Upgraded Protocol for Microplastics’ Extraction from the Soil Matrix by Sucrose Density Gradient Centrifugation
Abstract
1. Introduction
1.1. Isolation of Microplastic Particles
1.2. Density Separation
2. Materials and Methods
2.1. Pretreatment
2.2. Density Separation
2.3. Soil Organic Matter Removal
2.4. Validation
3. Results
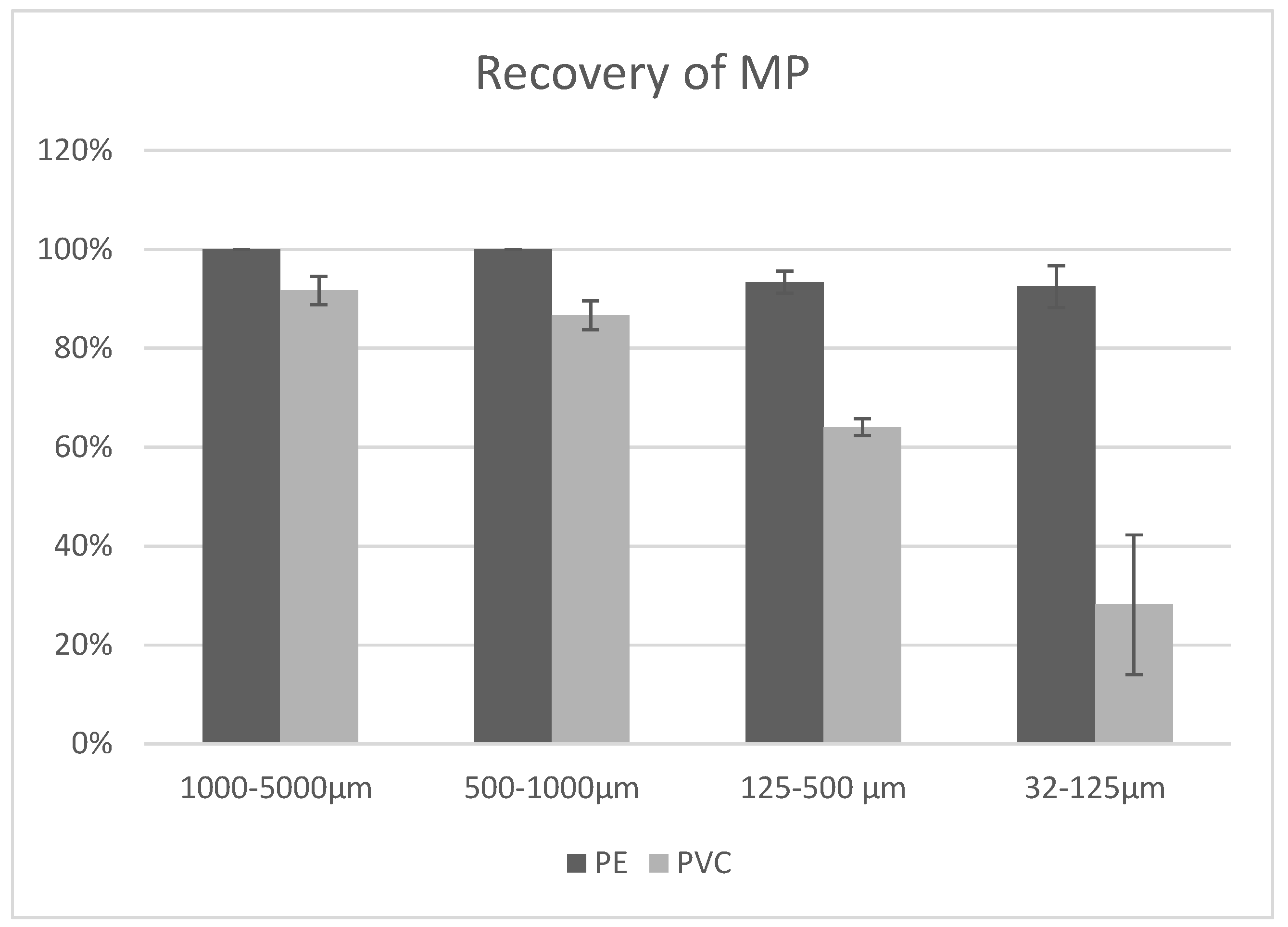
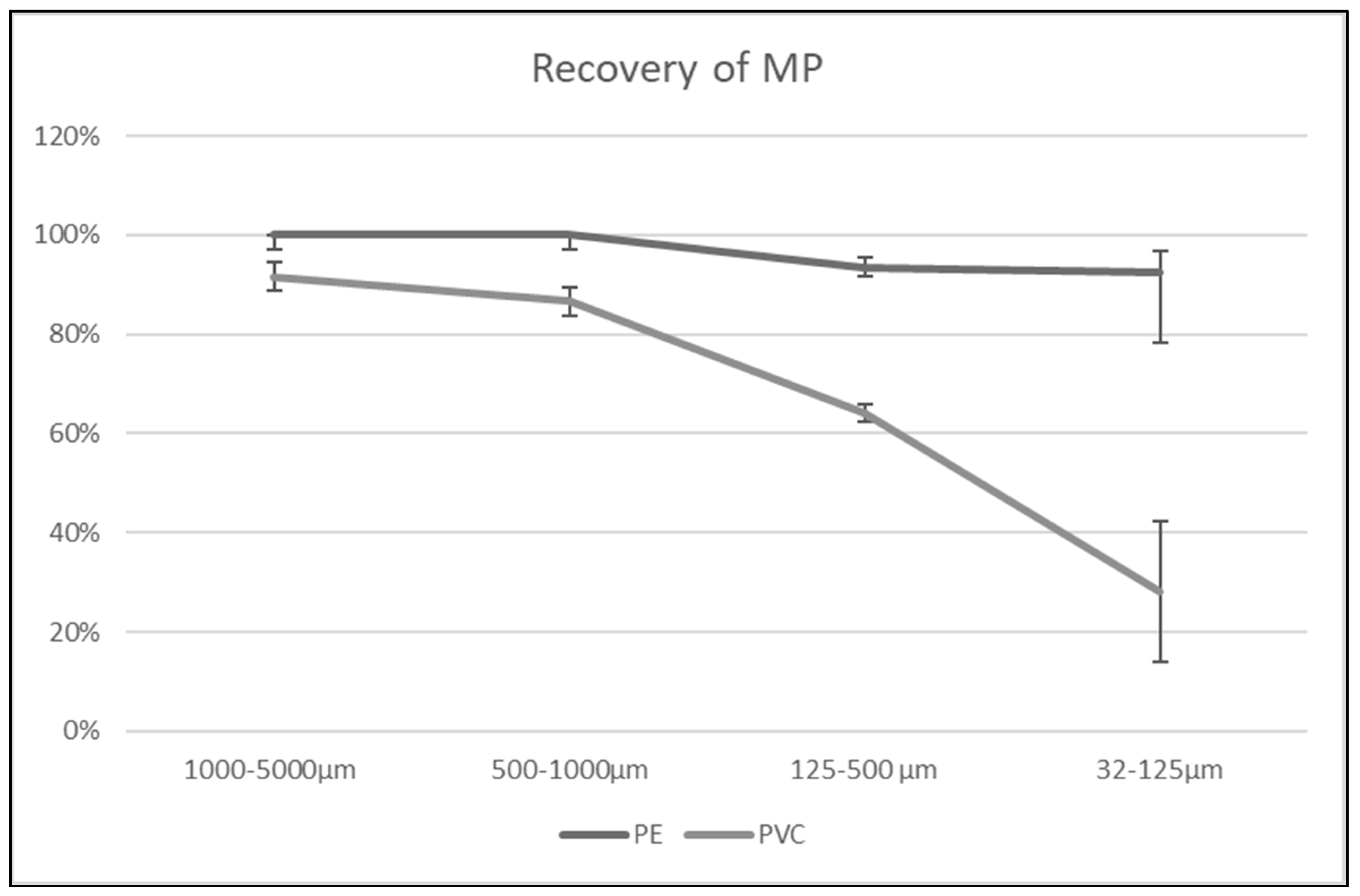
3.1. Validation Results
3.1.1. Spiking of Quartz Sand with MPs
3.1.2. Spiking of Soil Columns with MPs
4. Discussion
4.1. Pretreatment
4.2. Density Separation
4.3. Soil Organic Matter Removal
4.4. Validation
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| MP | Microplastic |
| PP | Polypropylene |
| PE | Polyethylene |
| PS | Polystyrene |
| PA | Polyamide (Nylon) |
| PC | Polycarbonate |
| ABS | Acrylonitrile Butadiene Styrene |
| PMMA | Polymethyl Methacrylate (Acrylic) |
| PET | Polyethylene Terephthalate |
| POM | Polyoxymethylene (Acetal) |
| PVC | Polyvinyl Chloride |
| HDPE | High-Density Polyethylene |
| AMF | Arbuscular Mycorrhizal Fungi |
| SOM | Soil Organic Matter |
| RSD | Relative Standard Deviation |
References
- Sharma, M.D.; Elanjickal, A.I.; Mankar, J.S.; Krupadam, R.J. Assessment of cancer risk of microplastics enriched with polycyclic aromatic hydrocarbons. J. Hazard. Mater. 2020, 398, 122994. [Google Scholar] [CrossRef] [PubMed]
- Plastics Europe. Plastics—The Facts 2019: An Analysis of European Plastics Production, Demand, and Waste Data. Plastics Europe. 2018. Available online: https://www.plasticseurope.org (accessed on 20 December 2024).
- Alimi, O.S.; Budarz, J.F.; Hernandez, L.M.; Tufenkji, N. Microplastics and nanoplastics in aquatic environments: Aggregation, deposition, and enhanced contaminant transport. Environ. Sci. Technol. 2018, 52, 1704–1724. [Google Scholar] [CrossRef] [PubMed]
- Colton, J.B.; Knapp, F.D.; Burns, B.R. Plastic particles in surface waters of the northwestern Atlantic. Science 1974, 185, 491–497. [Google Scholar] [CrossRef] [PubMed]
- Horton, A.A.; Walton, A.; Spurgeon, D.J.; Lahive, E.; Svendsen, C. Microplastics in freshwater and terrestrial environments: Evaluating the current understanding to identify the knowledge gaps and future research priorities. Sci. Total Environ. 2017, 586, 127–141. [Google Scholar] [CrossRef]
- Rillig, M.C. Microplastic in terrestrial ecosystems and the soil? Environ. Sci. Technol. 2012, 46, 6453–6454. [Google Scholar] [CrossRef]
- Rillig, M.C.; Lehmann, A. Microplastic in terrestrial ecosystems. Science 2020, 368, 1430–1431. [Google Scholar] [CrossRef]
- de Souza Machado, A.A.; Lau, C.W.; Kloas, W.; Bergmann, J.; Bachelier, J.B.; Faltin, E.; Becker, R.; Gorlich, A.S.; Rillig, M.C. Microplastics can change soil properties and affect plant performance. Environ. Sci. Technol. 2019, 53, 6044–6052. [Google Scholar] [CrossRef]
- Huang, Y.; Zhao, Y.; Wang, J.; Zhang, M.; Jia, W.; Qin, X. LDPE microplastic films alter microbial community composition and enzymatic activities in soil. Environ. Pollut. 2019, 254 Pt A, 112983. [Google Scholar] [CrossRef]
- Zhu, D.; Chen, Q.L.; An, X.L.; Yang, X.R.; Christie, P.; Ke, X.; Zhu, Y.G. Exposure of soil collembolans to microplastics perturbs their gut microbiota and alters their isotopic composition. Soil Biol. Biochem. 2018, 116, 302–310. [Google Scholar] [CrossRef]
- Zhu, F.; Zhu, C.; Wang, C.; Gu, C. Occurrence and ecological impacts of microplastics in soil systems: A review. Bull. Environ. Contam. Toxicol. 2019, 102, 741–749. [Google Scholar] [CrossRef]
- Liu, S.-L.; Jian, M.-F.; Zhou, L.-Y.; Li, W.-H.; Wu, X.-E.; Rao, D. Pollution characteristics of microplastics in migratory bird habitats located within Poyang Lake wetlands. Environ. Sci. 2019, 40, 2639–2646. [Google Scholar] [CrossRef]
- Rillig, M.C.; Hoffmann, M.; Lehmann, A.; Liang, Y.; Lück, M.; Augustin, J. Microplastic fibers affect dynamics and intensity of CO2 and N2O fluxes from soil differently. Microplastics Nanoplastics 2021, 1, 3. [Google Scholar] [CrossRef]
- Wang, F.Y.; Zhang, X.Q.; Zhang, S.Q.; Zhang, S.W.; Sun, Y.H. Interactions of microplastics and cadmium on plant growth and arbuscular mycorrhizal fungal communities in an agricultural soil. Chemosphere 2020, 254, 126791. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Ge, J.; Yu, X.; Li, H. Environmental fate and impacts of microplastics in soil ecosystems: Progress and perspective. Sci. Total Environ. 2020, 708, 134841. [Google Scholar] [CrossRef]
- Zhang, G.S.; Zhang, F.; Li, X.T. Effects of polyester microfibers on soil physical properties: Perception from a field and a pot experiment. Sci. Total Environ. 2019, 670, 1–7. [Google Scholar] [CrossRef]
- Zhang, P.; Huang, P.; Sun, H.; Ma, J.; Li, B. The structure of agricultural microplastics (PT, PU, and UF) and their sorption capacities for PAHs and PHE derivates under various salinity and oxidation treatments. Environ. Pollut. 2020, 257, 113525. [Google Scholar] [CrossRef]
- Wang, C.; Wang, L.; Ok, Y.S.; Tsang, D.C.W.; Hou, D. Soil plastisphere: Exploration methods, influencing factors, and ecological insights. J. Hazard. Mater. 2022, 430, 128503. [Google Scholar] [CrossRef]
- Wan, Y.; Wu, C.; Xue, Q.; Hui, X. Effects of plastic contamination on water evaporation and desiccation cracking in soil. Sci. Total Environ. 2019, 654, 576–582. [Google Scholar] [CrossRef]
- Cao, J.; Chen, P.; Li, Y.; Fang, H.; Gu, X.; Li, Y. Effect of plastic film residue on vertical infiltration under different initial soil moisture contents and dry bulk densities. Water 2020, 12, 1346. [Google Scholar] [CrossRef]
- Shashank, B.S.; Kuntikana, G.; Jiang, N.J.; Singh, D. Investigations on biosorption and biogenic calcite precipitation in sands. Soil Use Manag. 2020, 36, 438–446. [Google Scholar] [CrossRef]
- Cao, D.; Wang, X.; Luo, X.; Liu, G.; Zheng, H. Effects of polystyrene microplastics on the fitness of earthworms in an agricultural soil. IOP Conf. Ser. Earth Environ. Sci. 2017, 61, 012148. [Google Scholar] [CrossRef]
- Rillig, M.C.; Ingraffia, R.; Machado, A.A.d.S. Microplastic incorporation into soil in agroecosystems. Front. Plant Sci. 2017, 8, 1805. [Google Scholar] [CrossRef] [PubMed]
- Qi, Y.; Yang, X.; Pelaez, A.M.; Lwanga, E.H.; Beriot, N.; Gertsen, H.; Garbeva, P.; Geissen, V. Macro- and micro- plastics in soil-plant system: Effects of plastic mulch film residues on wheat (Triticum aestivum) growth. Sci. Total Environ. 2018, 645, 1048–1056. [Google Scholar] [CrossRef]
- Gong, J.; Xie, P. Research progress in sources, analytical methods, eco-environmental effects, and control measures of microplastics. Chemosphere 2020, 254, 126790. [Google Scholar] [CrossRef]
- Zhou, C.-Q.; Lu, C.-H.; Mai, L.; Bao, L.-J.; Liu, L.-Y.; Zeng, E.Y. Response of rice (Oryza sativa L.) roots to nanoplastic treatment at seedling stage. J. Hazard. Mater. 2021, 401, 123412. [Google Scholar] [CrossRef]
- Li, Q.; Zeng, A.; Jiang, X.; Gu, X. Are microplastics correlated to phthalates in facility agriculture soil? J. Hazard. Mater. 2021, 412, 125164. [Google Scholar] [CrossRef]
- Ren, Z.; Gui, X.; Xu, X.; Zhao, L.; Qiu, H.; Cao, X. Microplastics in the soil-groundwater environment: Aging, migration, and co-transport of contaminants—A critical review. J. Hazard. Mater. 2021, 419, 126455. [Google Scholar] [CrossRef]
- Lan, T.; Wang, T.; Cao, F.; Yu, C.; Chu, Q.; Wang, F. A comparative study on the adsorption behavior of pesticides by pristine and aged microplastics from agricultural polyethylene soil films. Ecotoxicol. Environ. Saf. 2021, 209, 111781. [Google Scholar] [CrossRef]
- van Wezel, A.P.; van Vlaardingen, P.; Posthumus, R.; Crommentuijn, G.H.; Sijm, D. Environmental risk limits for two phthalates, with special emphasis on endocrine disruptive properties. Ecotoxicol. Environ. Saf. 2000, 46, 305–321. [Google Scholar] [CrossRef]
- Chen, Y.; Wu, C.; Zhang, H.; Lin, Q.; Hong, Y.; Luo, Y. Empirical estimation of pollution load and contamination levels of phthalate esters in agricultural soils from plastic film mulching in China. Environ. Earth Sci. 2013, 70, 239–247. [Google Scholar] [CrossRef]
- Sun, H.; Lei, C.; Yuan, Y.; Xu, J.; Han, M. Nanoplastic impacts on the foliar uptake, metabolism and phytotoxicity of phthalate esters in corn (Zea mays L.) plants. Chemosphere 2022, 304, 135309. [Google Scholar] [CrossRef] [PubMed]
- Fan, W.; Qiu, C.; Qu, Q.; Hu, X.; Mu, L.; Gao, Z.; Tang, X. Sources and identification of microplastics in soils. Soil Environ. Health 2023, 1, 100019. [Google Scholar] [CrossRef]
- Möller, J.N.; Löder, M.G.J.; Laforsch, C. Finding microplastics in soils: A review of analytical methods. Environ. Sci. Technol. 2020, 54, 2078–2090. [Google Scholar] [CrossRef] [PubMed]
- Claessens, M.; Van Cauwenberghe, L.; Vandegehuchte, M.B.; Janssen, C.R. New techniques for the detection of microplastics in sediments and field collected organisms. Mar. Pollut. Bull. 2013, 70, 227–233. [Google Scholar] [CrossRef]
- Hu, B. Magnetic Density Separation of Polyolefin Wastes. Ph.D. Thesis, Delft University of Technology, Delft, The Netherlands, 2014. [Google Scholar] [CrossRef]
- Crichton, E.M.; Noël, M.; Gies, E.A.; Ross, P.S. A novel, density-independent and FTIR-compatible approach for the rapid extraction of microplastics from aquatic sediments. Anal. Methods 2017, 9, 1419–1428. [Google Scholar] [CrossRef]
- Felsing, S.; Kochleus, C.; Buchinger, S.; Brennholt, N.; Stock, F.; Reifferscheid, G. A new approach in separating microplastics from environmental samples based on their electrostatic behavior. Environ. Pollut. 2018, 234, 279–287. [Google Scholar] [CrossRef]
- Hengstmann, E.; Tamminga, M.; Bruch, C.V.; Fischer, E.K. Microplastic in beach sediments of the Isle of Rügen (Baltic Sea): Implementing a novel glass elutriation column. Mar. Pollut. Bull. 2018, 126, 263–274. [Google Scholar] [CrossRef]
- Grbic, J.; Nguyen, B.; Guo, E.; You, J.B.; Sinton, D.; Rochman, C.M. Magnetic extraction of microplastics from environmental samples. Environ. Sci. Technol. Lett. 2019, 6, 68–72. [Google Scholar] [CrossRef]
- Yang, L.; Zhang, Y.; Kang, S.; Wang, Z.; Wu, C. Microplastics in soil: A review on methods, occurrence, sources, and potential risk. Sci. Total Environ. 2021, 780, 146546. [Google Scholar] [CrossRef]
- Imhof, H.K.; Laforsch, C.; Wiesheu, A.C.; Schmid, J.; Anger, P.M.; Niessner, R.; Ivleva, N.P. A novel, highly efficient method for the separation and quantification of plastic particles in sediments of aquatic environments. Limnol. Oceanogr. Methods 2012, 10, 524–537. [Google Scholar] [CrossRef]
- Mani, T.; Frehland, S.; Kalberer, A.; Burkhardt-Holm, P. Using castor oil to separate microplastics from four different environmental matrices. Anal. Methods 2019, 11, 1788–1794. [Google Scholar] [CrossRef]
- Enders, K.; Tagg, A.S.; Labrenz, M. Evaluation of electrostatic separation of microplastics from mineral-rich environmental samples. Front. Environ. Sci. 2020, 8, 112. [Google Scholar] [CrossRef]
- Liu, M.; Lu, S.; Chen, Y.; Cao, C.; Bigalke, M.; He, D. Analytical methods for microplastics in environments: Current advances and challenges. In The Handbook of Environmental Chemistry; Springer: Berlin/Heidelberg, Germany, 2020; pp. 3–24. [Google Scholar]
- Van Cauwenberghe, L.; Devriese, L.; Galgani, F.; Robbens, J.; Janssen, C.R. Microplastics in sediments: A review of techniques, occurrence and effects. Mar. Environ. Res. 2015, 111, 5–17. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.; Kim, S.; Sin, A.; Kim, G.; Khan, S.; Nadagouda, M.N.; Sahle-Demessie, E.; Han, C. Pretreatment methods for monitoring microplastics in soil and freshwater sediment samples: A comprehensive review. Sci. Total Environ. 2023, 871, 161718. [Google Scholar] [CrossRef]
- Plastics Europe. Plastics—The Facts 2020: An Analysis of European Plastics Production, Demand, and Waste Data. Plastics Europe. 2020. Available online: https://plasticseurope.org/knowledge-hub/plastics-the-facts-2020/ (accessed on 20 December 2024).
- Scheurer, M.; Bigalke, M. Microplastics in Swiss floodplain soils. Environ. Sci. Technol. 2018, 52, 3591–3598. [Google Scholar] [CrossRef]
- Liu, M.; Song, Y.; Lu, S.; Qiu, R.; Hu, J.; Li, X.; Bigalke, M.; Shi, H.; He, D. A method for extracting soil microplastics through circulation of sodium bromide solutions. Sci. Total Environ. 2019, 691, 341–347. [Google Scholar] [CrossRef]
- Gohla, J.; Bračun, S.; Gretschel, G.; Koblmüller, S.; Wagner, M.; Pacher, C. Potassium carbonate (K2CO3)—A cheap, non-toxic and high-density floating solution for microplastic isolation from beach sediments. Mar. Pollut. Bull. 2021, 170, 112618. [Google Scholar] [CrossRef]
- Uguagliati, F.; Zattin, M.; Waldschläger, K.; Ghinassi, M. Optimising microplastic polyethylene terephthalate fibre extraction from sediments: Tailoring a density-separation procedure for enhanced recovery and reliability. Sci. Total Environ. 2024, 957, 177483. [Google Scholar] [CrossRef]
- Masura, J.; Baker, J.; Foster, G.; Arthur, C. Laboratory Methods for the Analysis of Microplastics in the Marine Environment: Recommendations for Quantifying Synthetic Particles in Waters and Sediments; NOAA Tech Memo NOS-OR&R-48; NOAA Marine Debris Division: Silver Spring, MD, USA, 2015. [Google Scholar]
- Maes, T.; Jessop, R.; Wellner, N.; Haupt, K.; Mayes, A.G. A rapid-screening approach to detect and quantify microplastics based on fluorescent tagging with Nile Red. Sci. Rep. 2017, 7, 44501. [Google Scholar] [CrossRef]
- Thomas, D.; Schütze, B.; Heinze, W.M.; Steinmetz, Z. Sample preparation techniques for the analysis of microplastics in soil—A review. Sustainability 2020, 12, 9074. [Google Scholar] [CrossRef]
- Zubkov, M.B.; Esiukova, E.E. Microplastics in a Marine Environment: Review of Methods for Sampling, Processing, and Analyzing Microplastics in Water, Bottom Sediments, and Coastal Deposits. Oceanology 2018, 58, 137–143. [Google Scholar] [CrossRef]
- Quinn, B.; Murphy, F.; Ewins, C. Validation of density separation for the rapid recovery of microplastics from sediment. Anal. Methods 2017, 9, 1491–1498. [Google Scholar] [CrossRef]
- Liu, M.; Song, Y.; Lu, S.; Lei, L.; Hu, J.; Lv, W.; Zhou, W.; Cao, C.; Shi, H.; Yang, X.; et al. Microplastic and mesoplastic pollution in farmland soils in suburbs of Shanghai, China. Environ. Pollut. 2018, 242, 855–862. [Google Scholar] [CrossRef] [PubMed]
- Van Cauwenberghe, L.; Vanreusel, A.; Mees, J.; Janssen, C.R. Microplastic pollution in deep-sea sediments. Environ. Pollut. 2013, 182, 495–499. [Google Scholar] [CrossRef]
- Kedzierski, M.; Le Tilly, V.; César, G.; Sire, O.; Bruzaud, S. Efficient microplastics extraction from sand. A cost effective methodology based on sodium iodide recycling. Mar. Pollut. Bull. 2017, 115, 120–129. [Google Scholar] [CrossRef]
- Nuelle, M.-T.; Dekiff, J.H.; Remy, D.; Fries, E. A new analytical approach for monitoring microplastics in marine sediments. Environ. Pollut. 2014, 184, 161–169. [Google Scholar] [CrossRef]
- Han, X.; Lu, X.; Vogt, R.D. An optimized density-based approach for extracting microplastics from soil and sediment samples. Environ. Pollut. 2019, 254, 113009. [Google Scholar] [CrossRef]
- Dekiff, J.H.; Remy, D.; Klasmeier, J.; Fries, E. Occurrence and spatial distribution of microplastics in sediments from Norderney. Environ. Pollut. 2014, 186, 248–256. [Google Scholar] [CrossRef]
- Hurley, R.R.; Lusher, A.L.; Olsen, M.; Nizzetto, L. Validation of a method for extracting microplastics from complex, organic-rich, environmental matrices. Environ. Sci. Technol. 2018, 52, 7409–7417. [Google Scholar] [CrossRef]
- Frère, L.; Paul-Pont, I.; Rinnert, E.; Petton, S.; Jaffré, J.; Bihannic, I.; Soudant, P.; Lambert, C.; Huvet, A. Influence of environmental and anthropogenic factors on the composition, concentration and spatial distribution of microplastics: A case study of the Bay of Brest (Brittany, France). Environ. Pollut. 2017, 225, 211–222. [Google Scholar] [CrossRef]
- van Den Berg, P.; Huerta-Lwanga, E.; Corradini, F.; Geissen, V. Sewage sludge application as a vehicle for microplastics in eastern Spanish agricultural soils. Environ. Pollut. 2020, 261, 114198. [Google Scholar] [CrossRef] [PubMed]
- Corradini, F.; Meza, P.; Eguiluz, R.; Casado, F.; Huerta-Lwanga, E.; Geissen, V. Evidence of microplastic accumulation in agricultural soils from sewage sludge disposal. Sci. Total Environ. 2019, 671, 411–420. [Google Scholar] [CrossRef]
- Corradini, F.; Casado, F.; Leiva, V.; Huerta-Lwanga, E.; Geissen, V. Microplastics occurrence and frequency in soils under different land uses on a regional scale. Sci. Total Environ. 2021, 752, 141917. [Google Scholar] [CrossRef]
- Zhou, B.; Wang, J.; Zhang, H.; Shi, H.; Fei, Y.; Huang, S.; Tong, Y.; Wen, D.; Luo, Y.; Barceló, D. Microplastics in agricultural soils on the coastal plain of Hangzhou Bay, east China: Multiple sources other than plastic mulching film. J. Hazard. Mater. 2020, 388, 121814. [Google Scholar] [CrossRef]
- Zhou, Q.; Zhang, H.; Fu, C.; Zhou, Y.; Dai, Z.; Li, Y.; Tu, C.; Luo, Y. The distribution and morphology of microplastics in coastal soils adjacent to the Bohai Sea and the Yellow Sea. Geoderma 2018, 322, 201–208. [Google Scholar] [CrossRef]
- Zhou, Y.; Liu, X.; Wang, J. Characterization of microplastics and the association of heavy metals with microplastics in suburban soil of central China. Sci. Total Environ. 2019, 694, 133798. [Google Scholar] [CrossRef]
- Oehl, F.; Sieverding, E.; Ineichen, K.; Mäder, P.; Boller, T.; Wiemken, A. Impact of land use intensity on the species diversity of arbuscular mycorrhizal fungi in agroecosystems of Central Europe. Appl. Environ. Microbiol. 2003, 69, 2816–2824. [Google Scholar] [CrossRef]
- Shamini, S.; Amutha, K. Techniques for extraction of arbuscular mycorrhizal fungi spores. Int. J. Front. Sci. Technol. 2014, 2, 1–6. [Google Scholar]
- Dane, J.H.; Topp, G.C. (Eds.) Methods of Soil Analysis: Part 4 Physical Methods; SSSA Book Series No. 5; Soil Science Society of America: Madison, WI, USA, 2002; p. 1692. [Google Scholar]
- Vermaire, J.C.; Pomeroy, C.; Herczegh, S.M.; Haggart, O.; Murphy, M. Microplastic abundance and distribution in the open water and sediment of the Ottawa River, Canada, and its tributaries. Facets 2017, 2, 301–314. [Google Scholar] [CrossRef]
- Garces-Ordóñez, O.; Castillo-Olaya, V.A.; Granados-Briceño, A.F.; García, L.M.B.; Díaz, L.F.E. Marine litter and microplastic pollution on mangrove soils of the Ciénaga Grande de Santa Marta, Colombian Caribbean. Mar. Pollut. Bull. 2019, 145, 455–462. [Google Scholar] [CrossRef]
- Zhang, G.S.; Liu, Y.F. The distribution of microplastics in soil aggregate fractions in southwestern China. Sci. Total Environ. 2018, 642, 12–20. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Yang, X.; Gertsen, H.; Peters, P.; Salánki, T.; Geissen, V. A simple method for the extraction and identification of light-density microplastics from soil. Sci. Total Environ. 2018, 616–617, 1056–1065. [Google Scholar] [CrossRef] [PubMed]
- Hagen, N.; Tadokoro, T. The rainbow beam experiment: Direct visualization of dipole scattering and optical rotatory dispersion. In Proceedings of the SPIE, Polarization Science and Remote Sensing IX, San Diego, CA, USA, 11–15 August 2019; Volume 11132, p. 111320E. [Google Scholar] [CrossRef]
- Grause, G.; Kuniyasu, Y.; Chien, M.F.; Inoue, C. Separation of microplastic from soil by centrifugation and its application to agricultural soil. Chemosphere 2022, 288, 132654. [Google Scholar] [CrossRef]
- Rühlmann, J.; Körschens, M.; Graefe, J. A new approach to calculate the particle density of soils considering properties of the soil organic matter and the mineral matrix. Geoderma 2006, 130, 272–283. [Google Scholar] [CrossRef]
- Tagg, A.S.; Harrison, J.P.; Ju-Nam, Y.; Sapp, M.; Bradley, E.L.; Sinclair, C.J.; Ojeda, J.J. Fenton’s reagent for the rapid and efficient isolation of microplastics from wastewater. Chem. Commun. 2017, 53, 372–375. [Google Scholar] [CrossRef]
- Herrera, A.; Garrido-Amador, P.; Martínez, I.; Samper, M.D.; Lopez-Martínez, J.; Gómez, M.; Packard, T.T. Novel methodology to isolate microplastics from vegetal-rich samples. Mar. Pollut. Bull. 2018, 129, 61–69. [Google Scholar] [CrossRef]
- Bläsing, M.; Amelung, W. Plastics in soil: Analytical methods and possible sources. Sci. Total Environ. 2018, 612, 422–435. [Google Scholar] [CrossRef]
- Ragoobur, D.; Huerta-Lwanga, E.; Somaroo, G.D. Microplastics in agricultural soils, wastewater effluents and sewage sludge in Mauritius. Sci. Total Environ. 2021, 798, 149326. [Google Scholar] [CrossRef]
- Duan, Z.; Zhao, S.; Zhao, L.; Duan, X.; Xie, S.; Zhang, H.; Liu, Y.; Peng, Y.; Liu, C.; Wang, L. Microplastics in Yellow River Delta wetland: Occurrence, characteristics, human influences, and marker. Environ. Pollut. 2020, 258, 113232. [Google Scholar] [CrossRef]
- Huang, Y.; Liu, Q.; Jia, W.; Yan, C.; Wang, J. Agricultural plastic mulching as a source of microplastics in the terrestrial environment. Environ. Pollut. 2020, 260, 114096. [Google Scholar] [CrossRef]
- Kim, S.-K.; Kim, J.-S.; Lee, H.; Lee, H.-J. Abundance and characteristics of microplastics in soils with different agricultural practices: Importance of sources with internal origin and environmental fate. J. Hazard. Mater. 2021, 403, 123997. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Wu, J.; Zhao, X.; Gu, X.; Ji, R. Separation and identification of microplastics from soil and sewage sludge. Environ. Pollut. 2019, 254, 113076. [Google Scholar] [CrossRef] [PubMed]
- Lv, W.; Zhou, W.; Lu, S.; Huang, W.; Yuan, Q.; Tian, M.; Lv, W.; He, D. Microplastic pollution in rice-fish co-culture system: A report of three farmland stations in Shanghai, China. Sci. Total Environ. 2019, 652, 1209–1218. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Reverón, R.; González-Sálamo, J.; Hernández-Sánchez, C.; González-Pleiter, M.; Hernández-Borges, J.; Díaz-Peña, F.J. Recycled wastewater as a potential source of microplastics in irrigated soils from an arid-insular territory (Fuerteventura, Spain). Sci. Total Environ. 2022, 817, 152830. [Google Scholar] [CrossRef]

| Method | Advantages | Limitations | References |
|---|---|---|---|
| Manual extraction | Simple | Time-consuming | Möller et al. [34] |
| Cheap | Size-limited | ||
| Misidentification | |||
| Electrostatic separation | High recovery | Must be verified for small-sized MPs | Felsing et al. [38] |
| Simple | Sample preparation | ||
| Fast | Potentially inapplicable for cohesive samples | ||
| Unaffected by plastic properties | |||
| Froth flotation | Large sample | Time-consuming | Imhof et al. [42] |
| Low recovery | |||
| Polymer-dependent | |||
| Magnetic extraction | Separates small-sized MPs | Time-consuming | Grbic et al. [40] |
| Cheap | Low recovery | ||
| Polymer-dependent | |||
| Sample damage | |||
| Elutriation | Fast | Low recovery | Claessens et al. [35] |
| Simple | Sand bycatch | Hengstmann et al. [39] | |
| Cheap | |||
| Vertical density gradient separation | Applicable within the recycling industry | Must be further examined | Hu et al. [36] |
| Potentially financially unjustifiable | |||
| Oil separation | High recovery | Size-limited | Crichton et al. [37] |
| Simple | Lower recovery for low-density MPs | Yang et al. [41] | |
| Fast | Interference with spectroscopic identification methods | ||
| Cheap |
| Solution | Density [g/cm3] | Polymer(s) | Recovery | Reference |
|---|---|---|---|---|
| H2O | 1 | PP, PE | 40–90% | Quinn et al. [57] |
| NaCl | 1.2 | PS, PP, PE, PA, PC, ABS, PMMA | 69–98% | Liu et al. [58] |
| NaBr | 1.4–1.6 | PA, PP, PE, PET, POM, PVC, PC, ABS, PMMA, PS | 85–100% | M. Liu et al. [50] |
| NaI hc | 1.6–1.8 | PVC | 98–100% | Cauwenberghe [59] |
| CaCl2 | 1.3–1.5 | MPs | 93–98% | Scheurer and Bigalke [49] |
| ZnCl2 hc | 1.37 | PA, PE | 85–98% | Maes et al. [54] |
| ZnBr2 hc | 1.7 | PE, HDPE, PS, PVC, PET | 95–100% | Quinn et al. [57] |
| K2CO3 | 1.54 | PVC | 90% | Gohla et al. [51] |
| SPT | up to 3.1 | PET | 86–99% | Uguagliati et al. [52] |
| LMT c | 1.62 | PE | 81% | Masura et al. [53] |
| Sava | Danube | Morava | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Replicate | 1 | 2 | 3 | 1 | 2 | 3 | 1 | 2 | 3 | |
| MP [g] | 1000–3000 μm | 2.0457 | 2.2122 | 2.2800 | 2.5873 | 2.4185 | 2.6097 | 2.8718 | 2.7567 | 3.0712 |
| 500–1000 μm | 0.9708 | 0.9462 | 1.0192 | 1.0403 | 1.0119 | 1.0542 | 1.0659 | 1.1145 | 1.1358 | |
| Sava | Danube | Morava | RSD | Average Recovery [%] | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Replicate | 1 | 2 | 3 | 1 | 2 | 3 | 1 | 2 | 3 | [%] | ||
| 1000–3000 μm | [g] | 1.9280 | 2.0658 | 2.0774 | 2.3038 | 2.1856 | 2.4459 | 2.7376 | 2.5659 | 2.9327 | 2.40 | 92.86 ± 2.23 |
| [%] | 94.25 | 93.38 | 91.11 | 89.04 | 90.37 | 93.73 | 95.32 | 93.08 | 95.49 | |||
| 500–1000 μm | [g] | 0.8682 | 0.8718 | 0.9239 | 0.8884 | 0.8953 | 0.9769 | 0.9334 | 1.0104 | 1.0340 | 2.58 | 89.78 ± 2.31 |
| [%] | 89.43 | 92.14 | 90.65 | 85.39 | 88.48 | 92.66 | 87.57 | 90.66 | 91.03 | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Grujić, T.; Saljnikov, E.; Stefanović, S.; Lazović, V.; Belanović Simić, S.; Marjanović, Ž. Upgraded Protocol for Microplastics’ Extraction from the Soil Matrix by Sucrose Density Gradient Centrifugation. Soil Syst. 2025, 9, 66. https://doi.org/10.3390/soilsystems9030066
Grujić T, Saljnikov E, Stefanović S, Lazović V, Belanović Simić S, Marjanović Ž. Upgraded Protocol for Microplastics’ Extraction from the Soil Matrix by Sucrose Density Gradient Centrifugation. Soil Systems. 2025; 9(3):66. https://doi.org/10.3390/soilsystems9030066
Chicago/Turabian StyleGrujić, Tara, Elmira Saljnikov, Slobodan Stefanović, Vojislav Lazović, Snežana Belanović Simić, and Žaklina Marjanović. 2025. "Upgraded Protocol for Microplastics’ Extraction from the Soil Matrix by Sucrose Density Gradient Centrifugation" Soil Systems 9, no. 3: 66. https://doi.org/10.3390/soilsystems9030066
APA StyleGrujić, T., Saljnikov, E., Stefanović, S., Lazović, V., Belanović Simić, S., & Marjanović, Ž. (2025). Upgraded Protocol for Microplastics’ Extraction from the Soil Matrix by Sucrose Density Gradient Centrifugation. Soil Systems, 9(3), 66. https://doi.org/10.3390/soilsystems9030066









