1. Introduction
Bernard Palissy (1510–1590) is one of the most famous French Renaissance ceramists. His masterpieces called the Rustique Figulines are ceramic plates, vases or architectural elements adorned with molded animals (reptiles, fishes, shells, etc.) or plants laying on a rockery-style base [
1]. To enhance the realistic appearance, Palissy was using colorful lead glazes to decorate the ceramic pieces. The artist was able to deal with a dozen of glazes of different colors and transparencies (and thus different chemical compositions and firing behaviors) to obtain at the end a single object illuminated by a harmonious set of glazes cohabiting perfectly together, without any elaboration defects (burr, cracks, etc.). In his writings, Palissy highlights how these results have been achievable only after long years of sacrifice and hard labor [
2]. Consequently, he always refuses to reveal his recipe secrets: very few information about the elaboration process of his glazed ceramics (raw materials, firing parameters) are available in his publications.
In the 1980s, Palissy workshop in the Tuileries garden (Paris) was excavated in the context of the Louvre Pyramid construction. Inside, a lot of glazed ceramics fragments including crockery and architectural elements, plus some materials used for their production (clay molds, saggars, etc.) were found [
3]. These precious archaeological samples represent a wealth of information for scientists. Glazes and pastes microstructures, including the glaze-paste interface area on which we are going to focus in this paper, were thoroughly investigated to obtain information on the ceramist elaboration method [
1,
4,
5,
6]. More generally, detailed investigation of ancient ceramics provides valuable information on the raw materials used, their provenance, their thermal history, and thus gives us a glimpse on arts and crafts of the past. For this purpose, scientists often proceed by comparing archaeological pieces with replicas elaborated under controlled conditions in the laboratory to precise the artist manufacturing procedure [
7].
Why looking especially at the glaze-paste interface region? First it should be recalled that during the firing of glazed ceramic, strong interactions occur between glaze and paste which are going to modify the chemistries of both parts [
8]. Due to the presence of concentration gradients at the interface, the diffusion process occurs between the thin liquid layer of melted glaze (~100 µm) and the paste. In the case of transparent high lead glazes for instance, lead (Pb) will diffuse from the glaze to the paste whereas aluminum (Al), silicon (Si), iron (Fe), titanium (Ti), alkalis (Na, K), and alkaline elements (Mg, Ca) will diffuse from the paste to the glaze. The diffusion process is accompanied by a digestion mechanism: the hot liquid melt induces a decomposition of the paste components. This digestion-diffusion process leads afterwards to the development of new devitrification crystallizations creating the so-called interface area [
9].
It is important to note that the interface definition may vary depending on the authors. For instance, Coentro et al. (2017) define the interface as the “layer with mineral inclusions that result from the reaction between the glaze and the ceramic body” [
10]. Furthermore, Molera et al. (2001) distinguish between the body part digested by the melt and the interface layer which is formed by small crystallites of K-Pb feldspars [
9]. Consequently, one should always consider the interface definition before comparing the interface extension obtained in different studies. In the present study, the interface extension will be defined as the sum of both the modified paste (part of the paste that has reacted with the melt) and the interfacial crystals regions extensions.
Numerous studies conducted on glazed ceramic replicas have demonstrated that the interface extension and microstructure depend on the following parameters: glaze and paste chemical composition, firing temperature, firing time, cooling rate, use of single- or double-firing method [
9,
11,
12]. Thus, investigating the interface allows gathering information on all these parameters which help to understand Palissy’s elaboration process.
In the case of transparent high lead glazes, it was found that an increase in firing temperature, duration, and a decrease in cooling rate induces a larger interface. Besides, the same glaze applied to different types of paste (illitic, kaolinitic, calcareous) leads to different interfaces thicknesses. Concerning the glaze chemistry, one major question is to determine to what extent the aluminum (Al) commonly detected in the glazes is due to the deliberate addition of clay by the ceramist and to what extent it is due to diffusion process from the body during the firing [
8]. Experiments recently conducted using two Palissy-style honey glazes replicas (one with 6%
wt alumina and one without) applied on the same white body (kaolinitic) revealed that the final glazes were both containing 6%
wt alumina after the thermal process [
6]. This result reveals how important the Al diffusion process is during the firing and that there seems to be a saturation limit. Examining the interface of these samples which have not yet been analyzed would be very useful to know if Palissy was intentionally adding Al or not in his glazing mixture. Lastly, several authors have tried to relate the interface expansion with the firing method (single or double) but no evident relationships have been found since two decades [
8,
13,
14]. Luckily we know that Palissy was very probably using the double firing method [
2,
4].
In addition to the interface expansion, the chemistry and the morphology of the interfacial crystals are also highly related to the above-mentioned parameters. In the case of a lead glaze applied on a calcareous paste, wollastonite microcrystals CaSiO
3 were often detected using scanning electron microscopy coupled to an X-ray energy dispersive spectrometer (SEM-EDX) [
15]. Besides, various other crystals such as andradite Ca
3Fe
2Si
3O
12, magnesioferrite MgFe
2O
4, diopside CaMgSi
2O
6, etc. were also identified using Raman spectroscopy and synchrotron micro X-ray diffraction (syn-µ-XRD) [
10,
16]. In the case of low calcareous pastes (such as those used by Palissy [
17]), a solid solution of K-Pb feldspars (K
x, Pb
y, Ca
z)Al
αSi
βO
δ microcrystals was mostly evidenced using SEM-EDX [
18,
19,
20] and in one study using Raman spectroscopy [
10]. In particular, a corpus composed of Palissy glazed ceramics samples (honey iron-colored glaze on white kaolinitic body) was characterized by SEM and K-Pb feldspars crystals were described in all the examined interfaces [
6].
Here one could realize that most of the interfacial compounds have been identified using SEM-EDX point analysis on micro-sized crystals. Although relatively simple to implement, this technique has several drawbacks: the EDX probed volume (~1 µm
3) is often too large for submicro- and nano-sized crystals which have been evidenced in the interface area [
16] and which thus have not been characterized, EDX quantitative analysis can only be achieved on homogeneous sample (yet the microcrystals are sometimes heterogeneous at the microscale), and finally this method does not provide a direct structural analysis. As shown above, some studies have thus turned to structural techniques such as Raman spectroscopy or syn-µ-XRD [
21,
22]. Both are very powerful techniques as they can detect sub micrometric species even if the beam is much larger (few microns for Raman and few tens of microns for syn-µ-XRD). However, the spatial resolution information is lost, and these techniques are still not perfectly adapted for nanoscale studies.
Hence in the present study, a multi-scale analysis has been developed including transmission electron microscopy (TEM) and associated techniques (electron diffraction, chemical analysis). This analytical strategy is used to characterize the morphology, chemistry, and microstructure of Palissy glazed ceramics interfaces areas (focusing on honey glaze on white body system) down to the nanoscale. In parallel, the influence of some elaboration parameters on the interface (firing time, cooling rate and addition of Al in the glazing mixture) is assessed through the preparation of Palissy-style replicas samples. Finally, archaeological and replicas samples are compared to shed some light on how Palissy was proceeding to achieve such masterpieces.
2. Materials and Methods
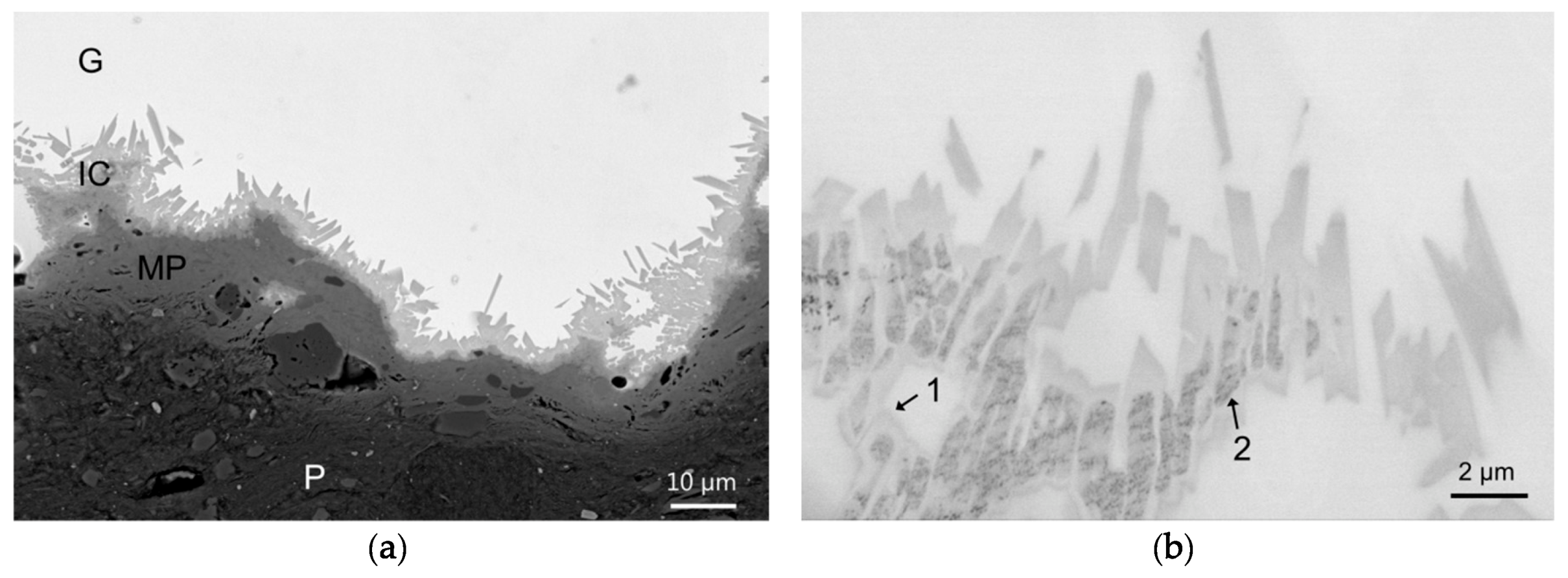
Four archaeological glazed ceramic shards (honey glaze on white body) coming from Palissy workshop in Paris (Tuileries) were selected. Three-millimeter sized fragments of these shards (EP422, EP470, and EP600) were embedded in cross-sections using acrylic resin before being mechanically polished. The last one (EP2319) was directly polished using a Leica TXC ion polisher (8 kV, 3 mA, 4 h) in order to investigate the capabilities of this facility to prepare glazed ceramic specimen compared to traditional preparation method (embedding and mechanical polishing).
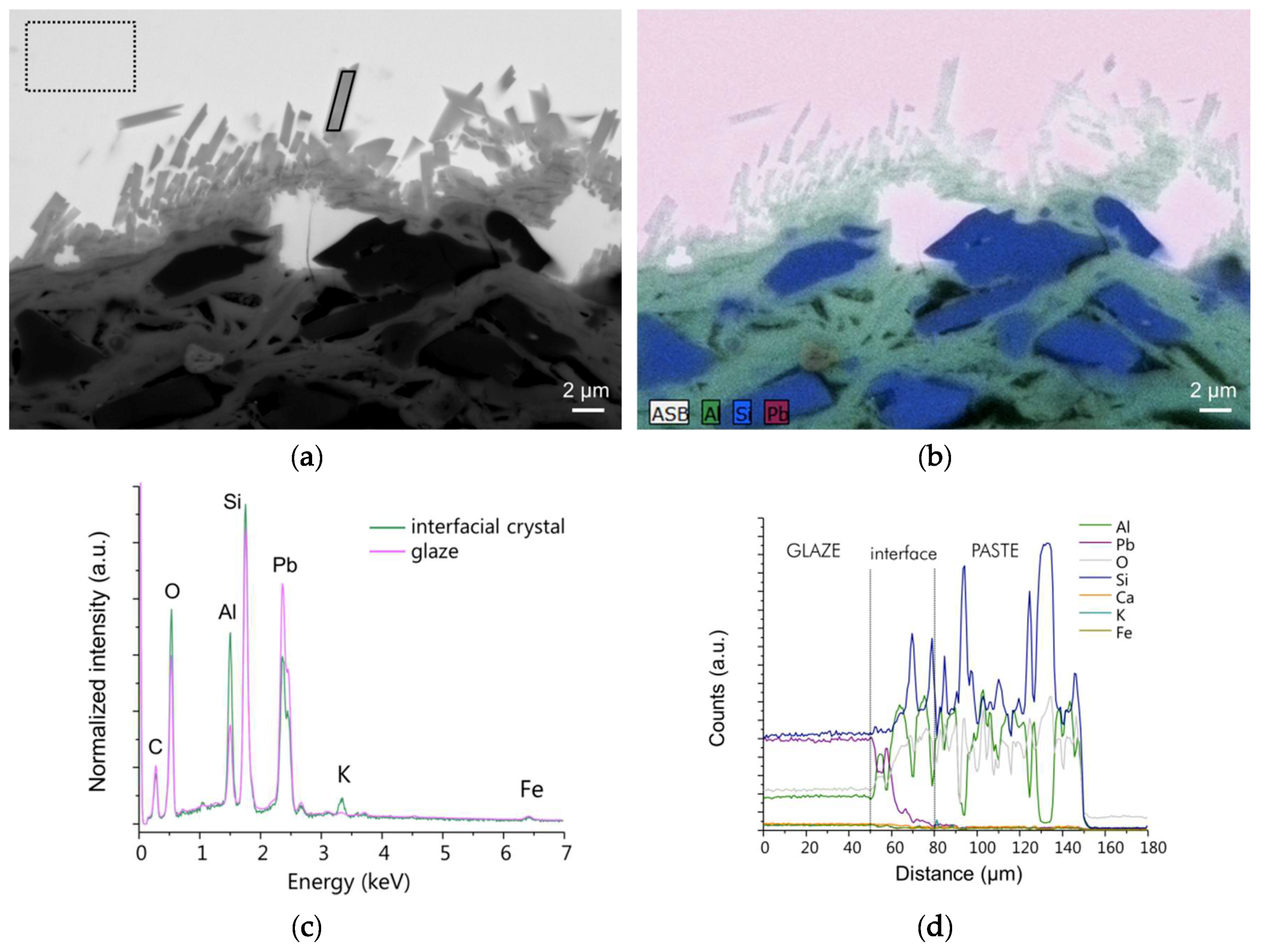
The samples were first observed using optical microscopy to examine the morphology at the sub-milliscale. They were then carbon coated and analyzed using a scanning electron microscope (SEM) FEG Zeiss Ultra55 (7–15 kV) coupled to a Bruker Quantax Energy Dispersive X-ray (EDX) detector to investigate the morphology and the qualitative chemical composition of the glaze-paste interface at the microscale. Imaging was essentially performed in backscattered electron mode (BSE). Hypermaps were acquired from which elementary maps, spectra, and profiles can be extracted afterwards.
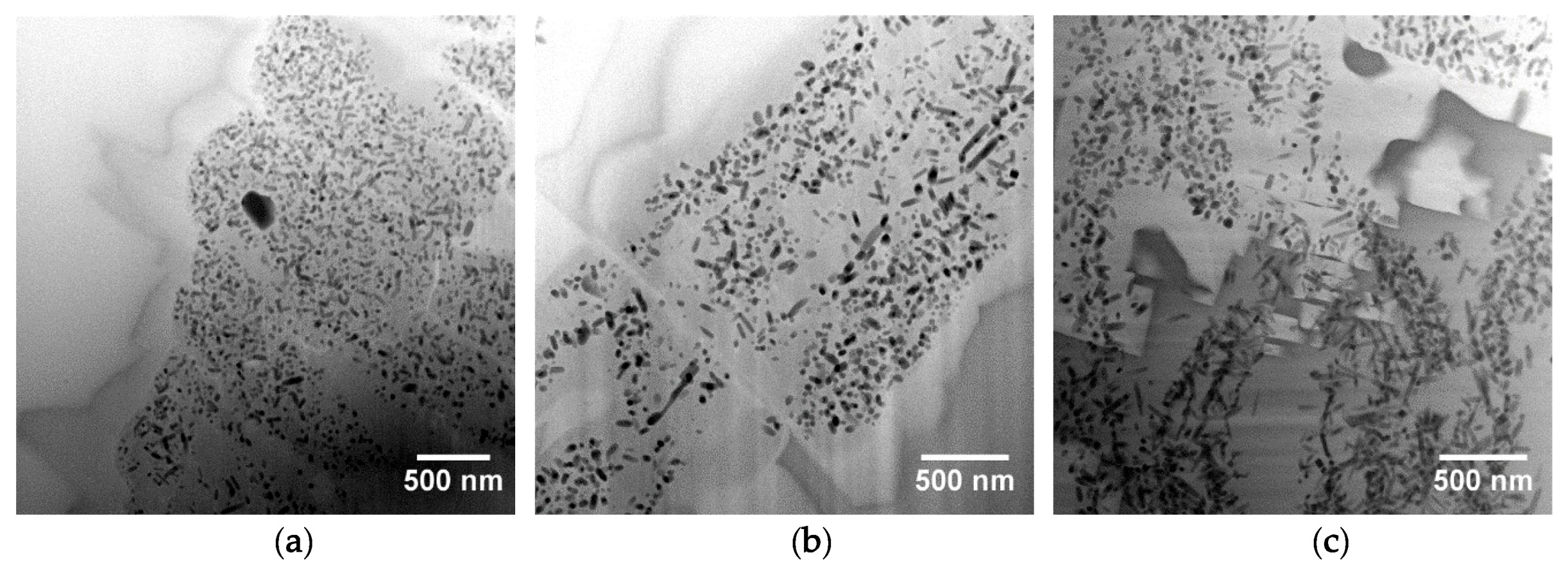
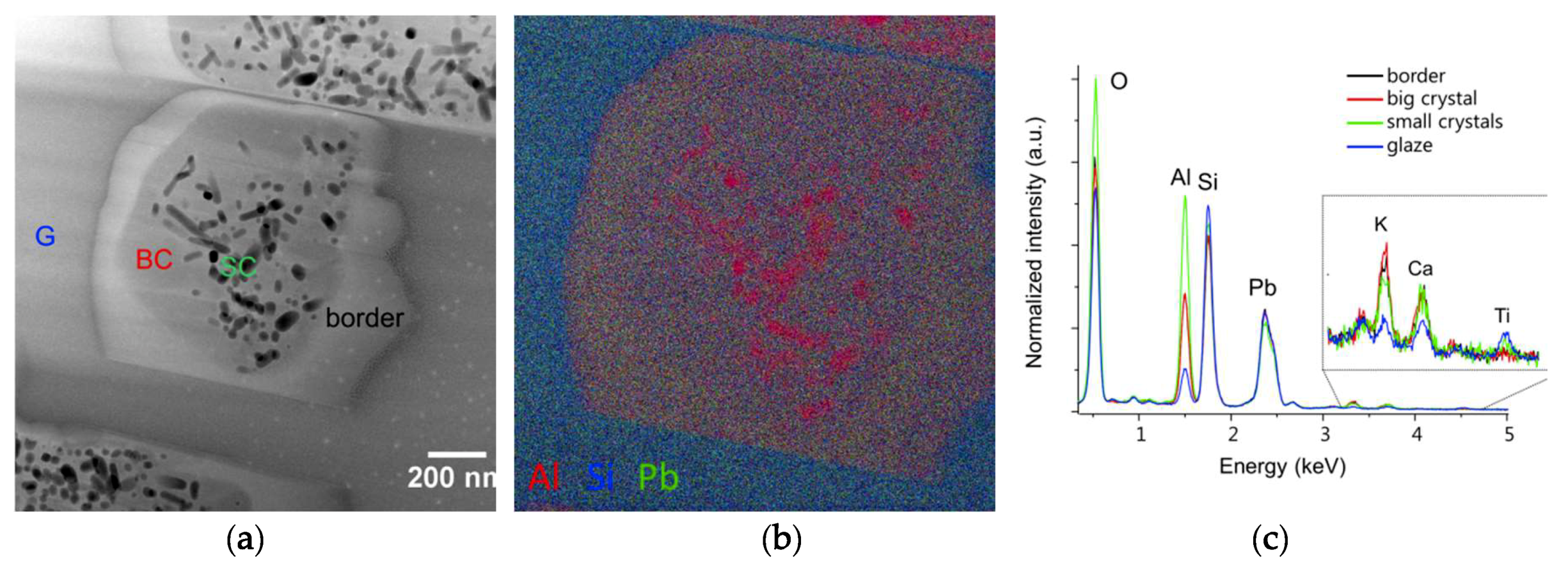
As the samples show nano-sized heterogeneities, a Focused Ion Beam (FIB) section (about 15 µm 5 µm 100 nm) was prepared in the interface area of one sample (EP2319) with a FEI Strata DB235 (IEMN) before being analyzed using a transmission electron microscope (TEM) JEOL2100F at 300 kV equipped with a scanning transmission electron microscope (STEM) device and a Si(Li) EDX detector. TEM coupled to selected area electron diffraction (SAED) and high-resolution TEM (HRTEM) imaging were performed to characterize the morphology, the crystallinity, and the structure of the interfacial compounds. STEM coupled to high angle annular dark field (HAADF) imaging (providing Z contrast images) and EDX analysis were used to examine the morphology and correlate it to the chemical composition of the interface compounds at the nanoscale. Images, SAED, and HRTEM data were treated using Image J whereas EDX hypermaps were treated using Analysis Station and Origin64 software.
In parallel, glazed ceramic replicas were elaborated taking into account the chemical compositions previously measured on a corpus of archeological honey glazed samples [
6,
7]. A kaolinitic clay originating from Provins in France (RP299) with a mineralogy very similar to those of Palissy white pastes was used to prepare these replicas (
Table 1).
Following the procedure detailed in [
6], 4 kg of clay was mixed with 0.4 kg of Fontainebleau sand (used as filler) and 6 L of water using a planetary mill for 30 min. The liquid mixture was then dried in a plaster shell before being worked and shaped into clay tiles (3
3
0.5 cm
3). The tiles were dried two weeks in the air after which they were fired at 1000 °C during 10 min (heating rate 50 °C·h
−1 until 400 °C, 100 °C·h
−1 until 1000 °C, cooling rate 50 °C·h
−1 until room temperature). A double firing procedure was chosen as Palissy was probably using this method. The paste is first fired alone (obtention of a biscuit); then glazing mixture is applied on the dried biscuit and the whole is fired a second time to produce the glazed ceramic.
Concerning the type of glazing mixture, previous examination of honey glazes replicas elaborated with either oxides powders or glass frit suggests that Palissy was most likely working with a glass frit [
6]. Indeed, glaze elaborated using oxides powders is very heterogeneous after firing: un-melted particles from the raw materials and cristobalite crystals are observed, contrary to glaze elaborated using a frit which has a more homogeneous appearance, similar to that of Palissy honey glazes. Furthermore, frit residues and crucibles were found in Palissy Parisian workshop during the 1980s excavations. Consequently, in the present study, a glass frit was elaborated by mixing γ-Al
2O
3 (99.99%, fine, Baikowski), SiO
2 (99.5%, <50 µm, Alfa Aesar), α-Fe
2O
3 (99%, <40 µm, Prolabo), and PbO (99.9%, <10 µm, Sigma Aldrich) in the proportions indicated in
Table 2 which correspond to the average of the chemical compositions measured on the archaeological honey glazes composing the corpus above mentioned [
6].
To study the effect of adding (or not) aluminum in the glazing mixture on the interface extension and microstructure, a second type of frit was elaborated by removing aluminum from the initial mix.
The powder mixtures were first fired in Pt or Pt/Au crucibles at 1050 °C for 30 min (heating rate 7 °C·h−1). Unfortunately, both melts were too viscous at that time and cannot be casted. The glass was thus re-fired for 10 min at 1200 °C. A first quench was then performed on a metallic plate at room temperature. To increase homogeneity, the glass was then roughly crushed before being replaced in the crucibles in the oven for 30 min at 1200 °C. A thin ( <40 µm) glass powder easily dispersible in water was prepared. To make the glazed ceramic replicas, 2.5 g of frit were mixed with 2.0 g of water and applied on the dried biscuits. For comparison, one sample was prepared using the frit without aluminum.
The samples were then fired at 1000 °C (heating rate 100 °C·h
−1) under different conditions. 1000 °C was chosen as an in-depth mineralogical study conducted by Wallez and Coquinot (detailed in [
6] (p. 35–37)) on Palissy pastes has demonstrated that his white pastes were fired between 950 and 1040 °C.
- -
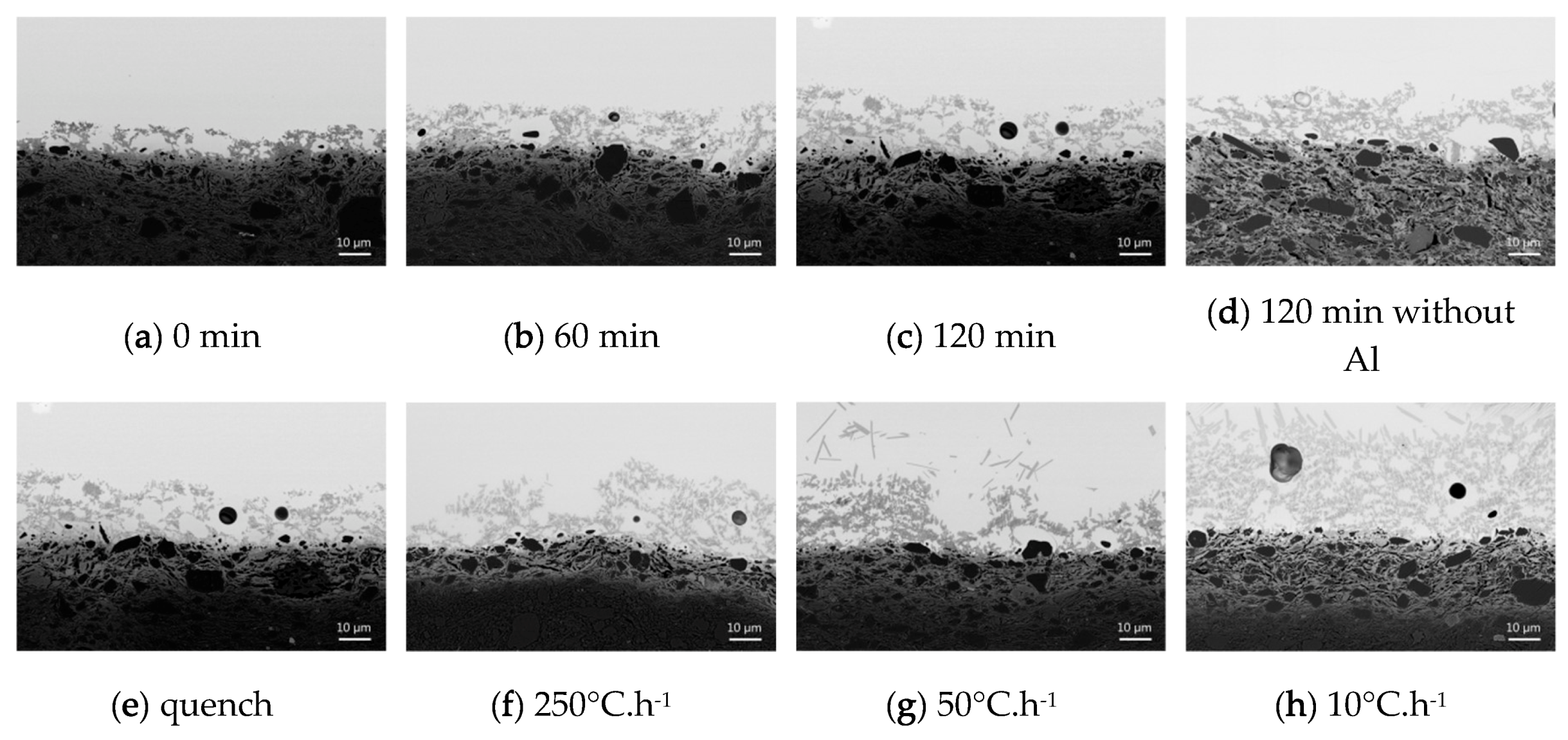
Five samples were quenched at different times (0, 10, 30, 60, 120 min) after reaching 1000 °C to examine the influence of the firing duration on the interface area.
- -
Three samples fired during 120 min were cooled using different rates to look at the influence of cooling rate on the interface area (10, 50, 250 °C·h−1 and quench). 50° C·h−1 corresponds approximatively to a slow cooling in the oven.
- -
The sample prepared using the frit without Al2O3 was fired during 120 min and slowly cooled in the oven (50 °C·h−1).
The quenching was performed in a metallic plate covered with a thin layer of water (lower than the thickness of the ceramic samples) at the bottom so that the ceramic substrates were cooled down rapidly.
After the firing process, 3 3 0.5 cm3 glazed ceramic tiles (with a honey transparent glaze covering a white body) were obtained. A millimeter-sized fragment of glazed ceramic was collected from each tile. The fragments were then ion polished using the above-mentioned facility and parameters. They were then analyzed following exactly the same procedure as the one used for archaeological samples.
4. Discussion: Comparison between Archaeological and Replicas Interfaces
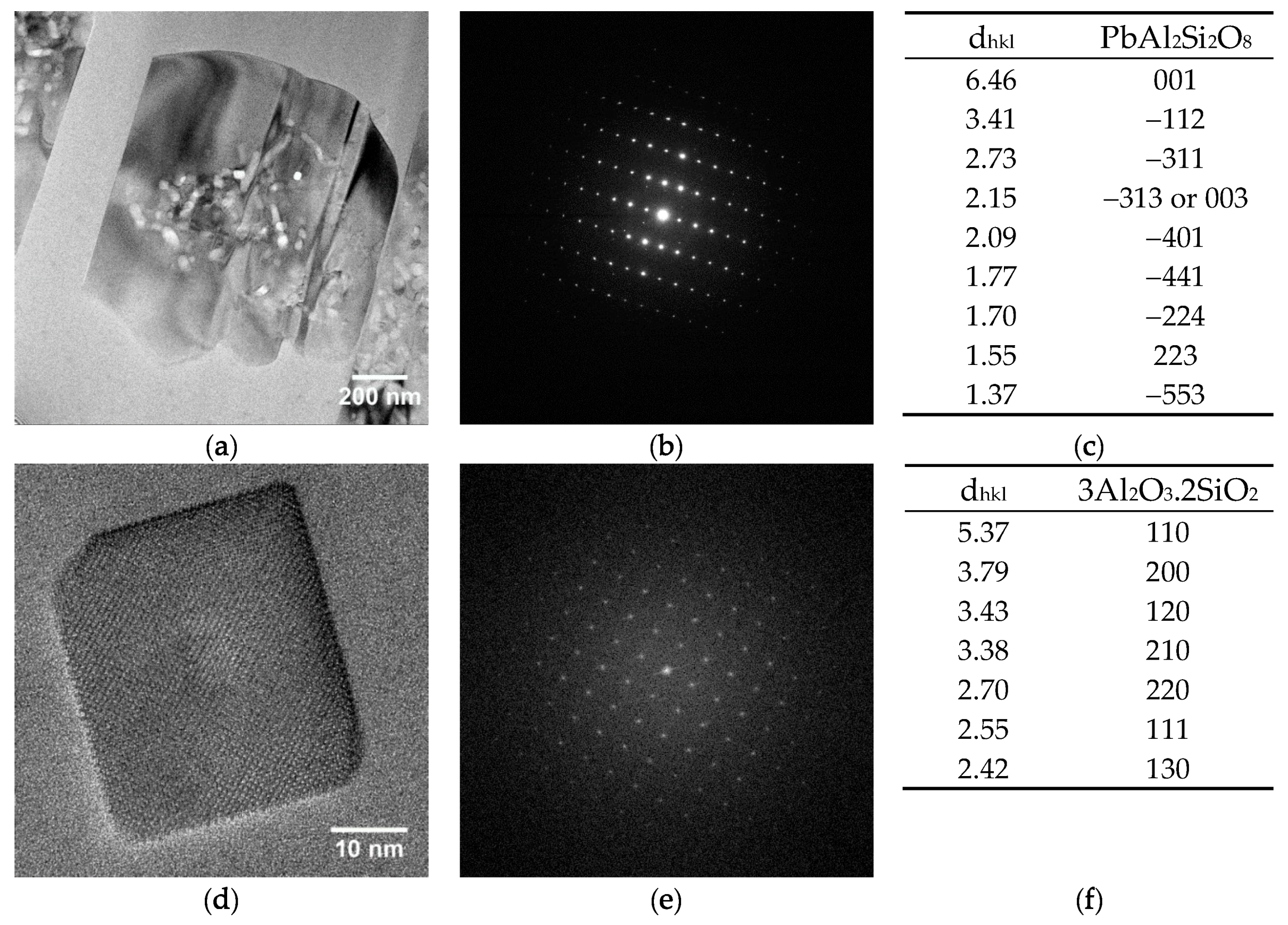
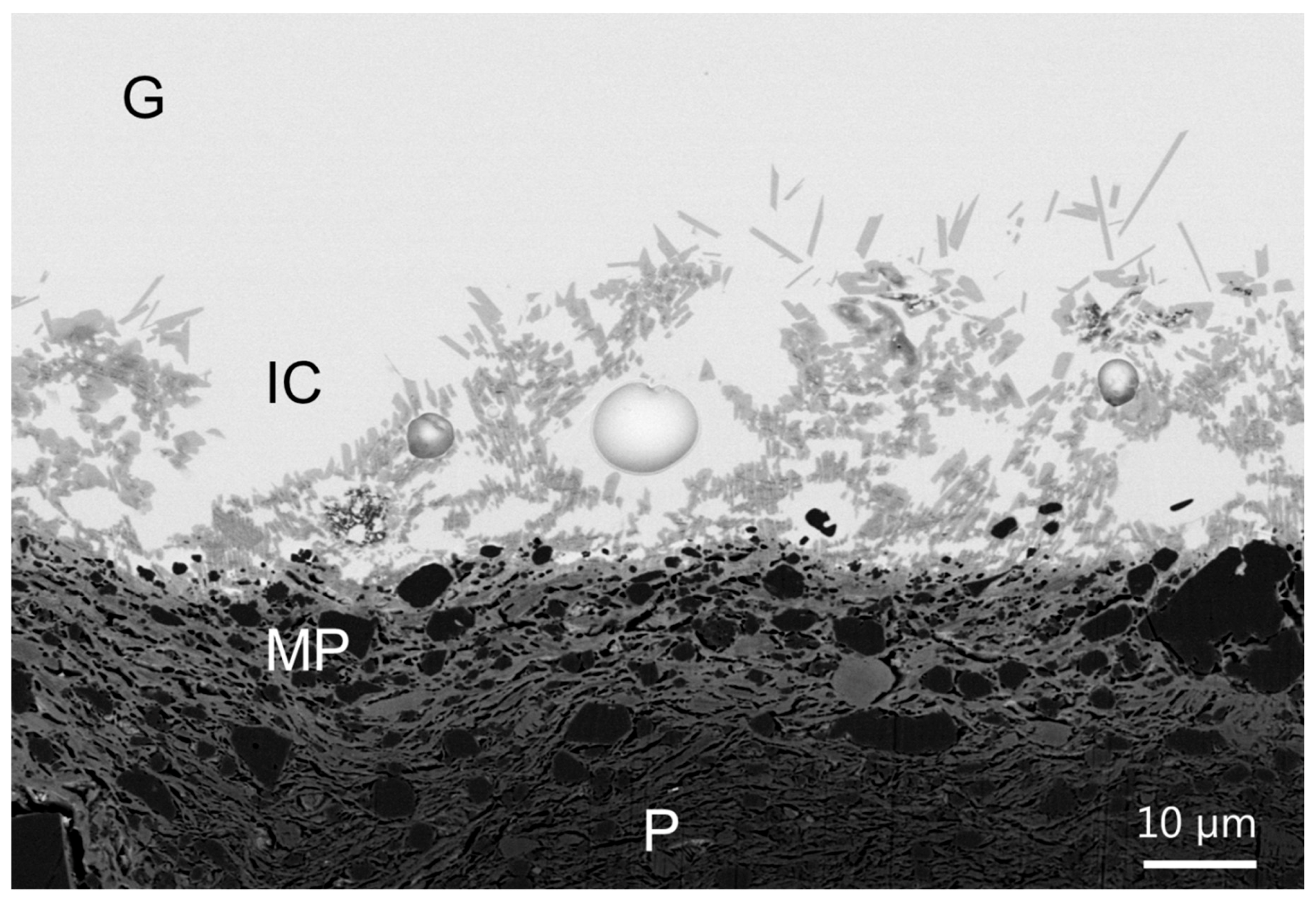
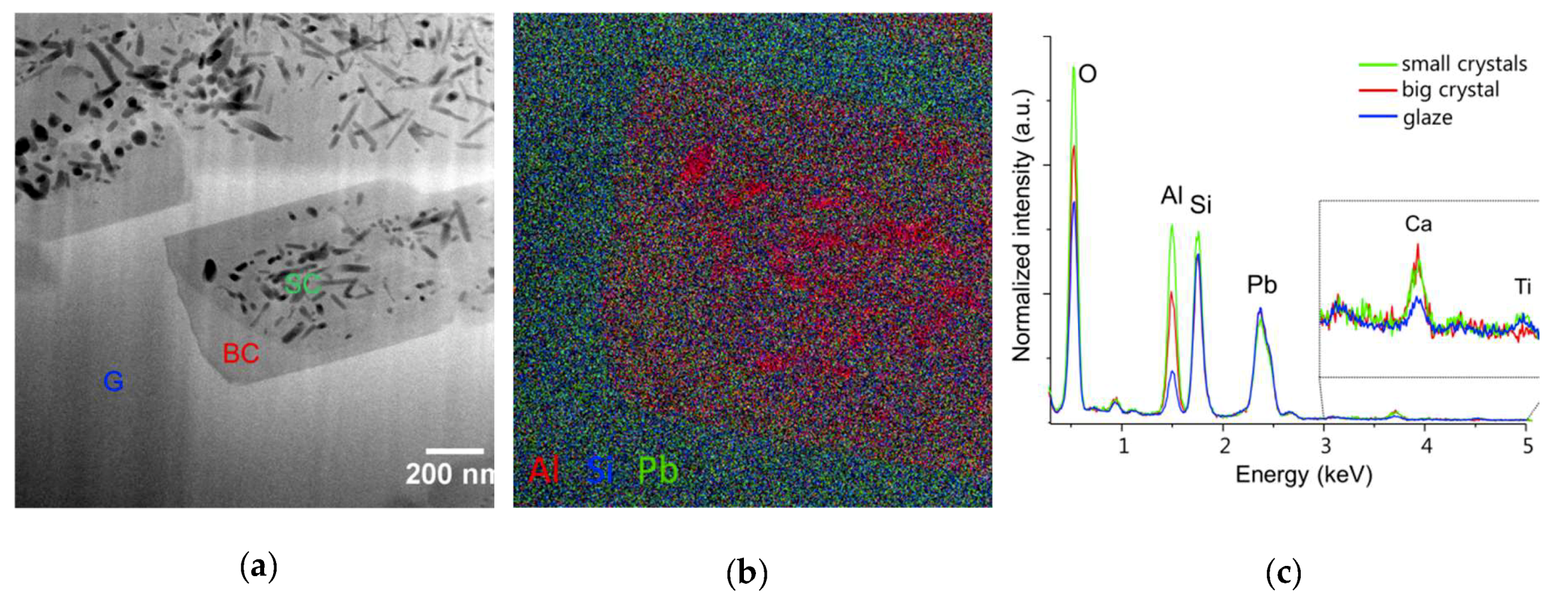
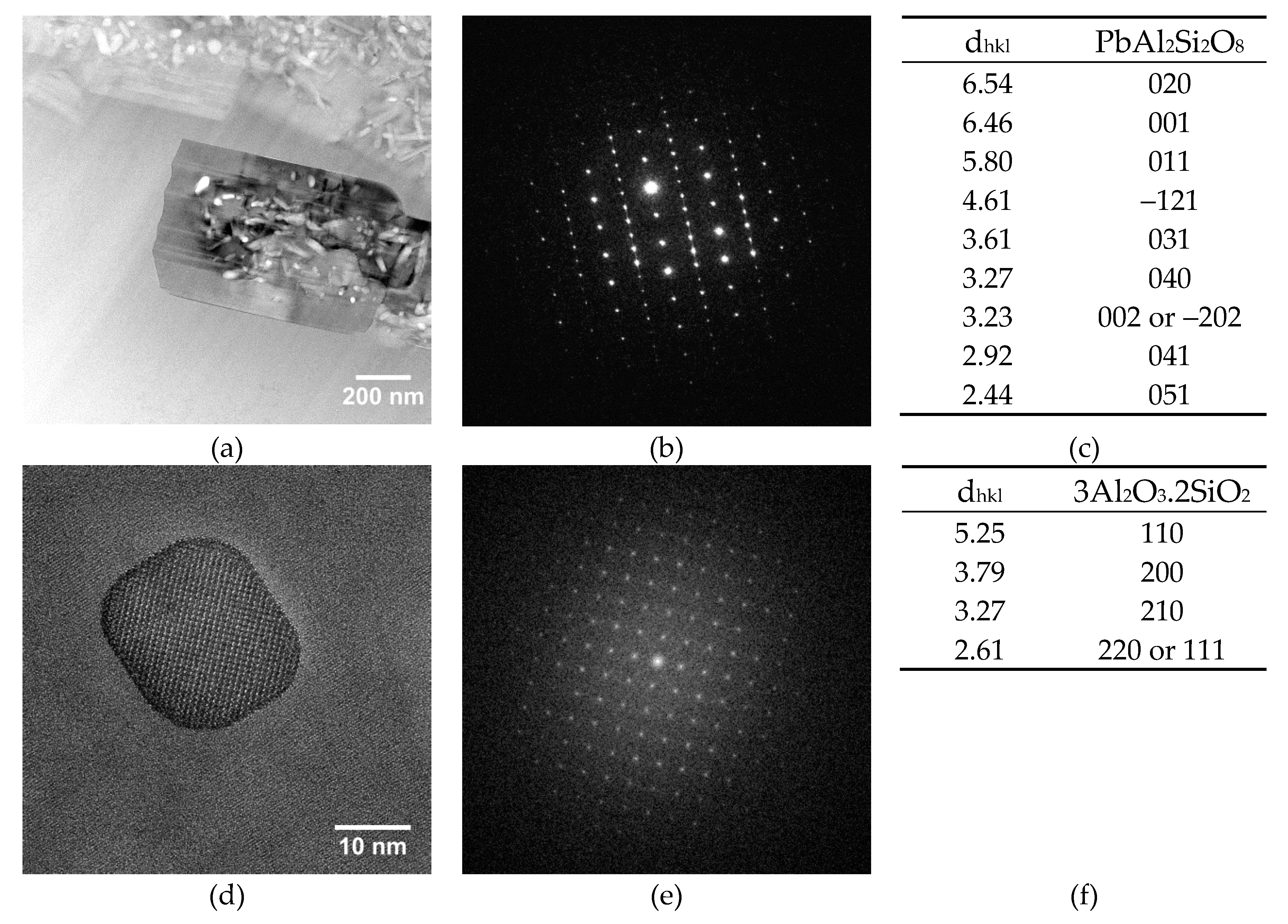
Both archaeological and replicas interfaces have comparable micro- and nano-structures. A modified paste area plus an interfacial crystals layer composed of lead feldspars monocrystals incorporating mullite monocrystals have been evidenced in both cases. The feldspars PbAl
2Si
2O
8 observed in archaeological interfaces contain small amounts of K and Ca (less than a few %
wt) and sometime Ti in trace amounts. These three elements have probably diffused from the paste during the firing and entered the feldspars crystal structure, considering their size compatibility with Pb
2+ ion (K
+ = 1.5 Å; Ca
2+ = 1.14 Å, Ti
2+ = 1 Å, and Pb
2+ = 1.33 Å). The lead feldspars observed in the replicas have the same composition except that they contain no K. This might be explained by the fact that the white paste of EP2319 sample contains slightly more K than the white paste used for the replicas (0.90 vs. 0.50 %
wt of K
2O). A similar type of feldspars of average composition (K
0.85Pb
0.12 Ca
0.03)Al
1.08 Si
2.92O
8 have been previously observed at the body-glaze interfaces of a corpus of Hispano-Moresque pottery [
18]. The interfacial crystals chemical composition is, in fact, a solid solution ranging between lead feldspars PbAl
2Si
2O
8, anorthite CaAl
2Si
2O
8, and K-feldspars KAlSi
3O
8.
Increasing the firing time and decreasing the cooling rate increase significantly the interface extension and the feldspars crystals become larger and more rectangular. These results are consistent with observations made by other authors [
9,
12] and can be explained by the fact that by staying longer at high temperature, the melt is less viscous. Thus, it penetrates more into the paste and crystallization process is promoted. The mullite crystals size seems not affected by these parameters’ modification. This tends to indicate that at least a part of the mullite crystals incorporated inside the feldspars are originating from the body [
6], rather than being newly formed compounds crystallizing during the thermal process.
Removing alumina from the glazing mixture induces an opaque and matte visual appearance linked to the crystallization of trydimite in the glaze. Furthermore, it increases significantly the modified paste extension to about 100 µm. Yet Palissy honey glazes (on white body) always have a transparent and brilliant aspect with no crystallization, and the interfaces are always measuring between 10–30 µm thick (in particular the modified paste area is about 10 µm). The ceramist was thus very probably adding intentionally Al into the glazing mixture (in the form of clay), knowing that the presence of Al increases the melt viscosity and thus reduces any potential crystallization process in the glaze [
23]. Another argument in favor of this hypothesis is that the amount of Al
2O
3 measured in different colored glazes (including yellow, grey, green, brown, etc.) decorating two authenticated Rustiques Figulines was found to be varying between 0.8 to 11%
wt, depending on the color [
5]. This result reinforces the hypothesis that the ceramist was controlling very precisely the amount of Al added in the glazing mixture, or else it would have been in the same order of magnitude on the whole object (only diffusing from the paste).
If we suppose that Palissy was working at 1000 °C and consider the interface extension, we can deduce that he was probably working with a firing time of 1 h. However, hematite crystallizations stable only between 915 and 950 °C were recently evidenced in some of Palissy honey iron-rich glazes (3.2–4.9 %
wt Fe
2O
3) applied on red pastes [
6]. This result indicates that Palissy may have also worked with a temperature lower than 1000 °C. In addition, preliminary tests conducted on a replica fired at 900 °C and quenched immediately revealed an interface extension of about 2–3 µm using SEM. Therefore, if Palissy was working at lower temperatures than 1000 °C, he was probably using a firing time longer than 1 h which constitutes quite a technical achievement at his time. The cooling in the oven was then leading to the growth of an interface of 10–30 µm with rectangular sharp-edged feldspars crystals.
5. Conclusions
Bernard Palissy white body-honey glaze interface areas (10–30 µm thick) were characterized down to the nanoscale: a modified paste region on which interfacial sharp-edged microcrystals are growing have been observed. These crystals have been identified as lead feldspars microcrystals (Pbx, Cay, Tiz, K1-x-y-z)Al2Si2O8 incorporating mullite 3Al2O3.2SiO2 nanocrystals.
Replica samples fired at 1000 °C show different interface extensions and crystal shapes depending on the elaboration conditions used. The importance of adding Al to the glaze mixture was demonstrated since otherwise an opaque matte glaze was produced and the interface extension was much wider (~100 µm) than the archaeological ones. Besides, it was found that longer firing times and slower cooling rates lead to wider interfaces and more well-formed sharp-edged crystals. At 1000 °C, a firing time of 1 h followed by a cooling in the oven (50 °C·h−1) lead to an interface extension and microstructure comparable to those of Palissy samples.
Finally, from a methodological point of view, the multi-scale analytical procedure including TEM and associated techniques has proved its worth. It has now to be applied for the investigation of others colored glazes and pastes to gain a deeper understanding of the elaboration conditions used in Palissy workshop.