TPA and PET Photo-Degradation by Heterogeneous Catalysis Using a (Al2O3)0.75TiO2 Coating
Abstract
1. Introduction
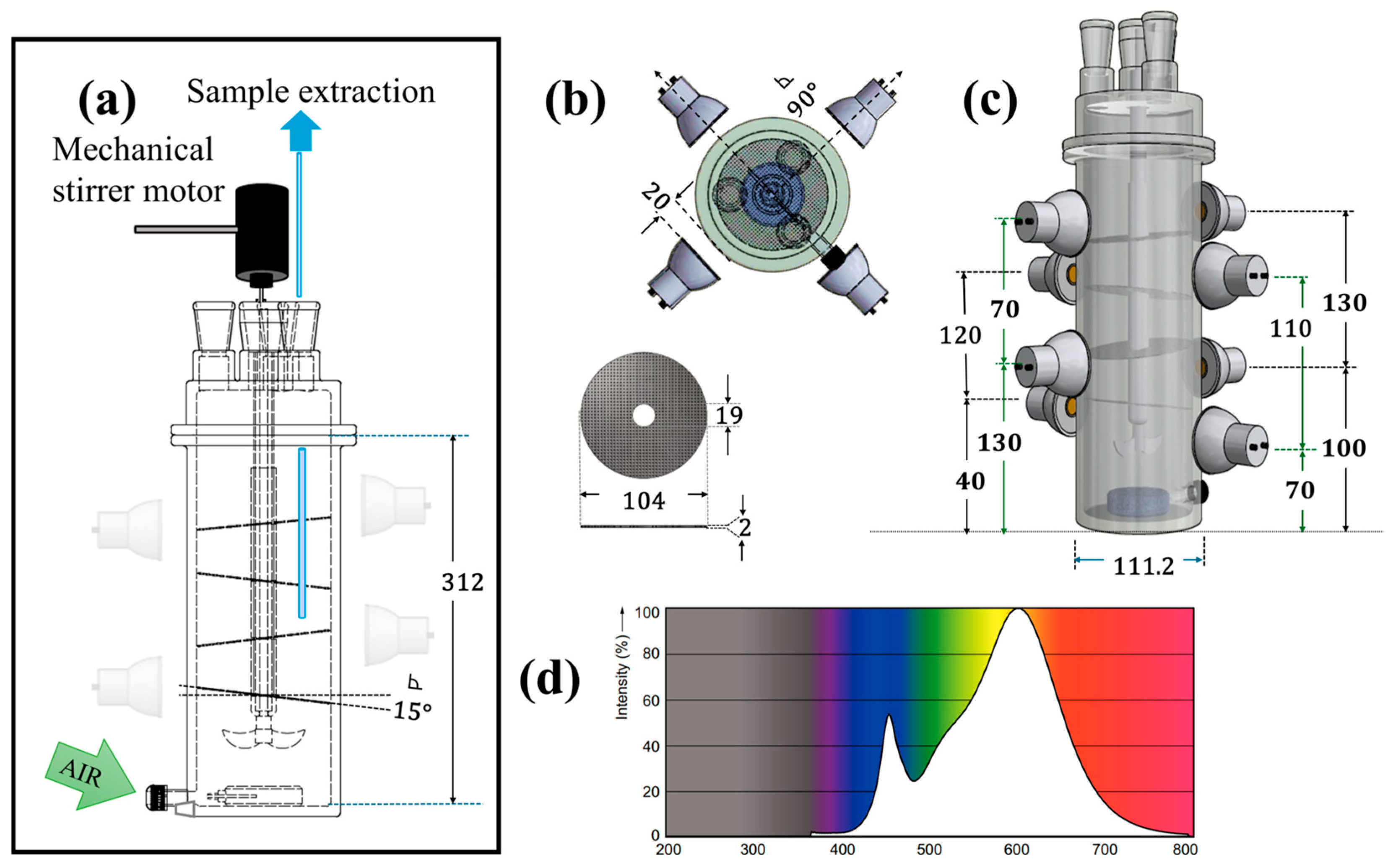
2. Materials and Methods
2.1. Coating Preparation by the Sol-Gel Route
2.2. Structural Characterization Measurements
2.3. Photocatalytic Degradation
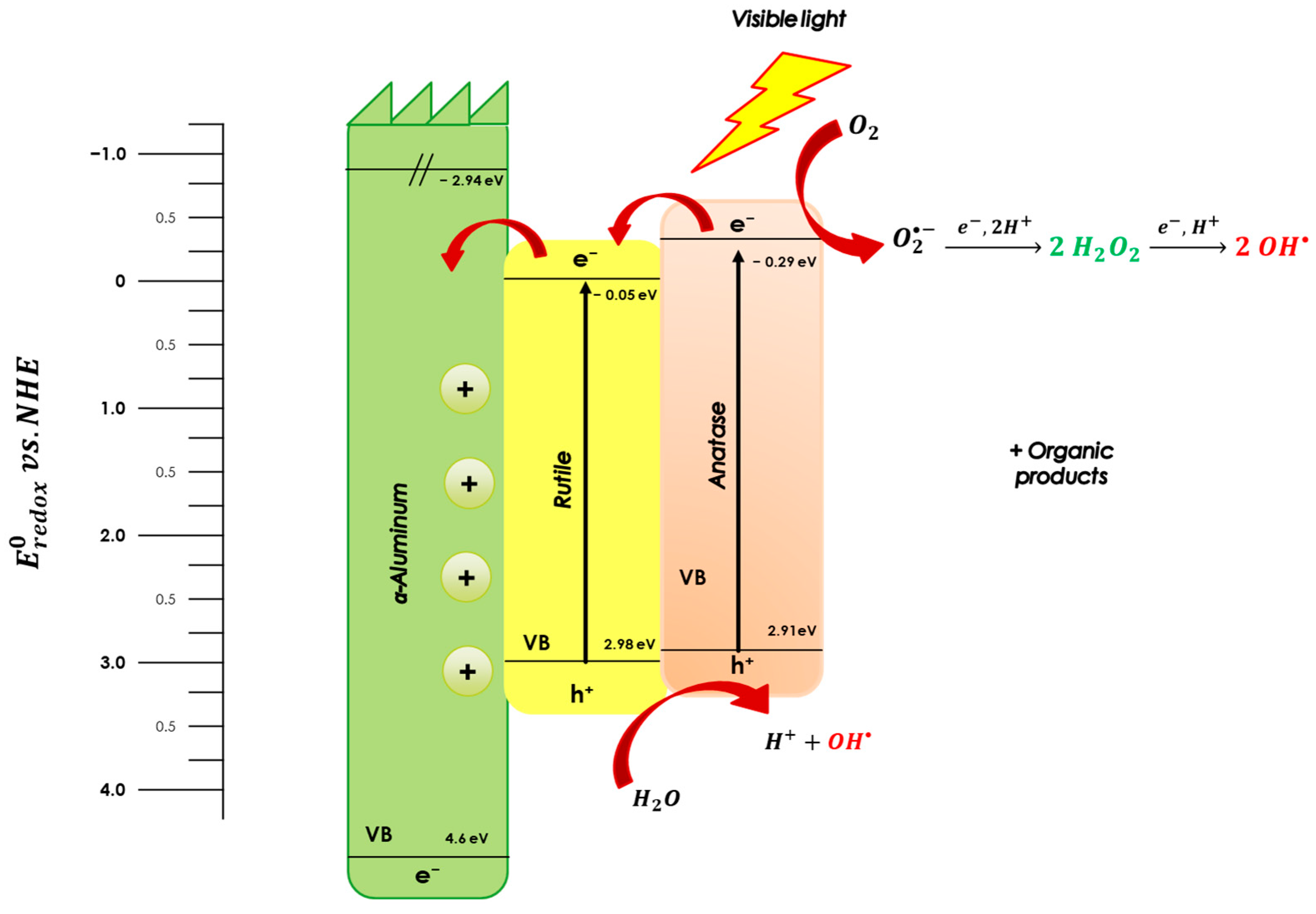
3. Results and Discussion
3.1. (Al2O3)0.75TiO2 Coating Characterization
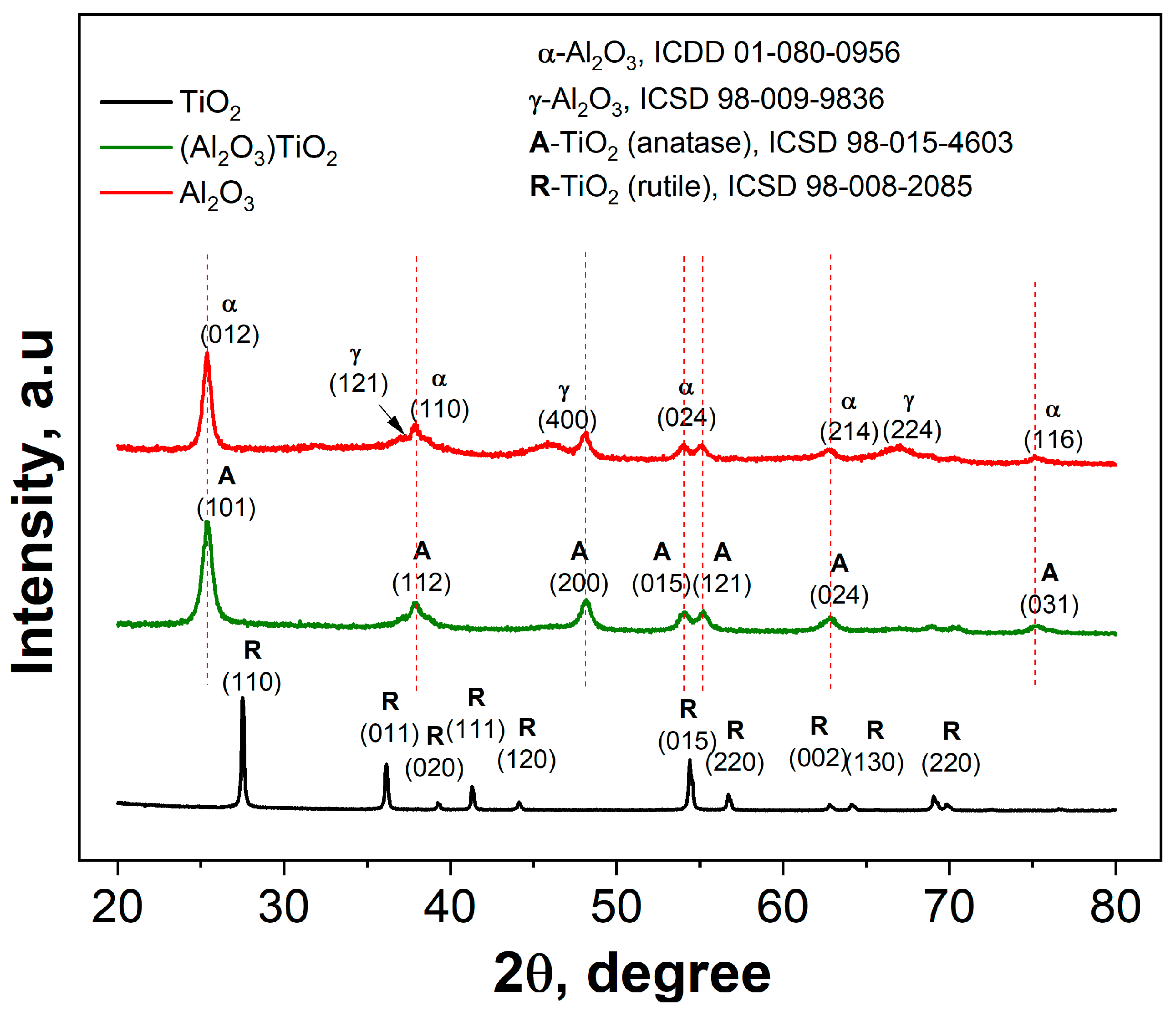
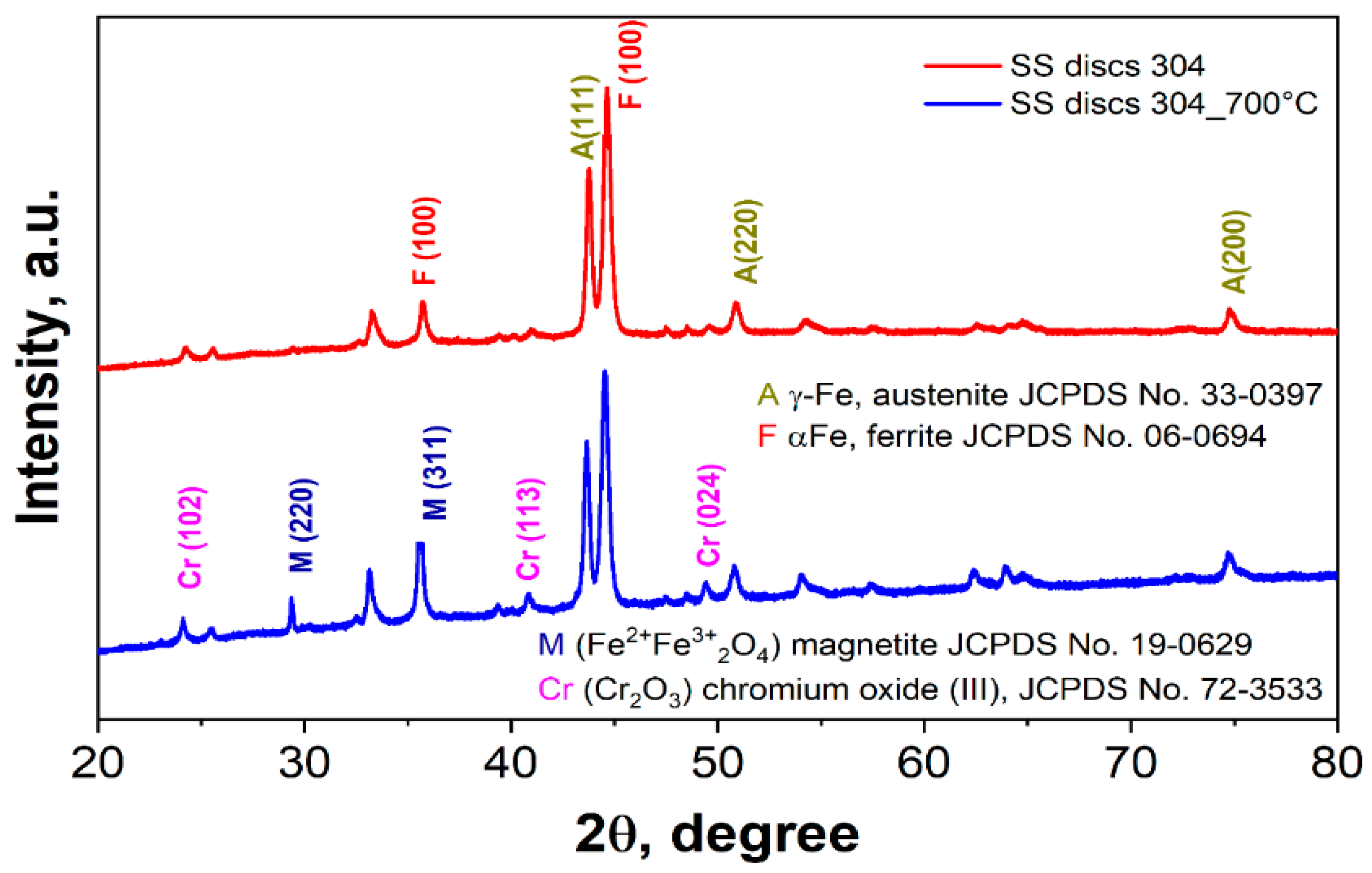
3.1.1. X-Ray Diffraction
3.1.2. Energy Dispersive Spectroscopy: Elemental Analysis
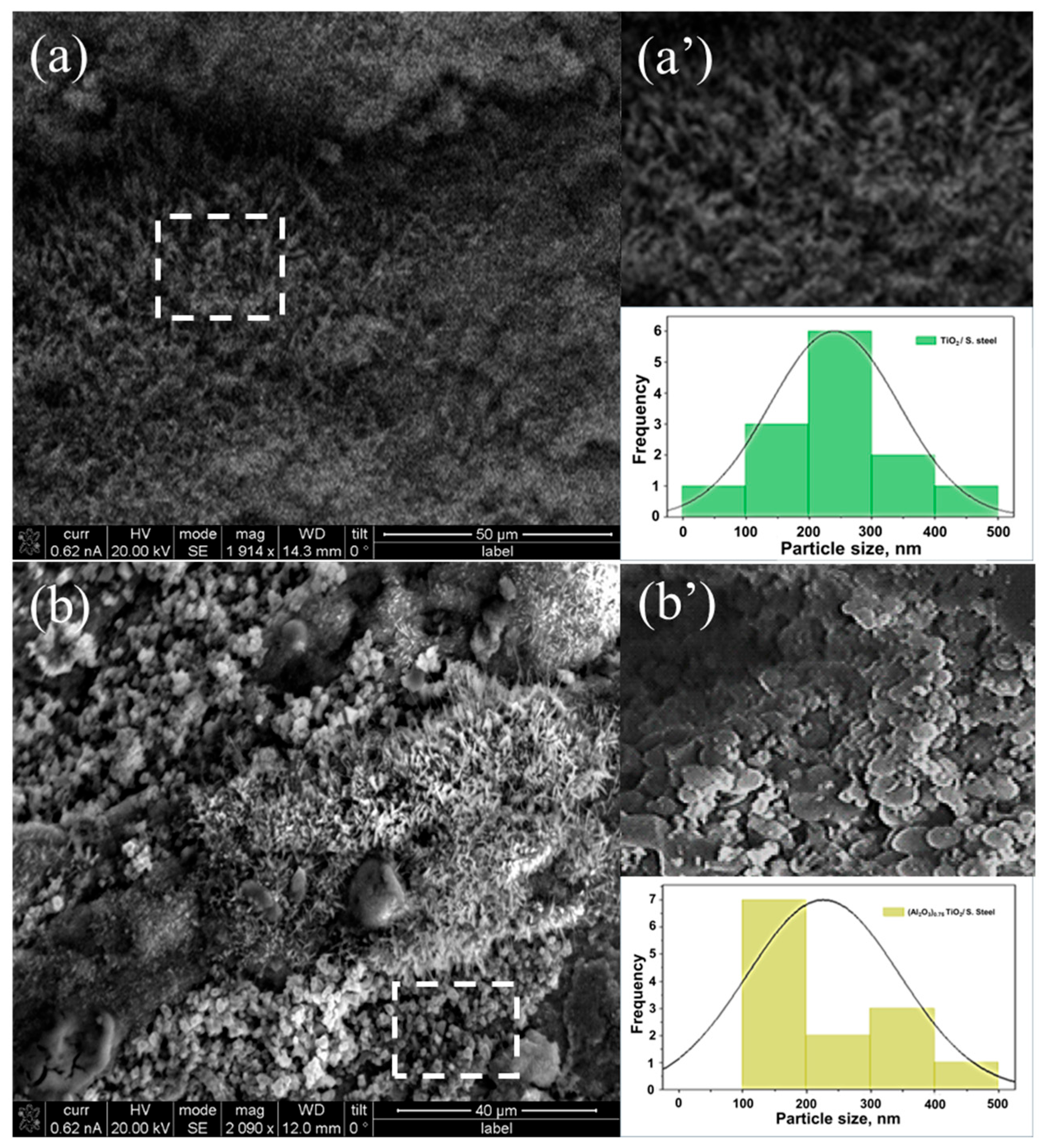
3.1.3. Scanning Electron Microscopy, Morphology and Particle Size
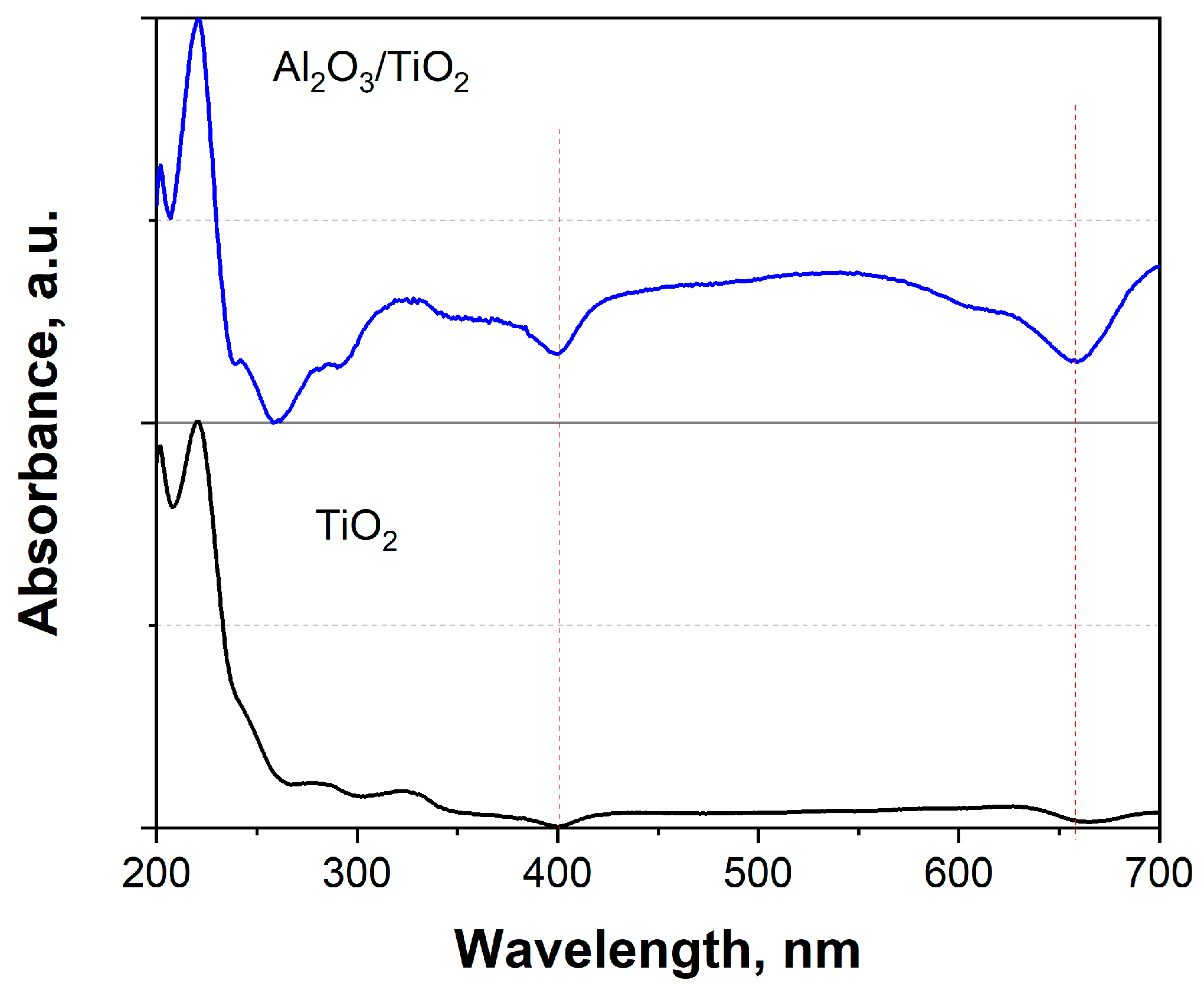
3.1.4. UV–Visible Spectroscopy: Optical Properties and Determination of the Forbidden Band Energy
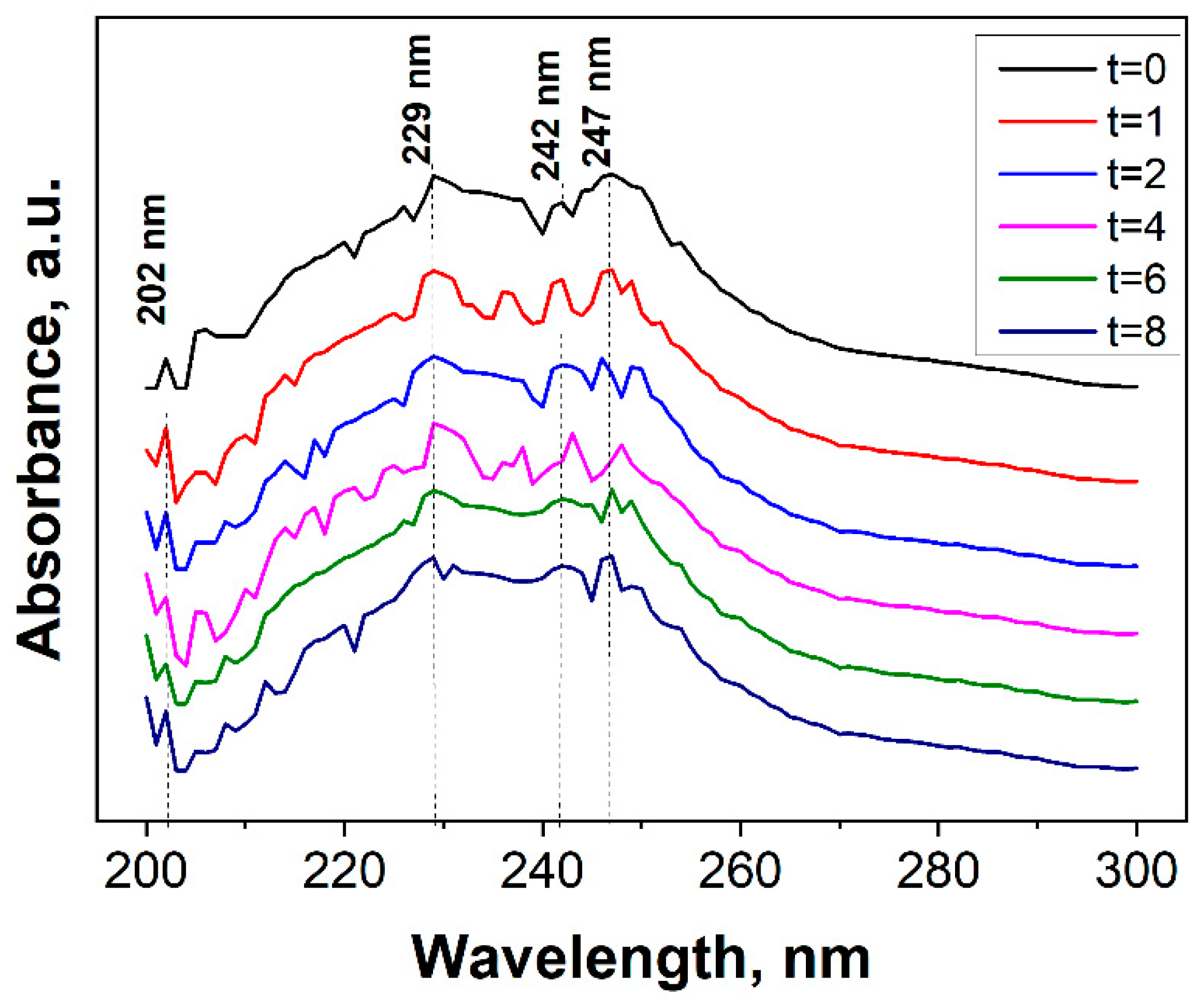
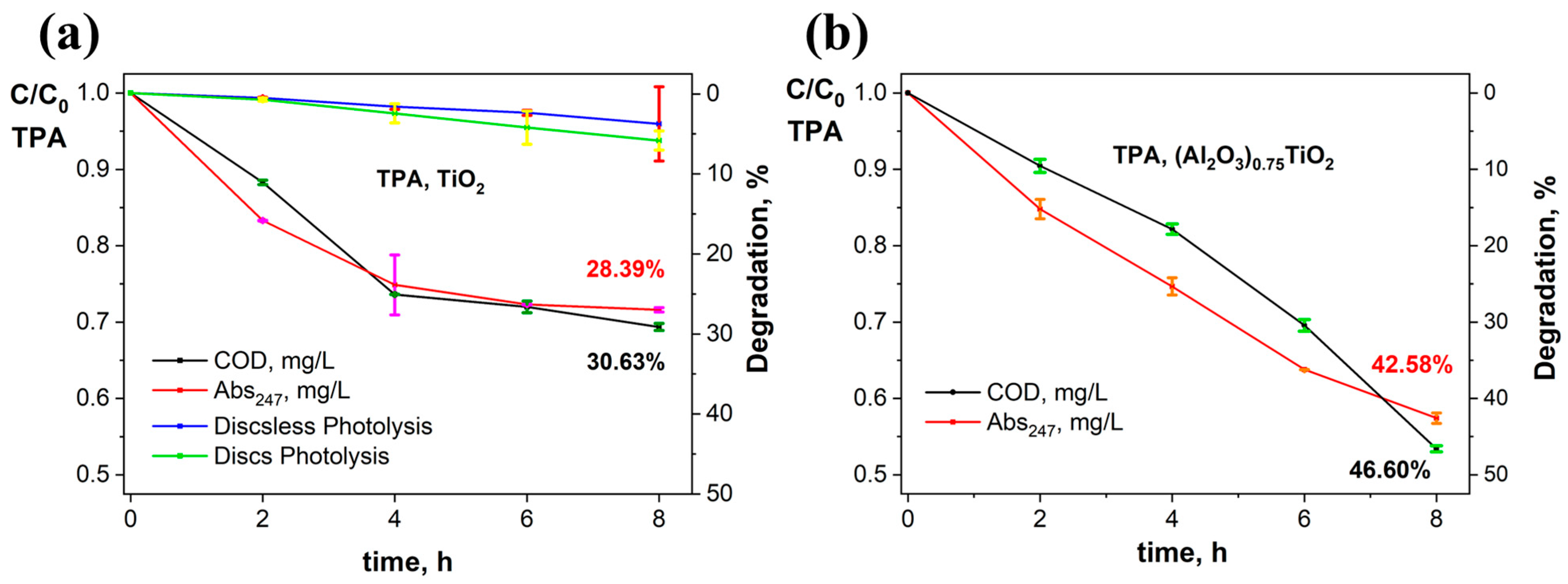
3.2. Evaluation of the Photocatalytic Efficiency in the Degradation of Terephthalic Acid by the Immobilized (Al2O3)0.75TiO2 System
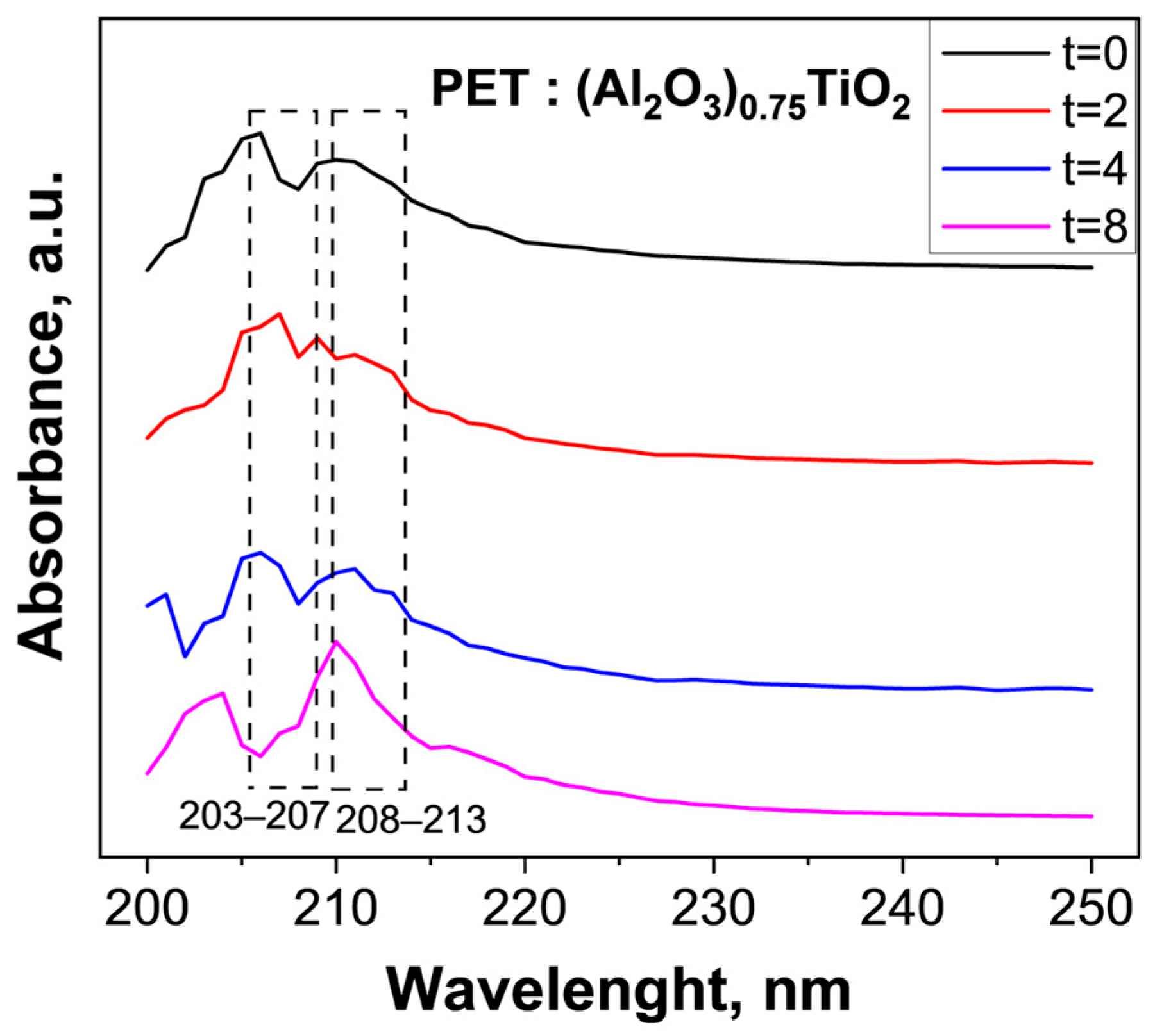
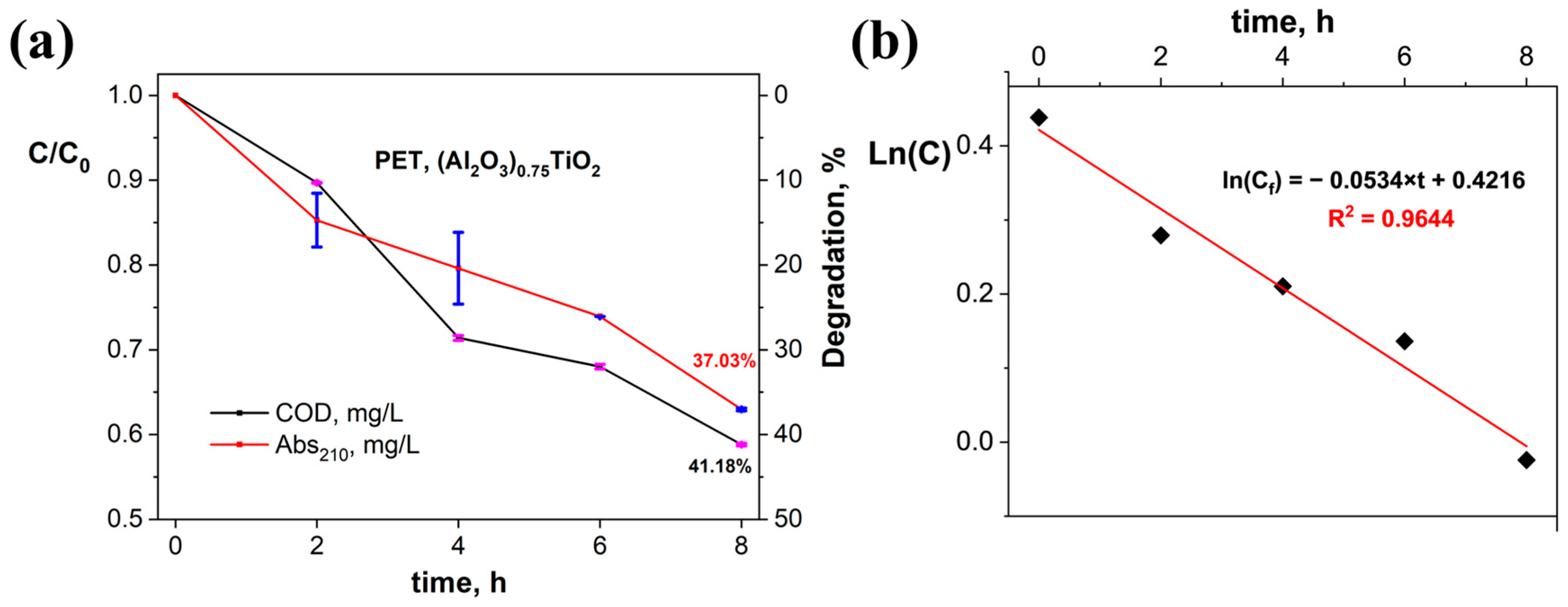
3.3. Evaluation of the Photocatalytic Efficiency of PET Degradation by the Immobilized (Al2O3)0.75TiO2 System
3.4. ANOVA Analysis
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Peng, Y.; Yang, J.; Deng, C.; Deng, J.; Shen, L.; Fu, Y. Acetolysis of waste polyethylene terephthalate for upcycling and life-cycle assessment study. Nat. Commun. 2023, 14, 3249. [Google Scholar] [CrossRef] [PubMed]
- Fang, P.; Liu, B.; Xu, J.; Zhou, Q.; Zhang, S.; Ma, J.; Lu, X. High-efficiency glycolysis of poly(ethylene terephthalate) by sandwich-structure polyoxometalate catalyst with two active sites. Polym. Degrad. Stab. 2018, 156, 22–31. [Google Scholar] [CrossRef]
- Xiao, Y.; Luo, W.P.; Zhang, X.Y.; Guo, C.C.; Liu, Q.; Jiang, G.F.; Li, Q.H. Aerobic Oxidation of p-Toluic Acid to Terephthalic Acid over T(p-Cl)PPMnCl/Co(OAc)2 Under Moderate Conditions. Catal. Lett. 2010, 134, 155–161. [Google Scholar] [CrossRef]
- Zhang, H.; Wang, Y.; Li, X.; Deng, K.; Yu, H.; Xu, Y.; Wang, H.; Wang, Z.; Wang, L. Electrocatalytic upcycling of polyethylene terephthalate plastic to formic acid coupled with energy-saving hydrogen production over hierarchical Pd-doped NiTe nanoarrays. Appl. Catal. B Environ. 2024, 340, 123236. [Google Scholar] [CrossRef]
- Ma, K.; Li, X.; Bao, L.; Li, X.; Cui, Y. The performance and bacterial community shifts in an anaerobic-aerobic process treating purified terephthalic acid wastewater under influent composition variations and ambient temperatures. J. Clean. Prod. 2020, 276, 124190. [Google Scholar] [CrossRef]
- Billings, A.; Jones, K.C.; Pereira, M.G.; Spurgeon, D.J. Plasticisers in the terrestrial environment: Sources, occurrence and fate. Environ. Chem. 2021, 18, 111–130. [Google Scholar] [CrossRef]
- Chang, B.V.; Yang, C.M.; Cheng, C.H.; Yuan, S.Y. Biodegradation of phthalate esters by two bacteria strains. Chemosphere 2004, 55, 533–538. [Google Scholar] [CrossRef]
- Kleerebezem, R.; Beckers, J.; Hulshoff Pol, L.W.; Lettinga, G. High rate treatment of terephthalic acid production wastewater in a two-stage anaerobic bioreactor. Biotechnol. Bioeng. 2005, 91, 169–179. [Google Scholar] [CrossRef]
- Sannino, D.; Vaiano, V.; Sacco, O.; Morante, N.; De Guglielmo, L.; Di Capua, G.; Femia, N. Visible Light Driven Degradation of Terephthalic Acid: Optimization of Energy Demand by Light Modulation Techniques. J. Photocatal. 2021, 2, 49–61. [Google Scholar] [CrossRef]
- Li, H.; Zhang, W.; Xia, D.; Ye, L.; Ma, W.; Li, H.; Li, Q.; Wang, Y. Improved anaerobic degradation of purified terephthalic acid wastewater by adding nanoparticles or co-substrates to facilitate the electron transfer process. Environ. Sci. Nano 2022, 9, 1011–1024. [Google Scholar] [CrossRef]
- Muñoz Sierra, J.D.; Lafita, C.; Gabaldón, C.; Spanjers, H.; van Lier, J.B. Trace metals supplementation in anaerobic membrane bioreactors treating highly saline phenolic wastewater. Bioresour. Technol. 2017, 234, 106–114. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Wu, J.; Li, R.; Kim, D.-H.; Bi, X.; Zhang, G.; Jiang, B.; Yong Ng, H.; Shi, X. Novel intertidal wetland sediment-inoculated moving bed biofilm reactor treating high-salinity wastewater: Metagenomic sequencing revealing key functional microorganisms. Bioresour. Technol. 2022, 348, 126817. [Google Scholar] [CrossRef]
- Ma, X.; Li, X.; Wang, X.; Liu, G.; Zuo, J.; Wang, S.; Wang, K. Impact of salinity on anaerobic microbial community structure in high organic loading purified terephthalic acid wastewater treatment system. J. Hazard. Mater. 2020, 383, 121132. [Google Scholar] [CrossRef]
- Zhang, H.; Tian, L.; Zhang, Z.; Han, J.; Wu, Z.; Wei, Z.; Wang, S.; Cao, Y.; Zhang, S.; Zhang, Y. Effective degradation of phenol by in-situ photocatalytic-Fenton-like technology with BiVO4/Bi2WO6/Ti3C2 QDs. Surf. Interfaces 2024, 55, 105315. [Google Scholar] [CrossRef]
- Fuentes, I.; Julia, L.R.; Hugo, T.; José, M.R.-H.; Isaac, C.; Poznyak, T. Terephthalic acid decomposition by photocatalytic ozonation with VxOy/ZnO under different UV-A LEDs distributions. Chem. Eng. Commun. 2020, 207, 263–277. [Google Scholar] [CrossRef]
- Zhu, Y.; Luo, H.; Wang, T.; Zhou, J.; Zhu, Y.; Hou, J. Advanced treatment of landfill leachate by catalytic ozonation with MnCeOx/γ-Al2O3 catalyst. Surf. Interfaces 2024, 46, 104113. [Google Scholar] [CrossRef]
- Jin, Y.; Gao, Y.; Pang, D.; Li, J.; Wang, X.; Chen, G.; Wang, W.; Mao, Y. Fe3C-modified carbon composites for boosted peroxymonosulfate activation and organic pollutant decontamination: Contributions of dual radical and nonradical pathways. Surf. Interfaces 2024, 46, 104085. [Google Scholar] [CrossRef]
- Žerjav, G.; Albreht, A.; Vovk, I.; Pintar, A. Revisiting terephthalic acid and coumarin as probes for photoluminescent determination of hydroxyl radical formation rate in heterogeneous photocatalysis. Appl. Catal. A Gen. 2020, 598, 117566. [Google Scholar] [CrossRef]
- Sacco, O.; Vaiano, V.; Rizzo, L.; Sannino, D. Photocatalytic activity of a visible light active structured photocatalyst developed for municipal wastewater treatment. J. Clean. Prod. 2018, 175, 38–49. [Google Scholar] [CrossRef]
- Jiang, Q.; Liu, J.; Qi, T.; Liu, Y. Enhanced visible-light photocatalytic activity and antibacterial behaviour on fluorine and graphene synergistically modified TiO2 nanocomposite for wastewater treatment. Environ. Technol. 2022, 43, 3821–3834. [Google Scholar] [CrossRef]
- Merino, N.; Qu, Y.; Deeb, R.A.; Hawley, E.L.; Hoffmann, M.R.; Mahendra, S. Degradation and Removal Methods for Perfluoroalkyl and Polyfluoroalkyl Substances in Water. Environ. Eng. Sci. 2016, 33, 615–649. [Google Scholar] [CrossRef]
- Byrne, C.; Subramanian, G.; Pillai, S.C. Recent advances in photocatalysis for environmental applications. J. Environ. Chem. Eng. 2018, 6, 3531–3555. [Google Scholar] [CrossRef]
- Al Miad, A.; Saikat, S.P.; Alam, M.K.; Sahadat Hossain, M.; Bahadur, N.M.; Ahmed, S. Metal oxide-based photocatalysts for the efficient degradation of organic pollutants for a sustainable environment: A review. Nanoscale Adv. 2024, 6, 4781–4803. [Google Scholar] [CrossRef]
- Krishnan, A.; Swarnalal, A.; Das, D.; Krishnan, M.; Saji, V.S.; Shibli, S.M.A. A review on transition metal oxides based photocatalysts for degradation of synthetic organic pollutants. J. Environ. Sci. 2024, 139, 389–417. [Google Scholar] [CrossRef]
- Wang, Y.; Xu, M.; Li, J.; Zhang, T. Photocatalytic Degradation of Organic Pollutants Using Al/TiO2 Composites Under Visible Light. Environ. Eng. Sci. 2024, 41, 204–215. [Google Scholar] [CrossRef]
- Ran, Y.; Zhong, J.; Li, J.; Li, M.; Tian, C. Substantially boosted photocatalytic detoxification activity of TiO2 benefited from Eu doping. Environ. Technol. 2023, 44, 1313–1321. [Google Scholar] [CrossRef]
- Zhang, Q.; Li, R.; Li, Z.; Li, A.; Wang, S.; Liang, Z.; Liao, S.; Li, C. The dependence of photocatalytic activity on the selective and nonselective deposition of noble metal cocatalysts on the facets of rutile TiO2. J. Catal. 2016, 337, 36–44. [Google Scholar] [CrossRef]
- Yakdoumi, F.Z.; Hadj-Hamou, A.S. Effectiveness assessment of TiO2-Al2O3 nano-mixture as a filler material for improvement of packaging performance of PLA nanocomposite films. J. Polym. Eng. 2020, 40, 848–858. [Google Scholar] [CrossRef]
- Karunakaran, C.; Magesan, P.; Gomathisankar, P.; Vinayagamoorthy, P. Absorption, emission, charge transfer resistance and photocatalytic activity of Al2O3/TiO2 core/shell nanoparticles. Superlattices Microstruct. 2015, 83, 659–667. [Google Scholar] [CrossRef]
- Magnone, E.; Kim, M.K.; Lee, H.J.; Park, J.H. Facile synthesis of TiO2-supported Al2O3 ceramic hollow fiber substrates with extremely high photocatalytic activity and reusability. Ceram. Int. 2021, 47, 7764–7775. [Google Scholar] [CrossRef]
- Martinez-Gómez, C.; Rangel-Vazquez, I.; Zarraga, R.; del Ángel, G.; Ruíz-Camacho, B.; Tzompantzi, F.; Vidal-Robles, E.; Perez-Larios, A. Photodegradation and Mineralization of Phenol Using TiO2Coated γ-Al2O3: Effect of Thermic Treatment. Processes 2022, 10, 1186. [Google Scholar] [CrossRef]
- Zhu, Y.; Ning, Y.; Li, L.; Chen, Z.; Li, H.; Zhang, Y. Effective removal of hexavalent chromium from aqueous system by biochar-supported titanium dioxide (TiO2). Environ. Chem. 2022, 19, 432–445. [Google Scholar] [CrossRef]
- Kanakaraju, D.; Motti, C.A.; Glass, B.D.; Oelgemöller, M. Photolysis and TiO2-catalysed degradation of diclofenac in surface and drinking water using circulating batch photoreactors. Environ. Chem. 2014, 11, 51–62. [Google Scholar] [CrossRef]
- Zhuang, Q.; Shi, K.; Wang, J.; Zhou, H.; Zhao, P.; Lou, Y. Revolutionizing pollution control with innovative CuO@TiO2 nanocomposite for enhanced photocatalytic degradation and antimicrobial efficacy. Surf. Interfaces 2024, 55, 105410. [Google Scholar] [CrossRef]
- Yu, X.; Wang, S.; Zhang, Y.; Yu, X.; Zhao, X.; Gao, H.; Yang, H.; Fang, L.; Syed, A. Biochar coupling induced charge transfer switching of a NiAl2O4/NiCr2O4 photocatalyst to enhance photocatalytic degradation of tetracycline hydrochloride. Surf. Interfaces 2024, 54, 105222. [Google Scholar] [CrossRef]
- Duan, L.; Wang, B.; Heck, K.; Guo, S.; Clark, C.A.; Arredondo, J.; Wang, M.; Senftle, T.P.; Westerhoff, P.; Wen, X.; et al. Efficient Photocatalytic PFOA Degradation over Boron Nitride. Environ. Sci. Technol. Lett. 2020, 7, 613–619. [Google Scholar] [CrossRef]
- Samy, M.; Ibrahim, M.G.; Fujii, M.; Diab, K.E.; ElKady, M.; Gar Alalm, M. CNTs/MOF-808 painted plates for extended treatment of pharmaceutical and agrochemical wastewaters in a novel photocatalytic reactor. Chem. Eng. J. 2021, 406, 127152. [Google Scholar] [CrossRef]
- Ibarra, I.C.R.; Dubé, I.Z.; Sánchez, M.F.G. 1. MX2019002787—Reactor Fotocatalítico para la Degradación de Contaminantes Orgánicos a Presiones por Encima de los Valores Ambientales. 2019002787. 2020. Available online: https://www.wipo.int/ipcpub/?symbol=B01J0003000000&menulang=es&lang=es (accessed on 1 August 2024).
- Camacho-González, M.A.; Lijanova, I.V.; Reyes-Miranda, J.; Sarmiento-Bustos, E.; Quezada-Cruz, M.; Vera-Serna, P.; Barrón-Meza, M.Á.; Garrido-Hernández, A. High Photocatalytic Efficiency of Al2O3-TiO2 Coatings on 304 Stainless Steel for Methylene Blue and Wastewater Degradation. Catalysts 2023, 13, 1351. [Google Scholar] [CrossRef]
- Habibi, S.; Jamshidi, M. Sol–gel synthesis of carbon-doped TiO2 nanoparticles based on microcrystalline cellulose for efficient photocatalytic degradation of methylene blue under visible light. Environ. Technol. 2020, 41, 3233–3247. [Google Scholar] [CrossRef]
- Farhadian Azizi, K.; Bagheri-Mohagheghi, M.-M. Transition from anatase to rutile phase in titanium dioxide (TiO2) nanoparticles synthesized by complexing sol–gel process: Effect of kind of complexing agent and calcinating temperature. J. Sol-Gel Sci. Technol. 2013, 65, 329–335. [Google Scholar] [CrossRef]
- Zhu, L.; Lu, Q.; Lv, L.; Wang, Y.; Hu, Y.; Deng, Z.; Lou, Z.; Hou, Y.; Teng, F. Ligand-free rutile and anatase TiO2 nanocrystals as electron extraction layers for high performance inverted polymer solar cells. RSC Adv. 2017, 7, 20084–20092. [Google Scholar] [CrossRef]
- Sadeq, Z.S.; Mahdi, Z.F.; Hamza, A.M. Low cost, fast and powerful performance interfacial charge transfer nanostructured Al2O3 capturing of light photocatalyst eco-friendly system using hydrothermal method. Mater. Lett. 2019, 254, 120–124. [Google Scholar] [CrossRef]
- Xu, L.; Song, H.; Chou, L. Facile synthesis of nano-crystalline alpha-alumina at low temperature via an absolute ethanol sol–gel strategy. Mater. Chem. Phys. 2012, 132, 1071–1076. [Google Scholar] [CrossRef]
- Askeland, D. Ciencia e Ingeniería de Materiales, 3rd ed.; Thomson, Ed.; Cengage Learning, Inc.: Toronto, ON, Canada, 2012. [Google Scholar]
- Miglierini, M.B.; Pašteka, L.; Cesnek, M.; Kmječ, T.; Bujdoš, M.; Kohout, J. Influence of surface treatment on microstructure of stainless steels studied by Mössbauer spectrometry. J. Radioanal. Nucl. Chem. 2019, 322, 1495–1503. [Google Scholar] [CrossRef]
- Zhang, X.; Yang, S.; Li, J.; Wu, J. Temperature-dependent evolution of oxide inclusions during heat treatment of stainless steel with yttrium addition. Int. J. Miner. Metall. Mater. 2020, 27, 754–763. [Google Scholar] [CrossRef]
- da Trindade, C.d.M.; da Silva, S.W.; Bortolozzi, J.P.; Banús, E.D.; Bernardes, A.M.; Ulla, M.A. Synthesis and characterization of TiO2 films onto AISI 304 metallic meshes and their application in the decomposition of the endocrine-disrupting alkylphenolic chemicals. Appl. Surf. Sci. 2018, 457, 644–654. [Google Scholar] [CrossRef]
- Oladijo, O.P.; Popoola, A.P.I.; Booi, M.; Fayomi, J.; Collieus, L.L. Corrosion and mechanical behaviour of Al2O3.TiO2 composites produced by spark plasma sintering. South Afr. J. Chem. Eng. 2020, 33, 58–66. [Google Scholar] [CrossRef]
- Liu, X.; Wan, Y.; Zhang, X. Preparation and Corrosion Properties of TiO2-SiO2-Al2O3 Composite Coating on Q235 Carbon Steel. Coatings 2023, 13, 1994. [Google Scholar] [CrossRef]
- Brinker, C.J.; Scherer, G.W. Sol-Gel Science: The Physics and Chemistry of Sol-Gel Processing; Academic Press: Cambridge, MA, USA, 2013; ISBN 0080571034. [Google Scholar]
- Tiss, B.; Zayoud, W.; Sekrafi, H.E.; Bouguila, N.; Cristea, D.; Croitoru, C.; Velicu, L.; Tiron, V.; Prepelita, P.; Craciun, V.; et al. Enhancing photocatalysis through annealing: Unveiling the role of physical properties in photocatalytic behavior. Mater. Chem. Phys. 2024, 313, 128665. [Google Scholar] [CrossRef]
- Ahmad, I.; Zou, Y.; Yan, J.; Liu, Y.; Shukrullah, S.; Naz, M.Y.; Hussain, H.; Khan, W.Q.; Khalid, N.R. Semiconductor photocatalysts: A critical review highlighting the various strategies to boost the photocatalytic performances for diverse applications. Adv. Colloid Interface Sci. 2023, 311, 102830. [Google Scholar] [CrossRef]
- Akkaya Arıer, U.O.; Tepehan, F.Z. Influence of Al2O3:TiO2 ratio on the structural and optical properties of TiO2–Al2O3 nano-composite films produced by sol gel method. Compos. Part B Eng. 2014, 58, 147–151. [Google Scholar] [CrossRef]
- Kolesnik, O. UV-Vis Spectrum of Terephthalic Acid. SIELC Technol. Available online: https://sielc.com/uv-vis-spectrum-of-terephthalic-acid (accessed on 1 August 2024).
- Skoog, D.A.; Holler, F.J.; Crouch, S.R. Principles of Instrumental Analysis. Cengage Learning. 2017. Available online: https://books.google.com.mx/books?id=D13EDQAAQBAJ (accessed on 1 August 2024).
- Zielińska-Jurek, A.; Wysocka, I.; Janczarek, M.; Stampor, W.; Hupka, J. Preparation and characterization of Pt–N/TiO2 photocatalysts and their efficiency in degradation of recalcitrant chemicals. Sep. Purif. Technol. 2015, 156, 369–378. [Google Scholar] [CrossRef]
- Agostini, I.; Ciuffi, B.; Gallorini, R.; Rizzo, A.M.; Chiaramonti, D.; Rosi, L. Recovery of Terephthalic Acid from Densified Post-consumer Plastic Mix by HTL Process. Molecules 2022, 27, 7112. [Google Scholar] [CrossRef]
- Shafaei, A.; Nikazar, M.; Arami, M. Photocatalytic degradation of terephthalic acid using titania and zinc oxide photocatalysts: Comparative study. Desalination 2010, 252, 8–16. [Google Scholar] [CrossRef]
- Mahmoodi, N.M.; Arami, M. Bulk phase degradation of Acid Red 14 by nanophotocatalysis using immobilized titanium(IV) oxide nanoparticles. J. Photochem. Photobiol. A Chem. 2006, 182, 60–66. [Google Scholar] [CrossRef]
- Miwa, T.; Kaneco, S.; Katsumata, H.; Suzuki, T.; Ohta, K.; Chand Verma, S.; Sugihara, K. Photocatalytic hydrogen production from aqueous methanol solution with CuO/Al2O3/TiO2 nanocomposite. Int. J. Hydrog. Energy 2010, 35, 6554–6560. [Google Scholar] [CrossRef]
- Ali, I.; Suhail, M.; Alothman, Z.A.; Alwarthan, A. Recent advances in syntheses, properties and applications of TiO2 nanostructures. RSC Adv. 2018, 8, 30125–30147. [Google Scholar] [CrossRef]
- Sotiles, A.R.; Barbosa, L.V.; Vedovato, Y.P.; Wypych, F.; de Faria, E.H. Effect of Zn2Cr layered double hydroxide on photocatalytic hydroxylation of terephthalic acid and photodegradation of sodium diclofenac. Catal. Today 2023, 423, 114287. [Google Scholar] [CrossRef]
- Tawfik, M.; Eskander, S. Recycling of Polyethylene Terephthalate Plastic Wastes based on unsaturated Diol. KGK Rubberpoint 2015, 68, 21–27. [Google Scholar]
- Kulkarni, R.M.; Patwardhan, N.S.; Iyer, P.B.; Bharadwaj, T.D. A review on microbial bioremediation of polyethylene terephthalate microplastics. Environ. Qual. Manag. 2024, 34, e22264. [Google Scholar] [CrossRef]


| Experiment | Sum of Squares | Degree of Freedom | Mean Square | Fvalue | pvalue | Significance |
|---|---|---|---|---|---|---|
| TPA_TiO2_DQO | 12,102.83 | 4 | 3025.71 | 3272.21 | <0.05 | Optimal |
| TPA_TiO2_Abs247 | 0.12 | 4 | 0.03 | 116.60 | <0.05 | Optimal |
| TPA_(Al2O3)0.75TiO2_DQO | 25,757.96 | 4 | 6439.49 | 4164.36 | <0.05 | Optimal |
| TPA_(Al2O3)0.75TiO2_Abs247 | 0.21 | 4 | 0.05 | 1847.31 | <0.05 | Optimal |
| PET_(Al2O3)0.75TiO2_DQO | 308,610.69 | 4 | 77,152.67 | 22,932.53 | <0.05 | Optimal |
| PET_(Al2O3)0.75TiO2_Abs210 | 0.35 | 4 | 0.09 | 126.28 | <0.05 | Optimal |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Camacho-González, M.A.; Hernández-Reyes, A.; Garrido-Hernández, A.; Olivares-Xometl, O.; Likhanova, N.V.; Lijanova, I.V. TPA and PET Photo-Degradation by Heterogeneous Catalysis Using a (Al2O3)0.75TiO2 Coating. Surfaces 2025, 8, 34. https://doi.org/10.3390/surfaces8020034
Camacho-González MA, Hernández-Reyes A, Garrido-Hernández A, Olivares-Xometl O, Likhanova NV, Lijanova IV. TPA and PET Photo-Degradation by Heterogeneous Catalysis Using a (Al2O3)0.75TiO2 Coating. Surfaces. 2025; 8(2):34. https://doi.org/10.3390/surfaces8020034
Chicago/Turabian StyleCamacho-González, Mónica A., Alberto Hernández-Reyes, Aristeo Garrido-Hernández, Octavio Olivares-Xometl, Natalya V. Likhanova, and Irina V. Lijanova. 2025. "TPA and PET Photo-Degradation by Heterogeneous Catalysis Using a (Al2O3)0.75TiO2 Coating" Surfaces 8, no. 2: 34. https://doi.org/10.3390/surfaces8020034
APA StyleCamacho-González, M. A., Hernández-Reyes, A., Garrido-Hernández, A., Olivares-Xometl, O., Likhanova, N. V., & Lijanova, I. V. (2025). TPA and PET Photo-Degradation by Heterogeneous Catalysis Using a (Al2O3)0.75TiO2 Coating. Surfaces, 8(2), 34. https://doi.org/10.3390/surfaces8020034






