1. Introduction
Alder buckthorn (
Frangula alnus Mill.) is a small tree growing naturally in Europe, North Africa, and North–West Asia. It thrives in different environments, both very wet and very dry, and is common in meadows and deciduous forests in Estonia, especially in the western part of the country [
1]. The leaves of alder buckthorn are shiny on the surface, and the flowers grow into pea-sized berries that are green at first, then ripen to red and finally black (
Figure 1). Its berries, leaves, and bark have been historically used for textile dyeing [
2,
3]. The focus of this article is on colours achievable from alder buckthorn bark.
The chemical composition of alder buckthorn bark is well-studied, as it is used for medical purposes [
4,
5,
6]. The main colouring compounds are anthraquinones. Three aglycones—chrysophanol, emodin, and physcion, often occurring together—are present in the bark. In the fresh bark, these anthraquinones are found mostly in combination with sugars as glycosides (e.g., frangulins A and B, glycofrangulins A and B), but the amount of glycosides decreases when the dried bark is stored [
2,
7]. Within one year of keeping, enzymatic processes transform the glycosides mentioned above into emodin, a source of red colour [
8].
The colours achievable from alder buckthorn bark are reported to be yellow, brown, and red [
2,
7,
9,
10]. To dye yellow or brown with alder buckthorn bark, the historic recipes mention boiling the bark in water and using alum. To obtain reddish shades, boiling the bark with the addition of alkali to the dye bath or soaking dyed yarns in lye after dyeing is instructed.
The earliest method of dyeing red with alder buckthorn bark is known from the south of Holland. It includes soaking a year-old dry bark in wood ash lye for nine days and then soaking the yarn for a few days in the warm dye bath. This description, which has been previously attributed to Johann C. Leuchs, who published it in 1825 [
11], was probably first reported by the Swedish scholar Johan Fischerström in 1761 [
12] as the description in Leuchs’ book is almost verbatim to Fischerström’s. It is a typical example of early 19th-century scientific literature paying little attention to referencing. The description of the Dutch method places the tradition of dyeing red with alder buckthorn bark in Europe firmly in the 18th century and more than 60 years earlier than previously reported [
2].
Estonian historical sources from the 19th and early 20th century reveal another, rather peculiar way of dyeing woollen yarn red with alder buckthorn bark, involving pre-treating alder buckthorn bark by rotting. To rot the alder buckthorn bark, the bark was left on the ground or dug underground for shorter or longer periods. This article introduces descriptions of this method in Estonian published and unpublished historical sources, as well as practical experiments that were conducted based on this information. With the experiments, the effects on colours achievable with alder buckthorn bark after shorter and longer-term rotting both on and under the ground were tested. Woollen yarns were dyed with rotted bark using the boiling method and tested for alkaline pH sensitivity and light fastness. Using HPLC-DAD, it was investigated whether the pretreatment of the bark affected not only the colour but also the dye composition of the dyed wool.
2. Materials and Methods
2.1. Published and Unpublished Written Sources
There are records describing the Estonian tradition of rotting alder buckthorn bark in published as well as unpublished sources. The only published report can be found in a book of the early 19th century (
Table 1, [
13]). In unpublished archival sources, there are five reports from the late 19th and early 20th centuries (
Table 1, [
14,
15,
16,
17,
18]).
The published report can be found in Johann Christoph Petri’s book “Ehstland und die Ehsten”, from 1802 [
13]. A similar description was brought forward by Wilhelm Christian Friebe in his book “Oekonomisch-technische Flora für Liefland, Ehstland und Kurland” [
19], discussed previously in connection to Estonian traditions [
20]. However, Friebe described the method as being used by Finnish women, and that the bark of common buckthorn (
Rhamnus cathartica, L.) was processed in this way. Considering that Petri’s book was published three years earlier than Friebe’s, it is very probable that it actually was an Estonian, not Finnish tradition, and that alder buckthorn bark, not common buckthorn bark, was pretreated by rotting. Moreover, common buckthorn is rare in Finland, growing only in Åland and the southwestern archipelago of Finland [
21].
It is not clear why Friebe made such changes in his writing, as the similarities in the two descriptions leave no doubt that he references Petri’s description of an Estonian dye method. It is nevertheless possible that a similar way of processing alder buckthorn was also used in Finland. An ethnographic observation made in 1908 in Uusikirkko in Finland reports that old-fashioned wool skirts (
hurstut, i.e., a wraparound skirt) were dyed red with alder buckthorn bark [
22]. Finnish ethnographic information was mainly collected in the 1930s and 1940s, when dyeing with natural dyes was already an almost lost craft. It is difficult to know how much the published dye books and dye workshops had spread dye knowledge amongst the Finnish rural inhabitants by that time. The ethnographic recipes, however, contain information about rotting bark that is not mentioned in the dye books. The only published mention of rotted bark is from an article in the 1918
Käsiteollisuus craft journal. That recipe instructed rotting alder buckthorn bark in a heap of nettle (
Urtica dioica) to produce red hues on wool yarns [
23]. Therefore, more detailed research into Finnish sources is needed.
The information in five Estonian unpublished sources was collected in the late 19th century and early 20th century (
Table 1, [
14,
15,
16,
17,
18]). At the end of the 19th century, when conscious gathering of all kinds of Estonian folklore started, only fragmental information about traditional plant dyeing reached the archives. There never was systematic data collection about the use of natural dyes in Estonia at the time when people still remembered the traditions. Therefore, having information from five different sources is significant. In two sources, it is specified that rotting of alder buckthorn bark was in use at least 50 years earlier, placing it around the middle of the 19th century [
15,
17]. Also, this tradition seems to have spread all over Estonia, as reports were collected from various parts of the country. In addition to the archival texts, the Estonian National Museum has two historic textiles—a pickup woven belt (ERM 10057, Martna parish) and a striped skirt (ERM 13475, Peetri parish)—from the 19th century in their collection, that were acquired with the information that alder buckthorn bark was the dye source for red yarns (
Figure 2).
Although chemical analysis revealed that redwood and cochineal were the sources of red dyes in these textiles [
24], the initial attribution of the red colour to alder buckthorn bark indicates that it was known as a source of red in these parishes as well. An in-depth analysis of the Estonian sources was published previously [
20].
2.2. Rotting Experiments
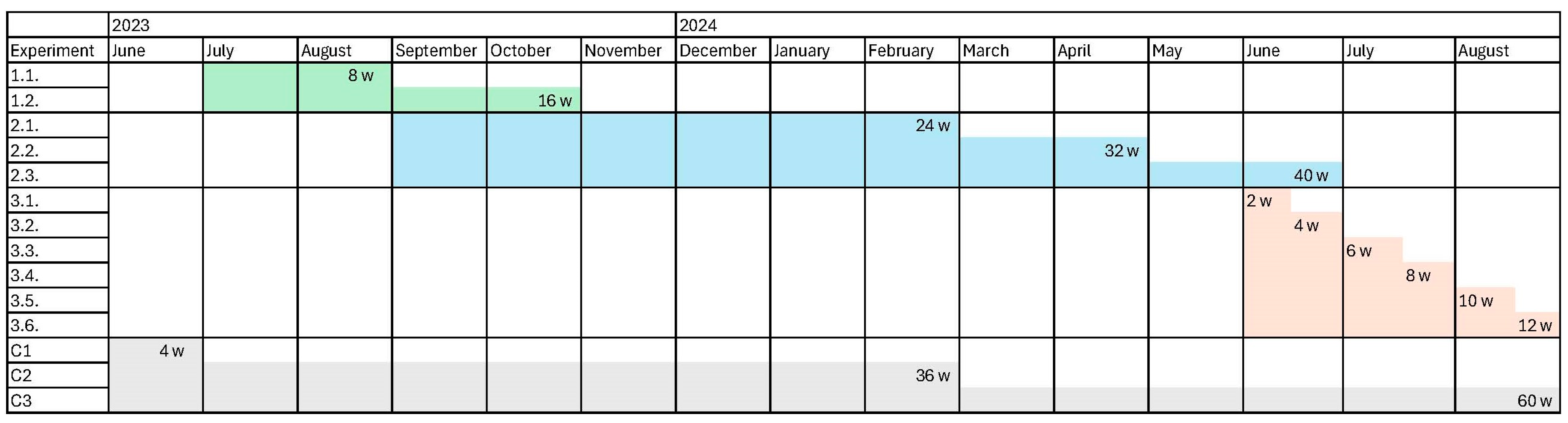
To evaluate the effect of rotting on colours achievable with alder buckthorn bark, three rotting experiments of varying lengths taking place during warmer and colder seasons were conducted in 2023 and 2024 (
Table 2,
Figure 3). The time points for the experiments were chosen based on the information provided in the archival records (
Table 1). It was taken into consideration that the seasons rotting took place, and rotting durations described in the sources varied. The rotting supposedly lasted from two weeks [
15] to several months [
16] and even up to a year [
14]. It was indicated that short-term rotting occurred during the warmer season, while the bark was left to rot long-term during the cold season (i.e., over winter). In the first experiment, intended to provide proof of concept, periods for rotting were chosen that stayed between the minimum and maximum rotting times mentioned in the archives. Rotting bark for 2 and 4 months was tested in this experiment, respectively. In the second experiment, long-term rotting over winter was tested. In the third experiment, short-term rotting during summer was tested.
In this article, we use the term rotting for the decomposition of the bark. The process was not analysed in more detail because of the complexity of bark decomposition and the wide range of biochemical and microbiological processes as well as seasonal temperature and moisture level changes involved in the process. As seasonal temperature differences are referred to in the text, the average outdoor air temperatures in Estonia during the experiments are shown in
Figure A1,
Appendix A.
The first preliminary rotting experiment, testing proof of concept, took 16 weeks (
Table 2, 1.1, 1.2). Based on the positive results of this experiment, two experiments lasting for 40 weeks and 12 weeks, testing long-term rotting over winter and short-term rotting in summer, respectively, followed (
Table 2, 2.1–2.3, 3.1–3.6). On-the-ground and underground rotting were tested simultaneously in each experiment, and 22 rotted bark samples were collected and used for dyeing. In addition, dried 4-, 36-, and 60-week-old unrotted barks were used for control dyeings (
Table 2, C1–C3). In summary, twenty-five differently treated bark samples were used in the dyeing experiments (
Table 2).
Alder buckthorn bark used in the experiments was collected from Seliste and Mereäärse villages in Southwest Estonia in June 2023 (
Table 2, 1.1–2.6) and in May–June 2024 (
Table 2, 3.1–3.12) at the time when the tree sap was running. Alder buckthorn trees of 2–4 cm in diameter near the ground were cut down, a thin strip of the bark was cut off lengthwise along the tree trunk with a short-bladed knife, and the rest of the bark was peeled off (
Figure 4).
After peeling, the bark was dried indoors on perforated racks in thin layers (
Figure 4). The drying lasted until the bark pieces broke easily when bent, indicating a moisture level of less than 15% [
25]. The weight of the bark decreased by about 45% during the drying. Part of the bark collected in June 2023 was saved for control dyeings (
Table 2, C1–C3) and kept in a cardboard box at room temperature until use.
Dense mesh polyester bags were used for storing bark during the rotting except in experiment 1.1. The bark of this first experiment was rotted in cotton bags in 800 g batches with the aim of replicating a possible historic way of storing the bark during rotting. As the bag disintegrated already during the first 8-week-long underground rotting and made the collection of the bark difficult, the bark was transferred to dense mesh polyester bags, and the rotting of experiment 1.2 was finished. The rotting of the bark in experiments 2 and 3 was conducted only in polyester bags. The bark was rotted in individual 300 g batches and collected after the respective time periods. The impact of changing the bag material mid-experiment and using polyester bags instead of cotton bags in the following experiments was not evaluated.
The bags with bark kept on the ground were stored behind an outbuilding along a tree-shaded wall, covered with a wide mesh net to prevent them from being scattered by animals (
Figure 5). For rotting underground, bags with bark were dug into the ground in an area previously used as a chicken yard, with fertile, humus-rich soil. The pH of the soil was 7.6, slightly alkaline. The bags were placed in a shallow, 10–15 cm deep hole and covered with soil from the same hole (
Figure 5). The corners of the hole were marked with stakes for ease of collection.
After the bags were collected, the bark was taken out of the bags, spread on dehydrator grates, and dried at 40 °C for at least 4 h or until completely dry to touch.
2.3. Dyeing Experiments
The same protocol of extracting the dye was used for all dye baths. The ratio of wool to bark was 1:2. The weighed-out bark was broken down somewhat by crushing it between hands. Tap water with a general hardness of 12 dH (i.e., a moderate water hardness) was used for the dye baths. The bark was covered with water using a 1:40 liquor ratio and soaked at room temperature overnight. The dye bath was then slowly heated to 85 °C and the temperature was maintained for 45 min. The dye bath was allowed to cool until the next day. Then the dye bath was strained, and the bark was discarded. Alum-mordanted (15% alum to the weight of textile) as well as unmordanted 100% wool yarns were dyed in the dye bath. It was slowly heated to 85 °C and the temperature was maintained for 45 min. The dyed materials were left to cool in the dye bath. The dyed and cooled materials were removed from the bath, rinsed, and dried.
2.4. Aftertreatment with Wood Ash Lye
As the oldest Estonian source indicated soaking the alder buckthorn bark-dyed yarns in wood ash lye, the effect of this aftertreatment was tested. Wood ash lye was prepared with hardwood (birch, common ash) ashes. A total of 1 kg of ashes taken from a wood-fired oven were covered with ten litres of boiling water and left to settle for two days at room temperature. After that, the clear lye liquid was poured off the ashes. The pH of the wood ash lye was 13.
A sample from each dye bath was used to evaluate the effect of wood ash lye. The dyed materials were covered with lye and left to soak at room temperature for 15 min. The ratio of yarn to lye was 1:20. After the soak, the materials were removed from the lye bath, rinsed thoroughly, and dried.
2.5. Light Fastness Testing
Light fastness testing was used to assess the viability of the dyestuffs by testing the dyed yarns to light against a known standard. The most common form of testing—the blue wool scale—was used. This consists of eight different samples of wool, dyed with synthetic dyes that have a known reaction to light and are totally reliable. The fastness ratings range from 1 (very low light fastness) to 8 (very high light fastness). These samples have been designed to fade in a geometric manner on exposure to light—each standard takes twice as long to fade to a certain level as the one below it [
26]. Historically, the dyes would have been rated by eye and durability in the wearing; here, they were tested against modern calculated standards. The standard used for lightfastness tests was ISO 105-BO2:2014 [
27].
Samples of the dyed yarns were wrapped around a non optically brightened white card. Part of the specimens were masked and the remaining part was exposed to a Xenon arc light representing D65 (natural daylight) using a James Heal Trufade light fastness tester (James Heal, Halifax, UK) along with blue standard samples. The samples were removed after specific periods of time (8, 16, 32, 64 h) and CIEL*a*b* readings were taken on a Verivide Digieye (D65 light source, 10° angle) using Digi Production software (VeriVide, Leicester, UK).
CIE, the International Commission on Illumination, devised a method of expressing colour as 3 values—L*, for perceptual lightness; a* and b* for the 4 colours of human vision (red, green, blue and yellow) where a given numerical change corresponds to a perceived change by the eye [
28].
Delta E (∆E
76) was calculated using the following equation:
The delta E calculations then gave the grade for fading to light.
2.6. HPLC-DAD Analysis
2.6.1. Sampling and Sample Preparation
HPLC analyses were performed to assess the impact of the pretreatment by rotting of alder buckthorn bark on or underground on the resulting dye composition in wool reference samples. The primary objective was to explore the correlation between this pretreatment and the emergence of redder colour hues. For this purpose, wool samples dyed with fresh air-dried alder buckthorn bark (
Table 2, experiment C1) were compared to samples dyed with bark that had undergone rotting on or underground for 2 and 4 months (
Table 2, experiments 1.1 and 1.2, respectively). Both dyeings with unmordanted and alum-mordanted wool were tested, to see if the mordanting affects not only the colour but also the dye composition in the fibres.
All yarns had undergone the same dyeing and post-treatment with wood ash lye as mentioned previously in paragraphs 2.3 and 2.4. For each of the woollen test yarns, a sample of 3–4 mm was taken. Dyes were recovered from the fibres by extraction in a solution of 250 μL ultrapure water/pure methanol (HPLC grade > 99.8%)/37% hydrochloric acid (1/1/2,
v/
v/
v) for 10 min at 105 °C, after which the samples were filtered and vacuum evaporated (SP Genevac EZ-2 Plus solvent evaporation system, Sysmex, Terhulpen, Belgium). The ultrapure water (18.2MΩ.cm resistivity at 25 °C) was obtained using the Aquadem-Purelab Flex 2 water purification system (Veolia Water STI, Tienen, Belgium). The residue was dissolved in 30/30 μL methanol/ultrapure water, of which 20 μL was injected into the chromatographic system. All reagents used were bought at VWR International (Leuven, Belgium). This extraction method, equally employed to analyse dyes from historical and archaeological Estonian textiles [
24], primarily targets dyes in their aglycone form. It is effective for analyzing mordant dyes, such as anthraquinones, which are the principal chromophores responsible for red shades in textiles.
2.6.2. Dye Analysis Protocol
The chromatographic analyses were conducted using the ACQUITY Arc system (Waters NV, Antwerp, Belgium) equipped with an autosampler and an automatic injection module and a column heater/cooler. The system was coupled to a photodiode array detector (2998PDA, Waters NV, Antwerp, Belgium) with analytical low dispersion flow cell (patented TaperSlit
TM4 flow cell, Waters NV, Antwerp, Belgium), which scanned absorbance over a wavelength range of 200–800 nm. Separation was achieved using a LiChrosorb RP-18 end-capped column (VWR International, Leuven, Belgium). The mobile phase consisted of three eluents: (A) pure methanol (HPLC grade, >99.8%), (B) a pure methanol/ultrapure water mixture (1:9,
v/
v), and (C) a 5% phosphoric acid solution (85 wt%
pro analisi) (VWR International, Leuven, Belgium). The mobile phase was delivered at a flow rate of 0.2 mL/min in gradient mode: 0–3 min: 23%A/67%B/10%C; 3–29 min: linear-gradient to 90%A/0%B/10%C; and re-equilibration between 30 and 35 min at 23%A/67%B/10%C [
29]. Data acquisition and processing were performed using Empower 3 system software (Waters NV, Antwerp, Belgium). The chromophores in the dye extracts were identified by comparing their absorption spectra with a user-generated reference database. This database contains spectral data from the dye constituents of both commercially and non-commercially available natural dye sources, encompassing a broad range of mordant, direct, and indigoid dyes derived from plant and animal origins.
3. Results
3.1. Rotting Alder Buckthorn Bark
The effect of rotting was visible in all samples, even when the rotting took only 2 weeks. The grey-and-yellow colour of the unrotted bark (
Figure 4) turned blackish-brown and purplish in places. Some rotted bark samples were covered with mold (
Figure 6). The bottom sides of the bags, which had been lying on the ground, were visibly brownish-red in colour. Also, traces of animal activity could be detected, as some small holes had been chewed into some of the bags (
Figure 6). In terms of structure, the rotted barks were papery and fibrous after drying, showing obvious signs of decomposition (
Figure 7). The rotted bark had no unpleasant smell.
3.2. Colours Achieved with Unrotted and Rotted Alder Buckthorn Bark
The colours obtained from the control dyeings are shown in
Figure 8. The yarns from the first control dyeing, i.e., dyed with unrotted bark kept at room temperature for 1 month, were a dull brownish-yellow colour. Control dyeing of bark that had dried for 8 months gave a slightly brighter shade, and the result of the last control dyeing of 14-month-old bark was an even brighter yellow, indicating a change in the dye composition of the bark taking place over time. The difference in tone obtained between unmordanted and mordanted yarns was barely noticeable.
The lye treatment changed the colour of the yarns towards brown in the first and second control dyeing, but the yellow yarns from the third control dyeing turned brick red during the lye soak. This corresponds well with the historic recipes instructing using at least a year-old bark for dyeing red with alder buckthorn bark [
12].
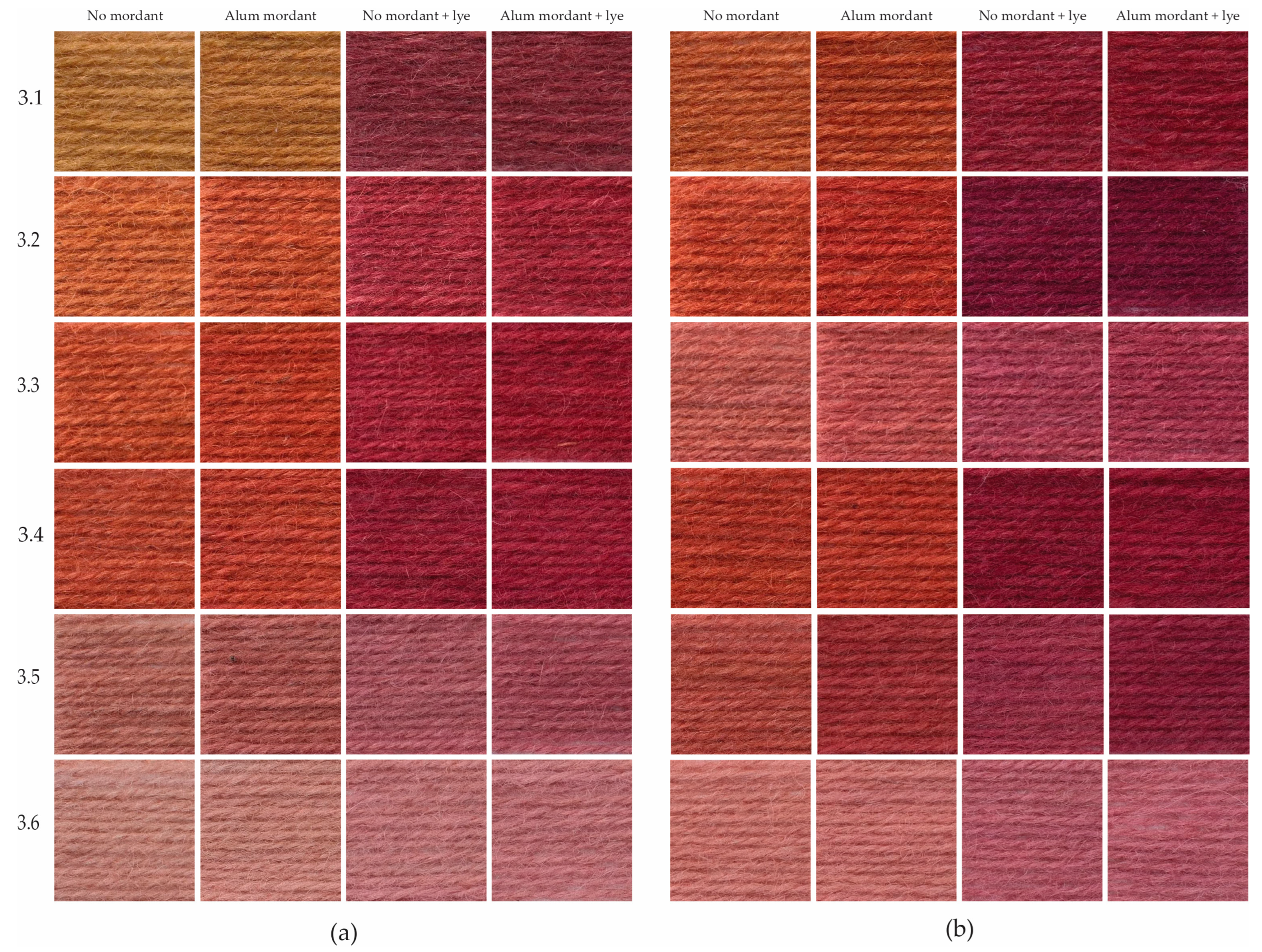
The results of yarns dyed during the first, preliminary rotting experiment are displayed in
Figure 9.
The yarns from the first rotting experiment dyed with bark rotted for 4 weeks on the ground dyed yellow, but the yarns rotted underground dyed bright brick red, giving proof of concept. After four more weeks, the on-the-ground rotted bark also dyed the wool yarns red, as did the underground rotted bark. The soak in wood ash lye revealed the dye’s sensitivity to pH change, turning the colour of yarns from yellow and orangey-red to darker orange, red, and purple. The colour changes after the lye soak of yarns dyed with either older dried bark or rotted bark may be attributed to the colouring compounds obtaining a negative charge because of ionization caused by the high pH, or it can indicate a possible change in colourant composition [
30].
The results of yarns dyed during the second rotting experiment meant for testing rotting during the colder season and lasting up to 10 months, are shown in
Figure 10.
Only light shades of orange and pink were dyed with barks that were rotted on the ground over winter, but the bark rotted underground dyed bright reds even after 6 and 8 months of rotting, only losing the depth of shade after 10 months of rotting. The visibly different results in shades can be attributed to the different environmental conditions in which the barks were kept, with the fluctuating outside temperatures and humidity being possible major influencing factors. For example, the end period of rotting of sample 2.3 reached the warmer weather of May–July.
Figure 11 shows the results of the third rotting experiment that took place during the warm season and lasted 12 weeks. It demonstrates that the degradation of the bark, resulting in the red yarns, happens faster with underground rotting than on-the-ground rotting. It is also noticeable that the loss of brightness and strength in colour appears faster with bark rotted on the ground. But, already after 4 weeks of keeping the alder buckthorn bark on the ground in summer, red shades can be dyed with the boiling method. It corresponds well to one archival report which says that to dye red, the alder buckthorn bark should be kept for a few weeks in a moist place [
15].
3.3. Light Fastness
Light fastness ratings of all the dyed samples on a scale from 1 (very low light fastness) to 8 (very high light fastness) are shown in
Table 3.
Modern synthetic dyes are expected to give a lightfastness result of 4, 5, or above [
31]; some of these samples achieve these results. For richness of colour rotting under the ground was mostly beneficial (
Figure 9,
Figure 10 and
Figure 11), although there was no significant improvement in fastness to light for many samples.
In most cases, the alum mordant and the lye after rinse did not improve the result; however, for the underground 4 weeks (experiment 3.2), there was a marked improvement with the addition of the lye after rinse. In most other cases, the lye appears to be more detrimental than beneficial. Longer rotting duration significantly reduced the depth of colour (e.g., experiments 2.2 and 2.3) and the fastness of the dye as well. The best light fastness was achieved with the shortest rotting time (e.g., 2 weeks, experiment 3.1).
The control samples gave average light fastness results around a value of 3, but not the richer shades of red that were achieved with rotting.
In some experiments, processing the bark either on the ground or underground for the same time gave quite different results in shades of colour; nevertheless, the fastness results were the same for the yarns. An example of this is experiment 2.1, but it can be noted for many of the other pairs of samples as well.
3.4. HPLC-DAD Analysis
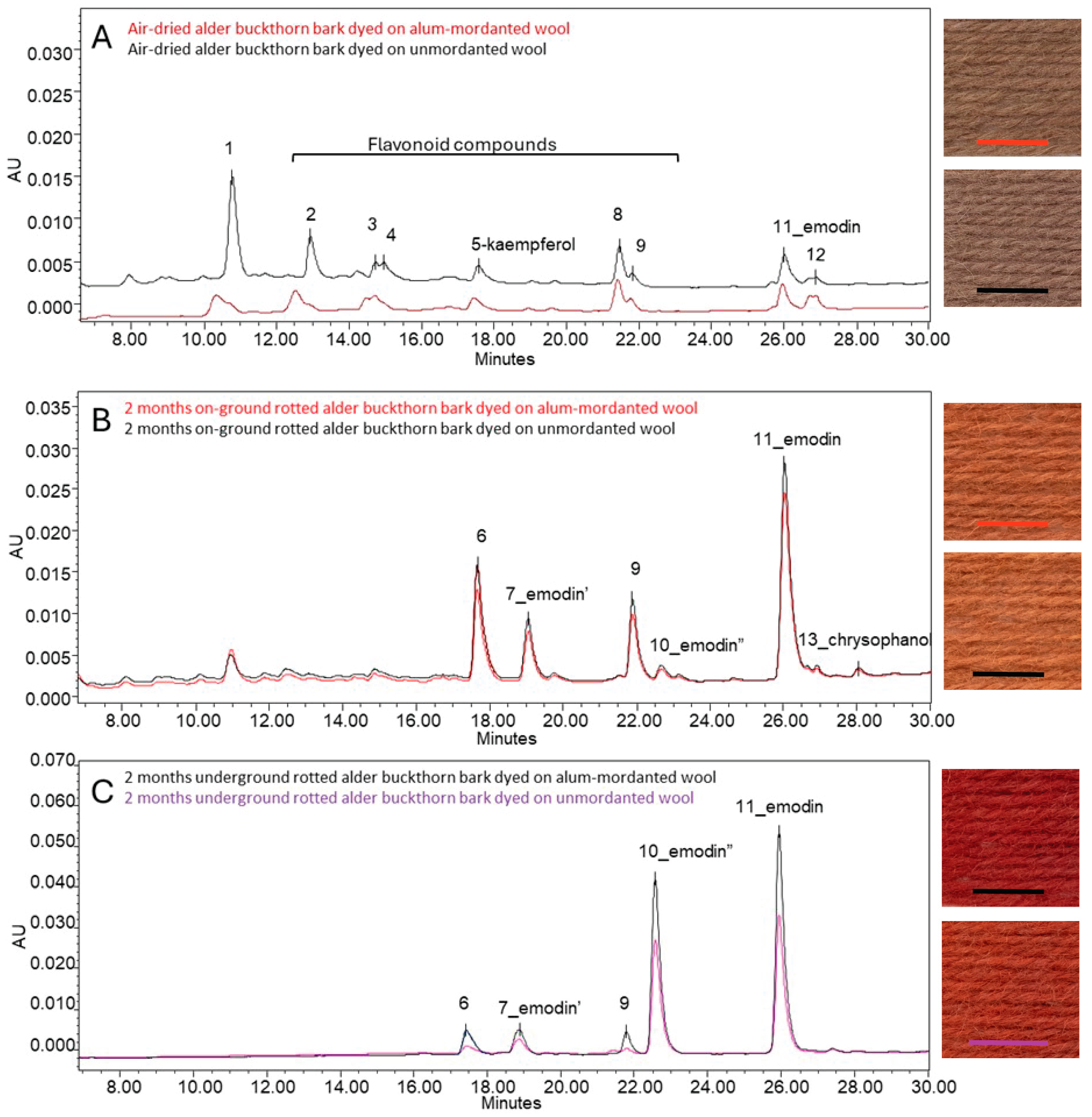
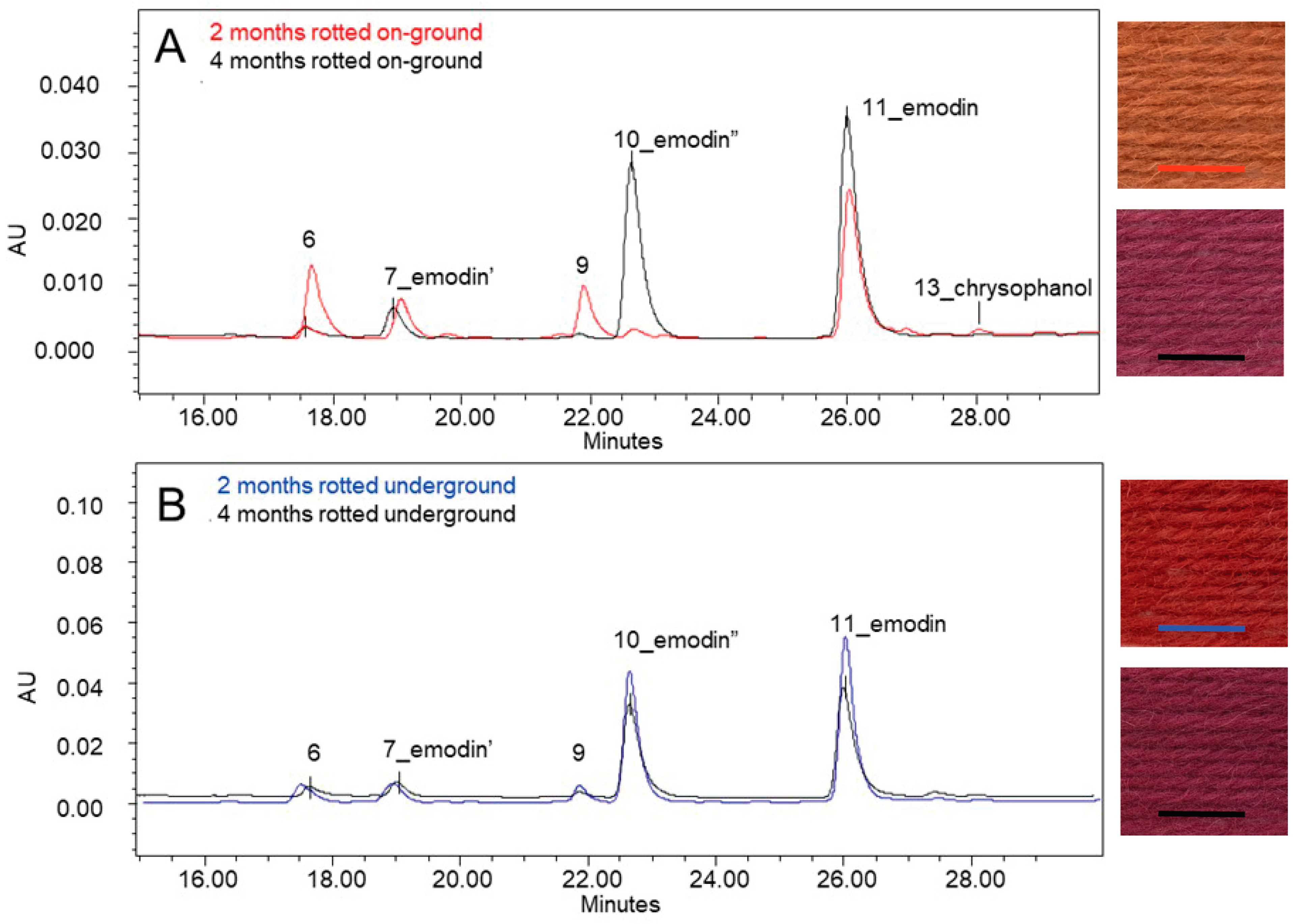
Dyeing wool with alder buckthorn bark that was air-dried immediately after collection resulted in a visually light brown colour (
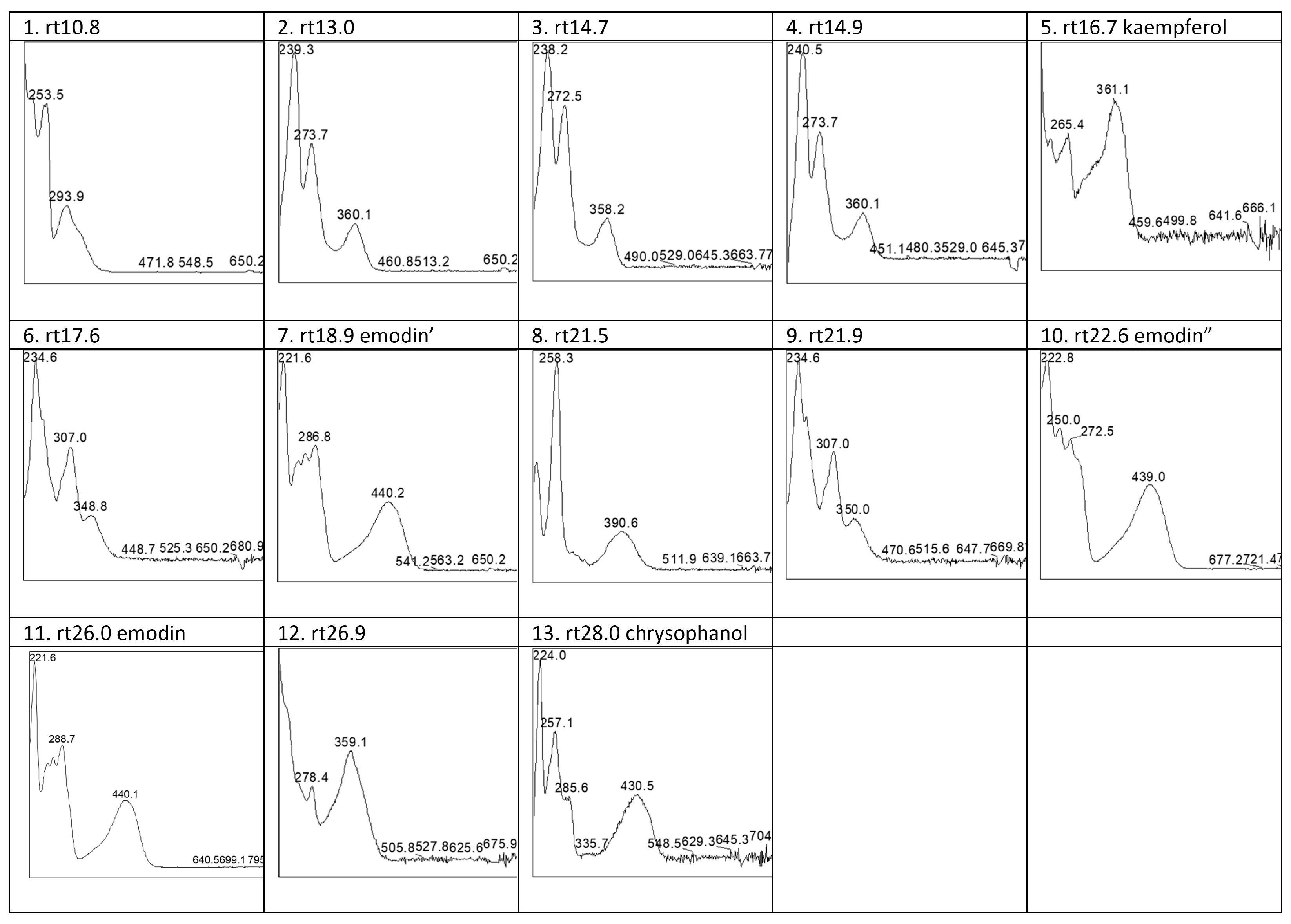
Figure 8(C1)). HPLC analyses of the acid extracts of these wool samples showed the presence of the flavonol compound kaempferol (3,4′,5,7-tetrahydroxyflavone) (5) and the anthraquinone emodin (6-methyl-1,3,8-trihydroxyanthraquinone) (11) (
Table 4, with absorption spectra in
Figure 12; chromatograms see
Figure 13A). In addition, several other compounds with absorption maxima in the visible wavelength range between 294 and 391 nm were observed (
Table 4, compounds 1–4, 8–9, 12). Based on a comparison with known reference spectra from the in-house database, no match was found for these to determine their nature. The UV/Vis absorption maxima of these compounds indicate, with the exception of the colourless compound (1), that it concerns flavonoid compounds [
32].
Figure 13B shows the dye composition of the unmordanted and alum-mordanted wool dyed with alder buckthorn bark after 2 months of rotting on the ground. Compared to the wool dyed with the freshly dried buckthorn bark (
Figure 13A), most flavonoid compounds disappeared after 2 months of outdoor decomposition, highlighting their instability. However, two more flavonoid compounds, the yellow compounds 6 and 9, eluting at 17.6 and 21.9 min under the applied chromatographic protocol, were clearly present. The samples also contain a significant amount of emodin, along with some chrysophanol (1,8-dihydroxy-3-methyl-anthraquinone) (13). In addition, two emodin-related compounds were observed, appearing at retention times of 18.9 and 22.6 min, respectively (see
Table 4,
Figure 12). The first, marked as emodin’ (7) had the same absorption spectrum as emodin, while the other, marked as emodin” (10), had the same absorption in the visible wavelengths but displayed slight differences in the UV region. No corresponding UV/Vis absorption spectra were found that allowed for further identification or could explain the variation in the UV range. It is possible that these compounds are glycosides of emodin. Glycosidic bonds are generally cleaved during acid hydrolysis, but such compounds can still be detected upon incomplete hydrolysis. However, the lack of conclusive evidence leaves open the possibility of other emodin-related structures. A more in-depth study of the mechanisms going on during the decomposition of the bark resulting in these two compounds, although very interesting, was beyond the scope of this study.
A very similar dye composition—alongside emodin (11), the presence of flavonoid compounds (6 and 9) and emodin-related compounds (7 and 10)—was detected in the analysed unmordanted and alum-mordanted wool samples dyed with bark rotted underground for 2 months (
Figure 13C). However, clear differences were observed in the relative proportions of the compounds, when comparing both samples dyed with 2 months on the ground and underground rotted bark.
Table 5 gives the relative ratios of the peak areas, measured at 255 nm wavelength, of the compounds (6, 7, 9 and 10) compared to emodin (11), for the samples dyed with alder buckthorn bark on alum-mordanted and unmordanted wool. The samples dyed with bark that had decomposed on the ground for 2 months show a significantly higher relative amount of flavonoid compounds (6 and 9) and of the emodin’ compound (7) compared to the samples dyed with bark that rotted underground for the same period. It concerns relative amounts of, respectively, 0.5, 0.3, and 0.3 on the ground to 0.1, 0.1, and 0.1 underground. On the contrary, a pronounced higher relative amount of the emodin” compound (10) is obtained from the samples dyed with 2 months of underground rotted bark, compared to the on-ground rotted bark for the same period (0.1 on-ground to 0.8 underground).
The use of alum-mordanted or unmordanted wool for dyeing with 2 months on-the-ground or underground rotted alder buckthorn bark demonstrates to have no significant influence on the dye composition detected in the dye extracts, nor on their relative proportions (
Figure 13B,C;
Table 5).
While the higher relative amounts of these flavonoids correlated well with the more orange colour obtained with the 2-month on-ground-rotted bark, the much lower relative amounts of these yellow compounds along with the high relative proportion of the emodin” compounds seem to be well correlated with the bright red colour obtained with the underground-rotted bark.
Dyeing with buckthorn bark after rotting on the ground for 4 instead of 2 months resulted in a colour change from orange to red (
Figure 9). This colour shift is associated with an altered dye composition of the samples (
Figure 14A). HPLC analyses revealed that the longer the bark rotted on the ground, the lower the relative amounts of the yellow compounds (6 and 9), respectively, going from 0.5 to 0.0 and from 0.3 to 0.0, and of the emodin’ compound (7), going from 0.3 to 0.1. At the same time, the relative amount of the emodin” compound (10) increased notably (from 0.1 to 0.8) (
Table 5). A similar red colour shift was observed in wool samples dyed with buckthorn bark after 2 and 4 months of underground rotting (
Figure 9). Dye analyses of these wool samples showed a very consistent dye composition, including the relative ratios of the detected compounds in relation to emodin (
Figure 14B,
Table 5). Moreover, the results corresponded very closely to those obtained from wool dyed with the 4-month on-the-ground rotted bark (
Figure 14A). Similar to the HPLC analyses of the samples dyed with the 2-month rotten bark, the use of alum-mordanted or unmordanted wool did not significantly affect the dye composition (
Table 5).
4. Discussion
The results from rotting experiments with alder buckthorn bark based on Estonian traditions showed that the process, initiating the decomposition of alder buckthorn bark, both above and below ground, strongly affected colours achievable with the boiling method. Decaying triggered a colour change, resulting in orange, pink, and bright red shades on dyed wool that notably differed from the brown and yellow shades achieved with dried bark. Soak in wood ash lye demonstrated the dye’s sensitivity to pH change, turning warm orangey-red hues purplish.
One of the main focuses of the experiments in this study was rotting duration. In addition, a wide range of other variables can impact the bark degradation process and the outcome of colour shades achievable when dyeing with rotted alder buckthorn bark. For example, the thickness of the bark, the size of bark pieces used for rotting, and the amount of bark being rotted in one batch, as well as environmental factors such as soil and rain pH values, temperature and moisture fluctuations, and the amount of sunlight when decomposing the bark on the ground can influence the outcome. Testing the impact of these variables in more controlled experiments, as well as applying different dyeing protocols, are avenues for future research.
The light fastness of yarns dyed with unrotted alder buckthorn bark was moderate, 2–3 on the scale of 1–8. The values varied markedly between yarns dyed with rotted alder buckthorn bark, reaching light fastness from very low to good. The highest light fastness was obtained by yarns dyed with the shortest time of rotting (two to four weeks); therefore, if good light fastness is required, a shorter rotting time is preferential. Longer rotting durations significantly reduced the depth of colour and light fastness. Although only light fastness was assessed in this study, additional fastness tests like wash and rub fastness should be carried out if rotted alder buckthorn bark dyed materials are considered for modern applications.
Both unmordanted and alum-mordanted yarns dyed woollen yarn red with little difference in the shade of colour between them. Therefore, rotted alder buckthorn bark contains substantive red dyes attaching well to unmordanted wool. This would have been an important characteristic in the past, considering its use by the rural population, who did not necessarily have access to alum and other mordants. Analysis of archival sources indicates that dyeing red with rotted alder buckthorn bark in Estonia can be dated to the middle of the 19th century and possibly even earlier [
20]. Rural Estonians belonged to the poorest class of inhabitants in Estonia at that time—Estonians were serfs ruled by a small, mostly German upper class until the first quarter of the 19th century [
33]. Money-earning opportunities for Estonians living in the countryside were limited, and their purchasing power in the first half of the 19th century was often very low. Although mordants like alum and imported red dyes were occasionally bought for dyeing textiles, money was preferably spent on more essential goods like salt and iron [
34]. So, they probably appreciated a local dye material that could be gathered for free and used to dye bright and reasonably colourfast red shades without the need to purchase alum.
Whereas the analyses of wool dyed with fresh air-dried alder buckthorn bark showed alongside emodin primarily flavonoid dye components including kaempferol, analysis of the dyed wool samples showed that the rotting of the bark is initially reflected by a loss of the associated unstable flavonoid compounds and that the colour shift obtained can be highly associated with the presence of prominent amounts of emodin and two emodin-related compounds, one of which is likely released during rotting. The analyses of wool dyed with rotted alder buckthorn bark were under the applied chromatographic conditions mainly characterised by a reduced presence to complete absence of flavonoid compounds and by the presence of emodin and two other anthraquinone emodin-related compounds. One of them, the emodin” compound (10), became increasingly prominent in analyses of wool dyed with bark that had undergone longer periods of rotting. The main difference observed in the dye composition between wool extracts from the on-the-ground and underground rotting processes was that the degradation of the flavonoid compounds and the formation of the emodin-related derivative (10) occurred more rapidly when the bark was rotted underground. This was already evident in the samples dyed with bark that had rotted underground for 2 months, whereas these changes were only observed after 4-month on-the-ground rotting period. Finally, no significant differences in the relative proportions of dye compounds were found in wool dyed with alder buckthorn bark that was rotted for 4 months, regardless of whether the rotting took place on or underground.
The Estonian tradition of rotting alder buckthorn bark, a local historical practice to obtain red, is an important finding that sheds light on the historical dyeing traditions of rural inhabitants of the Eastern Baltic area. Considering the vividness of the colour shades obtained and the availability of the dye material, red colour dyed with rotted alder buckthorn bark could become an exciting new historical dye material for natural colour enthusiasts. As the experiments presented in this paper showed, only two weeks of rotting underground were needed to change the colour achieved with alder buckthorn bark from brownish yellow to red. In comparison, the unprocessed bark had to be at least a year old to dye red. Even then, soaking in the highly alkaline wood ash lye was needed, which can damage the wool. Rotted alder buckthorn bark can be used in workshops to teach natural dyeing and introduce historical craft methods. Bark rotted and dried in preparation is simple to transport, and parallel dye baths of rotted and unrotted bark using the relatively fast boiling method can effectively demonstrate the impact of this pretreatment.