Isotope Detection in Microwave-Assisted Laser-Induced Plasma
Abstract
1. Introduction
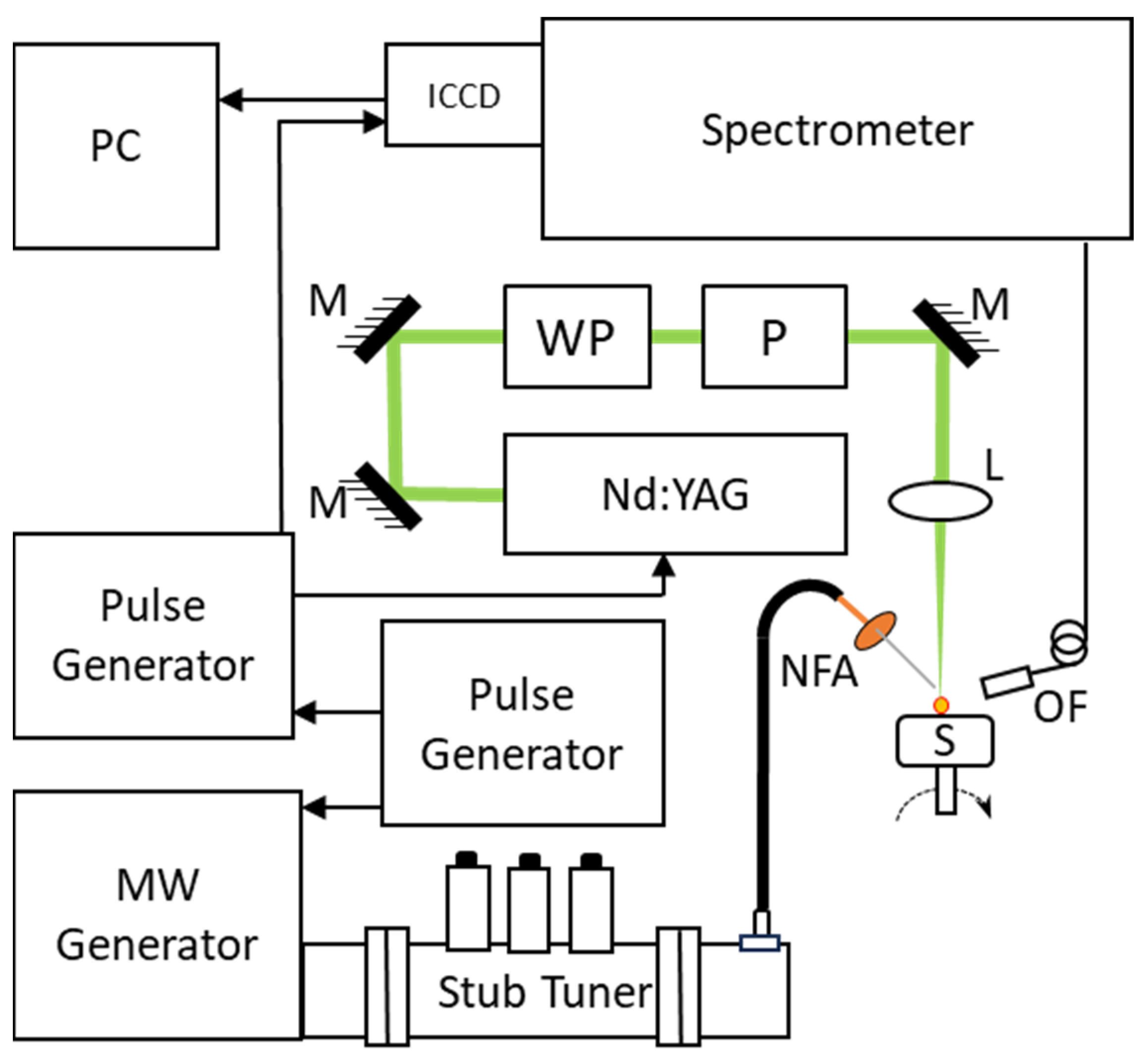
2. Experimental Arrangement
3. Results
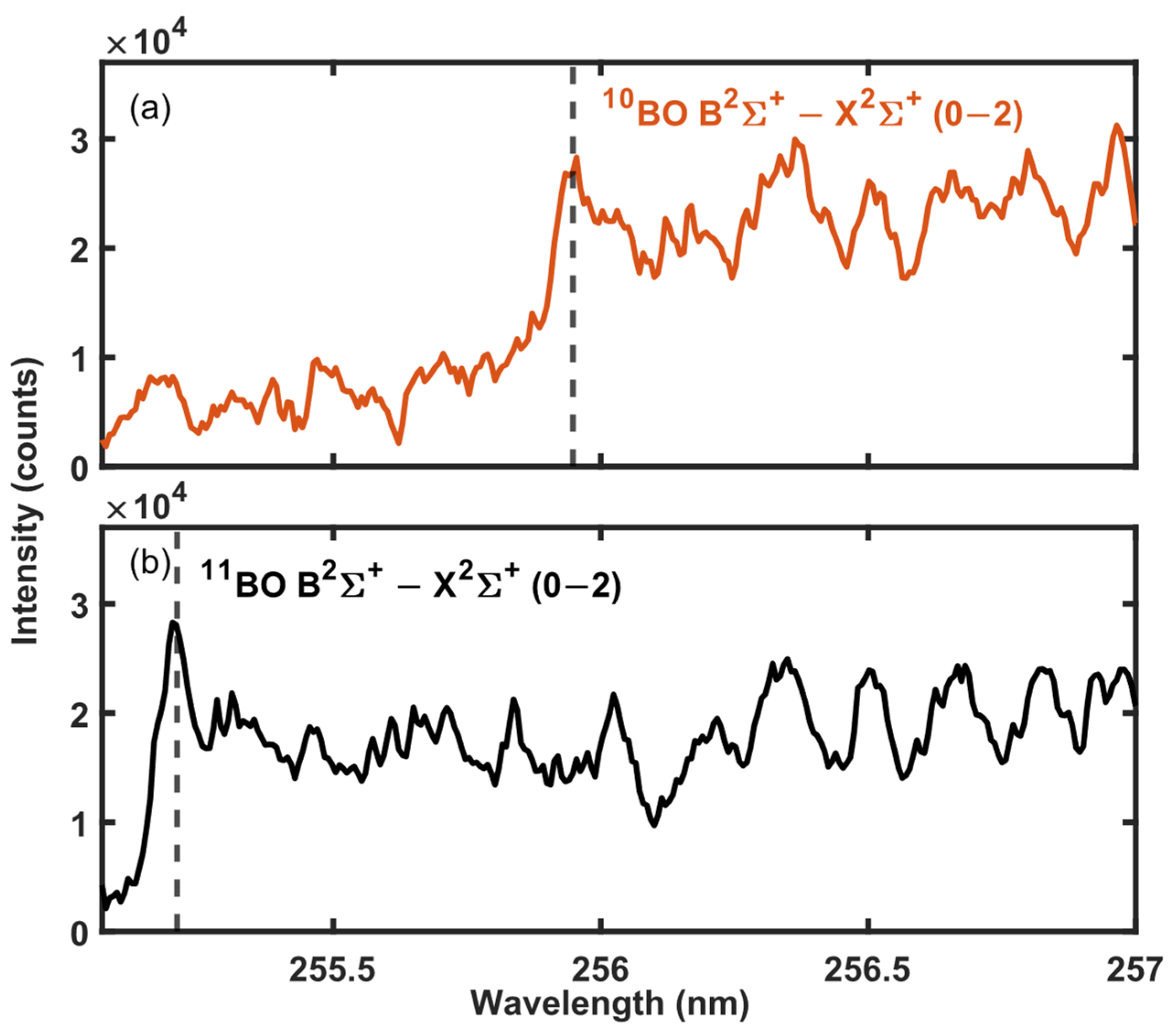
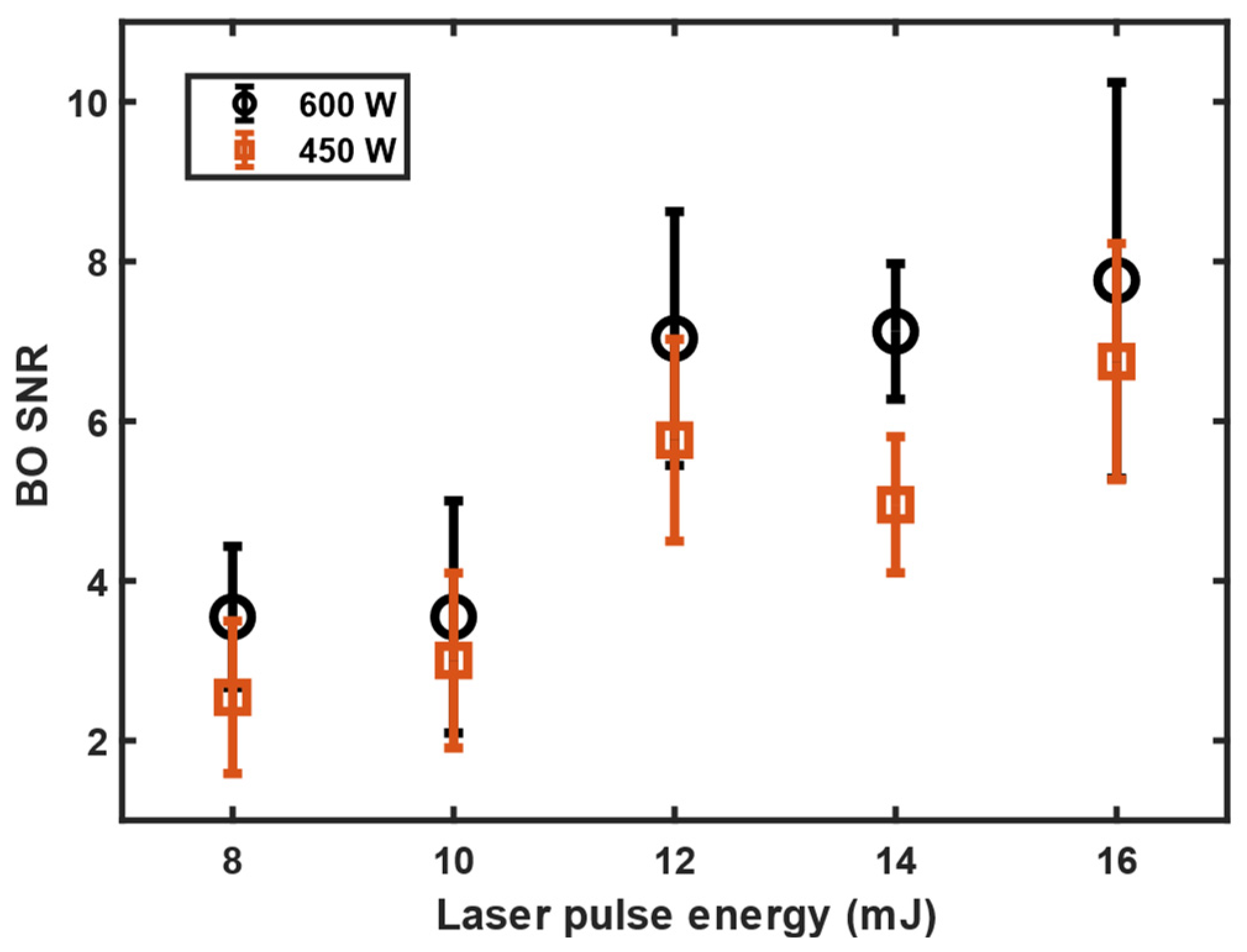
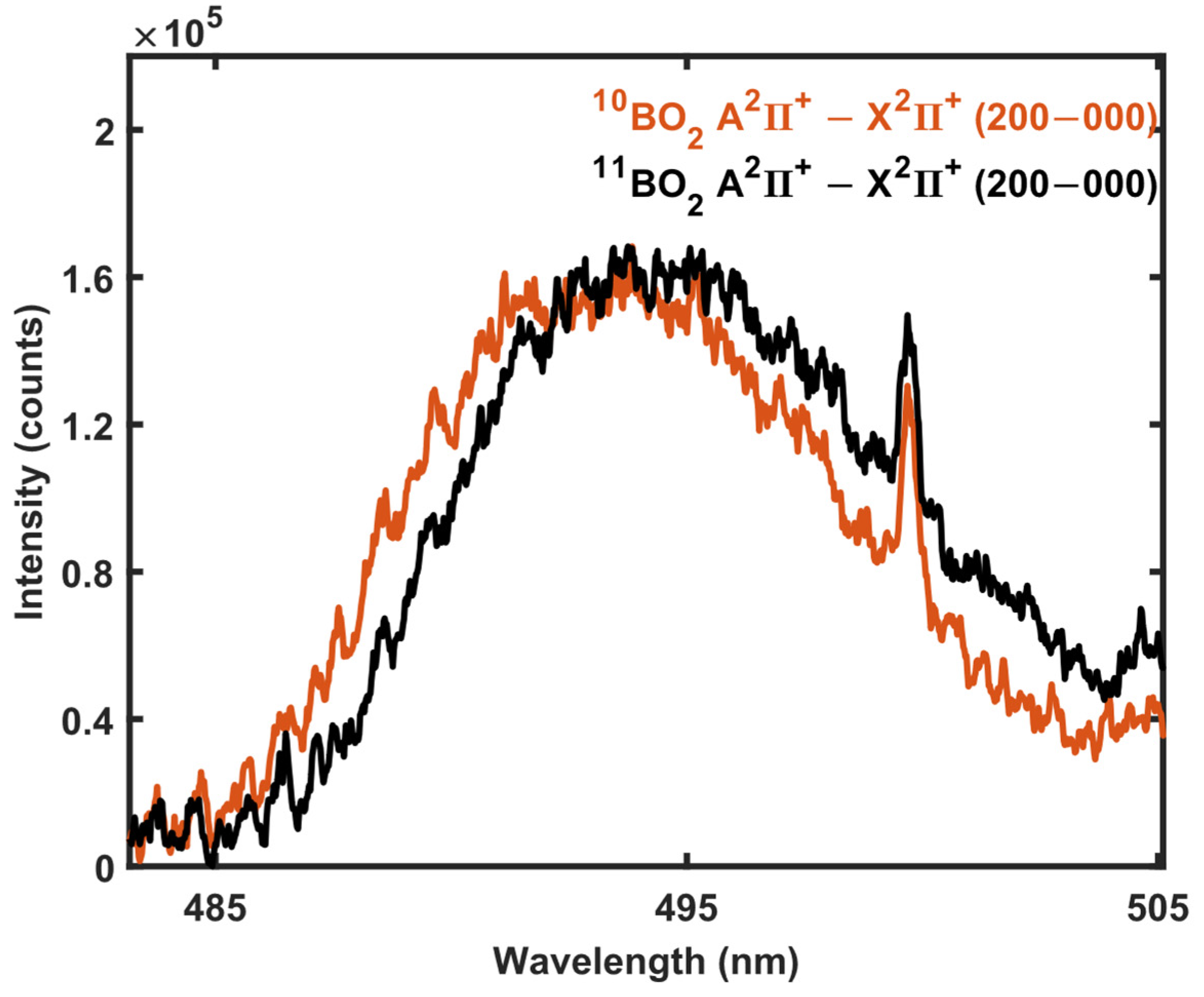
3.1. 11B and 10B Isotope Detection
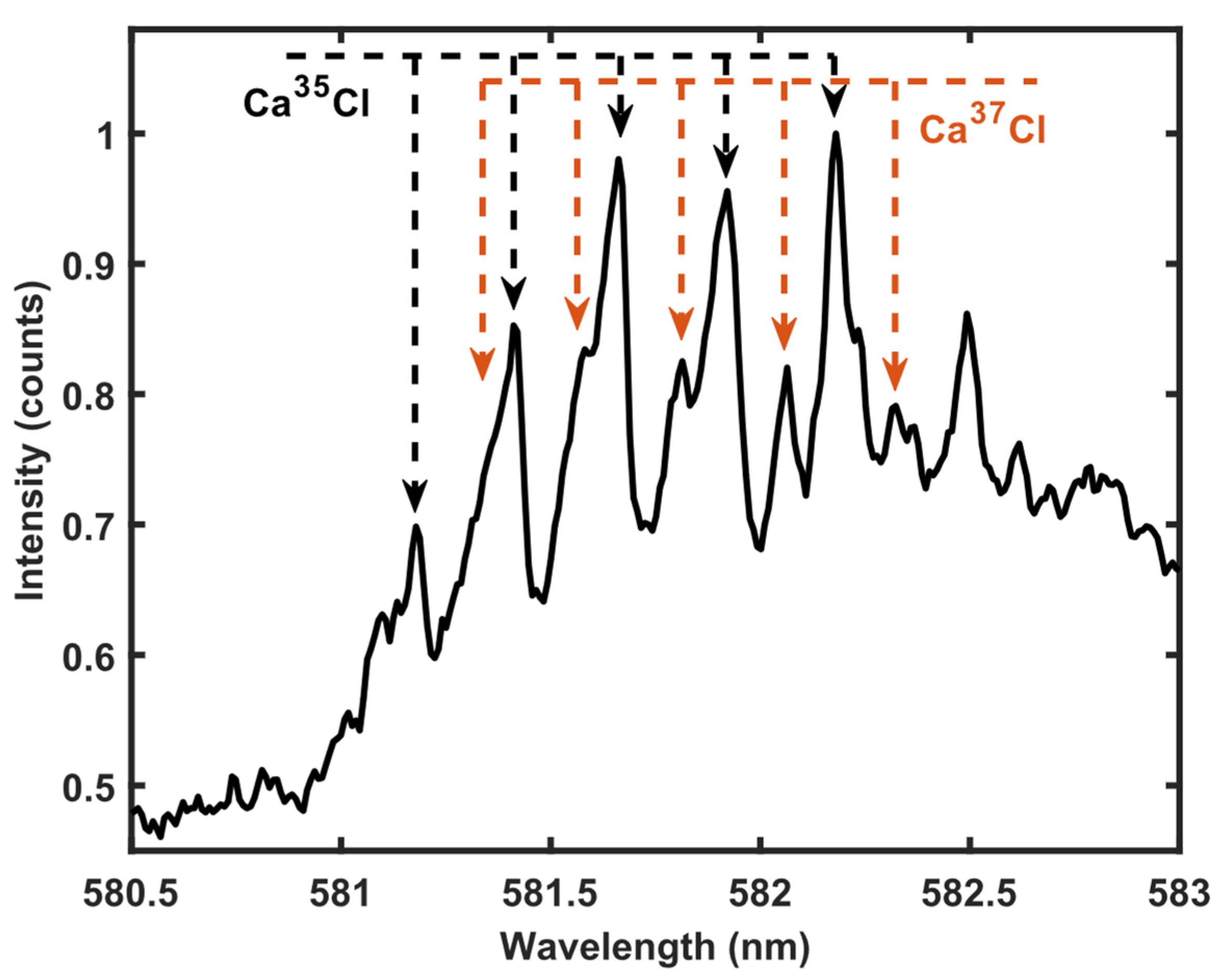
3.2. 35Cl and 37Cl Isotope Detection
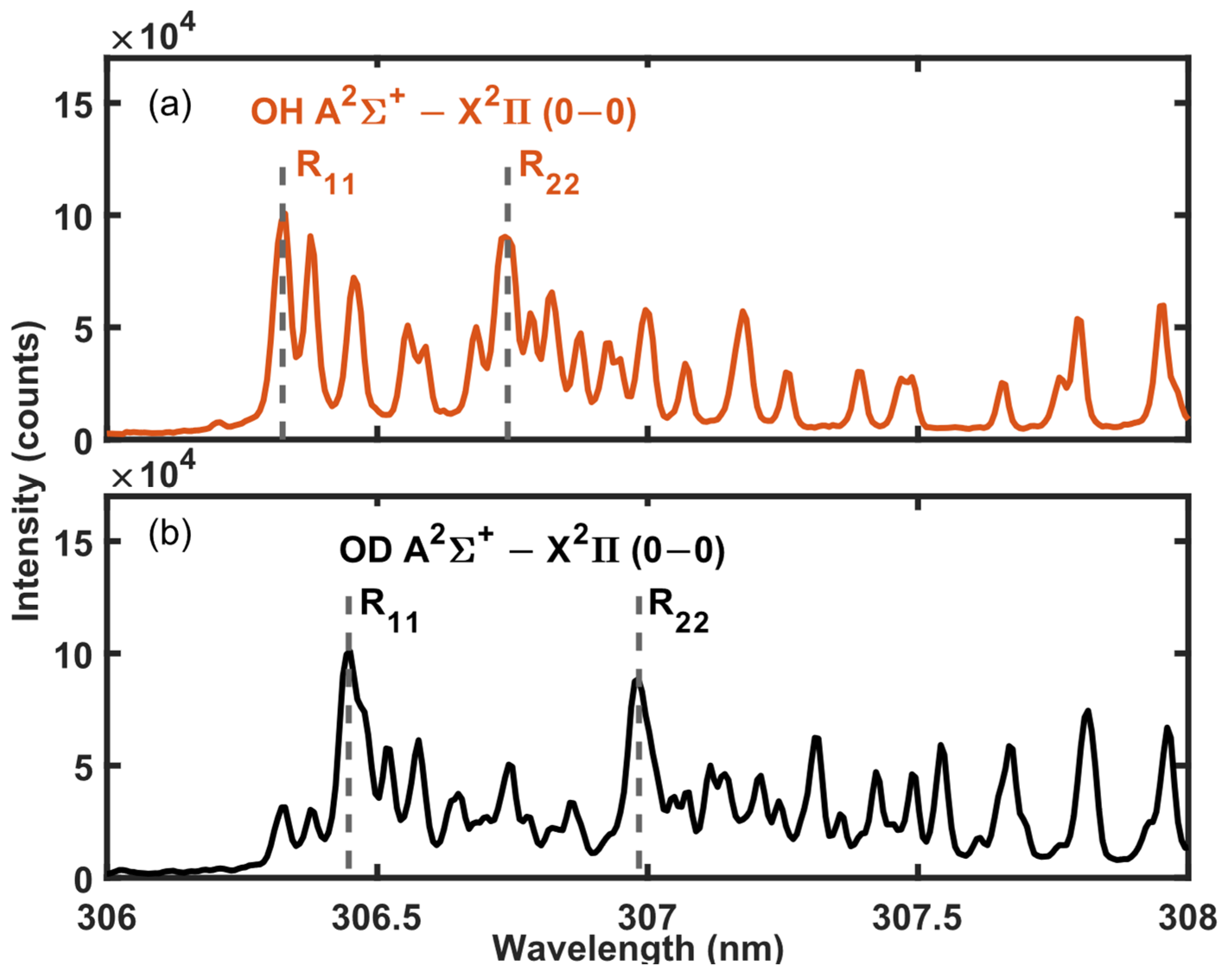
3.3. 1H and 2H Isotope Detection
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hahn, D.W.; Omenetto, N. Laser-Induced Breakdown Spectroscopy (LIBS), Part II: Review of Instrumental and Methodological Approaches to Material Analysis and Applications to Different Fields. Appl. Spectrosc. 2012, 66, 347–419. [Google Scholar] [CrossRef] [PubMed]
- Hahn, D.W.; Omenetto, N. Laser-induced breakdown spectroscopy (LIBS), part I: Review of basic diagnostics and plasma–particle interactions: Still-challenging issues within the analytical plasma community. Appl. Spectrosc. 2010, 64, 335A–366A. [Google Scholar] [CrossRef] [PubMed]
- Guezenoc, J.; Gallet-Budynek, A.; Bousquet, B. Critical review and advices on spectral-based normalization methods for LIBS quantitative analysis. Spectrochim. Acta Part B At. Spectrosc. 2019, 160, 105688. [Google Scholar] [CrossRef]
- Bol’Shakov, A.A.; Mao, X.; Perry, D.L.; Russo, R.E. Laser Ablation Molecular Isotopic Spectrometry for rare isotopes of the light elements. Spectroscopy 2014, 29, 30–39. [Google Scholar]
- Fernández-Menéndez, L.J.; Méndez-López, C.; Abad, C.; Fandiño, J.; González-Gago, C.; Pisonero, J.; Bordel, N. A critical evaluation of the chlorine quantification method based on molecular emission detection in LIBS. Spectrochim. Acta Part B At. Spectrosc. 2022, 190, 106390. [Google Scholar] [CrossRef]
- Moros, J.; Laserna, J. Laser-Induced Breakdown Spectroscopy (LIBS) of Organic Compounds: A Review. Appl. Spectrosc. 2019, 73, 963–1011. [Google Scholar] [CrossRef]
- Russo, R.E.; Bol’shakov, A.A.; Mao, X.; McKay, C.P.; Perry, D.L.; Sorkhabi, O. Laser ablation molecular isotopic spectrometry. Spectrochim. Acta Part B At. Spectrosc. 2011, 66, 99–104. [Google Scholar] [CrossRef]
- Gaudiuso, R.; Dell’Aglio, M.; De Pascale, O.; Senesi, G.S.; De Giacomo, A. Laser induced breakdown spectroscopy for elemental analysis in environmental, cultural heritage and space applications: A review of methods and results. Sensors 2010, 10, 7434–7468. [Google Scholar] [CrossRef]
- Bol’Shakov, A.A.; Mao, X.; González, J.J.; Russo, R.E. Laser ablation molecular isotopic spectrometry (LAMIS): Current state of the art. J. Anal. At. Spectrom. 2015, 31, 119–134. [Google Scholar] [CrossRef]
- Dong, M.; Mao, X.; Gonzalez, J.J.; Lu, J.; Russo, R.E. Carbon Isotope Separation and Molecular Formation in Laser-Induced Plasmas by Laser Ablation Molecular Isotopic Spectrometry. Anal. Chem. 2013, 85, 2899–2906. [Google Scholar] [CrossRef]
- Sarkar, A.; Mao, X.; Chan, G.C.-Y.; Russo, R.E. Laser ablation molecular isotopic spectrometry of water for 1D2/1H1 ratio analysis. Spectrochim. Acta Part B At. Spectrosc. 2013, 88, 46–53. [Google Scholar] [CrossRef]
- Mao, X.; Chan, G.C.-Y.; Choi, I.; Zorba, V.; Russo, R.E. Combination of atomic lines and molecular bands for uranium optical isotopic analysis in laser induced plasma spectrometry. J. Radioanal. Nucl. Chem. 2017, 312, 121–131. [Google Scholar] [CrossRef]
- Silverman, S.N.; Phillips, A.A.; Weiss, G.M.; Wilkes, E.B.; Eiler, J.M.; Sessions, A.L. Practical considerations for amino acid isotope analysis. Org. Geochem. 2022, 164, 104345. [Google Scholar] [CrossRef]
- Di, Y.; Krestianinov, E.; Zink, S.; Amelin, Y. High-precision multidynamic Sr isotope analysis using thermal ionization mass spectrometer (TIMS) with correction of fractionation drift. Chem. Geol. 2021, 582, 120411. [Google Scholar] [CrossRef]
- Gillespie, J.; Nemchin, A.A.; Kinny, P.D.; Martin, L.; Aleshin, M.; Roberts, M.P.; Ireland, T.R.; Whitehouse, M.J.; Jeon, H.; Cavosie, A.J.; et al. Strontium isotope analysis of apatite via SIMS. Chem. Geol. 2020, 559, 119979. [Google Scholar] [CrossRef]
- Vanhaecke, F.; Degryse, P. Isotopic Analysis: Fundamentals and Applications Using ICP-MS; John Wiley & Sons: Hoboken, NJ, USA, 2012. [Google Scholar]
- Bol’Shakov, A.A.; Mao, X.; Russo, R.E. Spectral emission enhancement by an electric pulse for LIBS and LAMIS. J. Anal. At. Spectrom. 2017, 32, 657–670. [Google Scholar] [CrossRef]
- Mao, X.; Bol’Shakov, A.A.; Perry, D.L.; Sorkhabi, O.; Russo, R.E. Laser Ablation Molecular Isotopic Spectrometry: Parameter influence on boron isotope measurements. Spectrochim. Acta Part B At. Spectrosc. 2011, 66, 604–609. [Google Scholar] [CrossRef]
- Choi, S.-U.; Han, S.-C.; Lee, J.-Y.; Yun, J.-I. Isotope analysis of iron on structural materials of nuclear power plants using double-pulse laser ablation molecular isotopic spectrometry. J. Anal. At. Spectrom. 2021, 36, 1287–1296. [Google Scholar] [CrossRef]
- Wakil, M.A.; Alwahabi, Z.T. Gated and non-gated silver detection using microwave-assisted laser induced breakdown spectroscopy. J. Anal. At. Spectrom. 2021, 36, 185–193. [Google Scholar] [CrossRef]
- Liu, Y.; Baudelet, M.; Richardson, M. Elemental analysis by microwave-assisted laser-induced breakdown spectroscopy: Evaluation on ceramics. J. Anal. At. Spectrom. 2010, 25, 1316–1323. [Google Scholar] [CrossRef]
- Khumaeni, A.; Motonobu, T.; Katsuaki, A.; Masabumi, M.; Ikuo, W. Enhancement of LIBS emission using antenna-coupled microwave. Opt. Express 2013, 21, 29755–29768. [Google Scholar] [CrossRef] [PubMed]
- Oba, M.; Miyabe, M.; Akaoka, K.; Wakaida, I. Development of microwave-assisted, laser-induced breakdown spectroscopy without a microwave cavity or waveguide. Jpn. J. Appl. Phys. 2020, 59, 062001. [Google Scholar] [CrossRef]
- Wall, M.; Sun, Z.; Alwahabi, Z.T. Quantitative detection of metallic traces in water-based liquids by microwave-assisted laser-induced breakdown spectroscopy. Opt. Express 2016, 24, 1507–1517. [Google Scholar] [CrossRef] [PubMed]
- Viljanen, J.; Zhao, H.; Zhang, Z.; Toivonen, J.; Alwahabi, Z. Real-time release of Na, K and Ca during thermal conversion of biomass using quantitative microwave-assisted laser-induced breakdown spectroscopy. Spectrochim. Acta Part B At. Spectrosc. 2018, 149, 76–83. [Google Scholar] [CrossRef]
- Viljanen, J.; Sun, Z.; Alwahabi, Z.T. Microwave assisted laser-induced breakdown spectroscopy at ambient conditions. Spectrochim. Acta Part B At. Spectrosc. 2016, 118, 29–36. [Google Scholar] [CrossRef]
- Ikeda, Y.; Ofosu, J.A.; Wakaida, I. Development of microwave-enhanced fibre-coupled laser-induced breakdown spectroscopy for nuclear fuel debris screening at Fukushima. Spectrochim. Acta Part B At. Spectrosc. 2020, 171, 105933. [Google Scholar] [CrossRef]
- Ikeda, Y.; Soriano, J.K.; Kawahara, N.; Wakaida, I. Spatially and temporally resolved plasma formation on alumina target in microwave-enhanced laser-induced breakdown spectroscopy. Spectrochim. Acta Part B At. Spectrosc. 2022, 197, 106533. [Google Scholar] [CrossRef]
- Wakil, M.; Alwahabi, Z.T. Quantitative fluorine and bromine detection under ambient conditions via molecular emission. J. Anal. At. Spectrom. 2020, 35, 2620–2626. [Google Scholar] [CrossRef]
- Wakil, M.A.; Alwahabi, Z.T. Microwave-assisted laser induced breakdown molecular spectroscopy: Quantitative chlorine detection. J. Anal. At. Spectrom. 2019, 34, 1892–1899. [Google Scholar] [CrossRef]
- Ikeda, Y.; Soriano, J.K. Microwave-enhanced laser-induced air plasma at atmospheric pressure. Opt. Express 2022, 30, 33756. [Google Scholar] [CrossRef]
- Ali, M.; Alamri; Jan, V.; Zeyad, T.A. Properties of Microwave-Assisted Laser-Induced Breakdown Plasma. Spectrochim. Act. B, 2003; Submitted. [Google Scholar]
- Chen, S.J.; Iqbal, A.; Wall, M.; Fumeaux, C.; Alwahabi, Z.T. Design and application of near-field applicators for efficient microwave-assisted laser-induced breakdown spectroscopy. J. Anal. At. Spectrom. 2017, 32, 1508–1518. [Google Scholar] [CrossRef]
- Akpovo, C.A.; Helms, L.; Profeta, L.T.; Johnson, L. Multivariate determination of 10B isotopic ratio by laser-induced breakdown spectroscopy using multiple BO molecular emissions. Spectrochim. Acta Part B At. Spectrosc. 2019, 162, 105710. [Google Scholar] [CrossRef]
- Amiri, S.H.; Darbani, S.M.R.; Saghafifar, H. Detection of BO2 isotopes using laser-induced breakdown spectroscopy. Spectrochim. Acta Part B At. Spectrosc. 2018, 150, 86–91. [Google Scholar] [CrossRef]
- Mélen, F.; Dubois, I.; Bredohl, H. The AX and BX transitions of BO. J. Phys. B At. Mol. Phys. 1985, 18, 2423. [Google Scholar] [CrossRef]
- Johns, J. The absorption spectrum of BO2. Can. J. Phys. 1961, 39, 1738–1768. [Google Scholar] [CrossRef]
- Pasternack, L. Gas-phase modeling of homogeneous boron/oxygen/hydrogen/carbon combustion. Combust. Flame 1992, 90, 259–268. [Google Scholar] [CrossRef]
- Berglund, M.; Wieser, M.E. Isotopic compositions of the elements 2009 (IUPAC Technical Report). Pure Appl. Chem. 2011, 83, 397–410. [Google Scholar] [CrossRef]
- Stewart, M.A.; Spivack, A.J. The stable-chlorine isotope compositions of natural and anthropogenic materials. Rev. Mineral. Geochem. 2004, 55, 231–254. [Google Scholar] [CrossRef]
- Pirali, T.; Serafini, M.; Cargnin, S.; Genazzani, A.A. Applications of Deuterium in Medicinal Chemistry. J. Med. Chem. 2019, 62, 5276–5297. [Google Scholar] [CrossRef]
- Belete, T.M. Recent Updates on the Development of Deuterium-Containing Drugs for the Treatment of Cancer. Drug Des. Dev. Ther. 2022, 16, 3465–3472. [Google Scholar] [CrossRef] [PubMed]
- De Feyter, H.M.; Behar, K.L.; Corbin, Z.A.; Fulbright, R.K.; Brown, P.B.; McIntyre, S.; Nixon, T.W.; Rothman, D.L.; de Graaf, R.A. Deuterium metabolic imaging (DMI) for MRI-based 3D mapping of metabolism in vivo. Sci. Adv. 2018, 4, eaat7314. [Google Scholar] [CrossRef] [PubMed]
- Atzrodt, J.; Derdau, V.; Kerr, W.J.; Reid, M. Deuterium- and Tritium-Labelled Compounds: Applications in the Life Sciences. Angew. Chem. Int. Ed. 2018, 57, 1758–1784. [Google Scholar] [CrossRef] [PubMed]
- Spagnolo, D.A.; Miller, A.I. The CECE Alternative for Upgrading/Detritiation in Heavy Water Nuclear Reactors and for Tritium Recovery in Fusion Reactors. Fusion Technol. 1995, 28, 748–754. [Google Scholar] [CrossRef]
- Stark, G.; Brault, J.; Abrams, M. Fourier-transform spectra of the A 2 Σ+–X 2 Π Δv = 0 bands of OH and OD. JOSA B 1994, 11, 3–32. [Google Scholar] [CrossRef]
- Choi, S.-U.; Han, S.-C.; Yun, J.-I. Hydrogen isotopic analysis using molecular emission from laser-induced plasma on liquid and frozen water. Spectrochim. Acta Part B At. Spectrosc. 2019, 162, 105716. [Google Scholar] [CrossRef]
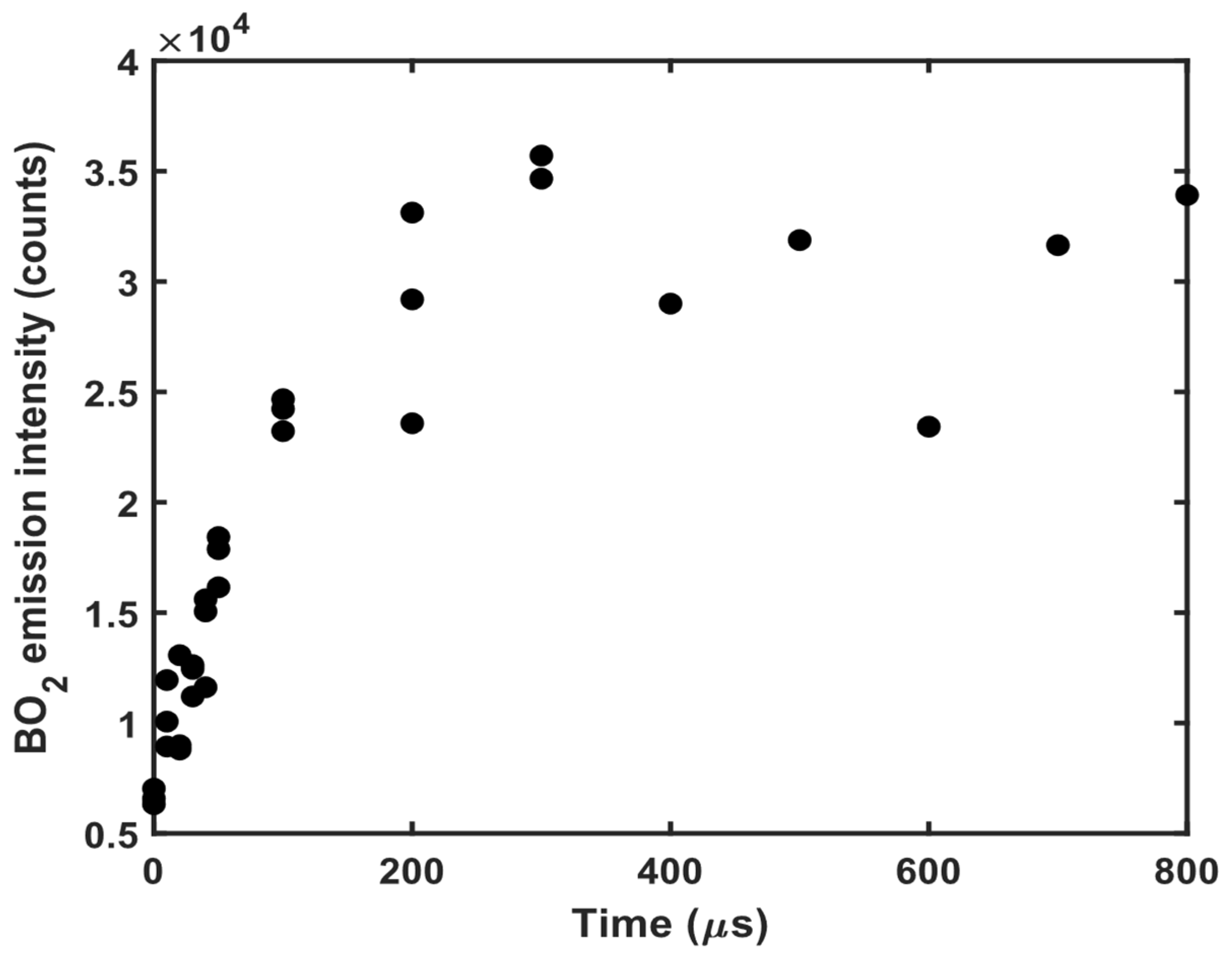
| B + O2 ⇌ BO2 | (1) |
| BO + O + M⇌ BO2 + M | (2) |
| BO + O2 ⇌ BO2 + O | (3) |
| B2O3 + M ⇌ BO + BO2 + M | (4) |
| B2O3 + O ⇌ 2BO2 | (5) |
| 2B2O3 + O2 ⇌ 4BO2 | (6) |
| B2O3 + BO ⇌ B2O2 + BO2 | (7) |
| B2O2 + O ⇌ BO + BO2 | (8) |
| B2O2 + O2 ⇌ 2BO2 | (9) |
| BO + OH ⇌ BO2 + H | (10) |
| BO + CO2 ⇌ BO2 + CO | (11) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alamri, A.M.; Viljanen, J.; Kwong, P.; Alwahabi, Z.T. Isotope Detection in Microwave-Assisted Laser-Induced Plasma. Plasma 2023, 6, 466-477. https://doi.org/10.3390/plasma6030032
Alamri AM, Viljanen J, Kwong P, Alwahabi ZT. Isotope Detection in Microwave-Assisted Laser-Induced Plasma. Plasma. 2023; 6(3):466-477. https://doi.org/10.3390/plasma6030032
Chicago/Turabian StyleAlamri, Ali M., Jan Viljanen, Philip Kwong, and Zeyad T. Alwahabi. 2023. "Isotope Detection in Microwave-Assisted Laser-Induced Plasma" Plasma 6, no. 3: 466-477. https://doi.org/10.3390/plasma6030032
APA StyleAlamri, A. M., Viljanen, J., Kwong, P., & Alwahabi, Z. T. (2023). Isotope Detection in Microwave-Assisted Laser-Induced Plasma. Plasma, 6(3), 466-477. https://doi.org/10.3390/plasma6030032





