A Comparison of the Fading of Dyestuffs as Textile Colourants and Lake Pigments
Abstract
1. Introduction
2. Materials and Methods
2.1. Light Exposure
2.2. Samples
2.2.1. Paint Samples
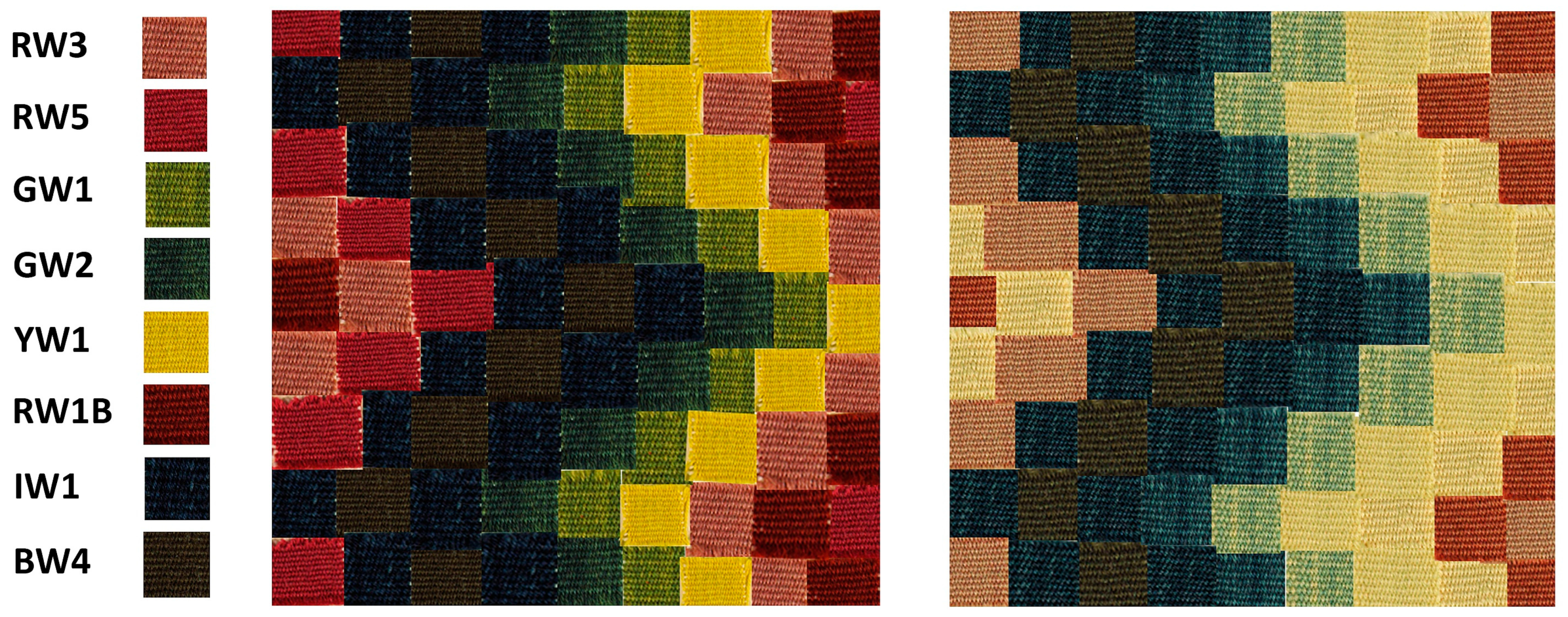
2.2.2. Textile Samples
2.3. Colour Measurement
2.4. Exposure Half-Lives
3. Results
3.1. Half-Lives
3.2. Red Pigments and Dyes
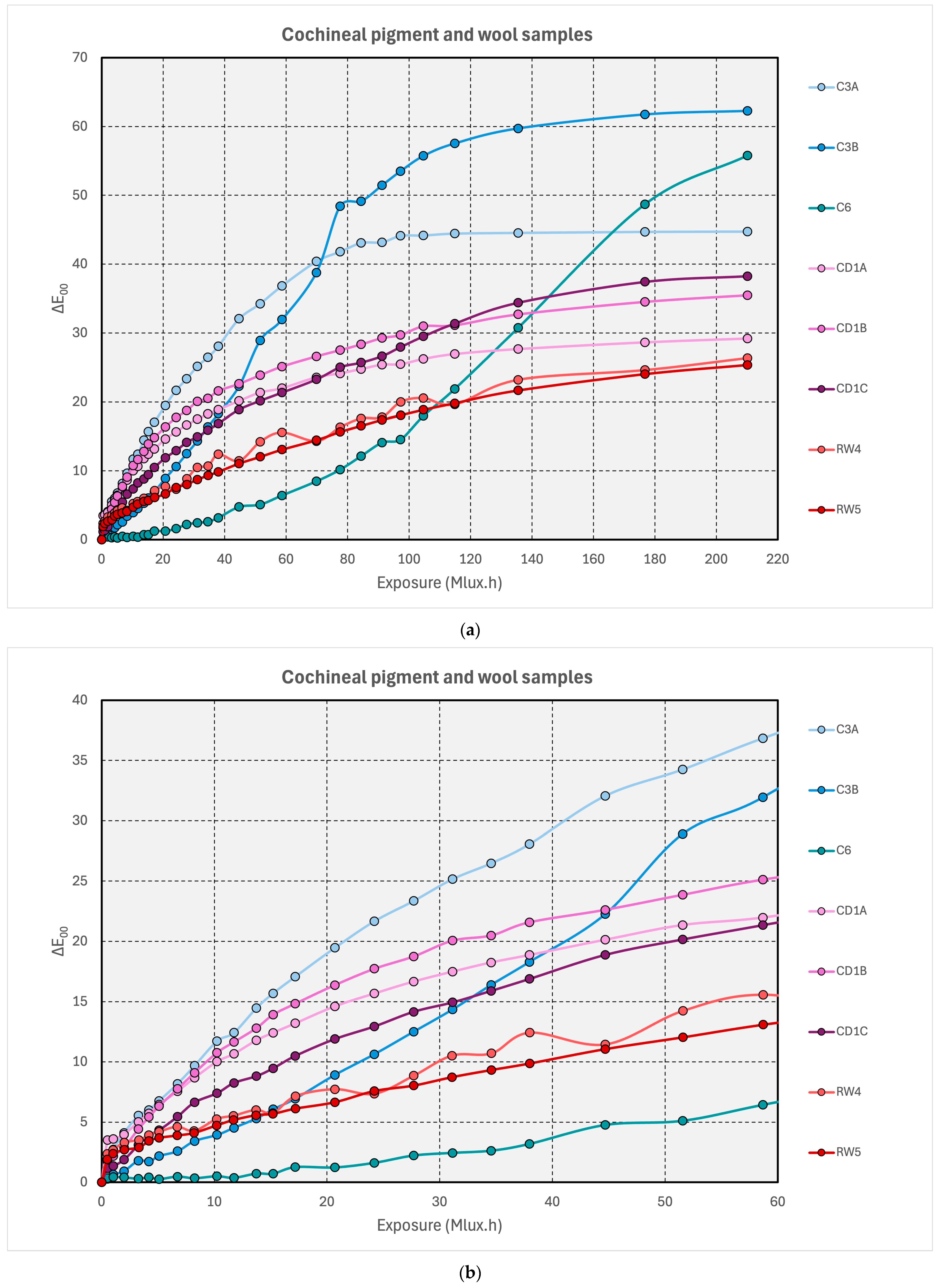
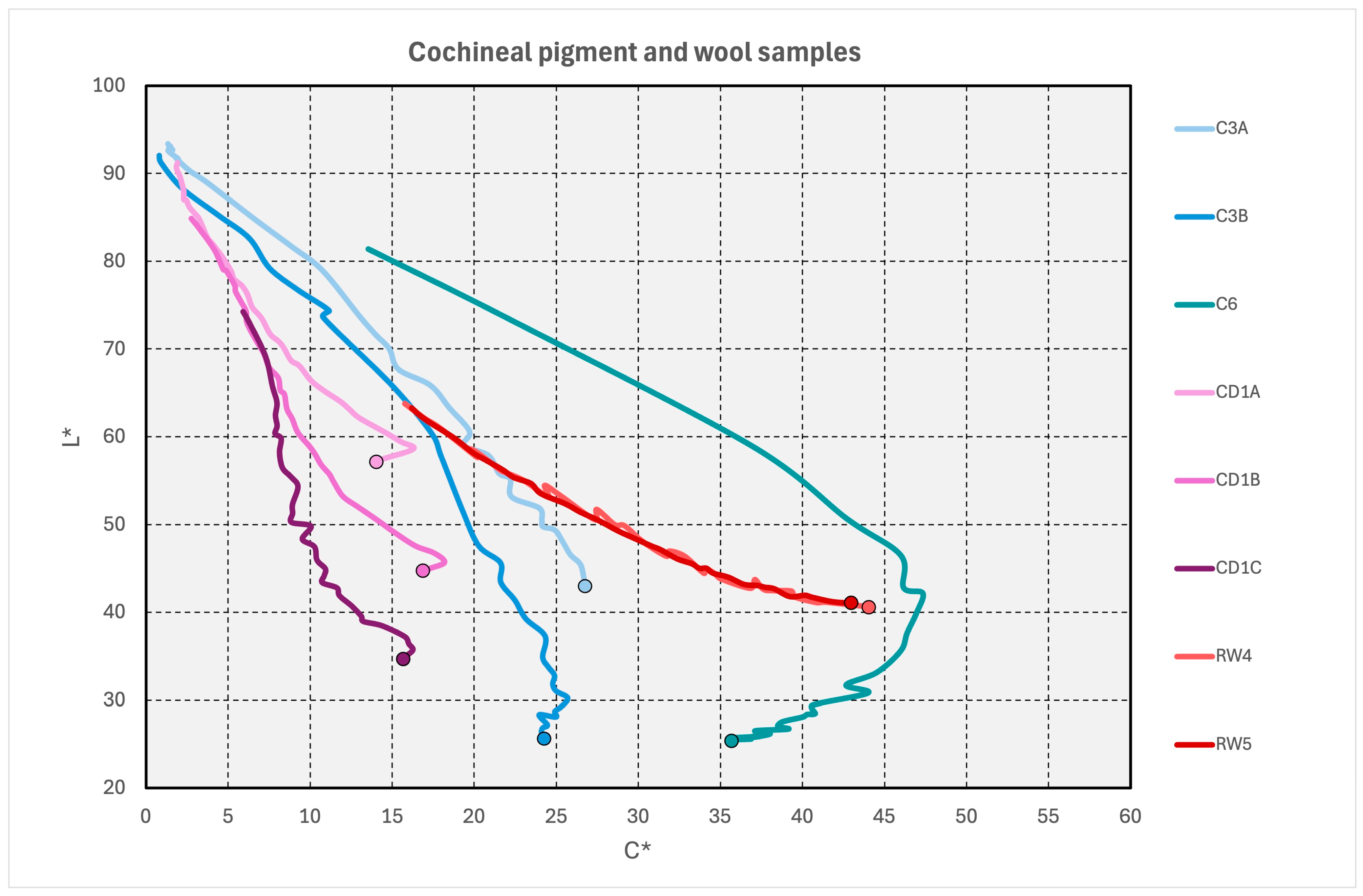
3.2.1. Cochineal-Containing Samples
3.2.2. Red Pigments
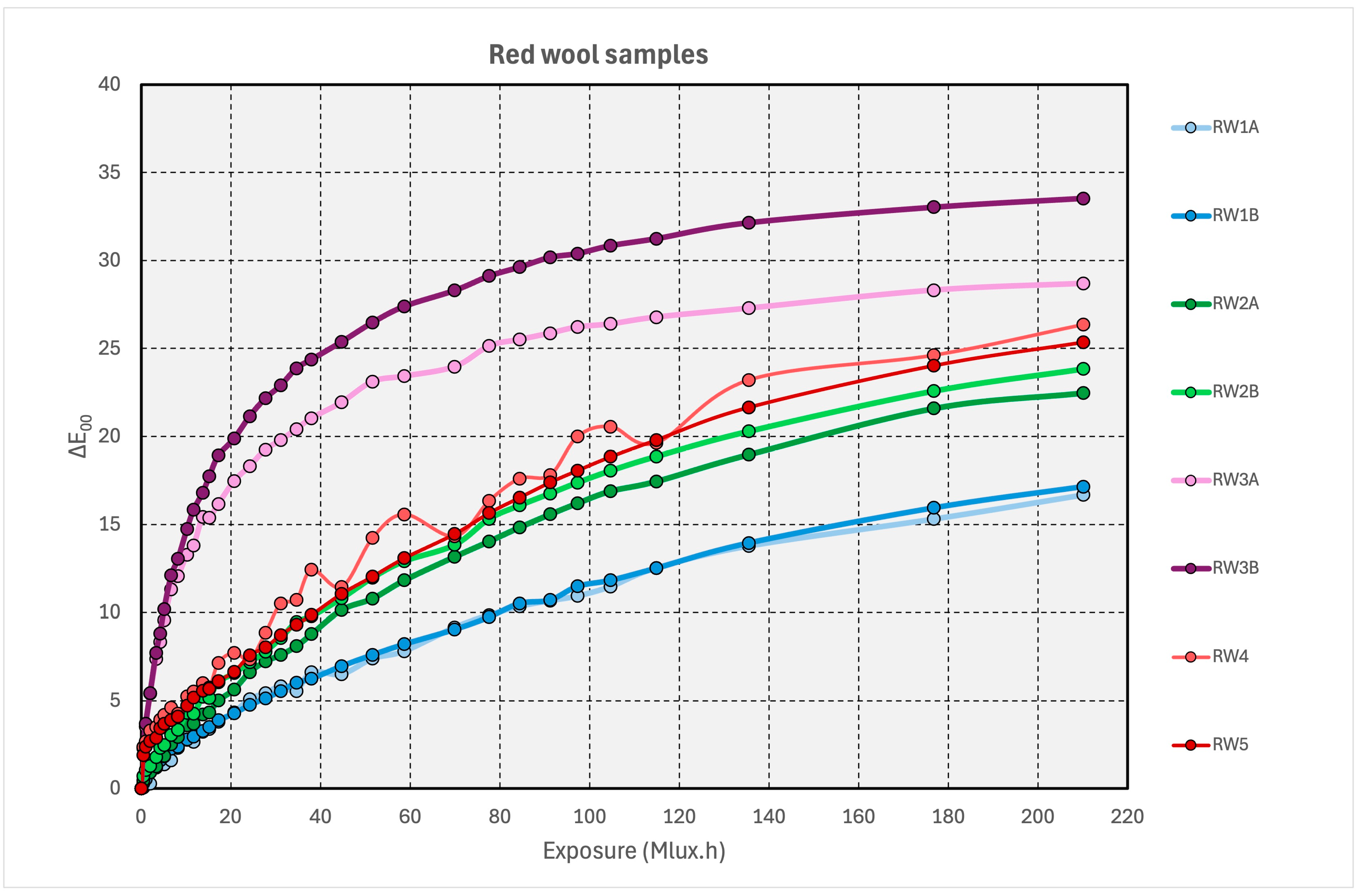
3.2.3. Red Dyes on Wool
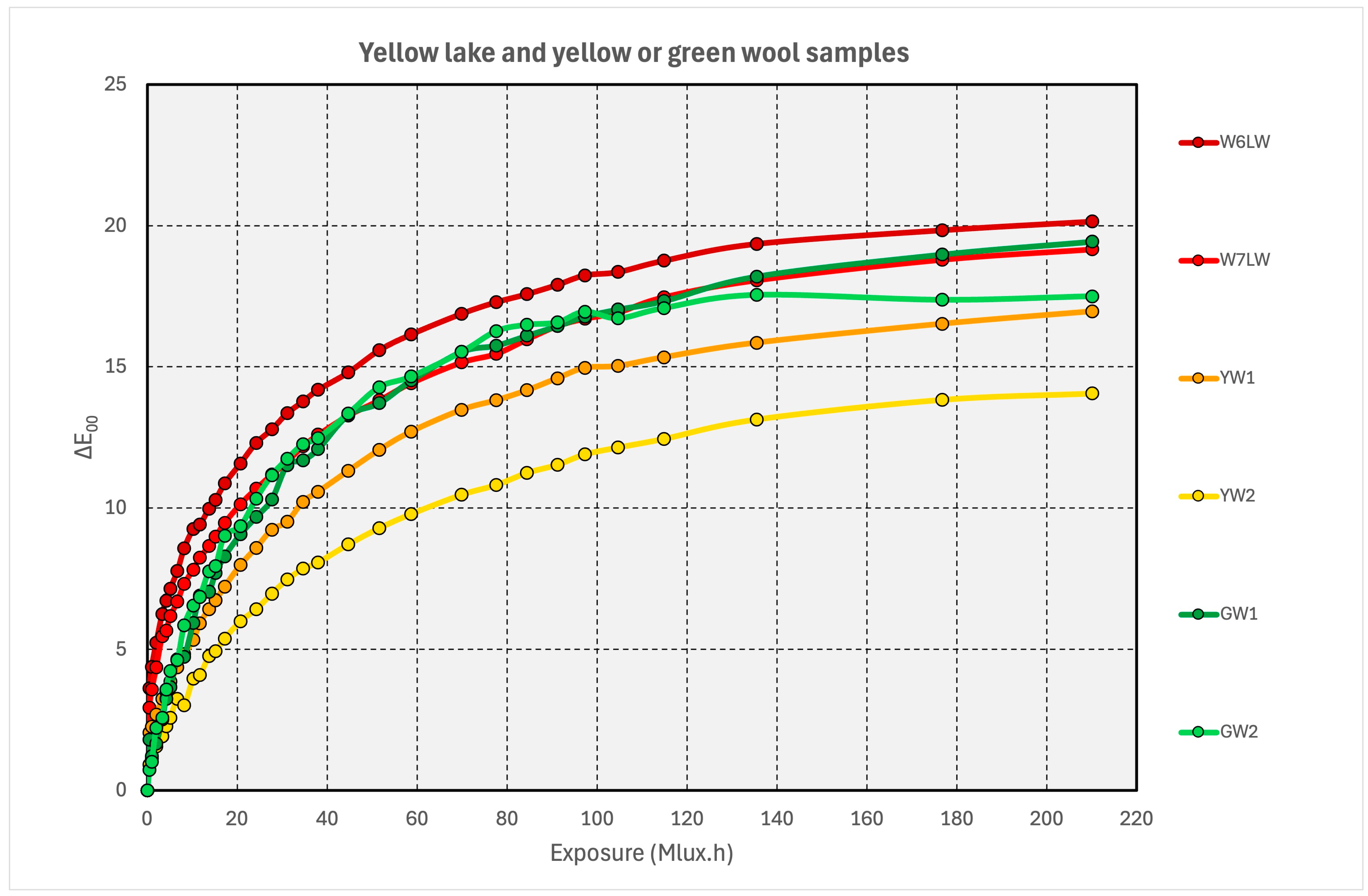
3.3. Yellow Pigments, Yellow Wools, and Green Wools
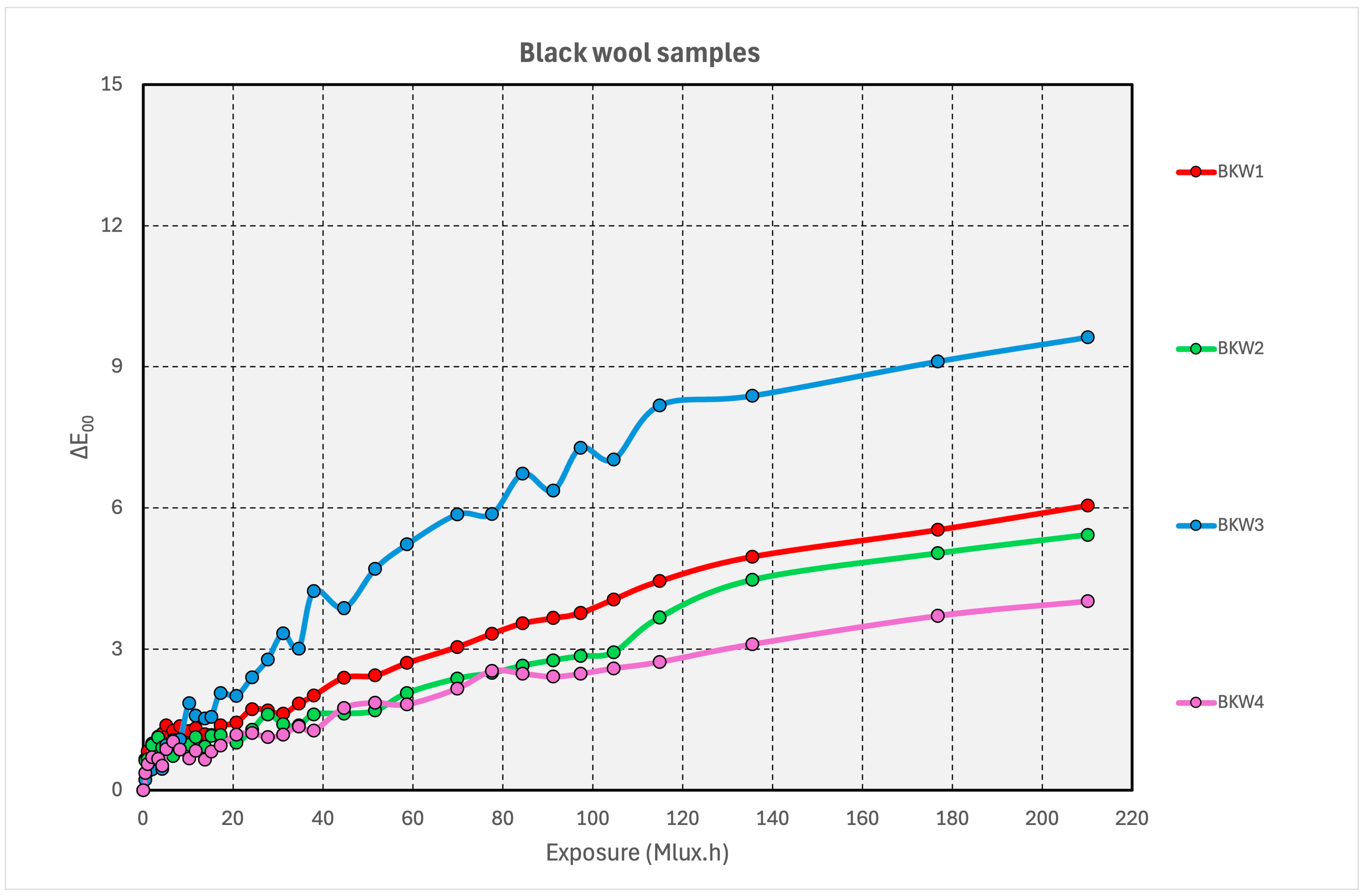
3.4. Black Dyes on Wool
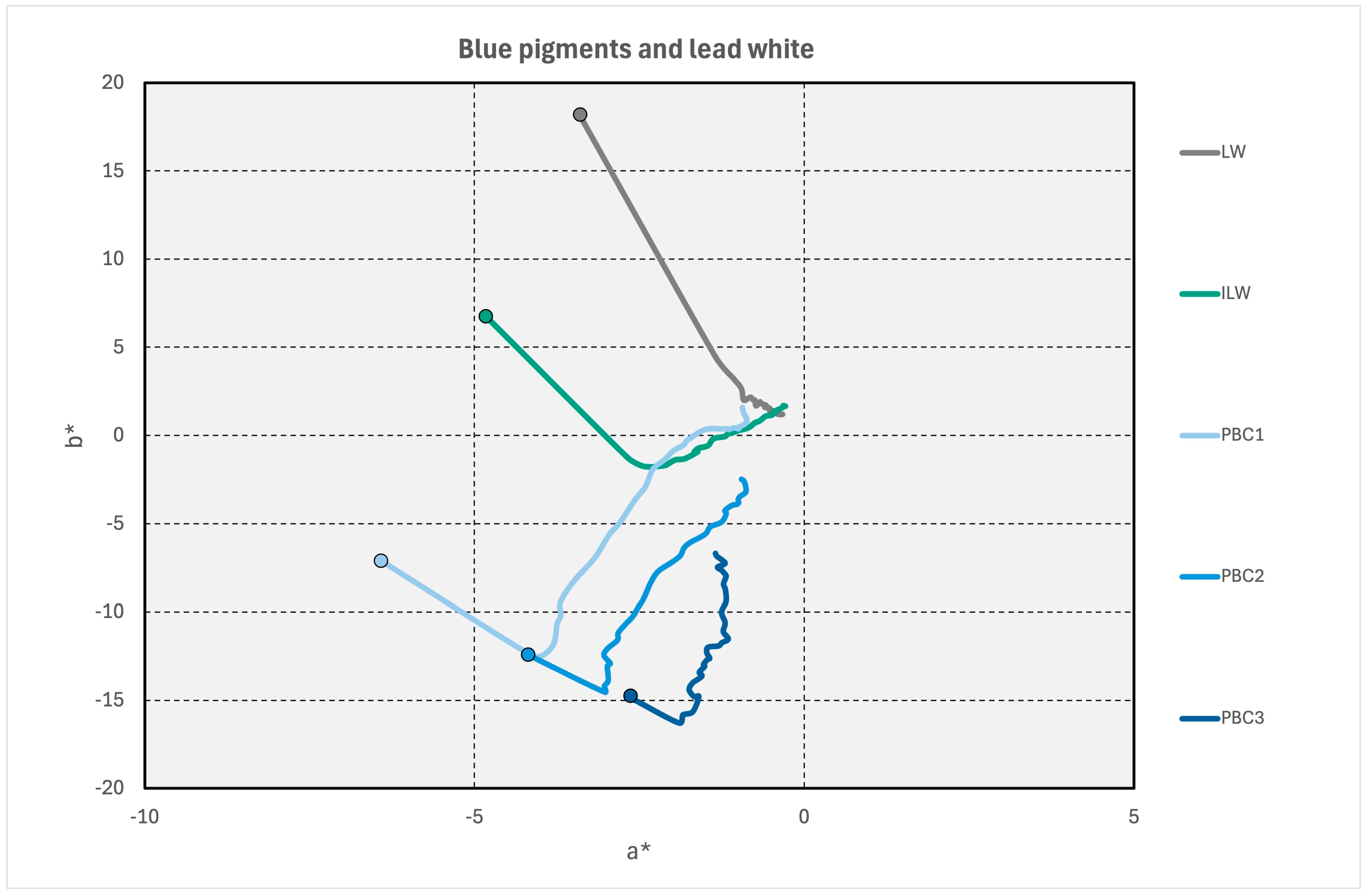
3.5. Admixtures with Lead White
3.6. The Dyeing Process
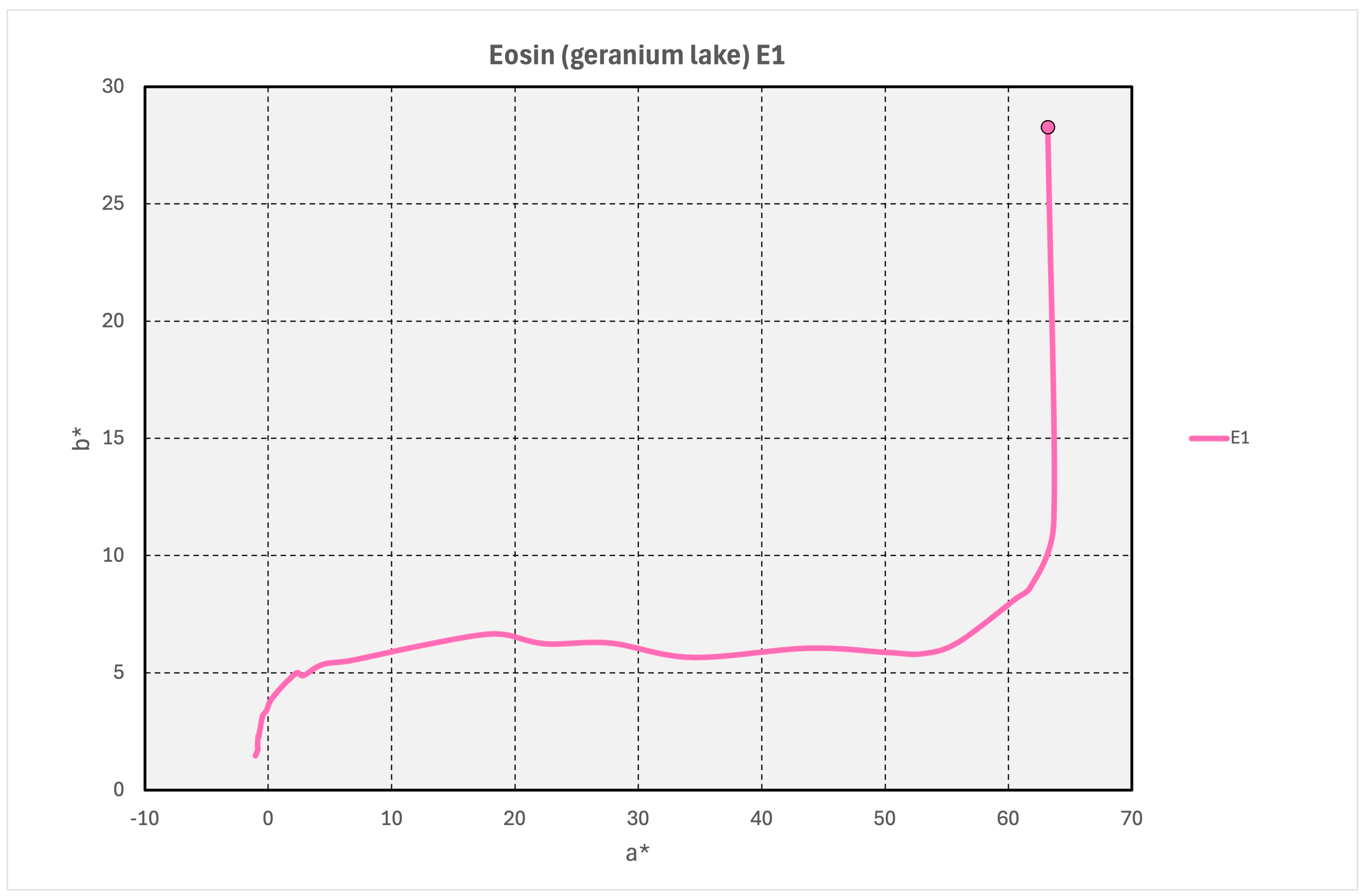
3.7. Eosin
4. Discussion and Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Saunders, D.; Kirby, J. Light-induced Colour Changes in Red and Yellow Lake Pigments. Nat. Gallery Tech. Bull. 1994, 15, 79–97. Available online: https://www.nationalgallery.org.uk/research/research-resources/technical-bulletin/technical-bulletin-volume-15 (accessed on 14 January 2025).
- Kirby, J.; Saunders, D. Sixteenth- to Eighteenth-century Green Colours in Landscape and Flower Paintings: Composition and Deterioration. Stud. Conserv. 1998, 43 (Suppl. 1), 155–159. [Google Scholar] [CrossRef]
- Padfield, T.; Landi, S.B. The Light-Fastness of the Natural Dyes. Stud. Conserv. 1966, 11, 181–196. [Google Scholar] [CrossRef]
- Duff, D.G.; Sinclair, R.S.; Stirling, D. Light-induced Colour Changes of Natural Dyes. Stud. Conserv. 1977, 22, 161–169. [Google Scholar] [CrossRef]
- Crews, P.C. The Influence of Mordant on the Lightfastness of Yellow Natural Dyes. J. Am. Inst. Conserv. 1982, 21, 43–58. [Google Scholar] [CrossRef]
- Crews, P.C. The Fading Rates of some Natural Dyes. Stud. Conserv. 1987, 32, 65–72. [Google Scholar] [CrossRef]
- Johnston-Feller, R.; Bailie, C.W. An Analysis of the Optics of Paint Glazes: Fading. Stud. Conserv. 1982, 27 (Suppl. 1), 180–185. [Google Scholar] [CrossRef]
- Johnston-Feller, R.; Feller, R.L.; Bailie, C.W.; Curran, M. The Kinetics of Fading: Opaque Paint Films Pigmented with Alizarin Lake and Titanium Dioxide. J. Am. Inst. Conserv. 1984, 23, 114–129. [Google Scholar] [CrossRef]
- Johnston-Feller, R.M. Reflections on the Phenomenon of Fading. J. Coat. Technol. 1986, 58, 33–50. [Google Scholar]
- Saunders, D.; Kirby, J. A Comparison of Light-induced Damage under Common Museum Illuminants. In ICOM Committee for Conservation 15th Triennial Meeting, New Delhi, India, 22–26 September 2008; Bridgland, J., Ed.; ICOM: Paris, France, 2008; Volume 2, pp. 766–774. Available online: www.icom-cc-publications-online.org/1935/A-comparison-of-light-induced-damage-under-common-museum-illuminants (accessed on 30 October 2024).
- Kirby, J.; Saunders, D.; Spring, M. Proscribed Pigments in Northern European Renaissance Paintings and the Case of Paris Red. Stud. Conserv. 2006, 51 (Suppl. 2), 236–243. [Google Scholar] [CrossRef]
- Kirby, J.; van Bommel, M.; Verhecken, A.; Spring, M.; Vanden Berghe, I.; Stege, H.; Richter, M. Natural Colorants for Dyeing and Lake Pigments: Practical Recipes and Their Historical Sources; Archetype Publications Ltd.: London, UK, 2014; ISBN 978-190-949-215-8. [Google Scholar]
- Burnstock, A.; Lanfear, I.; van den Berg, K.J.; Carlyle, L.; Clarke, M.; Hendriks, E.; Kirby, J. Comparison of the Fading and Surface Deterioration of Red Lake Pigments in Six Paintings by Vincent van Gogh with Artificially Aged Paint Reconstructions. In ICOM-Committee for Conservation 14th Triennial Meeting, The Hague, 12–16 September 2005; Verger, I., Ed.; James & James/Earthscan: London, UK, 2005; Volume 1, pp. 459–466. Available online: www.icom-cc-publications-online.org/2081/Comparison-of-the-fading-and-surface-deterioration-of-red-lake-pigments-in-six-paintings-by-Vincent-van-Gogh-with-artificially-aged-paint-reconstructions (accessed on 14 January 2025).
- Boutet, C. Traité de Mignature, Pour Apprendre Aisément à Peindre sans Maistre..., 3rd ed.; chez Christophe Ballard: Paris, France, 1678; pp. 12–13, 139–141. [Google Scholar]
- Rahn Phillips, C. The growth and composition of trade in the Iberian empires, 1450–1750. In The Rise of Merchant Empires: Long-Distance Trade in the Early Modern World, 1350–1750; Tracy, J.D., Ed.; Cambridge University Press: Cambridge, UK, 1993; pp. 34–101. ISBN (digital ed.) 978-051-156-308-9. [Google Scholar] [CrossRef]
- Marichal Salinas, C. Mexican cochineal, local technologies and the rise of global trade from the sixteenth to the nineteenth centuries. In Global History and New Polycentric Approaches: Europe, Asia and the Americas in a World Network System; Perez Garcia, M., De Sousa, L., Eds.; Palgrave MacMillan: Singapore, 2018; pp. 255–273. ISBN 978-981-10-4052-8. ISBN digital 978-981-10-4053-5. [Google Scholar] [CrossRef]
- Kirby, J.; Higgitt, C.; Spring, M. Madder lakes of the 15th–17th centuries: Variability of the dyestuff content. In The Diversity of Dyes in History and Archaeology; Kirby, J., Ed.; Archetype Publications Ltd.: Lindon, UK, 2017; pp. 148–161. ISBN 978-190-949-253-0. [Google Scholar]
- Quye, A.; Hallett, K.; Herrero Carretero, C. Wrought in Gold and Silk: Preserving the Art of Historic Tapestries; National Museums Scotland: Edinburgh, Scotland, 2009; ISBN 978-190-526-715-6. [Google Scholar]
- Hacke, M. Investigation into the Nature and Ageing of Tapestry Materials. Ph.D. Thesis, University of Manchester, Manchester, UK, 2006. [Google Scholar]
- Duffus, P.; Carr, C.; Vlachou-Mogire, C.; Potluri, P.; Margetts, L. Manufacture, analysis and conservation strategies for tapestries. In A Woven Alliance: Tapestry Yesterday, Today and for Tomorrow; Symposium of the ICON Textile Group, 21 September 2012, The Dovecot Studios, Edinburgh; (unpaginated; 3rd article in collection); Lennard, F., McClean, L., Eds.; Institute of Conservation: London, UK, 2013. [Google Scholar]
- University of Manchester. News, 10 April 2009. Scientists ‘Virtually Restore’ 16th-Century Tapestry at Hampton Court Palace. Available online: https://www.manchester.ac.uk/about/news/scientists-virtually-restore-16th-century-tapestry-at-hampton-court-palace/ (accessed on 15 June 2025).
- Perkins, R. Tapestries Revealed: Novel Methods of Characterisation, Conservation and Presentation. Ph.D. Thesis, University of Manchester, Manchester, UK, 2011. [Google Scholar]
- Frencken, H.G.T. T’bouck van Wondre, 1513; Roermond: Drukkerij H; Timmermans: Roermond, The Netherlands, 1934. [Google Scholar]
- De Nie, W.L.J. De Ontwikkeling der Noord-Nederlandsche Textielververij van de Veertiende tot de Achttiende Eeuw; N.V. Boek-en Steendrukkerij Eduard Ijdo: Leiden, The Netherlands, 1937. [Google Scholar]
- Rosetti, G. The Plictho of Gioanventura Rosetti: Instructions in the Art of the Dyers of Woolen Cloths, Linens, Cottons, and Silk by the Great Art as Well as By the Common. Translation of the First Edition of 1548; Edelstein, S.M., Borghetty, H.C., Eds.; MIT Press: Cambridge, MA, USA; London, UK, 1969. [Google Scholar]
- CIE (Commission Internationale de l’Éclairage). Recommendations on Uniform Color Spaces, Color-Difference Equations, Psychometric Color Terms; Publication no. 15, sup. 2; Bureau central de la CIE: Paris, France, 1978. [Google Scholar]
- CIE (Commission Internationale de l’Éclairage). Colorimetry—Part 6: CIEDE2000 Colour-Difference Formula. CIE Technical Report 11664-6:2022; CIE Central Bureau: Vienna, Austria, 2022. [Google Scholar]
- Giles, C.H.; McKay, R.B. The Lightfastness of Dyes: A Review. Text. Res. J. 1963, 33, 528–577. [Google Scholar] [CrossRef]
- Giles, C.H. The Fading of Colouring Matters. Stud. Conserv. 1965, 9 (Suppl. 1), 8–26. [Google Scholar] [CrossRef]
- De Keijzer, M.; Proaño Gaibor, A.N.; Geldof, M. Cornelis Drebbel’s scarlet. In Dyes in History and Archaeology, 35/36; Kirby, J., Ed.; Archetype Publications Ltd.: London, UK, 2021; pp. 1–14. ISBN 978-190-949-281-3. [Google Scholar]
- Gent, A.; Roy, A.; Morrison, R. Practice makes Imperfect: Reynolds’s Painting Technique. Nat. Gallery Tech. Bull. 2014, 35, 12–30. Available online: https://www.nationalgallery.org.uk/research/research-resources/technical-bulletin/technical-bulletin-volume-35 (accessed on 14 January 2025).
- Rader Bowers, L.M.; Schmidtke Sobeck, S.J. Impact of Medium and Ambient Environment on the Photodegradation of Carmine in Solution and Paints. Dye. Pigm. 2016, 127, 18–24. [Google Scholar] [CrossRef]
- Pirok, B.W.J.; Moro, G.; Meekel, N.; Berbers, S.V.J.; Schoenmakers, P.J.; van Bommel, M.R. Mapping Degradation Pathways of Natural and Synthetic Dyes with LC-MS: Influence of Solvent on Degradation Mechanisms. J. Cult. Herit. 2019, 38, 29–36. [Google Scholar] [CrossRef]
- Peggie, D.A.; Kirby, J.; Poulin, J.; Genuit, W.; Romanuka, J.; Wills, D.F.; De Simone, A.; Hulme, A.N. Historical Mystery Solved: A Multi-Analytical Approach to the Identification of a Key Marker for the Historical Use of Brazilwood (Caesalpinia spp.) in Paintings and Textiles. Anal. Methods 2018, 10, 617–623. [Google Scholar] [CrossRef]
- Willemen, H.; van den Meijdenberg, G.J.P.; van Beek, T.A.; Derksen, G.C.H. Comparison of Madder (Rubia tinctorum L.) and Weld (Reseda luteola L.) Total Extracts and their Individual Dye Compounds with Regard to their Dyeing Behaviour, Colour, and Stability towards Light. Color. Technol. 2018, 135, 40–47. [Google Scholar] [CrossRef]
- Peggie, D.A.; Kirby, J. The use and identification of red lake pigments in paintings by Rembrandt. In Rembrandt Now: Technical Practice, Conservation and Research; Spring, M., Roy, A., Eds.; The National Gallery with Archetype Publications: London, UK, 2022; pp. 71–83. Available online: https://www.nationalgallery.org.uk/research/research-resources/research-papers/rembrandt-now (accessed on 21 January 2025).
- Cardon, D. Natural Dyes: Sources, Tradition, Technology and Science; Archetype Publications Ltd.: London, UK, 2007; pp. 422–427. ISBN 190-498-200-. [Google Scholar]
- Luhamaa, L.; Rammo, R.; Bamford, D.; Vanden Berghe, I.; Veenhoven, J.; Wright, K.; Räisänen, R. Rotting for Red: Archival, Experimental and Analytical Research on Estonian Traditions of Decomposing Alder Buckthorn Bark Before Dyeing. Heritage 2025, 8, 220. [Google Scholar] [CrossRef]
- Merrifield, M.P. Original Treatises Dating from the XIIth to XVIIIth Centuries on the Arts of Painting; John Murray: London, UK, 1849; pp. ccxxxii–ccxxxiii. [Google Scholar]
- Neven, S. The Strasbourg Manuscript: A Medieval Tradition of Artists’ Recipe Collections (1400–1570); Archetype Publications Ltd.: London, UK, 2016; pp. 120–121. ISBN 978-190-949-241-7. [Google Scholar]
- Berger, E. Quellen für Maltechnik Während der Renaissance und Deren Folgezeit (XVI–XVIII Jahrhundert) in Italien, Spanien, den Niederlanden, Deutschland, Frankreich und England Nebst dem de Mayerne Manuskript; Georg, D.W., Ed.; Callwey: Munich, Germany, 1901; pp. 128–129, 138–139, 266–269, 306–307, 310–313, 316–325, 336–337. [Google Scholar]
- Favaro, G.; Clementi, C.; Romani, A.; Vickackaite, V. Acidichromism and Ionochromism of Luteolin and Apigenin, the Main Components of the Naturally Occurring Yellow Weld: A Spectrophotometric and Fluorimetric Study. J. Fluoresc. 2007, 17, 707–714. [Google Scholar] [CrossRef]
- De La Codre, H.; Marembert, C.; Claisse, P.; Daniel, F.; Chapoulie, R.; Servant, L.; Mounier, A. Non-invasive Characterization of Yellow Dyes in Tapestries of the 18th Century: Influence of Composition on Degradation. Color. Res. Appl. 2021, 46, 613–622. [Google Scholar] [CrossRef]
- Villela, A.; van Vuuren, M.S.A.; Willemen, H.M.; Derksen, G.C.H.; van Beek, T.A. Photo-stability of a Flavonoid Dye in Presence of Aluminium Ions. Dye. Pigm. 2019, 162, 222–231. [Google Scholar] [CrossRef]
- Clementi, C.; Doherty, B.; Gentili, P.L.; Miliani, C.; Romani, A.; Brunetti, B.G.; Sgamellotti, A. Vibrational and Electronic Properties of Painting Lakes. Appl. Phys. A 2008, 92, 25–33. [Google Scholar] [CrossRef]
- Groeneveld, I.; Kanelli, M.; Ariese, F.; van Bommel, M.R. Parameters That Affect the Photodegradation of Dyes and Pigments in Solution and on Substrate—An Overview. Dye. Pigm. 2023, 210, 110999. [Google Scholar] [CrossRef]
- Anselmi, C.; Capitani, D.; Tintaru, A.; Doherty, B.; Sgamellotti, A.; Miliani, C. Beyond the Color: A Structural Insight to Eosin-based Lakes. Dyes Pigm. 2017, 140, 297–311. [Google Scholar] [CrossRef]
- Chieli, A.; Miliani, C.; Degano, I.; Sabatini, F.; Tognotti, P.; Romani, A. New Insights into the Fading Mechanism of Geranium Lake in Painting Matrix. Dyes Pigm. 2020, 181, 108600. [Google Scholar] [CrossRef]
- Sabatini, F.; Eis, E.; Degano, I.; Thoury, M.; Bonaduce, I.; Lluveras-Tenorio, A. The Issue of Eosin Fading: A Combined Spectroscopic and Mass Spectrometric Approach Applied to Historical Lakes. Dye. Pigm. 2020, 180, 108436. [Google Scholar] [CrossRef]
- Geldof, M.; de Keijzer, M.; van Bommel, M.; Pilz, K.; Salvant, J.; van Keulen, H.; Megens, L. Van Gogh’s geranium lake. In Van Gogh’s Studio Practice; Vellekoop, M., Geldof, M., Hendriks, E., Jansen, L., de Tagle, A., Eds.; Van Gogh Museum: Amsterdam, The Netherlands; Mercatorfonds: Brussels, Belgium, 2013; pp. 268–289. [Google Scholar]
- Geldof, M.; Proaño Gaibor, A.N.; Ligterink, F.; Hendriks, E.; Kirchner, E. Reconstructing Van Gogh’s Palette to Determine the Optical Characteristics of his Paints. Herit. Sci. 2018, 6, 17. [Google Scholar] [CrossRef]
- Centeno, S.A.; Hale, C.; Carò, F.; Cesaratto, A.; Shibayama, N.; Delaney, J.; Dooley, K.; van der Snickt, G.; Janssens, K.; Stein, S.A. Van Gogh’s Irises and Roses: The Contribution of Chemical Analyses and Imaging to the Assessment of Color Changes in the Red Lake Pigments. Herit. Sci. 2017, 5, 18. [Google Scholar] [CrossRef]
- Monticolo, G. I Capitolari delle Arti Veneziane; Forzan e C.; Tipografi del Senato: Rome, Italy, 1896; Volume 1, p. 142. [Google Scholar]
- Espinas, G.; Pirenne, H. Recueil de D’cuments Relat’fs à L’histoire de L’industrie Drapière en Flandre. Première Partie, Des Origines à L’époque Bourguignonne; Librairie Kiessling et Cie, P; Imbreghts Successeur: Brussels, Belgium, 1920; pp. 479–480. [Google Scholar]
- Wilson, H.; Cruickshank, P.; Hacke, M.; Stacey, R.; Carr, C.; Daniels, V.; Rigout, M. Investigation of Non-aqueous Remedial Treatments for Iron-tannate Dyed Textiles. In ICOM-Committee for Conservation, 16th Triennial Meeting, Lisbon, Portugal, 19–23 September 2011 Critério Artes; Gráficas, L., Ed.; ICOM Committee for Conservation: Lisbon, Portugal; Available online: www.icom-cc-publications-online.org/1132/Investigation-of-non-aqueous-remedial-treatments-for-iron-tannate-dyed-textiles (accessed on 14 January 2025).
- Standen, E.A. European Post-Medieval Tapestries and Related Hangings in the Metropolitan Museum of Art; Metropolitan Museum of Art: New York, NY, USA, 1985; Volume 2, pp. 688–691. [Google Scholar]
- Hermens, E.; Wallert, A. The Pekstok papers, lake pigments, prisons and paint-mills. In Looking Through Paintings: The Study of Painting Techniques and Materials in Support of Art Historical Research; Hermens, E., Ouwerkerk, A., Costaras, N., Eds.; Archetype Publications: London, UK, 1998; pp. 269–294. [Google Scholar]












| Code | Pigments (in Linseed Oil Medium) † |
|---|---|
| C3A | Cochineal C3 (Sn substrate) + lead white 1:20 w/w |
| C3B | Cochineal C3 (Sn substrate) + lead white 1:8 w/w |
| C6 | Cochineal C6 (Sn substrate + starch extender) |
| CD1A | Cochineal CD1 (Al substrate) + lead white 1:40 w/w |
| CD1B | Cochineal CD1 (Al substrate) + lead white 1:20 w/w |
| CD1C | Cochineal CD1 (Al substrate) + lead white 1:8 w/w |
| M7A | Madder lake from plant M7i |
| MD6A | Madder lake from wool MD6ai |
| E1 | Eosin lake E1 |
| B10A | ‘Brazilwood’ BBr 10 * + lead white 1:40 w/w |
| B10B | ‘Brazilwood’ BBr 10 * + lead white 1:10 w/w |
| B10C | ‘Brazilwood’ BBr 10 * |
| VE | Vermilion |
| LW | Lead white |
| ILW | Indigo + lead white |
| PBC1 | Prussian blue SC + lead white 1:400 w/w |
| PBC2 | Prussian blue SC + lead white 1:100 w/w |
| PBC3 | Prussian blue SC + lead white 1:20 w/w |
| SG | Sap green lake |
| INY | Indigo + Naples yellow |
| BLUW | Buckthorn lake BuDS3 + ultramarine + lead white |
| GLW | Gamboge + lead white 1:3 v/v |
| W6LW | Weld lake W6 (Al substrate) + lead white 1:3 v/v |
| W7LW | Weld lake W7 (Al/Ca substrate) + lead white 1:3 v/v |
| Code | Textiles (MODHT) |
|---|---|
| RW1A | Madder (mordanted + semelwater * and alum; dyed + semelwater) |
| RW1B | Madder (mordanted + semelwater and alum; dyed + semelwater; treated + K2CO3) |
| RW2A | Madder (mordanted + oak galls then + alum; dyed) |
| RW2B | Madder (mordanted + oak galls then + alum; dyed; treated + K2CO3) |
| RW3A | Brazilwood (mordanted + alum; dyed) |
| RW3B | Brazilwood (mordanted + alum; dyed; treated + K2CO3) |
| RW4 | Cochineal (mordanted + alum, D-tartaric acid, salt and sandalwood; dyed + starch, salt and turmeric) |
| RW5 | Cochineal (mordanted + alum, D-tartaric acid, salt and sandalwood; dyed + gum arabic, alum, salt and turmeric) |
| BKW1 | Black (mordanted + oak galls; dyed + Fe(II) sulfate; all hanks rinsed together) |
| BKW2 | Black (mordanted + oak galls; dyed + Fe(II) sulfate; hanks rinsed separately) |
| BKW3 | Black (mordanted + alder bark; dyed + Fe(II) sulfate) |
| BKW4 | Black (mordanted + oak galls; dyed + Cu(II) + Fe(II) sulfates) |
| YW1 | Weld (mordanted + alum; dyed + K2CO3) |
| YW2 | Dyer’s broom (mordanted + alum; dyed + K2CO3) |
| GW1 | Green (weld as in YW1, overdyed with woad indigo) |
| GW2 | Green (woad indigo, overdyed with weld as in YW1) |
| IW1 | Indigo (all woad indigo dyed by the late John Edmonds) |
| Sample | Sample Code | Half-Life (Mlux·h) |
|---|---|---|
| Cochineal C3 (Sn substrate) + lead white 1:20 w/w | C3A | 27 |
| Cochineal C3 (Sn substrate) + lead white 1:8 w/w | C3B | 80 |
| Cochineal CD1 (Al substrate) + lead white 1:40 w/w | CD1A | 26 |
| Cochineal CD1 (Al substrate) + lead white 1:20 w/w | CD1B | 29 |
| Cochineal CD1 (Al substrate) + lead white 1:8 w/w | CD1C | 46 |
| Madder lake from wool MD6ai | MD6A | 114 |
| Madder lake from plant M7i | M7A | 120 |
| Eosin lake E1 | E1 | 8 |
| ‘Brazilwood’ BBr 10 + lead white 1:40 w/w | B10A | 6 |
| ‘Brazilwood’ BBr 10 + lead white 1:10 w/w | B10B | 12 |
| ‘Brazilwood’ BBr 10 | B10C | 36 |
| Weld lake W6 (Al substrate) + lead white 1:3 v/v | W6LW | 25 |
| Weld lake W7 (Al/Ca substrate) + lead white 1:3 v/v | W7LW | 29 |
| Madder (mordanted + semelwater and alum; dyed + semelwater) | RW1A | 59 |
| Madder (mordanted + semelwater and alum; dyed + semelwater; treated + K2CO3) | RW1B | 60 |
| Madder (mordanted + oak galls then + alum; dyed) | RW2A | 55 |
| Madder (mordanted + oak galls then + alum; dyed; treated + K2CO3) | RW2B | 52 |
| Brazilwood (mordanted + alum; dyed) | RW3A | 21 |
| Brazilwood (mordanted + alum; dyed; treated + K2CO3) | RW3B | 21 |
| Cochineal (mordanted + alum, D-tartaric acid, salt and sandalwood; dyed + starch, salt and turmeric) | RW4 | 53 |
| Cochineal (mordanted + alum, D-tartaric acid, salt and sandalwood; dyed + gum arabic, alum, salt and turmeric) | RW5 | 43 |
| Weld (mordanted +alum; dyed + K2CO3) | YW1 | 30 |
| Dyer’s broom (mordanted +alum; dyed + K2CO3) | YW2 | 32 |
| Green (weld as in YW1, overdyed with woad indigo) | GW1 | 27 |
| Green (woad indigo, overdyed with weld as in YW1) | GW2 | 24 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kirby, J.; Saunders, D. A Comparison of the Fading of Dyestuffs as Textile Colourants and Lake Pigments. Heritage 2025, 8, 260. https://doi.org/10.3390/heritage8070260
Kirby J, Saunders D. A Comparison of the Fading of Dyestuffs as Textile Colourants and Lake Pigments. Heritage. 2025; 8(7):260. https://doi.org/10.3390/heritage8070260
Chicago/Turabian StyleKirby, Jo, and David Saunders. 2025. "A Comparison of the Fading of Dyestuffs as Textile Colourants and Lake Pigments" Heritage 8, no. 7: 260. https://doi.org/10.3390/heritage8070260
APA StyleKirby, J., & Saunders, D. (2025). A Comparison of the Fading of Dyestuffs as Textile Colourants and Lake Pigments. Heritage, 8(7), 260. https://doi.org/10.3390/heritage8070260







