Use of Alkali in Traditional Dyeing Technologies with Plants
Abstract
1. Introduction
2. Materials and Methods
2.1. Informative Sources and Materials
2.1.1. Information from Written Sources
2.1.2. Dye Plants
2.2. Chemicals
2.3. Methods
2.3.1. Dyeing Experiments
- (1)
- Without mordanting;
- (2)
- Premordanted with alum and cream of tartar (8 g KAl(SO4)2 + 7 g KC4H5O6/100 g fiber);
- (3)
- Premordanted with tin salt (5 g SnCl2 · 2H2O/100 g fiber);
- (4)
- Mordanted with copper vitriol (copper (II) sulfate CuSO4 · 5H2O) during dyeing;
- (5)
- Mordanted with iron vitriol or copperas (iron (II) sulfate FeSO4 · 7H2O) during dyeing.
2.3.2. Dyestuff Extraction Methods
2.3.3. Instrumentation
3. Results
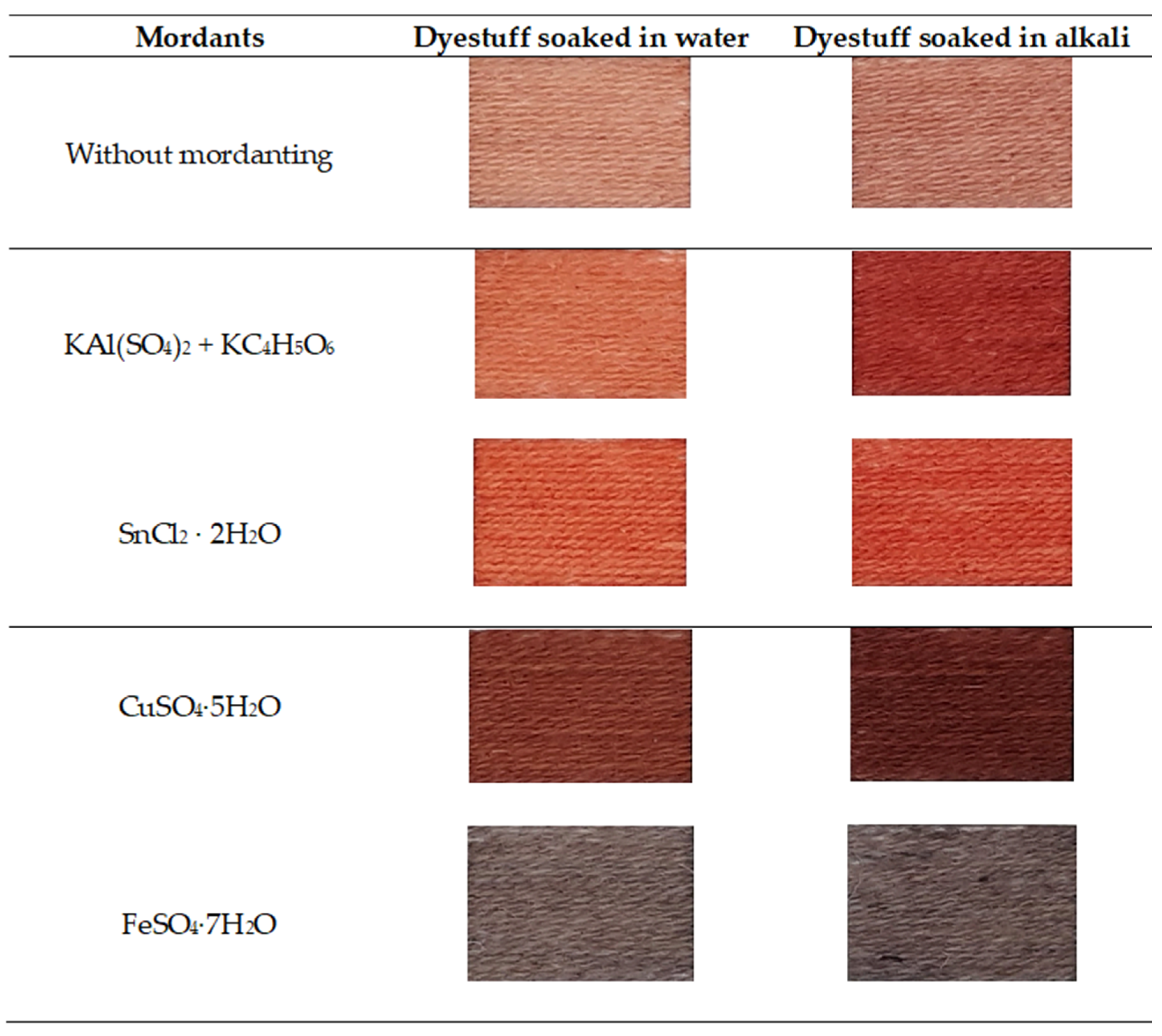
3.1. Color Tones
3.1.1. Colors Obtained with R. acetosa
3.1.2. Colors Obtained with P. erecta
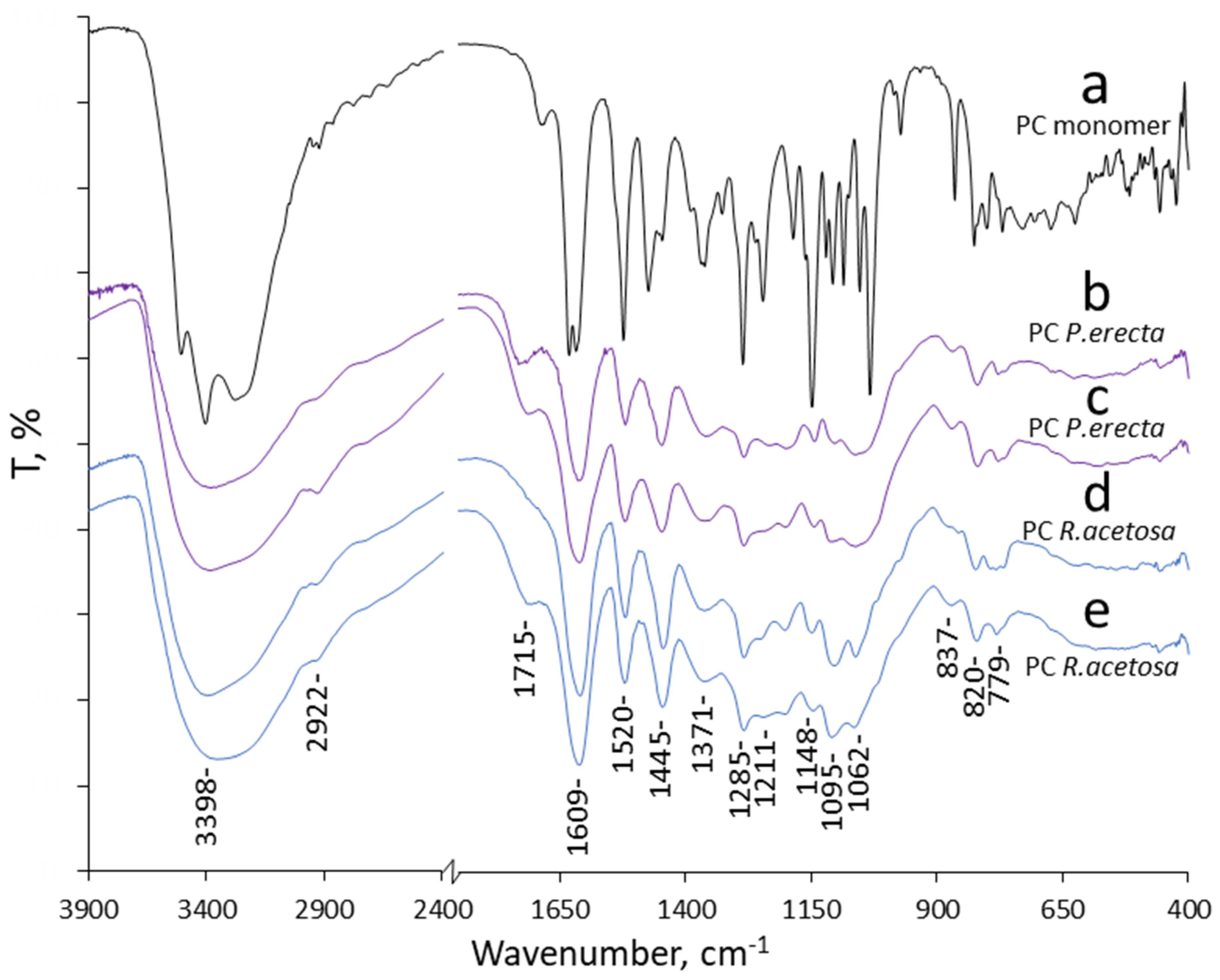
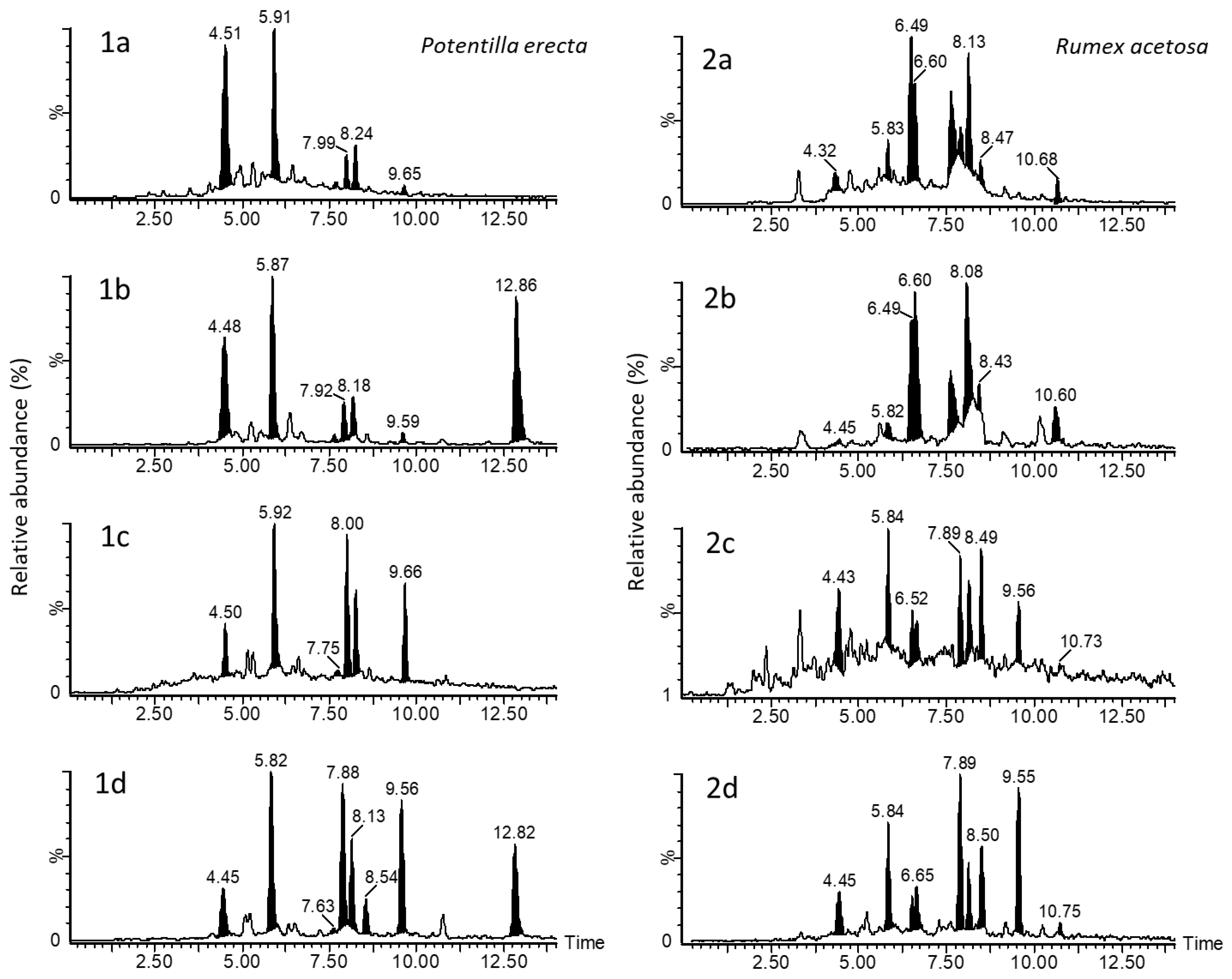
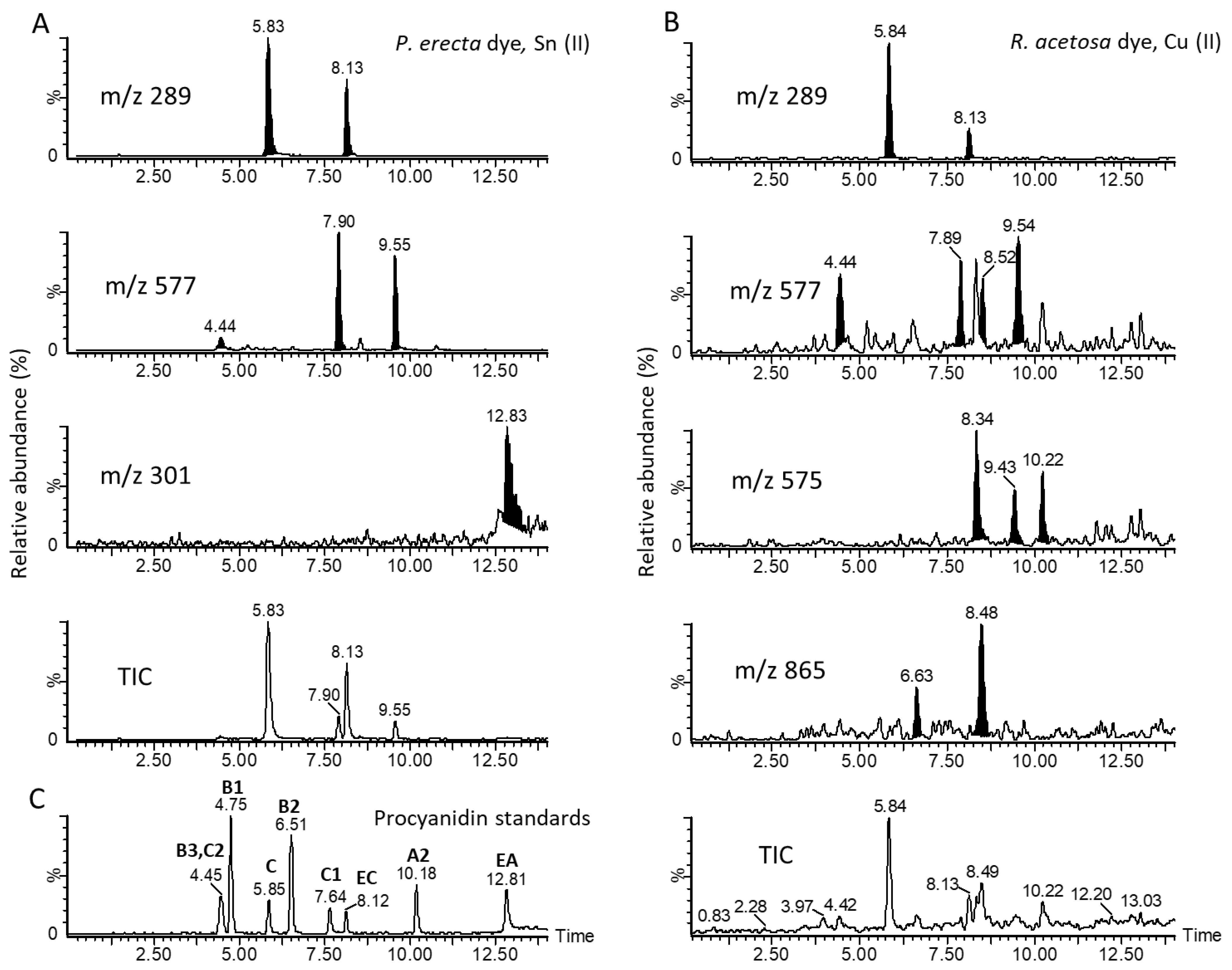
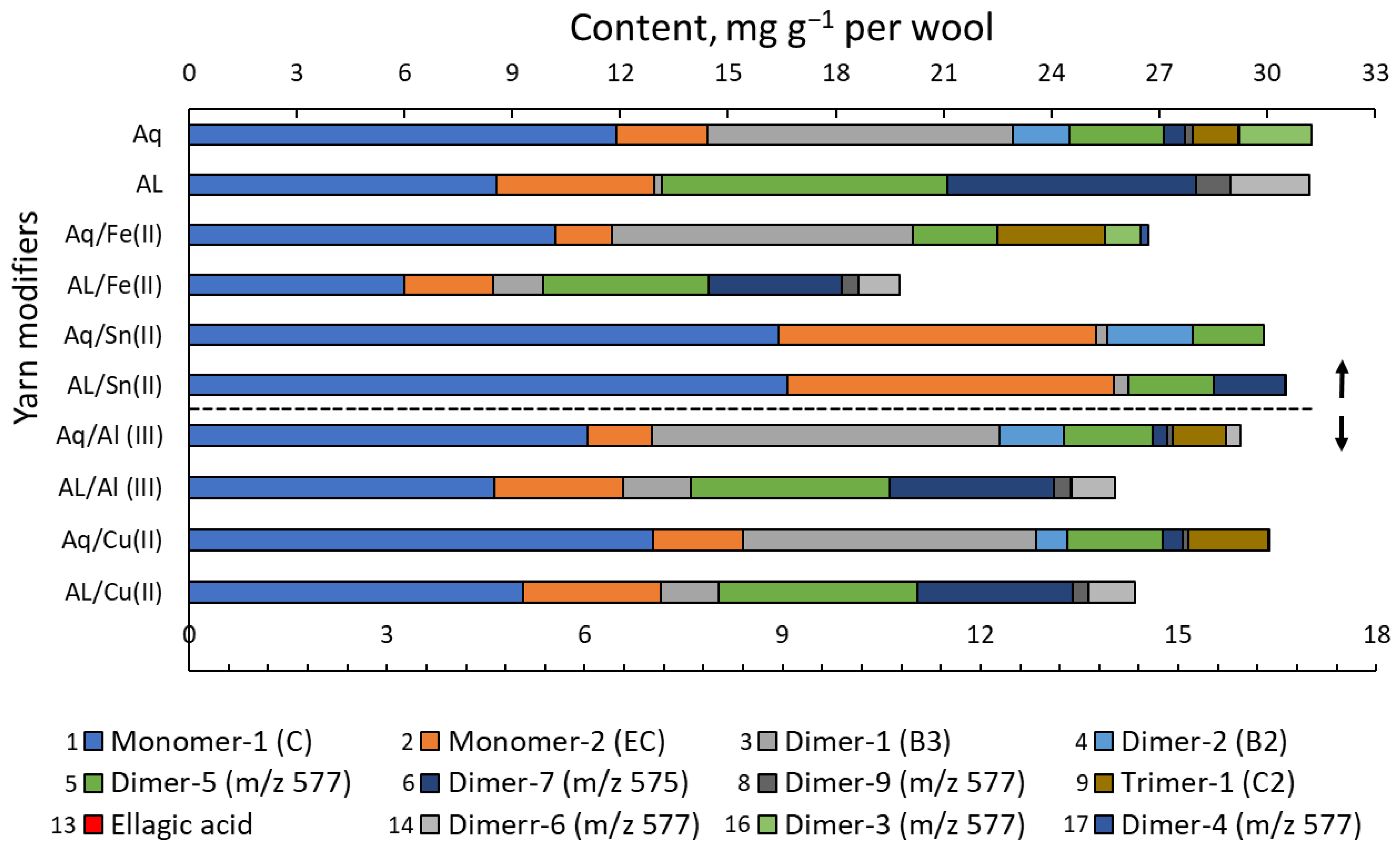
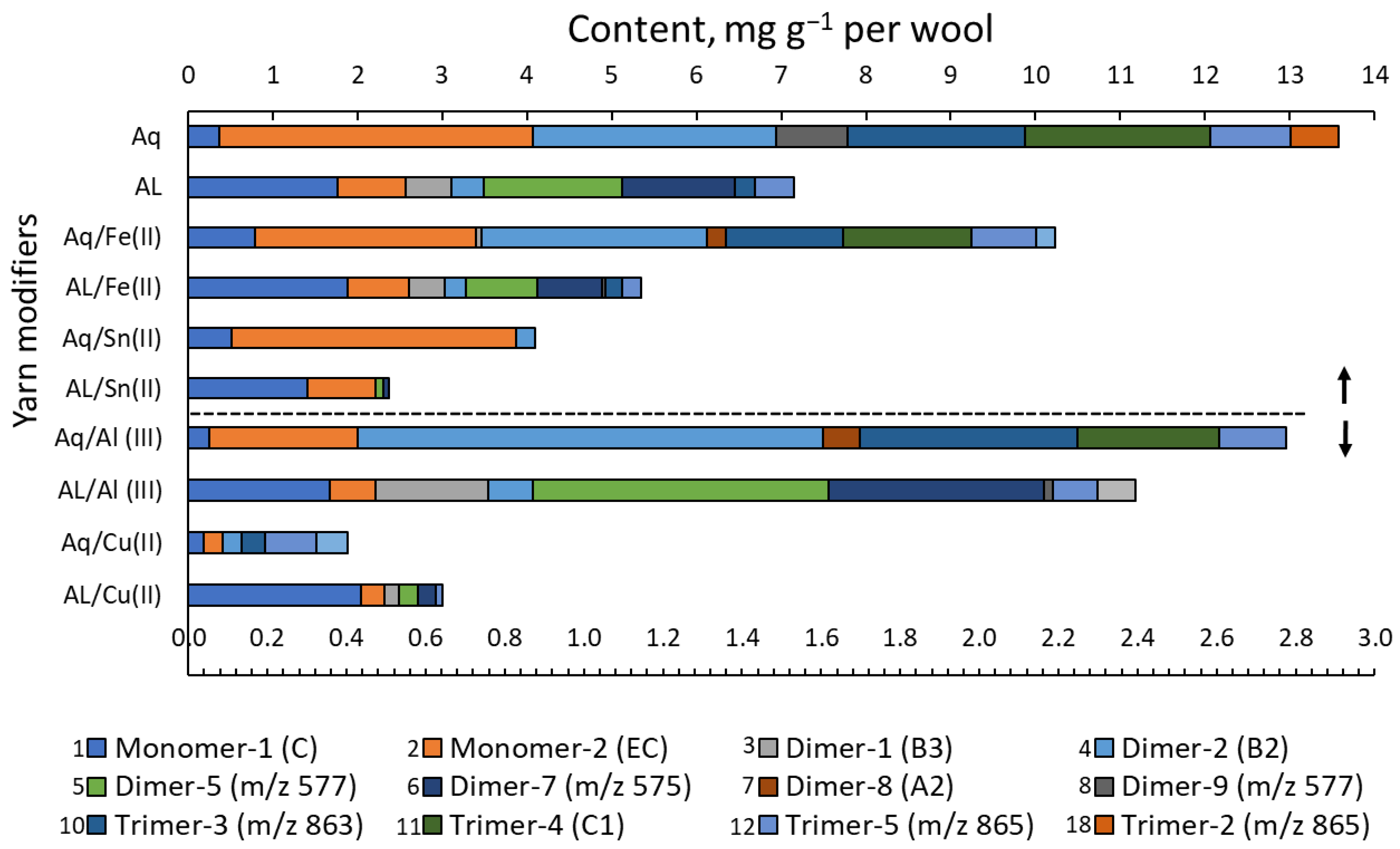
3.2. Chemical Analyses
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| LNVM ZAE | The scientific collection of the Latvian National History Museum, the Department of Ethnography |
Appendix A


References
- Samanta, A.K.; Adwaita, K. Dyeing of textiles with natural dyes. In Natural Dyes; Perrin Akçakoca Kumbasar, E., Ed.; InTech: Rijeka, Croatia, 2011; pp. 212–222. [Google Scholar]
- Pastoureau, M. Red: The History of a Color; Princeton University Press: Princeton, NJ, USA; Oxford, UK, 2017. [Google Scholar]
- Board of Trustees of the Royal Botanic Gardens. Potentilla erecta (L.) Raeusch. Distribution. Plants of the World Online. Available online: https://powo.science.kew.org/taxon/urn:lsid:ipni.org:names:60457410-2 (accessed on 27 May 2025).
- Board of Trustees of the Royal Botanic Gardens. Rumex acetosa L. Distribution. Plants of the World Online. Available online: https://powo.science.kew.org/taxon/urn:lsid:ipni.org:names:332105-2 (accessed on 27 May 2025).
- Regulations of the Cabinet of Ministers of Latvia No. 396 Noteikumi par Īpaši Aizsargājamo Sugu un Ierobežoti Izmantojamo Īpaši Aizsargājamo Sugu Sarakstu. Available online: https://likumi.lv/doc.php?id=12821 (accessed on 26 May 2025).
- Tomczyk, M.; Latté, K.P. Potentilla—A review of its phytochemical and pharmacological profile. J. Ethnopharmacol. 2009, 122, 184–204. [Google Scholar] [CrossRef] [PubMed]
- Vennat, B.; Bos, M.A.; Pourrat, A.; Bastide, P. Procyanidins from tormentil: Fractionation and study of the anti-radical activity towards superoxide anion. Biol. Pharm. Bull. 1994, 17, 1613–1615. [Google Scholar] [CrossRef] [PubMed]
- Vasas, A.; Orbán-Gyapai, O.; Hohmann, J. The genus Rumex: Review of traditional uses, phytochemistry and pharmacology. J. Ethnopharmacol. 2015, 175, 198–228. [Google Scholar] [CrossRef] [PubMed]
- Feduraev, P.; Skrypnik, L.; Nebreeva, S.; Dzhobadze, G.; Vatagina, A.; Kalinina, E.; Pungin, A.; Maslennikov, P.; Riabova, A.; Krol, O.; et al. Variability of phenolic compound accumulation and antioxidant activity in wild plants of some Rumex species (Polygonaceae). Antioxidants 2022, 11, 311. [Google Scholar] [CrossRef]
- Palomino, E.; Rahme, L.; Káradóttir, K.M.; Kokita, M.; Freysteinsson, S.P. Traditional Fish Leather Dyeing Methods with Indigenous Arctic Plants. Heritage 2024, 7, 3643–3663. [Google Scholar] [CrossRef]
- Nørtoft, M. The red-blue conundrum: An archaeo-linguistic approach to red dyes and blue flowers in prehistory. Archaeol. Text. Rev. 2017, 44–66. Available online: https://atnfriends.com/index.php/2017/12/01/atr-59 (accessed on 16 June 2025).
- Vajanto, K. Dyes and Dyeing Methods in Late Iron Age Finland. Academic Ph.D. Dissertation, University of Helsinki, Helsinki, Finland, 2016. ISBN 978-951-51-1790-8. Available online: http://hdl.handle.net/10138/159210 (accessed on 28 May 2025).
- Karlsone, A. The tradition of using natural dyes in Latvia. In Dyes in History and Archaeology; Kirby, J., Ed.; Archetype Publications: London, UK, 2021; Volume 35/36, pp. 62–69. ISBN 978-1-909492-81-3. [Google Scholar]
- LNVM ZAE, 47: The National History Museum of Latvia, the Ethnographic Expedition Materials of the Monuments Board, Dyeing, Washing, Bleaching, Folder 47, Collected in 1924–1942.
- LNVM ZAE, 35: The National History Museum of Latvia, the Ethnographic Expedition Materials of the Monuments Board, Female Folk Dress, Folder 35, Collected in 1924–1942.
- Vilde, P.E. Latviešu Ārste. 1769, 18, 2. Available online: http://www.periodika.lv/periodika2-viewer/?lang=fr#issue:25744 (accessed on 16 June 2025).
- Saržants, K. Par krāsošanu. Etnogrāfiskas ziņas par latviešiem. Dienas Lapas pielikums 1893, 4, 58–59. Available online: http://www.periodika.lv/periodika2-viewer/?lang=fr#issue:1079629 (accessed on 16 June 2025).
- Zilumkalns, A. Par krāsām un krāsošanu. Etnogrāfiskas ziņas par latviešiem. Dienas Lapas pielikums 1894, 6, 93. Available online: http://www.periodika.lv/periodika2-viewer/?lang=fr#issue:1074440 (accessed on 16 June 2025).
- Skruzitis, M. Latweeschu tautas apģehrbs savā vehsturiskā attihstihbā un nozihmē. Austrums 1895, 1, 10–13. Available online: https://periodika.lv/periodika2-viewer/?lang=fr#panel:pa|issue:1246799|article:DIVL285 (accessed on 26 March 2025).
- Skruzitis, M. Par latweeschu senehjo krahsoschanas mahkslu. Tehvija 1902, 21, 2–3, 22, 2–3; 23, 1–2. Available online: https://periodika.lv/periodika2-viewer/?lang=fr#panel:pa|issue:143751|article:DIVL39; https://periodika.lv/periodika2-viewer/?lang=fr#panel:pa|issue:95457|article:DIVL31; https://periodika.lv/periodika2-viewer/?lang=fr#panel:pp|issue:183800|article:DIVL15 (accessed on 26 March 2025).
- Bielenstein, M. Die Altlettischen Färbmethoden. Studien zur Indogermanischen Altertumskunde; Ernst Plates: Riga, Latvia, 1935. [Google Scholar]
- Hayward, M.; Riello, G.; Rublack, U. (Eds.) A revolution in Colour. Natural Dyes and Dress in Europe, c. 1400–1800; Bloomsbury Academic: London, UK, 2024; ISBN 978-350-40562-2. [Google Scholar]
- Coles, J.M. Experimental Archaeology; Academic Press: London, UK, 1979. [Google Scholar]
- Andersson Strand, E.B.; Munkholt, C.; Mannering, U.; Gleba, M. North European Symposium for Archaeological Textiles X; Oxbow Books: Oxford, UK, 2009. [Google Scholar]
- Mari, A.; Eletto, D.; Pizza, C.; Montoro, P.; Piacente, S. Integrated mass spectrometry approach to profile proanthocyanidins occurring in food supplements: Analysis of Potentilla erecta L. rhizomes. Food Chem. 2013, 141, 4171–4178. [Google Scholar] [PubMed]
- Bicker, J.; Petereit, F.; Hensel, A. Proanthocyanidins and a phloroglucinol derivative from Rumex acetosa L. Fitoterapia 2009, 80, 483–495. [Google Scholar] [CrossRef] [PubMed]
- Molnar, M.; Kovać, M.J.; Pavić, V. A comprehensive analysis of diversity, structure, biosynthesis and extraction of biologically active tannins from various plant-based materials using deep eutectic solvents. Molecules 2024, 29, 2615. [Google Scholar] [CrossRef] [PubMed]
- Latté, K.P. Potentilla erecta—Das Aufrechte Fingerkraut. Z. Für Phytother. 2006, 27, 198–206. [Google Scholar] [CrossRef]
- Sentkowska, A.; Pyrzynska, K. HILIC chromatography: Powerful technique in the analysis of polyphenols. In Polyphenols in Plants, Isolation, Purification and Extract Preparation; Watson, R.R., Ed.; Academic Press: Cambridge, MA, USA, 2019; pp. 341–351. [Google Scholar] [CrossRef]
- Kennedy, J.A. Proanthocyanidins. Extraction, purification and determination of subunit composition by HPLC. In Current Protocols in Food Analytical Chemistry; John Wiley & Sons, Ltd.: New York, NY, USA, 2003; pp. 1–11. [Google Scholar] [CrossRef]
- Chen, X.; Song, H.; Zhou, S.; Yuan, C.; Li, J. Exploring separation patterns and mechanisms of proanthocyanidins in grape seeds and pomace with diverse molecular weights, compositions, and structures. Food Chem. X 2023, 20, 101008. [Google Scholar] [CrossRef]
- Luca, S.V.; Bujor, A.; Miron, A.; Aprotosoaie, A.C.; Skalicka-Woźniak, K.; Trifan, A. Preparative separation and bioactivity of oligomeric proanthocyanidins. Phytochem. Rev. 2020, 19, 1093–1140. [Google Scholar] [CrossRef]
- Skruzitis, M. Par latweeschu citreizejo krahsoschanas mahkslu. Lihdums 1916, 202, 6. Available online: https://periodika.lv/periodika2-viewer/?lang=fr#panel:pa|issue:888847|article:DIVL119|page:5|block:P5_TB00001 (accessed on 26 March 2025).
- Paegle, E. Krāsojamie stādi. Latv. Saule 1923, 4, 35. Available online: https://periodika.lv/periodika2-viewer/?lang=fr#panel:pa|issue:1150476|article:DIVL96|page:7 (accessed on 28 March 2025).
- Liepiņa, A. Krāsošana stādu vielām. Zeltene 1931, 7, 26. Available online: https://periodika.lv/periodika2-viewer/?lang=fr#panel:pa|issue:766813|article:DIVL282 (accessed on 28 March 2025).
- LNVM ZAE, 35, Daugavpils apr. Jamuižas pag., Tekla Butlers (1873) (National History Museum of Latvia, Daugavpils Region, Jasmuiza Parish, the Narrator Was Born in 1873), 1933.
- Retels, P. Latvijas krāsojošie augi. Zeltene 1927, 12, 25. Available online: https://periodika.lv/periodika2-viewer/?lang=fr#panel:pa|issue:970221|article:DIVL415 (accessed on 28 March 2025).
- [Anonim] Augu izmantošana krāsošanā. Tēvija 1943, 164, 4. Available online: https://periodika.lv/periodika2-viewer/?lang=fr#panel:pa|issue:1113716|article:DIVL357 (accessed on 28 March 2025).
- Karlsone, A.; Luhamaa, L. Traditional dyeing technologies in Latvia and Estonia. In DHA 40 Dyes in History & Archaeology; British Museum: London, UK, 2021; p. 16. Available online: https://www.dyesinhistoryandarchaeology.com/resources/Programme/DHA40_AbstractsBook.pdf (accessed on 14 June 2025).
- Ķeņģe, M. Rudenī vācamie krāsaugi. Lauku Sēta 1940, 1–2, 17. Available online: https://periodika.lv/periodika2-viewer/?lang=fr#panel:pp|issue:798948|article:DIVL175 (accessed on 27 May 2025).
- Karlsone, A. Technology of Dyeing beyond Text. Heritage 2024, 7, 2668–2681. [Google Scholar] [CrossRef]
- Cardon, D. Natural Dye: Sources, Tradition, Technology and Science; Archetype Publications: London, UK, 2007; p. 409. ISBN 978-1-904982-00-5. [Google Scholar]
- Pāvuliņa, K. Krustpils. In Latvju Raksti; Valstspapīru spiestuve: Riga, Latvia, 1924–1931; pp. 4–172. [Google Scholar]
- Pāvuliņa, K. Kā vecos laikos veļu mazgāja un gludināja. Zeltene 1933, 10, 14–15. Available online: https://periodika.lv/periodika2-viewer/?lang=fr#panel:pp|issue:927705|article:DIVL151 (accessed on 27 May 2025).
- Gong, Y.; Fang, F.; Zhang, X.; Liu, B.; Luo, H.; Li, Z.; Zhang, X.; Zhang, Z.; Pang, X. B Type and Complex A/B Type Epicatechin Trimers Isolated from Litchi pericarp Aqueous Extract Show High Antioxidant and Anticancer Activity. Int. J. Mol. Sci. 2018, 19, 301. [Google Scholar] [CrossRef]
- Degano, I.; Lucejko, J.; Colombini, M.J. The unprecedented identification of Safflower dyestuff in a 16th century tapestry through the application of a new reliable diagnostic procedure. Cult. Herit. 2011, 12, 295–299. [Google Scholar] [CrossRef]
- Robertson, A.R. The CIE 1976 Color-Difference Formulae. Color Res. Appl. 1977, 2, 7–11. [Google Scholar] [CrossRef]
- Ramos-Tejada, M.M.; Durán, J.D.G.; Ontiveros-Ortega, A.; Espinosa-Jimenez, M.; Perea-Carpio, R.; Chibowski, E. Investigation of alumina/(+)-catechin system properties. Part I: A study of the system by FTIR-UV–Vis spectroscopy. Colloids Surf. B Biointerfaces 2002, 24, 297–308. [Google Scholar] [CrossRef]
- Liang, T.; Jiao, S.; Jing, P. Molecular interaction between pectin and catechin/procyanidin in simulative juice model: Insights from spectroscopic, morphology, and antioxidant activity. J. Food Sci. 2021, 86, 2445–2456. [Google Scholar] [CrossRef]
- Ku, C.S.; Mun, S.P. Characterization of proanthocyanidin in hot water extract isolated from Pinus radiata bark. Wood Sci. Technol. 2007, 41, 235–247. [Google Scholar] [CrossRef]
- Khanal, R.C.; Howard, L.R.; Prior, R.L. Effect of heating on the stability of grape and blueberry pomace procyanidins and total anthocyanins. Food Res. Int. 2010, 43, 1464–1469. [Google Scholar] [CrossRef]
- Chang, M.; Sun, X.; Guo, X.; Bai, H.; Liu, R.; Jin, Q.; Wang, X. Composition and antioxidant study of procyanidins from peanut skins. J. Food Meas. Charact. 2020, 14, 2781–2789. [Google Scholar] [CrossRef]
- Zuccari, G.; Baldassari, S.; Ailuno, G.; Turrini, F.; Alfei, S.; Caviglioli, G. Formulation strategies to improve oral bioavailability of ellagic acid. Appl. Sci. 2020, 10, 3353. [Google Scholar] [CrossRef]
- Taddei, P.; Montia, P.; Freddi, G.; Araic, T.; Tsukada, M. Binding of Co(II) and Cu(II) cations to chemically modified wool fibres: An IR investigation. J. Mol. Struct. 2003, 650, 105–113. [Google Scholar]
- Zhu, Q.Y.; Holt, R.R.; Lazarus, S.A.; Ensunsa, J.L.; Hammerstone, J.F.; Schmitz, H.H.; Keen, C.L. Stability of the Flavan-3-ols Epicatechin and Catechin and Related Dimeric Procyanidins Derived from Cocoa. J. Agric. Food Chem. 2002, 50, 1700–1705. [Google Scholar] [CrossRef]
- Suvanto, J.; Nohynek, L.; Seppänen-Laakso, T.; Heiko, R.; Juha-Pekka, S.; Puupponen-Pimiä, R. Variability in the production of tannins and other polyphenols in cell cultures of 12 Nordic plant species. Planta 2017, 246, 227–241. [Google Scholar] [CrossRef]
- Linh, H.T. Natural dyes in eastern asia (Vietnam and neighbouring countries). In Handbook of Natural Colorants; Bechtold, T., Mussak, R., Eds.; John Wiley & Sons, Ltd.: Chichester, UK, 2009; pp. 65–72. [Google Scholar] [CrossRef]
- Hsu, F.-L.; Nonaka, G.; Nishioka, I. Tannins and Related Compounds. XXXIII. Isolation and Characterization of Procyanidins in Dioscorea cirrhosa LOUR. Chem. Pharm. Bull. 1985, 33, 3293–3298. [Google Scholar] [CrossRef][Green Version]
- Yanfei, R.; Jixian, G.; Fubang, W.; Zheng, L.; Jianfei, Z.; Ranran, F.; Jiangfei, L. Effect of dye bath pH on dyeing and functional properties of wool fabric dyed with tea extract. Dye. Pigment. 2016, 134, 334–341. [Google Scholar] [CrossRef]
- Yu, K.; Song, Y.; Lin, J.; Dixon, R.A. The complexities of proanthocyanidin biosynthesis and its regulation in plants. Plant Commun. 2023, 4, 100498. [Google Scholar] [CrossRef]
- Porter, L.J. Structure and Chemical Properties of the Condensed Tannins. In Plant Polyphenols; Springer: Boston, MA, USA, 1992; pp. 245–258. [Google Scholar] [CrossRef]
- Ebrahimnejad, H.; Burkholz, T.; Jacob, C. Flavanols and proanthocyanidins. In Recent Advances in Redox Active Plant and Microbial Products, 1st ed.; Jacob, C., Kirsch, G., Slusarenko, A.J., Winyard, P.G., Burkholz, T., Eds.; Springer: Dordrecht, The Netherlands, 2014; pp. 211–232. [Google Scholar] [CrossRef]
- Pauk, V.; Barták, P.; Lemr, K. Characterization of natural organic colorants in historical and art objects by high-performance liquid chromatography. J. Sep. Sci. 2014, 37, 3393–3410. [Google Scholar] [CrossRef]
- Zhang, X.; Laursen, R.A. Development of Mild Extraction Methods for the Analysis of Natural Dyes in Textiles of Historical Interest Using LC-Diode Array Detector-MS. Anal. Chem. 2005, 77, 2022–2025. [Google Scholar] [CrossRef] [PubMed]
- Vajanto, K. Textile standards in experimental archaeology. In Focus on Archaeological Textiles: Multidisciplinary Approaches (MASF 3); Lipkin, S., Vajanto, K., Eds.; Archaeological Society of Finland: Helsinki, Finland, 2014; pp. 62–75. [Google Scholar]
- Pīgozne, I. Sarkans, dzeltens, brūns: Par krāsu vizuālajām un mītiskajām robežām latviešu folklorā un senajā apģērbā. Letonica 2012, 3, 99–116. Available online: https://lulfmi.lv/files/letonica/Letonica2012_3.pdf (accessed on 30 March 2025).
- Telichowska, A.; Kobus-Cisowska, J.; Szulc, P. Phytopharmacological possibilities of bird cherry Prunus padus L. and Prunus serotina L. species and their bioactive phytochemicals. Nutrients 2020, 12, 1966. [Google Scholar] [CrossRef] [PubMed]
- Laitinen, M.L.; Julkunen-Tiitto, R.; Rousi, M. Variation in phenolic compounds within a birch (Betula pendula) population. J. Chem. Ecol. 2000, 26, 1609–1622. [Google Scholar] [CrossRef]



| Mordants | Dyestuff Soaked in Water | Dyestuff Soaked in Alkali |
|---|---|---|
| Without mordanting |  |  |
| KAl(SO4)2 + KC4H5O6 |  |  |
| SnCl2 · 2H2O |  |  |
| CuSO4·5H2O |  |  |
| FeSO4·7H2O |  |  |
| Mordants | Dyestuff Soaked in Water | Dyestuff Soaked in Alkali |
|---|---|---|
| Without mordanting |  |  |
| KAl(SO4)2 + KC4H5O6 |  |  |
| SnCl2 · 2H2O |  |  |
| CuSO4·5H2O |  |  |
| FeSO4·7H2O |  |  |
| Eluent | Fraction | Potentilla erecta | Rumex acetosa | ||
|---|---|---|---|---|---|
| Solution of Dyeing | |||||
| Alkaline-Aqueous | Aqueous | Alkaline-Aqueous | Aqueous | ||
| H2O | 1 | 220.9 ± 10.8 | 88.6 ± 4.2 | 47.1 ± 1.6 | 43.1 ± 1.8 |
| 1.0% FA in MeOH | 2 | 131.2 ± 6.8 | 84.1 ± 4.1 | 21.5 ± 0.9 | 40.9 ± 1.5 |
| DCM/MeOH/H2O | 3 | 67.9 ± 3.7 | 43.7 ± 2.0 | 12.2 ± 0.4 | 14.1 ± 0.7 |
| Species | Polymeric PCs | Abbr. | RT | [M−H]− | Content, mg g−1 DM | |
|---|---|---|---|---|---|---|
| Aqueous | Alkaline | |||||
| R. a. | C → B → C → B → C | C2 | 4.4 | 865 | <LOQ | <LOQ |
| P. e. | 11.3 ± 1.5 | <LOQ | ||||
| R. a. | C → B → C | B3 | 4.5 | 577 | <LOQ | 1.0 ± 0.1 |
| P. e. | 38.7 ± 1.8 | <LOQ | ||||
| R. a. | C | C | 5.8 | 289 | 2.8 ± 0.7 | 1.5 ± 0.1 |
| P. e. | 39.1 ± 1.9 | 18.8 ± 0.5 | ||||
| R. a. | EC → B → EC | B2 | 6.5 | 577 | 18.9 ± 1.4 | 0.6 ± 0.1 |
| P. e. | <LOQ | <LOQ | ||||
| R. a. | (EC)C → B → (EC)C → A → (EC)C | 6.6 | 863 | 10.8 ± 1.3 | <LOQ | |
| P. e. | <LOQ | <LOQ | ||||
| R. a. | EC → B → EC → B → EC | C1 | 7.7 | 865 | 18.0 ± 1.2 | <LOQ |
| P. e. | <LOQ | <LOQ | ||||
| R. a. | (E)C → A → (E)C | 7.9 | 575 | <LOQ | 1.3 ± 0.1 | |
| P. e. | 6.4 ± 0.6 | 23.1 ± 0.6 | ||||
| R. a. | EC | EC | 8.2 | 289 | 17.0 ± 1.4 | 1.0 ± 0.1 |
| P. e. | 8.9 ± 0.5 | 10.7 ± 0.5 | ||||
| R. a. | (E)C → B → (E)C → A → (E)C | 8.5 | 863 | 3.2 ± 0.3 | 1.0 ± 0.1 | |
| P. e. | <LOQ | <LOQ | ||||
| R. a. | (E)C → A → (E)C | 9.6 | 575 | <LOQ | 0.8 ± 0.1 | |
| P. e. | <LOQ | 17.8 ± 1.2 | ||||
| R. a. | (E)C → B → (E)C | 10.7 | 577 | 2.6 ± 0.3 | <LOQ | |
| P. e. | <LOQ | <LOQ | ||||
| R. acetosa | Total procyanidins (mg g−1 DM of dye) | 73.3 ± 9.7 | 7.2 ± 0.7 | |||
| P. erecta | 104.4 ± 6.5 | 81.8 ± 2.8 | ||||
| Mordant | Dye Typ | Rumex acetosa | Potentilla erecta | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| L* | a* | b* | c* | h° | ΔE | L* | a* | b* | c* | h° | ΔE | ||
| Unused | Aq | 77.28 | 12.05 | 39.98 | 41.75 | 73.23 | 41.75 | 77.59 | 14.86 | 38.94 | 41.68 | 69.11 | 41.79 |
| AL/Aq | 71.06 | 14.55 | 30.58 | 33.87 | 64.56 | 35.41 | 60.05 | 24.66 | 27.85 | 37.19 | 48.47 | 50.93 | |
| CuSO4 | Aq | 35.88 | 19.37 | 35.07 | 40.06 | 61.09 | 71.62 | 51.18 | 10.08 | 17.86 | 20.51 | 60.57 | 49.68 |
| AL/Aq | 16.57 | 25.62 | 21.38 | 33.37 | 39.85 | 87.24 | 29.65 | 20.77 | 21.13 | 29.63 | 45.49 | 73.49 | |
| FeSO4 | Aq | 53.13 | −0.33 | 18.02 | 18.03 | 91.06 | 46.46 | 55.86 | 9.57 | 3.57 | 10.22 | 20.47 | 43.78 |
| AL/Aq | 34.63 | 2.02 | 6.65 | 6.95 | 73.11 | 63.58 | 49.90 | 16.26 | −1.08 | 16.30 | 356.19 | 51.94 | |
| SnCl2 | Aq | 83.07 | 13.20 | 42.09 | 44.12 | 72.59 | 41.40 | 79.82 | 14.59 | 24.54 | 28.55 | 59.27 | 30.37 |
| AL/Aq | 69.74 | 31.70 | 25.45 | 40.65 | 38.76 | 47.66 | 51.21 | 32.54 | 27.95 | 42.89 | 40.66 | 61.84 | |
| KAl(SO4)2 | Aq | 88.70 | 5.90 | 31.53 | 32.07 | 79.41 | 27.80 | 83.30 | 9.37 | 25.60 | 27.26 | 69.04 | 26.56 |
| AL/Aq | 73.69 | 26.26 | 23.74 | 35.40 | 42.12 | 40.85 | 39.95 | 27.31 | 20.58 | 34.20 | 36.99 | 66.45 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Karlsone, A.; Kviesis, J. Use of Alkali in Traditional Dyeing Technologies with Plants. Heritage 2025, 8, 264. https://doi.org/10.3390/heritage8070264
Karlsone A, Kviesis J. Use of Alkali in Traditional Dyeing Technologies with Plants. Heritage. 2025; 8(7):264. https://doi.org/10.3390/heritage8070264
Chicago/Turabian StyleKarlsone, Anete, and Jorens Kviesis. 2025. "Use of Alkali in Traditional Dyeing Technologies with Plants" Heritage 8, no. 7: 264. https://doi.org/10.3390/heritage8070264
APA StyleKarlsone, A., & Kviesis, J. (2025). Use of Alkali in Traditional Dyeing Technologies with Plants. Heritage, 8(7), 264. https://doi.org/10.3390/heritage8070264









