Deciphering Ageing Effects in Green-Dyed English Wool Carpet Yarns from the 1840s
Abstract
1. Introduction
2. Materials and Methods
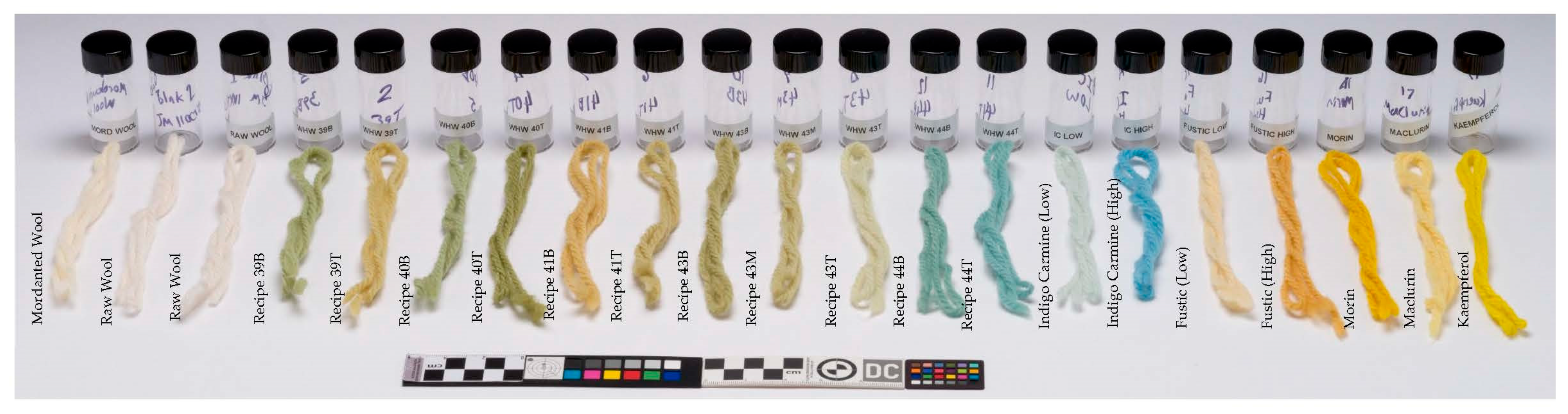
2.1. Materials
2.2. Methods
3. Results
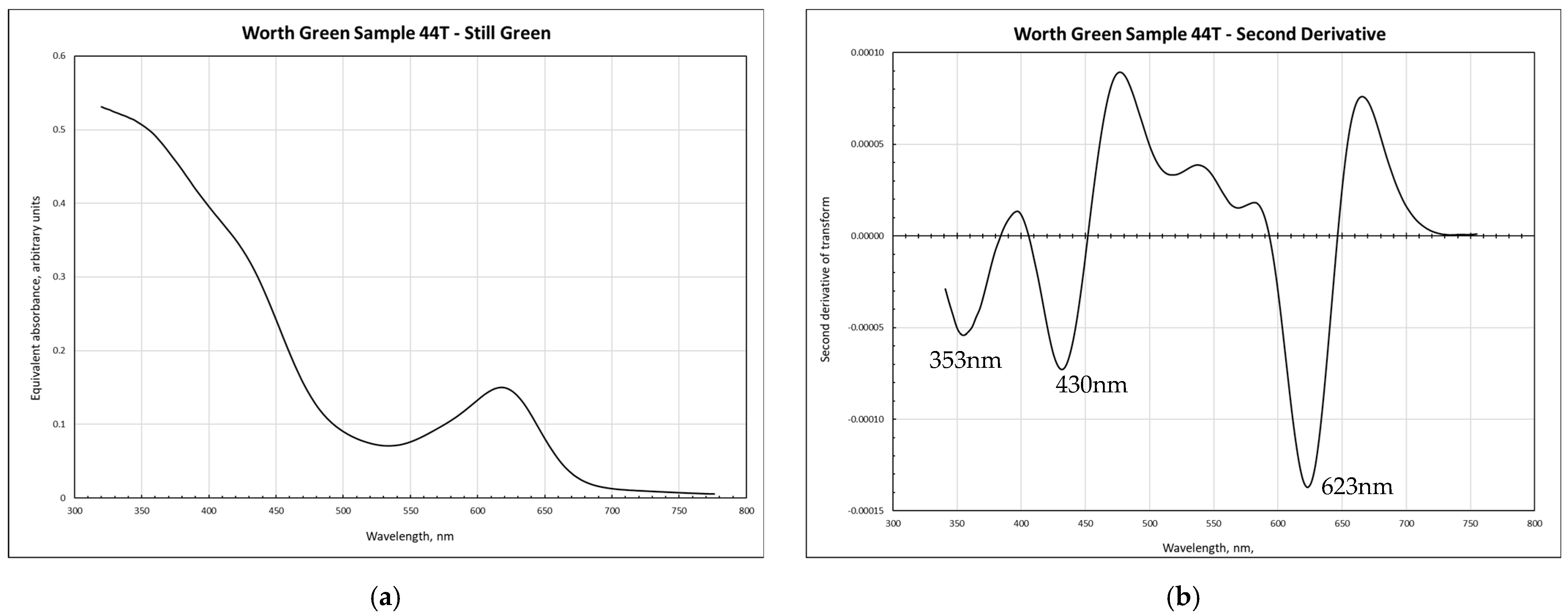
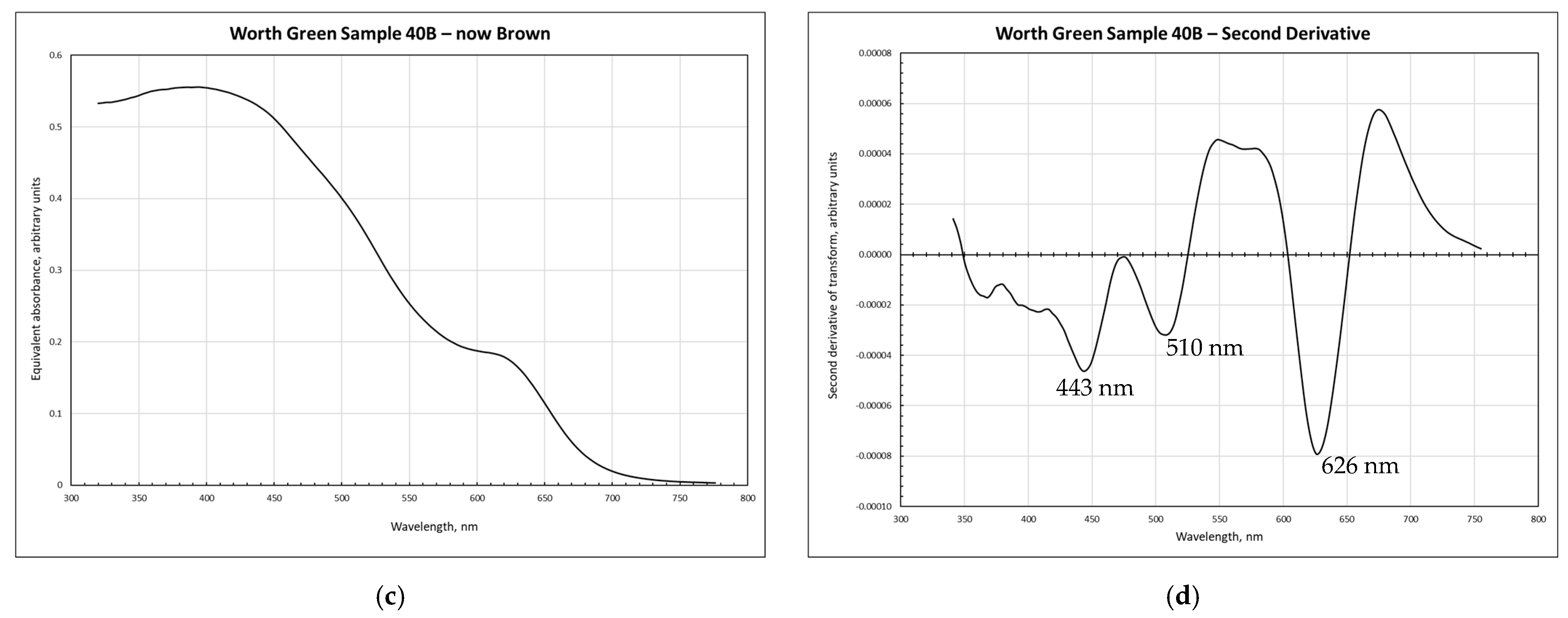
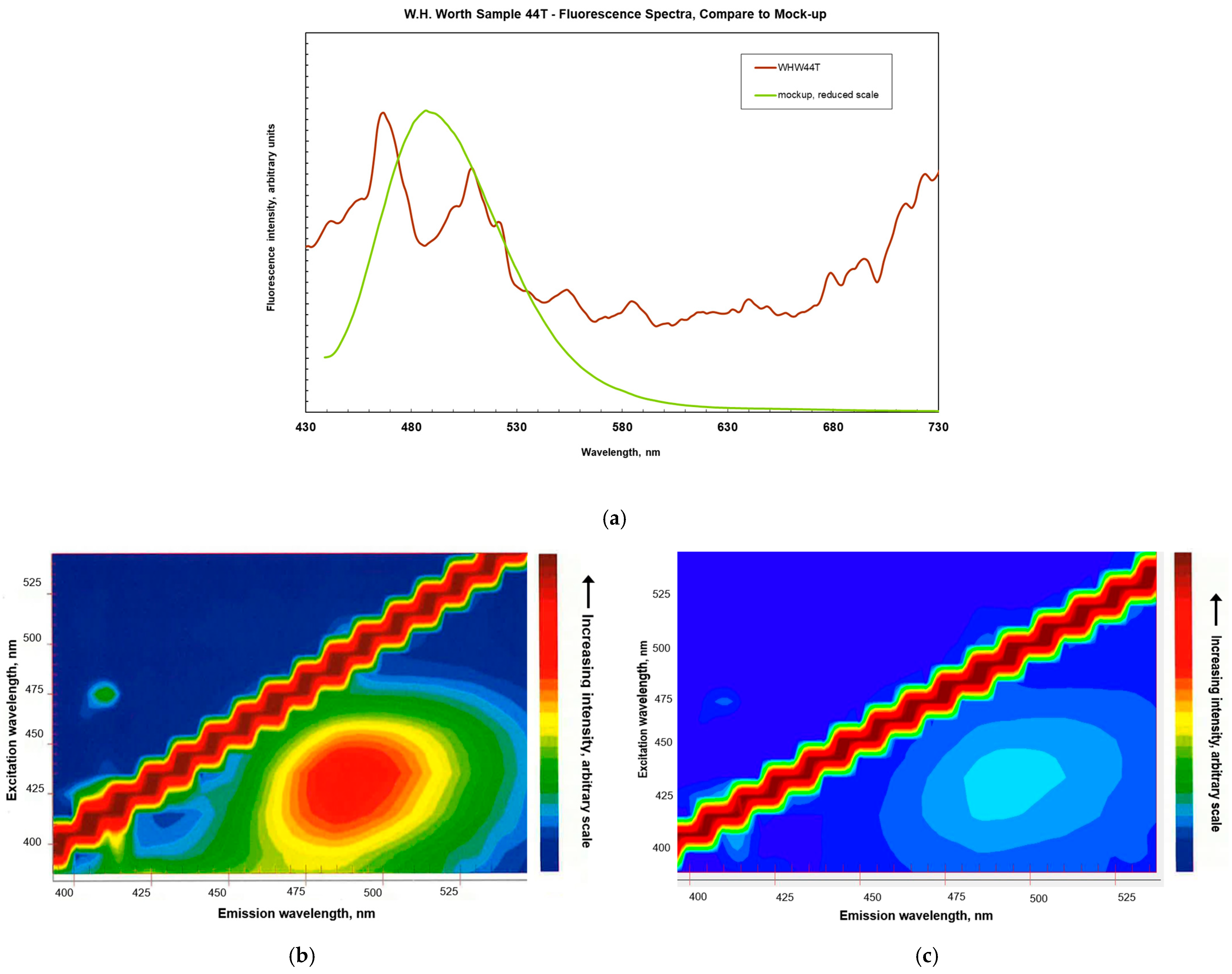
3.1. Spectroscopy
3.2. Variations in the Dyebath Recipes
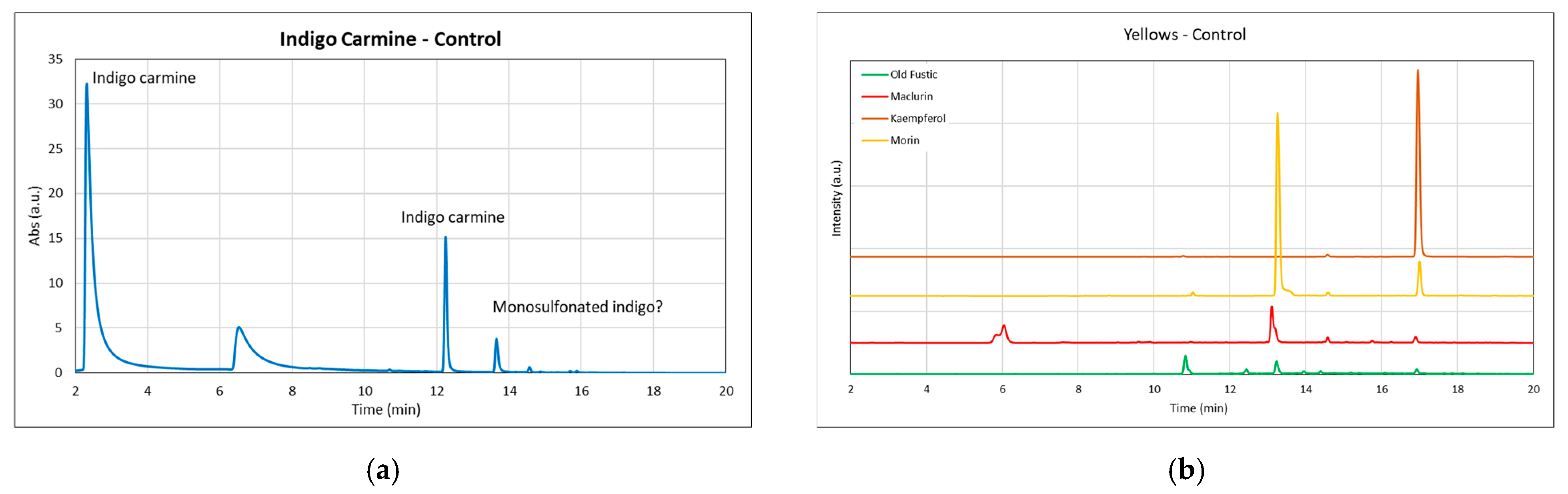
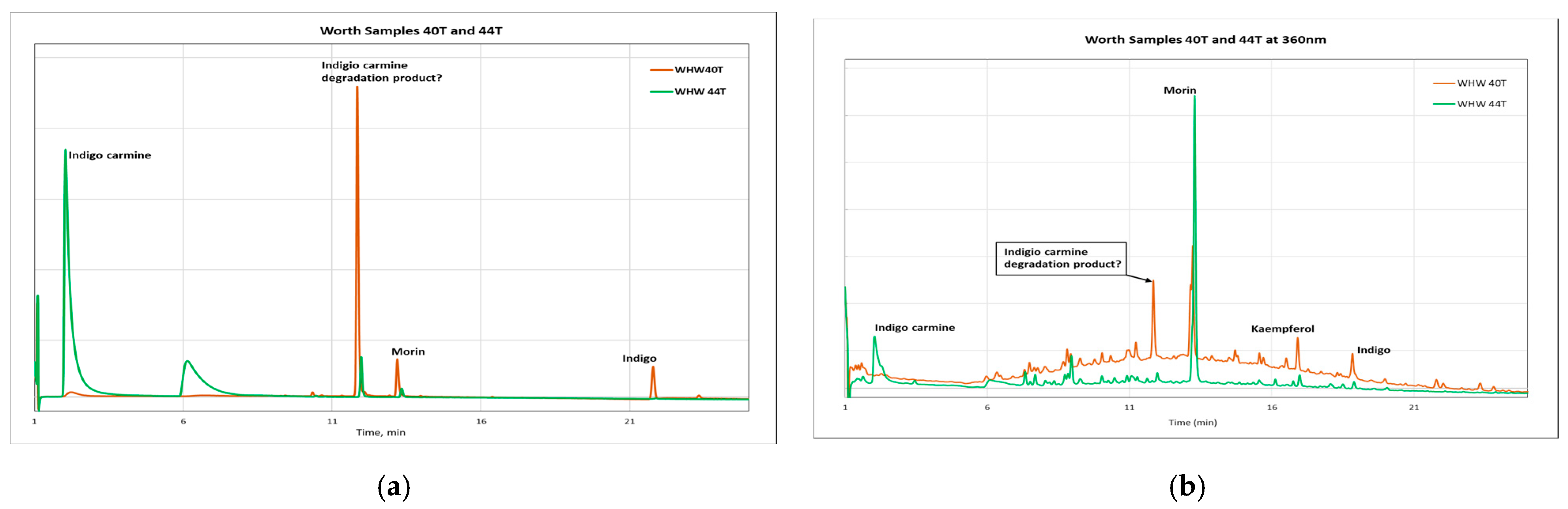
3.3. HPLC-DAD
4. Discussion
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
Appendix A.1. Analytical Methods
Appendix A.1.1. Fibre Optic Reflectance Spectroscopy (FORS)
Appendix A.1.2. Fluorescence Spectroscopy
Appendix A.1.3. High Performance Liquid Chromatography with Diode Array Detection (HPLC-DAD)
References
- Schaeffer, T.T.M. Worth’s woollen yarns: A Victorian book of dyed samples with recipes. In The Diversity of Dyes in History and Archaeology; Kirby, J., Ed.; Archetype Publications: London, UK, 2017; pp. 304–316. [Google Scholar]
- Hofenck de Graaff, J. The Colourful Past: Origins, Chemistry and Identification of Natural Dyestuffs, London and Riggisberg; Archetype Publications and Abegg-Stiftung: London, UK, 2004; pp. 258–260. [Google Scholar]
- Napier, J. A Manual of Dyeing and Dyeing Receipts: Comprising a System of Elementary Chemistry as Applied to Dyeing; Charles Griffin & Co.: Glasgow, UK, 1853; p. 300. [Google Scholar]
- Maccarelli, L.; Schaeffer, T. Recipes, Colourants, Carpets: The use of natural dyestuffs by a mid—Victorian English carpet manufacturer. Dye. Hist. Archaeol. 2023, 37/40, 156–168. [Google Scholar]
- De Keijzer, M.; van Bommel, M.; Hofmann-de Keijzer, R.; Knaller, R.; Oberhumer, E. Indigo carmine: Understanding a problematic blue dye. In 24th Biennial IIC Congress: The Decorative: Conservation and the Applied Arts. Stud. Conserv. 2012, 57, S87–S95. [Google Scholar] [CrossRef]
- Colombini, M.P.; Andreotti, A.; Baraldi, C.; Degano, I.; Lucejko, J. Color fading in textiles: A model study of the decomposition of natural dyes. Microchem. J. 2007, 85, 174–182. [Google Scholar] [CrossRef]
- Hoefener, S.; Kooijman, P.C.; Groen, J.; Ariese, F.; Visscher, L. Fluorescence behavior of (selected) flavonols: A combined experimental and computational study. Phys. Chem. Chem. Phys. 2013, 15, 12572–12581. [Google Scholar] [CrossRef] [PubMed]
- Feller, R.L. Appendix A. Photochemical effects in the discoloration of wool. In Accelerated Aging: Photochemical and Thermal Aspects; The Getty Conservation Institute: Los Angeles, CA, USA, 1994; pp. 169–176. [Google Scholar]
- Mathieson, S.; Ballard, M. The effect of moth resistance agents on the lightfastness of Natural Dyes. In Proceedings of the AIC Annual Meeting, Richmond, VA, USA, 29 May–3 June 1990. [Google Scholar]
- Mathieson, S. Changes in Natural Dyes on Wool Caused by Exposure to Moth Resistance Vapors, Either Naphthalene or p-Dichlorobenzene, in Closed Glass Jar for 5 Months in the Dark. Master’s Thesis, Fashion Institute of Technology (FIT), New York, NY, USA, 1989. [Google Scholar]








| Green Yarn Sample | Dyes Component | Mordant | ||||
|---|---|---|---|---|---|---|
| Old Fustic | Mixture | Extract of Indigo | Alum | Vitriol | ||
| WHW 39T 1 |  | X | X | X | ||
| WHW 39B |  | X | X | X | ||
| WHW 40T |  | X | X | X | ||
| WHW 40B |  | X | X | X | ||
| WHW 41T |  | X | X | X | ||
| WHW 41B |  | X | X | X | ||
| WHW 42T |  | No Text | No Text | No Text | No Text | No Text |
| WHW 42B |  | No Text | No Text | No Text | No Text | No Text |
| WHW 43T |  | X | X | X | ||
| WHW 43M |  | X | X | X | ||
| WHW 43B |  | X | X | X | X | |
| WHW 44T |  | X | X | X | ||
| WHW 44B |  | X | X | X | X | |
| WHW 45 |  | No Text | No Text | No Text | No Text | No Text |
| WHW 88 |  | Number 16 Green 120 lbs of Worsted | ||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Schaeffer, T.T.; Mobberley, J.; Maccarelli, L. Deciphering Ageing Effects in Green-Dyed English Wool Carpet Yarns from the 1840s. Heritage 2025, 8, 216. https://doi.org/10.3390/heritage8060216
Schaeffer TT, Mobberley J, Maccarelli L. Deciphering Ageing Effects in Green-Dyed English Wool Carpet Yarns from the 1840s. Heritage. 2025; 8(6):216. https://doi.org/10.3390/heritage8060216
Chicago/Turabian StyleSchaeffer, Terry T., Jacob Mobberley, and Laura Maccarelli. 2025. "Deciphering Ageing Effects in Green-Dyed English Wool Carpet Yarns from the 1840s" Heritage 8, no. 6: 216. https://doi.org/10.3390/heritage8060216
APA StyleSchaeffer, T. T., Mobberley, J., & Maccarelli, L. (2025). Deciphering Ageing Effects in Green-Dyed English Wool Carpet Yarns from the 1840s. Heritage, 8(6), 216. https://doi.org/10.3390/heritage8060216









