Enhanced Cycling Stability of High-Voltage Sodium-Ion Batteries via DFEC-Driven Fluorinated Interface Engineering
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation of Electrolytes
2.2. Preparation and Electrochemical Test of Electrodes
2.3. Electrochemical Characterization
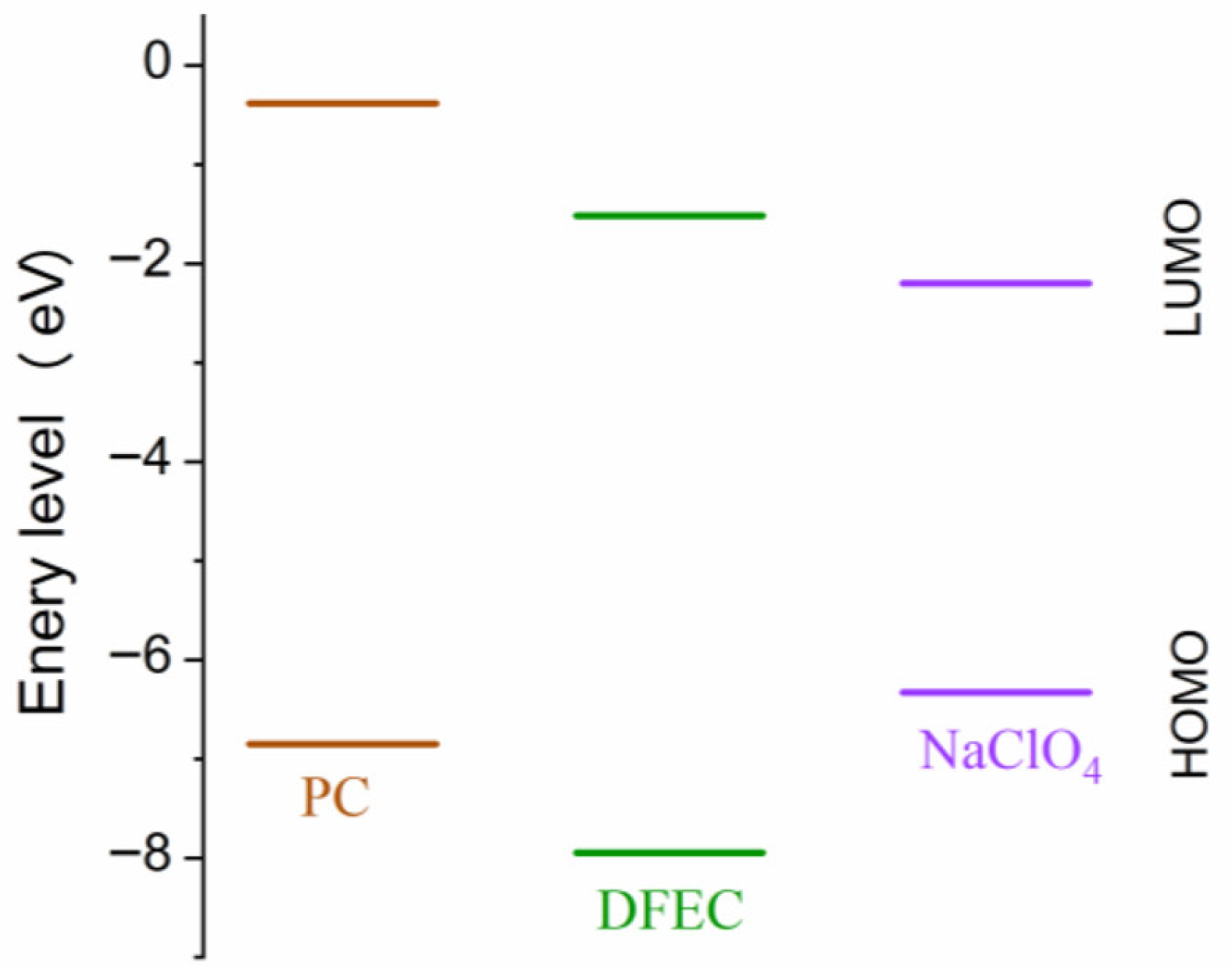
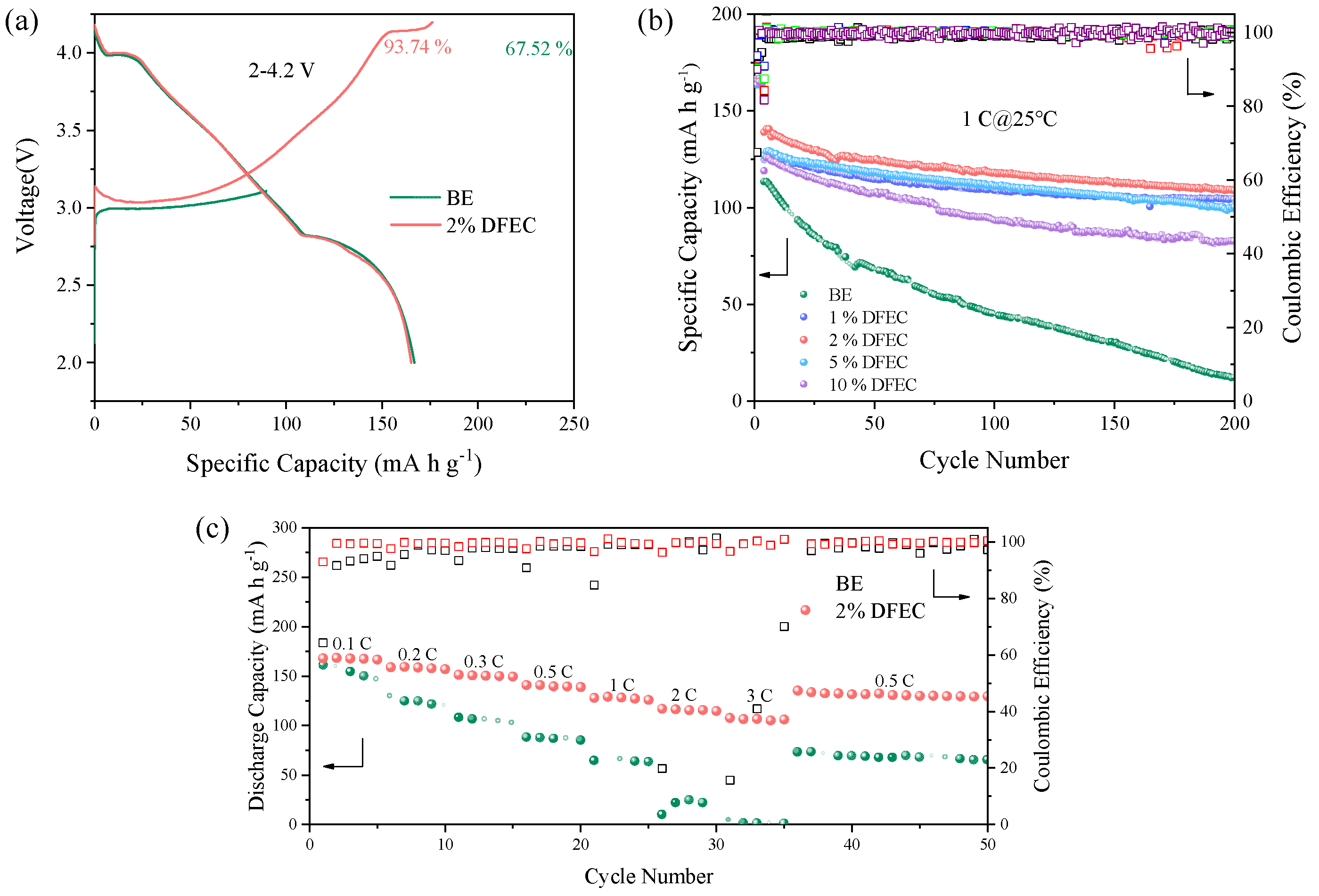
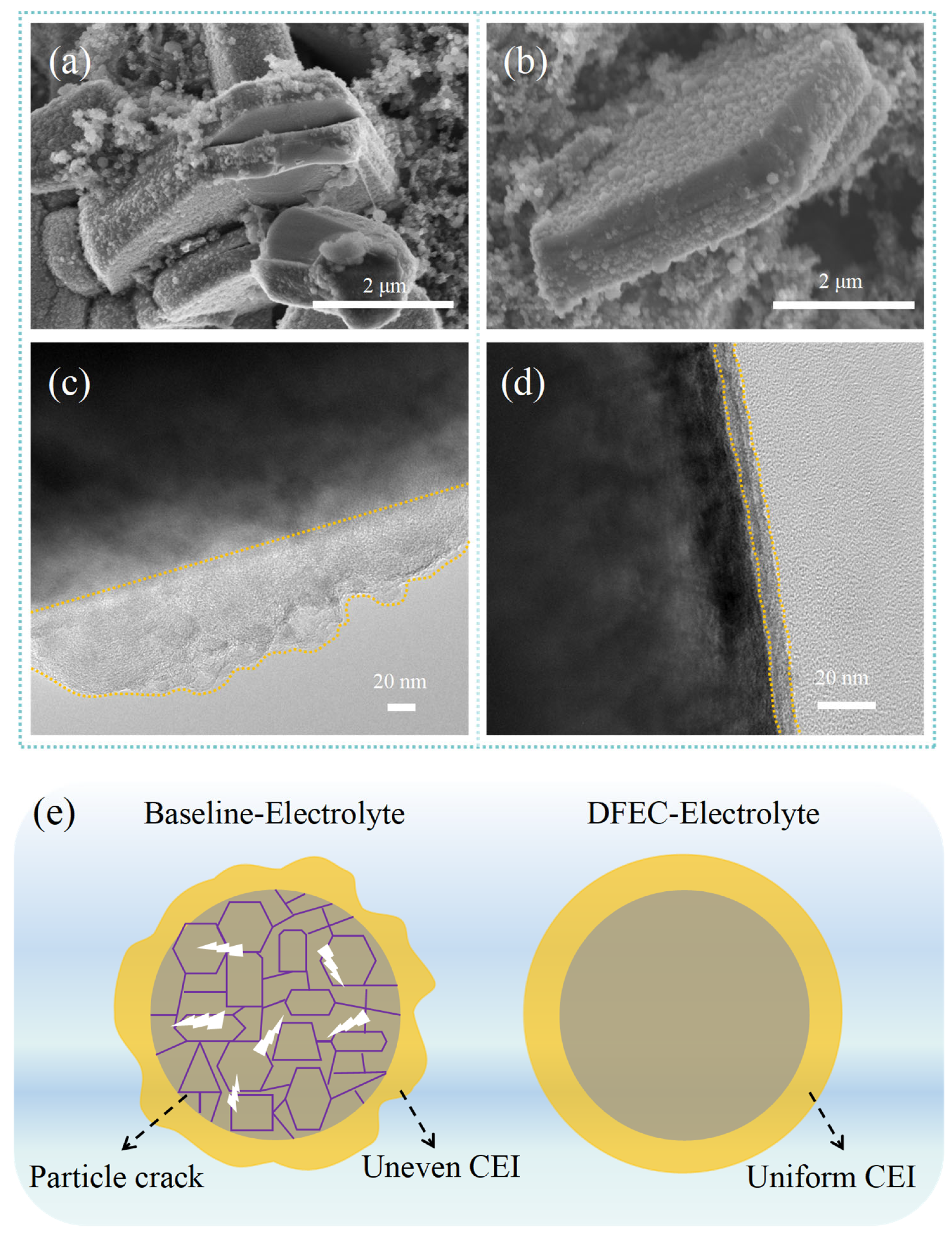
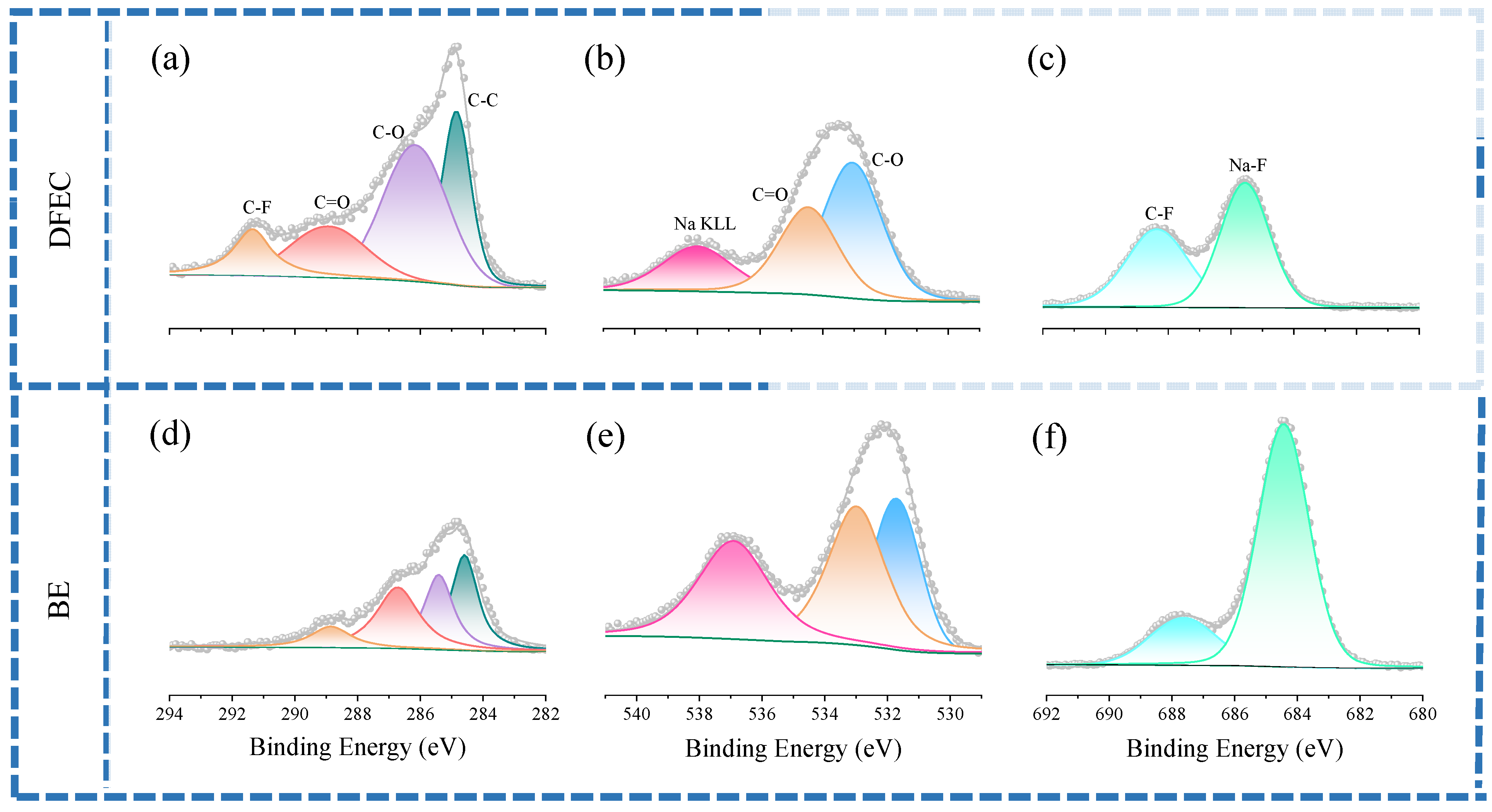
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| SIBs | Sodium-ion batteries |
| CEI | Cathode electrolyte interphase |
| DFEC | Difluoroethylene carbonate |
| LIBs | Lithium-ion batteries |
| TMSI | Trimethoxymethylsilane |
| FEC | Fluoroethylene carbonate |
| VC | Vinylene carbonate |
| PES | Prop-1-ene-1,3-sultone |
| SA | Succinic anhydride |
| DGA | Diglycolic anhydride |
| DFT | Density functional theory |
| HOMO | Highest occupied molecular orbital |
| ICE | Initial coulombic efficiency |
| SEM | Scanning electron microscopy |
| TEM | Transmission electron microscope |
| XPS | X-ray photoelectron spectroscopy |
References
- Yin, D.Y.; Ni, J.M.; Shi, X.Y.; Liu, H.; Lv, M.; Shen, W.; Zhang, G.X. Dynamic Overcharge Performance and Mechanism of Lithium-Ion Batteries during High-Temperature Calendar Aging. ACS Appl. Energy Mater. 2025, 8, 3491–3499. [Google Scholar] [CrossRef]
- Han, X.P.; Zhang, Z.X.; Han, M.Y.; Cui, Y.R.; Sun, J. Fabrication of Red Phosphorus Anode for Fast-Charging Lithium-Ion Batteries based on TiN/TiP2-Enhanced Interfacial Kinetics. Energy Storage Mater. 2020, 26, 147–156. [Google Scholar] [CrossRef]
- Feng, X.N.; Ren, D.S.; He, X.M.; Ouyang, M.G. Mitigating Thermal Runaway of Lithium-Ion Batteries. Joule 2020, 4, 743–770. [Google Scholar] [CrossRef]
- Liu, H.Q.; Gao, X.; Chen, J.; Gao, J.Q.; Yin, S.Y.; Zhang, S.; Yang, L.; Fang, S.S.; Mei, Y.; Xiao, X.H. Reversible OP4 Phase in P2-Na2/3Ni1/3Mn2/3O2 Sodium Ion Cathode. J. Power Sources 2021, 508, 230324. [Google Scholar] [CrossRef]
- Boivin, E.; House, R.A.; Marie, J.-J.; Bruce, P.G. Controlling Iron Versus Oxygen Redox in the Layered Cathode Na0.67Fe0.5Mn0.5O2: Mitigating Voltage and Capacity Fade by Mg Substitution. Adv. Energy Mater. 2022, 12, 2200702. [Google Scholar] [CrossRef]
- Song, T.F.; Chen, L.; Gastol, D.; Dong, B.; Marco, J.F.; Berry, F.; Slater, P.; Reed, D.; Kendrick, E. High-Voltage Stabilization of O3-Type Layered Oxide for Sodium-Ion Batteries by Simultaneous Tin Dual Modification. Chem. Mater. 2022, 34, 4153–4165. [Google Scholar] [CrossRef]
- Deng, J.Q.; Luo, W.-B.; Lu, X.; Yao, Q.R.; Wang, Z.M.; Liu, H.-K.; Zhou, H.Y.; Dou, S.-X. High Energy Density Sodium-ion Battery with Industrially Feasible and Air-Stable O3-Type Layered Oxide Cathode. Adv. Energy Mater. 2018, 8, 1701610. [Google Scholar] [CrossRef]
- Lu, J.L.; Zhang, J.W.; Huang, Y.Y.; Zhang, Y.; Yin, Y.S.; Bao, S. Advances on Layered Transition-Metal Oxides for Sodium-Ion Batteries: A Mini Review. Fronit. Energy Res. 2023, 11, 1246327. [Google Scholar] [CrossRef]
- Usiskin, R.; Lu, Y.X.; Popovic, J.; Law, M.; Balaya, P.; Hu, Y.-S.; Maier, J. Fundamentals, Status and Promise of Sodium-Based Batteries. Nat. Rev. Mater. 2021, 6, 1020–1035. [Google Scholar] [CrossRef]
- Liu, Q.N.; Hu, Z.; Chen, M.Z.; Zou, C.; Jin, H.L.; Wang, S.; Chou, S.-L.; Dou, S.-X. Recent Progress of Layered Transition Metal Oxide Cathodes for Sodium-Ion Batteries. Small 2019, 15, 1805381. [Google Scholar] [CrossRef]
- Gao, R.M.; Zheng, Z.J.; Wang, P.F.; Wang, C.Y.; Ye, H.; Cao, F.F. Recent Advances and Prospects of Layered Transition Metal Oxide Cathodes for Sodium-Ion Batteries. Energy Storage Mater. 2020, 30, 9–26. [Google Scholar] [CrossRef]
- Liu, Y.-F.; Han, K.; Peng, D.N.; Kong, L.-Y.; Su, Y.; Li, H.-W.; Hu, H.-Y.; Li, J.-Y.; Wang, H.-R.; Fu, Z.-L.; et al. Layered Oxide Cathodes for Sodium-Ion Batteries: From Air Stability, Interface Chemistry to Phase Transition. InfoMat 2023, 5, e12422. [Google Scholar] [CrossRef]
- Zhao, Q.Q.; Wang, R.R.; Gao, M.; Butt, F.K.; Jia, J.F.; Wu, H.S.; Zhu, Y.Q. Interfacial Engineering of The Layered Oxide Cathode Materials for Sodium-Ion Battery. Nano Res. 2023, 17, 1441–1464. [Google Scholar] [CrossRef]
- Wang, P.F.; You, Y.; Yin, Y.X.; Guo, Y.G. Layered Oxide Cathodes for Sodium-Ion Batteries: Phase Transition, Air Stability, and Performance. Adv. Energy Mater. 2018, 8, 1701912. [Google Scholar] [CrossRef]
- Mao, Y.; Gong, H.C.; Wang, X.Y.; Cao, Y.; Wang, S.W.; Ma, K.; Fuku, X.G.; Zhou, C.Y.; Sun, J. Stabilization of Phase Transition Process and Lattice Oxygen of O3-Layered Oxide Cathode for Sodium-Ion Battery via Dual-Doping Strategy. Adv. Energy Mater. 2025, 15, 2502592. [Google Scholar] [CrossRef]
- Chang, Y.X.; Liu, X.; Xie, Z.Y.; Jin, Z.A.; Guo, Y.; Zhang, X.; Zhang, J.; Zheng, L.R.; Hong, S.; Xu, S.; et al. Manipulating Thermodynamics and Crystal Structure Modulates P2/O3 Biphasic Layered Oxide Cathodes for Sodium-Ion Batteries. Energy Storage Mater. 2025, 74, 103972. [Google Scholar] [CrossRef]
- Guo, J.; Zheng, J.; Zhou, W.; Long, X.; Zhou, X.; Cha, W.; Feng, L.; Hao, Y.; Ni, W.; Li, Y.; et al. In Situ Intergrowth Tunnel/P3 Cathode Enhancing High Voltage Stability Toward High Energy Sodium-Ion Batteries. Adv. Funct. Mater. 2025, 35, 2500604. [Google Scholar] [CrossRef]
- Ke, J.; Su, L. Advancing High-Voltage Cathodes for Sodium-Ion Batteries: Challenges, Material Innovations and Future Directions. Energy Storage Mater. 2025, 76, 104133. [Google Scholar] [CrossRef]
- Liu, X.Y.; Gong, H.C.; Han, C.Y.; Cao, Y.; Li, Y.T.; Sun, J. Barium Ions Act as Defenders to Prevent Water from Entering Prussian Blue Lattice for Sodium-Ion Battery. Energy Storage Mater. 2023, 57, 118–124. [Google Scholar] [CrossRef]
- Qian, J.F.; Wu, C.; Cao, Y.L.; Ma, Z.F.; Huang, Y.H.; Ai, X.P.; Yang, H.X. Prussian Blue Cathode Materials for Sodium-Ion Batteries and Other Ion Batteries. Adv. Energy Mater. 2018, 8, 1702619. [Google Scholar] [CrossRef]
- Chen, J.S.; Li, W.; Mahmood, A.; Pei, Z.X.; Zhou, Z.; Chen, X.C.; Chen, Y. Prussian Blue, Its Analogues and Their Derived Materials for Electrochemical Energy Storage and Conversion. Energy Storage Mater. 2020, 25, 585–612. [Google Scholar] [CrossRef]
- Liu, X.Y.; Cao, Y.; Sun, J. Defect Engineering in Prussian Blue Analogs for High-Performance Sodium-Ion Batteries. Adv. Energy Mater. 2022, 12, 2202532. [Google Scholar] [CrossRef]
- Xiang, X.D.; Zhang, K.; Chen, J. Recent Advances and Prospects of Cathode Materials for Sodium-Ion Batteries. Adv. Mater. 2015, 27, 5343–5364. [Google Scholar] [CrossRef] [PubMed]
- Senthilkumar, B.; Murugesan, C.; Sharma, L.; Lochab, S.; Barpanda, P. An Overview of Mixed Polyanionic Cathode Materials for Sodium-Ion Batteries. Small Methods 2019, 3, 1800253. [Google Scholar] [CrossRef]
- He, L.; Li, H.X.; Ge, X.C.; Li, S.H.; Wang, X.; Wang, S.; Zhang, L.Y.; Zhang, Z.A. Iron-Phosphate-Based Cathode Materials for Cost-Effective Sodium-Ion Batteries: Development, Challenges, and Prospects. Adv. Mater. Interfaces 2022, 9, 2200515. [Google Scholar] [CrossRef]
- Niu, Y.B.; Zhang, Y.; Xu, M.W. A Review on Pyrophosphate Framework Cathode Materials for Sodium-Ion Batteries. J. Mater. Chem. A 2019, 7, 15006. [Google Scholar] [CrossRef]
- Yang, W.; Liu, Q.; Zhao, Y.S.; Zhao, Y.S.; Mu, D.B.; Tan, G.Q.; Gao, H.C.; Li, L.; Chen, R.J.; Wu, F. Progress on Fe-Based Polyanionic Oxide Cathodes Materials toward Grid-Scale Energy Storage for Sodium-Ion Batteries. Small Methods 2022, 6, 2200555. [Google Scholar] [CrossRef]
- Chen, J.W.; Adit, G.; Li, L.; Zhang, Y.X.; Cuua, D.H.C.; Lee, P.S. Optimization Strategies Toward Functional Sodium-Ion Batteries. Energy Environ. Mater. 2023, 6, e12633. [Google Scholar] [CrossRef]
- Kim, H.; Kim, H.; Ding, Z.; Lee, M.H.; Lim, K.; Yoon, G.; Kang, K. Recent Progress in Electrode Materials for Sodium-Ion Batteries. Adv. Energy Mater. 2016, 6, 1600943. [Google Scholar] [CrossRef]
- Ren, H.X.; Li, Y.; Ni, Q.; Bai, Y.; Zhao, H.C.; Wu, C. Unraveling Anionic Redox for Sodium Layered Oxide Cathodes: Breakthroughs and Perspectives. Adv. Mater. 2022, 34, 2106171. [Google Scholar] [CrossRef]
- Yu, Y.; Ning, D.; Li, Q.Y.; Franz, A.; Zheng, L.R.; Zhang, N.; Ren, G.X.; Schumacher, G.; Liu, X.F. Revealing the Anionic Redox Chemistry in O3-Type Layered Oxide Cathode for Sodium-Ion Batteries. Energy Storage Mater. 2021, 38, 130–140. [Google Scholar] [CrossRef]
- Sun, C.; Zhang, L.L.; Deng, Z.R.; Yan, B.; Gao, L.; Yang, X.L. PTFE-Derived Carbon-Coated Na3V2(PO4)2F3 Cathode Material for High-Performance Sodium Ion Battery. Electrochim. Acta 2022, 432, 141187. [Google Scholar] [CrossRef]
- Tang, L.B.; Liu, X.H.; Li, Z.; Pu, X.M.; Zhang, J.H.; Xu, Q.J.; Liu, H.M.; Wang, Y.G.; Xia, Y.Y. CNT-Decorated Na4Mn2Co(PO4)2P2O7 Microspheres as a Novel High-Voltage Cathode Material for Sodium-Ion Batteries. ACS Appl. Mater. Interfaces 2019, 11, 27813–27822. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.L.; Liu, J.; Wei, C.; Sun, P.P.; Gao, L.; Ding, X.K.; Liang, G.; Yang, X.L.; Huang, Y.H. N/P-Dual-Doped Carbon-Coated Na3V2(PO4)2O2F Microspheres as a High-Performance Cathode Material for Sodium-Ion Batteries. ACS Appl. Mater. Interfaces 2020, 12, 3670–3680. [Google Scholar] [CrossRef]
- Zhang, Y.X.; Liu, G.Q.; Su, C.; Liu, G.Y.; Suun, H.D.; Qiao, D.L.; Wen, L. Study on the Influence of Cu/F dual-doping on the Fe-Mn Based Compound as Cathode Material for Sodium Ion Batteries. J. Power Sources 2022, 536, 231511. [Google Scholar] [CrossRef]
- Liu, Z.B.; Zhou, C.J.; Liu, J.; Yang, L.C.; Liu, J.W.; Zhu, M. Phase Tuning of P2/O3-Type Layered Oxide Cathode for Sodium Ion Batteries via a Simple Li/F Co-Doping Route. Chem. Eng. J. 2022, 431, 134273. [Google Scholar] [CrossRef]
- Fan, Y.; Ye, X.C.; Yang, X.F.; Guan, L.Y.; Chen, C.H.; Wang, H.; Ding, X. Zn/Ti/F Synergetic-Doped Na0.67Ni0.33Mn0.67O2 for Sodium-Ion Batteries with High Energy Density. J. Mater. Chem. A 2023, 11, 3608. [Google Scholar] [CrossRef]
- Zhang, Z.S.; Liu, Y.Q.; Liu, Z.X.; Li, H.L.; Huang, Y.L.; Liu, W.J.; Ruan, D.S.; Cai, X.; Yu, X.Y. Dual-Strategy of Cu-Doping and O3 Biphasic Structure Enables Fe/Mn-Based Layered Oxide for High-Performance Sodium-Ion Batteries Cathode. J. Power Sources 2023, 567, 232930. [Google Scholar] [CrossRef]
- Sun, Y.; Wang, H.; Meng, D.C.; Li, X.Q.; Liao, X.Z.; Che, H.Y.; Cui, G.J.; Yu, F.P.; Yang, W.M.; Li, L.S.; et al. Degradation Mechanism of O3-Type NaNi1/3Fe1/3Mn1/3O2 Cathode Materials During Ambient Storage and Their In Situ Regeneration. ACS Appl. Energy Mater. 2021, 4, 2061–2067. [Google Scholar] [CrossRef]
- Li, R.R.; Gao, J.; Li, J.P.; Huang, H.; Li, X.L.; Wang, W.L.; Zheng, L.R.; Hao, S.M.; Qiu, J.S.; Zhou, W.D. An Undoped Tri-Phase Coexistent Cathode Material for Sodium-Ion Batteries. Adv. Funct. Mater. 2022, 32, 2205661. [Google Scholar] [CrossRef]
- Liu, X.H.; Zhao, J.H.; Dong, H.H.; Zhang, L.L.; Zhang, H.; Gao, Y.; Zhou, X.Z.; Zhang, L.H.; Li, L.; Liu, Y.; et al. Sodium Difluoro (Oxalato) Borate Additive-Induced Robust SEI and CEI Layers Enable Dendrite-Free and Long-Cycling Sodium-Ion Batteries. Adv. Funct. Mater. 2024, 34, 2402310. [Google Scholar] [CrossRef]
- Zhang, J.Y.; Ma, S.Y.; Zhang, J.H.; Zhang, J.; Wang, X.; Wen, L.F.; Tang, G.C.; Hu, M.X.; Wang, E.X.; Chen, W.H. Critical Review on Cathode Electrolyte Interphase Towards Stabilization for Sodium-Ion Batteries. Nano Energy 2024, 128, 109814. [Google Scholar] [CrossRef]
- Cheng, F.Y.; Hu, J.; Zhang, W.; Guo, B.Y.; Yu, P.; Sun, X.L.; Peng, J. Reviving Ether-Based Electrolytes for Sodium-Ion Batteries. Energy Environ. Sci. 2025, 18, 6874. [Google Scholar] [CrossRef]
- Cui, Y.W.; Ni, Y.X.; Wang, Y.K.; Wang, L.Y.; Yang, W.X.; Wu, S.; Xie, W.W.; Zhang, K.; Yan, Z.H.; Chen, J. A Temperature-Adapted Ultraweakly Solvating Electrolyte for Cold-Resistant Sodium-Ion Batteries. Adv. Energy Mater. 2025, 15, 2405363. [Google Scholar]
- Liu, Y.M.; Zhu, L.J.; Wang, E.H.; An, Y.; Liu, Y.T.; Shen, K.E.; He, M.X.; Jia, Y.F.; Ye, G.; Xiao, Z.T.; et al. Electrolyte Engineering with Tamed Electrode Interphases for High-Voltage Sodium-Ion Batteries. Adv. Mater. 2024, 36, 2310051. [Google Scholar] [CrossRef] [PubMed]
- Zheng, X.Y.; Cao, Z.; Gu, Z.Y.; Huang, L.Q.; Sun, Z.H.; Zhao, T.; Yu, S.J.; Wu, X.-L.; Luo, W.; Huang, Y.H. Toward High Temperature Sodium Metal Batteries via Regulating the Electrolyte/Electrode Interfacial Chemistries. ACS Energy Lett. 2022, 7, 2032–2042. [Google Scholar] [CrossRef]
- Dai, P.; Shi, C.-G.; Huang, Z.; Wu, X.-H.; Deng, Y.-P.; Fu, J.; Xie, Y.X.; Fan, J.J.; Shen, S.Y.; Shen, C.-H.; et al. A New Film-forming Electrolyte Additive in Enhancing the Interface of Layered Cathode and Cycling Life of Sodium Ion Batteries. Energy Storage Mater. 2023, 56, 551–561. [Google Scholar] [CrossRef]
- Che, H.Y.; Yang, X.R.; Wang, H.; Liao, X.-Z.; Zhang, S.S.; Wang, C.S.; Ma, Z.-F. Long Cycle Life of Sodium-Ion Pouch Cell Achieved by Using Multiple Electrolyte Additives. J. Power Sources 2018, 407, 173–179. [Google Scholar] [CrossRef]
- Wu, S.L.; Su, B.Z.; Ni, K.; Pan, F.; Wang, C.L.; Zhang, K.L.; Yu, D.Y.; Zhu, Y.W.; Zhang, W.J. Fluorinated Carbonate Electrolyte with Superior Oxidative Stability Enables Long-Term Cycle Stability of Na2/3Ni1/3Mn2/3O2 Cathodes in Sodium-Ion Batteries. Adv. Energy Mater. 2021, 11, 2002737. [Google Scholar] [CrossRef]
- Wu, M.L.; Zhang, B.; Ye, Y.H.; Fu, L.; Xie, H.L.; Jin, H.Z.; Tang, Y.G.; Wang, H.Y.; Sun, D. Anion-Induced Uniform and Robust Cathode-Electrolyte Interphase for Layered Metal Oxide Cathodes of Sodium Ion Batteries. ACS Appl. Mater. Interfaces 2024, 16, 15586–15595. [Google Scholar] [CrossRef]
- Fan, J.J.; Dai, P.; Shi, C.G.; Wen, Y.F.; Luo, C.X.; Yang, J.; Song, C.; Huang, L.; Sun, S.G. Synergistic Dual-Additive Electrolyte for Interphase Modification to Boost Cyclability of Layered Cathode for Sodium Ion Batteries. Adv. Funct. Mater. 2021, 31, 2010500. [Google Scholar] [CrossRef]
- Liu, X.; Zhang, M.; Ping, R.; Chen, Y.; Hu, X. A Study on the Effects of Vinylene Carbonate and Fluoroethylene Carbonate on Thermal Runaway in Lithium-Ion Batteries. J. Power Sources 2025, 649, 237428. [Google Scholar] [CrossRef]
- Zhang, Q.M.; Wang, Z.X.; Li, X.H.; Guo, H.J.; Peng, W.J.; Wang, J.X.; Yan, G.C. Comparative Study of 1,3-Propane Sultone, Prop-1-Ene-1,3-Sultone and Ethylene Sulfate as Film-Forming Additives for Sodium Ion Batteries. J. Power Sources 2022, 541, 231726. [Google Scholar] [CrossRef]
- Nimkar, A.; Shpigel, N.; Malchik, F.; Bublil, S.; Fan, T.J.; Penki, T.R.; Tsubery, M.N.; Aurbach, D. Unraveling the Role of Fluorinated Alkyl Carbonate Additives in Improving Cathode Performance in Sodium-Ion Batteries. ACS Appl. Mater. Interfaces 2021, 13, 46478–46487. [Google Scholar] [CrossRef]
- She, S.X.; Zhou, Y.F.; Hong, Z.J.; Huang, Y.H.; Wu, Y.J. Effect of Fluoroethylene Carbonate Electrolyte Additives on the Electrochemical Performance of Nickel-Rich NCM Ternary Cathodes. ACS Appl. Energy Mater. 2023, 6, 7289–7297. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, X.; Yao, Y.; Liu, X. Enhanced Cycling Stability of High-Voltage Sodium-Ion Batteries via DFEC-Driven Fluorinated Interface Engineering. Reactions 2025, 6, 52. https://doi.org/10.3390/reactions6040052
Li X, Yao Y, Liu X. Enhanced Cycling Stability of High-Voltage Sodium-Ion Batteries via DFEC-Driven Fluorinated Interface Engineering. Reactions. 2025; 6(4):52. https://doi.org/10.3390/reactions6040052
Chicago/Turabian StyleLi, Xin, Yali Yao, and Xinying Liu. 2025. "Enhanced Cycling Stability of High-Voltage Sodium-Ion Batteries via DFEC-Driven Fluorinated Interface Engineering" Reactions 6, no. 4: 52. https://doi.org/10.3390/reactions6040052
APA StyleLi, X., Yao, Y., & Liu, X. (2025). Enhanced Cycling Stability of High-Voltage Sodium-Ion Batteries via DFEC-Driven Fluorinated Interface Engineering. Reactions, 6(4), 52. https://doi.org/10.3390/reactions6040052






