An Archaeometric Analysis of Black-Appearing Iron Age Glass Beads from Vinha das Caliças 4 (Portugal)
Abstract
1. Introduction
1.1. Historical Context
1.2. Black and Black-Appearing Glass
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. Optical Microscopy
2.2.2. μ-XRD
2.2.3. VP-SEM-EDS
2.2.4. LA-ICP-MS
3. Results and Discussion
3.1. Typology and Bead Manufacture
3.1.1. Typology
3.1.2. Bead Manufacture
3.2. State of Preservation
3.3. Glassmaking Technology
3.3.1. Fluxing Agent
3.3.2. Network Former
3.3.3. Stabilizer
3.4. Colouring Technology
3.4.1. Black-Appearing Glass
3.4.2. White Glass
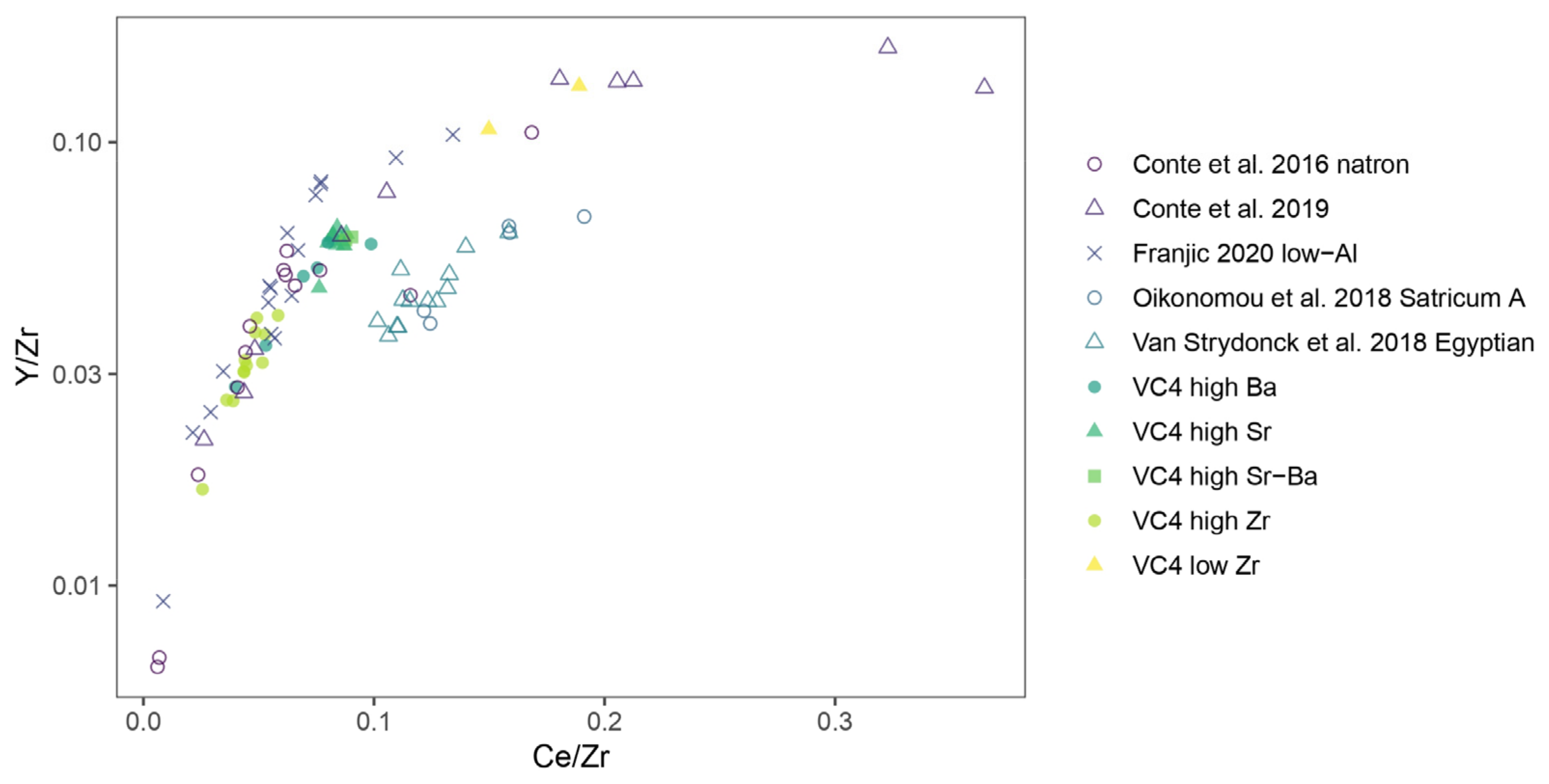
3.5. Silica Sources
4. Final Remarks and Implications for the Understanding of Iron Age Glass Trade Networks in the Mediterranean
- Black-appearing beads from Southern Portugal are (predominately) low-Al natron glass coloured with iron;
- Trace element and REE related to the silica source show more similarities with glasses thought to have been produced in Egypt, than other known glassmaking regions in the (wider) Mediterranean;
- Different iron colourants, were consistently used in the production of black-appearing beads;
- There is a link between bead typology and the type of iron colourant used.
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
| 1 | |
| 2 | “Phoenicians” is an ethnonym taken from the ancient Greek to designate the people inhabiting Levantine city-states like Tyre, Byblos, and Sidon. During the 1st millennium BCE, these cities saw a rapid colonial expansion across the Mediterranean and established a wide-spread network of maritime trade and production of luxury goods like dyes, textiles, and glass. There is, however, little evidence for their cultural, political, linguistic or religious centralisation. For further discussion, see references [26,27,28,29,30,31]. |
| 3 | A full morphometric and typological study of the assemblage is underway. So far, 77 occurrences of beads with one or more wavy line decorations were documented in a set of 133 beads initially described as black-appearing. |
| 4 | 28 occurrences among 133 observed black-appearing beads. |
| 5 | Diameter, measured as the largest perceived dimension of the bead when the apex (perforation) of the bead is observed. |
| 6 | 19 occurrences among 133 observed black-appearing beads. |
References
- Gratuze, B. Les premiers verres au natron retrouvés en Europe occidentale: Composition chimique et chronotypologie. In Annales du 17° Congrès de l’Association Internationale pour Histoire du Verre/Annales of the 17th Congress of the International Association for the History of Glass; Janssens, K., Degryse, P., Cosyns, P., Caen, J., Dack, L.V., Eds.; Antwerp University Press: Antwerp, Belgium, 2009; pp. 8–14, 47–54. [Google Scholar]
- Reade, W.; Freestone, I.; Bourke, S. Innovation and continuity in Bronze and Iron Age glass from Pella in Jordan. In Annales 17° Congrès de l’Association Internationale pour Histoire du Verre. Annales of the 17th Congress of the International Association for the History of Glass; Janssens, K., Degryse, P., Cosyns, P., Caen, J., Dack, L.V., Eds.; University Press Antwerp: Antwerp, Belgium, 2009; pp. 47–54. [Google Scholar]
- Gomes, F.B. Early Iron Age “black” Glass in Southwestern Iberia: Typology, Distribution and Context. Zephyrus 2021, 87, 125–144. [Google Scholar] [CrossRef]
- Henderson, J. Electron Probe Microanalysis of Mixed-Alkali Glasses. Archaeometry 1988, 30, 77–91. [Google Scholar] [CrossRef]
- Rehren, T.; Rosenow, D. Three Millennia of Egyptian Glassmaking. In Mobile Technologies in the Ancient Sahara and Beyond; Duckworth, C., Cuénod, A., Mattingly, D., Eds.; Cambridge University Press: Cambridge, UK, 2020; pp. 423–450. [Google Scholar]
- Schlick-Nolte, B.; Werthmann, R. Glass Vessels from the Burial of Nesikhons. J. Glass Stud. 2003, 45, 11–34. [Google Scholar]
- Dardeniz, G. Was Ancient Egypt the Only Supplier of Natron? New Res. Reveals Major Anatol. Depos. Anatolica 2015, 41, 191–202. [Google Scholar]
- Brill, R.H. Chemical Analyses of Early Glasses; Tables of Analyses; Corning Museum of Glass: Corning, NY, USA, 1999; Volume 2. [Google Scholar]
- Arletti, R.; Ferrari, D.; Vezzalini, G. Pre-Roman Glass from Mozia (Sicily-Italy): The First Archaeometrical Data. J. Archaeol. Sci. 2012, 39, 3396–3401. [Google Scholar] [CrossRef]
- Arletti, R.; Maiorano, C.; Ferrari, D.; Vezzalini, G.; Quartieri, S. The First Archaeometric Data on Polychrome Iron Age Glass from Sites Located in Northern Italy. J. Arhceol. Sci. 2010, 37, 703–712. [Google Scholar] [CrossRef]
- Arletti, R.; Rivi, L.; Ferrarri, D.; Vezzalini, G. The Mediterranean Group II: Analyses of Vessels from Etruscan Contexts in Northern Italy. J. Arhceol. Sci. 2011, 38, 2094–2100. [Google Scholar] [CrossRef]
- Van Strydonck, M.; Gratuze, B.; Rolland, J.; De Mulder, G. An Archaeometric Study of Some Pre-Roman Glass Beads from Son Mas (Mallorca, Spain). J. Archaeol. Sci. Rep. 2018, 17, 491–499. [Google Scholar] [CrossRef]
- Panighello, S.; Orsega, E.F.; Elteren, J.T.; Šelih, V. Analysis of Polychrome Iron Age Glass Vessels from Mediterranean I, II and III Groups by LA-ICP-MS. J. Archaeol. Sci. 2012, 39, 2945–2955. [Google Scholar] [CrossRef]
- Conte, S.; Arletti, R.; Henderson, J.; Degryse, P.; Blomme, A. Different Glassmaking Technologies in the Production of Iron Age Black Glass from Italy and Slovakia. Archaeol. Anthropol. Sci. 2018, 10, 503–521. [Google Scholar] [CrossRef]
- Blomme, A.; Degryse, P.; Dotsika, E.; Ingnatiadou, D.; Longinelli, A.; Silvestri, A. Provenance of Polychrome and Colourless 8th–4th Century BC Glass from Pieria, Greece: A Chemical and Isotopic Approach. J. Archaeol. Sci. 2017, 78, 134–146. [Google Scholar] [CrossRef]
- Shortland, A.J.; Schroeder, H. Analysis of First Millennium BC Glass Vessels and Beads from the Pichvnari Necropolis, Georgia. Archaeometry 2009, 51, 947–965. [Google Scholar] [CrossRef]
- Rolland, J. Le Verre de l’Europe Celtique. Approches Archéométriques, Technologiques et Sociales d’un Artisanat Du Prestige Au Second Âge Du Fer; Sidestone Press: Leiden, The Netherlands, 2021; ISBN 978-90-8890-995-5. [Google Scholar]
- Rolland, J.; Venclová, N. Iron Age Glass-working in Moravia, Central Europe: New Archaeometric Research on Raw Glass and Waste—3rd–First Century BC. Archaeol. Anthropol. Sci. 2021, 13, 124. [Google Scholar] [CrossRef]
- Šmit, Ž.; Laharnar, B.; Turk, P. Analysis of Prehistoric Glass from Slovenia. J. Archaeol. Sci. Rep. 2020, 29, 102114. [Google Scholar] [CrossRef]
- Oikonomou, A.; Henderson, J.; Gnade, M.; Chenery, S.; Zacharias, N. An Archaeometric Study of Hellenistic Glass Vessels: Evidence for Multiple Sources. Archaeol. Anthropol. Sci. 2018, 10, 97–110. [Google Scholar] [CrossRef]
- Ham-Meert, A.; Dillis, S.; Blomme, A.; Cahill, N.; Claeys, P.; Elsen, J.; Eremin, K.; Gerdes, A.; Steuwe, C.; Roeffaers, M.; et al. A unique recipe for glass beads at Iron Age Sardis. J. Archaeol. Sci. 2019, 108, 109474. [Google Scholar] [CrossRef]
- Conte, S.; Arletti, R.; Mermati, F.; Gratuze, B. Unravelling the Iron Age Glass Trade in Southern Italy: The First Trace-Element Analyses. Eur. J. Mineral. 2016, 28, 409–433. [Google Scholar] [CrossRef]
- Tzankova, N.; Mihaylov, P. Chemical Characterization of Glass Beads from the Necropolis of DrenDelyan (6th–4th Century BC), Southwest Bulgaria. Geol. Balc. 2019, 48, 31–50. [Google Scholar] [CrossRef]
- Schmidt, K. Glass and Glass Production in the Near East during the Iron Age: Evidence from Objects, Texts and Chemical Analysis; Archaeopress Publishing Ltd.: Oxford, UK, 2019; ISBN 978-1-78969-155-9. [Google Scholar]
- Purowski, T.; Syta, O.; Wagner, B. Between East and West: Glass Beads from the Eighth to Third Centuries Bce from Poland. Archaeometry 2020, 62, 752–773. [Google Scholar] [CrossRef]
- Quinn, J. Search for the Phoenicians; Princeton University Press: Princeton, NJ, USA, 2018; ISBN 978-0-691-17527-0. [Google Scholar]
- Aubet, M.E. Phoenicians Abroad: From Merchant Venturers to Colonists. In Eurasia at the Dawn of History: Urbanization and Social Change; Krausse, D., Fernández-Götz, M., Eds.; Cambridge University Press: Cambridge, UK, 2017; pp. 254–264. ISBN 978-1-107-14740-9. [Google Scholar]
- Aubet, M.E. The Phoenicians and the West: Politics, Colonies and Trade, 2nd ed.; Cambridge University Press: Cambridge, UK, 2001. [Google Scholar]
- Amadasi Guzzo, M.G. The Language. In The Oxford Handbook of the Phoenician and Punic Mediterranean; Doak, B.R., López-Ruiz, C., Eds.; Oxford University Press: Oxford, UK, 2019; ISBN 978-0-19-049934-1. [Google Scholar]
- Xella, P. Religion. In The Oxford Handbook of the Phoenician and Punic Mediterranean; Doak, B.R., López-Ruiz, C., Eds.; Oxford University Press: Oxford, UK, 2019; ISBN 978-0-19-049934-1. [Google Scholar]
- Bellard, C.G. Agriculture. In The Oxford Handbook of the Phoenician and Punic Mediterranean; Doak, B.R., López-Ruiz, C., Eds.; Oxford University Press: Oxford, UK, 2019; ISBN 978-0-19-049934-1. [Google Scholar]
- Arruda, A.M. Los Fenicios en Portugal. Fenicios e indígenas en el centro y sur de Portugal (siglos VIII-VI a.C).; Universidad Pompeo Fabra: Barcelona, Spain, 1999. [Google Scholar]
- Arruda, A.M. O 1.o milénio a.n.e. no Centro e no Sul de Portugal: Leituras possíveis no início de um novo século. Arqueólogo Port. 2005, 4, 9–156. [Google Scholar]
- Gomes, F. Mediterranean goods in “Post-Orientalizing” funerary contexts of Southern Portugal: Some remarks on consumption, peripherality and cultural identity. In Actas del XVIII Congreso Internacional de Arqueología Clásica: Centro y Perifería en el Mundo Clásico; Martínez, J.M.Á., Basarrate, T.N., Llanza, I.R., Eds.; National Museum of Roman Art: Mérida, Spain, 2015; Volume 1, pp. 435–438. [Google Scholar]
- Costa, M.; Barrulas, P.; Arruda, A.M.; Dias, L.; Barbosa, R.; Vadenabeele, P.; Mirão, J. An Insight into the Provenance of the Phoenician-Punic Glass Beads of the Necropolis of Vinha Das Caliças (Beja, Portugal). Archaeol. Anthropol. Sci. 2021, 13, 17. [Google Scholar] [CrossRef]
- García-Heras, M.; Rincón, J.M.; Jimeno, A.; Villegas, M.A. Pre- Roman Coloured Glass Beads from the Iberian Peninsula: A Chemico- Physical Characterisation Study. J. Archaeol. Sci. 2005, 32, 727–738. [Google Scholar] [CrossRef]
- Gomes, F.B. Mediterranean Exotica and the Fabric of Early Iron Age Society in Western Iberia (8th–5th Centuries BCE). Layers Archeol. Territ. Contesti 2023, 8, 1–19. [Google Scholar] [CrossRef]
- Dietler, M. Colonial Encounters in Iberia and the Western Mediterranean: An Exploratory Framework. In Colonial Encounters in Ancient Iberia: Phoenician, Greek, and Indigenous Relations; Dietler, M., López-Ruiz, C., Eds.; University of Chicago Press: Chicago, IL, USA, 2009; pp. 3–48. [Google Scholar]
- Fletcher, R.N. Opening the Mediterranean: Assyria, the Levant and the Transformation of Early Iron Age Trade. Antiquity 2012, 86, 211–220. [Google Scholar] [CrossRef]
- Wagner, C.G.; Alvar, J. La colonización agrícola en la península ibérica: Estado de la cuestión y nuevas perspectivas. In Ecohistoria del Paisaje Agrario: La Agricultura Fenicio-Púnica en el Mediterráneo; Bellard, C.G., Ed.; Universidad de Valencia/Universitat de València: Valencia, Spain, 2003; pp. 187–204. ISBN 978-84-370-5508-4. [Google Scholar]
- Eshel, T.; Erel, Y.; Yahalom-Mack, N.; Tirosh, O.; Gilboa, A. Lead Isotopes in Silver Reveal Earliest Phoenician Quest for Metals in the West Mediterranean. Proc. Natl. Acad. Sci. USA 2019, 116, 6007–6012. [Google Scholar] [CrossRef] [PubMed]
- Arruda, A.M. The Phoenicians in Portugal. In The Oxford Handbook of the Phoenician and Punic Mediterranean; López-Ruiz, C., Doak, B.R., Eds.; Oxford University Press: Oxford, UK, 2019. [Google Scholar]
- Soares, A.M.M.; Arruda, A.M.; Barcleó, J.A.; Bogdanovic, I.; Morell, B. A cronologia de radiocarbono para a Idade do Ferro Orientalizante no território português. Uma leitura crítica dos dados arqueométricos e arqueológicos. In IberCrono 2016 Cronometrías Para la Historia de la Península Ibérica (Chronometry for the History of the Iberian Peninsula); CEUR: Barcelona, Spain, 2017; Volume 2024, pp. 235–259. [Google Scholar]
- López Castro, J.L. The Iberian Peninsula. In The Oxford Handbook of the Phoenician and Punic Mediterranean; Doak, B.R., López-Ruiz, C., Eds.; Oxford University Press: Oxford, UK, 2019; ISBN 978-0-19-049934-1. [Google Scholar]
- Gomes, F.B. The West Writes Back: Cultural Contact and Identity Constructs in Southern Portuguese Late Bronze Age/Early Iron Age. In The Mediterranean Mirror: Cultural Contacts in the Mediterranean Sea between 1200 and 750 B.C.; Babbi, A., Bubbenheimer-Erhart, F., Marín-Aguilera, B., Mühl, S., Eds.; Römisch-Germanisches Zentralmuseum: Mainz, Germany, 2015; pp. 305–318. [Google Scholar]
- Arruda, A.M. Necrópoles proto-históricas do sul de Portugal: O mundo oriental e orientalizante. O mundo funerario. In Actas de III Seminario Internacional sobre Temas Fenicos, Alicante; González Prats, A., Pellicer Catalán, M., Eds.; Generalitat Valenciana: Alicante, Spain, 2004; pp. 457–494. [Google Scholar]
- Gomes, F.B.; Arruda, A.M. On the Edge of History? The Early Iron Age of Southern Portugal, between Texts and Archaeology. World Archaeol. 2019, 50, 764–780. [Google Scholar] [CrossRef]
- Berrocal-Rangel, L.; Silva, C.A. O Castro dos Ratinhos (Barragem de Alqueva, Moura): Excava-Cões Num Povoado Proto-Histórico do Guadiano, 2004–2007; O Archeólogo Português Suplemento; Museu Nacional de Arqueologia: Lisboa, Portugal, 2010; ISBN 978-972-9257-25-4. [Google Scholar]
- Gomes, F.B. Cuentas de vidrio oculadas de la Edad del Hierro del sur de Portugal (ss. VII–II a. n. e.). Trab. Prehist. 2023, 80, e17. [Google Scholar] [CrossRef]
- Arruda, A.; Barbosa, R.; Gomes, F.; Sousa, E. A Necrópole de Vinha das Caliças (Beja, Portugal). In Sidereum Ana III-El río Guadiana y Tartessos; Ávila, J.J., Ed.; Consórcio de la Ciudad Monumental Histórico-Artística y Arqueológica de Mérida: Mérida, Mexico, 2016; pp. 187–225. [Google Scholar]
- Gomes, F.B. O mundo funerário da I Idade do Ferro no Sul do actual território português: Notas para uma síntese. Arqueol. História 2014, 66–67, 47–62. [Google Scholar]
- Santos, F.J.; Antunes, A.S.; Deus, M.G. A necrópole de Palhais (Beringel, Beja). In Sidereum Ana III-El río Guadiana y Tartessos; Ávila, J.J., Ed.; Consórcio de la Ciudad Monumental Histórico-Artística y Arqueológica de Mérida: Mérida, Mexico, 2017; pp. 227–262. [Google Scholar]
- Costa, M.; Arruda, A.M.; Dias, L.; Barbosa, R.; Mirão, J.; Vandenabeele, P. The Combined Use of Raman and Micro-X-Ray Diffraction Analysis in the Study of Archaeological Glass Beads. J. Raman Spectrosc. 2019, 50, 250–261. [Google Scholar] [CrossRef]
- Biron, I.; Chopinet, M.H. Colouring, Decolouring, and Opacifying of Glass. In Modern Methods for Analysing Archaeological and Histroical Glass; Janssens, K., Ed.; John Wiley & Sons, Ltd.: Chichester, UK, 2013; Volume 1, pp. 49–65. [Google Scholar]
- Cosyns, P. The Production, Distribution and Consumption of Black Glass in the Roman Empire during the 1st–5th Century AD: An Archaeological, Archaeometric and Historical Approach, Faculteit Letteren en Wijsbegeerte; Vrije Universiteit Brussel: Brussel, Belgium, 2011. [Google Scholar]
- Van Der Linden, V.; Cosyns, P.; Schalm, O.; Cagno, S.; Nys, K.; Janssens, K.; Nowak, A.; Wagner, B.; Bulska, E. Deeply Coloured and Black Glass in the Northern Provinces of the Roman Empire: Differences and Similarities in Chemical Composition Before and After AD150*. Archaeometry 2009, 51, 822–844. [Google Scholar] [CrossRef]
- Cagno, S.; Cosyns, P.; Nys, K.; Janssens, K. Black-Appearing Roman Glass. In Modern Methods for Analysing Archaeological and Historical Glass; Janssens, K., Ed.; John Wiley & Sons, Ltd.: Chichester, UK, 2013; Volume 1, pp. 369–386. [Google Scholar]
- Lankton, J.W.; Pulak, C.; Gratuze, B. Glass Ingots from the Uluburun Shipwreck: Glass by the Batch in the Late Bronze Age. J. Archaeol. Sci. Rep. 2022, 42, 103354. [Google Scholar] [CrossRef]
- Nicholson, P.T.; Gerisch, R.; Brook, E.; Jackson, C.M. Brilliant Things for Akhenaten: The Production of Glass, Vitreous Materials and Pottery at Amarna Site O45.1; Excavation Memoir, Egypt Exploration Society: London, UK; David Brown Book Co.: Oakville, CT, USA, 2007; ISBN 978-0-85698-178-4. [Google Scholar]
- Shortland, A.J.; Eremin, K. The Analysis of Second Millennium Glass from Egypt and Mesopotamia, Part 1: New Wds Analyses. Archaeometry 2006, 48, 581–603. [Google Scholar] [CrossRef]
- Kemp, V.; Delbey, T.; Shortland, A. The Dating and Provenance of Glass Fragments from the Site of Serabit El-Khâdim, Sinai. J. Archaeol. Sci. Rep. 2023, 49, 103920. [Google Scholar] [CrossRef]
- Franjić, A. Iron Age Glass Technology in South East Europe; UCL (University College London): London, UK, 2020. [Google Scholar]
- Cagno, S.; Cosyns, P.; Der Linden, V.V.; Schalm, O.; Izmer, A.; Deconinck, I.; Vanhaecke, F.; Nowak, A.; Wagner, B.; Bulska, E.; et al. Composition Data of a Large Collection of Black-Appearing Roman Glass. Open J. Archaeom. 2013, 1, e22. [Google Scholar] [CrossRef]
- Cagno, S.; Cosyns, P.; Izmer, A.; Vanhaecke, F.; Nys, K.; Jansens, K. Deeply Colored and Black-Appearing Roman Glass: A Continued Research. J. Archaeol. Sci. 2014, 42, 128–139. [Google Scholar] [CrossRef]
- Pelicano, A. Práticas Funerárias na I idade do Ferro. O Caso de Vinha das Caliças 4, Trigaches. Master’s Thesis, Universidade de Coimbra, Coimbra, Portugal, 2016. [Google Scholar]
- Costa, M.; Arruda, A.M.; Barbosa, R.; Barrulas, P.; Vandenabeele, P.; Mirão, J. A Micro-Analytical Study of the Scarabs of the Necropolis of Vinha Das Caliças (Portugal). Microsc. Microanal. 2019, 25, 214–220. [Google Scholar] [CrossRef] [PubMed]
- Costa, M.; Barrulas, P.; Arruda, A.M.; Barbosa, R.; Vandenabeele, P.; Mirão, J. New Approaches for the Study of Faience Using Beads from Southern Portugal. J. Archaeol. Sci. Rep. 2022, 46, 103703. [Google Scholar] [CrossRef]
- Ngan-Tillard, D.J.M.; Huisman, D.J.; Corbella, F.; Van Nass, A. Over the Rainbow? Micro-CT Scanning to Non-Destructively Study Roman and Early Medieval Glass Bead Manufacture. J. Archaeol. Sci. 2018, 98, 7–21. [Google Scholar] [CrossRef]
- Medeghini, L.; Botticelli, M.; Cadena-Irizar, A.C.; Lepri, B.; Ferrandes, A.F.; Costa, M.; Barrulas, P. Blue Shadows of Roman Glass Artefacts. Microchem. J. 2022, 179, 107526. [Google Scholar] [CrossRef]
- McGloin, J. Of Beads and Burials: A Microwear and Experimental Study of Early Medieval Glass and Amber Beads from the Merovingian Site of Lent-Lentseveld. J. Archaeol. Sci. 2021. [Google Scholar] [CrossRef]
- Purowski, T. Identifying Bronze Age Glass Production Centres through Bead-Making Techniques. Archeol. Pol. 2022, 67, 61–80. [Google Scholar] [CrossRef]
- Venclová, N. Prehistoric Glass in Bohemia; Archeologický ústav ČSAV: Praha, Czech Republic, 1990. [Google Scholar]
- Yatsuk, O.; Gorghinian, A.; Fiocco, G.; Davit, P.; Francone, S.; Serges, A.; Koch, L.; Re, A.; Lo Giudice, A.; Ferretti, M.; et al. Ring-Eye Blue Beads in Iron Age Central Italy—Preliminary Discussion of Technology and Possible Trade Connections. J. Archaeol. Sci. Rep. 2023, 47, 103763. [Google Scholar] [CrossRef]
- Koch, L.C. Zur Herstellungstechnik Der Perlen. In Früheisenzeitliches Glas und Glasfunde Mittelitaliens: Eine Übersicht von der Villanovazeit bis zum Orientalizzante und eine Analyse der Glasperlen als Grabbeigabe des Gräberfeldes Quattro Fontanili in Veji; Bochumer Forschungen zur ur- und frühgeschichtlichen Archäologie; Verlag Marie Leidorf: Rahden, Germany, 2011; pp. 28–31. [Google Scholar]
- Cox, G.A.; Ford, B.A. The Long-Term Corrosion of Glass by Ground-Water. J. Mater. Sci. 1993, 28, 5637–5647. [Google Scholar] [CrossRef]
- Fernández-Navarro, J.-M.; Villegas, M.-Á. What Is Glass? Janssens, K., Ed.; John Wiley & Sons, Ltd.: Chichester, UK, 2013; Volume 1. [Google Scholar]
- Melcher, M.; Schreiner, M. Glass Degradation by Liquids and Atmospheric Agents. In Modern Methods for Analysing Archaeological and Histroical Glass; Janssens, K., Ed.; John Wiley & Sons, Ltd.: Chichester, UK, 2013; Volume 1, pp. 609–652. [Google Scholar]
- Gratuze, B.; Janssens, K. Provenance Analysis of Glass Artefacts. In Comprehensive Analytical Chemistry; Jansens, K., Grieken, R., Eds.; Elsevier Science: Amsterdam, The Netherlands, 2004; Volume 42, pp. 663–712. [Google Scholar]
- Stern, W.B. Phosphate: A Neglected Argument in Studies of Ancient Glass Technology. Swiss J. Geosci. 2017, 110, 725–740. [Google Scholar] [CrossRef]
- Eremin, K.; Degryse, P.; Erb-Satullo, N.; Ganio, M.; Greene, J.; Shortland, A.; Walton, M.; Stager, L. Iron Age Glass Beads from Carthage. In Historical Technology, Materials, and Conservation: SEM and Microanalysis; Meeks, N.D., Meek, A., Eds.; Archetype Publications and the British Museum: London, UK, 2012; pp. 30–35. [Google Scholar]
- Brems, D.; Degryse, P. Trace Element Analysis in Provenancing Roman Glass-Making. Archaeometry 2014, 56, 116–136. [Google Scholar] [CrossRef]
- Agua, F.; Conde, J.F.; Kobylińska, U.; Kobyliński, Z.; García-Heras, M.; Villegas, M.Á. Chemical–Physical Characterisation of Early Iron Age Glass Beads from Central Europe. Bol. Soc. Esp. Cerámica Vidr. 2017, 56, 119–130. [Google Scholar] [CrossRef]
- Braun, C. Analyse von Gläsern Aus Der Hallstattzeit Mit Einem Excurs Über Römische Fenstergläser. In Glasperlen der vorrömischen Eisenzeit Vol. I. Marburger Studien zur Vor- und Frühgeschichte 5; Haevernick, T.E., Frey, O.-H., Eds.; Verlag Phillip von Zabern: Mainz am Rhein, Germany, 1983; pp. 129–175. [Google Scholar]
- Conte, S.; Matarese, I.; Vezzalini, G.; Pacciarelli, M.; Scarano, T.; Vanzetti, A.; Gratuze, B.; Arletti, R. How Much Is Known about Glassy Materials in Bronze and Iron Age Italy? New Data and General Overview. Archaeol. Anthropol. Sci. 2019, 11, 1813–1841. [Google Scholar] [CrossRef]
- Degryse, P.; Shortland, A.J. Interpreting Elements and Isotopes in Glass: A Review. Archaeometry 2020, 62, 117–133. [Google Scholar] [CrossRef]
- Foy, D.; Picon, M.; Vichy, M. Verres Omeyyades et Abbasides d’origine Egyptienne: Les temoignages de l’archéologie et de l’archéometrie. In Annales du 15e Congrès de l’Association Internationale pour l’Histoire du Verre; AIHV: Nottingham, UK, 2003; Volume 15, pp. 138–143. [Google Scholar]
- Brems, D.; Degryse, P.; Hasendoncks, F.; Gimeno, D.; Silvestri, A.; Vassilieva, E.; Luypaers, S.; Honings, J. Western Mediterranean Sand Deposits as Raw Material for Roman Glass Production. J. Archaeol. Sci. 2012, 39, 2897–2907. [Google Scholar] [CrossRef]
- Brems, D.; Degryse, P. Western Mediterranean Sands for Ancient Glass Making. In Glass Making in the Greco-Roman World: Results of the ARCHGLASS Project; Degryse, P., Ed.; Leuven university Press: Leuven, Belgium, 2015; pp. 27–50. [Google Scholar]
- Turner, W.E.S. Studies in Ancient Glasses and Glass Making Processes. Part V. Raw Materials and the Melting Process. J. Soc. Glass Technol. 1956, 40, 277–300. [Google Scholar]
- Cormier, L. Glasses: Aluminosilicates. In Encyclopedia of Materials: Technical Ceramics and Glasses; Pomeroy, M., Ed.; Elsevier: Oxford, UK, 2021; pp. 496–518. ISBN 978-0-12-822233-1. [Google Scholar]
- Freestone, I.C.; Leslie, K.A.; Thirlwall, M.; Gorin-Rosen, Y. Strontium Isotopes in the Investigation of Early Glass Production: Byzantine and Early Islamic Glass from the Near East*. Archaeometry 2003, 45, 19–32. [Google Scholar] [CrossRef]
- Martignier, A.; Filella, M.; Pollok, K.; Melkonian, M.; Bensimon, M.; Barja, F.; Langenhorst, F.; Jaquet, J.-M.; Ariztegui, D. Marine and Freshwater Micropearls: Biomineralization Producing Strontium-Rich Amorphous Calcium Carbonate Inclusions Is Widespread in the Genus Tetraselmis (Chlorophyta). Biogeosciences 2018, 15, 6591–6605. [Google Scholar] [CrossRef]
- Otter, L.M.; Agbaje, O.B.A.; Kilburn, M.R.; Lenz, C.; Henry, H.; Trimby, P.; Hoppe, P.; Jacob, D.E. Insights into Architecture, Growth Dynamics, and Biomineralization from Pulsed Sr-Labelled Katelysia Rhytiphora Shells (Mollusca, Bivalvia). Biogeosciences 2019, 16, 3439–3455. [Google Scholar] [CrossRef]
- Wedepohl, K.H. The Chemical Composition Including the Rare Earth Elements of the Three Major Glass Types of Europe and the Orient Used in Late Antiquity and the Middle Ages. Chem. Erde 2011, 71, 289–296. [Google Scholar] [CrossRef]
- Silvestri, A.; Molin, G.; Salviulo, G. The Colourless Glass of Iulia Felix. J. Archaeol. Sci. 2008, 35, 331–341. [Google Scholar] [CrossRef]
- Foster, H.E.; Jackson, C.M. The Composition of Late Romano-British Colourless Vessel Glass: Glass Production and Consumption. J. Archaeol. Sci. 2010, 37, 3068–3080. [Google Scholar] [CrossRef]
- Freestone, I.C. The Recycling and Reuse of Roman Glass: Analytical Approaches. J. Glass Stud. 2015, 57, 29–40. [Google Scholar]
- Freestone, I.C.; Ponting, M.; Hughes, M.J. The Origins of Byzantine Glass from Maroni Petrera, Cyprus. Archaeometry 2002, 44, 257–272. [Google Scholar] [CrossRef]
- Gliozzo, E. The Composition of Colourless Glass: A Review. Archaeol. Anthropol. Sci. 2017, 9, 455–483. [Google Scholar] [CrossRef]
- Bliem, R.; Pavelec, J.; Gamba, O.; McDermott, E.; Wang, Z.; Gerhold, S.; Wagner, M.; Osiecki, J.; Schulte, K.; Schmid, M.; et al. Adsorption and Incorporation of Transition Metals at the Magnetite Fe3O4 (001) Surface. Phys. Rev. B 2015, 92, 075440. [Google Scholar] [CrossRef]
- Zhong, Y.; Liang, X.; Tan, W.; Zhong, Y.; He, H.; Zhu, J.; Yuan, P.; Jiang, Z. A Comparative Study about the Effects of Isomorphous Substitution of Transition Metals (Ti, Cr, Mn, Co and Ni) on the UV/Fenton Catalytic Activity of Magnetite. J. Mol. Catal. Chem. 2013, 372, 29–34. [Google Scholar] [CrossRef]
- Giannini, R.; Freestone, I.C.; Shortland, A.J. European Cobalt Sources Identified in the Production of Chinese Famille Rose Porcelain. J. Archaeol. Sci. 2017, 80, 27–36. [Google Scholar] [CrossRef]
- Gratuze, B.; Pactat, I.; Schibille, N. Changes in the Signature of Cobalt Colorants in Late Antique and Early Islamic Glass Production. Minerals 2018, 8, 225. [Google Scholar] [CrossRef]
- Rodríguez González, E.; Celestino Pérez, S.; Medina Sánchez, M.C.; Zucchiatti, A.; Barrio Martín, J. Trade with the West. Glass Bowls of Eastern Mediterranean Origin Found in the Courtyard of the Casas Del Turuñuelo Site (Guareña, Badajoz, Spain): Archaeological Context, Analysis and Conservation. J. Archaeol. Sci. Rep. 2023, 50, 104029. [Google Scholar] [CrossRef]
- Oikonomou, A.; Triantafyllidis, P. An Archaeometric Study of Archaic Glass from Rhodes, Greece: Technological and Provenance Issues. J. Archaeol. Sci. Rep. 2018, 22, 493–505. [Google Scholar] [CrossRef]
- Franjic, A.; Freestone, I.; Križ, B.; Stipančić, P. The Spectrometric Analysis of Iron Age Glass Beads from Novo Mesto, Slovenia|Spektrometrične Analize Železnodobnih Steklenih Jagod Iz Novega Mesta, Slovenija. Stud. Univ. Hered. Znan. Rev. Za Raziskave Teor. Kult. Dediščine 2022, 10, 23–29. [Google Scholar] [CrossRef]
- Schibille, N.; De Juan Ares, J.; Casal García, M.T.; Guerrot, C. Ex Novo Development of Lead Glassmaking in Early Umayyad Spain. Proc. Natl. Acad. Sci. USA 2020, 117, 16243–16249. [Google Scholar] [CrossRef]
- Miazga, B.; Duma, P.; Cembrzyński, P.; Matyszczak, M.; Piekalski, J. Analytical Studies on Medieval Lead Ingots from Wrocław and Kraków (Poland): A Step towards Understanding Bulk Trade of Lead from Kraków and Silesia Upland Pb–Zn Deposits. Herit. Sci. 2022, 10, 184. [Google Scholar] [CrossRef]
- Wedepohl, K.H.; Baumann, A. Isotope Composition of Medieval Lead Glasses Reflecting Early Silver Production in Central Europe. Miner. Deposita 1997, 32, 292–295. [Google Scholar] [CrossRef]
- Pactat, I.; Guérit, M.; Simon, L.; Gratuze, B.; Raux, S.; Aunay, C. Evolution of Glass Recipes during the Early Middle Ages in France: Analytical Evidence of Multiple Solutions Adapted to Local Contexts. In Annales du 20e congrès de l’Association Internationale Pour l’Histoire du Verre: Fribourg/Romont, 7–11 Septembre 2015; Wolf, S., De Pury-Gysel, A., Eds.; Verlag Marie Leidorf GmbH: Rahden, Germany, 2017; pp. 334–340. [Google Scholar]
- Anguilano, L.; Rehren, T.; Müller, W.; Rothenberg, B. The Importance of Lead in the Silver Production at Riotinto (Spain). ArcheoSciences Rev. Archéom. 2010, 34, 269–276. [Google Scholar] [CrossRef]
- Kaufman, B.; Docter, R.; Fischer, C.; Chelbi, F.; Maraoui Telmini, B. Ferrous Metallurgy from the Bir Massouda Metallurgical Precinct at Phoenician and Punic Carthage and the Beginning of the North African Iron Age. J. Archaeol. Sci. 2016, 71, 33–50. [Google Scholar] [CrossRef][Green Version]
- Lahlil, S.; Biron, I.; Cotte, M.; Susini, J.; Menguy, N. Synthesis of Calcium Antimonate Nano-Crystals by the 18th Dynasty Egyptian Glassmakers. Appl. Phys. Mater. Sci. Process. 2010, 98, 1–8. [Google Scholar] [CrossRef]
- Lahlil, S.; Biron, I.; Galoisy, L.; Morin, G. Rediscovering Ancient Glass Technologies through the Examination of Opacifier Crystals. Appl. Phys. Mater. Sci. Process. 2008, 92, 109–116. [Google Scholar] [CrossRef]
- Vandini, M.; Fiorentino, S. From Crystals to Color: A Compendium of Multi-Analytical Data on Mineralogical Phases in Opaque Colored Mosaic Tesserae. Minerals 2020, 10, 609. [Google Scholar] [CrossRef]
- Shortland, J. The Use and Origin of Antimonate Colorants in Early Egyptian Glass. Archaeometry 2002, 44, 517–530. [Google Scholar] [CrossRef]
- Lahlil, S.; Biron, I.; Cotte, M.; Susini, J. New Insight on the in Situ Crystallization of Calcium Antimonate Opacified Glass during the Roman Period. Appl. Phys. Mater. Sci. Process. 2010, 100, 683–692. [Google Scholar] [CrossRef]
- Truffa Giachet, M.; Gratuze, B.; Ozainne, S.; Mayor, A.; Huysecom, E. A Phoenician Glass Eye Bead from 7th–5th c. Cal BCE Nin-Bèrè 3, Mali: Compositional Characterisation by LA–ICP–MS. J. Archaeol. Sci. Rep. 2019, 24, 748–758. [Google Scholar] [CrossRef]
- Shortland, A.; Rogers, N.; Eremin, K. Trace Element Discriminants between Egyptian and Mesopotamian Late Bronze Age Glasses. J. Archaeol. Sci. 2007, 34, 781–789. [Google Scholar] [CrossRef]
- Freestone, I.; Gorin-Rosen, Y.; Hughes, M.J. Primary glass from israel and the Production of Glass in Late Antiquity and the Early Islamic Period, La Route du verre. Ateliers primaires et secondaires du second millénaire av. J.-C. au Moyen Âge. Colloque organisé en 1989 par l’Association française pour l’Archéologie du Verre (AFAV). Lyon Maison Orient Méditerranée Jean Pouilloux 2000, 33, 65–83. [Google Scholar]
- Schibille, N.; Gratuze, B.; Ollivier, E.; Blondeau, É. Chronology of Early Islamic Glass Compositions from Egypt. J. Archaeol. Sci. 2019, 104, 10–18. [Google Scholar] [CrossRef]
- Wedepohl, K.H. The Composition of the Continental Crust. Cosmochim. Acta 1995, 59, 1217–1232. [Google Scholar] [CrossRef]
- Blomme, A.; Elsen, J.; Brems, D.; Shortland, A.; Dotsika, E.; Degryse, P. Tracing the Primary Production Location of Core-Formed Glass Vessels, Mediterranean Group I. J. Archaeol. Sci. Rep. 2016, 5, 1–9. [Google Scholar] [CrossRef]
- D’Oriano, C.; Da Pelo, S.; Podda, F.; Cioni, R. Laser-Ablation Inductively Coupled Plasma Mass Spectrometry (LA-ICP-MS): Setting Operating Conditions and Instrumental Performance. Period. Mineral. 2008, 77, 65–74. [Google Scholar] [CrossRef]
- Pearce, N.J.; Perkins, W.T.; Westgate, J.A.; Gorton, M.P.; Jackson, S.E.; Neal, C.R.; Chenery, S.P. A Compilation of New and Published Major and Trace Element Data for NIST SRM 610 and NIST SRM 612 Glass Reference Materials. Geostand. Newsl. 1997, 21, 115–144. [Google Scholar] [CrossRef]


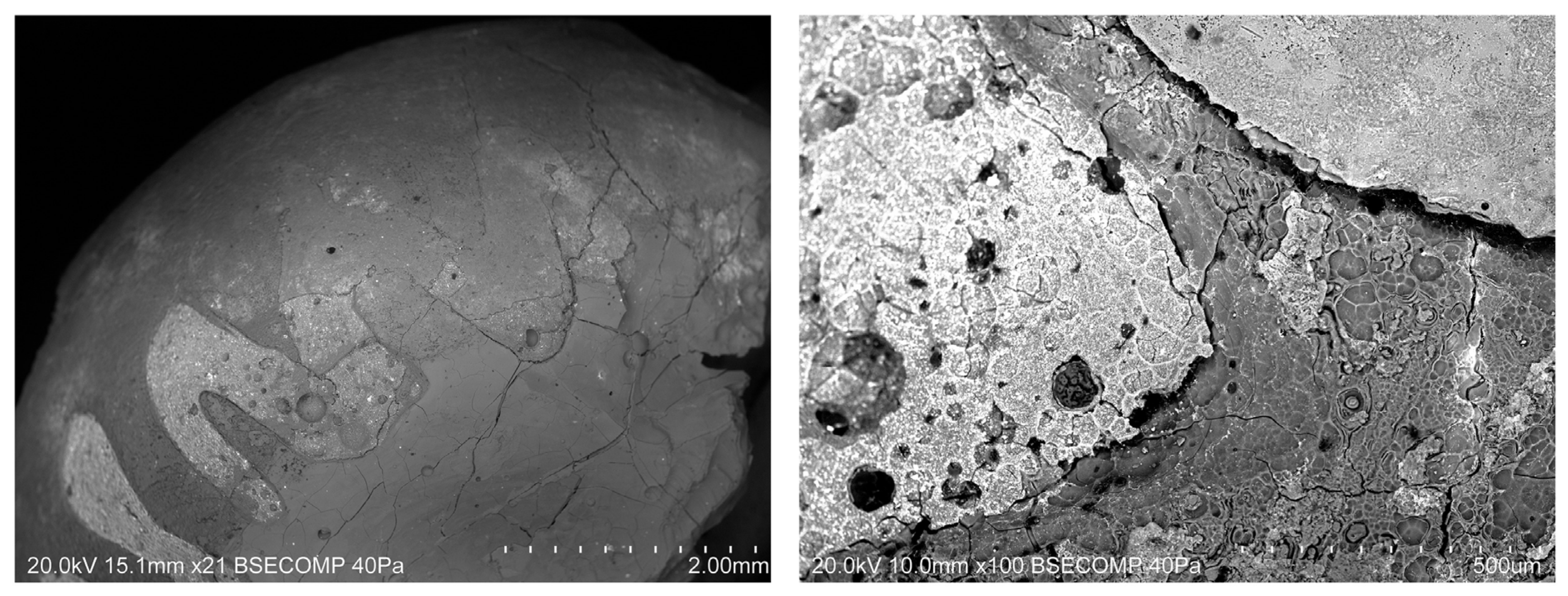
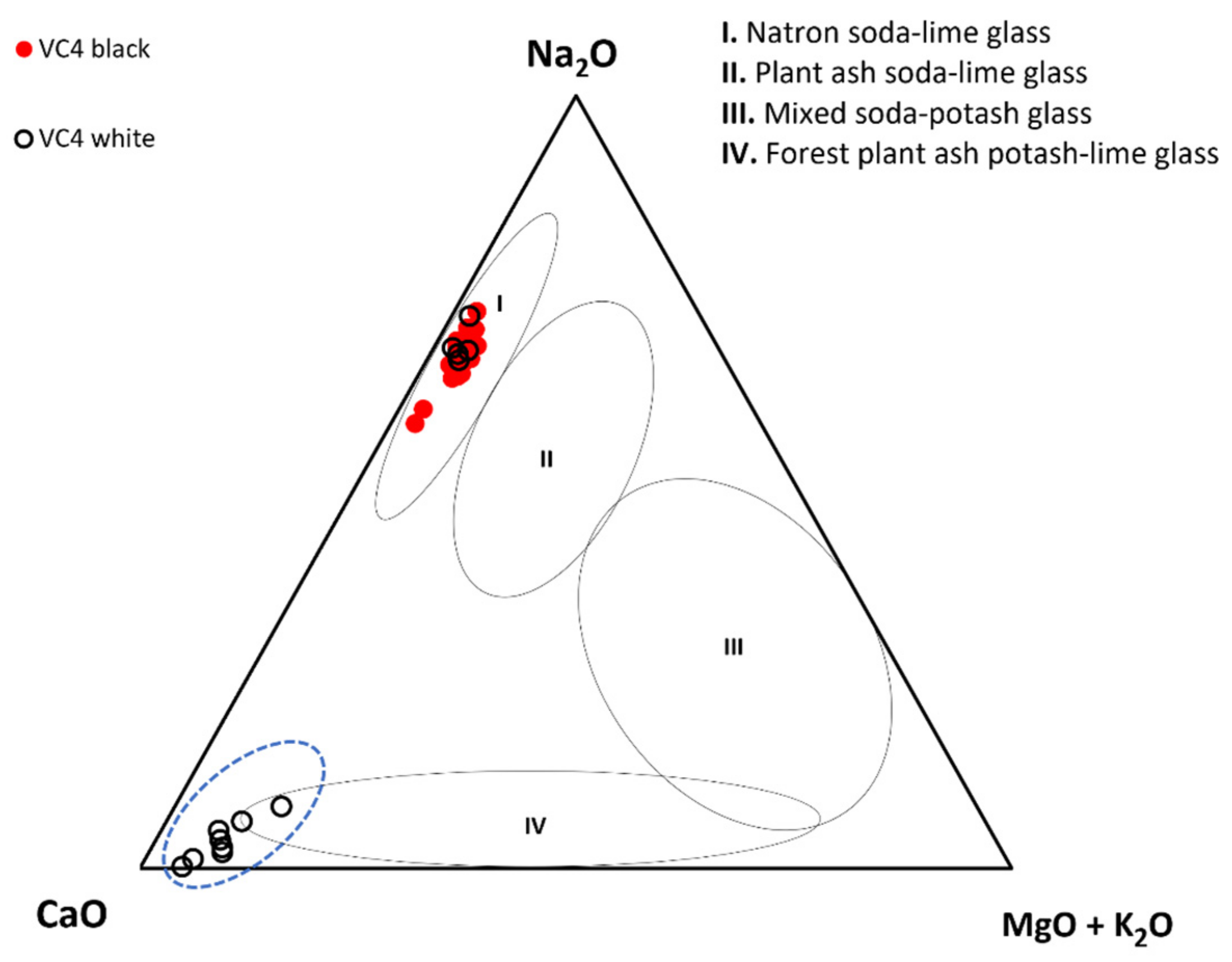
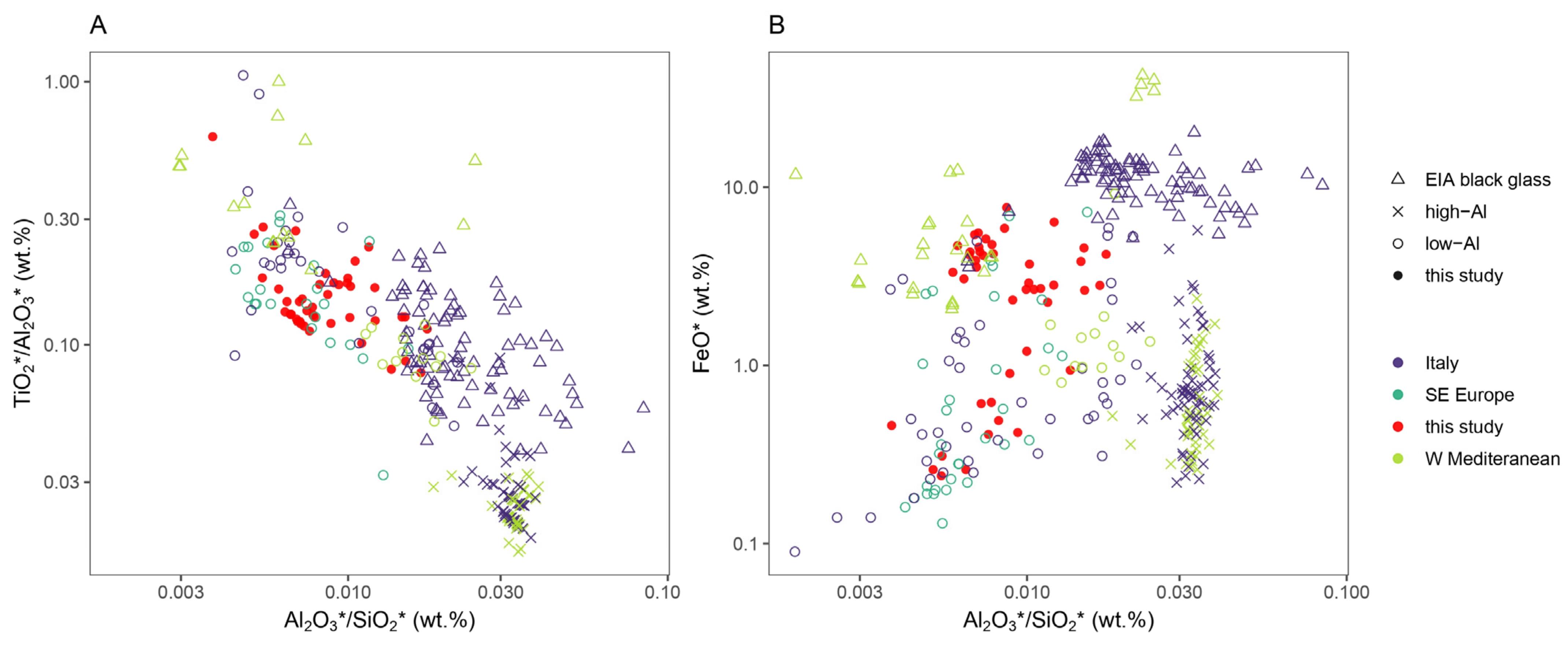
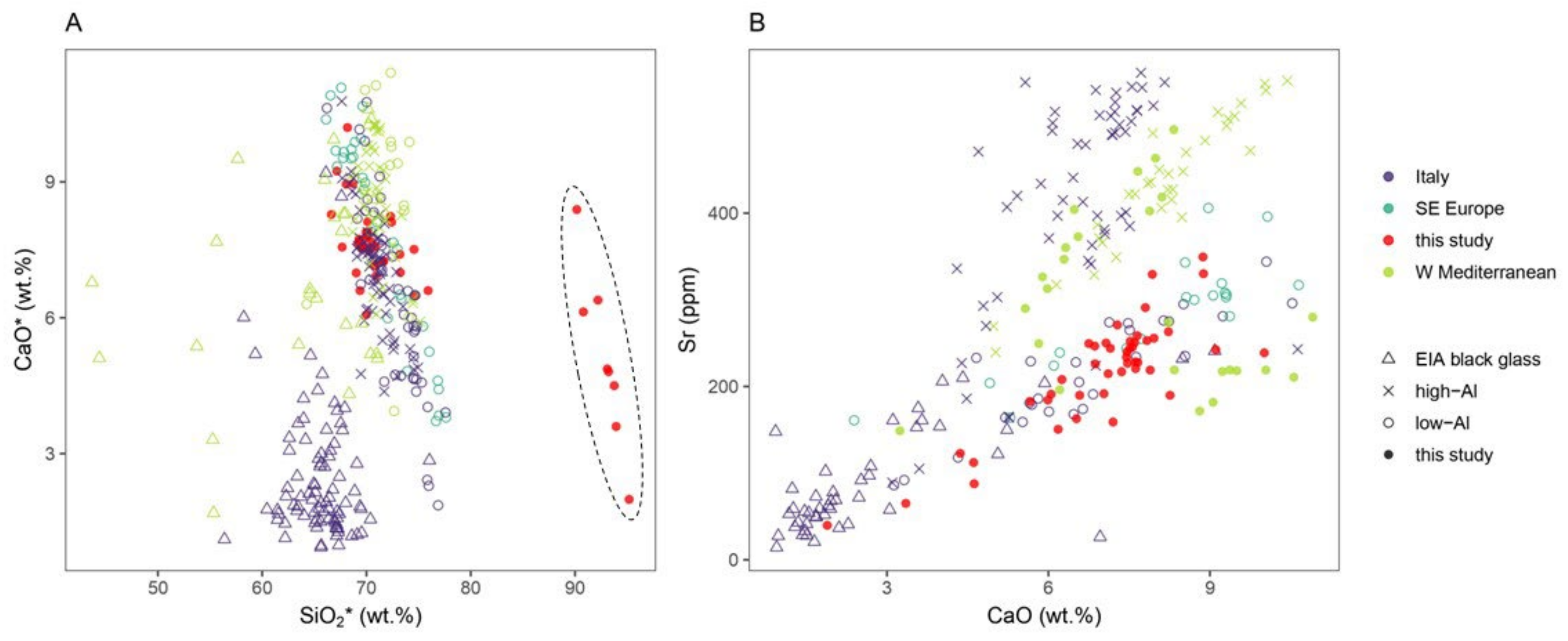

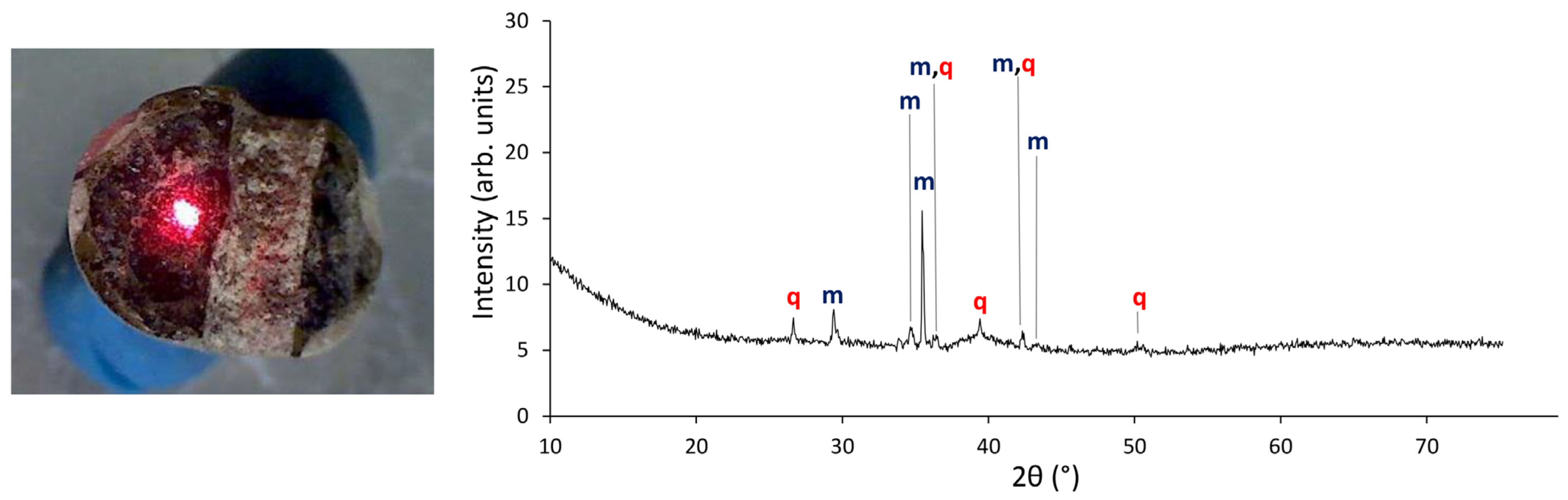

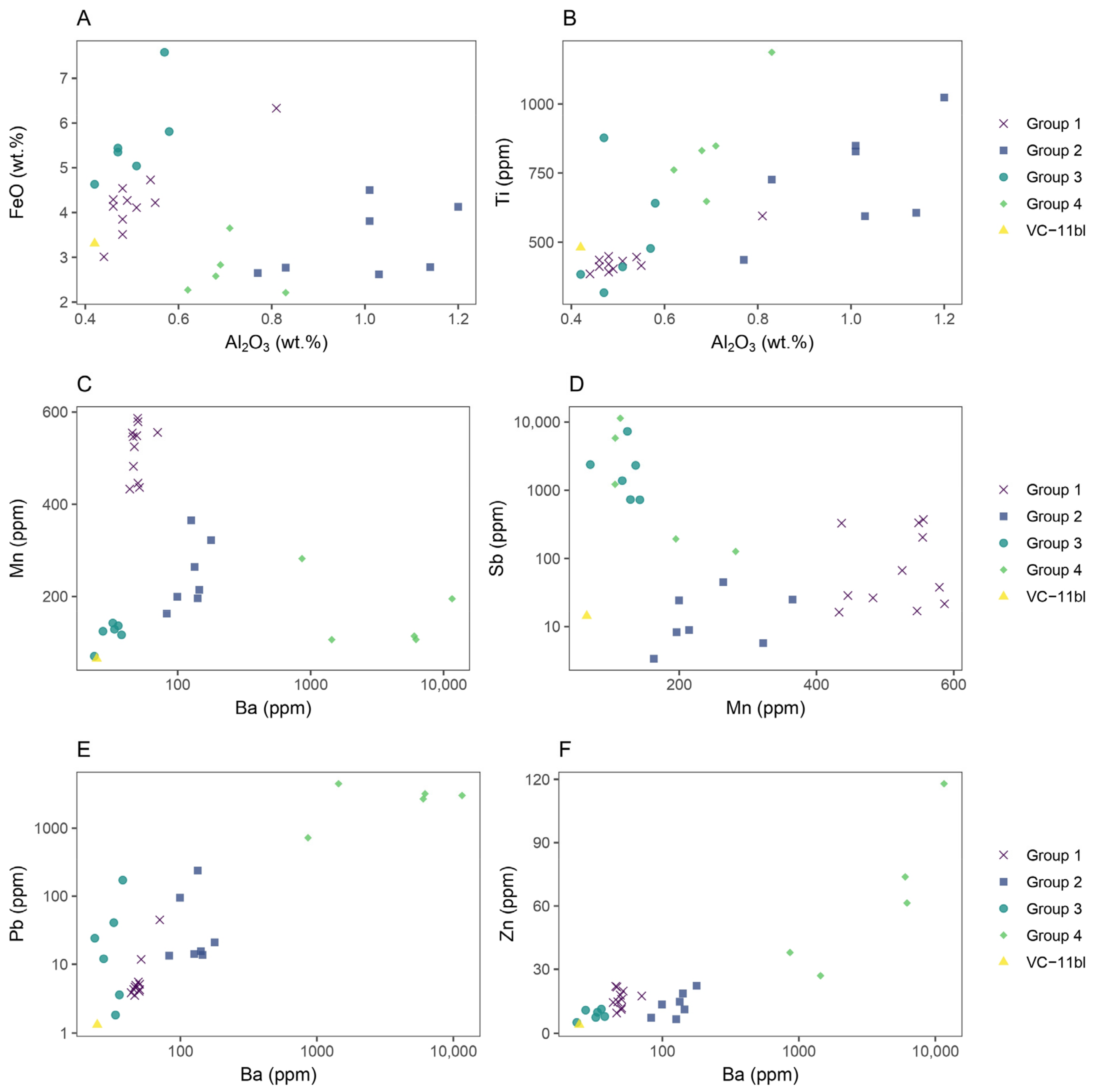
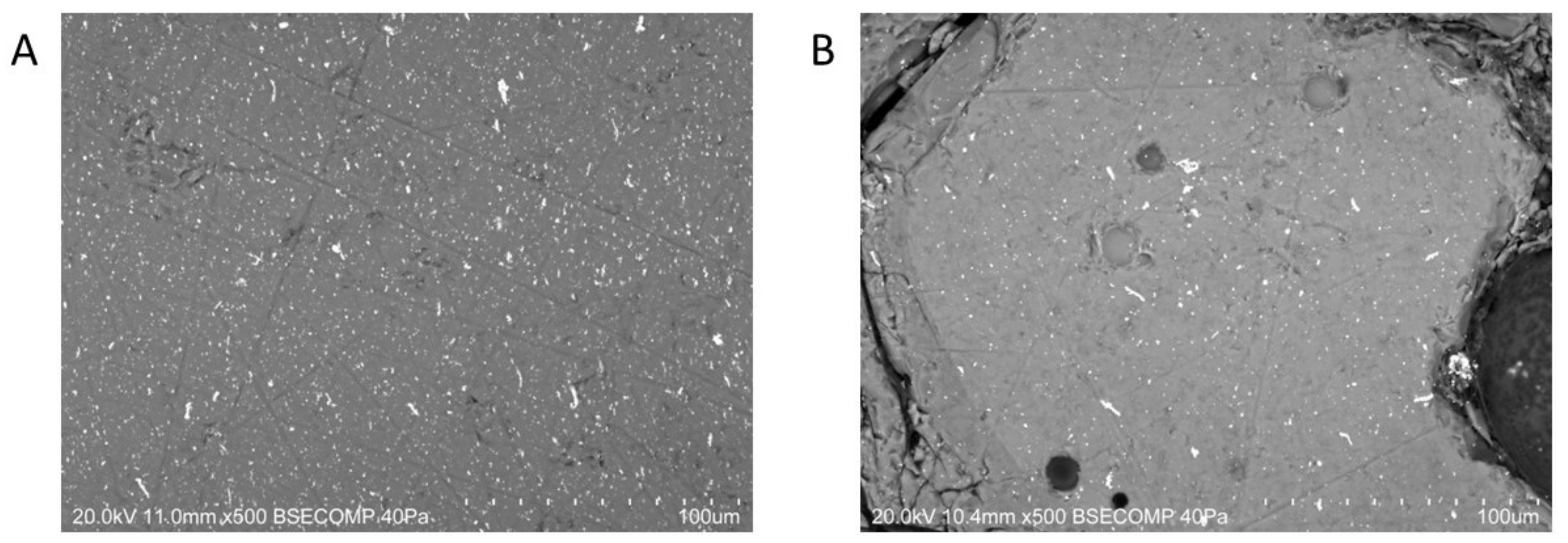



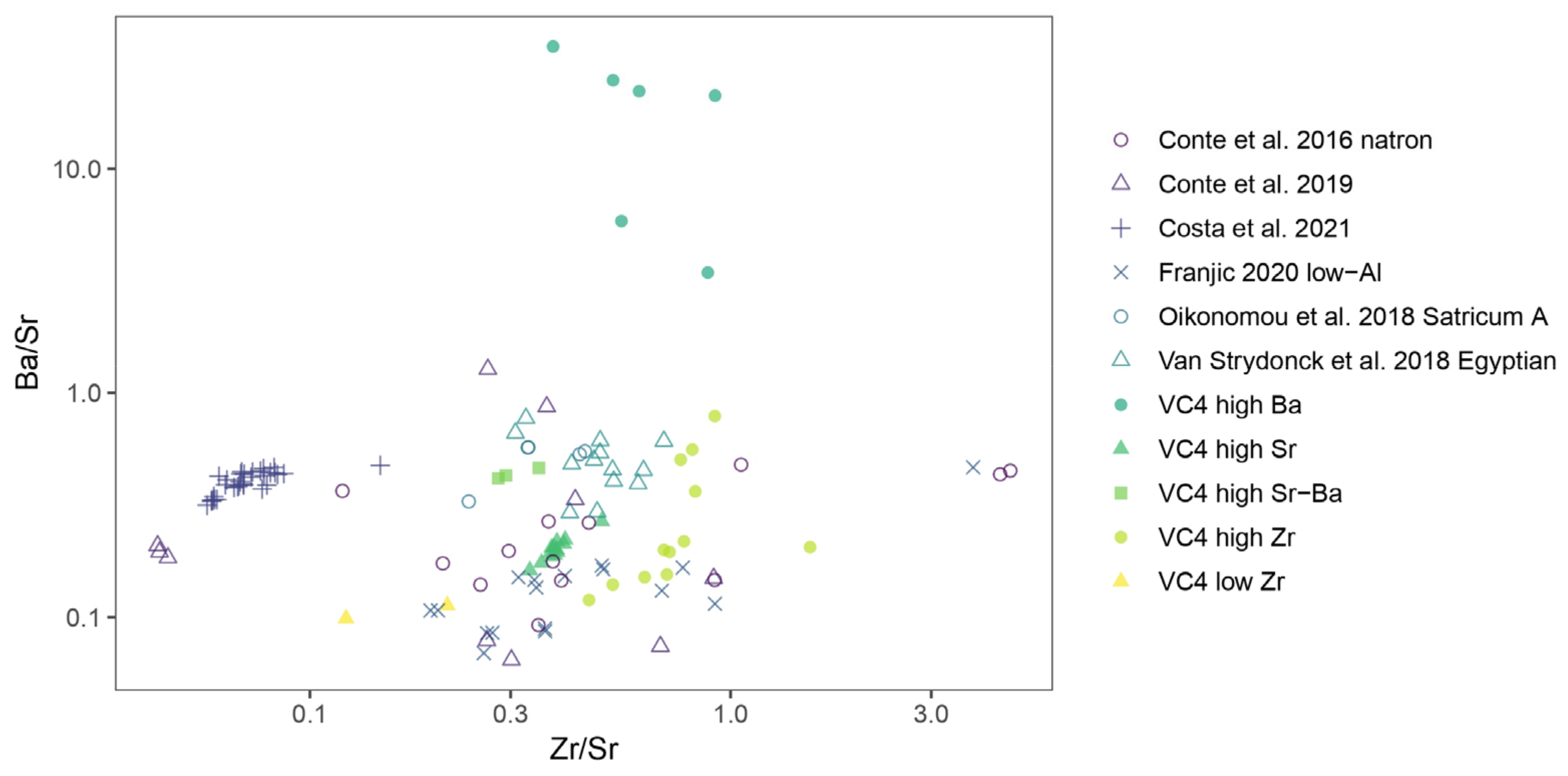
| Laser energy output | 100% |
| Analysis mode | spot |
| Spot size | 50 μm |
| Repetition rate | 20 Hz |
| Burst count | 600 |
| Carrier gas (He) flow | 0.8 L/min |
| Run | 55 s |
| Gas blank | 15 s |
| Ablation | 30 s |
| Washout | 10 s |
| Acquisition mode | time-resolved analysis (TRA) |
| Scan type | MS/MS |
| Plasma parameters | |
| RF power | 1200 |
| RF matching | 1.25 V |
| Sample depth | 4.0 mm |
| Carrier gas (He) | 0.6 L/min |
| Plasma gas (Ar) | 15 L/min |
| Analysis mode | no gas |
| Dwell times | |
| 2 ms | 23Na; 28Si 56Fe; 57Fe |
| 5 ms | 24Mg; 27Al; 39K; 43Ca; 44Ca; 55Mn |
| 10 ms | 47Ti; 51V; 52Cr; 59Co; 60Ni; 63Cu; 66Zn; 85Rb; 88Sr; 137Ba; 208Pb |
| 20 ms | 75As; 89Y; 90Zr; 93Nb; 118Sn; 121Sb; 139La; 140Ce; 141Pr; 146Nd; 147Sm; 153Eu; 157Gd; 159Tb; 163Dy; 165Ho; 166Er; 169Tm; 172Yb; 175Lu; 178Hf; 181Ta; 209Bi; 232Th; 238U |
| Sample | Colour | Na2O | MgO | Al2O3 | SiO2 * | P2O5 * | SO3 * | Cl * | K2O | CaO | TiO2 | MnO | FeO | CoO | CuO | SnO2 | Sb2O3 | PbO |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| VC-11bl | “black” | 16.6 | 0.4 | 0.4 | 71.2 | <0.1 | 0.6 | <0.1 | 0.2 | 7.2 | 0.1 | <0.1 | 3.3 | <0.1 | <0.1 | <0.1 | <0.1 | <0.1 |
| VC-11w | white | 16.3 | 0.3 | 0.4 | 69.6 | <0.1 | <0.1 | 0.8 | 0.1 | 6.1 | <0.1 | <0.1 | 0.2 | <0.1 | <0.1 | <0.1 | 6.0 | <0.1 |
| VC-112bl | “black” | 16.5 | 0.4 | 0.5 | 68.3 | <0.1 | 0.3 | 1.1 | 0.6 | 7.6 | <0.1 | <0.1 | 4.5 | <0.1 | <0.1 | <0.1 | <0.1 | <0.1 |
| VC-16bl | “black” | 16.2 | 0.4 | 0.5 | 69.2 | 0.1 | 0.4 | 0.9 | 0.5 | 7.4 | <0.1 | <0.1 | 4.3 | <0.1 | <0.1 | <0.1 | <0.1 | <0.1 |
| VC-16w | white | 0.2 | 0.3 | 0.9 | 90.4 | 0.4 | 0.2 | 0.3 | <0.1 | 1.9 | 0.2 | <0.1 | 1.1 | <0.1 | <0.1 | <0.1 | 4.1 | <0.1 |
| VC-24bl | “black” | 15.6 | 0.4 | 0.5 | 68.7 | 0.4 | 0.2 | 0.9 | 0.3 | 7.6 | <0.1 | <0.1 | 5.0 | <0.1 | <0.1 | <0.1 | 0.3 | <0.1 |
| VC-26bl | “black” | 17.1 | 0.5 | 0.8 | 68.8 | <0.1 | 1.5 | 0.3 | 0.4 | 7.7 | 0.1 | <0.1 | 2.8 | <0.1 | <0.1 | <0.1 | <0.1 | <0.1 |
| VC-26w | white | <0.1 | 0.4 | 0.3 | 88.8 | <0.1 | 0.3 | 1.0 | <0.1 | 8.3 | 0.2 | <0.1 | 0.5 | <0.1 | <0.1 | <0.1 | <0.1 | <0.1 |
| VC-28bl | “black” | 17.3 | 0.5 | 1.0 | 68.1 | <0.1 | <0.1 | 0.7 | 0.6 | 8.9 | <0.1 | <0.1 | 2.6 | <0.1 | <0.1 | <0.1 | <0.1 | <0.1 |
| VC-28w | white | <0.1 | 0.4 | 0.5 | 89.2 | <0.1 | 0.2 | 0.3 | <0.1 | 6.2 | 0.1 | <0.1 | 0.3 | <0.1 | <0.1 | <0.1 | 2.6 | <0.1 |
| VC-30bl | “black” | 16.6 | 0.4 | 0.6 | 68.5 | 0.3 | 0.7 | <0.1 | 0.2 | 6.5 | 0.1 | <0.1 | 5.8 | <0.1 | <0.1 | <0.1 | <0.1 | <0.1 |
| VC-36bl | “black” | 17.8 | 0.5 | 1.0 | 66.8 | 0.2 | 0.3 | 0.7 | 0.5 | 7.5 | 0.1 | <0.1 | 4.5 | <0.1 | <0.1 | <0.1 | <0.1 | <0.1 |
| VC-39bl | “black” | 17.1 | 0.5 | 1.0 | 68.8 | <0.1 | 0.1 | 0.2 | 0.6 | 7.6 | 0.1 | <0.1 | 3.8 | <0.1 | <0.1 | <0.1 | <0.1 | <0.1 |
| VC-45bl | “black” | 16.9 | 0.4 | 1.2 | 67.9 | 0.4 | 0.9 | 0.2 | 0.8 | 6.9 | 0.1 | <0.1 | 4.1 | <0.1 | <0.1 | <0.1 | <0.1 | <0.1 |
| VC-46bl | “black” | 16.3 | 0.5 | 0.5 | 68.9 | <0.1 | 0.3 | 0.4 | 0.6 | 7.5 | <0.1 | <0.1 | 4.7 | <0.1 | <0.1 | <0.1 | <0.1 | <0.1 |
| VC-46w | white | 16.8 | 0.5 | 0.4 | 68.4 | <0.1 | <0.1 | 1.2 | 0.5 | 7.8 | <0.1 | <0.1 | 0.2 | <0.1 | <0.1 | <0.1 | 4.1 | <0.1 |
| VC-47bl | “black” | 17.0 | 0.4 | 0.5 | 69.2 | <0.1 | 0.2 | 0.3 | 0.5 | 7.5 | <0.1 | <0.1 | 4.1 | <0.1 | <0.1 | <0.1 | <0.1 | <0.1 |
| VC-48bl | “black” | 17.1 | 0.5 | 0.5 | 69.1 | <0.1 | <0.1 | 0.5 | 0.8 | 7.5 | <0.1 | <0.1 | 3.9 | <0.1 | <0.1 | <0.1 | <0.1 | <0.1 |
| VC-48w | white | 14.5 | 0.5 | 0.7 | 71.9 | <0.1 | <0.1 | 0.7 | 0.4 | 6.3 | 0.1 | <0.1 | 0.4 | <0.1 | 0.2 | <0.1 | 4.4 | <0.1 |
| VC-51bl | “black” | 17.7 | 0.4 | 0.6 | 69.1 | <0.1 | 0.6 | 0.5 | 0.5 | 6.8 | 0.1 | <0.1 | 2.3 | <0.1 | <0.1 | <0.1 | 0.6 | 0.3 |
| VC-51w | white | 14.6 | 0.4 | 0.7 | 70.7 | <0.1 | 0.6 | 0.6 | 0.3 | 7.2 | 0.2 | <0.1 | 2.6 | <0.1 | <0.1 | <0.1 | 1.4 | 0.2 |
| VC-64bl | “black” | 16.5 | 0.6 | 0.8 | 66.2 | 0.2 | 0.1 | 0.1 | 0.6 | 8.2 | 0.1 | <0.1 | 6.3 | <0.1 | <0.1 | <0.1 | <0.1 | <0.1 |
| VC-67bl | “black” | 16.9 | 0.4 | 0.4 | 69.8 | 0.2 | 0.8 | 0.5 | 0.4 | 7.5 | <0.1 | <0.1 | 3.0 | <0.1 | <0.1 | <0.1 | <0.1 | <0.1 |
| VC-95bl | “black” | 17.1 | 0.4 | 0.5 | 69.1 | <0.1 | <0.1 | 1.1 | 0.2 | 6.0 | 0.1 | <0.1 | 5.4 | <0.1 | <0.1 | <0.1 | 0.2 | <0.1 |
| VC-97bl | “black” | 14.3 | 0.5 | 0.6 | 66.2 | <0.1 | <0.1 | 0.4 | 0.2 | 9.1 | <0.1 | <0.1 | 7.6 | <0.1 | <0.1 | <0.1 | 1.0 | <0.1 |
| VC-77bl | “black” | 16.0 | 0.5 | 0.6 | 70.0 | <0.1 | <0.1 | <0.1 | 0.6 | 7.9 | <0.1 | <0.1 | 4.2 | <0.1 | <0.1 | <0.1 | <0.1 | <0.1 |
| VC-77w | white | 0.1 | 0.4 | 0.7 | 90.8 | <0.1 | <0.1 | 0.2 | <0.1 | 4.4 | <0.1 | <0.1 | 0.4 | <0.1 | <0.1 | <0.1 | 2.9 | <0.1 |
| VC-81b | blue | 16.9 | 0.4 | 0.6 | 71.1 | <0.1 | 0.7 | 0.2 | 0.6 | 8.0 | 0.1 | <0.1 | 0.5 | <0.1 | 0.7 | <0.1 | <0.1 | <0.1 |
| VC-94bl | “black” | 14.6 | 0.5 | 0.5 | 66.9 | <0.1 | <0.1 | 1.4 | 0.2 | 10.0 | <0.1 | <0.1 | 5.4 | <0.1 | <0.1 | <0.1 | 0.4 | <0.1 |
| VC-96bl | “black” | 15.1 | 0.6 | 0.8 | 71.8 | <0.1 | 0.3 | 0.9 | 0.4 | 6.9 | 0.2 | <0.1 | 2.2 | <0.1 | <0.1 | <0.1 | 0.2 | 0.5 |
| VC-109bl | “black” | 17.0 | 0.4 | 0.7 | 68.0 | 0.2 | 0.7 | 0.4 | 0.5 | 7.3 | 0.1 | <0.1 | 2.6 | <0.1 | <0.1 | <0.1 | 1.2 | 0.2 |
| VC-109w | white | 0.2 | 0.4 | 0.7 | 88.1 | 0.2 | 0.7 | 0.4 | <0.1 | 4.6 | <0.1 | <0.1 | 0.6 | <0.1 | <0.1 | <0.1 | 4.0 | <0.1 |
| VC-110bl | “black” | 16.4 | 0.4 | 0.8 | 70.2 | <0.1 | 1.9 | <0.1 | 0.4 | 7.1 | <0.1 | <0.1 | 2.7 | <0.1 | <0.1 | <0.1 | <0.1 | <0.1 |
| VC-110w | white | 0.2 | 0.3 | 0.8 | 87.3 | <0.1 | 2.1 | <0.1 | <0.1 | 3.4 | <0.1 | <0.1 | 0.8 | <0.1 | 0.2 | <0.1 | 4.6 | <0.1 |
| VC-115bl | “black” | 16.8 | 0.4 | 0.7 | 68.5 | <0.1 | 0.5 | 0.5 | 0.4 | 7.9 | <0.1 | <0.1 | 2.8 | <0.1 | <0.1 | <0.1 | <0.1 | 0.3 |
| VC-118 | “black” | 16.5 | 0.4 | 0.7 | 69.8 | <0.1 | 0.4 | 0.7 | 0.4 | 7.1 | 0.1 | <0.1 | 3.7 | <0.1 | <0.1 | <0.1 | <0.1 | <0.1 |
| VC-123bl | “black” | 16.1 | 0.5 | 0.5 | 69.5 | <0.1 | 0.2 | 0.4 | 0.8 | 7.8 | <0.1 | <0.1 | 4.1 | <0.1 | <0.1 | <0.1 | <0.1 | <0.1 |
| VC-123w | white | 0.3 | 0.4 | 1.1 | 83.8 | <0.1 | 0.1 | 0.3 | <0.1 | 5.7 | <0.1 | <0.1 | 0.9 | <0.1 | 0.1 | <0.1 | 7.1 | <0.1 |
| VC-129bl | “black” | 15.9 | 0.4 | 0.4 | 69.8 | <0.1 | 0.1 | 1.2 | 0.2 | 7.0 | <0.1 | <0.1 | 4.6 | <0.1 | <0.1 | <0.1 | <0.1 | <0.1 |
| VC-129w | white | 14.5 | 0.3 | 0.4 | 65.4 | 0.1 | 0.3 | 1.3 | 0.1 | 6.6 | <0.1 | <0.1 | 0.2 | <0.1 | <0.1 | <0.1 | 10.6 | <0.1 |
| VC-132bl | “black” | 16.8 | 0.4 | 0.5 | 68.6 | <0.1 | <0.1 | 0.9 | 0.7 | 7.7 | <0.1 | <0.1 | 4.3 | <0.1 | <0.1 | <0.1 | <0.1 | <0.1 |
| VC-141bl | “black” | 16.4 | 0.4 | 0.5 | 69.6 | <0.1 | 0.2 | 1.1 | 0.6 | 7.6 | <0.1 | <0.1 | 3.5 | <0.1 | <0.1 | <0.1 | <0.1 | <0.1 |
| VC-141w | white | 0.1 | 0.4 | 0.6 | 89.6 | <0.1 | <0.1 | 0.1 | <0.1 | 4.6 | 0.1 | <0.1 | 0.6 | <0.1 | 0.2 | <0.1 | 3.6 | <0.1 |
| VC-142bl | “black” | 17.7 | 0.5 | 1.1 | 67.4 | <0.1 | 0.8 | <0.1 | 0.6 | 8.9 | <0.1 | <0.1 | 2.8 | <0.1 | <0.1 | <0.1 | <0.1 | <0.1 |
| Decoration Type | Na2O | MgO | Al2O3 | SiO2 | P2O5 * | SO3 * | Cl * | K2O | CaO | TiO2 | MnO | FeO | CoO | CuO | SnO2 | Sb2O3 | PbO | ||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| black Group 1 (N = 12) | wavy line | avg | 16.53 | 0.46 | 0.52 | 68.94 | 0.04 | 0.23 | 0.55 | 0.61 | 7.64 | 0.07 | 0.06 | 4.27 | >0.01 | 0.01 | >0.01 | 0.01 | >0.01 |
| SD | 0.36 | 0.05 | 0.10 | 1.04 | 0.08 | 0.22 | 0.37 | 0.12 | 0.25 | 0.01 | 0.01 | 0.83 | >0.01 | 0.01 | >0.01 | 0.02 | >0.01 | ||
| black Group 2 (N = 7) | “pseudo-eye” | avg | 17.18 | 0.47 | 1.00 | 68.27 | 0.10 | 0.81 | 0.29 | 0.55 | 7.78 | 0.11 | 0.03 | 3.32 | >0.01 | 0.02 | >0.01 | >0.01 | >0.01 |
| SD | 0.48 | 0.05 | 0.15 | 1.10 | 0.17 | 0.70 | 0.28 | 0.12 | 0.80 | 0.03 | 0.01 | 0.80 | >0.01 | 0.01 | >0.01 | >0.01 | 0.01 | ||
| black Group 3 (N = 6) | “eye” | avg | 15.67 | 0.42 | 0.50 | 68.20 | 0.12 | 0.17 | 0.86 | 0.23 | 7.71 | 0.09 | 0.02 | 5.64 | >0.01 | >0.01 | >0.01 | 0.33 | >0.01 |
| SD | 1.08 | 0.07 | 0.06 | 1.37 | 0.17 | 0.29 | 0.51 | 0.05 | 1.56 | 0.03 | 0.01 | 1.03 | >0.01 | >0.01 | >0.01 | 0.33 | 0.01 | ||
| black Group 4 (N = 5) | various | avg | 16.63 | 0.44 | 0.71 | 69.43 | 0.05 | 0.48 | 0.60 | 0.42 | 7.18 | 0.13 | 0.02 | 2.71 | >0.01 | 0.01 | >0.01 | 0.39 | 0.27 |
| SD | 0.94 | 0.06 | 0.08 | 1.46 | 0.11 | 0.16 | 0.19 | 0.05 | 0.47 | 0.04 | 0.01 | 0.58 | >0.01 | 0.01 | >0.01 | 0.51 | 0.14 | ||
| outlier | other | VC-11bl | 16.63 | 0.36 | 0.42 | 71.21 | >0.01 | 0.55 | >0.01 | 0.16 | 7.20 | 0.10 | 0.01 | 3.31 | >0.01 | >0.01 | >0.01 | >0.01 | >0.01 |
| SD | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lončarić, V.; Arruda, A.M.; Barrulas, P.; Costa, M. An Archaeometric Analysis of Black-Appearing Iron Age Glass Beads from Vinha das Caliças 4 (Portugal). Heritage 2024, 7, 1265-1297. https://doi.org/10.3390/heritage7030061
Lončarić V, Arruda AM, Barrulas P, Costa M. An Archaeometric Analysis of Black-Appearing Iron Age Glass Beads from Vinha das Caliças 4 (Portugal). Heritage. 2024; 7(3):1265-1297. https://doi.org/10.3390/heritage7030061
Chicago/Turabian StyleLončarić, Valentina, Ana Margarida Arruda, Pedro Barrulas, and Mafalda Costa. 2024. "An Archaeometric Analysis of Black-Appearing Iron Age Glass Beads from Vinha das Caliças 4 (Portugal)" Heritage 7, no. 3: 1265-1297. https://doi.org/10.3390/heritage7030061
APA StyleLončarić, V., Arruda, A. M., Barrulas, P., & Costa, M. (2024). An Archaeometric Analysis of Black-Appearing Iron Age Glass Beads from Vinha das Caliças 4 (Portugal). Heritage, 7(3), 1265-1297. https://doi.org/10.3390/heritage7030061








