Unveiling Polychrome Printing Methods on Textiles: Preliminary Results from the Mariano Fortuny y Madrazo Collection in Venice
Abstract
1. Introduction
Mariano Fortuny y Madrazo and His Patents
2. Materials and Methods
- −
- A/P (azelaic to palmitic acid) to distinguish between egg paint (A/P < 0.3) and oil paint (A/P > 1) [16];
- −
- P/S (palmitic to stearic acid) to identify the type of oil used, as SFA does not change during film formation processes;
- −
- A/Sub (azelaic to suberic acid) to provide an assessment of any pre-heating processes that may have occurred in the preparation of the oil;
- −
- D/P, the ratio of dicarboxylic acids (suberic, sebacic and azelaic) to palmitic acid, which gives a good estimate of the degree of drying of the oil over time, since the percentage of dicarboxylic acids increases as the fatty di-acids are formed during auto-oxidation.
- −
- O/S (oleic to stearic acid), an index of oxidation used to define the maturity of a film (values around 0.1–0.2 are common for aged samples).
3. Results
3.1. Design, Pattern and Print Papers
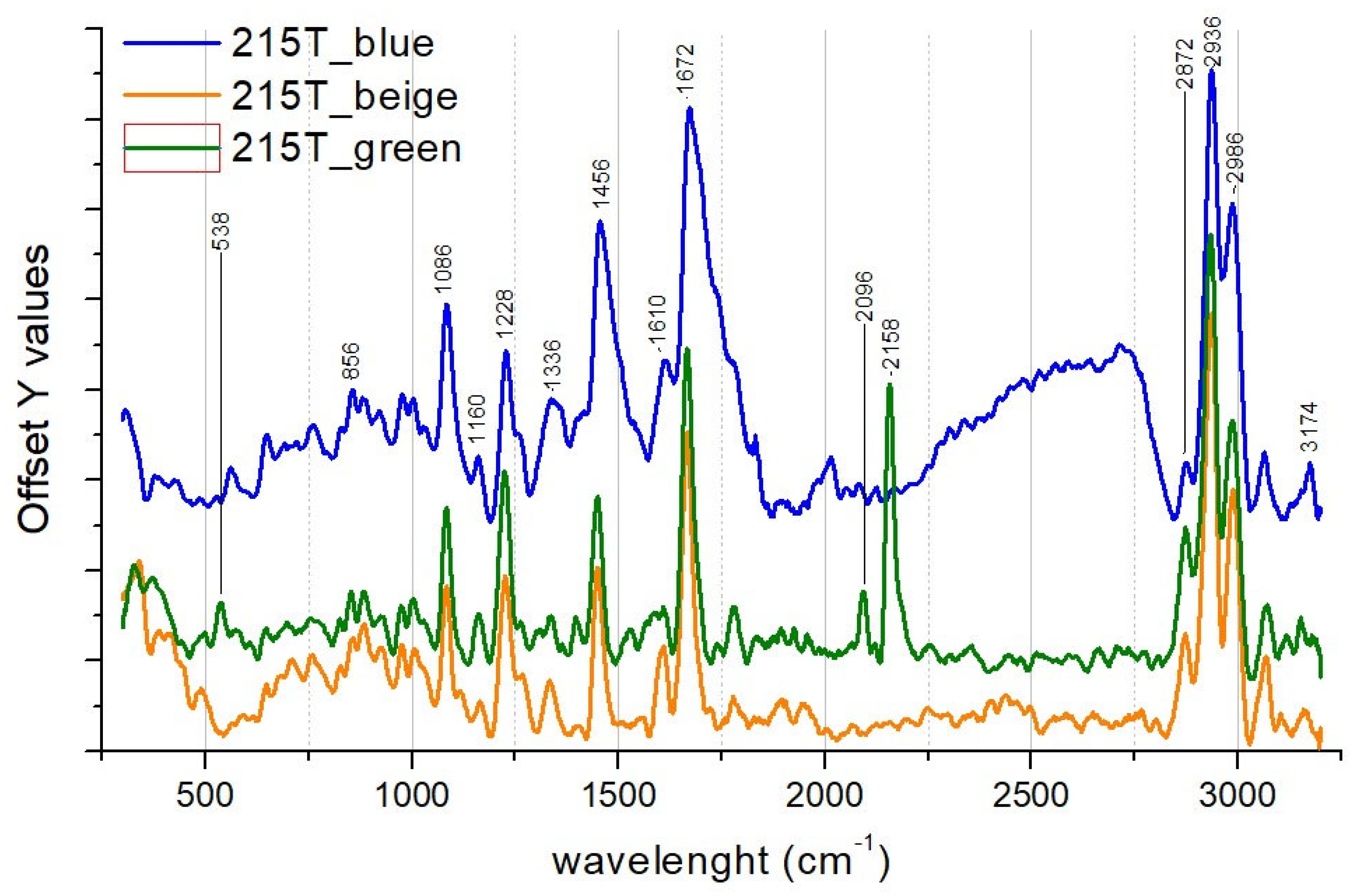
3.2. Polychrome Textiles
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jacobs, F.F. Textile Printing: Materials, Methods and Formulae; Literary Licensing, LLC.: Whitefish, MT, USA, 2012. [Google Scholar]
- Tikkanen, A. Batik Indonesian, Wax-Resist, Textiles. Available online: https://www.britannica.com/technology/batik (accessed on 10 December 2023).
- Martinsen, H.E.H. Fashionable Chemistry: The History of Printing Cotton in France in the Second Half of the Eighteenth and First Decades of the Nineteenth Century. Ph.D. Thesis, University of Toronto, Toronto, ON, Canada, 2015. [Google Scholar]
- Group of Four Art Déco Pochoir. Available online: https://www.dimanoinmano.it/en/cp230217/art/twentieth-century/group-of-four-art-deco-pochoir (accessed on 10 December 2023).
- Morini, E. Storia Della Moda: 18.–21. Secolo; Skira: Lausanne, Switzerland, 2006. [Google Scholar]
- De Osma, G. Mariano Fortuny: His Life and Work; Victoria & Albert Museum: London, UK, 2015. [Google Scholar]
- Fortuny, M.M. Procédé d’impression Polychrome Sur Tissus, Papiers, etc. 1909. [Google Scholar]
- Fortuny, M.M. Procédé d’impression Sur Tissus, Papiers, etc. 1910. [Google Scholar]
- Franzini, C.; Romanelli, G. (Eds.) The Fortuny Museum in Palazzo Pesaro Degli Orfei, Venice; Skira: Venezia, Italy, 2008. [Google Scholar]
- Nuzzi, C. Fortuny Nella Belle Epoque; Electa: Stockholm, Sweden, 1984. [Google Scholar]
- Maino, M. Fortuny y La Escena: La Iluminación Como Terreno de Experimentación y Reforma. In Fortuny: El Mago de Venecia; Fundación Dialnet: Logroño, Spain, 2010; pp. 71–98. [Google Scholar]
- Deschodt, A.-M. Mariano Fortuny: Un Magicien de Venise; Éditions du Regard: Paris, France, 1979. [Google Scholar]
- Sache, I. Spain: 1938–1945. Available online: https://www.fotw.info/flags/es1938.html (accessed on 10 December 2023).
- Dino-Lite Ideal for Restoration of Ethnographic and Art Objects. Available online: https://www.dino-lite.eu/en/dino-lite-ideal-for-restoration-of-ethnographic-and-art-objects (accessed on 10 December 2023).
- Fuster-López, L.; Izzo, F.C.; Andersen, C.K.; Murray, A.; Vila, A.; Picollo, M.; Stefani, L.; Jiménez, R.; Aguado-Guardiola, E. Picasso’s 1917 Paint Materials and Their Influence on the Condition of Four Paintings. SN Appl. Sci. 2020, 2, 2159. [Google Scholar] [CrossRef]
- Izzo, F.C.; Ferriani, B.; Van den Berg, K.J.; Van Keulen, H.; Zendri, E. 20th Century Artists’ Oil Paints: The Case of the Olii by Lucio Fontana. J. Cult. Herit. 2014, 15, 557–563. [Google Scholar] [CrossRef]
- Izzo, F.C.; Zanin, C.; Keulen, H.V.; Roit, C.D. From Pigment to Paints: Studying Original Materials from the Atelier of the Artist Mariano Fortuny y Madrazo. Int. J. Conserv. Sci. 2017, 8, 547–564. [Google Scholar]
- Fuster-López, L.; Izzo, F.C.; Damato, V.; Yusà-Marco, D.J.; Zendri, E. An Insight into the Mechanical Properties of Selected Commercial Oil and Alkyd Paint Films Containing Cobalt Blue. J. Cult. Herit. 2019, 35, 225–234. [Google Scholar] [CrossRef]
- Eastaugh, N.; Walsh, V.; Chaplin, T.; Siddall, R. Pigment Compendium: A Dictionary and Optical Microscopy of Historical Pigments; Routledge: London, UK, 2024. [Google Scholar]
- Krekel, C. Chemische Struktur Historischer Eisengallustinten. Kohlhammer Stuttg. 1999, 10, 25–36. [Google Scholar]
- Zaccaron, S.; Potthast, A.; Henniges, U.; Draxler, J.; Prohaska, T.; McGuiggan, P. The Disastrous Copper. Comparing Extraction and Chelation Treatments to Face the Threat of Copper-Containing Inks on Cellulose. Carbohydr. Polym. 2019, 206, 198–209. [Google Scholar] [CrossRef] [PubMed]
- Hahn, O.; Kanngießer, B.; Malzer, W. X-Ray Fluorescence Analysis of Iron Gall Inks, Pencils and Coloured Crayons. Stud. Conserv. 2005, 50, 23–32. [Google Scholar] [CrossRef]
- Strlič, M.; Kolar, J.; Šelih, V.; Kocar, D.; Pihlar, B. A Comparative Study of Several Transition Metals in Fenton-like Reaction Systems at Circum-Neutral PH. Acta Chim. Slov. 2003, 50, 619–632. [Google Scholar]
- Fichera, G.V.; Malagodi, M.; Cofrancesco, P.; Weththimuni, M.L.; Guglieri, C.; Olivi, L.; Ruffolo, S.; Licchelli, M. Study of the Copper Effect in Iron-Gall Inks after Artificial Ageing. Chem. Pap. 2018, 72, 1905–1915. [Google Scholar] [CrossRef]
- Camacho, N.P.; Rinnerthaler, S.; Paschalis, E.P.; Mendelsohn, R.; Boskey, A.L.; Fratzl, P. Complementary Information on Bone Ultrastructure from Scanning Small Angle X-Ray Scattering and Fourier-Transform Infrared Microspectroscopy. Bone 1999, 25, 287–293. [Google Scholar] [CrossRef]
- Librando, V.; Minniti, Z.; Lorusso, S. Ancient and Modern Paper Characterization by FTIR and Micro-Raman Spectroscopy. Conserv. Sci. Cult. Herit. 2011, 11, 249–268. [Google Scholar]
- Tomasini, E.; Siracusano, G.; Maier, M.S. Spectroscopic, Morphological and Chemical Characterization of Historic Pigments Based on Carbon. Paths for the Identification of an Artistic Pigment. Microchem. J. 2012, 102, 28–37. [Google Scholar] [CrossRef]
- Tomasini, E.P.; Halac, E.B.; Reinoso, M.; Di Liscia, E.J.; Maier, M.S. Micro-Raman Spectroscopy of Carbon-based Black Pigments. J. Raman Spectrosc. 2012, 43, 1671–1675. [Google Scholar] [CrossRef]
- Rouchon, V.; Pellizzi, E.; Janssens, K. FTIR Techniques Applied to the Detection of Gelatine in Paper Artifacts: From Macroscopic to Microscopic Approach. Appl. Phys. A 2010, 100, 663–669. [Google Scholar] [CrossRef][Green Version]
- Izzo, F.C.; Kratter, M.; Nevin, A.; Zendri, E. A Critical Review on the Analysis of Metal Soaps in Oil Paintings. ChemistryOpen 2021, 10, 904–921. [Google Scholar] [CrossRef] [PubMed]
- Gunn, M.; Chottard, G.; Rivière, E.; Girerd, J.-J.; Chottard, J.-C. Chemical Reactions Between Copper Pigments and Oleoresinous Media. Stud. Conserv. 2002, 47, 12–23. [Google Scholar] [CrossRef]
- Prati, S.; Bonacini, I.; Sciutto, G.; Genty-Vincent, A.; Cotte, M.; Eveno, M.; Menu, M.; Mazzeo, R. ATR-FTIR Microscopy in Mapping Mode for the Study of Verdigris and Its Secondary Products. Appl. Phys. A 2016, 122, 10. [Google Scholar] [CrossRef]
- Santoro, C.; Zarkout, K.; Le Hô, A.-S.; Mirambet, F.; Gourier, D.; Binet, L.; Pagès-Camagna, S.; Reguer, S.; Mirabaud, S.; Du, Y.; et al. New Highlights on Degradation Process of Verdigris from Easel Paintings. Appl. Phys. A 2014, 114, 637–645. [Google Scholar] [CrossRef]
- Fleet, M.E. Infrared Spectra of Carbonate Apatites: Ν2-Region Bands. Biomaterials 2009, 30, 1473–1481. [Google Scholar] [CrossRef] [PubMed]
- Bell, I.M.; Clark, R.J.H.; Gibbs, P.J. Raman Spectroscopic Library of Natural and Synthetic Pigments (Pre- ≈ 1850 AD). Spectrochim. Acta A Mol. Biomol. Spectrosc. 1997, 53, 2159–2179. [Google Scholar] [CrossRef] [PubMed]
- Burgio, L.; Clark, R.J.H. Library of FT-Raman Spectra of Pigments, Minerals, Pigment Media and Varnishes, and Supplement to Existing Library of Raman Spectra of Pigments with Visible Excitation. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2001, 57, 1491–1521. [Google Scholar] [CrossRef]
- Gunasekaran, S.; Anbalagan, G.; Pandi, S. Raman and Infrared Spectra of Carbonates of Calcite Structure. J. Raman Spectrosc. 2006, 37, 892–899. [Google Scholar] [CrossRef]
- Osticioli, I.; Mendes, N.F.C.; Nevin, A.; Gil, F.P.S.C.; Becucci, M.; Castellucci, E. Analysis of Natural and Artificial Ultramarine Blue Pigments Using Laser Induced Breakdown and Pulsed Raman Spectroscopy, Statistical Analysis and Light Microscopy. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2009, 73, 525–531. [Google Scholar] [CrossRef] [PubMed]
- Caggiani, M.C.; Cosentino, A.; Mangone, A. Pigments Checker Version 3.0, a Handy Set for Conservation Scientists: A Free Online Raman Spectra Database. Microchem. J. 2016, 129, 123–132. [Google Scholar] [CrossRef]
- Innocenti, S.; Quintero Balbas, D.; Pezzati, L.; Fontana, R.; Striova, J. Portable Sequentially Shifted Excitation Raman Spectroscopy to Examine Historic Powders Enclosed in Glass Vials. Sensors 2022, 22, 3560. [Google Scholar] [CrossRef] [PubMed]
- Lazzari, M.; Chiantore, O. Drying and Oxidative Degradation of Linseed Oil. Polym. Degrad. Stab. 1999, 65, 303–313. [Google Scholar] [CrossRef]
- Pizzimenti, S.; Bernazzani, L.; Tinè, M.R.; Treil, V.; Duce, C.; Bonaduce, I. Oxidation and Cross-Linking in the Curing of Air-Drying Artists’ Oil Paints. ACS Appl. Polym. Mater. 2021, 3, 1912–1922. [Google Scholar] [CrossRef]
- Nardelli, F.; Martini, F.; Lee, J.; Lluvears-Tenorio, A.; La Nasa, J.; Duce, C.; Ormsby, B.; Geppi, M.; Bonaduce, I. The Stability of Paintings and the Molecular Structure of the Oil Paint Polymeric Network. Sci. Rep. 2021, 11, 14202. [Google Scholar] [CrossRef] [PubMed]
- Andreotti, A.; Bonaduce, I.; Colombini, M.P.; Gautier, G.; Modugno, F.; Ribechini, E. Combined GC/MS Analytical Procedure for the Characterization of Glycerolipid, Waxy, Resinous, and Proteinaceous Materials in a Unique Paint Microsample. Anal. Chem. 2006, 78, 4490–4500. [Google Scholar] [CrossRef] [PubMed]
- Colombini, P.M.P.; Modugno, F. Organic Mass Spectrometry in Art and Archaeology; John Wiley & Sons: Hoboken, NJ, USA, 2009. [Google Scholar]
- Eumelen, G.J.A.M.; Bosco, E.; Suiker, A.S.J.; Hermans, J.J. Chemo-Mechanical Model for Degradation of Oil Paintings by Amorphous and Crystalline Metal Soaps. Eur. J. Mech. A/Solids 2023, 97, 104827. [Google Scholar] [CrossRef]
- Beerse, M.; Keune, K.; Iedema, P.; Woutersen, S.; Hermans, J. Evolution of Zinc Carboxylate Species in Oil Paint Ionomers. ACS Appl. Polym. Mater. 2020, 2, 5674–5685. [Google Scholar] [CrossRef]
- Bona, M.; Isnardi, F.; Straneo, S. Manuale Di Tecnologia Tessile. Fibre Tessili. Filatura. Tessitura. Tessuti Vari. Taglio e Confezione. Tintura. Stampa. Finitura. Analisi e Controlli; Zanichelli: Bologna, Italy, 1991. [Google Scholar]
- Oktiani, R.; Ragadhita, R.; Nandiyanto, A.B.D.; Widiaty, I. Effect of Solvents and Fixation Agents on Colouring Batik by Guava Leaves Extract. J. Eng. Sci. Technol. 2020, 15, 2301–2308. [Google Scholar]
- Liu, J.; Guo, D.; Zhou, Y.; Wu, Z.; Li, W.; Zhao, F.; Zheng, X. Identification of Ancient Textiles from Yingpan, Xinjiang, by Multiple Analytical Techniques. J. Archaeol. Sci. 2011, 38, 1763–1770. [Google Scholar] [CrossRef]
- Asakura, T.; Ashida, J.; Yamane, T.; Kameda, T.; Nakazawa, Y.; Ohgo, K.; Komatsu, K. A Repeated β-Turn Structure in Poly(Ala-Gly) as a Model for Silk I of Bombyx Mori Silk Fibroin Studied with Two-Dimensional Spin-Diffusion NMR under off Magic Angle Spinning and Rotational Echo Double Resonance. J. Mol. Biol. 2001, 306, 291–305. [Google Scholar] [CrossRef]
- Monti, P.; Freddi, G.; Arosio, C.; Tsukada, M.; Arai, T.; Taddei, P. Vibrational Spectroscopic Study of Sulphated Silk Proteins. J. Mol. Struct. 2007, 834–836, 202–206. [Google Scholar] [CrossRef]
- Vahur, S.; Teearu, A.; Peets, P.; Joosu, L.; Leito, I. ATR-FT-IR Spectral Collection of Conservation Materials in the Extended Region of 4000–80 cm−1. Anal. Bioanal. Chem. 2016, 408, 3373–3379. [Google Scholar] [CrossRef]
- Peets, P.; Leito, I.; Pelt, J.; Vahur, S. Identification and Classification of Textile Fibres Using ATR-FT-IR Spectroscopy with Chemometric Methods. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2017, 173, 175–181. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, S.C.R.; Franchi, M.; Pais, A.A.C.C.; Seixas de Melo, J.S. The Chemistry behind the First Portuguese Postage Stamps (1853–1894). A Non-Destructive Analytical and Chemometric Analysis of Pigments, Fillers and Binders. Dye. Pigment. 2022, 205, 110519. [Google Scholar] [CrossRef]
- Prussian Blue Spectrum IRUG IMP00077. Spectrum Acquired in Transmission Mode by Beth Price, Philadelphia Museum of Art, Philadelphia, PA, USA. Available online: http://www.irug.org/jcamp-details?id=800 (accessed on 10 December 2023).
- Daher, C.; Drieu, L.; Bellot-Gurlet, L.; Percot, A.; Paris, C.; Le Hô, A. Combined Approach of FT-Raman, SERS and IR Micro-ATR Spectroscopies to Enlighten Ancient Technologies of Painted and Varnished Works of Art. J. Raman Spectrosc. 2014, 45, 1207–1214. [Google Scholar] [CrossRef]
- Moretti, G.; Gervais, C. Raman Spectroscopy of the Photosensitive Pigment Prussian Blue. J. Raman Spectrosc. 2018, 49, 1198–1204. [Google Scholar] [CrossRef]
- Conti, C.; Botteon, A.; Bertasa, M.; Colombo, C.; Realini, M.; Sali, D. Portable Sequentially Shifted Excitation Raman Spectroscopy as an Innovative Tool for in Situ Chemical Interrogation of Painted Surfaces. Analyst 2016, 141, 4599–4607. [Google Scholar] [CrossRef] [PubMed]
- Van Grieken, R.; Delalieux, F.; Gysels, K. Cultural Heritage and the Environment. Pure Appl. Chem. 1998, 70, 2327–2331. [Google Scholar] [CrossRef]
- Gervais, C.; Languille, M.-A.; Reguer, S.; Garnier, C.; Gillet, M. Light and Anoxia Fading of Prussian Blue Dyed Textiles. Herit. Sci. 2014, 2, 26. [Google Scholar] [CrossRef]


| Object | Object Code | Description | Analyses | ||||
|---|---|---|---|---|---|---|---|
| OM | XRF | ATR_FTIR | Raman | GCMS | |||
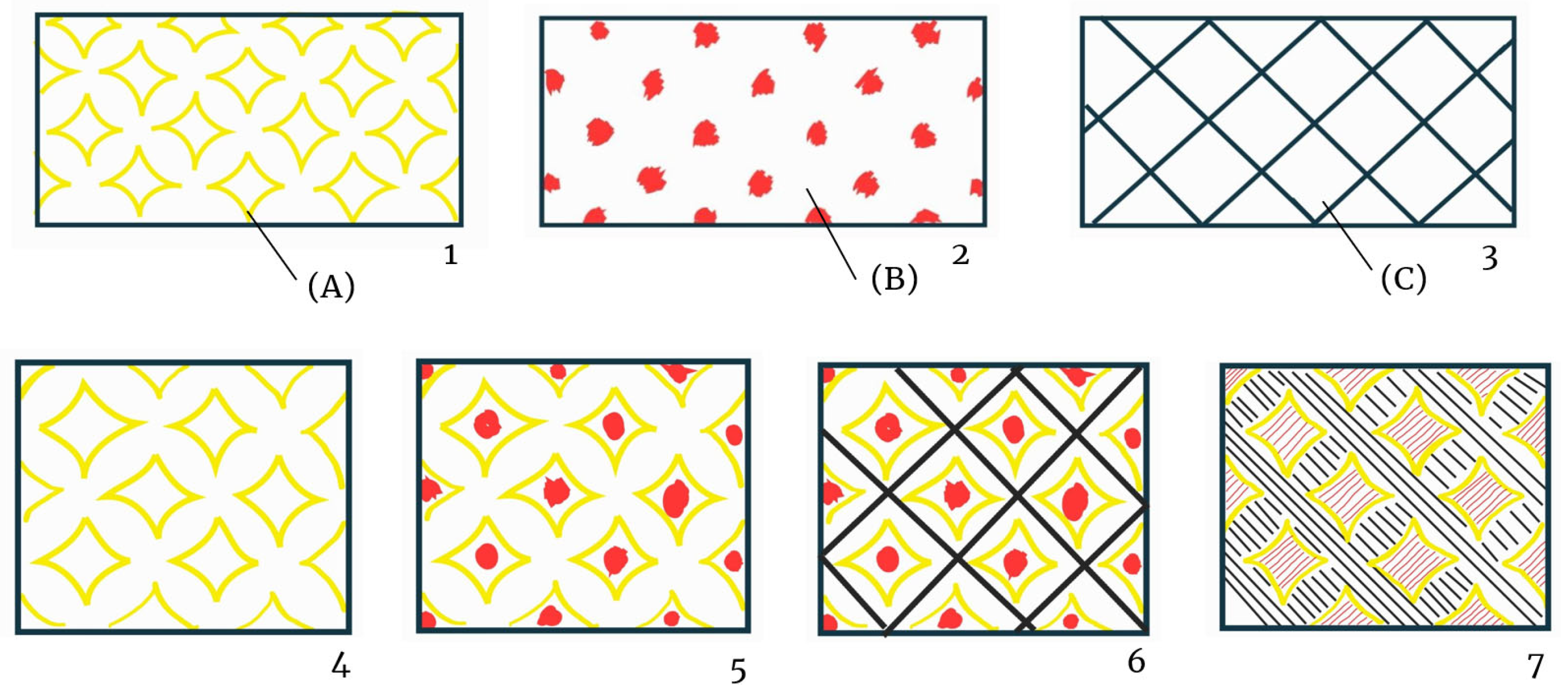
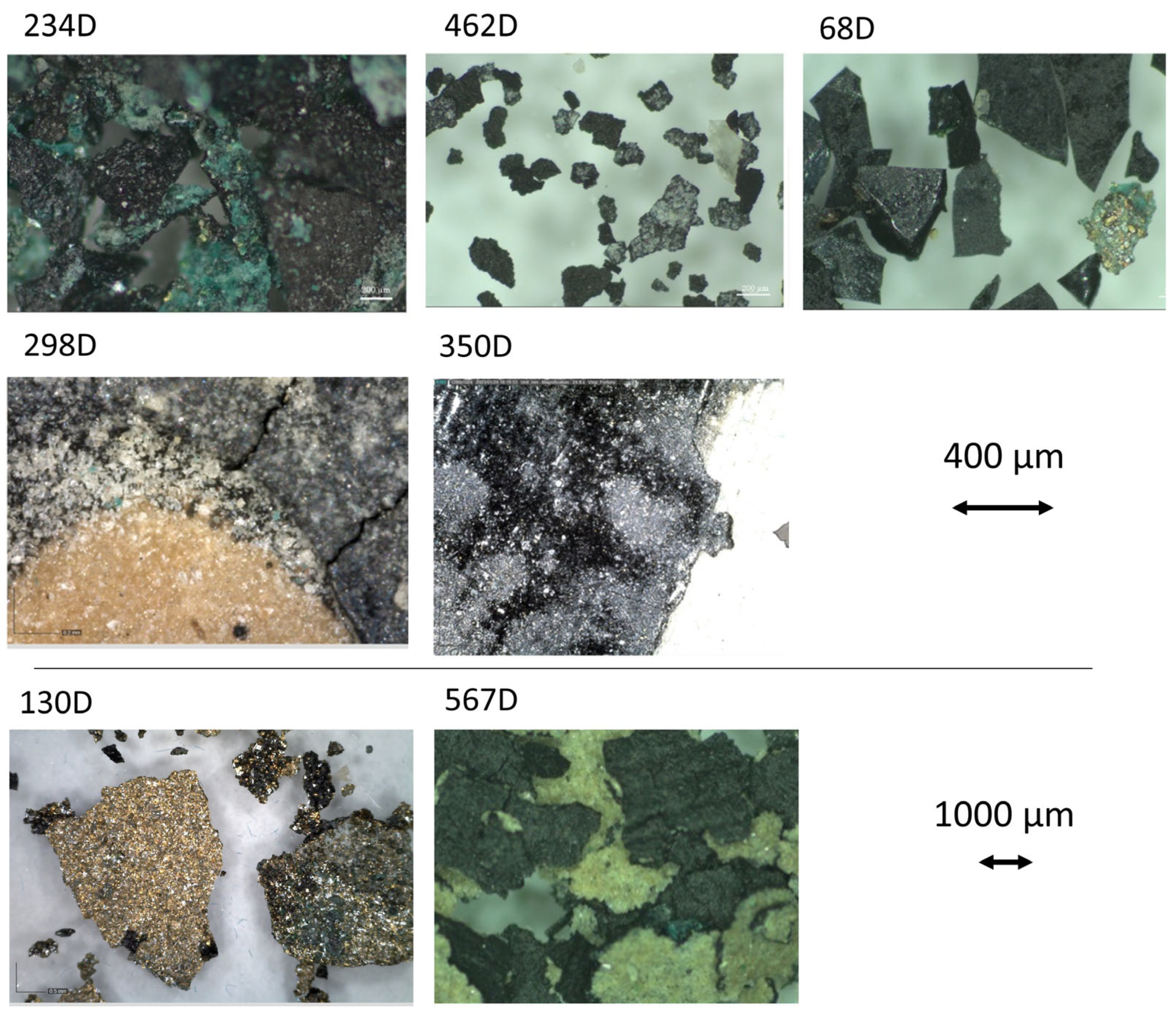
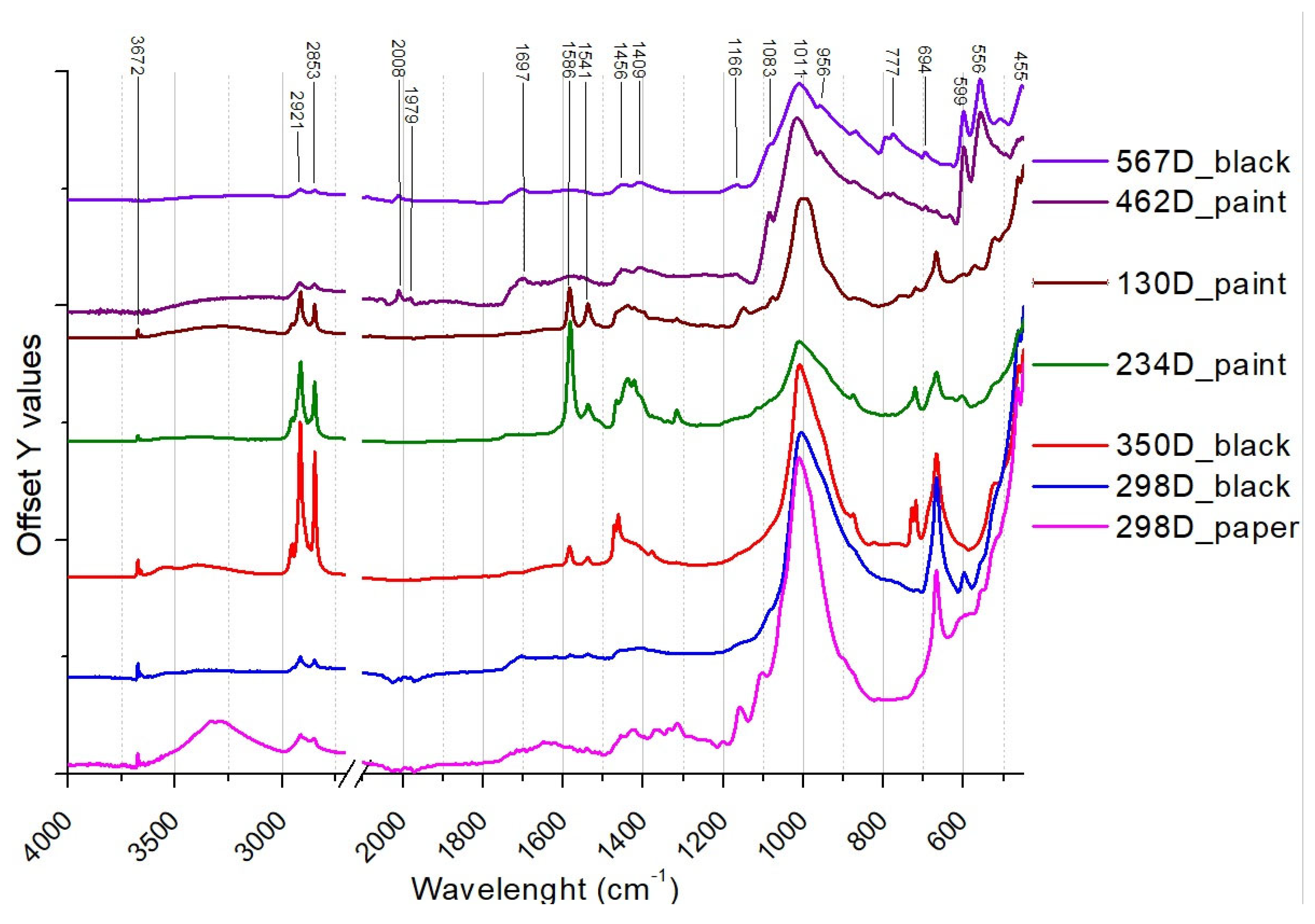
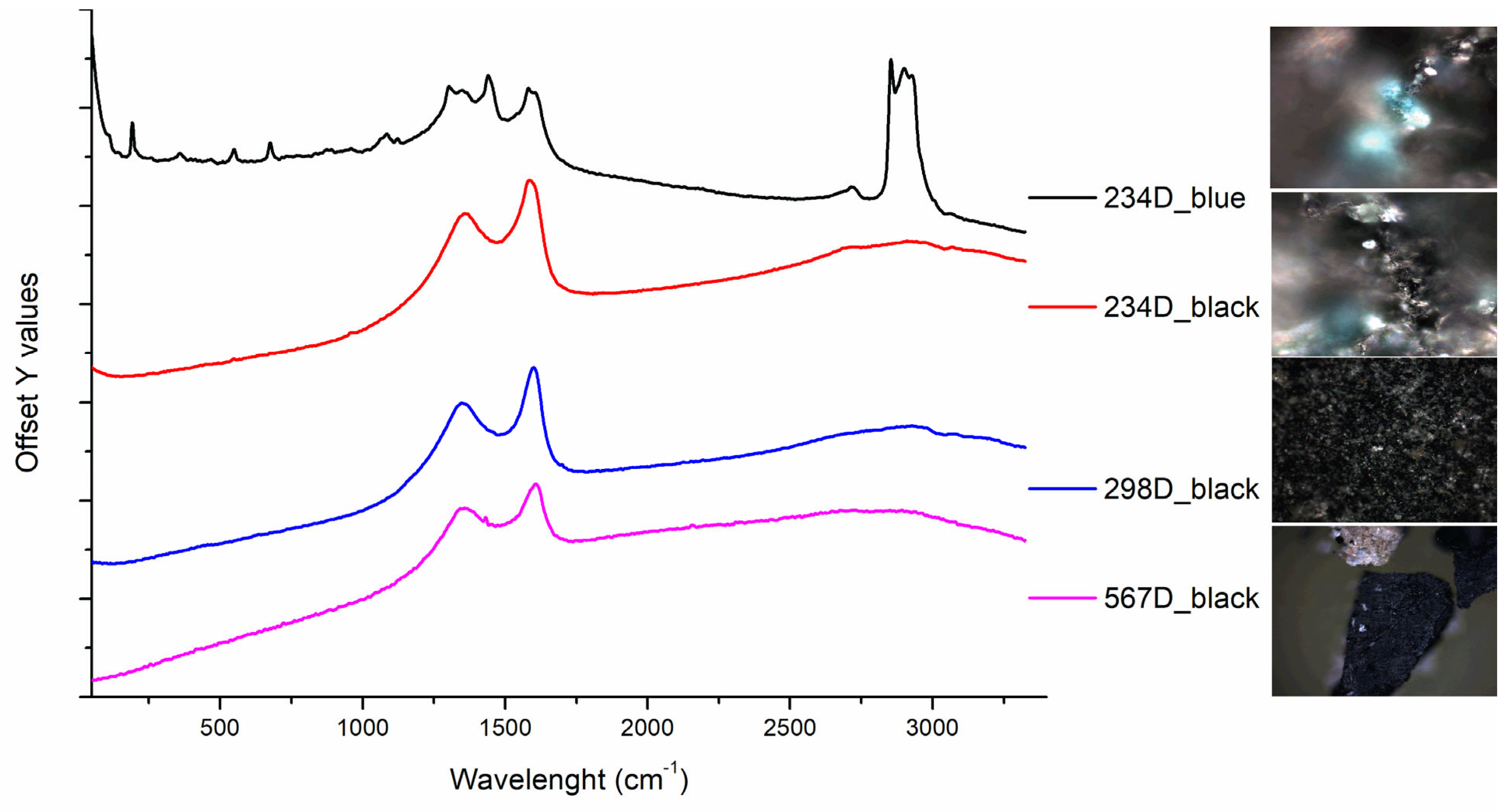
| Printed paper | 234 D | Printed paper with floral festoon motif. | x S | x | x * | x | |
| 462 D | Printed paper with a geometric floral motif, bordered by a square frame. | x S | x | x | |||
| 567 D | Paper printed with a geometric pattern of repeated triangles forming a circle. | x S | x | x * | x | ||
| 350 D | Glossy paper printed with a floral motif together with two mirrored deer in the centre of the design reminiscent of the style in Parisian Gothic churches. | x D | x | x | |||
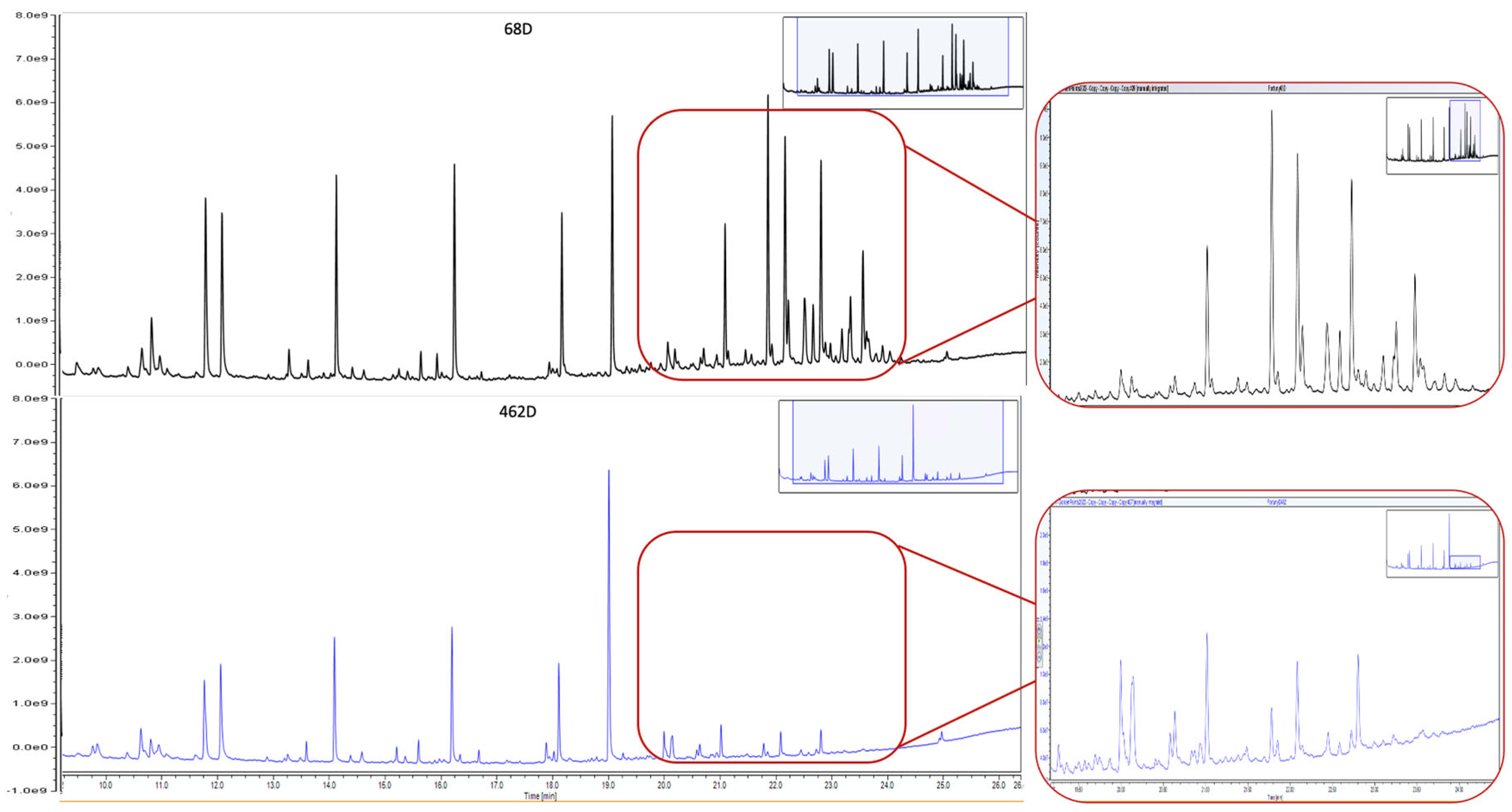
| 68 D | Printed paper with the coat of arms of the Franco regime in the 1938–1945 version according to the so-called ‘abbreviated’ model, which became very popular due to its wide circulation [13]. | x S | x | x | x | ||
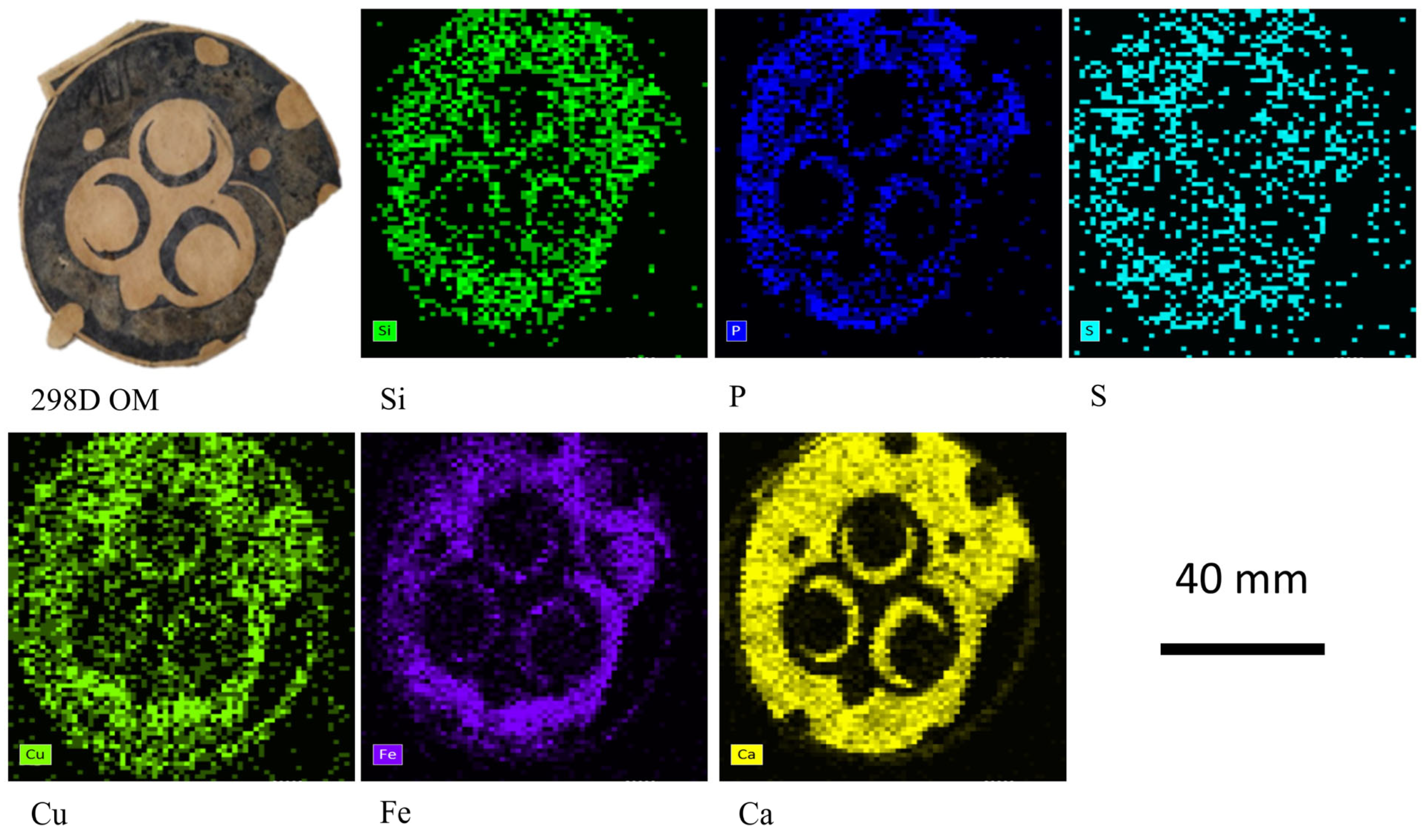
| 298 D | Fragment of paper printed with circular motifs and representations of small moons. | x D | x | x | x * | ||
| 130 D | Paper printed with stylised roses in arabesques. | x | x | x | |||
| Polychrome fabrics | 215 T | Printed fabric with a floral pattern, whose main design appears to be a stylised representation of a pineapple. | x T | x | x | ||
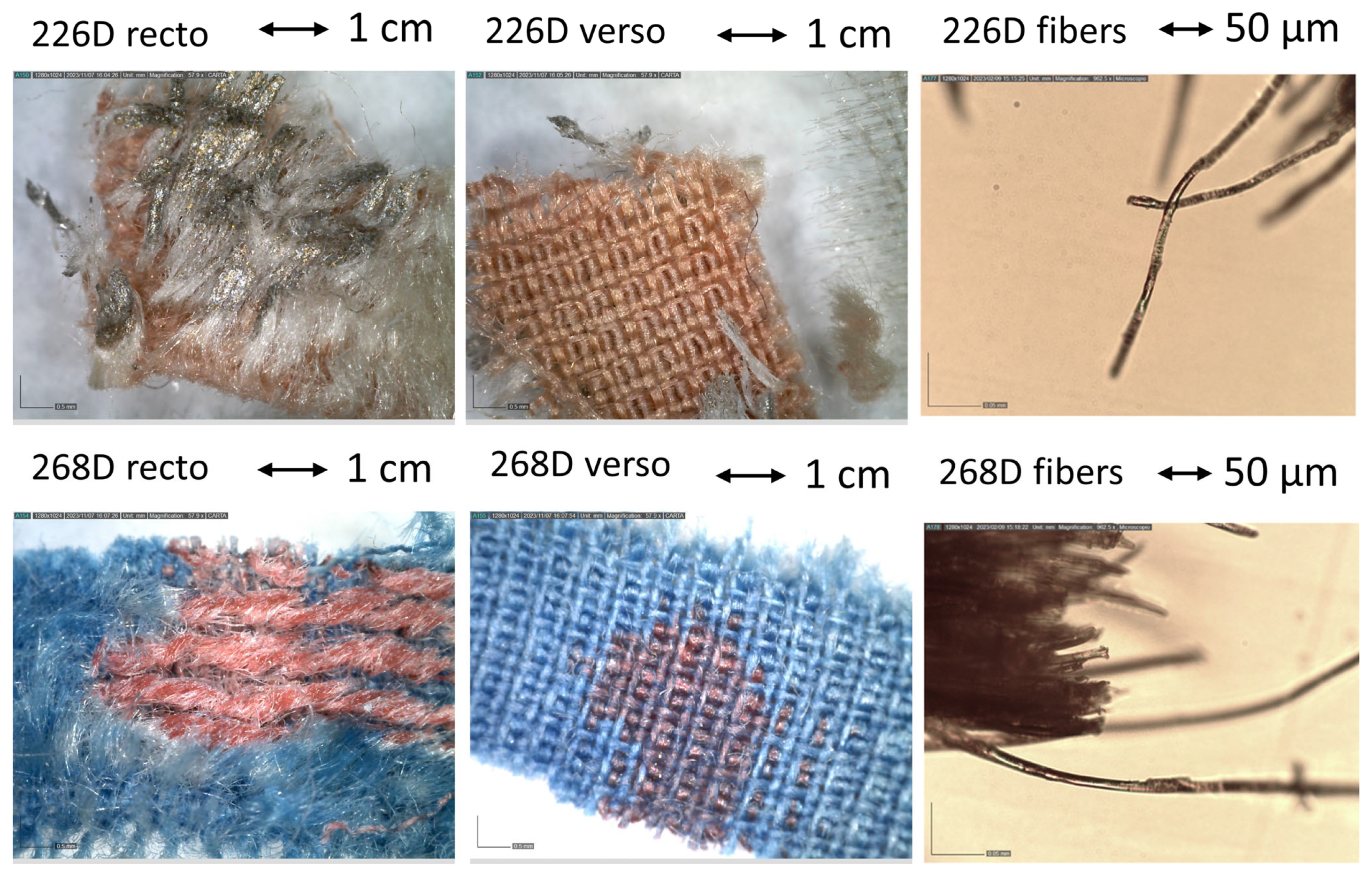
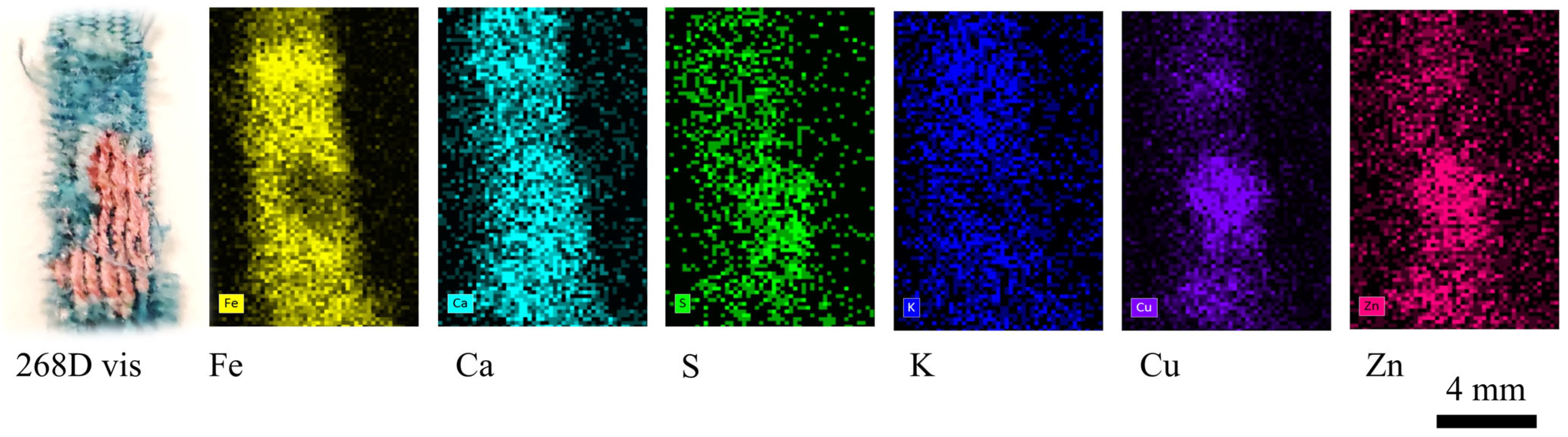
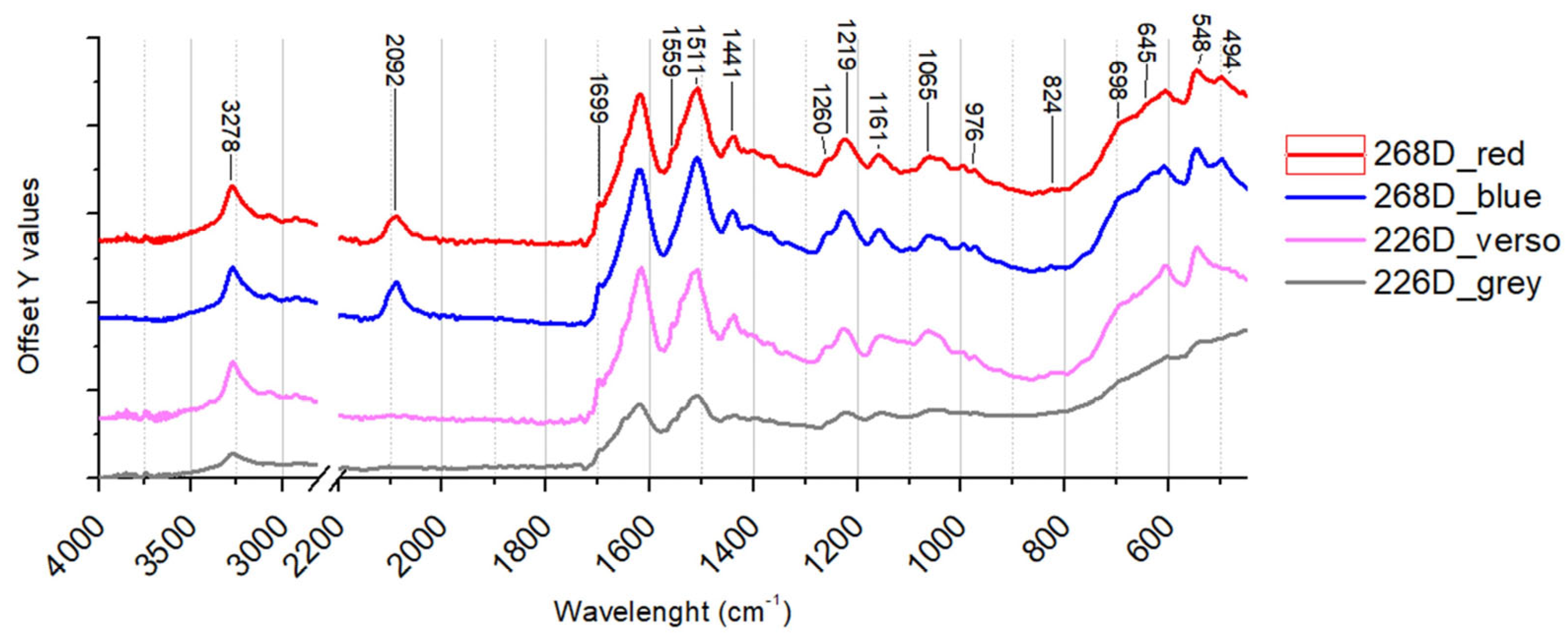
| 268 D | Fragment of blue textile with designs in red. | x T | x | x | x | ||
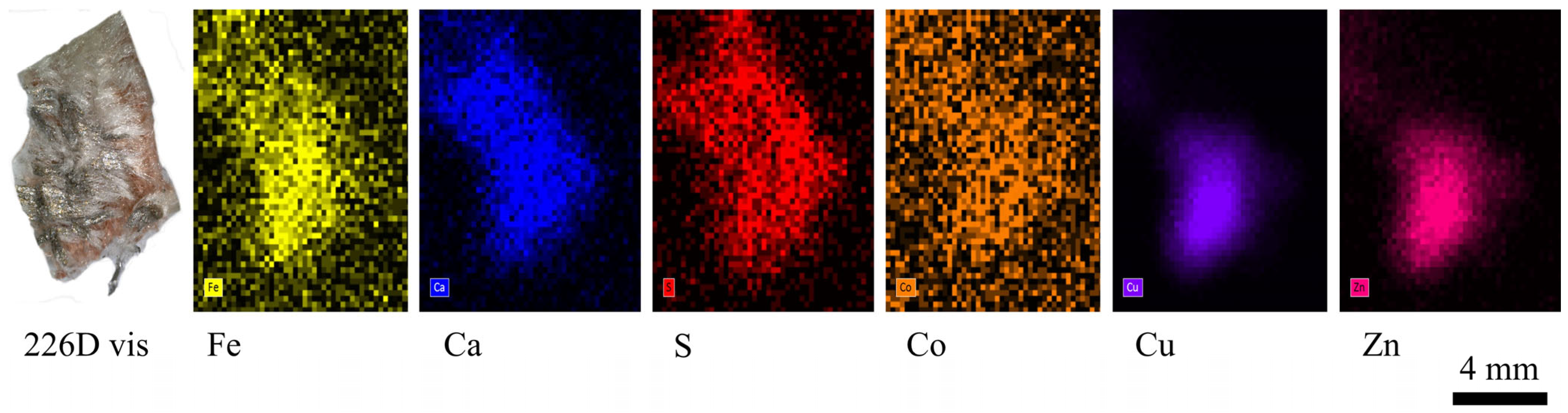
| 226 D | Orange-coloured fragment of fabric with a softer light-coloured part on the surface and a metallic grey corner. | x T | x | x | x | ||
| Identified Compound | m/z | Paint Samples Collected from | ||||
|---|---|---|---|---|---|---|
| 567D | 462D | 68D | 234D | |||
| Fatty acid and other compounds | Glycerol derivate | 89 | √ | √ | √ | √ |
| Glycerol derivate | 162 | √ | √ | √ | √ | |
| Nonanoic acid, 9-oxo ME | 172 | √ | √ | √ | √ | |
| Glycerol derivate | 75 | √ | √ | √ | √ | |
| Suberic acid diME | 202 | 6.91% | 6.00% | 9.57% | 2.20% | |
| Phthalic acid diME | 166 | √ | √ | √ | √ | |
| Glycerol derivate | 394 | √ | √ | √ | √ | |
| Lauric acid ME | 200 | √ | √ | √ | √ | |
| 10-Oxodecanoic acid ME | 200 | - | - | √ | - | |
| Azelaic acid diME | 216 | 29.38% | 25.54% | 10.88% | 9.13% | |
| Benzoic acid, 3,4-dimethoxy ME | 182 | - | √ | √ | - | |
| Caprylic acid ME | 144 | - | √ | √ | √ | |
| Sebacic acid diME | 230 | 3.64% | 1.46% | 3.46% | 0.81% | |
| Myristic acid ME | 242 | √ | √ | √ | √ | |
| Aleuritic acid ME | 304 | √ | √ | √ | √ | |
| Palmitic acid ME | 270 | 17.94% | 25.54% | 26.94% | 44.99% | |
| Margaric acid ME | 270 | - | √ | - | √ | |
| Elaidic acid ME | 296 | √ | √ | √ | √ | |
| Linoleic acid ME | 292 | 6.92% | 3.50% | 1.55% | 1.89% | |
| Oleic acid ME | 296 | 1.75% | 0.55% | 0.56% | 14.53% | |
| Linolenic acid ME | 294 | 0.05% | 0.30% | 0.27% | 0.01% | |
| Stearic acid ME | 298 | 20.94% | 19.22% | 20.90% | 24.53% | |
| Nonadecanoic acid ME (IS) | 312 | √ | √ | √ | √ | |
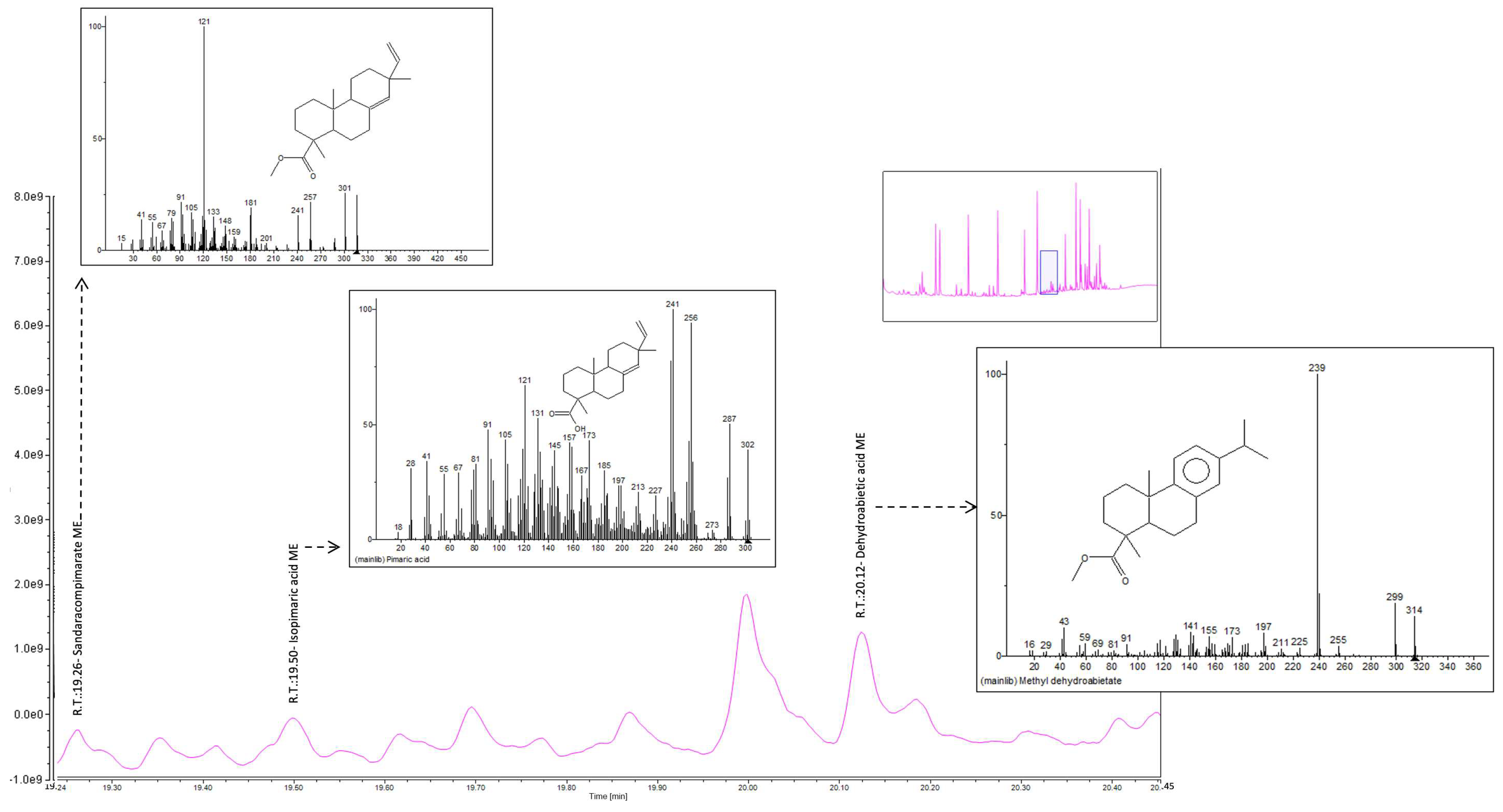
| Sandaracopimarate ME | 316 | √ | √ | √ | √ | |
| Isopimaric acid methyl ester | 316 | √ | √ | √ | √ | |
| Oxiraneoctanoic acid, 3-octyl ME | 312 | √ | √ | √ | √ | |
| Eicosanoic acid ME | 326 | - | - | - | √ | |
| Glycerol | 524 | √ | √ | √ | √ | |
| Dehydroabietic acid ME | 300 | √ | √ | √ | √ | |
| Octadecanoic acid, 9,10-epooxy ME | 312 | √ | √ | √ | - | |
| Arachidic acid ME | 312 | √ | - | √ | - | |
| Methyl 6- dehydrodehydroabietate | 306 | √ | - | - | - | |
| Tetradehydroabietic acid, 7-methoxy ME | 314 | √ | √ | √ | - | |
| Octadecanoic acid, 9,10-dihydroxy ME | 330 | √ | √ | √ | √ | |
| 10- Octadecanoic acid ME | 354 | √ | - | - | √ | |
| Behenic acid ME | 340 | √ | √ | √ | - | |
| 7-Oxoxdehydroabietic acid ME | 328 | √ | √ | √ | - | |
| 7,15-Dimethoxytetradehydroabuietic acid ME | 332 | - | √ | √ | √ | |
| 15-Hydroxy-7-oxodehydroabietic acid ME | 330 | - | - | √ | √ | |
| Octadecanoic acid, 9,10-oxo- ME | 310 | √ | - | √ | - | |
| Molar ratios among fatty acids | A/P | 1.64 | 1.01 | 0.92 | 0.20 | |
| P/S | 0.86 | 1.33 | 1.29 | 1.87 | ||
| A/Sub | 4.25 | 4.30 | 2.58 | 4.16 | ||
| O/S | 0.08 | 0.03 | 0.03 | 0.08 | ||
| D/P | 2.23 | 1.30 | 1.40 | 0.27 | ||
| %D | 39.93 | 33.24% | 37.77% | 12.14% | ||
| Object Code | OM | XRF | ATR-FTIR & Raman Spectroscopy Raman | GC–MS |
|---|---|---|---|---|
| 298D | White brilliant grains on the paper surface. Black areas. | Ca, Ti, Si, Fe, P, Cu | Bone Black (ivory), calcite, talc, zinc stearate, organic binder | n.d. |
| 350D | Shiny areas on the surface. Presence of a black thin coloured layer. | Si, Ca, Ti, Mn, Fe | Bone Black (ivory), calcite, talc, zinc stearate, organic binder | n.d. |
| 68D | Easily detachable thin paint layer composed of grains with sharply regular edges. The surface of the grains appears shiny, with intrusion of iridescent green-yellow grains. | n.d. | Bone Black (ivory), calcite, talc, zinc stearate, organic binder | Linseed oil, Colophony |
| 234D | Easily detachable thick paint layer composed of green grains, in particular dark green particles in the dark areas. | Si, Ca, Mn, Ti, S, Fe, Cu, Pb | Green copper pigments, linseed oil, calcite, talc, copper stearate, zinc stearate | Linseed oil, Colophony |
| 462D | Easily detachable thick paint layer composed of dark grains with irregular edges. White flakes like salts deposit over some grains. | n.d. | Bone Black (ivory), calcite, gypsum, linseed oil | Linseed oil, Colophony |
| 567D | Easily detachable thick paint layer composed of distinct colours on the front (black) and back sides (light- brown) over a waxy paper. | n.d. | Bone Black (ivory), organic binder | Linseed oil, Colophony |
| 130D | Layer of metallic paint, with iridescent and green grains that easily detach from the transparent paper. The paper has a waxy consistency. | Si, Ca, Cu, Ti, Fe, Zn | Green copper pigments, linseed oil, calcite, talc, copper stearate, zinc stearate, carboxylates | n.d. |
| 215T | A thick layer of paint covers a dyed velvet fabric. The fabric looks shiny. | n.d. | Silk, Linseed oil, Calcite, Prussian blue | n.d. |
| 226D | Elongated smooth fibres. | Cu, Zn, Ca | Silk | Traces of fatty acids |
| 268D | Elongated smooth fibres. | Fe, Ca | Silk, Prussian blue | Traces of fatty acids |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Farinelli, V.; Falchi, L.; da Roit, C.; Gnemmi, M.; Izzo, F.C. Unveiling Polychrome Printing Methods on Textiles: Preliminary Results from the Mariano Fortuny y Madrazo Collection in Venice. Heritage 2024, 7, 1298-1319. https://doi.org/10.3390/heritage7030062
Farinelli V, Falchi L, da Roit C, Gnemmi M, Izzo FC. Unveiling Polychrome Printing Methods on Textiles: Preliminary Results from the Mariano Fortuny y Madrazo Collection in Venice. Heritage. 2024; 7(3):1298-1319. https://doi.org/10.3390/heritage7030062
Chicago/Turabian StyleFarinelli, Virginia, Laura Falchi, Cristina da Roit, Margherita Gnemmi, and Francesca Caterina Izzo. 2024. "Unveiling Polychrome Printing Methods on Textiles: Preliminary Results from the Mariano Fortuny y Madrazo Collection in Venice" Heritage 7, no. 3: 1298-1319. https://doi.org/10.3390/heritage7030062
APA StyleFarinelli, V., Falchi, L., da Roit, C., Gnemmi, M., & Izzo, F. C. (2024). Unveiling Polychrome Printing Methods on Textiles: Preliminary Results from the Mariano Fortuny y Madrazo Collection in Venice. Heritage, 7(3), 1298-1319. https://doi.org/10.3390/heritage7030062







