Convenient Synthesis of Triphenylphosphine Sulfide from Sulfur and Triphenylphosphine
Abstract
:1. Introduction
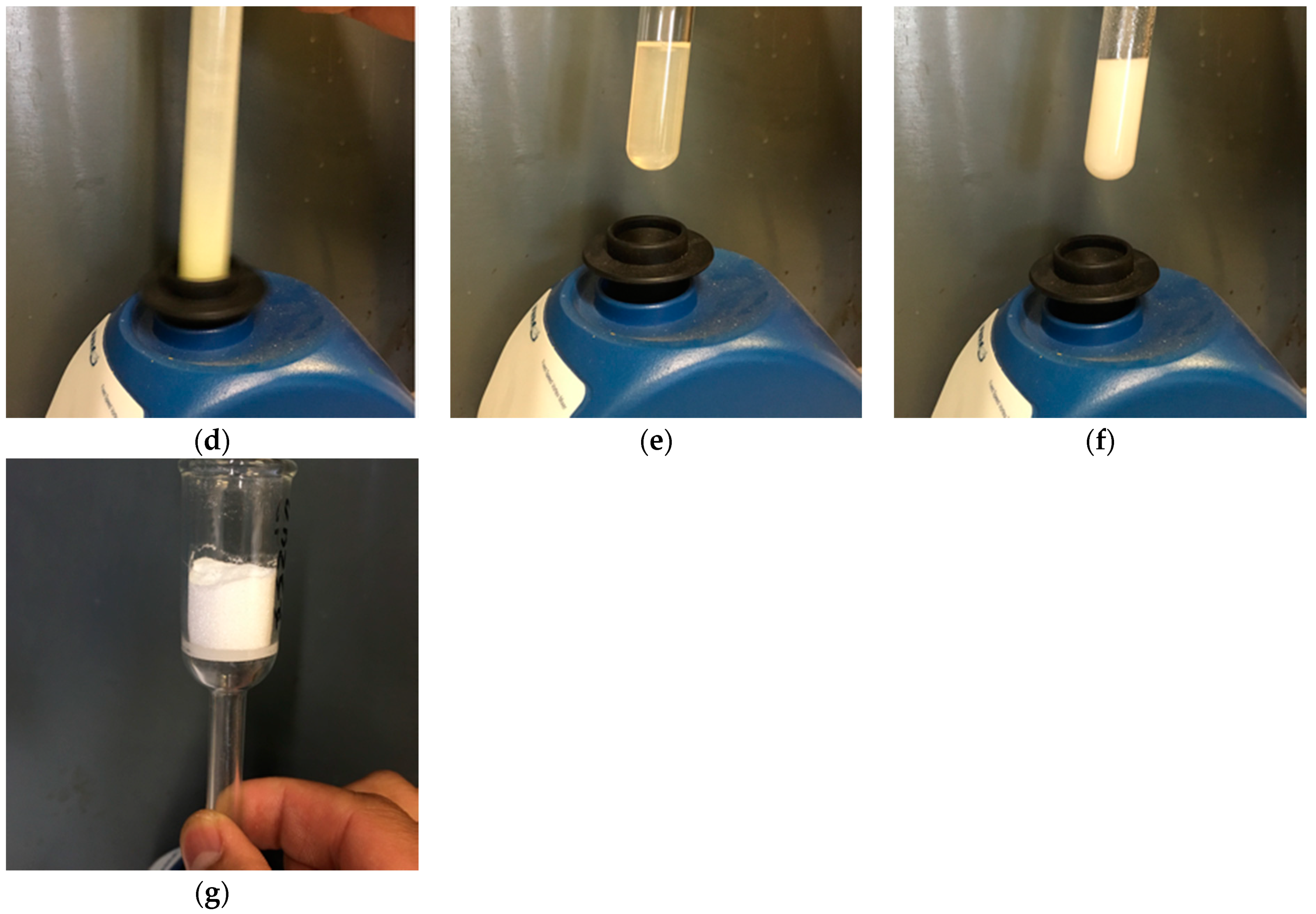
2. Experiments
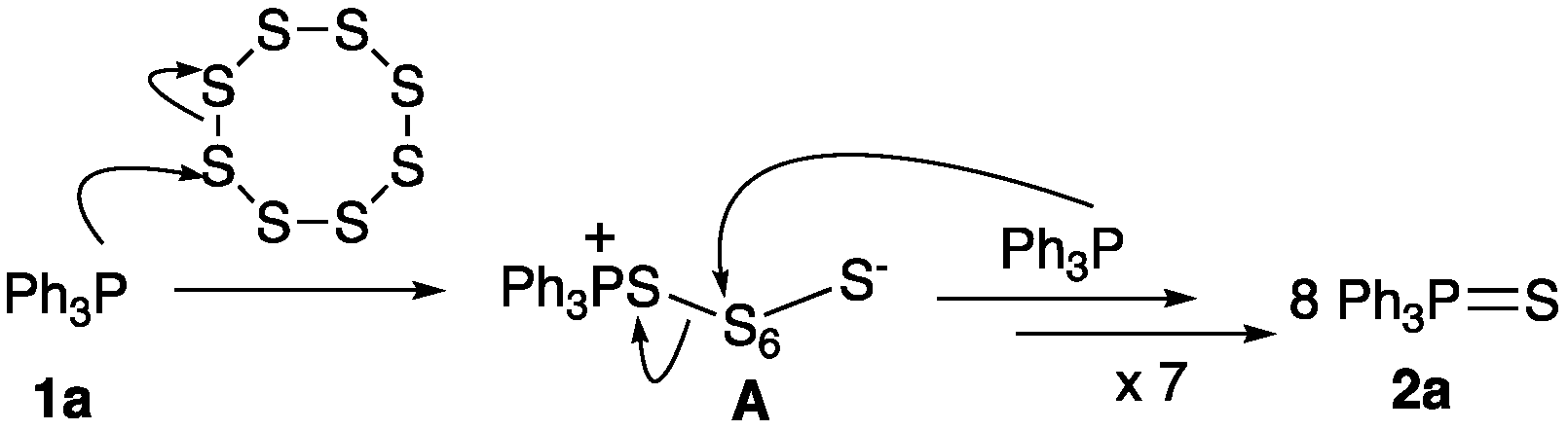
3. Results and Discussion
4. Conclusions
Supplementary Materials
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Castillo, M.; Rivero, I.A. Combinatorial synthesis of fluorescent trialkylphosphine sulfides as sensor materials for metal ions of environmental concern. ARKIVOC 2003, 11, 193. [Google Scholar] [CrossRef] [Green Version]
- Yang, J.; Stolee, J.A.; Jiang, H.; Xiao, L.; Kiesman, W.F.; Antia, F.D.; Fillon, Y.A.; Ng, A.; Shi, X. Solid-Phase Synthesis of Phosphorothioate Oligonucleotides Using Sulfurization Byproducts for in Situ Capping. J. Org. Chem. 2018, 83, 11577. [Google Scholar] [CrossRef] [PubMed]
- Svara, J.; Weferling, N.; Hofmann, T. Phosphorus Compounds, Organic. In Ullmann’s Encyclopedia of Industrial Chemistry; Wiley-VCH: Weinheim, Germany, 2006. [Google Scholar] [CrossRef]
- Lorsch, J.R.; Bartel, D.P.; Szostak, J.W. Reverse transcriptase reads through a 2′–5′ linkage and a 2′-thiophosphate in a template. Nucleic Acids Res. 1995, 23, 2811. [Google Scholar] [CrossRef]
- Bartlett, P.D.; Meguerian, G. Reactions of Elemental Sulfur. I. The Uncatalyzed Reaction of Sulfur with Triarylphosphines. J. Am. Chem. Soc. 1956, 78, 3710. [Google Scholar] [CrossRef]
- Guzaev, A.P. Reactivity of 3H-1,2,4-dithiazole-3-thiones and 3H-1,2-dithiole-3-thiones as sulfurizing agents for oligonucleotide synthesis. Tetrahedron Lett. 2011, 52, 434. [Google Scholar] [CrossRef]
- Sugimoto, H.; Tatemoto, S.; Toyota, K.; Ashikari, K.; Kubo, M.; Ogura, T.; Itoh, S. Oxo-sulfido- and oxo-selenido-molybdenum(vi) complexes possessing a dithiolene ligand related to the active sites of hydroxylases of molybdoenzymes: Low temperature preparation and characterization. Chem. Commun. 2013, 49, 4358. [Google Scholar] [CrossRef] [PubMed]
- Wada, M.; Kanzaki, M.; Fujiwara, M.; Kajihara, K.; Erabi, T. Some Unusual Properties of Tris(2,6-dimethoxyphenyl)phosphine Sulfide and the Related Compounds. Bull. Chem. Soc. Jpn. 1991, 64, 1782. [Google Scholar] [CrossRef]
- Hanusek, J.; Russell, M.A.; Laws, A.P.; Jansa, P.; Atherton, J.H.; Fettes, K.; Page, M.I. Mechanism of the sulfurisation of phosphines and phosphites using 3-amino-1,2,4-dithiazole-5-thione (xanthane hydride). Org. Biomol. Chem. 2007, 5, 478. [Google Scholar] [CrossRef] [PubMed]
- Kumar, R.; Kumar, S.; Pandey, M.K.; Kashid, V.S.; Radhakrishna, L.; Balakrishna, M.S. Synthesis of Phosphine Chalcogenides Under Solvent-Free Conditions Using a Rotary Ball Mill. Eur. J. Inorg. Chem. 2018, 8, 1028. [Google Scholar] [CrossRef]
- Nguyen, T.B. Recent Advances in the Synthesis of Heterocycles via Reactions Involving Elemental Sulfur. Adv. Synth. Catal. 2020, 362, 3448. [Google Scholar] [CrossRef]
- Nguyen, T.B. Recent Advances in Organic Reactions Involving Elemental Sulfur. Adv. Synth. Catal. 2017, 359, 1106. [Google Scholar] [CrossRef]
- Nguyen, T.B. Elemental sulfur and molecular iodine as efficient tools for carbon-nitrogen bond formation via redox reactions. Asian J. Org. Chem. 2017, 6, 477. [Google Scholar] [CrossRef]
- Nguyen, L.A.; Ngo, Q.A.; Retalleau, P.; Nguyen, T.B. Elemental Sulfur as Polyvalent Reagent in Redox Condensation with o-Chloronitrobenzenes and Benzaldehydes: Three-Component Access to 2-Arylbenzothiazoles. Green Chem. 2017, 19, 4289. [Google Scholar] [CrossRef]
- Nguyen, T.B.; Retailleau, P. Elemental Sulfur as Reaction Medium for the Synthesis of Fused Nitrogen Heterocycles by Oxidative Coupling between Cycloalkanones and Nitrogen Nucleophiles. Adv. Synth. Catal. 2017, 359, 3843. [Google Scholar] [CrossRef]
- Nguyen, T.B.; Retailleau, P. Base-Catalyzed Three-Component Reaction between Chalcones, Isothiocyanates and Sulfur: Access to Thiazole-2-thiones. Org. Lett. 2021, 23, 5344. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, T.B.; Mac, D.H.; Retailleau, P. Base-Catalyzed Three-Component Reaction of α-Cyanoacetates with Chalcones and Elemental Sulfur: Access to 2-Aminothiophenes Unobtainable via the Gewald Reaction. J. Org. Chem. 2021, 86, 9418. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, T.B.; Mac, D.H.; Retailleau, P. Room-Temperature Synthesis of Tetrasubstituted 1,3-Dithioles by Dimerizing Sulfuration of Chalcones with Elemental Sulfur. J. Org. Chem. 2020, 85, 13508. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, T.B.; Retailleau, P. Sulfur-Promoted Decarboxylative Sulfurative Hexamerization of Phenylacetic Acids: Direct Approach to Hexabenzylidyne Tetrasulfides. Org. Lett. 2019, 21, 279. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, T.B.; Retailleau, P. Sulfur-Promoted DABCO-Catalyzed Oxidative Trimerization of Phenylacetonitriles. J. Org. Chem. 2019, 84, 5907. [Google Scholar] [CrossRef] [PubMed]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nguyen, T.B. Convenient Synthesis of Triphenylphosphine Sulfide from Sulfur and Triphenylphosphine. Clean Technol. 2022, 4, 234-238. https://doi.org/10.3390/cleantechnol4020013
Nguyen TB. Convenient Synthesis of Triphenylphosphine Sulfide from Sulfur and Triphenylphosphine. Clean Technologies. 2022; 4(2):234-238. https://doi.org/10.3390/cleantechnol4020013
Chicago/Turabian StyleNguyen, Thanh Binh. 2022. "Convenient Synthesis of Triphenylphosphine Sulfide from Sulfur and Triphenylphosphine" Clean Technologies 4, no. 2: 234-238. https://doi.org/10.3390/cleantechnol4020013
APA StyleNguyen, T. B. (2022). Convenient Synthesis of Triphenylphosphine Sulfide from Sulfur and Triphenylphosphine. Clean Technologies, 4(2), 234-238. https://doi.org/10.3390/cleantechnol4020013




