | Zn2 | O4B | 1.988(12) | |||||
| P1 | O1 | 1.5052(14) | Zn2 | O4B | 1.988(12) | ||||||
| P1 | O2 | 1.5138(10) | 2x | P1 | O1 | 1.484(3) | |||||
| P1 | F1 | 1.5934(12) | P1 | O2 | 1.512(2) | ||||||
| P1 | O3 | 1.512(2) | |||||||||
| O−P1−O | 114.36(8)–114.85(5) | P1 | F1 | 1.575(2) | |||||||
| O−P1−F1 | 103.15(5)–104.34(7) | P2 | O4B | 1.469(12) | |||||||
| P2 | O5 | 1.488(3) | |||||||||
| P2 | O6 | 1.489(3) | |||||||||
| P2 | O4A | 1.532(7) | |||||||||
| P2 | F2 | 1.556(3) | |||||||||
| P3 | O7 | 1.472(3) | |||||||||
| P3 | O8A | 1.485(6) | |||||||||
| P3 | O9 | 1.498(3) | |||||||||
| P3 | F3 | 1.556(3) | |||||||||
| P3 | O8B | 1.597(13) | |||||||||
| O−P−O | 101.6(4)–127.3(10) | ||||||||||
| O−P−F | 86.8(9)–116.2(6) | ||||||||||
| D | H | A | D–H | H···A | D···A | D–H···A | D | H | A | D–H | H···A | D···A | D–H···A |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CdPO3F(H2O)2 | ZnPO3F(H2O)2.5 | ||||||||||||
| OW1 | H1 | OW2 | 0.83(2) | 1.99(2) | 2.7983(15) | 164(2) | O1 | H1 | O10 | 0.819(9) | 1.960(9) | 2.7778(11) | 177(2) |
| OW1 | H2 | O2 | 0.81(2) | 2.34(3) | 2.9231(14) | 129(2) | O1 | H2 | O5 | 0.819(10) | 2.004(10) | 2.8212(11) | 175(3) |
| OW1 | H2 | O1 | 0.81(2) | 2.36(3) | 2.9635(14) | 133(2) | O2 | H3 | O1 | 0.818(10) | 2.25(2) | 2.9854(14) | 149(3) |
| OW2 | H3 | O2 | 0.90(2) | 2.00(2) | 2.8740(15) | 162(2) | O2 | H4 | O4 | 0.814(10) | 2.496(15) | 3.2526(14) | 155(3) |
| OW2 | H3 | F1 | 0.90(2) | 2.57(3) | 3.1411(14) | 121(2) | O2 | H4 | O6 | 0.814(10) | 2.52(2) | 3.1410(13) | 134(2) |
| OW2 | H4 | O1 | 0.84(2) | 2.18(2) | 3.0032(15) | 167(2) | O4 | H5 | O2 | 0.823(9) | 1.992(11) | 2.8053(13) | 170(3) |
| OW2 | H4 | F1 | 0.84(2) | 2.60(2) | 3.0887(13) | 118(2) | O4 | H6 | O5 | 0.828(9) | 2.047(10) | 2.8707(11) | 173(2) |
| O6 | H7 | O8 | 0.816(9) | 1.900(10) | 2.7136(11) | 175(2) | |||||||
| (NH4)2Co(PO3F)2(H2O)2 | O6 | H8 | O7 | 0.818(9) | 2.018(10) | 2.8336(11) | 174(2) | ||||||
| N1H | H1 | O1 | 0.92(4) | 1.88(4) | 2.800(4) | 180(5) | O11 | H9 | O7 | 0.819(10) | 2.059(11) | 2.8641(11) | 168(3) |
| N1H | H2 | O2 | 0.86(4) | 2.22(3) | 2.924(3) | 139.9(8) | O11 | H10 | O2 | 0.822(10) | 2.059(10) | 2.8742(13) | 171(3) |
| N1H | H3 | O2 | 0.85(3) | 2.32(3) | 3.031(4) | 142(3) | |||||||
| O1W | H4 | O1 | 0.81(3) | 2.14(3) | 2.8702(14) | 149(3) | (NH4)2Ni(PO3F)2(H2O)6 | ||||||
| N1 | H1N | O3 | 0.849(12) | 2.159(12) | 2.9613(5) | 157.6(11) | |||||||
| (NH4)2Mg(PO3F)2(H2O)2 | N1 | H2N | O3 | 0.917(11) | 1.921(11) | 2.8283(4) | 169.8(10) | ||||||
| N1H | H1 | O1 | 0.90(4) | 1.90(4) | 2.801(5) | 175(5) | N1 | H3N | O1 | 0.896(12) | 1.921(12) | 2.8134(5) | 173.7(10) |
| N1H | H2 | O2 | 0.88(4) | 2.21(3) | 2.933(4) | 139.7(9) | N1 | H4N | O2 | 0.849(11) | 2.115(11) | 2.9053(5) | 154.7(10) |
| N1H | H3 | O2 | 0.82(3) | 2.34(4) | 3.040(4) | 143(4) | O4 | H5W | O3 | 0.834(10) | 1.866(10) | 2.6969(4) | 174.0(11) |
| O1W | H4 | O1 | 0.76(3) | 2.15(3) | 2.8732(16) | 160(4) | O4 | H6W | O1 | 0.826(11) | 1.938(11) | 2.7489(4) | 167.0(10) |
| O5 | H7W | O2 | 0.799(11) | 1.883(11) | 2.6792(4) | 173.7(11) | |||||||
| (NH4)2Mn(PO3F)2(H2O)2 | O5 | H8W | F1 | 0.804(11) | 2.010(11) | 2.8146(4) | 178.6(12) | ||||||
| O1W | H1W | O2 | 0.850(10) | 1.930(14) | 2.748(3) | 161(3) | O6 | H9W | O1 | 0.805(12) | 1.956(12) | 2.7589(4) | 175.3(11) |
| O1W | H2W | O2 | 0.849(10) | 2.44(3) | 3.103(3) | 136(4) | O6 | H10W | O2 | 0.789(11) | 2.001(11) | 2.7874(4) | 174.1(11) |
| N1H | H1N | O1 | 0.899(10) | 2.131(19) | 2.945(3) | 150(3) | |||||||
| N1H | H2N | F1 | 0.897(10) | 2.50(3) | 3.041(3) | 119(3) | NH4Cu2(H3O2)(PO3F)2 | ||||||
| N1H | H2N | O1W | 0.897(10) | 2.59(3) | 3.209(3) | 127(3) | O1H | H1O | F1 | 0.837(10) | 2.344(10) | 3.1797(18) | 177(4) |
| N1H | H3N | O2 | 0.902(10) | 1.909(11) | 2.810(3) | 176(3) | O1H | H2O | O1H | 0.999(10) | 1.50(4) | 2.489(3) | 169(20) |
| N1H | H4N | O3 | 0.900(10) | 1.912(12) | 2.803(3) | 170(3) | N1 | H1N | O2 | 0.8999(10) | 2.021(18) | 2.8448(10) | 152(3) |
| N1 | H2N | F1 | 0.9000(10) | 2.24(6) | 2.9730(13) | 139(8) | |||||||
| N1 | H2N | O1 | 0.9000(10) | 2.03(4) | 2.8554(14) | 152(8) | |||||||
| NH4Cr(PO3F)2(H2O)6 | |||||||||||||
| O2W | H1 | O1 | 0.864(16) | 1.753(16) | 2.6124(9) | 173.1(16) | (NH4)2Zn3(PO3F)4(H2O) | ||||||
| N1H | H2 | O1 | 0.95(4) | 2.00(4) | 2.9493(10) | 176(3) | N1H/O1W | H1 | F1 | 1.02(8) | 2.31(8) | 3.1703(11) | 141(6) |
| N1H/O1W | H1 | O1 | 1.02(8) | 2.40(8) | 3.007(3) | 117(6) | |||||||
| (NH4)2Zn(PO3F)2(H2O)0.2 | N1H/O1W | H1 | O1 | 1.02(8) | 2.38(9) | 3.190(4) | 136(7) | ||||||
| N1 | H1 | O7 | 0.897(10) | 1.874(16) | 2.756(5) | 167(5) | |||||||
| N1 | H2 | O1 | 0.891(10) | 2.17(3) | 2.955(5) | 147(5) | |||||||
| N1 | H3 | O3 | 0.898(10) | 2.130(18) | 3.000(4) | 163(5) | |||||||
| N1 | H4 | O6 | 0.894(10) | 1.992(14) | 2.878(5) | 171(5) | |||||||
| N2 | H5 | O6 | 0.897(10) | 2.015(18) | 2.890(4) | 164(5) | |||||||
| N2 | H6 | O2 | 0.896(10) | 2.16(2) | 3.000(4) | 156(4) | |||||||
| N2 | H6 | O9 | 0.896(10) | 2.74(4) | 3.321(5) | 123(4) | |||||||
| N2 | H7 | O6 | 0.899(10) | 1.925(13) | 2.817(5) | 172(5) | |||||||
| N2 | H8 | O8A | 0.897(10) | 1.96(2) | 2.811(8) | 158(5) | |||||||
| N2 | H8 | O8B | 0.897(10) | 2.49(4) | 3.28(3) | 148(5) | |||||||
| N3 | H11 | O1 | 0.894(10) | 2.08(3) | 2.869(5) | 146(4) | |||||||
| N3 | H9 | O7 | 0.899(10) | 1.89(2) | 2.743(5) | 158(5) | |||||||
| N3 | H10 | O4A | 0.897(10) | 2.165(16) | 3.055(9) | 171(4) | |||||||
| N3 | H10 | O8B | 0.897(10) | 2.55(5) | 3.03(2) | 114(4) | |||||||
| N3 | H12 | O1 | 0.892(10) | 1.949(13) | 2.837(5) | 173(5) | |||||||
| Vibrational Mode | Free Anion (C3v) | Site Symmetry (C1) | IR | Raman | |
|---|---|---|---|---|---|
| ν1 | ν(P–F) | A1 | A | 825 vs | 826 m |
| ν2 | νs(PO3) | A1 | A | 1006 vs | 1022 vs |
| ν3 | δ(FPO3) | A1 | A | ||
| ν4 | νas(PO3) | E | 2A | 1142 vs, 1106 vs | --- |
| ν5 | δ(PO3) | E | 2A | 560 sh, 541 s | 540 w |
| ν6 | ρ(PO3) | E | 2A | --- | 395 w |
| ν(OH) | 3496 vs, 3393 vs, 3223 sh | --- | |||
| δ(H2O) | 1648 sh, 1626 m | --- |
| Monofluorophosphate (Reference) | Space Group, Z | Site Symmetry PO3F Group(s) | D–H···F–P Hydrogen Bonding with D···F/Å and D–H···F/° | Corresponding Sulfate | Relationship | Remark |
|---|---|---|---|---|---|---|
| LiKPO3F(H2O) [36] | P21/c, 4 | 1 | 3.15; 120 | - | - | - |
| Li(NH4)PO3F [37] | P21/c, 4 | 1 | 2.98; 147 | Li(NH4)(SO4) P21cn, Z = 4; P21/c Z = 8; Pmcn, Z = 8. | - | Sulfate shows poly-morphism. |
| Na2PO3F [38] | P212121, 8 | 1, 1 | - | Na2SO4 Fddd, Z = 8; P63/mmc, Z = 2. | - | Sulfate shows dimorphism. |
| Na2PO3F(H2O)10 [39] | P21/c, 4 | 1 | 2.83, 149 3.01, 178 | Na2SO4(H2O)10 (Glauber salt) | Isotypic | - |
| NaK3(PO3F)2 [40] | Pm1, 1 | 3m | - | NaK3(SO4)2 (glaserite) | Isotypic | - |
| (NH4)Na(PO3F)(H2O) [41] | Pn, 2 | 1 | - | - | - | - |
| K2PO3F [6,42] | Pnam, 4 | m | - | K2SO4 (arcanite); high-temperature form: P63/mmc; Z = 2. | Isotypic | Isotypic with low-temperature form; |
| K3(PO3F)F [34] | I4/mcm, 4 | 2m | - | K3(PO3F)F | Isotypic, | PO3F disordered. Phase transition reported for the sulfate. |
| Rb2PO3F [43] | Pnma, 4 | m | - | Rb2SO4 | Isotypic | K2SO4 structure type. |
| Cs2PO3F [43] | Pnma, 4 | m | - | Cs2SO4 | Isotypic | K2SO4 structure type. |
| Cs3(NH4)2(HPO3F)3(PO3F) [44] | P21/c, 8 | 1,1,1,1 | - | - | - | - |
| (NH4)2(PO3F) [45] | Pna21, 4 | 1 | - | (NH4)2(SO4) | Isotypic | Ferroelectric phase; K2SO4 structutre type. |
| (NH4)2(PO3F)(H2O) [46,47,48] | P21/c, 4 | 1 | - | - | - | - |
| (NH4)2Mg(PO3F)2(H2O)2 [this work] | C2/m, 2 | m | - | - | - | - |
| CaPO3F(H2O)2 [46] | P, 2 | 1 | 3.12, 147 | CaPO4(H2O)2 (gypsum) C2/m, Z = 4. | - | - |
| SrPO3F [49] | P21/c, 4 | 1 | - | Barite-type SrSO4; Pnma, Z = 4; | - | SrPO3F adopts the monazite structure type. |
| SrPO3F(H2O) [49] | P21/c, 4 | 1 | 2.77, 108 2.95, 110 | - | - | X-ray powder data. |
| BaPO3F [50] | P21/c, 8 | 1, 1 | - | BaSO4 (barite), Pnma, Z = 4; F3m, Z = 4 (HT) | - | Sulfate shows dimorphism. |
| CsTi2F2(PO4)(PO3F)2 [51] | P2/c, 2 | 1 | - | - | - | |
| Cr2(PO3F)3(H2O)18.8 [this work] | P, 4 | 1, 1, 1, 1, 1, 1 | ? | - | - | H atoms not determined. |
| NH4Cr(PO3F)2(H2O)6 [this work] | Rm, 3 | 3m | - | - | - | - |
| MnPO3F(H2O)2 [52] | P, 2 | 1 | 2.91, 111 3.11, 128 3.18, 127 | - | - | - |
| Li3Mn(PO3F)2F2 [53] | P21/c, 2 | 1 | - | - | - | - |
| KMnF2(PO3F) [53] | P21/n, 4 | 1 | - | - | - | - |
| K2Mn3(HPO4)2(PO3F)F [54] | P21/c, 4 | 1 | 3.09, 142 2.93, 122 | - | - | - |
| Rb3Mn3(PO4)(PO3F)2F5 [53] | Cc, 4 | 1, 1 | - | - | - | - |
| Cs2Mn2F4(PO3F)2 [53] | P21, 2 | 1, 1 | - | - | - | - |
| (NH4)2Mn(PO3F)2(H2O)2 [this work] | P21/n, 2 | 1 | - | - | - | - |
| (NH4)Mn(PO3F)F2 [53,55] | P21/n, 4 | 1 | - | NH4Mn(SO4)F2, Pnna, Z = 8. | - | - |
| (NH4)2Mn3(HPO4)2(PO3F)F2 [54] | P21/c, 4 | 1 | N–H 3.11, 160 O–H 2.87, 104 | - | - | - |
| (NH4)Mn3(PO3F)2(H2PO4)F2 [54] | C2/c, 4 | 1 | O–H 2.97, 128 | - | - | Same unit cell as (NH4)Mn3(PO3F)2(PO2F2)F2 *. |
| (NH4)Mn3(PO3F)2(PO2F2)F2 [56] | C2/c, 4 | 1 | - | - | - | Same unit cell as (NH4)Mn3(PO3F)2(H2PO4)F2 *. |
| Ba2Mn2(PO3F)F6 [57] | P21/c, 4 | 1 | - | - | - | - |
| Fe2(PO3F)3 [33] | P63/m, 6 | m, m, m | - | Fe2(SO4)3, P21/n, Z = 4; R, Z = 6. | - | Sulfate shows dimorphism. |
| NaFe(PO3F)2 [33] | P21/c, 4 | 1, 1 | - | NaFe(SO4)2, C2/m, Z = 2 | - | - |
| KFe(PO3F)F2 [54] | P21/c, 4 | 1 | - | - | - | - |
| KFe2(PO2F2)(PO3F)2F2 [33] | P, 2 | 1, 1 | - | - | - | - |
| RbFe3(PO3F)((PO2)2(F1.5(OH)0.5)2)F2 [33] | C2/c, 4 | 1 | - | - | - | - |
| Cs2Fe2F3(PO3F)2(PO2F2) [33] | Aea2, 4 | 1 | - | - | - | - |
| (NH4)2Fe2(PO3F)2FCl2 [33] | Pca21, 4 | 1, 1 | - | - | - | H atoms not determined. |
| (NH4)3Fe(PO3F)2F2 [33] | P21/m, 4 | m, m | - | - | - | F and O wrongly assigned for P2. |
| CoPO3F(H2O)3 [58] | P, 2 | 1 | 2.98, 118 | - | - | - |
| (NH4)2Co(PO3F)2(H2O)2 [this work] | C2/m, 2 | m | - | - | - | - |
| (NH4)Co3(PO3F)2(PO2F2)F2 [56] | C2/c, 4 | 1 | 2.96, 115 | - | - | - |
| Ba2Co2(PO3F)F6 [55] | P21/c, 4 | 1 | - | - | - | - |
| (NH4)2(Ni(H2O)6)(PO3F)2 [15] | P21/c, 2 | 1 | 2.84, 176 | (NH4)2(Ni(H2O)6(SO4)2 | Isotypic | Picromerite structure type. |
| (NH4)2(Ni(H2O)6)(PO3F)2 [this work] | P21/c, 2 | 1 | 2.81, 179 | (NH4)2(Ni(H2O)6 (SO4)2 | Isotypic | - |
| Ba2Ni2(PO3F)F6 [57] | P21/c, 4 | 1 | - | - | - | - |
| CuPO3F(H2O)2 [59] | P21/c, 4 | 1 | - | - | - | - |
| Cu2K(OH)(PO3F)2(H2O) [30] | C2/m, 2 | m | 3.00, 174 | Cu2K(H3O2) (SO4)2 | Isotypic | |
| KCu3(PO2F2)(PO3F)2F2 [55] | C2/c, 4 | 1 | - | - | - | - |
| RbCu3(PO2F2)(PO3F)2F2 [55] | C2/c, 4 | 1 | - | - | - | - |
| NH4Cu2(H3O2)(PO3F)2 [this work] | C2/m. 2 | m | O–H 3.18, 177 N–H 2.97, 139 | -KCu2(H3O2)(SO4)2 | Natrochalcite structure type. | |
| (NH4)2(Cu(H2O)2(PO3F)2) [27] | C2/m, 2 | m | - | - | - | - |
| Ba2Cu2(PO3F)F6 [57] | P21/c, 4 | 1 | - | - | - | |
| ZnPO3F(H2O)2.5 [14] | P, 4 | 1, 1 | - | - | H atoms not reliably determined. | |
| ZnPO3F(H2O)2.5 [this work] | P, 4 | 1, 1 | - | - | - | - |
| (NH4)2Zn(PO3F)2(H2O)0.2 [this work] | C2/c, 12 | 1, 1, 1 | ? | - | - | H atoms of water not determined. |
| (NH4)2Zn3(PO3F)4(H2O) [this work] | I3d, 4 | 3 | (N,O)– 3.17, 141 | - | - | |
| SnPO3F [60] | P21/c, 4 | 1 | - | Barite-type SnSO4,Pnma, Z = 4 | - | - |
| Ag2PO3F [4] | C2/c, 8 | 1 | - | Ag2SO4, Fddd, Z = 8; P63/mmc, Z = 2 | - | Sulfate shows dimorphism. |
| (NH4)Ag3(PO3F)2 [61] | I2, 8 | 1, 1, 1, 1 | ? | - | H atoms not determined | |
| CdPO3F(H2O)2 [this work] | P-, 2 | 1 | 3.14, 121 3.09, 118 | - | - | |
| Hg2PO3F [21] | Ibam, 8 | m | - | Hg2SO4, P2/c, Z = 2 | - | - |
| Pb2PO3FCl2(H2O) [this work] | Pnma, 4 | m | ? | - | H atoms not determined. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Weil, M. Monofluorophosphates—New Examples and a Survey of the PO3F2− Anion. Chemistry 2021, 3, 45-73. https://doi.org/10.3390/chemistry3010005
Weil M. Monofluorophosphates—New Examples and a Survey of the PO3F2− Anion. Chemistry. 2021; 3(1):45-73. https://doi.org/10.3390/chemistry3010005
Chicago/Turabian StyleWeil, Matthias. 2021. "Monofluorophosphates—New Examples and a Survey of the PO3F2− Anion" Chemistry 3, no. 1: 45-73. https://doi.org/10.3390/chemistry3010005
APA StyleWeil, M. (2021). Monofluorophosphates—New Examples and a Survey of the PO3F2− Anion. Chemistry, 3(1), 45-73. https://doi.org/10.3390/chemistry3010005





