Study of Influential Parameters of the Caffeine Extraction from Spent Coffee Grounds: From Brewing Coffee Method to the Waste Treatment Conditions
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Plant Material
2.3. Storage of Spent Coffee Grounds (SCG)
2.4. Solid/Liquid Extraction—General Protocol
2.5. Liquid/Liquid Extraction–General Protocol
2.6. HPLC-UV Analysis
2.7. Statistical Analysis
3. Results and Discussion
3.1. Influence of Brewing Methods
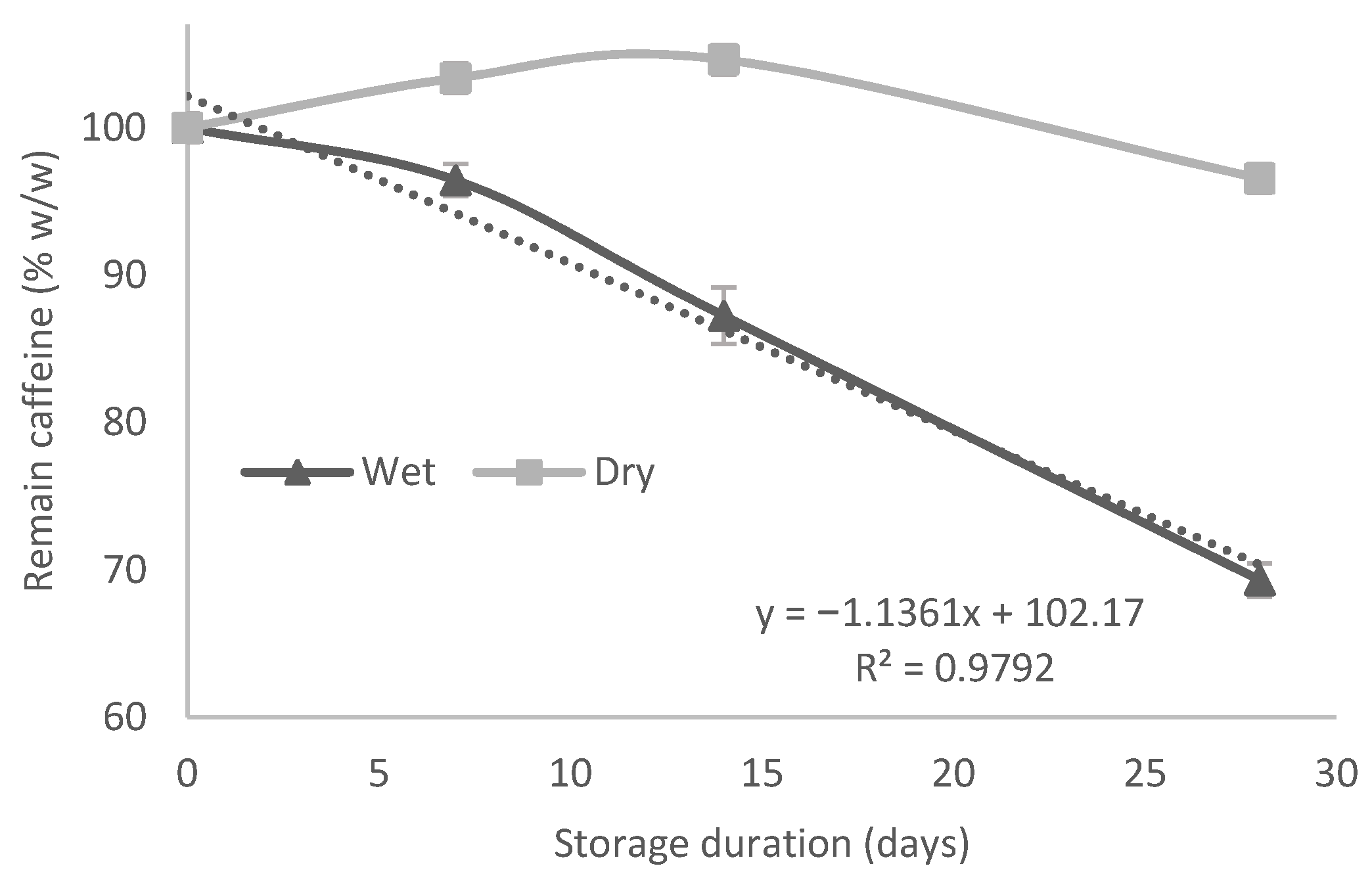
3.2. Influence of Spent Coffee Grounds Storage
3.3. Solid/Liquid Extraction Optimization
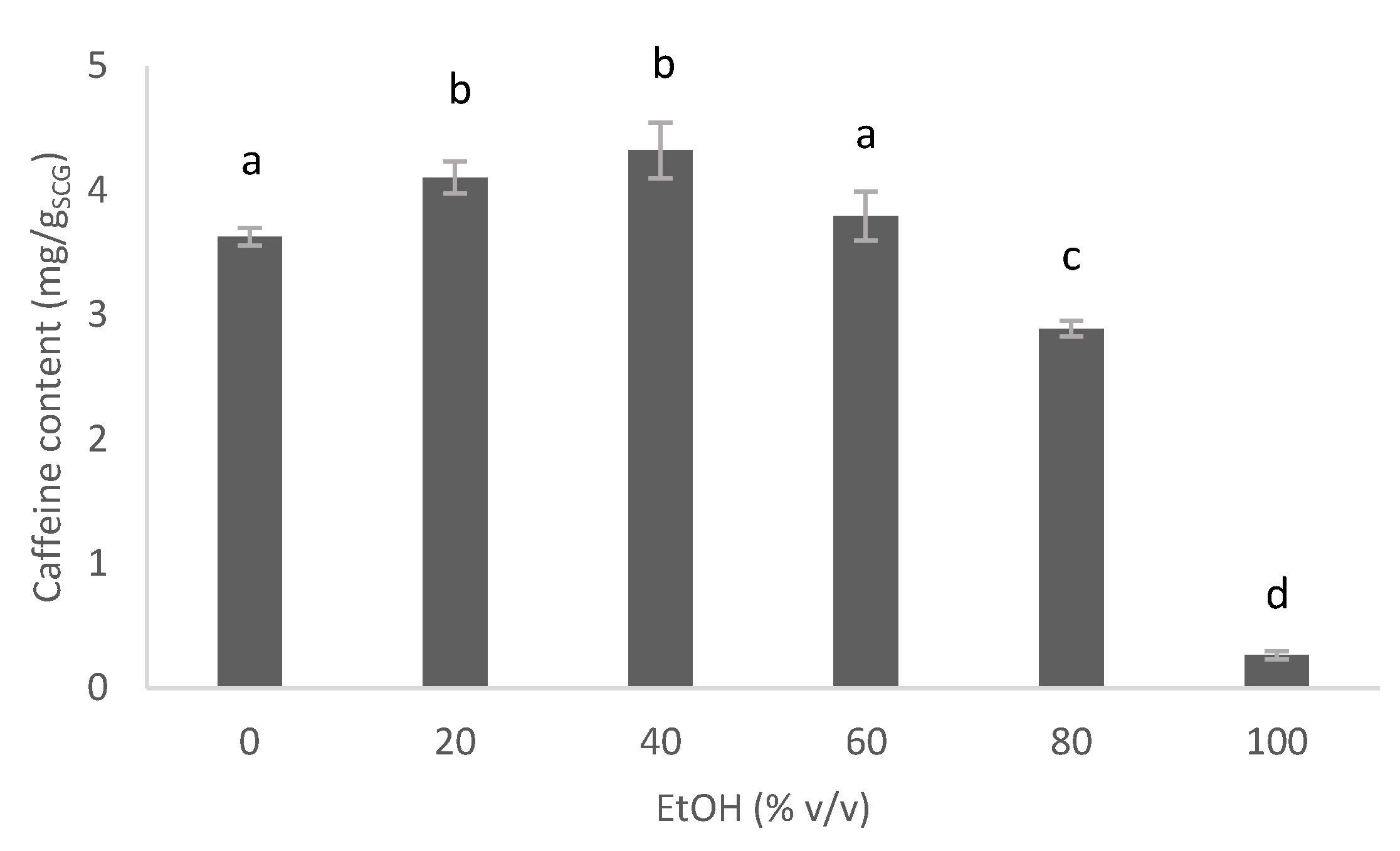
3.3.1. Influence of the Nature of the Solvent
3.3.2. Influence of the Extraction Temperature
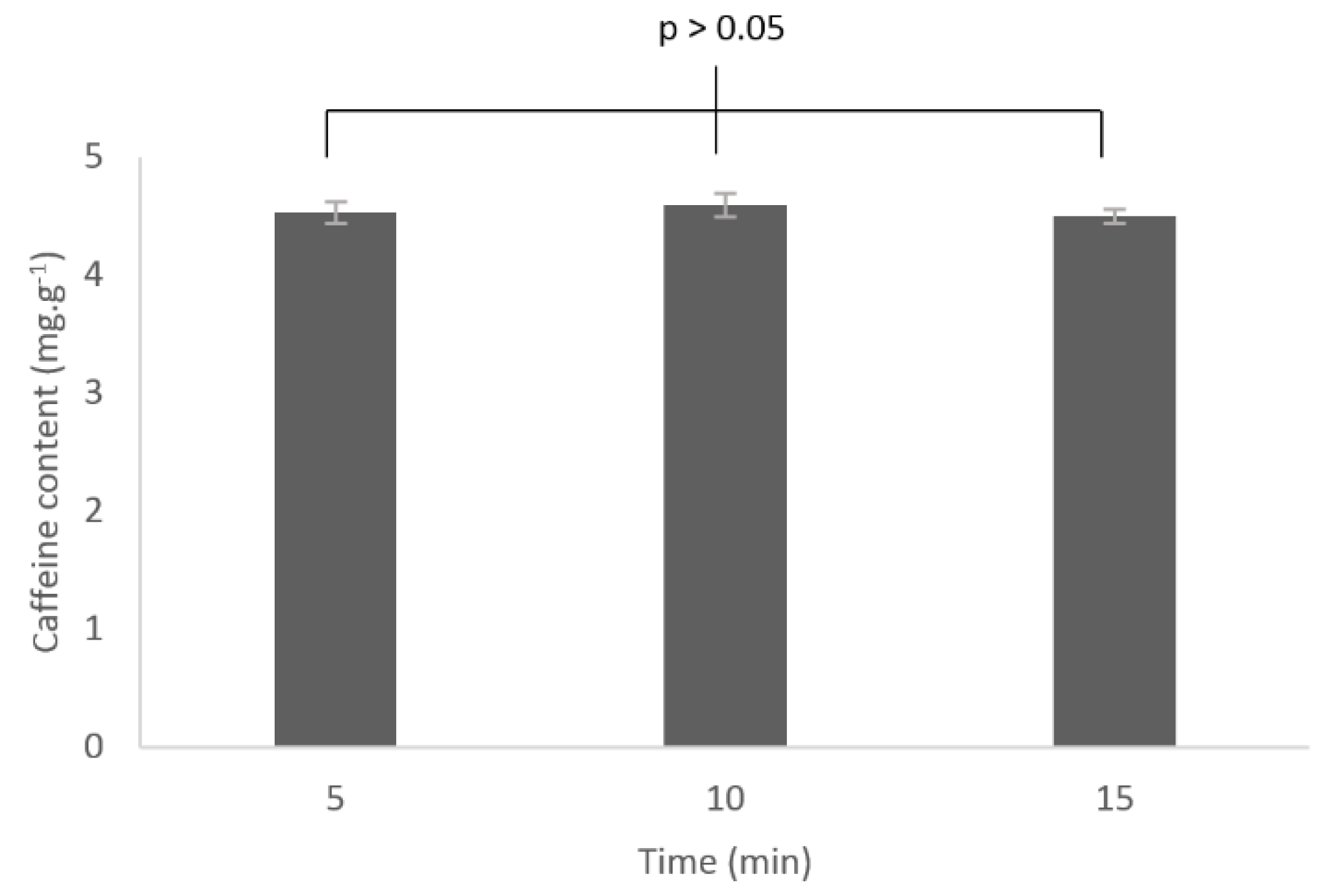
3.3.3. Influence of the Extraction Time
3.3.4. Influence of the Solid/Liquid Ratio
3.4. Purification of Caffeine by Liquid/Liquid Extraction
3.4.1. Influence of Different Organic Solvents for Liquid/Liquid Extraction of Caffeine
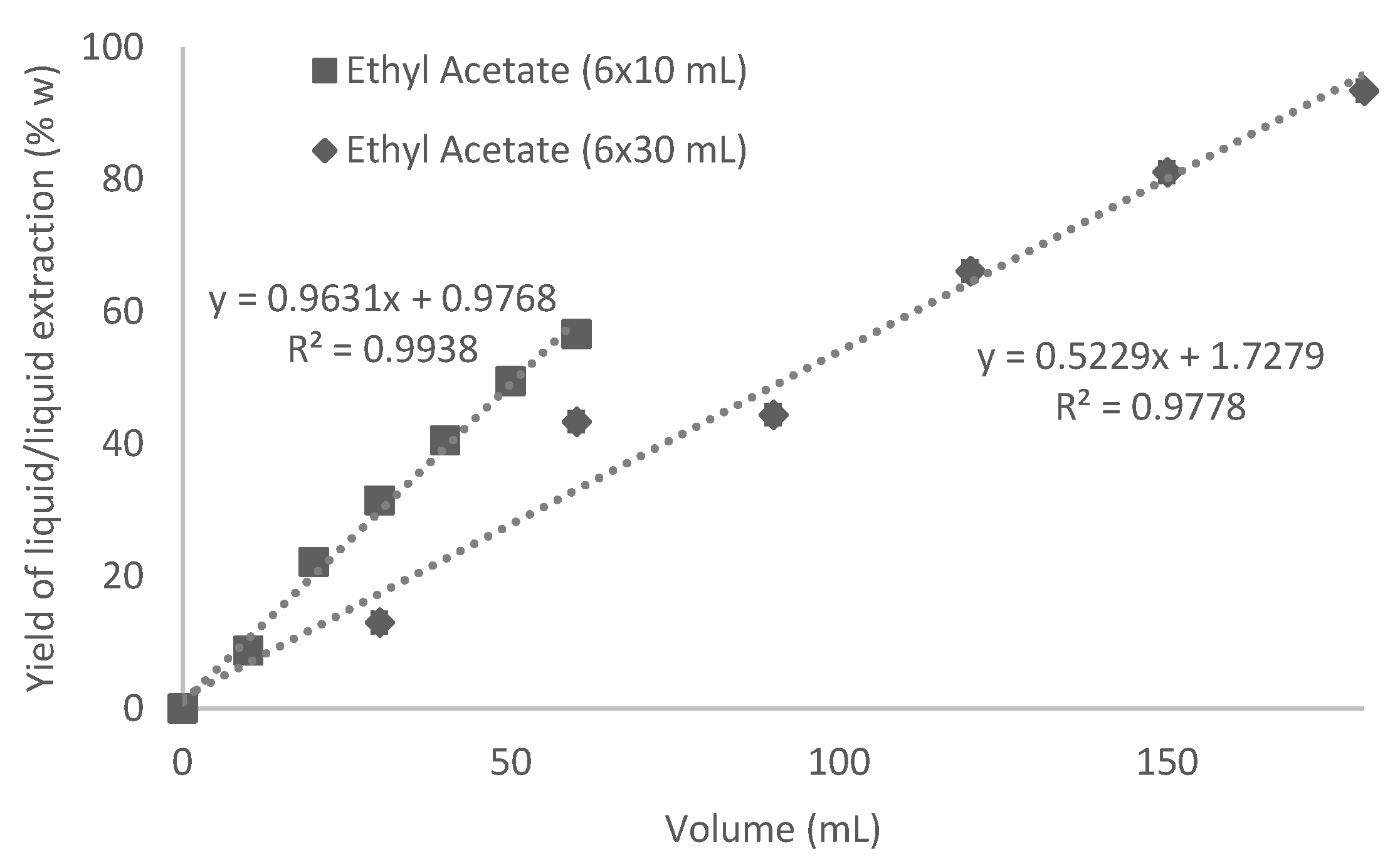
3.4.2. Influence of the Volume of Extraction Solvent
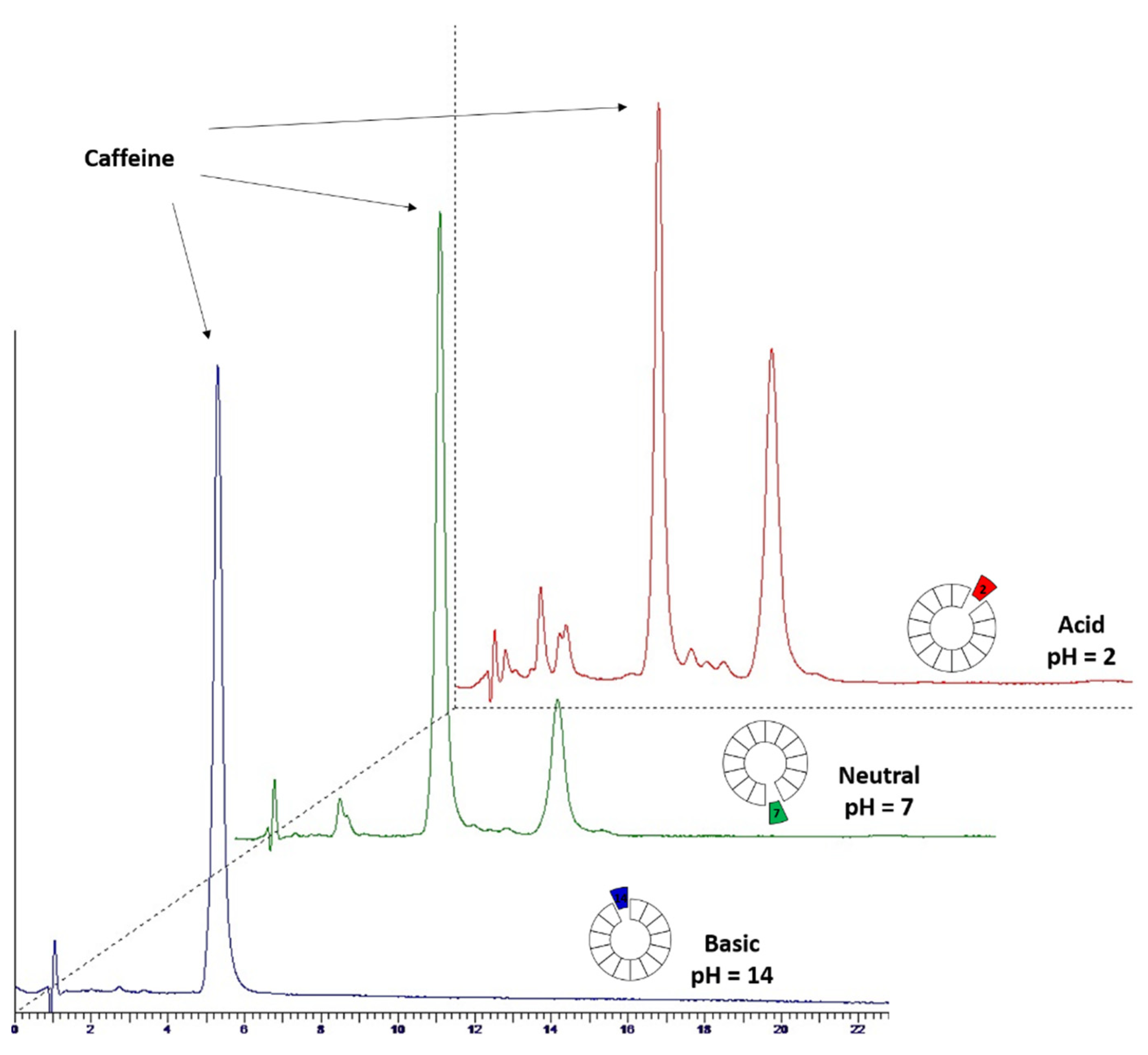
3.4.3. Influence of Aqueous Extract pH
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- International Coffee Organization. Available online: http://www.ico.org/ (accessed on 4 October 2020).
- Pfluger, R.A. Soluble coffee processing. In Solid Wastes Origin Collection Processing & Disposal. Cl Mantell; Wiley: New York, NY, USA, 1975. [Google Scholar]
- Liu, Y.; Tu, Q.; Knothe, G.; Lu, M. Direct transesterification of spent coffee grounds for biodiesel production. Fuel 2017, 199, 157–161. [Google Scholar] [CrossRef]
- Caetano, N.S.; Silva, V.F.; Mata, T.M. Valorization of coffee grounds for biodiesel production. Chem. Eng. Trans. 2012, 26. [Google Scholar] [CrossRef]
- Kwon, E.E.; Yi, H.; Jeon, Y.J. Sequential co-production of biodiesel and bioethanol with spent coffee grounds. Bioresour. Technol. 2013, 136, 475–480. [Google Scholar] [CrossRef] [PubMed]
- Choi, I.S.; Wi, S.G.; Kim, S.-B.; Bae, H.-J. Conversion of coffee residue waste into bioethanol with using popping pretreatment. Bioresour. Technol. 2012, 125, 132–137. [Google Scholar] [CrossRef] [PubMed]
- Obruca, S.; Benesova, P.; Petrik, S.; Oborna, J.; Prikryl, R.; Marova, I. Production of polyhydroxyalkanoates using hydrolysate of spent coffee grounds. Process Biochem. 2014, 49, 1409–1414. [Google Scholar] [CrossRef]
- Cruz, M.V.; Paiva, A.; Lisboa, P.; Freitas, F.; Alves, V.D.; Simões, P.; Barreiros, S.; Reis, M.A. Production of polyhydroxyalkanoates from spent coffee grounds oil obtained by supercritical fluid extraction technology. Bioresour. Technol. 2014, 157, 360–363. [Google Scholar] [CrossRef] [PubMed]
- Plaza, M.; González, A.; Pevida, C.; Pis, J.; Rubiera, F. Valorisation of spent coffee grounds as CO2 adsorbents for postcombustion capture applications. Appl. Energy 2012, 99, 272–279. [Google Scholar] [CrossRef]
- Cerino-Córdova, F.; Díaz-Flores, P.; García-Reyes, R.; Soto-Regalado, E.; Gómez-González, R.; Garza-González, M.; Bustamante-Alcántara, E. Biosorption of Cu (II) and Pb (II) from aqueous solutions by chemically modified spent coffee grains. Int. J. Environ. Sci. Technol. 2013, 10, 611–622. [Google Scholar] [CrossRef]
- Acevedo, F.; Rubilar, M.; Scheuermann, E.; Cancino, B.; Uquiche, E.; Garcés, M.; Inostroza, K.; Shene, C. Spent coffee grounds as a renewable source of bioactive compounds. J. Biobased Mater. Bioenergy 2013, 7, 420–428. [Google Scholar] [CrossRef]
- Magalhães, L.M.; Machado, S.; Segundo, M.A.; Lopes, J.A.; Páscoa, R.N. Rapid assessment of bioactive phenolics and methylxanthines in spent coffee grounds by FT-NIR spectroscopy. Talanta 2016, 147, 460–467. [Google Scholar] [CrossRef]
- Ribeiro, H.M.; Allegro, M.; Marto, J.; Pedras, B.; Oliveira, N.G.; Paiva, A.; Barreiros, S.; Gonçalves, L.D.M.; Simões, P. Converting spent coffee grounds into bioactive extracts with potential skin antiaging and lightening effects. Acs Sustain. Chem. Eng. 2018, 6, 6289–6295. [Google Scholar] [CrossRef]
- Scully, D.; Jaiswal, A.; Abu-Ghannam, N. An investigation into spent coffee waste as a renewable source of bioactive compounds and industrially important sugars. Bioengineering 2016, 3, 33. [Google Scholar] [CrossRef] [PubMed]
- Bravo, J.; Monente, C.; Juániz, I.; De Peña, M.P.; Cid, C. Influence of extraction process on antioxidant capacity of spent coffee. Food Res. Int. 2013, 50, 610–616. [Google Scholar] [CrossRef]
- Andrade, K.S.; Gonçalvez, R.T.; Maraschin, M.; Ribeiro-do-Valle, R.M.; Martínez, J.; Ferreira, S.R. Supercritical fluid extraction from spent coffee grounds and coffee husks: Antioxidant activity and effect of operational variables on extract composition. Talanta 2012, 88, 544–552. [Google Scholar] [CrossRef]
- Mussatto, S.I.; Ballesteros, L.F.; Martins, S.; Teixeira, J.A. Extraction of antioxidant phenolic compounds from spent coffee grounds. Sep. Purif. Technol. 2011, 83, 173–179. [Google Scholar] [CrossRef]
- Panusa, A.; Zuorro, A.; Lavecchia, R.; Marrosu, G.; Petrucci, R. Recovery of natural antioxidants from spent coffee grounds. J. Agric. Food Chem. 2013, 61, 4162–4168. [Google Scholar] [CrossRef]
- Getachew, A.T.; Cho, Y.J.; Chun, B.S. Effect of pretreatments on isolation of bioactive polysaccharides from spent coffee grounds using subcritical water. Int. J. Biol. Macromol. 2018, 109, 711–719. [Google Scholar] [CrossRef]
- Ballesteros, L.F.; Teixeira, J.A.; Mussatto, S.I. Extraction of polysaccharides by autohydrolysis of spent coffee grounds and evaluation of their antioxidant activity. Carbohydr. Polym. 2017, 157, 258–266. [Google Scholar] [CrossRef]
- Vandeponseele, A.; Draye, M.; Piot, C.; Chatel, G. Subcritical water and supercritical carbon dioxide: Efficient and selective eco-compatible solvents for coffee and coffee by-products valorization. Green Chem. 2020, 22, 8544–8571. [Google Scholar] [CrossRef]
- Juliano, L.M.; Griffiths, R.R. A critical review of caffeine withdrawal: Empirical validation of symptoms and signs, incidence, severity, and associated features. Psychopharmacology 2004, 176, 1–29. [Google Scholar] [CrossRef]
- McCusker, R.R.; Goldberger, B.A.; Cone, E.J. Caffeine content of energy drinks, carbonated sodas, and other beverages. J. Anal. Toxicol. 2006, 30, 112–114. [Google Scholar] [CrossRef] [PubMed]
- Renner, B.; Clarke, G.; Grattan, T.; Beisel, A.; Mueller, C.; Werner, U.; Kobal, G.; Brune, K. Caffeine accelerates absorption and enhances the analgesic effect of acetaminophen. J. Clin. Pharmacol. 2007, 47, 715–726. [Google Scholar] [CrossRef] [PubMed]
- Byun, S.-Y.; Kwon, S.-H.; Heo, S.-H.; Shim, J.-S.; Du, M.-H.; Na, J.-I. Efficacy of slimming cream containing 3.5% water-soluble caffeine and xanthenes for the treatment of cellulite: Clinical study and literature review. Ann. Dermatol. 2015, 27, 243–249. [Google Scholar] [CrossRef] [PubMed]
- Pietsch, A. Decaffeination—Process and Quality. In The Craft and Science of Coffee; Elsevier: Amsterdam, The Netherlands, 2017; pp. 225–243. [Google Scholar]
- Ramalakshmi, K.; Raghavan, B. Caffeine in coffee: Its removal. Why and how? Crit. Rev. Food Sci. Nutr. 1999, 39, 441–456. [Google Scholar] [CrossRef]
- Srdjenovic, B.; Djordjevic-Milic, V.; Grujic, N.; Injac, R.; Lepojevic, Z. Simultaneous HPLC determination of caffeine, theobromine, and theophylline in food, drinks, and herbal products. J. Chromatogr. Sci. 2008, 46, 144–149. [Google Scholar] [CrossRef]
- McCusker, R.R.; Goldberger, B.A.; Cone, E.J. Caffeine content of specialty coffees. J. Anal. Toxicol. 2003, 27, 520–522. [Google Scholar] [CrossRef] [PubMed]
- Caprioli, G.; Cortese, M.; Sagratini, G.; Vittori, S. The influence of different types of preparation (espresso and brew) on coffee aroma and main bioactive constituents. Int. J. Food Sci. Nutr. 2015, 66, 505–513. [Google Scholar] [CrossRef]
- Angeloni, G.; Guerrini, L.; Masella, P.; Bellumori, M.; Daluiso, S.; Parenti, A.; Innocenti, M. What kind of coffee do you drink? An investigation on effects of eight different extraction methods. Food Res. Int. 2019, 116, 1327–1335. [Google Scholar] [CrossRef]
- Ravindran, R.; Jaiswal, S.; Abu-Ghannam, N.; Jaiswal, A.K. Two-step sequential pretreatment for the enhanced enzymatic hydrolysis of coffee spent waste. Bioresour. Technol. 2017, 239, 276–284. [Google Scholar] [CrossRef]
- Vakalis, S.; Moustakas, K.; Benedetti, V.; Cordioli, E.; Patuzzi, F.; Loizidou, M.; Baratieri, M. The “COFFEE BIN” concept: Centralized collection and torrefaction of spent coffee grounds. Environ. Sci. Pollut. Res. 2019, 26, 35473–35481. [Google Scholar] [CrossRef]
- Batista, L.R.; Chalfoun, S.M.; Silva, C.F.; Cirillo, M.; Varga, E.A.; Schwan, R.F. Ochratoxin A in coffee beans (Coffea arabica L.) processed by dry and wet methods. Food Control 2009, 20, 784–790. [Google Scholar] [CrossRef]
- Dash, S.S.; Gummadi, S.N. Catabolic pathways and biotechnological applications of microbial caffeine degradation. Biotechnol. Lett. 2006, 28, 1993–2002. [Google Scholar] [CrossRef] [PubMed]
- Jian, K.; Tong, W.; Hongyuan, Z.; Leilei, S. The extraction and mass transfer process of soluble solids in Russian olive. Afr. J. Plant Sci. 2013, 7, 407–413. [Google Scholar] [CrossRef][Green Version]
- Fick, A.V. On liquid diffusion. Lond. Edinb. Dublin Philos. Mag. J. Sci. 1855, 10, 30–39. [Google Scholar] [CrossRef]
- Santana, Á.L.; Macedo, G.A. Effects of hydroalcoholic and enzyme-assisted extraction processes on the recovery of catechins and methylxanthines from crude and waste seeds of guarana (Paullinia cupana). Food Chem. 2019, 281, 222–230. [Google Scholar] [CrossRef] [PubMed]
- Bustamante, P.; Navarro, J.; Romero, S.; Escalera, B. Thermodynamic origin of the solubility profile of drugs showing one or two maxima against the polarity of aqueous and nonaqueous mixtures: Niflumic acid and caffeine. J. Pharm. Sci. 2002, 91, 874–883. [Google Scholar] [CrossRef] [PubMed]
- Zosel, K. Process for the Decaffeination of Coffee. U.S. Patent 42,606,39A, 7 April 1981. [Google Scholar]
- Todd, R.; Baroutian, S. A techno-economic comparison of subcritical water, supercritical CO2 and organic solvent extraction of bioactives from grape marc. J. Clean. Prod. 2017, 158, 349–358. [Google Scholar] [CrossRef]
- Shalmashi, A.; Golmohammad, F. Solubility of caffeine in water, ethyl acetate, ethanol, carbon tetrachloride, methanol, chloroform, dichloromethane, and acetone between 298 and 323 K. Lat. Am. Appl. Res. 2010, 40, 283. [Google Scholar]
- Sondheimer, E.; Covitz, F.; Marquisee, M.J. Association of naturally occurring compounds, the chlorogenic acid-caffeine complex. Arch. Biochem. Biophys. 1961, 93, 63–71. [Google Scholar] [CrossRef]
- Linares, A.R.; Hase, S.L.; Vergara, M.L.; Resnik, S.L. Modeling yerba mate aqueous extraction kinetics: Influence of temperature. J. Food Eng. 2010, 97, 471–477. [Google Scholar] [CrossRef]
- Shalmashi, A.; Abedi, M.; Golmohammad, F.; Eikani, M.H. Isolation of caffeine from tea waste using subcritical water extraction. J. Food Process Eng. 2010, 33, 701–711. [Google Scholar] [CrossRef]
- Deb, S.; Pou, K.J. A review of withering in the processing of black tea. J. Biosyst. Eng. 2016, 41, 365–372. [Google Scholar] [CrossRef]
- Bi, W.; Zhou, J.; Row, K.H. Decaffeination of coffee bean waste by solid-liquid extraction. Korean J. Chem. Eng. 2011, 28, 221–224. [Google Scholar] [CrossRef]
- Gerke, I.B.B.; Hamerski, F.; de Paula Scheer, A.; da Silva, V.R. Solid–liquid extraction of bioactive compounds from yerba mate (Ilex paraguariensis) leaves: Experimental study, kinetics and modeling. J. Food Process Eng. 2018, 41, e12892. [Google Scholar] [CrossRef]
- Hanson, C. Recent Advances in Liquid-Liquid Extraction; Elsevier: Amsterdam, The Netherlands, 2013. [Google Scholar]
- Lide, D. CRC Handbook of Chemistry and Physics: A Ready Reference Book; CRC Press: Boca Raton, FL, USA, 2002. [Google Scholar]
- Hildebrand, S. Standard Hildebrand values from Hansen. J. Paint Technol. 1967, 39, 505. [Google Scholar]
- Fedors, R.; Van Krevelen, D.; Hoftyzer, P.; Barton, A.C. Handbook of Solubility Parameters and Other Cohesion Parameters; Barton, A.F.M., Ed.; CRC Press: Boca Raton, FL, USA, 1983. [Google Scholar]
- Adjei, A.; Newburger, J.; Martin, A. Extended Hildebrand approach: Solubility of caffeine in dioxane–water mixtures. J. Pharm. Sci. 1980, 69, 659–661. [Google Scholar] [CrossRef]
- Mohammed, M.J.; Al-Bayati, F.A. Isolation, identification and purification of caffeine from Coffea arabica L. and Camellia sinensis L.: A combination antibacterial study. Int. J. Green Pharm. IJGP 2009, 3. [Google Scholar] [CrossRef]
- Ramón-Gonçalves, M.; Alcaraz, L.; Pérez-Ferreras, S.; León-González, M.E.; Rosales-Conrado, N.; López, F.A. Extraction of polyphenols and synthesis of new activated carbon from spent coffee grounds. Sci. Rep. 2019, 9, 1–11. [Google Scholar]
- Bouhlal, F.; Aqil, Y.; Chamkhi, I.; Belmaghraoui, W.; Labjar, N.; Hajjaji, S.E.; Benabdellah, G.A.; Aurag, J.; Lotfi, E.M.; Mahi, M.E. GC-MS Analysis, Phenolic Compounds Quantification, Antioxidant, and Antibacterial Activities of the Hydro-alcoholic Extract of Spent Coffee Grounds. J. Biol. Act. Prod. Nat. 2020, 10, 325–337. [Google Scholar] [CrossRef]
- Daglia, M.; Papetti, A.; Gregotti, C.; Bertè, F.; Gazzani, G. In vitro antioxidant and ex vivo protective activities of green and roasted coffee. J. Agric. Food Chem. 2000, 48, 1449–1454. [Google Scholar] [CrossRef]
- Maegawa, Y.; Sugino, K.; Sakurai, H. Identification of free radical species derived from caffeic acid and related polyphenols. Free Radic. Res. 2007, 41, 110–119. [Google Scholar] [CrossRef]
- Newton, D.W.; Kluza, R.B. pKa values of medicinal compounds in pharmacy practice. Drug Intell. Clin. Pharm. 1978, 12, 546–554. [Google Scholar] [CrossRef]
- Martin, A.; Swarbrick, J.; Cammarata, A. Physical Pharmacy; Lea & Febiger: Philadelphia, PA, USA, 1969. [Google Scholar]
- Švorc, L.U. Determination of caffeine: A comprehensive review on electrochemical methods. Int. J. Electrochem. Sci. 2013, 8, 5755–5773. [Google Scholar]
- Zuorro, A.; Lavecchia, R. Influence of extraction conditions on the recovery of phenolic antioxidants from spent coffee grounds. Am. J. Appl. Sci. 2013, 10, 478. [Google Scholar] [CrossRef]









| Kruskal-Wallis Chi-Squared | Degree of Freedom (fd) | Probability Value (p-Value) | |
|---|---|---|---|
| Solvent | 22.044 | 5 | 0.0005136 |
| Temperature | 20.022 | 3 | 0.000168 |
| Time | 6.1869 | 2 | 0.04535 |
| Solid/Liquid ratio | 9.0544 | 3 | 0.02858 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vandeponseele, A.; Draye, M.; Piot, C.; Chatel, G. Study of Influential Parameters of the Caffeine Extraction from Spent Coffee Grounds: From Brewing Coffee Method to the Waste Treatment Conditions. Clean Technol. 2021, 3, 335-350. https://doi.org/10.3390/cleantechnol3020019
Vandeponseele A, Draye M, Piot C, Chatel G. Study of Influential Parameters of the Caffeine Extraction from Spent Coffee Grounds: From Brewing Coffee Method to the Waste Treatment Conditions. Clean Technologies. 2021; 3(2):335-350. https://doi.org/10.3390/cleantechnol3020019
Chicago/Turabian StyleVandeponseele, Alexandre, Micheline Draye, Christine Piot, and Gregory Chatel. 2021. "Study of Influential Parameters of the Caffeine Extraction from Spent Coffee Grounds: From Brewing Coffee Method to the Waste Treatment Conditions" Clean Technologies 3, no. 2: 335-350. https://doi.org/10.3390/cleantechnol3020019
APA StyleVandeponseele, A., Draye, M., Piot, C., & Chatel, G. (2021). Study of Influential Parameters of the Caffeine Extraction from Spent Coffee Grounds: From Brewing Coffee Method to the Waste Treatment Conditions. Clean Technologies, 3(2), 335-350. https://doi.org/10.3390/cleantechnol3020019








