Multi-Analytical Characterization of Corvins’ Castle—Deserted Tower. Construction Materials and Conservation Tests
Abstract
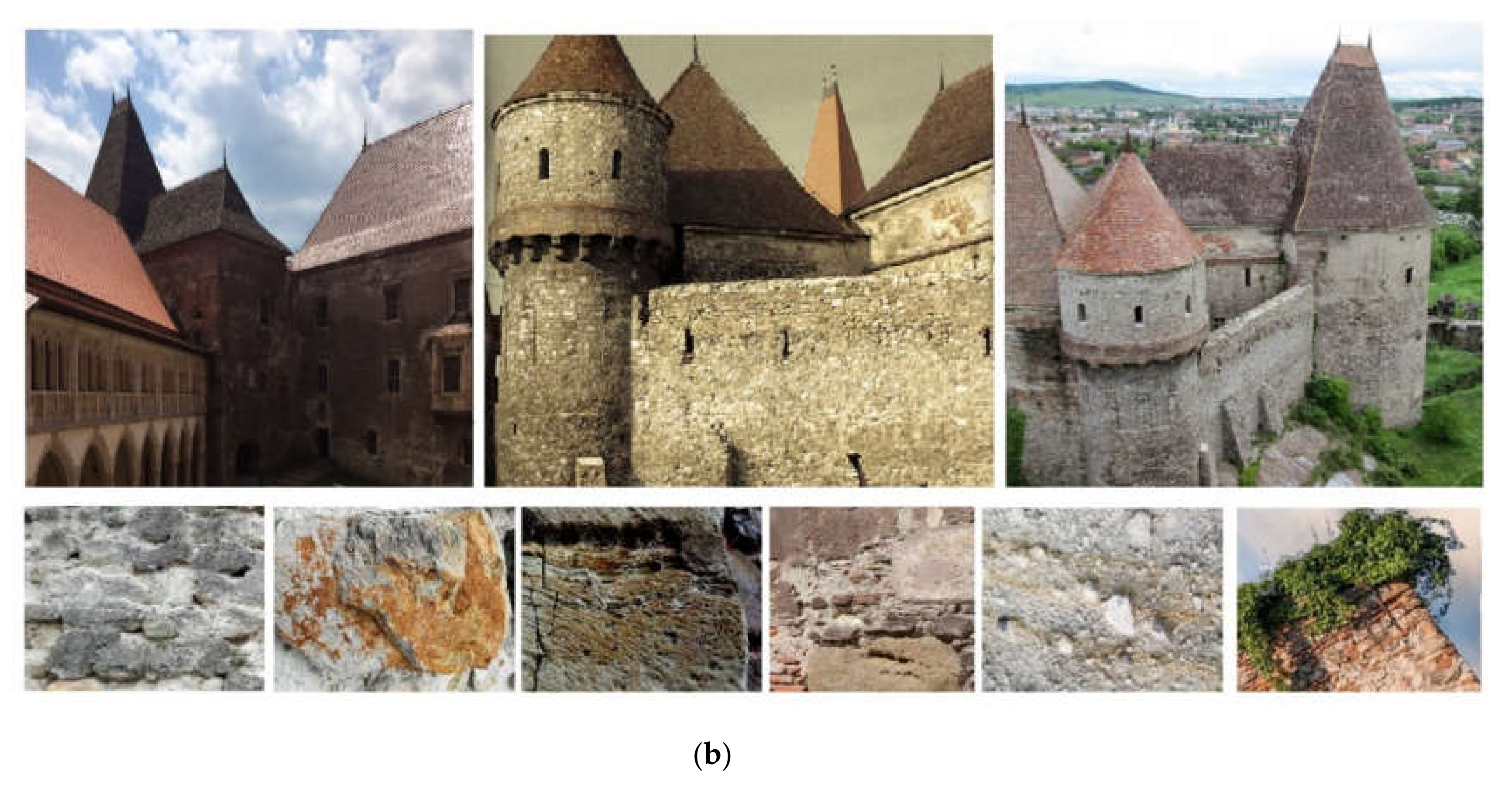
:1. Introduction and Historical Settings
2. Materials and Methods
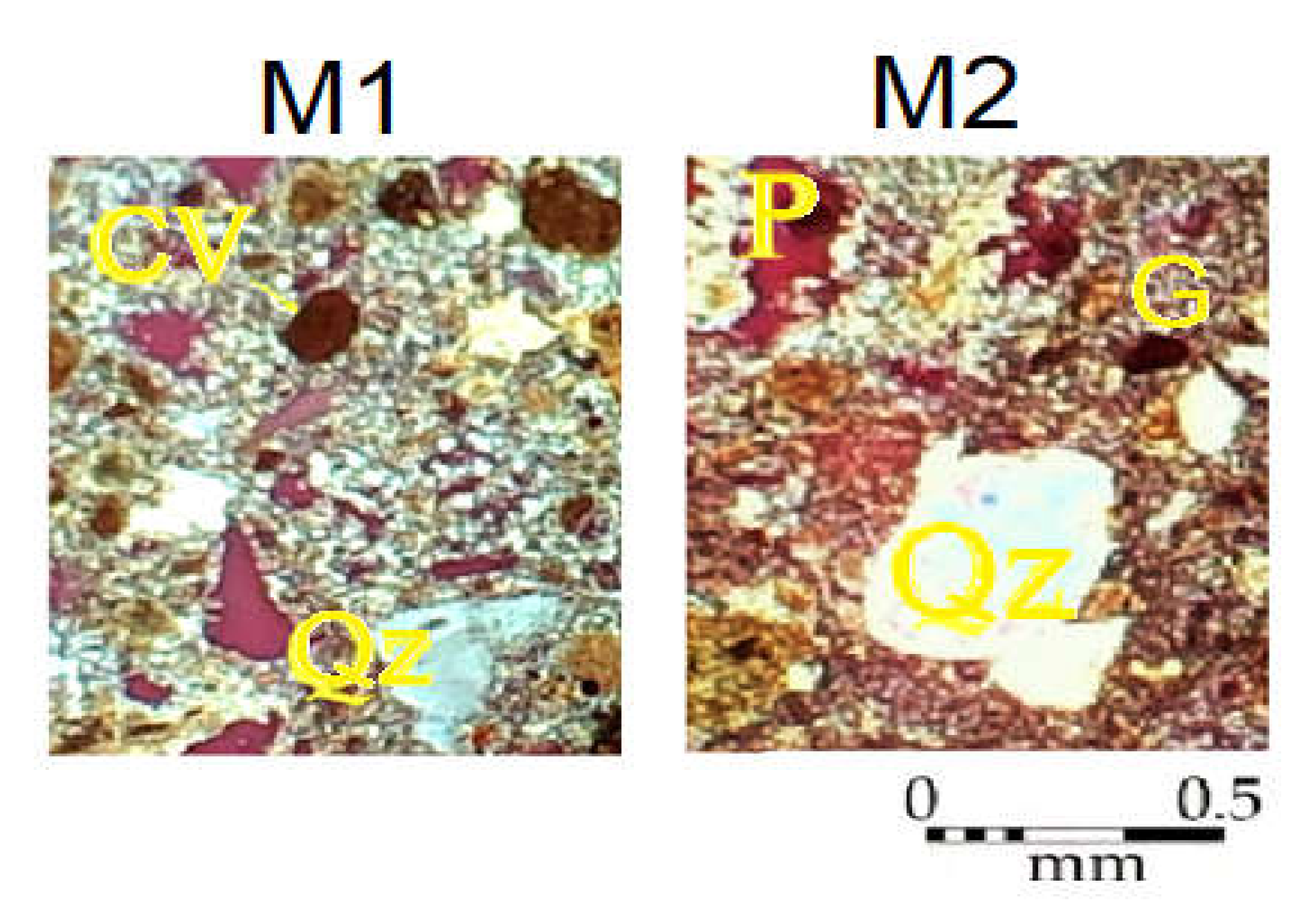
2.1. Construction Materials
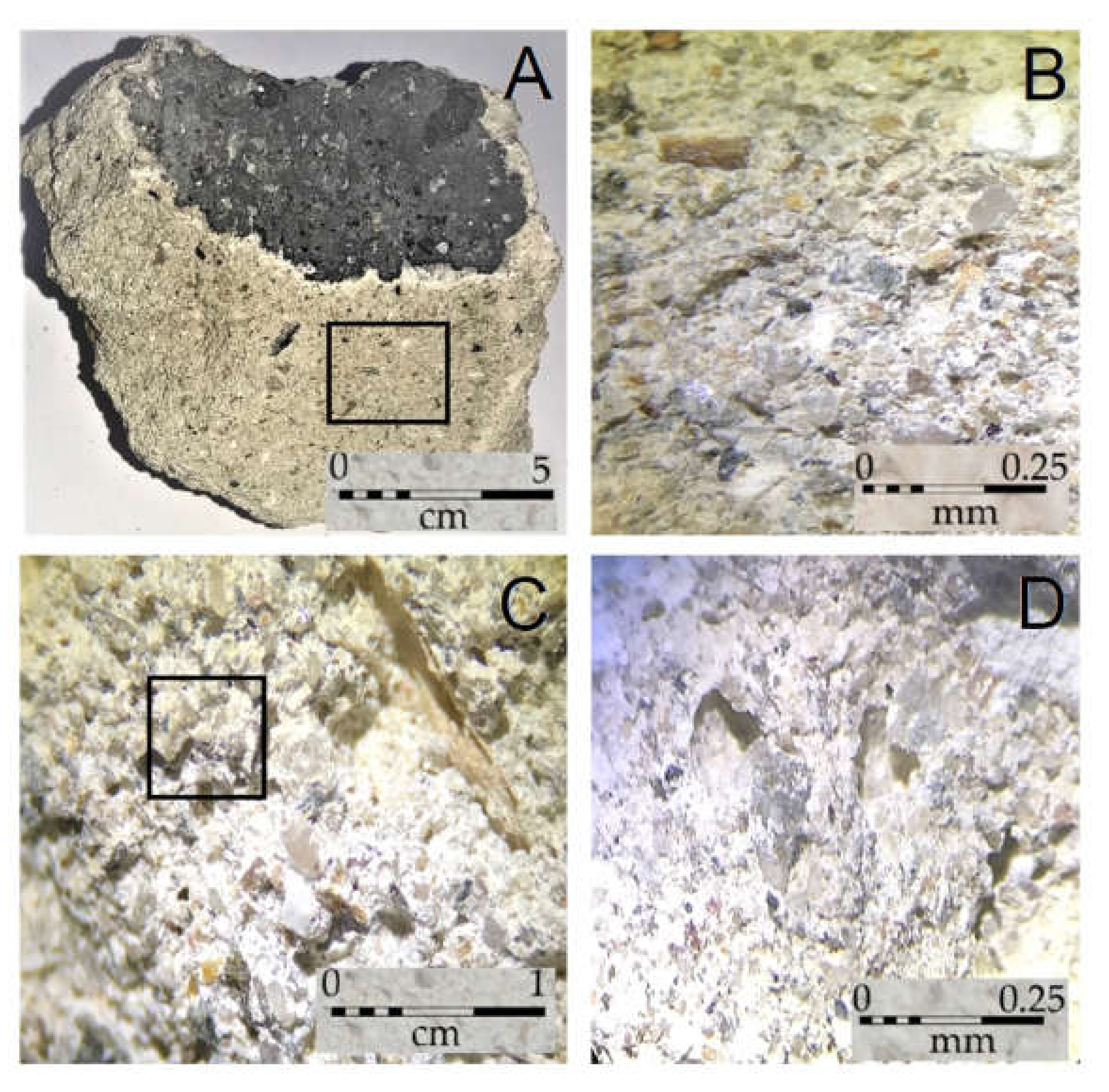
2.2. Sampling and Macroscopic Characterization
2.3. Testing Methods
2.4. Consolidant Derivatives and Application Method
2.5. Analytical Methods
3. Results
3.1. In Situ Measurements and Observations
3.2. Consolidant Applications
3.2.1. Conservation Proposals
3.2.2. Chromatic Parameters
3.2.3. Carbonation Process
3.2.4. Roughness Parameters
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Van den Eynde, V.C.; Mateos, F.J.; Paradelo, R. Degradability of building stone: Influence of the porous network on the rate of dissolution of carbonate and evaporitic rocks. J. Cult. Herit. 2013, 14, 89–96. [Google Scholar] [CrossRef] [Green Version]
- Gräf, V.; Jamek, M.; Rohatsch, A.; Tschegg, E. Effects of thermal-heating cycle treatment on thermal expansion behavior of different building stones. Int. J. Rock Mech. Min. Sci. 2013, 64, 228–235. [Google Scholar] [CrossRef]
- Bodochi, I. Castelul Corvinilor în secolul al XVII-lea (1). Corviniana 2008, XII, 207–232. [Google Scholar]
- Velescu, O. Castelul de la Hunedoara; Editura Meridiane: Monumentele Patriei Noastre: Bucuresti, Romania, 1961. [Google Scholar]
- Lupescu, R. Domeniul cetăţii Hunedoara în timpul Hunedorenilor. MedTrans 2001–2002, VVI, 7–34. [Google Scholar]
- Roman, C.C.; Diaconescu, D.; Tiplic, M. Archaeological excavations at Hunedoara—The Corvins’ Castle—The sacristy of the chapel. In Studii de Istorie Veche si Arheologie: Bibliotheca Archaeologica et Historica Corvinensis—IV; Matos, C., Ed.; Editura Mereamia Napocae: Hunedoara, Romania, 2004. [Google Scholar]
- Bogdan, C. Contribuţii arheologice la cunoaşterea evoluţiei Castelului Corvineştilor de la Hunedoara. BMI 1970, 39, 18–25. [Google Scholar]
- Vatasianu, V. Castelul Corvinilor din Hunedoara. Boabe de Grau 1933, 6, 420–431. [Google Scholar]
- Roman, C.C.; Tincu, S. Observations regarding the ecclesiastical complex from Hunedoara—The Corvin’s Castle. Sargetia Acata Musei Devensis Deva, Romania 2012, 3, 243–258. [Google Scholar]
- Schilling, R. Historical conclusions about the archaeological excavations at the medieval castle of Hunedoara. Bul. Monum. Istor. 1970, 2, 55. [Google Scholar]
- Ion, R.-M.; Iancu, L.; Grigorescu, R.M.; Tincu, S.; Vasilievici, G.; Ion, N.; Bucurică, I.A.; Teodorescu, S.; Dulama, I.D.; Ştirbescu, R.M.; et al. Arhaeometric investigations on ceramic materials from Hunedoara-the court area. J. Sci. Arts 2018, 18, 471–480. [Google Scholar]
- Ion, R.; Iancu, L.; Grigorescu, R.M.; Carutiu, D.T.; Tincu, S.; Ion, N.; Bucurică, I.A.; Teodorescu, S.; Dulama, I.D.; Ştirbescu, R.M.; et al. Arhaeometric Concepts and Methods of Intervention on Historical Monument Buildings. The Case of the Corvins’ Castle. IOP Conf. Ser. Mater. Sci. Eng. 2018, 374, 012073. [Google Scholar]
- Ion, R.-M.; Tincu, S.; Iancu, L.; Grigorescu, R.M. Investigations of the new gate tower from Corvins’ Castle. IOP Conf. Ser. Mater. Sci. Eng. 2019, 572, 012088. [Google Scholar] [CrossRef]
- Baglioni, P.; Giorgi, R. Soft and hard nanomaterials for restoration and conservation of cultural heritage. Soft Matter. 2006, 2, 293–303. [Google Scholar] [CrossRef] [PubMed]
- Ion, R.-M.; Iancu, L.; Vasilievici, G.; Grigore, M.E.; Andrei, R.E.; Radu, G.I.; Grigorescu, R.M.; Teodorescu, S.; Bucurica, I.A.; Ion, M.L.; et al. Ion-Substituted Carbonated Hydroxyapatite Coatings for Model Stone Samples. Coatings 2019, 9, 231. [Google Scholar] [CrossRef] [Green Version]
- Sabbioni, C. Mechanisms of air pollution damage to stone. In The Effects of Air Pollution on the Built Environment, Air Pollution Reviews; Brimblecombe, P., Ed.; Imperial College Press: London, UK, 2003; pp. 63–106. [Google Scholar]
- Frahm, E.; Doonan, R.C.P. The technological versus methodological revolution of portable XRF in archaeology. J. Archaeol. Sci. 2013, 40, 1425–1434. [Google Scholar] [CrossRef]
- Shackley, M.S. An Introduction to X-ray Fluorescence (XRF) Analysis in Archaeology. In X-ray Fluorescence Spectrometry (XRF) in Geoarchaeology; Shackley, M., Ed.; Springer: New York, NY, USA, 2011. [Google Scholar]
- Cechak, L.T.; Gerndt, J.; Kopecka, I.; Musılek, L. X-ray fluorescence in research on Czech cultural monuments. Nucl. Instrum. Methods Phys. Res. B 2004, 213, 735–740. [Google Scholar]
- Acun, S.; Ariolu, N. A Method for the Preservation and Restoration of the Stones Used in Historical Buildings. Archit. Sci. Rev. 2006, 49, 143–148. [Google Scholar] [CrossRef]
- Stuart, B.H. Analytical Techniques in Materials Conservation; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2007; ISBN 0-470-01280-3. [Google Scholar]
- Mazzetto, S.; Petruccioli, A. Methods and Techniques Used in Significant Restoration Projects in Qatar. Stud. Conserv. 2018, 63, 303–314. [Google Scholar] [CrossRef]
- ICOMOS. International Chapter for the Conservation and Restoration of Monumets and Sites (The Venice Chapter 1964). In Proceedings of the IInd International Congress of Architects and Technicians of Historic Monuments, Venice, Italy, 25–31 May 1964. [Google Scholar]
- Sandis, C. (Ed.) Cultural Heritage Ethics: Between Theory and Practice; Open Book Publishers: Cambridge, UK, 2014; ISBN 978-1-78374-067-3. [Google Scholar]
- Ricca, M.; Le Pera, E.; Licchelli, M.; Macchia, A.; Malagodi, M.; Randazzo, L.; Rovella, N.; Ruffolo, S.A.; Weththimuni, M.L.; La Russa, M.F. The CRATI Project: New Insights on the Consolidation of Salt Weathered Stone and the Case Study of San Domenico Church in Cosenza (South Calabria, Italy). Coatings 2019, 9, 330. [Google Scholar] [CrossRef] [Green Version]
- De los Santos, D.M.; Sanmartin, P.; Rivas, T.; Silva, B.; Mosquera, M.J. A new material with both. In Proceedings of the International Symposium on Stone Consolidation in Cultural Heritage–research and practice, Lisbon, Portugal, May 6–7 2008; Delgado, R., Mimoso, J.M., Eds.; Laboratório Nacional de Engenharia Civil: Lisbon, Portugal; pp. 455–462.
- Pinto, A.P.F.; Rodrigues, J.D. Consolidation of carbonate stones: Influence of treatment procedures on the strengthening action of consolidants. J. Cult. Herit. 2012, 13, 154–166. [Google Scholar] [CrossRef]
- Teutonico, J.; Charola, A.E.; De Witte, E.; Grassegger, G.; Koestler Robert, J.; Laurenzi Tabasso, M.; Sasse, H.R.; Snethlage, R. Group Report: How Can We Ensure the Responsible and Effective Use of Treatments (Cleaning, Consolidation, Protection)? In Saving Our Architectural Heritage: The Conservation of Historic Stone Structures; Dahlem Workshop Report ES20; John Wiley & Sons Ltd.: Hoboken, NJ, USA, 1997. [Google Scholar]
- Teutonico, J.M. A laboratory Manual for Architectural Conservators; ICCROM: Rome, Italy, 1988; p. 168. [Google Scholar]
- Drdácký, M.; Lesák, J.; Niedoba, K.; Valach, J. Peeling tests for assessing the cohesion and consolidation characteristics of mortar and render surfaces. Mater. Struct. 2015, 48, 1947–1963. [Google Scholar] [CrossRef]
- Scrivano, S.; Gaggero, L.; Gonzalez, A.Y.; Aguilar, J.G. Assessing surface weathering by revision and implememtation of the peeling test: In situ sampling and integrated analysis. J. Cult. Herit. 2017, 27, 88–96. [Google Scholar] [CrossRef]
- Becerra, J.; Ortiz, P.; Martín, J.M.; Zaderenko, A.P. Nanolimes doped with quantum dots for stone consolidation assessment. Constr. Build. Mater. 2019, 199, 581–593. [Google Scholar] [CrossRef]
- Wang, R.X.; Yuan, Z.C. The Application of Getting Rc Value from Point Load Test and Rebound Test by Tunnel Surrounding Rock Classification System. Appl. Mech. Mater. 2014, 580, 1116–1121. [Google Scholar] [CrossRef]
- BSI Group. SR EN 1015-10:2002/A1:2007—Methods for Testing Masonry Mortars—Part 10: Determining the Apparent Density of Hardened Mortar; British Standards Institution: London, UK, 1999. [Google Scholar]
- Japanese Standards Association (JSA). JIS Z 8729:2004. Color Specification—CIELAB and CIELUV Color Spaces; Japanese Standards Association (JSA): Tokyo, Japan, 2004. [Google Scholar]
- Codarcea, A.; Dimitrescu, R.; Gherasi, N.; Mureşan, M.; Mureşan, G.; Kräutner, H.; Kräutner, F.; Lupu, M.; Marinescu, F.; Savu, H.; et al. Geological Map of Romania: Deva Sheet, Scale 1:200.000; Geological Institute of Romania Printing House: Bucharest, Romania, 1967. [Google Scholar]
- Danciu, C.; Toderaș, M.; Lorinț, C.; Florea, A. Characterization and Classification of Andesites of Criscior and Albini from Southern Apuseni Mountains for Capitalization. Ann. Univ. Petrosani Min. Eng. 2018, 19, 61–69. [Google Scholar]
- Balintoni, I. Geotectonica Terenurilor Metamorfice din România; Ed. Carpatica: Cluj-Napoca, Romania, 1997; p. 176. [Google Scholar]
- Balintoni, I.; Iancu, V. Probleme de metamorfism, litostratigrafie si structura ale cristalinului din masivul Poiana Rusca. Studii si Cercetari de Geologie, Geofizica si Geografie, seria Geologie, Bucuresti 1986, 31, 51–67. [Google Scholar]
- Layani, J.; Mayer, I.; Cuisinier, F. Carbonated hydroxyapatites precipitated in the presence of Ti. J. Inorg. Biochem. 2000, 81, 57–63. [Google Scholar] [CrossRef]
- Rodrigues, J.D.; Grossi, A. Indicators and ratings for the compatibility assessment of conservation actions. J. Cult. Herit. 2007, 8, 32–43. [Google Scholar] [CrossRef]
- BSI. BS EN 12615:1999: Products and Systems for the Protection and Repair of Concrete Structures-Test Methods-Determination of Slant Shear Strength; BSI: London, UK, 1999. [Google Scholar]
- ISO. 4287:1997—Geometrical Product Specifications (GPS). Surface Texture. Profile method. Terms, Definitions and Surface Texture Parameters; International Organization for Standardization: Geneva, Switzerland, 1997. [Google Scholar]
- Curray, J.R. The analysis of two-dimensional orientation data. J. Geol. 1956, 64, 117–131. [Google Scholar] [CrossRef]
- Ion, R.-M.; Doncea, S.M.; Ion, M.L.; Rădiţoiu, V.; Amăriuţei, V. Surface investigations of old book paper treated with hydroxyapatite nanoparticles. Appl. Surf. Sci. 2013, 285, 27–32. [Google Scholar] [CrossRef]
- Ion, R.-M.; Turcanu-Caruţiu, D.; Fierăscu, R.C.; Fierăscu, I.; Bunghez, I.R.; Ion, M.L.; Teodorescu, S.; Vasilievici, G.; Rădiţoiu, V. Caoxite-hydroxyapatite composition as consolidating material for the chalk stone from Basarabi–Murfatlar churches ensemble. Appl. Surf. Sci. 2015, 358, 612–618. [Google Scholar] [CrossRef]
- Ion, R.-M.; Nyokong, T.; Nwahara, N.; Fierascu, I. Wood preservation with gold hydroxyapatite system. Herit. Sci. 2018, 6, 37. [Google Scholar] [CrossRef] [Green Version]
- Ion, R.-M.; Turcanu-Carutiu, D.; Fierascu, R.C.; Fierascual, I. Chalk stone restoration with hydroxyapatite–based nanoparticles. Sci. Bull. Valahia Univ. Mater. Mech. 2014, 9, 16–19. [Google Scholar]
- Wong, W.; Noor, A.-F.M. Synthesis and sintering-wet carbonation of nano-sized carbonated hydroxyapatite. Proc. Chem. 2016, 19, 98–105. [Google Scholar]
- Kovaleva, E.S.; Shabanov, M.P.; Putlyaev, V.I.; Tretyakov, Y.D.; Ivanov, V.K.; Silkin, N.I. Bioresorbable carbonated hydroxyapatite Ca10−xNax(PO4)6−x(CO3) x (OH)2 powders for bioactive materials preparation. Cent. Eur. J. Chem. 2009, 7, 168–174. [Google Scholar] [CrossRef]
- Price, C.; Ross, K. Technical appraisal of stone conservation techniques at Wells Cathedral. In Conservation of Building and Decorative Stone; Butterworth/Heinemann: London, UK, 1990. [Google Scholar]
- Jerome, P.S.; Weiss, N.R.; Gilbert, A.S.; Scott, J.A. Ethyl silicate as a treatment for marble: Conservation of St. John’s Hall, Fordham University. APT Bull. 1998, 29, 19–26. [Google Scholar]
- Weththimuni, M.L.; Licchelli, M.; Malagodi, M.; Rovella, N.; La Russa, M. Consolidation of bio-calcarenite stone by treatment based on diammonium hydrogenphosphate and calcium hydroxide nanoparticles. Measurement 2018, 127, 396–405. [Google Scholar] [CrossRef]
- Kim, H.-W.; Koh, Y.H.; Kong, Y.M.; Kang, K.G.; Kim, H.E. Strontium substituted calcium phosphate biphasic ceramics obtained by a powder precipitation method. J. Mater. Sci. Mater. Med. 2004, 15, 1129–1134. [Google Scholar] [CrossRef]
- Čadež, V.; Erceg, I.; Selmani, A.; Jurašin, D.D.; Šegota, S.; Lyons, D.M.; Kralj, D.; Sikirić, M.D. Amorphous calcium phosphate formation and aggregation process revealed by light scattering techniques. Crystals 2018, 8, 254. [Google Scholar] [CrossRef] [Green Version]


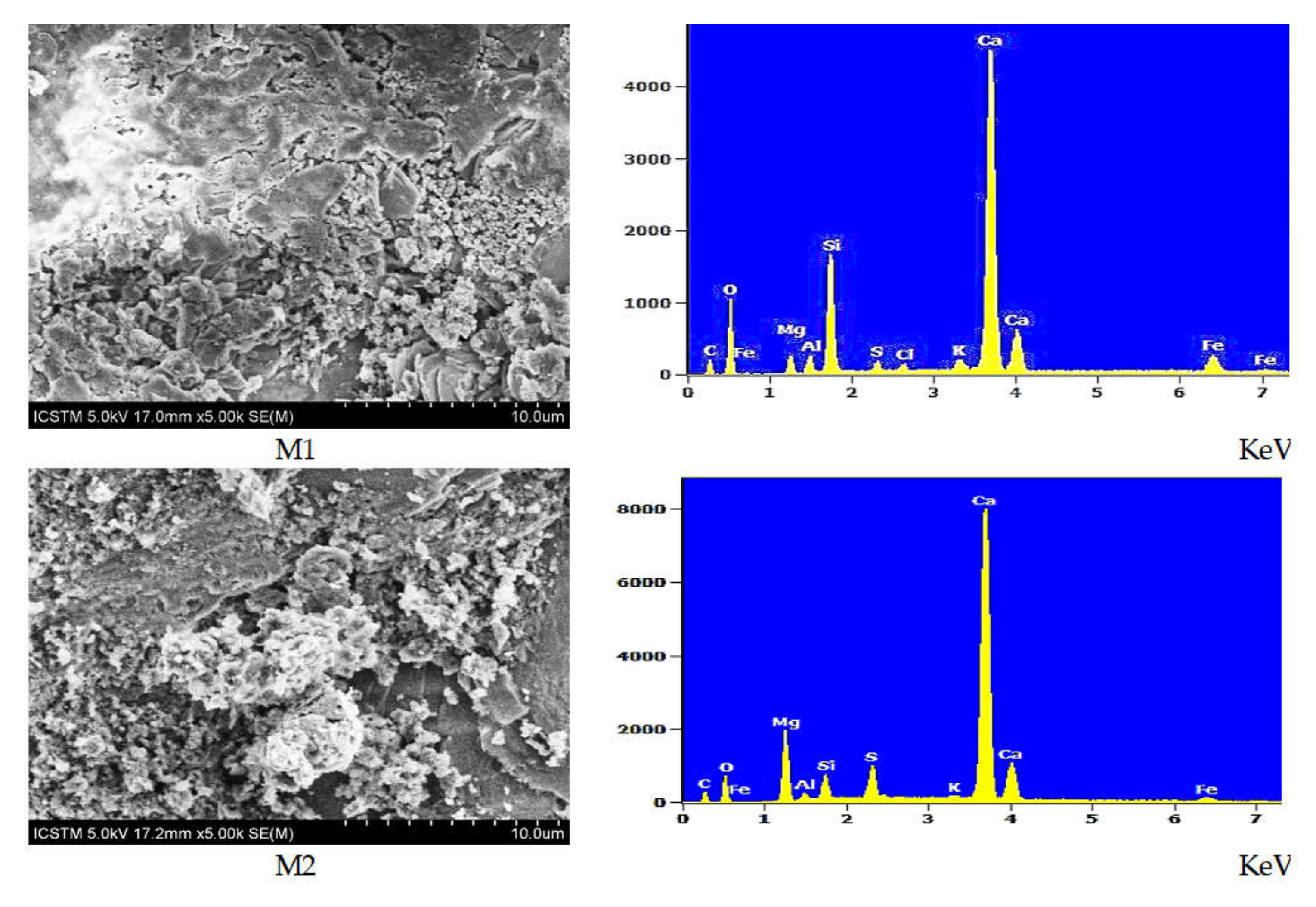

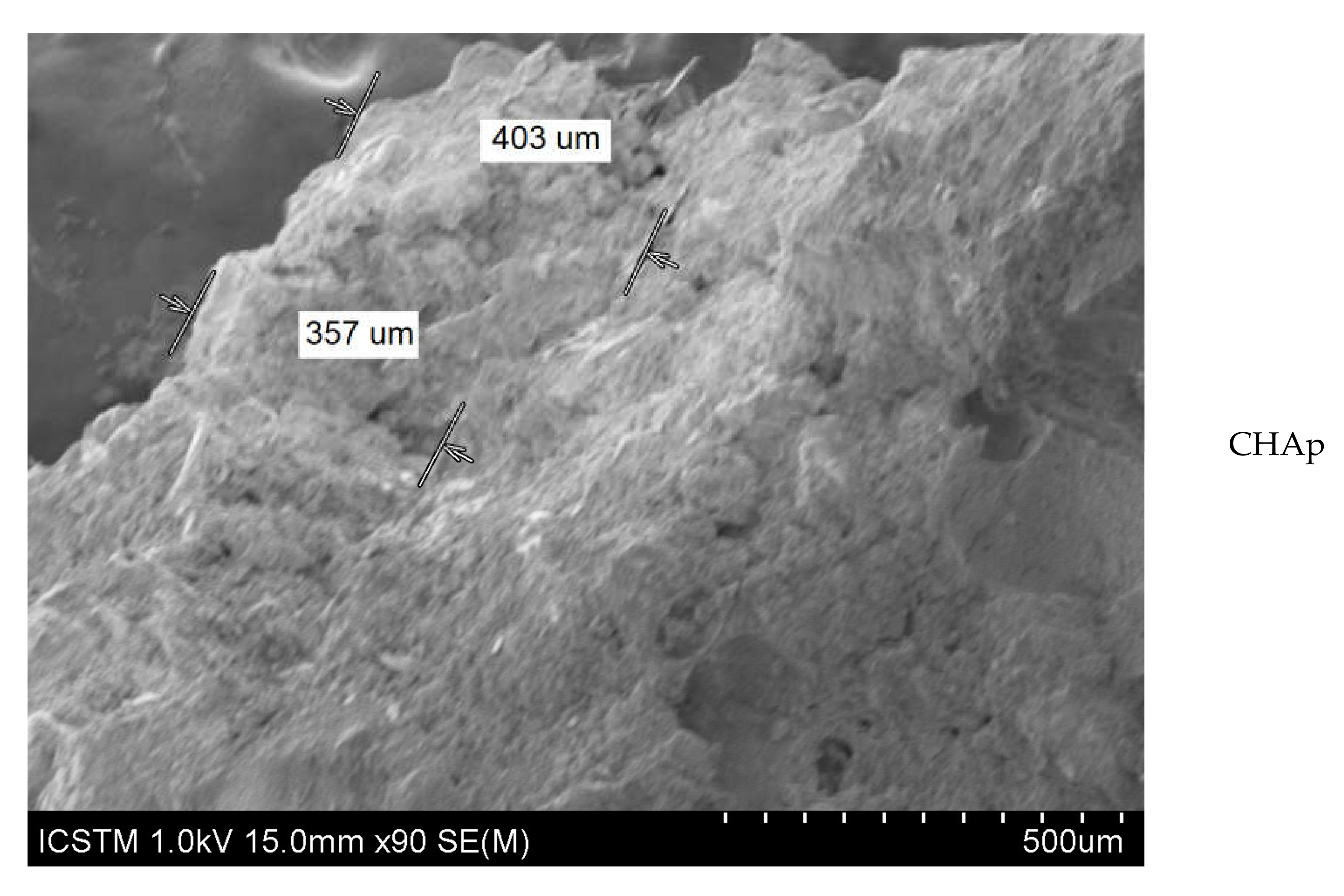

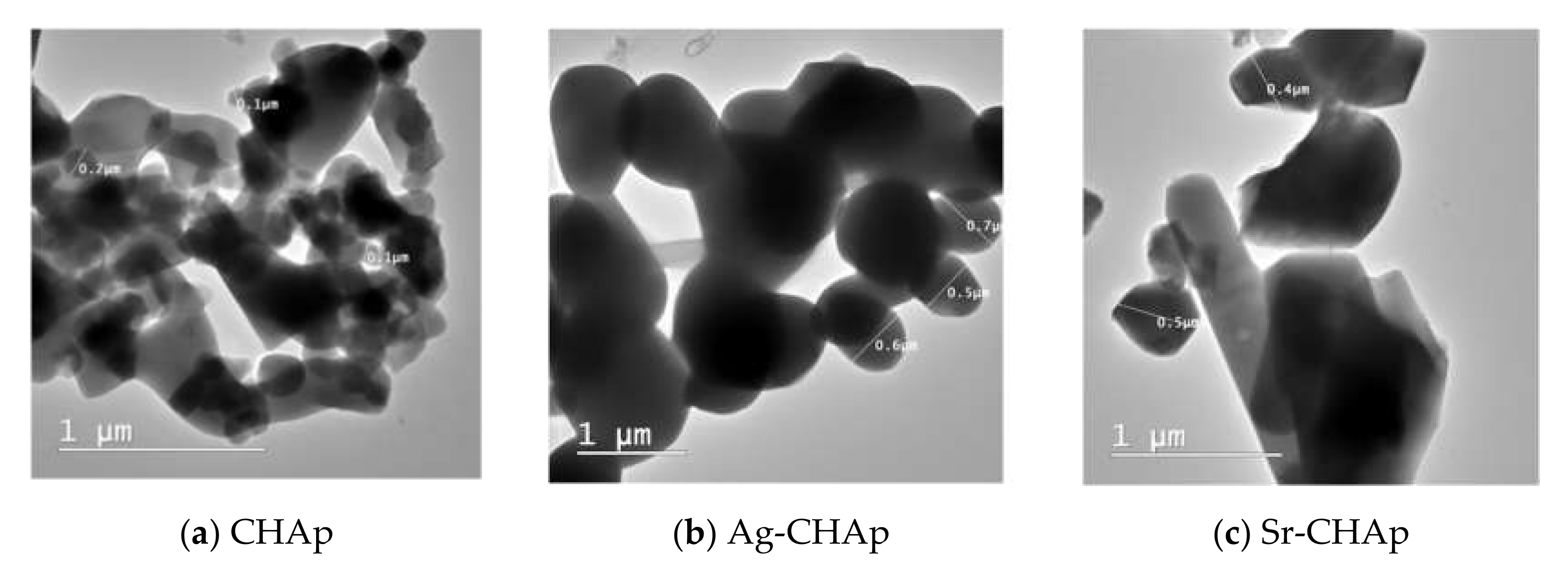


| Sample | Composition (wt % ± Error %) 1 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| C | O | Mg | Al | Si | S | Cl | K | Ca | Fe | |
| M1 | 5.68 ± 0.2 | 35.70 ± 0.54 | 1.57 ± 0.06 | 1.32 ± 0.04 | 8.42 ± 0.09 | 0.69 ± 0.03 | 0.48 ± 0.05 | 0.98 ± 0.04 | 38.64 ± 0.27 | 6.51 ± 0.31 |
| M2 | 5.95 ± 0.16 | 25.74 ± 0.46 | 8.58 ± 0.08 | 0.81 ± 0.04 | 0.81 ± 0.04 | 3.56 ± 0.05 | n.d. 2 | 0.27 ± 0.04 | 50.40 ± 0.28 | 2.23 ± 0.22 |
| No. | 2-Theta (deg.) | d (ang.) | Phase Name |
|---|---|---|---|
| 1. | 17.772 | 4.987 | Muscovite-2M1 (0,0,4) |
| 2. | 20.842 | 4.259 | Quartz, syn (1,0,0), Clinozoisite (0,0,2), Muscovite-2M1 (1,1,1), Berlinite HP, syn, berlinite, syn (1,0,0) |
| 3. | 23.732 | 3.746 | Muscovite-2M1 (0,2,3) |
| 4. | 26.607 | 3.347 | Quartz, syn (1,0,1), Clinozoisite (0,1,2), Muscovite-2M1 (0,2,4) |
| 5. | 25.502 | 3.241 | Graphite-2H (0,0,2), Clinozoisite (1,0,2), Berlinite HP, syn, berlinite, syn (0,0,3) |
| 6. | 29.970 | 2.979 | Clinozoisite (3,0,−2), Muscovite-2M1 (0,2,5) |
| 7. | 31.068 | 2.876 | Gypsum (1,1,−2), Calcite (0,0,6), Muscovite-2M1 (1,1,5), |
| 8. | 33.300 | 2.688 | Clinozoisite (1,0,−3), Muscovite-2M1 (0,2,6) |
| 9. | 35.300 | 2.541 | Gypsum (1,3,−2), Dolomite (0,1,5), Muscovite-2M1 (2,0,0) |
| 10. | 36.530 | 2.458 | Quartz, syn (1,1,0), Clinozoisite (2,0,2), Muscovite-2M1 (1,3,−3) |
| 11. | 39.402 | 2.285 | Calcite (1,1,3), Quartz, syn (1,0,2), Gypsum (1,5,1), Clinozoisite (0,2,2) |
| 12. | 42.407 | 2.130 | Quartz, syn (2,0,0), Muscovite-2M1 (2,2,2) |
| 13. | 43.110 | 2.097 | Calcite (2,0,2), Clinozoisite (0,0,4), Muscovite-2M1 (2,2,−4), Berlinite HP, syn, berlinite, syn (1,0,4) |
| 14. | 45.762 | 1.981 | Quartz, syn (2,0,1) |
| 15. | 47.530 | 1.911 | Calcite (0,2,4), Graphite-2H (1,0,1), Gypsum (1,7,−1), Clinozoisite (4,1,0), Muscovite-2M1 (2,2,−6) |
| 16. | 48.420 | 1.878 | Calcite (1,1,6), Gypsum (3,1,−1), Clinozoisite (2,0,−5), Muscovite-2M1 (2,2,5) |
| 17. | 50.103 | 1.819 | Quartz, syn (1,1,2), Clinozoisite (1,0,−5) |
| 18. | 54.826 | 1.673 | Quartz, syn (2,0,2), Muscovite-2M1 (1,3,−2), Gypsum (2,6,−2) |
| 19. | 59.936 | 1.542 | Quartz, syn (2,1,1), Clinozoisite (2,2,−5), Muscovite-2M1 (3,1,4), Dolomite (2,1,−2) |
| 20. | 67.677 | 1.383 | Quartz, syn (2,1,2), Gypsum (4,0,−2), Muscovite-2M1 (3,1,7), Dolomite (3,0,0) |
| 21. | 68.113 | 1.375 | Quartz, syn (2,0,3), Clinozoisite (0,3,4), Muscovite-2M1 (1,5,−9), Dolomite (0,1,11) |
| 22. | 81.160 | 1.184 | Quartz, syn (1,1,4), Muscovite-2M1 (4,0,−8) |
| Component | DT | DT + CHAp | DT + Ag-CHAp | DT + Sr-CHAp |
|---|---|---|---|---|
| wt% | ||||
| CaO | 21.3589 ± 0.044 | 44.0349 ± 0.0710 | 51.3650 ± 1.0360 | 61.0121 ± 1.6570 |
| SiO2 | 38.9495 ± 0.1324 | 21.2177 ± 0.0380 | 21.9194 ± 0.0490 | 16.1732 ± 0.0390 |
| MgO | 12.8629 ± 0.2506 | 7.9097 ± 0.2500 | 7.4038 ± 0.2480 | 7.7009 ± 0.1220 |
| SO3 | 7.7038 ± 0.0361 | 1.7943 ± 0.0098 | 0.9783 ± 0.0230 | 1.7084 ± 0.0180 |
| Al2O3 | 6.655 ± 0.0799 | 5.5471 ± 0.1320 | 6.3187 ± 0.0720 | 4.9450 ± 0.0920 |
| Na2O | n.d. | 1.7682 ± 0.0790 | 1.9661 ± 0.0670 | n.d. |
| Fe2O3 | 9.6387 ± 0.02983 | 2.2969 ± 0.0290 | 4.3919 ± 0.0430 | 3.3747 ± 0.0390 |
| K2O | 1.4488 ± 0.04470 | 1.6287 ± 0.0460 | 2.2576 ± 0.0270 | 1.9142 ± 0.0320 |
| P2O5 | 0.3077 ± 0.03871 | 0.1504 ± 0.0360 | 0.3241 ± 0.0210 | 0.5373 ± 0.0260 |
| Cl | 0.1567 ± 0.0098 | 0.3983 ± 0.0440 | 1.1004 ± 0.0040 | 0.7842 ± 0.0080 |
| ZrO2 | 0.0384 ± 0.0162 | 0.1669 ± 0.0290 | 0.0677 ± 0.0030 | 0.0485 ± 0.0050 |
| Ag2O | n.d. | n.d. | 1.1904 ± 1.2340 | n.d. |
| SrO | 0.0629 ± 0.0162 | n.d. | n.d. | 0.0610 ± 0.0050 |
| Other | 0.8167 ± 0.0511 | 3.087 ± 0.0120 | 0.717 ± 0.0400 | 1.7406 ± 0.0600 |
| Sample | PDI (DLS) | Size, nm (DLS) | Z mV (DLS) | Pore Diameter (nm) (BET) | Surface Area (m2/g) (BET) | Hydrodynamic Diameter Å (DLS) |
|---|---|---|---|---|---|---|
| CHAp | 0.830 | 955.5 | −25.6 | 4.2 | 5.8 | 285.7 |
| Ag-CHAp | 0.483 | 147 | −10.3; −30.3 | 4.085 | 4.02 | 307.2 |
| Sr-CHAp | 0.246 | 156.2 | −17.6 | 4.014 | 4.106 | 287.7 |
| Sample | Δbx | ΔEx |
|---|---|---|
| DT + CHAp | 1.95 | 3.45 |
| DT + Ag-CHAp | 1.81 | 4.05 |
| DT + Sr-CHAp | 3.17 | 4.32 |
| Sample | Rq | SA |
|---|---|---|
| Not-treated | 45.574 | 5.6089 |
| CHAp | 43.164 | 4.5657 |
| Ag-CHAp | 40.9166 | 8.9172 |
| Sr-CHAp | 41.1277 | 5.3291 |
| Sample | Absorption, % |
|---|---|
| DT | 13.4304 |
| DT + CHAp | 16.0617 |
| DT + Ag-CHAp | 13.7955 |
| DT + Sr-CHAp | 12.5797 |
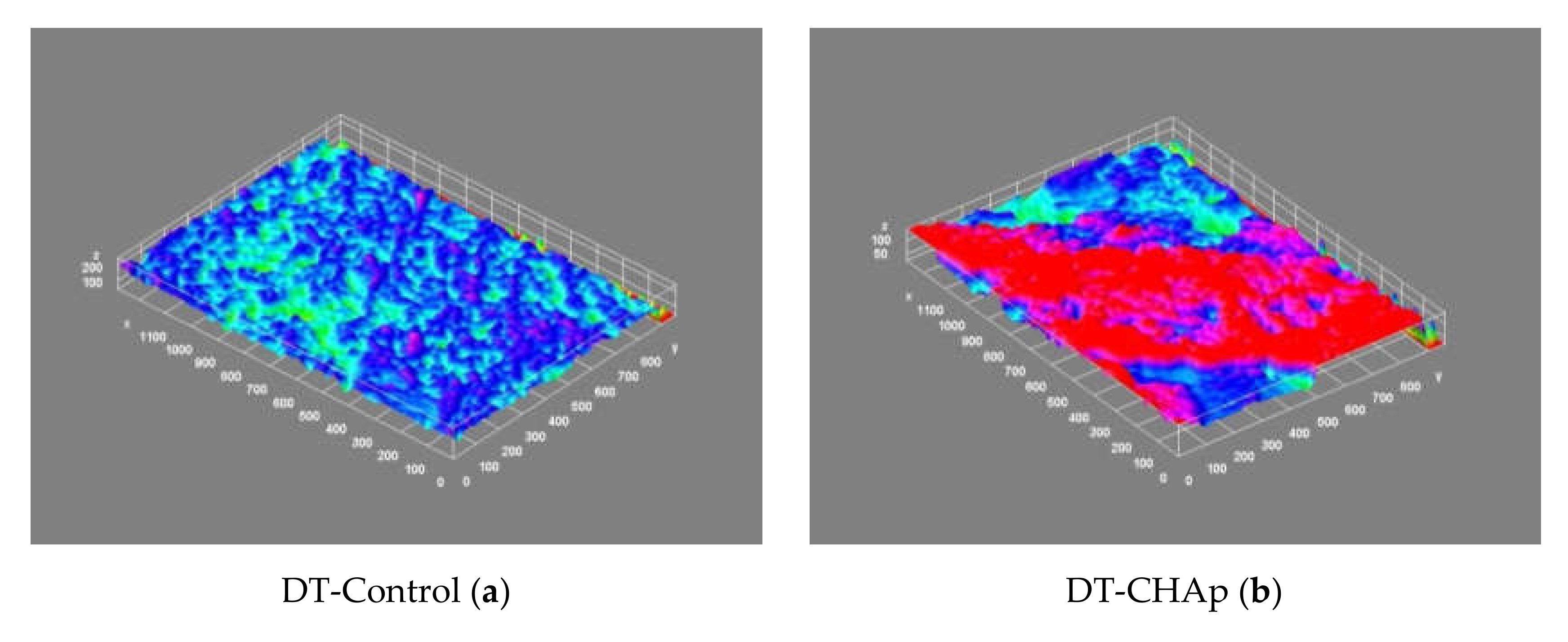
| Sample | MO (2X Magnification) | Image Reconstruction | Image 3D Reconstruction | DA, % | PC, % |
|---|---|---|---|---|---|
| Control |  |  | 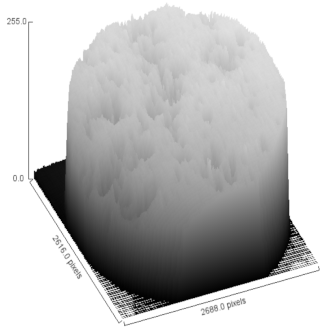 | 8.61 | - |
| CHAp |  |  | 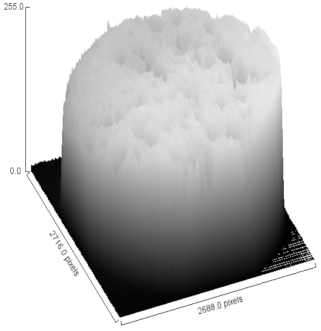 | 5.54 | 52.85 |
| Sr-CHAp |  |  | 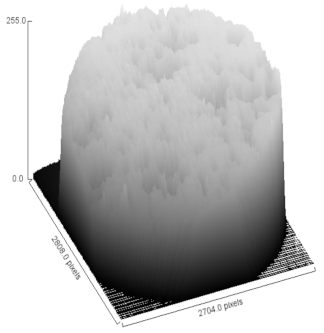 | 5.81 | 48.57 |
| Ag-CHAp | 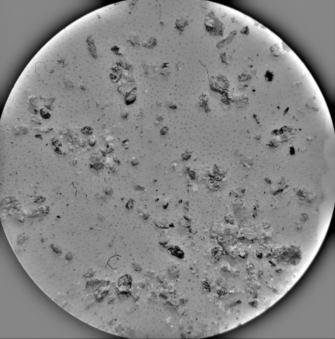 |  | 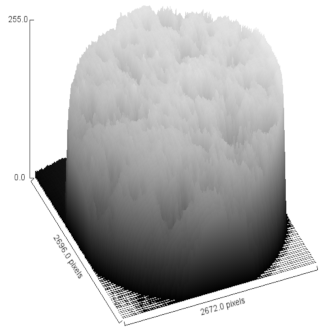 | 5.15 | 53.42 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ion, R.M.; Iancu, L.; David, M.E.; Grigorescu, R.M.; Trica, B.; Somoghi, R.; Vasile, S.F.; Dulama, I.D.; Gheboianu, A.I.; Tincu, S. Multi-Analytical Characterization of Corvins’ Castle—Deserted Tower. Construction Materials and Conservation Tests. Heritage 2020, 3, 941-964. https://doi.org/10.3390/heritage3030051
Ion RM, Iancu L, David ME, Grigorescu RM, Trica B, Somoghi R, Vasile SF, Dulama ID, Gheboianu AI, Tincu S. Multi-Analytical Characterization of Corvins’ Castle—Deserted Tower. Construction Materials and Conservation Tests. Heritage. 2020; 3(3):941-964. https://doi.org/10.3390/heritage3030051
Chicago/Turabian StyleIon, Rodica Mariana, Lorena Iancu, Madalina Elena David, Ramona Marina Grigorescu, Bogdan Trica, Raluca Somoghi, Sorina Florentina Vasile, Ioana Daniela Dulama, Anca Irina Gheboianu, and Sorin Tincu. 2020. "Multi-Analytical Characterization of Corvins’ Castle—Deserted Tower. Construction Materials and Conservation Tests" Heritage 3, no. 3: 941-964. https://doi.org/10.3390/heritage3030051









