Facile Elaboration of Wet Cellulose Film as Catalyst Support of MnOx Nanoparticles for the Catalytic Oxidation of Dyes in Absence of Light
Abstract
1. Introduction
2. Materials and Methods
3. Results and Discussion
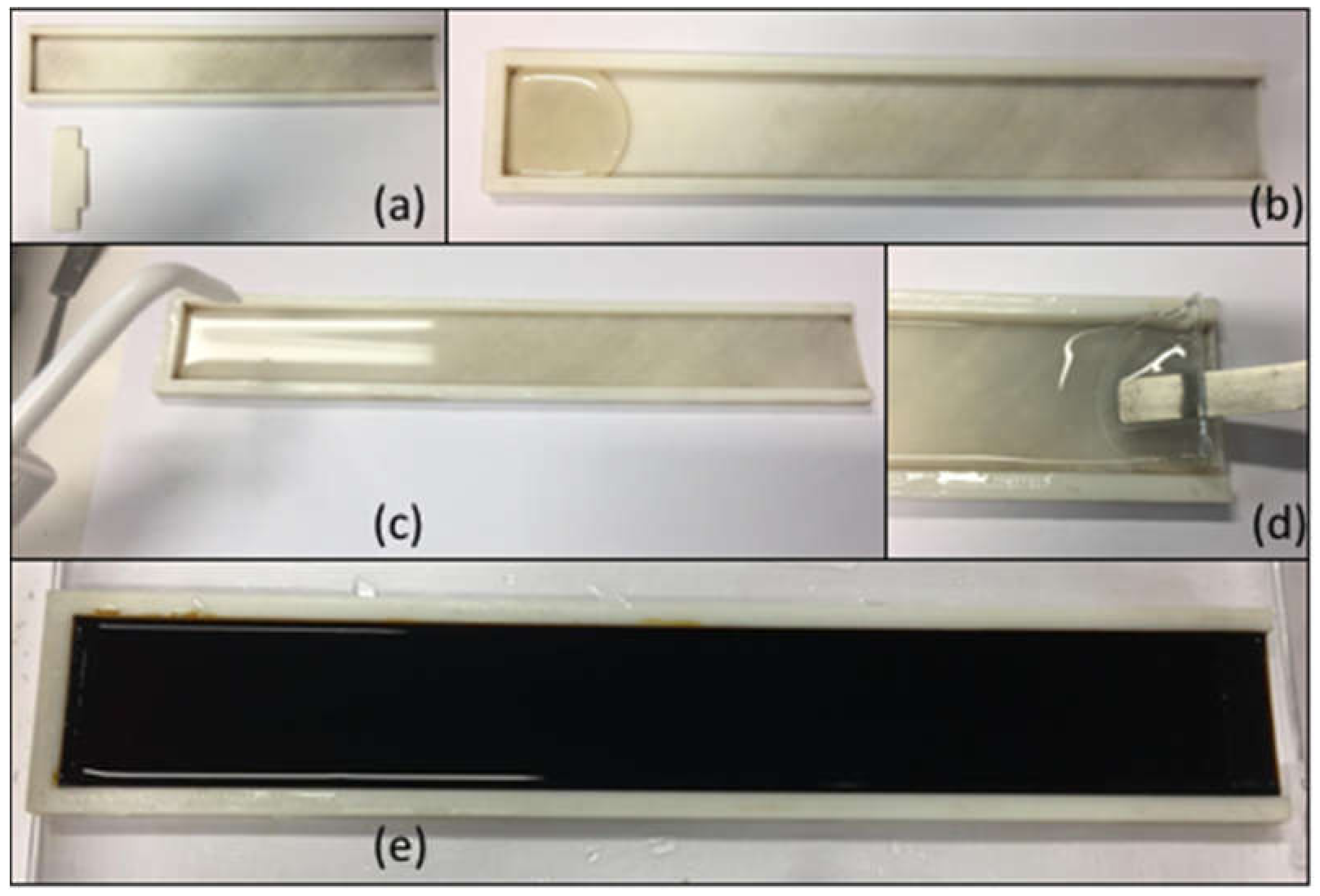
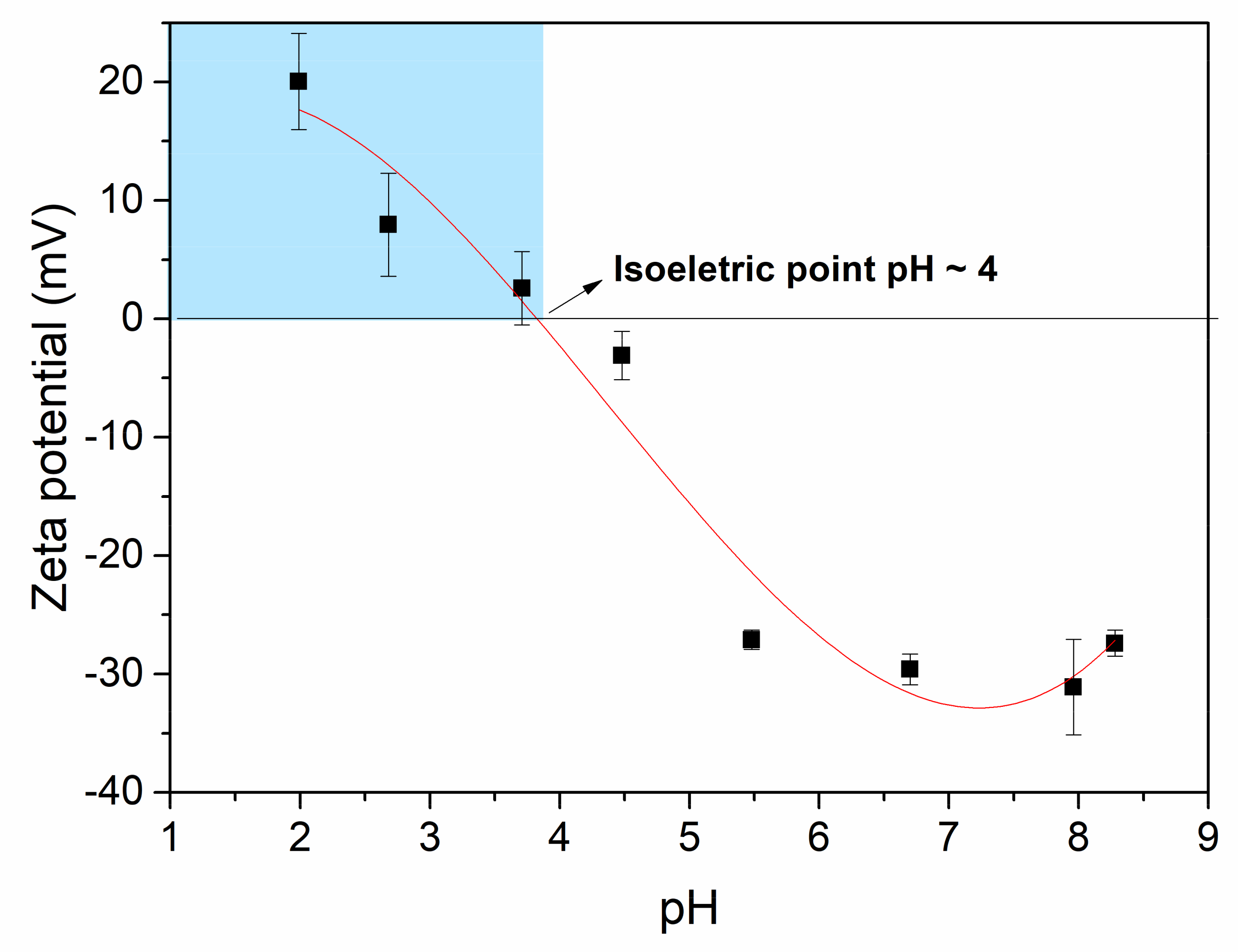
3.1. Film Characterization
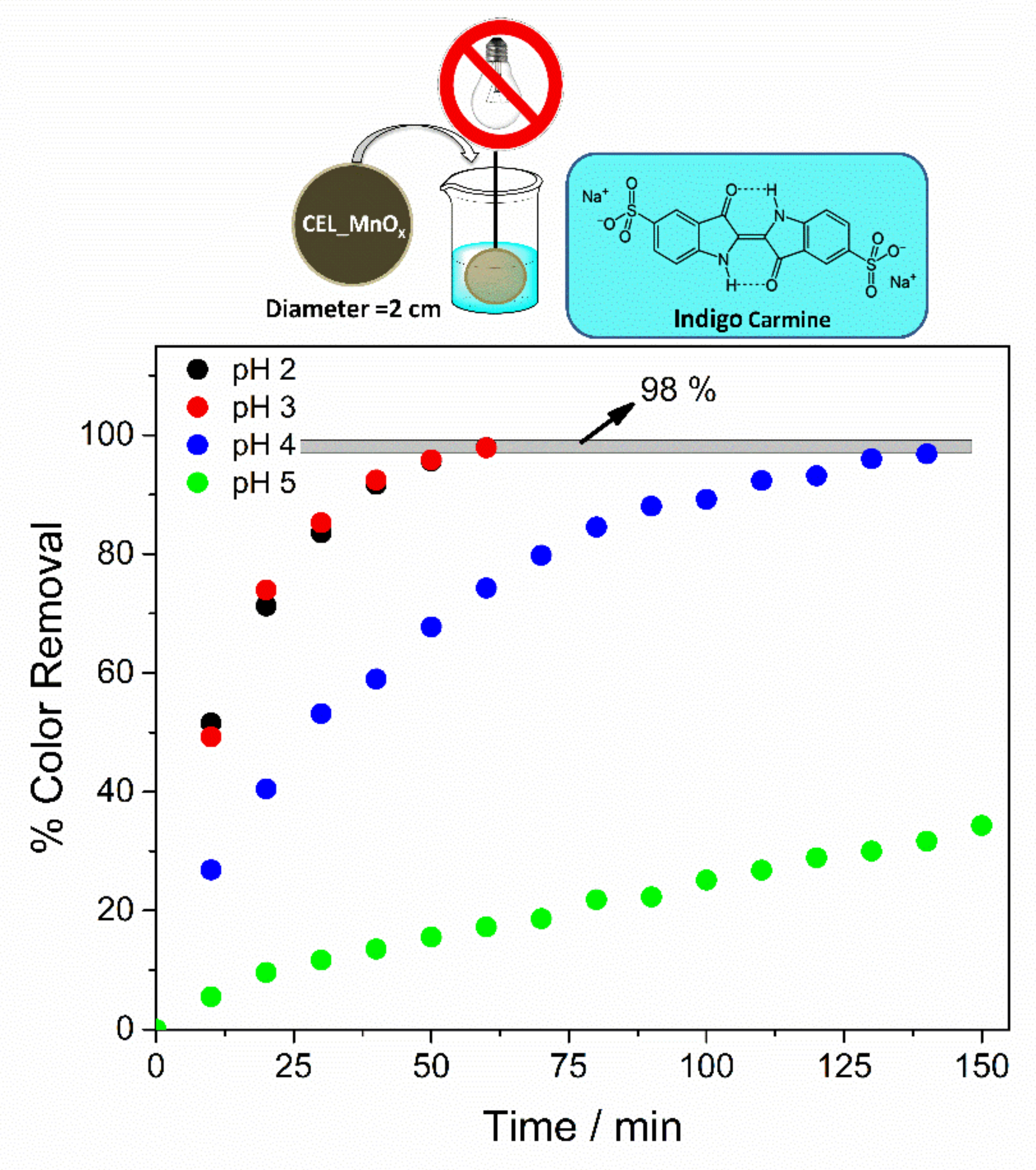
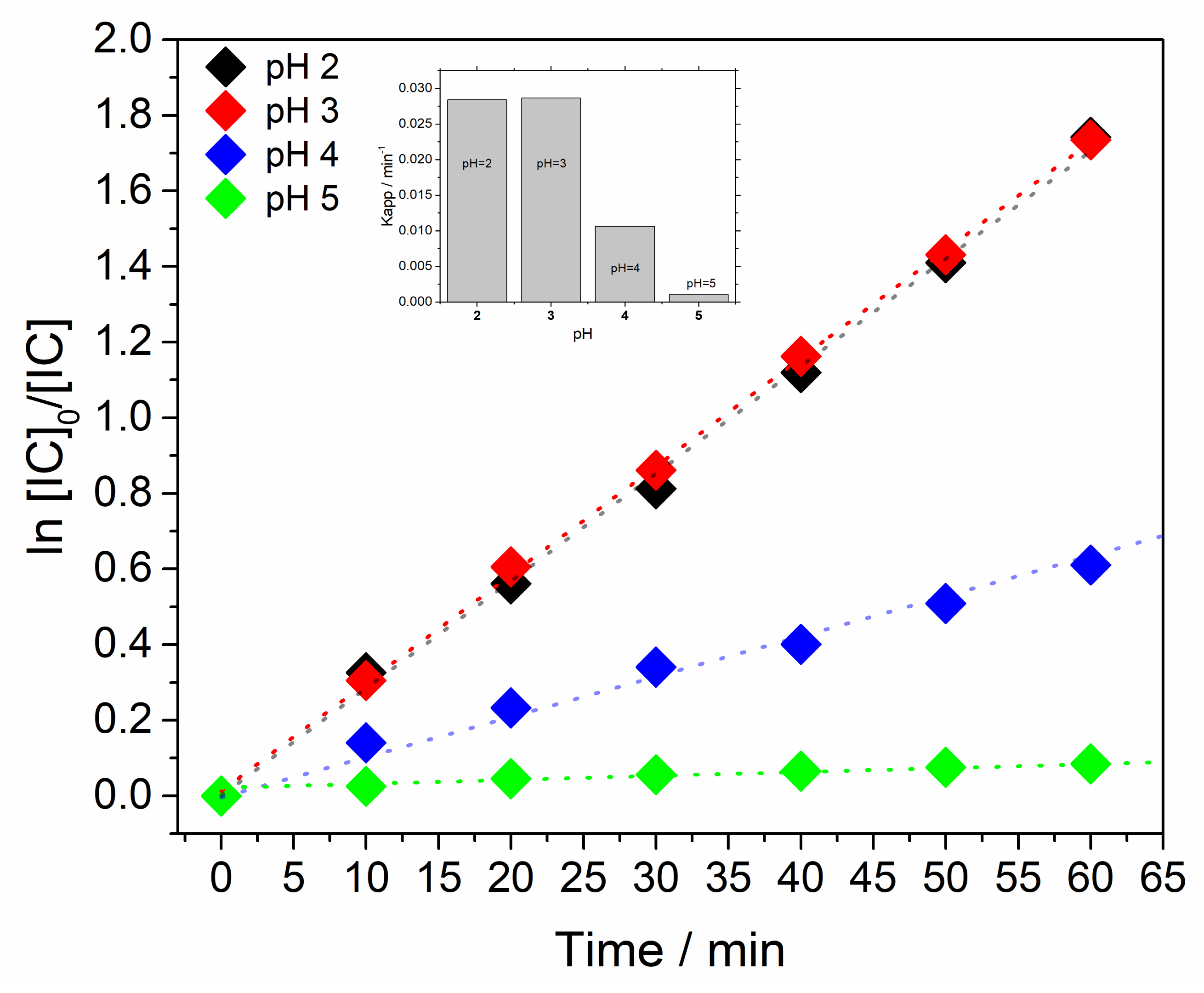
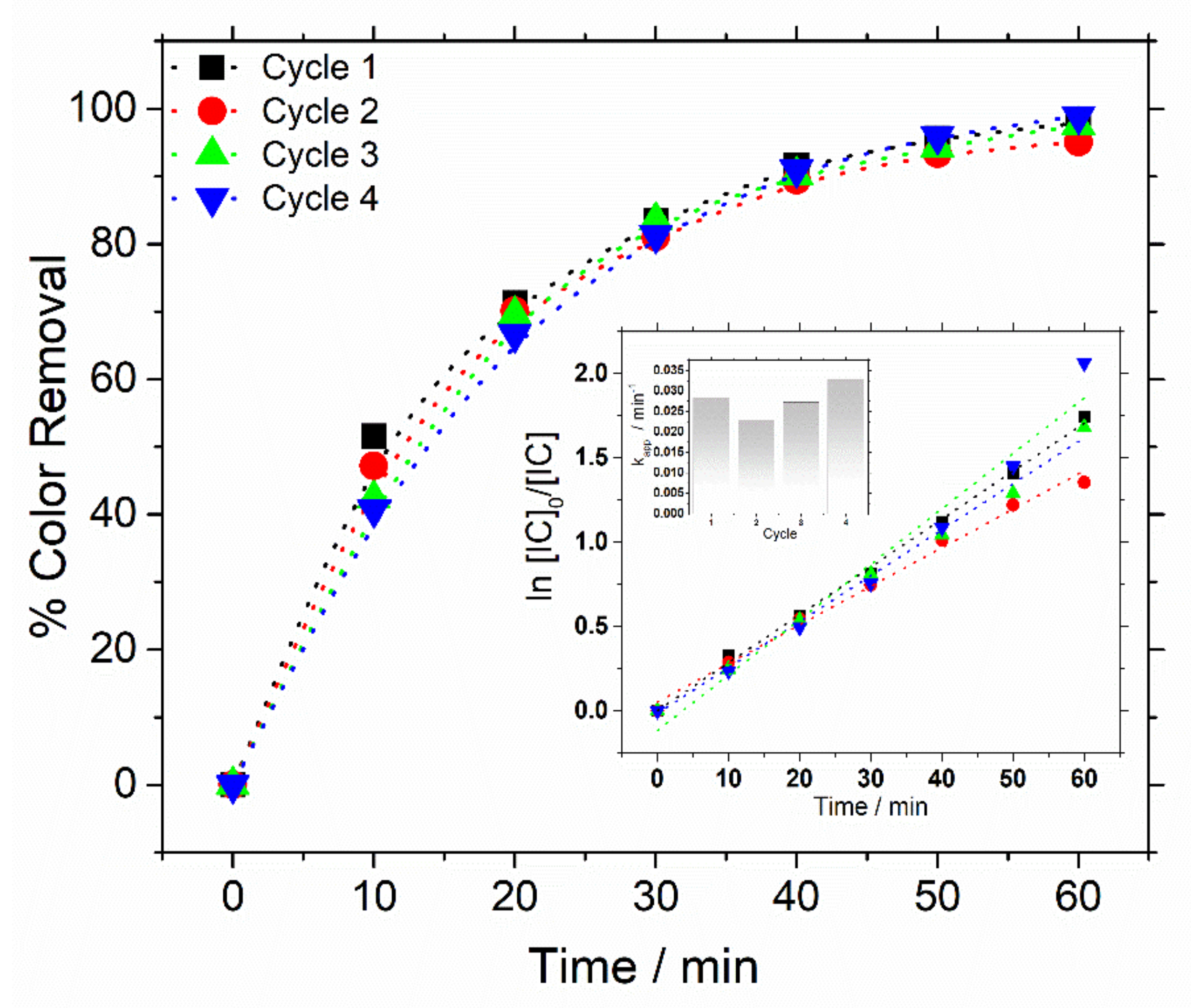
3.2. Catalytic Oxidation of Indigo Carmine Dye
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Dipika, J.; Malviya, A. Composites for wastewater purification: A review. Chemosphere 2020, 246, 125788. [Google Scholar] [CrossRef]
- Cana, O.T.; Kobyaa, M.; Demirbasb, E.; Bayramoglu, M. Treatment of the textile wastewaterby combined electrocoagulation. Chemosphere 2006, 62, 181–187. [Google Scholar] [CrossRef] [PubMed]
- Tak-Hyun, K.; Chulhwan, P.; Eung-Bai, S.; Sangyong, K. Decolorization of disperse and reactive dyes by continuous electrocoagulation process. Desalination 2002, 150, 165–175. [Google Scholar] [CrossRef]
- Ait Himi, M.; El Ghachtouli, S.; Amarray, A.; Zaroual, Z.; Bonnaillie, P.; Azzi, M. Nanostructured manganese oxide as an efficient eco-friendly catalyst for removing azo dye Calcon from water. Mater. Today 2021, 37, 3905–3912. [Google Scholar] [CrossRef]
- Islam, M.A.; Ali, I.; Karim, S.A.; Firoz, M.S.H.; Chowdhury, A.N.; Morton, D.W.; Angove, M.J. Angove. Removal of dye from polluted water using novel nano manganese oxide-based materials. J. Water Process Eng. 2019, 32, 100911. [Google Scholar] [CrossRef]
- Zhao, X.; Lv, L.; Pan, B.; Zhang, W.; Zhang, S.; Zhang, Q. Polymer-supported nanocomposites for environmental application: A review. Chem. Eng. J. 2011, 170, 381–394. [Google Scholar] [CrossRef]
- Wei, J.; Li, K.; Yu, H.; Yin, H.; Cohen Stuart, M.A.; Wang, J.; Zhou, S. Controlled Synthesis of Manganese Oxide Nanoparticles Encaged in Hollow Mesoporous Silica Nanoreactors and Their Enhanced Dye Degradation Activity. J. Am. Chem. Soc. 2020, 5, 6852–6861. [Google Scholar] [CrossRef] [PubMed]
- Su, Q.; Pan, B.; Pan, B.; Zhang, Q.; Zhang, W.; Lv, L.; Wang, X.; Wu, J.; Zhang, Q. Fabrication of polymer-supported nanosized hydrous manganese dioxide (HMO) for enhanced lead removal from waters. Sci. Total. Environ. 2009, 407, 5471–5477. [Google Scholar] [CrossRef]
- Nasrollahzadeh, M.; Shafiei, N.; Nezafat, Z.; Bidgoli, N.S.S.; Soleimani, F. Recent progresses in the application of cellulose, starch, alginate, gum, pectin, chitin and chitosan based (nano)catalysts in sustainable and selective oxidation reactions: A review. Carbohydr. Polym. 2020, 241, 116353. [Google Scholar] [CrossRef]
- Swatloski, R.P.; Spear, S.K.; Holbrey, J.D.; Rogers, R.D. Dissolution of Cellose with Ionic Liquids. J. Am. Chem. Soc. 2002, 124, 4974–4975. [Google Scholar] [CrossRef]
- Liu, Z.; Wang, H.; Li, Z.; Lu, X.; Zhang, X.; Zhang, S.; Zhou, K. Characterization of the regenerated cellulose films in ionic liquids and rheological properties of the solutions. Mater. Chem. Phys. 2011, 128, 220–227. [Google Scholar] [CrossRef]
- Peng, R.; Zhang, H.; Gui, L.; Wu, Z.; Yu, P.; Luo, Y. Facile Synthesis of MnO2@Cellulose Composite Film. Environ. Eng. Sci. 2019, 36, 583–588. [Google Scholar] [CrossRef]
- Oliveira, L.V.F.; Bennici, S.; Josien, L.; Limousy, L.; Bizeto, M.A.; Camilo, F.F. Free-standing cellulose film containing manganese dioxide nanoparticles and its use in discoloration of indigo carmine dye. Carbohydr. Polym. 2020, 230, 115621. [Google Scholar] [CrossRef]
- Sun, H.; Xu, K.; Huang, M.; Shang, Y.; She, P.; Yin, S.; Liu, Z. One-pot synthesis of ultrathin manganese dioxide nanosheets and their efficient oxidative degradation of Rhodamine B. Appl. Surf. Sci. 2015, 357, 69–73. [Google Scholar] [CrossRef]
- Sun, H.; Shang, Y.; Xu, K.; Tang, Y.; Li, J.; Liu, Z. MnO2 aerogels for highly efficient oxidative degradation of Rhodamine B. RSC Adv. 2017, 7, 30283–30288. [Google Scholar] [CrossRef]
- Zhang, Y.; Zheng, T.X.; Hu, Y.B.; Guo, X.L.; Peng, H.H.; Zhang, Y.X.; Feng, L.; Zheng, H.L. Delta manganese dioxide nanosheets decorated magnesium wire for the degradation of methyl orange. J. Colloid. Interf. Sc. 2017, 490, 226–232. [Google Scholar] [CrossRef] [PubMed]
- Islam, A.; Morton, D.W.; Johnson, B.B.; Mainali, B.; Angove, M.J. Manganese oxides and their application to metal ion and contaminant removal from wastewater. J. Water Process. Eng. 2018, 26, 264–280. [Google Scholar] [CrossRef]
- Brock, R.M.; Sanabria, M.; Suib, S.L.; Urban, V.; Thiyagarrajan, P.; Potter, D.I. Particle Size Control and Self-Assembly Processes in Novel Colloids of Nanocrystalline Manganese Oxide. J. Phys. Chem. B 1999, 103, 7416–7428. [Google Scholar] [CrossRef]
- Zhou, S.; Ray, A.K. Kinetic Studies for Photocatalytic Degradation of Eosin B on a Thin Film of Titanium Dioxide. Ind. Eng. Chem. Res. 2003, 42, 6020–6033. [Google Scholar] [CrossRef]
- Morimoto, T.; Kattika, S. Isoelectric point of manganese oxide. Bull. Chem. Soc. Jpn. 1974, 47, 1586–1588. [Google Scholar] [CrossRef]
- Gray, M.J.; Malati, M.A.; Rophael, M.W. The point of zero charge of manganese dioxides. J. Electroanal. Chem. 1978, 89, 135–140. [Google Scholar] [CrossRef]
- Biesinger, M.C.; Payne, B.P.; Grosvenor, A.P.; Lau, L.W.M.; Gerson, A.R.; Smart, R.S.C. Resolving surface chemical states in XPS analysis of first row transition metals, oxides and hydroxides: Cr, Mn, Fe, Co and Ni. Appl. Surf. Sci. 2011, 257, 2717–2730. [Google Scholar] [CrossRef]

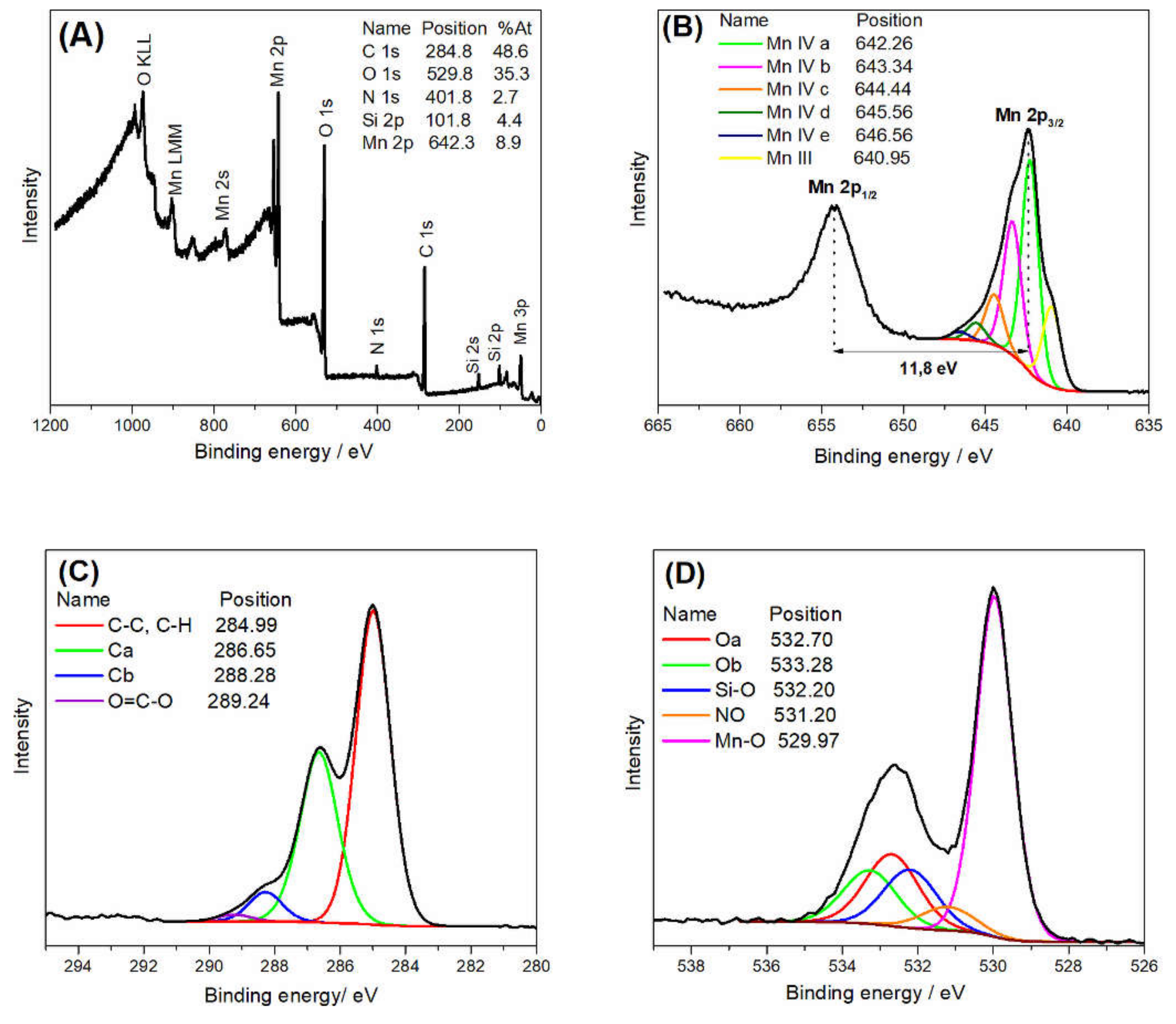
| Spectrum | Attribution | Binding Energy ± 0.1 (eV) | Atomic Concentration ± 0.05 (%) |
|---|---|---|---|
| C 1s | Ca | 286.65 | 16.77 |
| C 1s | Cb | 288.28 | 2.53 |
| O 1s | Oa | 532.70 | 5.85 |
| O 1s | Ob | 533.28 | 4.37 |
| O 1s | Si-O | 532.20 | 4.66 |
| O 1s | Mn-O | 529.97 | 19.24 |
| O 1s | NO | 531.20 | 1.92 |
| C 1s | C-C C-H | 284.99 | 27.83 |
| C 1s | O=C-O | 289.24 | 0.57 |
| N 1s | C-N | 400.00 | 0.79 |
| N 1s | NO | 402.39 | 1.92 |
| Si 2p | Si 2p3/2 Si(-O)2 | 102.22 | 4.66 |
| Mn 2p | Mn a (Mn IV) | 642.26 | 3.79 |
| Mn 2p | Mn b (Mn IV) | 643.34 | 2.59 |
| Mn 2p | Mn c (Mn IV) | 644.44 | 0.98 |
| Mn2p | Mn d (Mn IV) | 645.56 | 0.35 |
| Mn2p | Mn e (Mn IV) | 646.56 | 0.16 |
| Mn2p | Mn III | 640.95 | 1.02 |
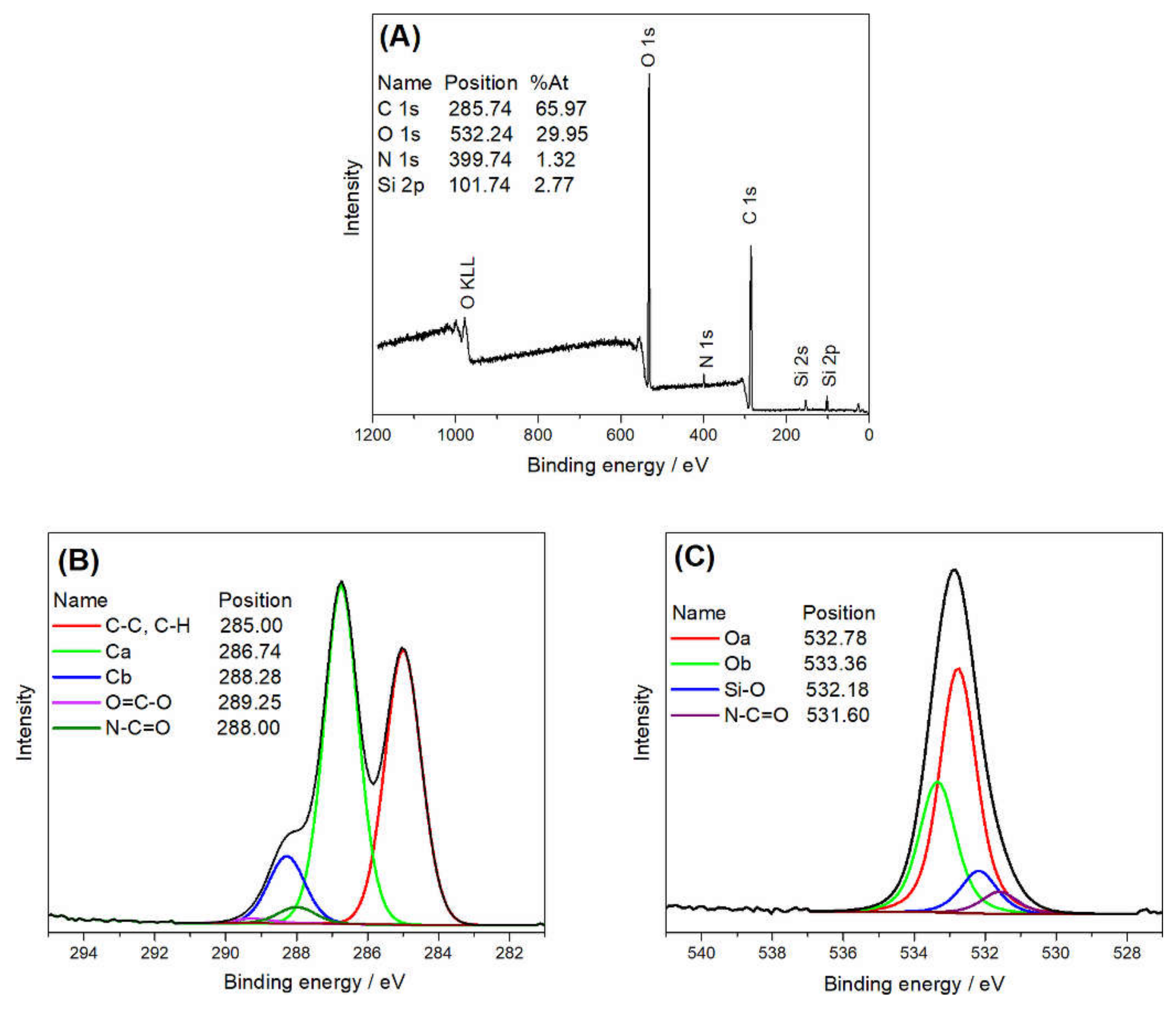
| Spectrum | Attribution | Binding Energy ± 0.1 (eV) | Atomic Concentration ± 0.05 (%) |
|---|---|---|---|
| C 1s | Ca | 286.74 | 30.02 |
| C 1s | Cb | 288.28 | 6.33 |
| O 1s | Oa | 532.78 | 17.59 |
| O 1s | Ob | 533.36 | 9.4 |
| O 1s | Si-O | 532.2 | 2.97 |
| O 1s | N-C=O | 531.6 | 1.53 |
| C 1s | C-C, C-H | 285 | 25.61 |
| C 1s | O=C-O | 289.25 | 0.44 |
| C 1s | O=C-N | 288 | 1.54 |
| N 1s | C-N | 400.15 | 1.58 |
| Si 2p | Si 2p3/2 Si(-O)2 | 102.18 | 2.98 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Oliveira, L.V.F.; Limousy, L.; Bennici, S.; Josien, L.; Hajjar-Garreau, S.; Goddard, M.-L.; Bizeto, M.A.; Camilo, F.F. Facile Elaboration of Wet Cellulose Film as Catalyst Support of MnOx Nanoparticles for the Catalytic Oxidation of Dyes in Absence of Light. Clean Technol. 2021, 3, 288-298. https://doi.org/10.3390/cleantechnol3020016
Oliveira LVF, Limousy L, Bennici S, Josien L, Hajjar-Garreau S, Goddard M-L, Bizeto MA, Camilo FF. Facile Elaboration of Wet Cellulose Film as Catalyst Support of MnOx Nanoparticles for the Catalytic Oxidation of Dyes in Absence of Light. Clean Technologies. 2021; 3(2):288-298. https://doi.org/10.3390/cleantechnol3020016
Chicago/Turabian StyleOliveira, Larissa V. F., Lionel Limousy, Simona Bennici, Ludovic Josien, Samar Hajjar-Garreau, Mary-Lorène Goddard, Marcos A. Bizeto, and Fernanda F. Camilo. 2021. "Facile Elaboration of Wet Cellulose Film as Catalyst Support of MnOx Nanoparticles for the Catalytic Oxidation of Dyes in Absence of Light" Clean Technologies 3, no. 2: 288-298. https://doi.org/10.3390/cleantechnol3020016
APA StyleOliveira, L. V. F., Limousy, L., Bennici, S., Josien, L., Hajjar-Garreau, S., Goddard, M.-L., Bizeto, M. A., & Camilo, F. F. (2021). Facile Elaboration of Wet Cellulose Film as Catalyst Support of MnOx Nanoparticles for the Catalytic Oxidation of Dyes in Absence of Light. Clean Technologies, 3(2), 288-298. https://doi.org/10.3390/cleantechnol3020016









