Electrochemical and Surface Analytical Study on the Role of Poly(butylene-succinate)-l-proline during Corrosion of Mild Steel in 1 M HCl
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and Chemicals
2.2. Mild Steel Coupons Preparation
2.3. Electrochemical Studies
2.4. Surface Analysis
3. Results and Discussion
3.1. Characterization of PBSLP
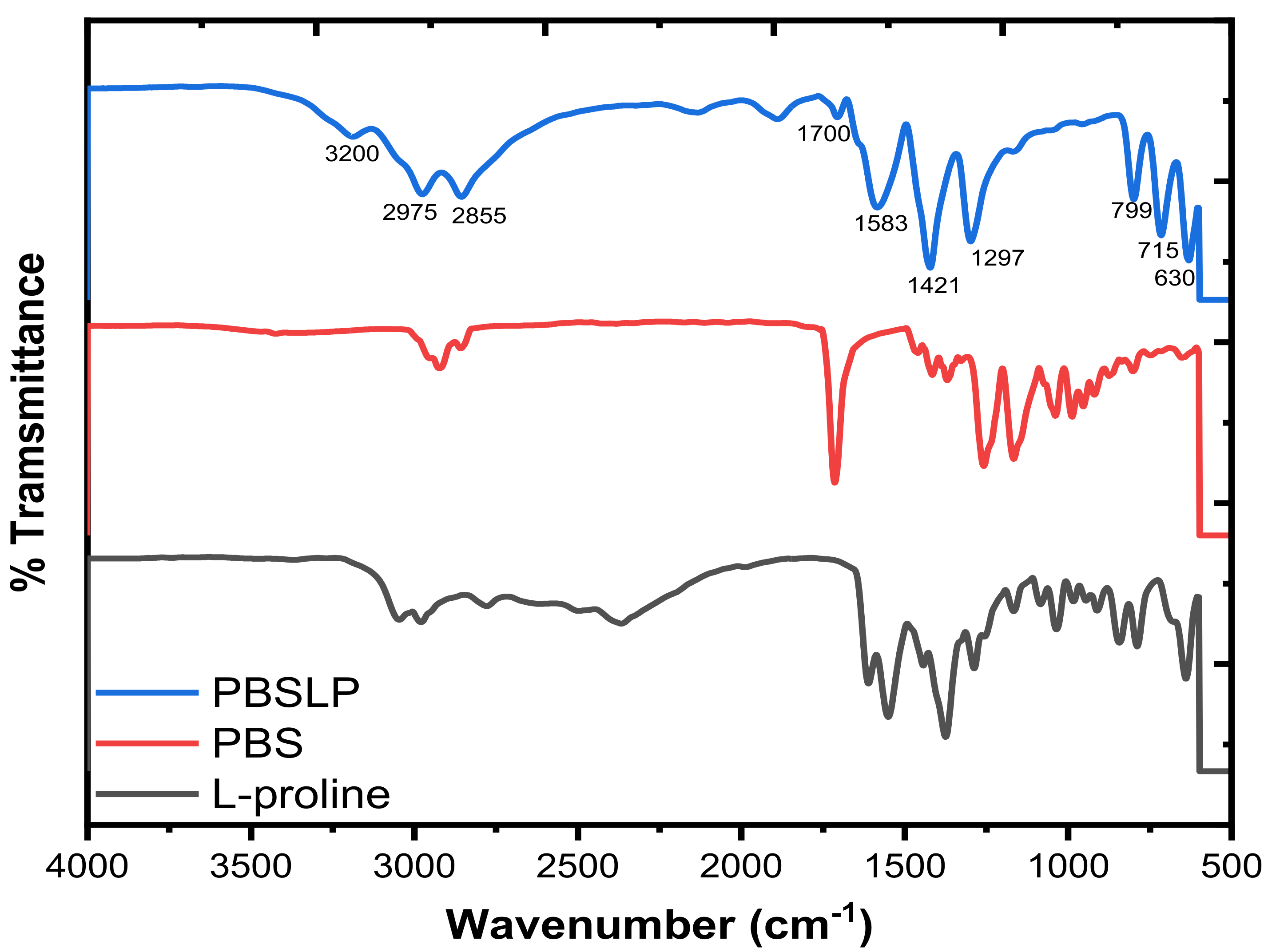
3.1.1. FTIR
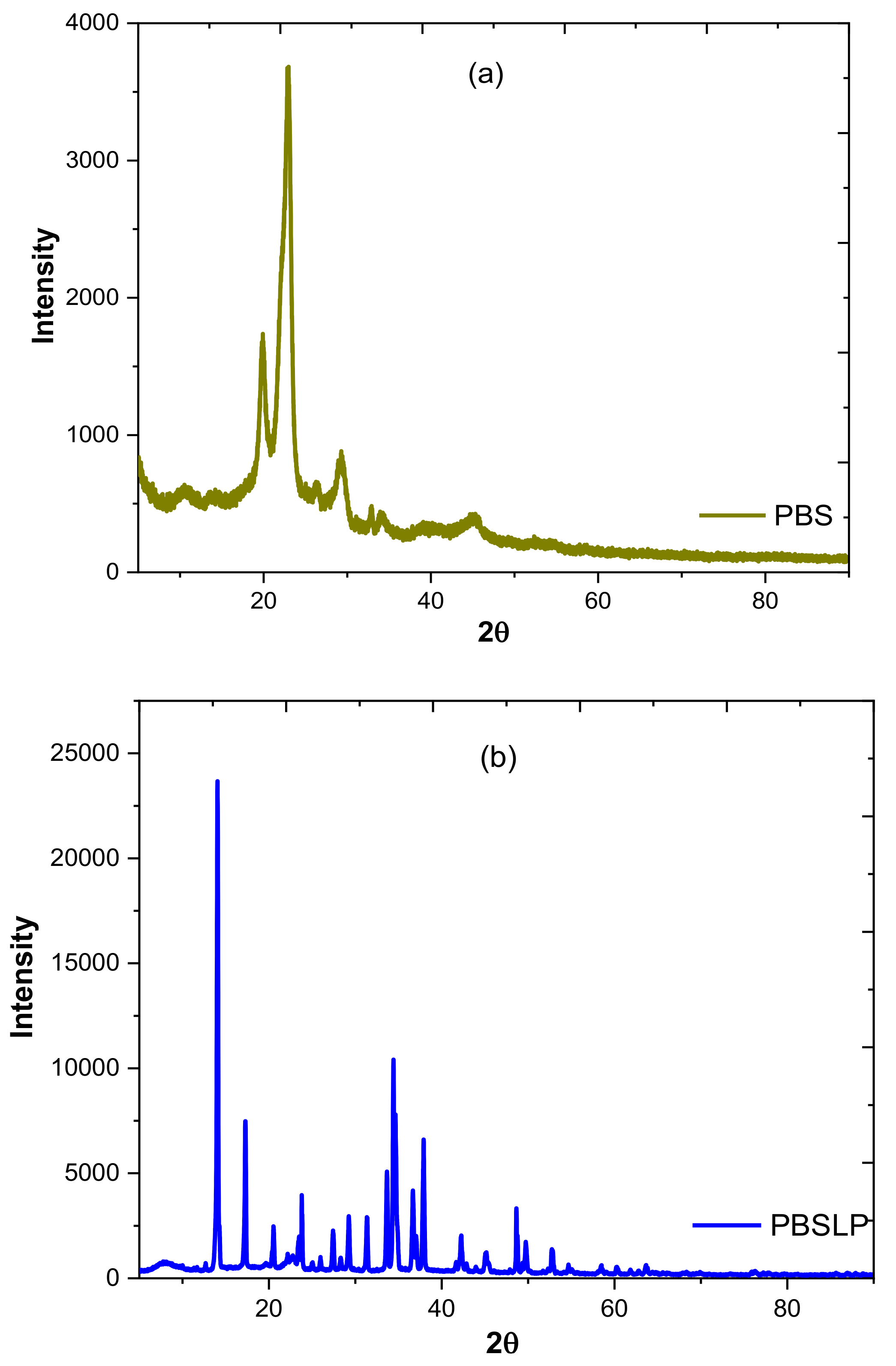
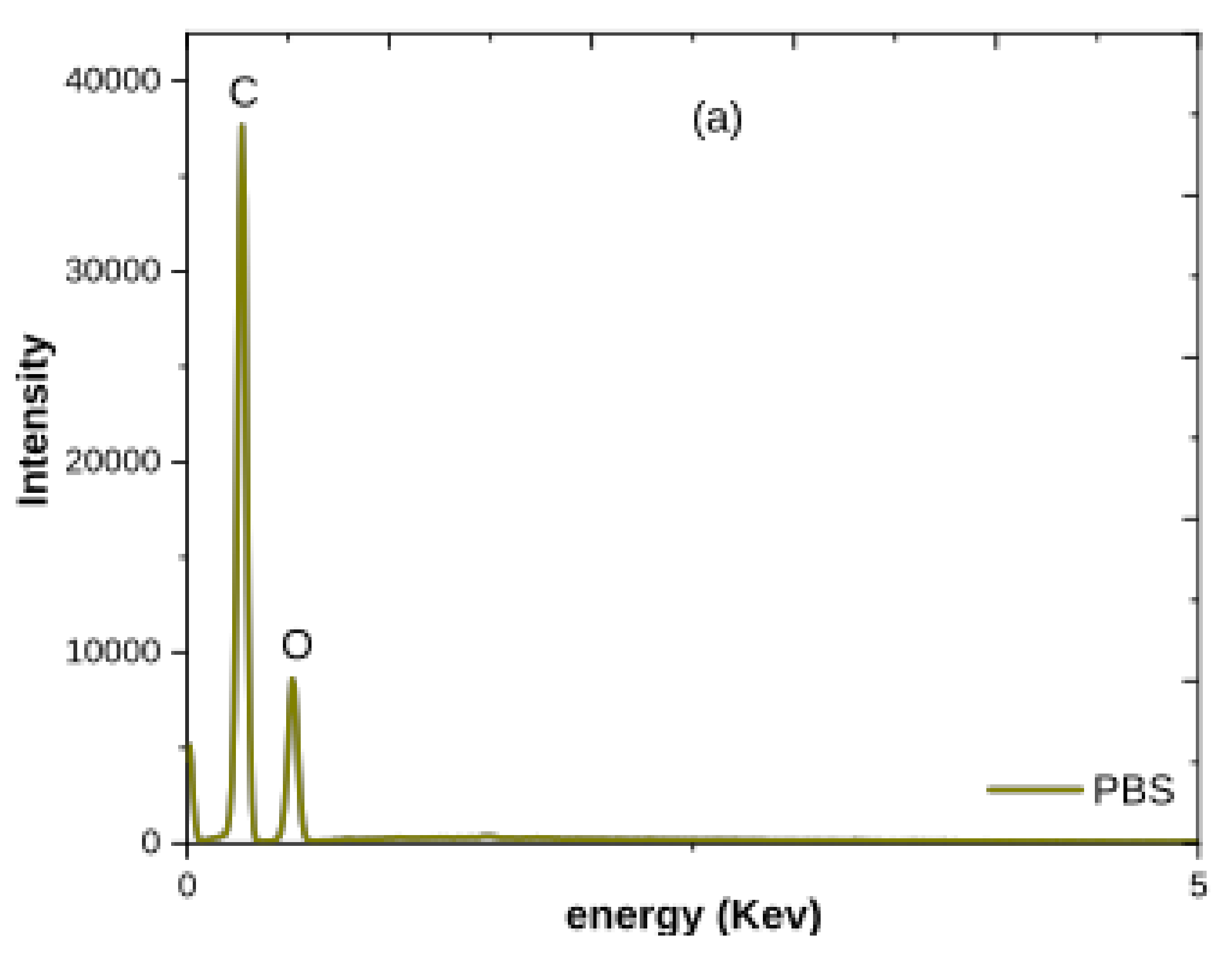
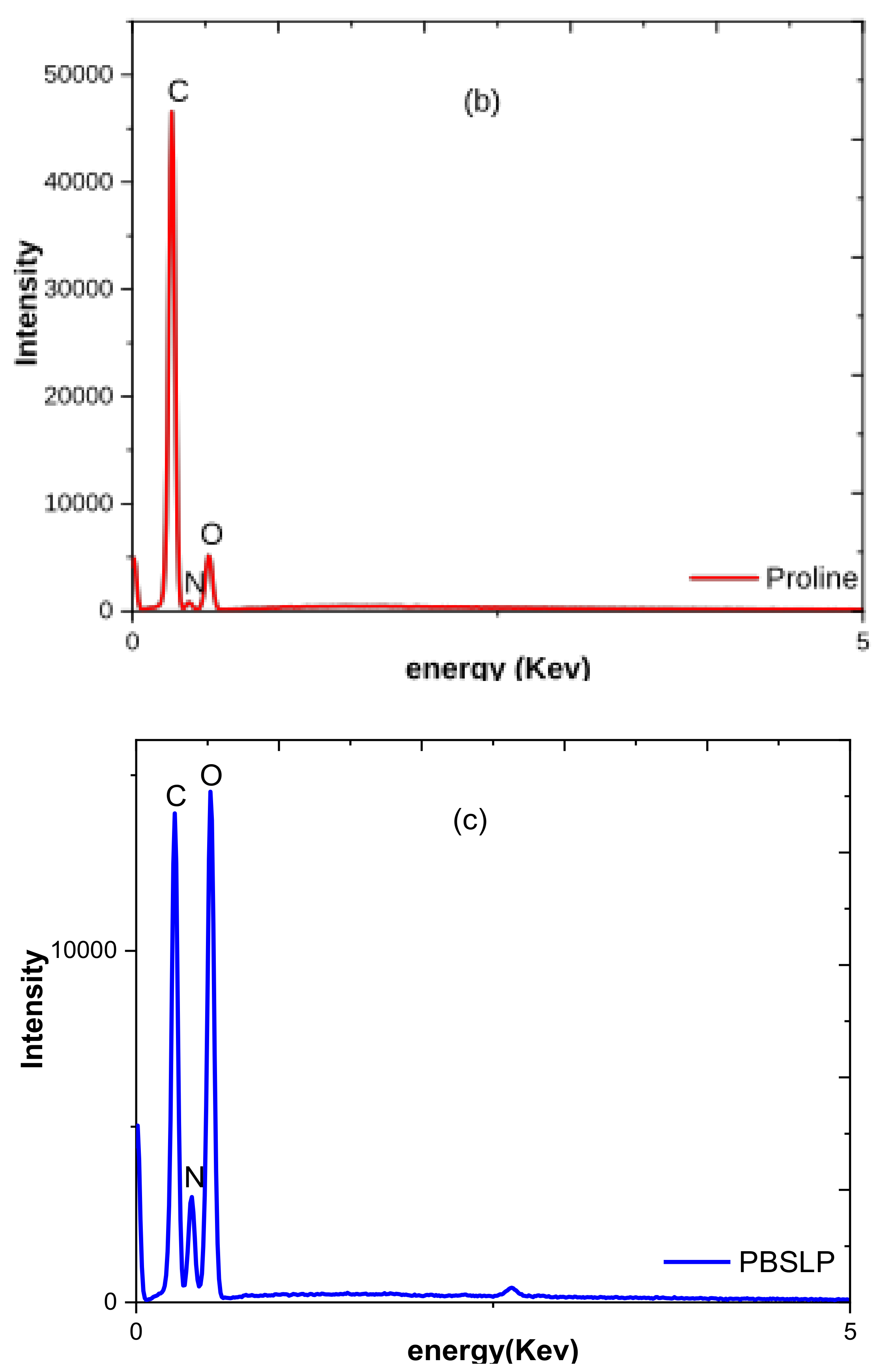
3.1.2. XRD and SEM–EDX
3.2. Electrochemical Analysis
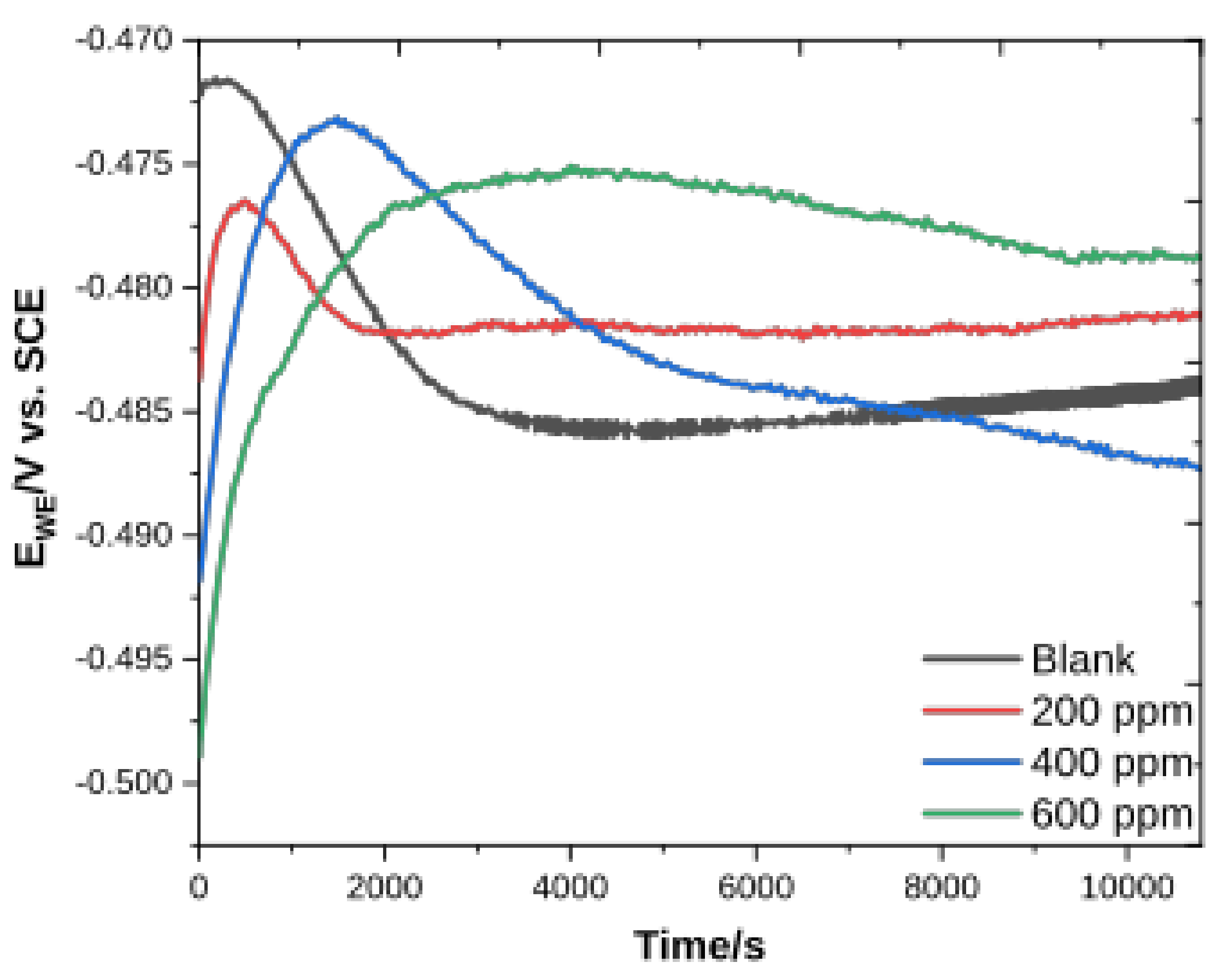
3.2.1. Potential vs. Time
3.2.2. Potentiodynamic Polarization
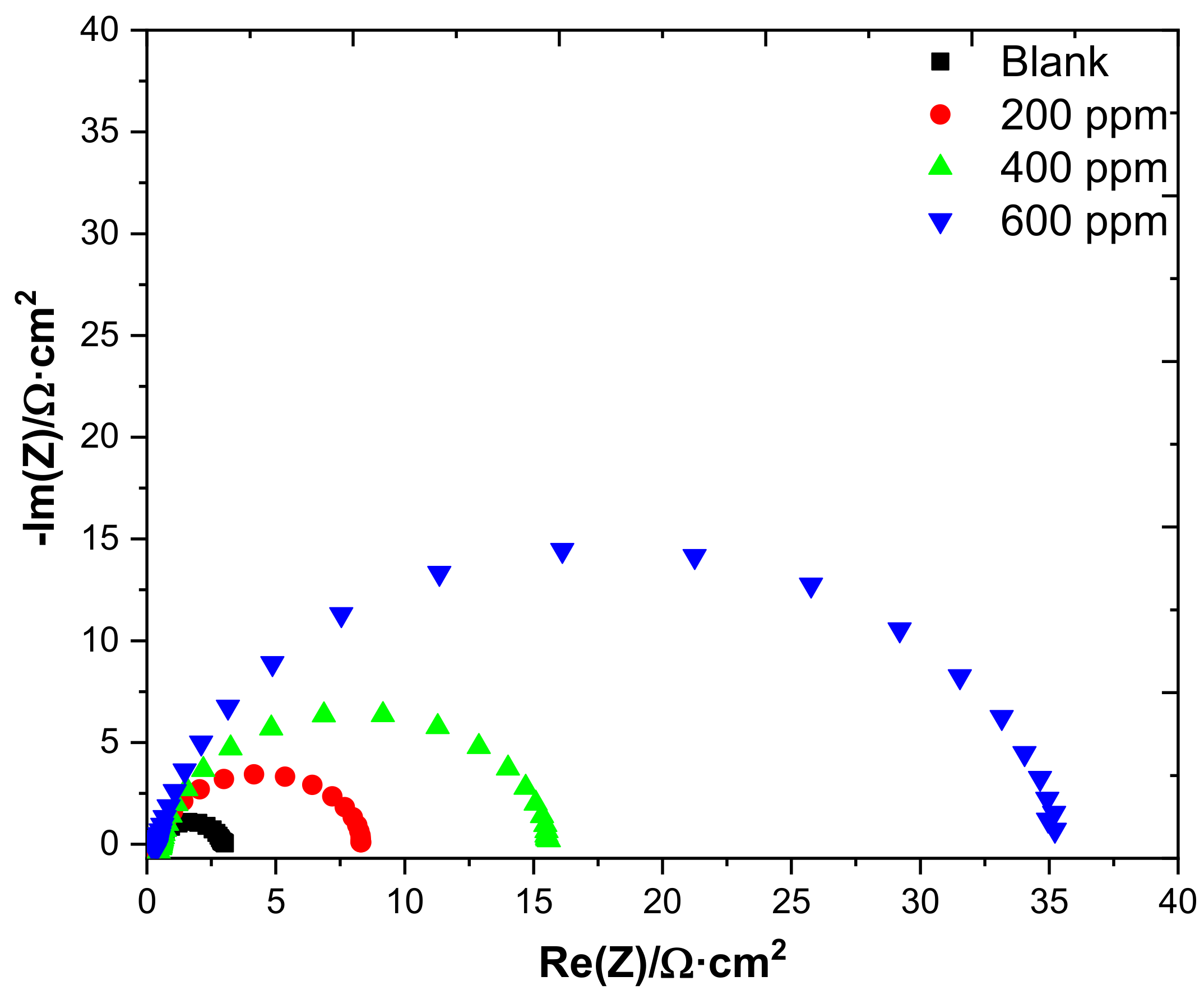
3.2.3. Electrochemical Impedance Spectroscopy (EIS)
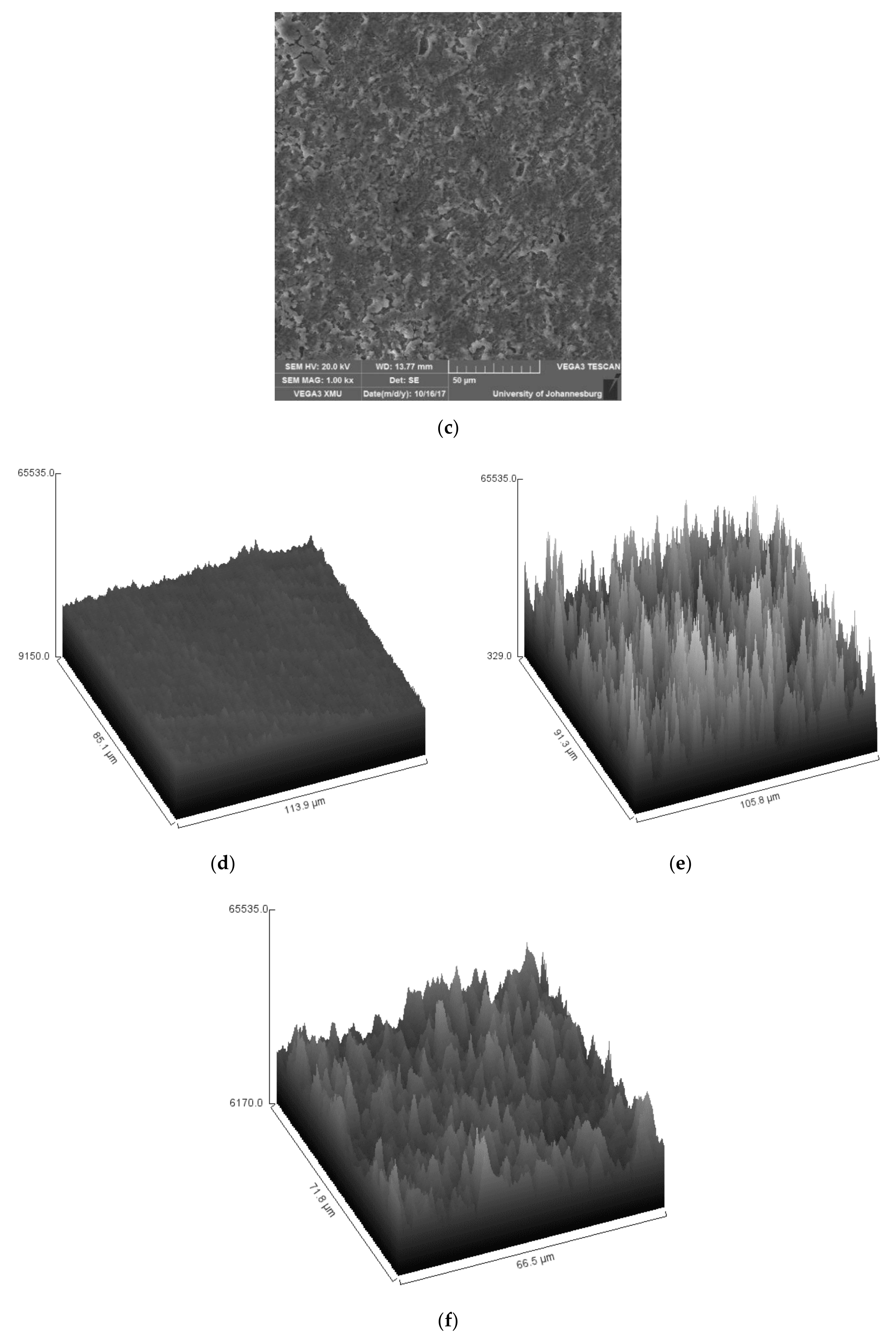
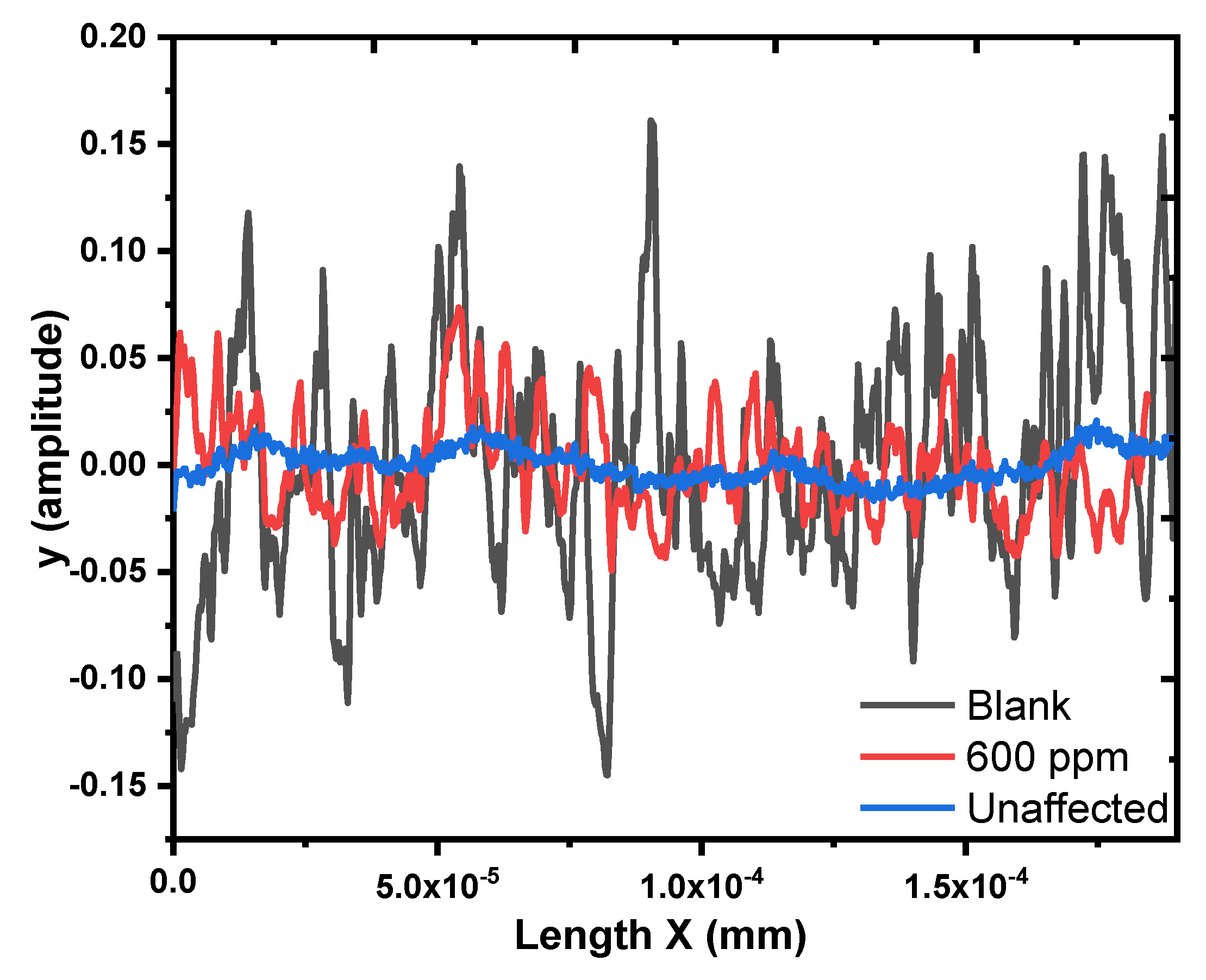
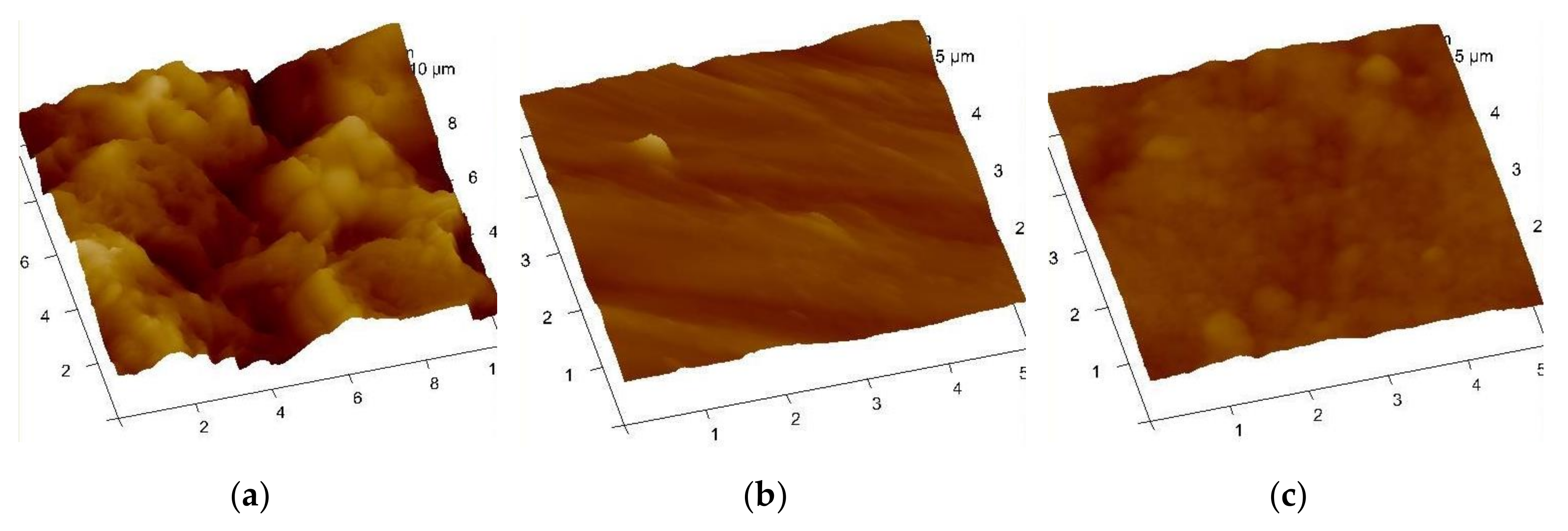
3.3. Surface Analysis
3.4. Adsorption Studies
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Kumari, P.P.; Rao, S.A.; Shetty, P. Corrosion Inhibition of Mild Steel in 2M HCl by a Schiff Base Derivative. Procedia Mater. Sci. 2014, 5, 499–507. [Google Scholar] [CrossRef]
- Mobin, M.; Parveen, M.; Rafiquee, M.Z.A. Synergistic effect of sodium dodecyl sulfate and cetyltrimethyl ammonium bromide on the corrosion inhibition behavior of l-methionine on mild steel in acidic medium. Arab. J. Chem. 2013, 10, S1364–S1372. [Google Scholar] [CrossRef]
- Obot, I.B.; Gasem, Z.M. Theoretical evaluation of corrosion inhibition performance of some pyrazine derivatives. Corros. Sci. 2014, 83, 359–366. [Google Scholar] [CrossRef]
- Yang, L.; Li, Y.; Qian, B.; Hou, B. Polyaspartic acid as a corrosion inhibitor for WE43 magnesium alloy. J. Magnes. Alloy. 2015, 3, 47–51. [Google Scholar] [CrossRef]
- Ahamad, I.; Quraishi, M.A. Bis (benzimidazol-2-yl) disulphide: An efficient water soluble inhibitor for corrosion of mild steel in acid media. Corros. Sci. 2009, 51, 2006–2013. [Google Scholar] [CrossRef]
- Hamadi, L.; Mansouri, S.; Oulmi, K.; Kareche, A. The use of amino acids as corrosion inhibitors for metals: A review. Egypt. J. Pet. 2018, 27, 1157–1165. [Google Scholar] [CrossRef]
- Askari, M.; Aliofkhazraei, M.; Gha, S.; Hajizadeh, A. Film former corrosion inhibitors for oil and gas pipelines—A technical review. J. Nat. Gas Sci. Eng. 2018, 58, 92–114. [Google Scholar] [CrossRef]
- Popoola, L.T.; Grema, A.S.; Latinwo, G.K.; Gutti, B.; Balogun, A.S. Corrosion problems during oil and gas production and its mitigation. Int. J. Ind. Chem. 2013, 4, 1–15. [Google Scholar] [CrossRef]
- Singh, P.; Quraishi, M.A.; Ebenso, E.E. Thiourea-Formaldehyde Polymer a New and Effective Corrosion Inhibitor for Mild Steel in Hydrochloric Acid Solution. Int. J. Electrochem. Sci. 2014, 9, 4900–4912. [Google Scholar]
- Obot, I.B.; Macdonald, D.D.; Gasem, Z.M. Density functional theory (DFT) as a powerful tool for designing new organic corrosion inhibitors. Part 1: An overview. Corros. Sci. 2015, 99, 1–30. [Google Scholar] [CrossRef]
- Sethuraman, M.G.; Aishwarya, V.; Kamal, C.; Edison, T.J.I. Studies on Ervatinine—The anticorrosive phytoconstituent of Ervatamia coronaria. Arab. J. Chem. 2013, 10, S522–S530. [Google Scholar] [CrossRef]
- Kumari, P.P.; Shetty, P.; Rao, S.A. Electrochemical measurements for the corrosion inhibition of mild steel in 1M hydrochloric acid by using an aromatic hydrazide derivative. Arab. J. Chem. 2013, 29. [Google Scholar] [CrossRef]
- Guo, L.; Xun, S.; Savas, K.; Yan-li, Z. Adsorption of Amino Acid Inhibitors on Iron Surface: A First-principles Investigation. Surf. Technol. 2017, 46, 228–234. [Google Scholar]
- Karthik, D.; Tamilvendan, D.; Prabhu, G.V. Study on the inhibition of mild steel corrosion by 1,3-bis-(morpholin-4-yl-phenyl-methyl)-thiourea in hydrochloric acid medium. J. Saudi Chem. Soc. 2014, 18, 835–844. [Google Scholar] [CrossRef]
- Markhali, B.P.; Naderi, R.; Mahdavian, M.; Sayebani, M.; Arman, S.Y. Electrochemical impedance spectroscopy and electrochemical noise measurements as tools to evaluate corrosion inhibition of azole compounds on stainless steel in acidic media. Corros. Sci. 2013, 75, 269–279. [Google Scholar] [CrossRef]
- Mobin, M.; Zehra, S.; Parveen, M. L-Cysteine as corrosion inhibitor for mild steel in 1 M HCl and synergistic effect of anionic, cationic and non-ionic surfactants. J. Mol. Liq. 2016, 216, 598–607. [Google Scholar] [CrossRef]
- el Ibrahimi, B.; Jmiai, A.; Bazzi, L.; el Issami, S. Amino Acids and their Derivatives as Corrosion Inhibitors for Metals and Alloys. Arab. J. Chem. 2017, 13, 740–771. [Google Scholar] [CrossRef]
- Tsoeunyane, M.G.; Makhatha, M.E.; Arotiba, O.A. Corrosion Inhibition of Mild Steel by Poly(butylene succinate)-L-histidine Extended with 1,6-diisocynatohexane Polymer Composite in 1 M HCl. Int. J. Corros. 2019, 2019. [Google Scholar] [CrossRef]
- Osório, W.R.; Freitas, E.S.; Garcia, A. EIS and potentiodynamic polarization studies on immiscible monotectic Al—In alloys. Electrochim. Acta. 2013, 102, 436–445. [Google Scholar] [CrossRef]
- Osorio, W.R.; Peixoto, L.C.; Moutinho, D.J.; Gomes, L.G.; Ferreira, I.L. Corrosion resistance of directionally solidified Al–6Cu–1Si and Al–8Cu–3Si alloys castings. Mater. Des. 2011, 32, 3832–3837. [Google Scholar] [CrossRef]
- Zhang, X.; Jiang, Z.H.; Yao, Z.P.; Song, Y.; Wu, Z.D. Effects of scan rate on the potentiodynamic polarization curve obtained to determine the Tafel slopes and corrosion current density. Corros. Sci. 2009, 51, 581–587. [Google Scholar] [CrossRef]
- Mccafferty, E. Validation of corrosion rates measured by the Tafel extrapolation method. Corros. Sci. 2005, 47, 3202–3215. [Google Scholar] [CrossRef]
- Karthikaiselvi, R.; Subhashini, S.; Rajalakshmi, R. Poly (vinyl alcohol—aniline) water soluble composite as corrosion inhibitor for mild steel in 1 M HCl. Arab. J. Chem. 2012, 5, 517–522. [Google Scholar] [CrossRef]
- Hegazy, M.A.; Abdallah, M.; Awad, M.K.; Rezk, M. Three novel di-quaternary ammonium salts as corrosion inhibitors for API X65 steel pipeline in acidic solution. Part I: Experimental results. Corros. Sci. 2014, 81, 54–64. [Google Scholar] [CrossRef]
- Hegazy, M.A.; Ahmed, H.M. Investigation of the inhibitive effect of p-substituted 4- (N,N,N-dimethyldodecylammonium bromide) benzylidene-benzene-2-yl-amine on corrosion of carbon steel pipelines in acidic medium. Corros. Sci. 2011, 53, 671–678. [Google Scholar] [CrossRef]
- Orazem, M.E.; Tribollet, B. Electrochemical Impedance Spectroscopy, 1st ed.; John Wiley & Sons Inc: Hoboken, NJ, USA, 2008. [Google Scholar]
- Wang, F.S.; Li, Y.; Zhang, Y.X.; Chen, G.Y. A method to select the optimal equivalent electrical circuit applied to study corrosion system of composite coating on magnesium alloy. Phys. Lett. Acs Lett. A 2020, 384, 126452. [Google Scholar] [CrossRef]
- Rodrigues, Q.; Padilha, G.S.; Bortolozo, A.D.; Osorio, W.R. Effect of sintering time on corrosion behavior of an Ag/Al/Nb/Ti/Zn alloy system. J. Alloys Compd. 2020, 834. [Google Scholar] [CrossRef]
- Satizabal, L.M.; Costa, D.; Moraes, P.B.; Bortolozo, A.D.; Osório, W.R. Microstructural array and solute content affecting electrochemical behavior of Sn-Ag and Sn-Bi alloys compared with a traditional Sn-Pb alloy. Mater. Chem. Phys. 2019, 223, 410–425. [Google Scholar] [CrossRef]
- Hirschorn, B.; Orazem, M.E.; Tribollet, B.; Vivier, V.; Frateur, I.; Musiani, M. Determination of effective capacitance and film thickness from constant-phase-element parameters. Electrochim. Acta 2010, 55, 6218–6227. [Google Scholar] [CrossRef]
- Hirschorn, B.; Orazem, M.E.; Tribollet, B.; Vivier, V.; Frateur, I.; Musiani, M. Constant-Phase-Element Behavior Caused by Resistivity Distributions in Films II. Applications. J. Electrochem. Soc. 2010, 157, C458–C463. [Google Scholar] [CrossRef]
- Genchev, G.; Gmbh, M.E. Sour Gas Corrosion—Corrosion of Steels and Other Metallic Materials in Aqueous Environments Containing H2S. In Encyclopedia of Interfacial Chemistry: Surface Science and Electrochemistry, part of “Reference Module in Chemistry, Molecular Sciences and Chemical Engineering”; Wandelt, K., Reedijk, J., Eds.; Elsevier: Amsterdam, The Netherlands, 2018. [Google Scholar] [CrossRef]
- Barsoukov, E.; Macdonald, J.R. Impedance Spectroscopy: Theory, Experiment, and Applications, 2nd ed.; John Wiley & Sons Inc.: Hoboken, NJ, USA, 2005. [Google Scholar]
- Ilman, M.N.; Kusmono. Analysis of internal corrosion in subsea oil pipeline. Case Stud. Eng. Fail. Anal. 2014, 2, 1–8. [Google Scholar] [CrossRef]
- Zarrouk, A.; Hammouti, B.; Zarrok, H.; Messali, M. Temperature Effect, Activation Energies and Thermodynamic Adsorption Studies of L-Cysteine Methyl Ester Hydrochloride As Copper Corrosion Inhibitor In Nitric Acid 2M. Int. J. Electrochem. Sci. 2011, 6, 6261–6274. [Google Scholar]
- Bouanis, M.; Tourabi, M.; Nyassi, A.; Zarrouk, A.; Jama, C.; Bentiss, F. Applied Surface Science Corrosion inhibition performance of HCl solution: Gravimetric, electrochemical and XPS studies. Appl. Surf. Sci. 2016, 389, 952–966. [Google Scholar] [CrossRef]
- Goldberg, S. Equations and Models Describing Adsorption Processes In Soils. In Chemical Processes in Soils; Sparks, D., Tabatabai, M., Eds.; Soil Science Society of America: Madison, WI, USA, 2005. [Google Scholar]
- Parekh, K.; Jauhari, S.; Upadhyay, R.V. Mechanism of acid corrosion inhibition using magnetic nano fluid. Adv. Nat. Sci. Nanosci. Nanotechnol. 2016, 7. [Google Scholar] [CrossRef]


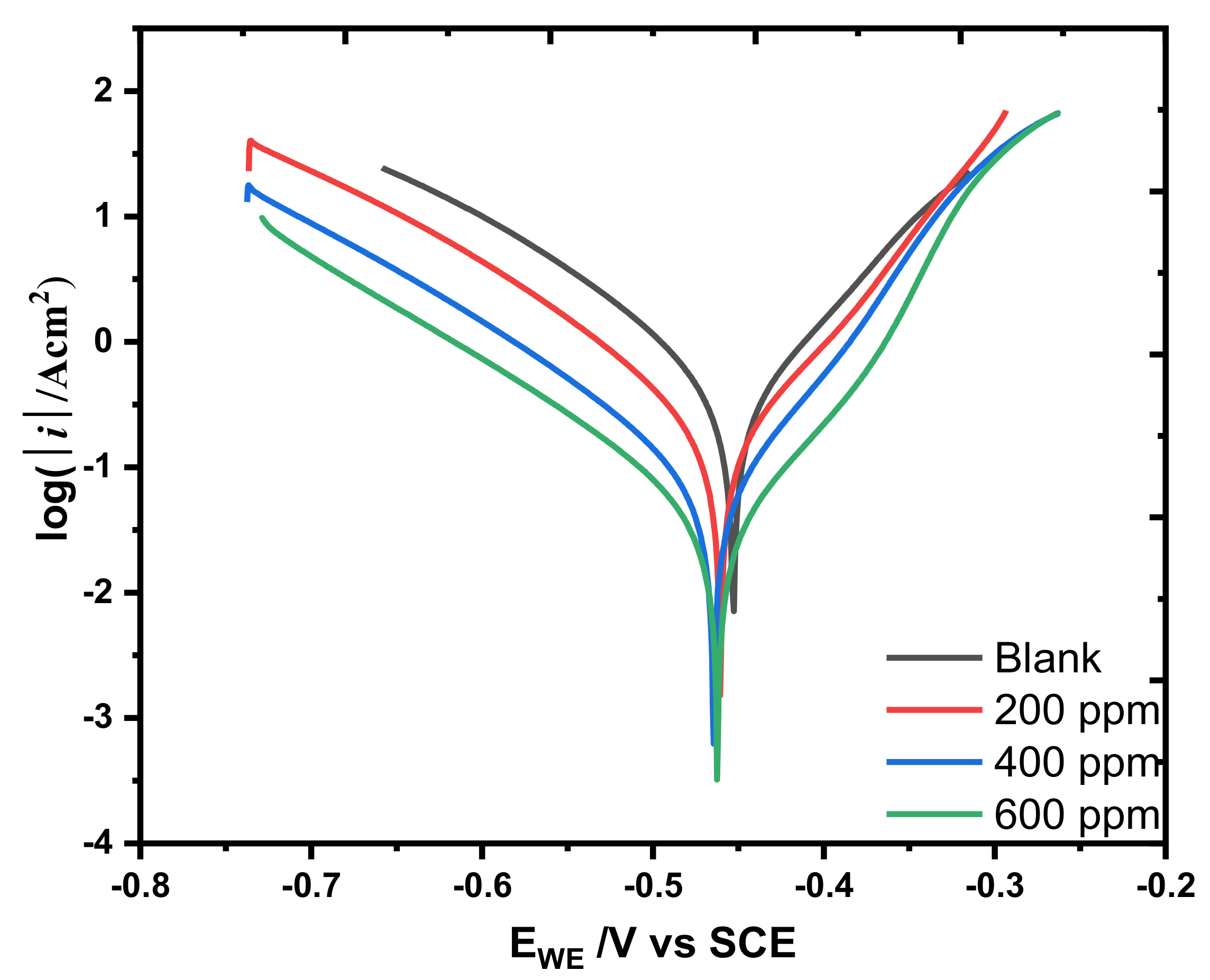
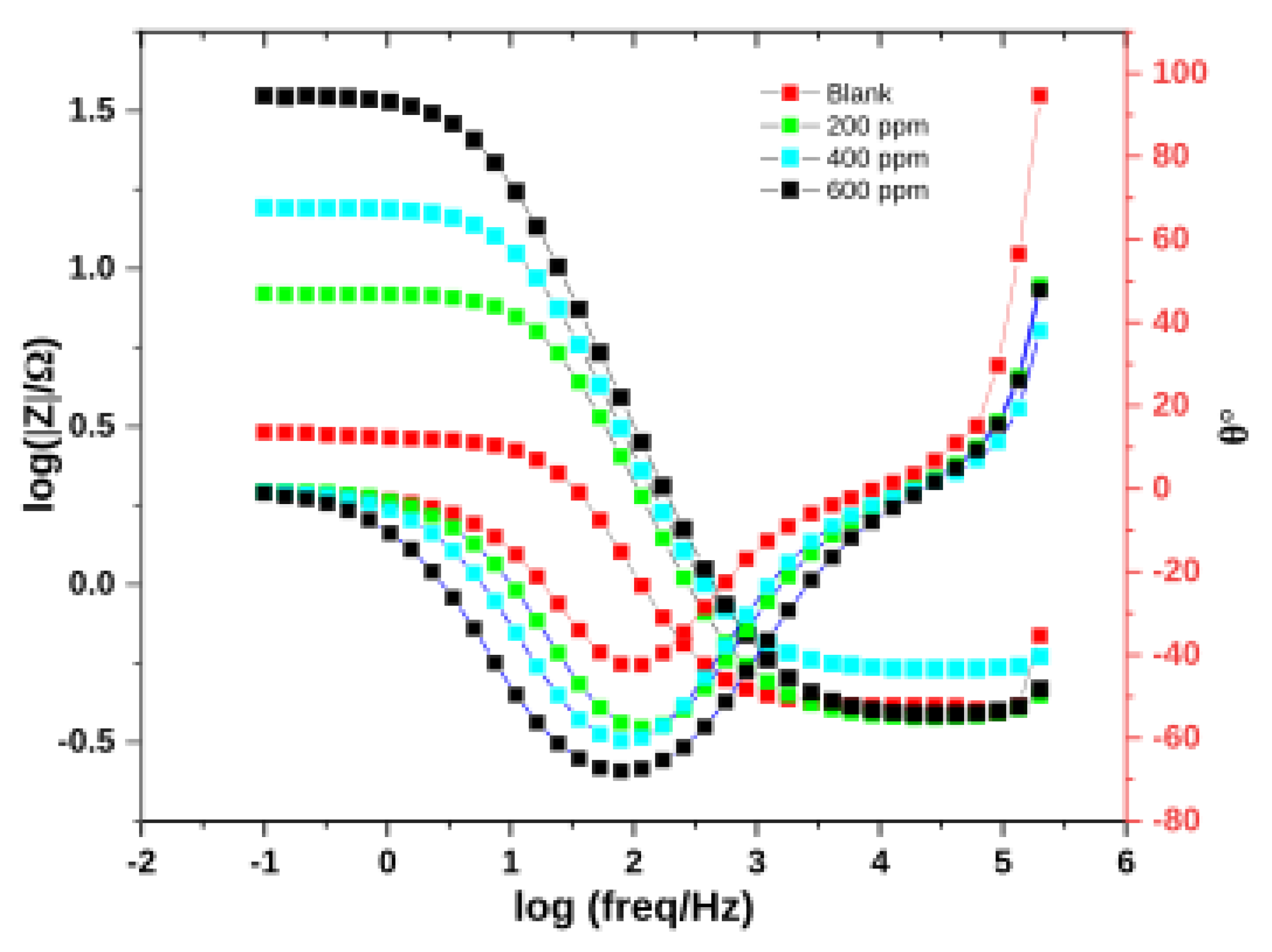


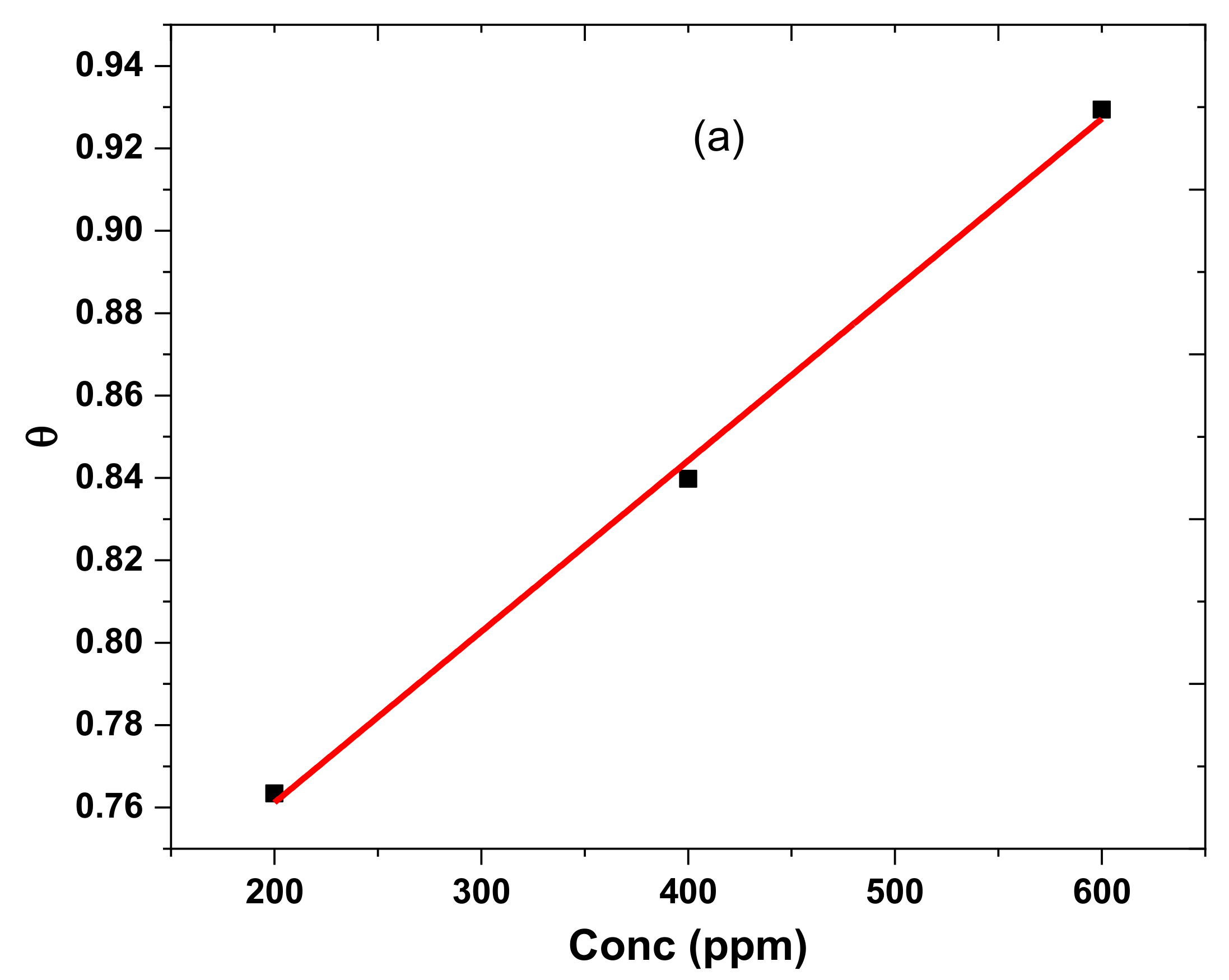
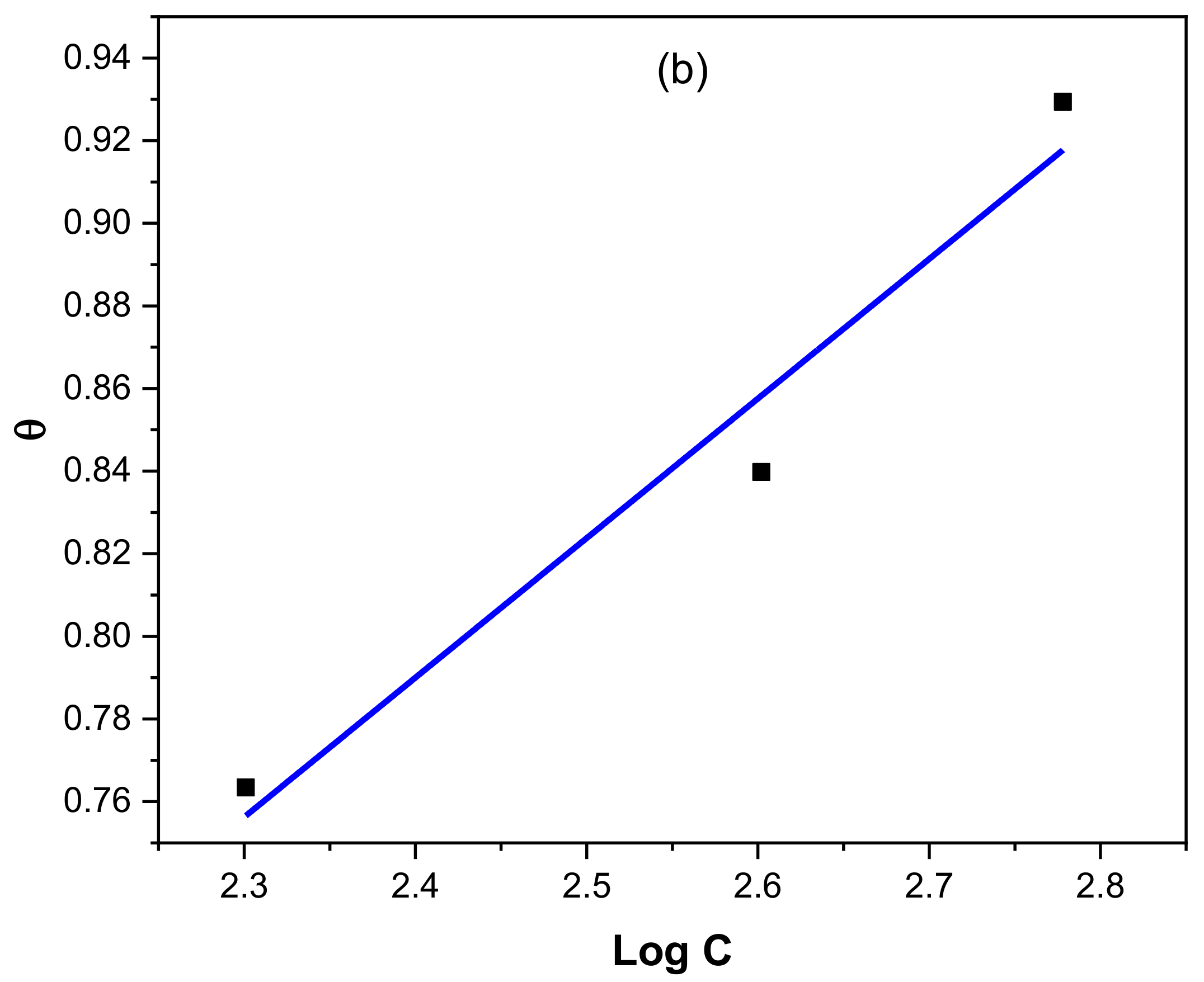
| Conc (ppm) | Ecorr/mV | icorr/Acm−2 | βA/(mV/Decade) | βC/(mV/Decade) | CR/mmpy | %IE | θ |
|---|---|---|---|---|---|---|---|
| Blank | −453.11 | 0.232 | 65.8 | 68.3 | 0.212 | - | - |
| 200 | −460.72 | 0.055 | 55.9 | 61.3 | 0.0502 | 76.34 | 0.766 |
| 400 | −463.93 | 0.037 | 50.6 | 62.4 | 0.0340 | 83.98 | 0.839 |
| 600 | −461.96 | 0.016 | 48.3 | 53.2 | 0.0150 | 92.94 | 0.929 |
| Conc/ppm | Rs/Ω | Q1/F.s^(α−1) | α1 | Rpor/Ω | Q2/F.s^(α−1) | α2 | Rct/Ω | %IE | χ2 |
|---|---|---|---|---|---|---|---|---|---|
| Blank | 0.4131 | 3.231 × 10−3 | 0.901 | 0.088 | 5.692 × 10−5 | 1 | 2.058 | - | 0.045 |
| 200 | 0.4785 | 1.971 × 10−3 | 0.894 | 0.154 | 0.5608 × 10−6 | 0.895 | 4.833 | 57 | 0.059 |
| 400 | 0.4343 | 1.251 × 10−3 | 0.898 | 0.267 | 1.251 × 10−7 | 0.898 | 15.13 | 86 | 0.024 |
| 600 | 0.3808 | 1.083 × 10−3 | 0.882 | 4.34 | 6.493 × 10−9 | 0.925 | 30.75 | 93 | 0.044 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tsoeunyane, G.M.; Makhatha, E.M. Electrochemical and Surface Analytical Study on the Role of Poly(butylene-succinate)-l-proline during Corrosion of Mild Steel in 1 M HCl. Chemistry 2020, 2, 900-917. https://doi.org/10.3390/chemistry2040057
Tsoeunyane GM, Makhatha EM. Electrochemical and Surface Analytical Study on the Role of Poly(butylene-succinate)-l-proline during Corrosion of Mild Steel in 1 M HCl. Chemistry. 2020; 2(4):900-917. https://doi.org/10.3390/chemistry2040057
Chicago/Turabian StyleTsoeunyane, George M., and Elizabeth M. Makhatha. 2020. "Electrochemical and Surface Analytical Study on the Role of Poly(butylene-succinate)-l-proline during Corrosion of Mild Steel in 1 M HCl" Chemistry 2, no. 4: 900-917. https://doi.org/10.3390/chemistry2040057
APA StyleTsoeunyane, G. M., & Makhatha, E. M. (2020). Electrochemical and Surface Analytical Study on the Role of Poly(butylene-succinate)-l-proline during Corrosion of Mild Steel in 1 M HCl. Chemistry, 2(4), 900-917. https://doi.org/10.3390/chemistry2040057





