Thermal Properties of MWCNT-rGO-MgO-Incorporated Alkali-Activated Engineered Composites
Abstract
1. Introduction
2. Experimental Program, Materials, and Mix Design
2.1. Materials and Properties
2.2. Mix Design and Mixing Procedure
2.3. Specimens, Test Methods, and Testing Procedures
3. Results and Discussion
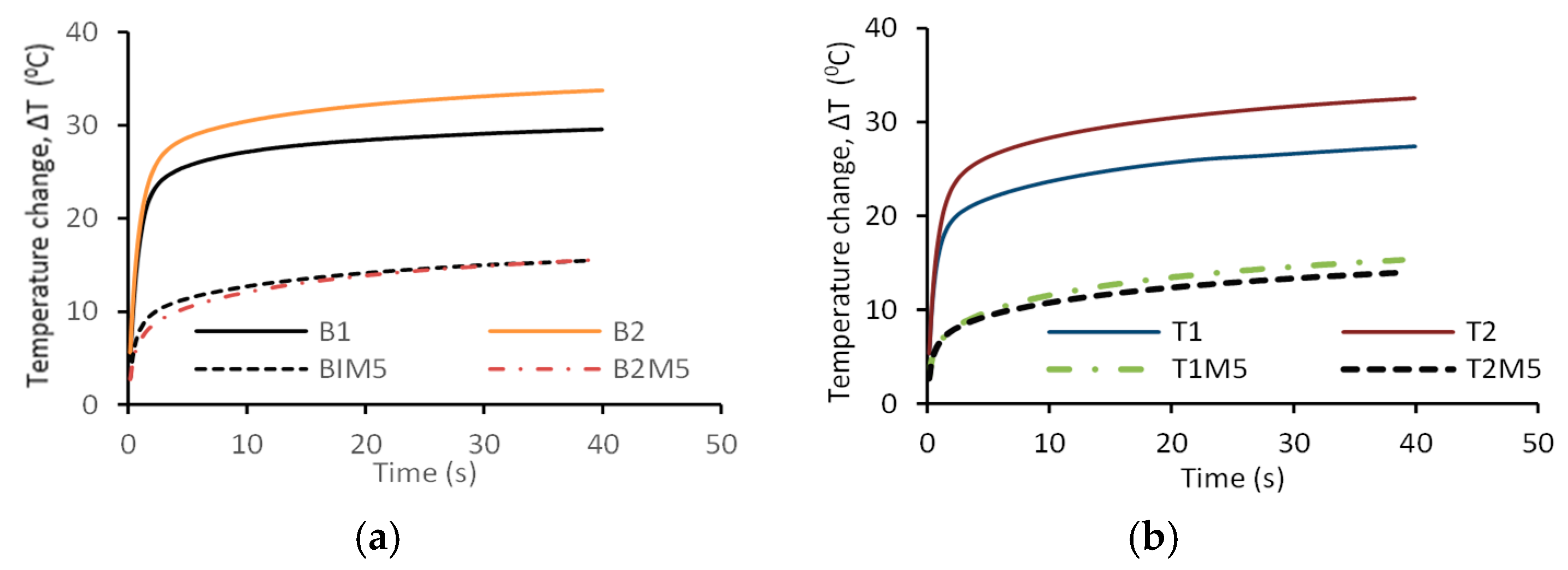
3.1. Effect of Reagent Type, MWCNT, rGO, and MgO Contents on Temperature Change of AAECs
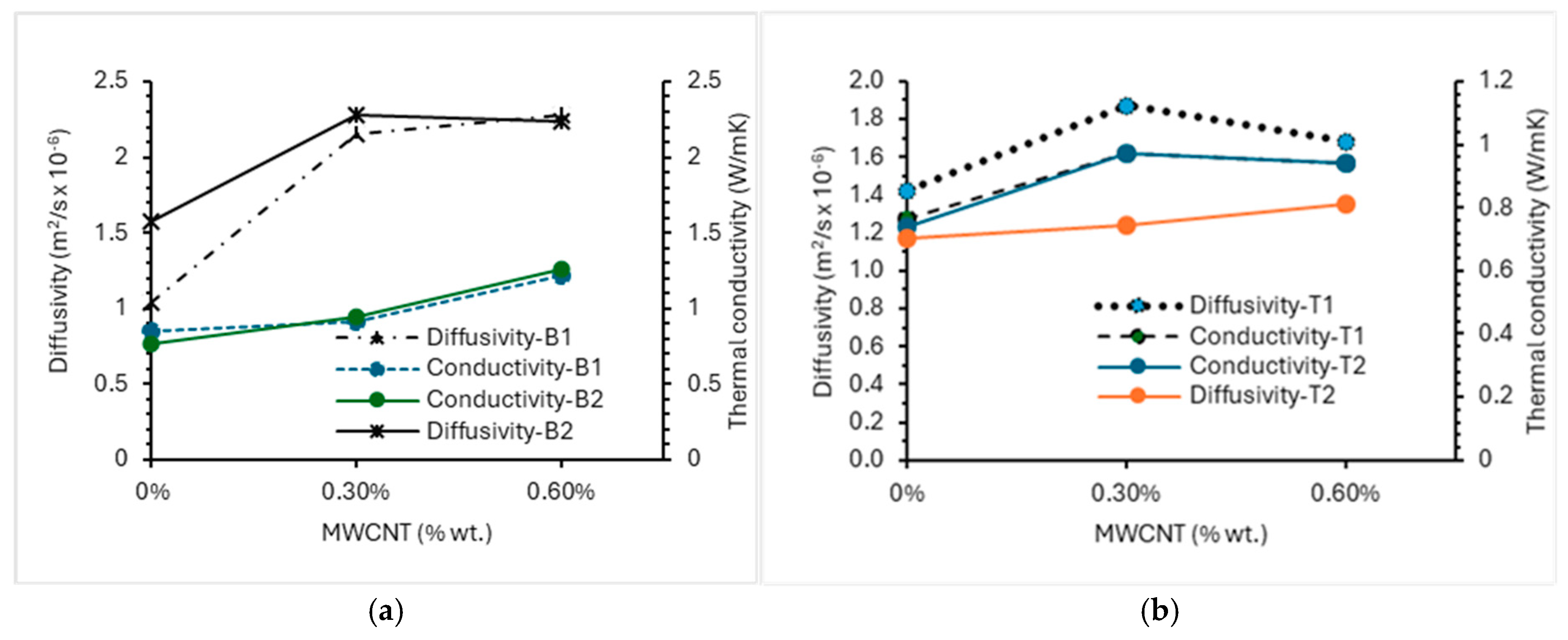
3.2. Effect of Reagent Type and MWCNT/rGO/MgO Dosages on the Thermal Conductivity/Diffusivity of AAECs
3.2.1. Control and MgO AAECs
3.2.2. MWCNT AAECs
3.2.3. rGO AAECs
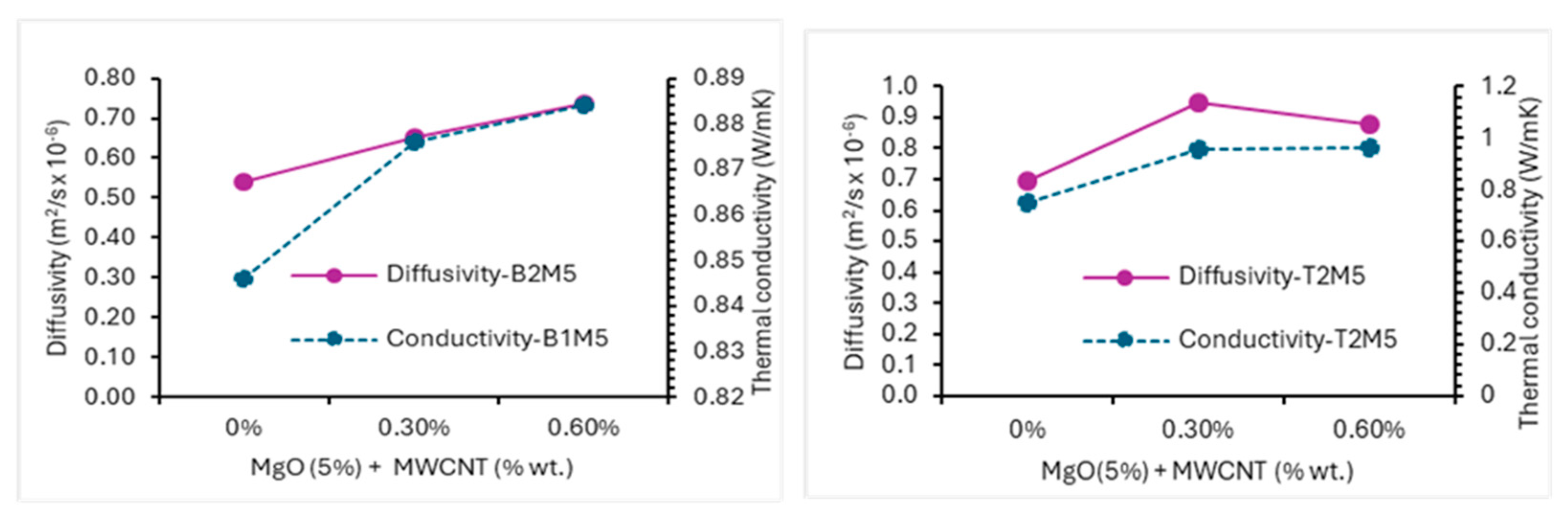
3.2.4. MgO-MWCNT/rGO AAECs
3.3. Thermal Conductivity vs. Dry Density of AAECs
4. Conclusions
- MWCNT/rGO/MgO AAECs showed lower steady-state temperature change (ΔT) than their control AAEC counterparts during the thermal conductivity test, with binary AAECs showing higher steady ΔT than their ternary counterparts.
- The thermal conductivity and diffusivity of AAECs increased with the increase in MWCNTs/rGO (0–6%) and MgO (0–5%). Binary and reagent 2 AAECs showed higher thermal conductivity than their ternary and reagent 1 counterparts.
- The thermal conductivity values of MgO AAECs were 0.44–10.1% higher than their control counterparts. Binary and reagent 2 control/MgO AAECs produced higher thermal conductivity than their ternary and reagent 1 counterparts.
- The thermal conductivity values of MWCNT AAECs were 16.6–41.7%, and 23.2–49.4% higher for 0.3% and 0.6%MWCNT contents, respectively, with respect to control AAECs.
- For rGO AAECs, increases of 3.6–27.7% and 2.1–28.6% in thermal conductivity were found for 0.3% and 0.6% rGO, respectively, compared to control counterparts. Thermal conductivity increases of 3.6–27.7% and 2.1–27.7% were observed for MgO-MWCNTs and MgO-rGO AAECs, respectively.
- MWCNT AAECs showed the highest thermal conductivity (0.91–1.26 W/mK) followed by rGO (0.87–1.02 W/mK), MgO-MWCNTs (0.88–0.96 W/mK), MgO-rGO (0.84–0.88 W/mK, MgO (0.75–0.85 W/mK), and control (0.74–0.84 W/mK) AAECs. MWCNTs were found to be more effective in increasing the thermal properties of AAECs.
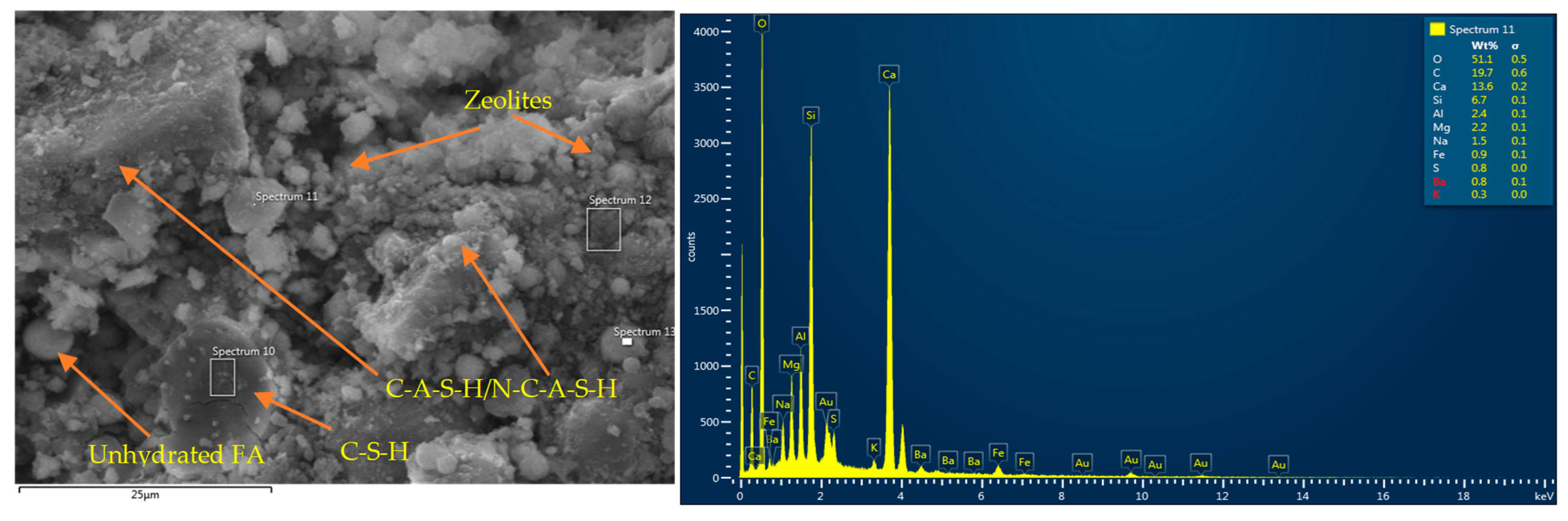
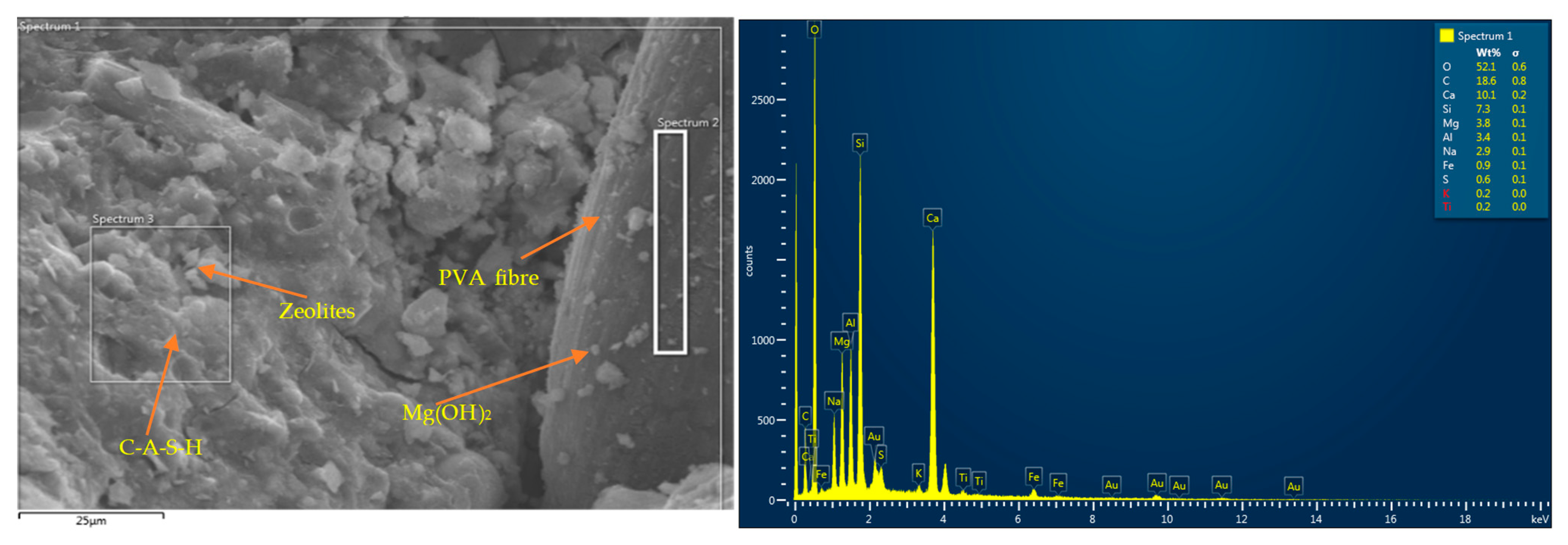
- Thermal conductivity enhancement occurred due to the development of additional crystalline reaction products in the case of binary/reagent 2 (C-S-H), MgO (M-S-H and Ht), and rGO (zeolite) in addition to basic C-A-S-H/N-C-A-S-H or C-A-S-H/C-S-H gels. Thermal conductivity enhancement was also related to nanomaterial–matrix bonding, matrix connectivity, and conductivity of nanomaterials.
- This study suggests that MWCNTs and rGO are effective in increasing the thermal conductivity of AAECs and can be used for producing MWCNT/rGO-incorporated conductive AAECs for construction applications.
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hossain, K.M.A.; Sood, D. The Strength and Fracture Characteristics of One-Part Strain-Hardening Green Alkali-Activated Engineered Composites. Materials 2023, 16, 5077. [Google Scholar] [CrossRef] [PubMed]
- Karakoc, M.B.; Türkmen, İ.; Maraş, M.M.; Kantarci, F.; Demirboğa, R. Sulfate resistance of ferrochrome slag based geopolymer concrete. Ceram. Int. 2016, 42, 1254–1260. [Google Scholar] [CrossRef]
- Romagnoli, M.; Leonelli, C.; Kamse, E.; Lassinantti Gualtieri, M. Rheology of geopolymer by DOE approach. Constr. Build. Mater. 2012, 36, 251–258. [Google Scholar] [CrossRef]
- Sood, D.; Hossain, K.M.A. Fresh State, Rheological and Microstructural Characteristics of Alkali-Activated Mortars Developed Using Novel Dry Mixing Technique under Ambient Conditions. Appl. Sci. 2021, 11, 8920. [Google Scholar] [CrossRef]
- Sood, D.; Hossain, K.M.A. Strength, Fracture and Durability Characteristics of Ambient Cured Alkali—Activated Mortars Incorporating High Calcium Industrial Wastes and Powdered Reagents. Crystals 2021, 11, 1167. [Google Scholar] [CrossRef]
- Sood, D.; Hossain, K.M.A. Strength, Shrinkage and Early Age Characteristics of One-Part Alkali-Activated Binders with High-Calcium Industrial Wastes, Solid Reagents and Fibers. J. Compos. Sci. 2021, 5, 315. [Google Scholar] [CrossRef]
- Sumajouw, D.M.J.; Hardjito, D.; Wallah, S.E.; Rangan, B.V. Fly ash-based geopolymer concrete: Study of slender reinforced columns. J. Mater. Sci. 2007, 42, 3124–3130. [Google Scholar] [CrossRef]
- Wang, W.; Fan, C.; Wang, B.; Zhang, X.; Liu, Z. Workability, rheology, and geopolymerization of fly ash geopolymer: Role of alkali content, modulus, and water–binder ratio. Constr. Build. Mater. 2023, 367, 130357. [Google Scholar] [CrossRef]
- Hoy, M.; Horpibulsuk, S.; Rachan, R.; Chinkulkijniwat, A.; Arulrajah, A. Recycled asphalt pavement—Fly ash geopolymers as a sustainable pavement base material: Strength and toxic leaching investigations. Sci. Total Environ. 2016, 573, 19–26. [Google Scholar] [CrossRef]
- Hossain, M.A.; Hossain, K.M.A. Rheological, fresh state, and strength characteristics of alkali-activated mortars incorporating MgO and carbon nanoparticles. Materials 2024, 17, 5931. [Google Scholar] [CrossRef]
- Jindal, B.B.; Sharma, R. The effect of nanomaterials on properties of geopolymers derived from industrial by-products: A state-of-the-art review. Constr. Build. Mater. 2020, 252, 119028. [Google Scholar] [CrossRef]
- Luz, G.; Gleize, P.J.P.; Batiston, E.R.; Pelisser, F. Effect of pristine and functionalized carbon nanotubes on microstructural, rheological, and mechanical behaviors of metakaolin-based geopolymer. Cem. Concr. Compos. 2019, 104, 103332. [Google Scholar] [CrossRef]
- Balandin, A.A.; Ghosh, S.; Bao, W.; Calizo, I.; Teweldebrhan, D.; Miao, F.; Lau, C.N. Superior thermal conductivity of single-layer graphene. Nano Lett. 2008, 8, 902–907. [Google Scholar] [CrossRef] [PubMed]
- Chen, I.; Wang, C.; Chen, C. Preparation of carbon nanotube (CNT) composites by polymer functionalized CNT under plasma treatment. Plasma Process. Polym. 2010, 7, 59–63. [Google Scholar] [CrossRef]
- Kim, P.; Shi, L.; Majumdar, A.; McEuen, P.L. Thermal Transport Measurements of Individual Multiwalled Nanotubes. Phys. Rev. Lett. 2001, 87, 215502. [Google Scholar] [CrossRef]
- Pop, E.; Mann, D.; Wang, Q.; Goodson, K.; Dai, H. Thermal conductance of an individual single-wall carbon nanotube above room temperature. Nano Lett. 2006, 6, 96–100. [Google Scholar] [CrossRef] [PubMed]
- Gonnet, P.; Liang, Z.; Choi, E.S.; Kadambala, R.S.; Zhang, C.; Brooks, J.S.; Wang, B.; Kramer, L. Thermal conductivity of magnetically aligned carbon nanotube buckypapers and nanocomposites. Curr. Appl. Phys. 2006, 6, 119–122. [Google Scholar] [CrossRef]
- Park, J.G.; Cheng, Q.; Lu, J.; Bao, J.; Li, S.; Tian, Y.; Liang, Z.; Zhang, C.; Wang, B. Thermal conductivity of MWCNT/epoxy composites: The effects of length, alignment and functionalization. Carbon 2012, 50, 2083–2090. [Google Scholar] [CrossRef]
- Fan, Z.-J.; Kai, W.; Yan, J.; Wei, T.; Zhi, L.-J.; Feng, J.; Ren, Y.; Song, L.-P.; Wei, F. Facile Synthesis of Graphene Nanosheets via Fe Reduction of Exfoliated Graphite Oxide. ACS Nano 2011, 5, 191–198. [Google Scholar] [CrossRef]
- Robinson, J.T.; Perkins, F.K.; Snow, E.S.; Wei, Z.; Sheehan, P.E. Reduced Graphene Oxide Molecular Sensors. Nano Lett. 2008, 8, 3137–3140. [Google Scholar] [CrossRef]
- Davoodabadi, M.; Liebscher, M.; Hampel, S.; Sgarzi, M.; Rezaie, A.B.; Wolf, D.; Cuniberti, G.; Mechtcherine, V.; Yang, J. Multi-walled carbon nanotube dispersion methodologies in alkaline media and their influence on mechanical reinforcement of alkali-activated nanocomposites. Compos. Part B Eng. 2021, 209, 108559. [Google Scholar] [CrossRef]
- Hu, J.; Wang, K.; Ge, Z. Study of concrete thermal properties for sustainable pavement design. J. Sustain. Cem. Based Mater. 2012, 1, 126–137. [Google Scholar] [CrossRef]
- Zhang, Z.; Provis, J.L.; Reid, A.; Wang, H. Mechanical, thermal insulation, thermal resistance and acoustic absorption properties of geopolymer foam concrete. Cem. Concr. Compos. 2015, 62, 97–105. [Google Scholar] [CrossRef]
- Wang, Y.; Xiong, W.; Tang, D.; Hao, L.; Li, Z.; Li, Y.; Cheng, K. Rheology effect and enhanced thermal conductivity of diamond/metakaolin geopolymer fabricated by direct ink writing. Rapid Prototyp. J. 2021, 27, 837–850. [Google Scholar] [CrossRef]
- Ali, M.F.; Vijayalakshmi Natrajan, M.M. A Review of Geopolymer Composite Thermal Properties. IOP Conf. Ser. Earth Environ. Sci. 2021, 822, 012051. [Google Scholar] [CrossRef]
- Zhao, Y.H. Effect of CNT/CNF on Thermal and Mechanical Properties of Cement Mortars. Adv. Mater. Res. 2014, 1049–1050, 234–237. [Google Scholar] [CrossRef]
- Hassanzadeh-Aghdam, M.K.; Mahmoodi, M.J.; Safi, M. Effect of adding carbon nanotubes on the thermal conductivity of steel fiber-reinforced concrete. Compos. Part B Eng. 2019, 174, 106972. [Google Scholar] [CrossRef]
- Hanjitsuwan, S.; Chindaprasirt, P.; Pimraksa, K. Electrical conductivity and dielectric property of fly ash geopolymer pastes. Int. J. Miner. Metall. Mater. 2011, 18, 94–99. [Google Scholar] [CrossRef]
- Hassan, A.A.A.; Lachemi, M.; Hossain, K.M.A. Effect of metakaolin on the rheology of self-consolidating concrete. In Design, Production and Placement of Self-Consolidating Concrete: Proceedings of SCC2010, Montreal, QC, Canada, 26–29 September 2010; Springer: Amsterdam, The Netherlands, 2010; pp. 103–112. [Google Scholar] [CrossRef]
- Litina, C.; Bumanis, G.; Anglani, G.; Dudek, M.; Maddalena, R.; Amenta, M.; Papaioannou, S.; Pérez, G.; Calvo, J.L.G.; Asensio, E.; et al. Evaluation of methodologies for assessing self-healing performance of concrete with mineral expansive agents: An interlaboratory study. Materials 2021, 14, 2024. [Google Scholar] [CrossRef]
- Shahedan, N.F.; Abdullah, M.M.A.B.; Mahmed, N.; Kusbiantoro, A.; Binhussain, M.; Zailan, S.N. Review on thermal insulation performance in various type of concrete. In AIP Conference Proceedings; AIP Publishing: Melville, NY, USA, 2017. [Google Scholar] [CrossRef]
- Kodur, V.; Khaliq, W. Effect of Temperature on Thermal Properties of Different Types of High-Strength Concrete. J. Mater. Civ. Eng. 2011, 23, 793–801. [Google Scholar] [CrossRef]
- Cao, Y.F.; Tao, Z.; Pan, Z.; Wuhrer, R. Effect of calcium aluminate cement on geopolymer concrete cured at ambient temperature. Constr. Build. Mater. 2018, 191, 242–252. [Google Scholar] [CrossRef]
- Feng, J.; Zhang, R.; Gong, L.; Li, Y.; Cao, W.; Cheng, X. Development of porous fly ash-based geopolymer with low thermal conductivity. Mater. Des. 2015, 65, 529–533. [Google Scholar] [CrossRef]
- Zhang, P.; Zheng, Y.; Wang, K.; Zhang, J. A review on properties of fresh and hardened geopolymer mortar. Compos. Part B Eng. 2018, 152, 79–95. [Google Scholar] [CrossRef]
- Liu, M.Y.J.; Alengaram, U.J.; Jumaat, M.Z.; Mo, K.H. Evaluation of thermal conductivity, mechanical and transport properties of lightweight aggregate foamed geopolymer concrete. Energy Build. 2014, 72, 238–245. [Google Scholar] [CrossRef]
- Kamseu, E.; Beleuk à Moungam, L.M.; Cannio, M.; Billong, N.; Chaysuwan, D.; Melo, U.C.; Leonelli, C. Substitution of sodium silicate with rice husk ash-NaOH solution in metakaolin based geopolymer cement concerning reduction in global warming. J. Clean. Prod. 2017, 142, 3050–3060. [Google Scholar] [CrossRef]
- Duxson, P.; Lukey, G.C.; van Deventer, J.S.J. Thermal Conductivity of Metakaolin Geopolymers Used as a First Approximation for Determining Gel Interconnectivity. Ind. Eng. Chem. Res. 2006, 45, 7781–7788. [Google Scholar] [CrossRef]
- Chen, L.; Wang, Z.; Wang, Y.; Feng, J. Preparation and Properties of Alkali Activated Metakaolin-Based Geopolymer. Materials 2016, 9, 767. [Google Scholar] [CrossRef]
- Duan, P.; Song, L.; Yan, C.; Ren, D.; Li, Z. Novel thermal insulating and lightweight composites from metakaolin geopolymer and polystyrene particles. Ceram. Int. 2017, 43, 5115–5120. [Google Scholar] [CrossRef]
- Henon, J.; Pennec, F.; Alzina, A.; Absi, J.; Smith, D.S.; Rossignol, S. Analytical and numerical identification of the skeleton thermal conductivity of a geopolymer foam using a multi-scale analysis. Comput. Mater. Sci. 2014, 82, 264–273. [Google Scholar] [CrossRef]
- Jaya, N.A.; Yun-Ming, L.; Cheng-Yong, H.; Abdullah, M.M.A.B.; Hussin, K. Correlation between pore structure, compressive strength and thermal conductivity of porous metakaolin geopolymer. Constr. Build. Mater. 2020, 247, 118641. [Google Scholar] [CrossRef]
- Medri, V.; Papa, E.; Mazzocchi, M.; Laghi, L.; Morganti, M.; Francisconi, J.; Landi, E. Production and characterization of lightweight vermiculite/geopolymer-based panels. Mater. Des. 2015, 85, 266–274. [Google Scholar] [CrossRef]
- Novais, R.M.; Senff, L.; Carvalheiras, J.; Seabra, M.P.; Pullar, R.C.; Labrincha, J.A. Sustainable and efficient cork—Inorganic polymer composites: An innovative and eco-friendly approach to produce ultra-lightweight and low thermal conductivity materials. Cem. Concr. Compos. 2019, 97, 107–117. [Google Scholar] [CrossRef]
- Aboulayt, A.; Gounni, A.; El Alami, M.; Hakkou, R.; Hannache, H.; Gomina, M.; Moussa, R. Thermo-physical characterization of a metakaolin-based geopolymer incorporating calcium carbonate: A case study. Mater. Chem. Phys. 2020, 252, 123266. [Google Scholar] [CrossRef]
- Du, F.-P.; Xie, S.-S.; Zhang, F.; Tang, C.-Y.; Chen, L.; Law, W.-C.; Tsui, C.-P. Microstructure and compressive properties of silicon carbide reinforced geopolymer. Compos. Part B Eng. 2016, 105, 93–100. [Google Scholar] [CrossRef]
- Subaer; van Riessen, A. Thermo-mechanical and microstructural characterisation of sodium-poly(sialate-siloxo) (Na-PSS) geopolymers. J. Mater. Sci. 2007, 42, 3117–3123. [Google Scholar] [CrossRef]
- Zhu, Y.; Qian, Y.; Zhang, L.; Bai, B.; Wang, X.; Li, J.; Bi, S.; Kong, L.; Liu, W.; Zhang, L. Enhanced thermal conductivity of geopolymer nanocomposites by incorporating interface engineered carbon nanotubes. Compos. Commun. 2021, 24, 100691. [Google Scholar] [CrossRef]
- Dong, A.; Duan, X.; Cheng, S.; Zhang, Z.; Yang, B.; Lian, Q.; Li, J.; Sun, Z.; Liu, Y.; Wong, C.-P. Enhanced thermal conductivity of natural rubber based thermal interfacial materials by constructing covalent bonds and three-dimensional networks. Compos. Part A Appl. Sci. Manuf. 2020, 135, 105928. [Google Scholar] [CrossRef]
- Saafi, M.; Andrew, K.; Tang, P.L.; McGhon, D.; Taylor, S.; Rahman, M.; Yang, S.; Zhou, X. Multifunctional properties of carbon nanotube/fly ash geopolymeric nanocomposites. Constr. Build. Mater. 2013, 49, 46–55. [Google Scholar] [CrossRef]
- Sherir, M.A.A.; Hossain, K.M.A.; Lachemi, M. Self-healing and expansion characteristics of cementitious composites with high volume fly ash and MgO-type expansive agent. Constr. Build. Mater. 2016, 127, 80–92. [Google Scholar] [CrossRef]
- Hossain, M.A.; Hossain, K.M.A. Physical, Compressive Strength, and Microstructural Characteristics of Alkali-Activated Engineered Composites Incorporating MgO, MWCNTs, and rGO. Appl. Sci. 2025, 15, 1712. [Google Scholar] [CrossRef]
- Hossain, M.A.; Hossain, K.M.A.; Manzur, T.; Hasan, M.J.; Sood, D. Fresh and hardened properties of engineered geopolymer composite with MgO. In Proceedings of the International Conference on Civil 2020, Structural and Transportation Engineering, Online, 1 November 2020. [Google Scholar] [CrossRef]
- Chen, J.; Akono, A.-T. Influence of multi-walled carbon nanotubes on the fracture response and phase distribution of metakaolin-based potassium geopolymers. J. Mater. Sci. 2021, 56, 19403–19424. [Google Scholar] [CrossRef]
- Buck, W.; Rudtsch, S. Thermal Properties. In Springer Handbook of Materials Measurement Methods; Czichos, H., Saito, T., Smith, L., Eds.; Springer: Berlin/Heidelberg, Germany, 2006; pp. 399–429. [Google Scholar] [CrossRef]
- McConnell, A.D.; Uma, S.; Goodson, K.E. Thermal conductivity of doped polysilicon layers. J. Microelectromechanical Syst. 2001, 10, 360–369. [Google Scholar] [CrossRef]
- Tritt, T.M. Thermal Conductivity: Theory, Properties, and Applications; Springer Science & Business Media: Berlin, Germany, 2005. [Google Scholar]
- Haha, M.B.; Lothenbach, B.; Le Saout, G.; Winnefeld, F. Influence of slag chemistry on the hydration of alkali-activated blast-furnace slag—Part I: Effect of MgO. Cem. Concr. Res. 2011, 41, 955–963. [Google Scholar] [CrossRef]
- Jin, F.; Abdollahzadeh, A.; Al-Tabbaa, A. Effect of different MgO on the hydration of MgO-activated granulated ground blastfurnace slag paste. In Proceedings of the Third International Conference on Sustainable Construction Materials and Technologies, Kyoto, Japan, 18–21 August 2013; Available online: http://www.claisse.info/Proceedings.htm (accessed on 25 June 2024).
- Sherir, M.A.; Hossain, K.M.; Lachemi, M. Permeation and Transport Properties of Self-Healed Cementitious Composite Produced with MgO Expansive Agent. J. Mater. Civ. Eng. 2018, 30, 04018291. [Google Scholar] [CrossRef]
- Rovnaník, P.; Šimonová, H.; Topolář, L.; Schmid, P.; Keršner, Z. Effect of Carbon Nanotubes on the Mechanical Fracture Properties of Fly Ash Geopolymer. Procedia Eng. 2016, 151, 321–328. [Google Scholar] [CrossRef]
- Li, C.; Sun, H.; Li, L. A review: The comparison between alkali-activated slag (Si + Ca) and metakaolin (Si + Al) cements. Cem. Concr. Res. 2010, 40, 1341–1349. [Google Scholar] [CrossRef]
- Cui, H.; Yan, X.; Tang, L.; Xing, F. Possible pitfall in sample preparation for SEM analysis—A discussion of the paper “Fabrication of polycarboxylate/graphene oxide nanosheet composites by copolymerization for reinforcing and toughening cement composites” by Lv et al. Cem. Concr. Compos. 2017, 77, 81–85. [Google Scholar] [CrossRef]
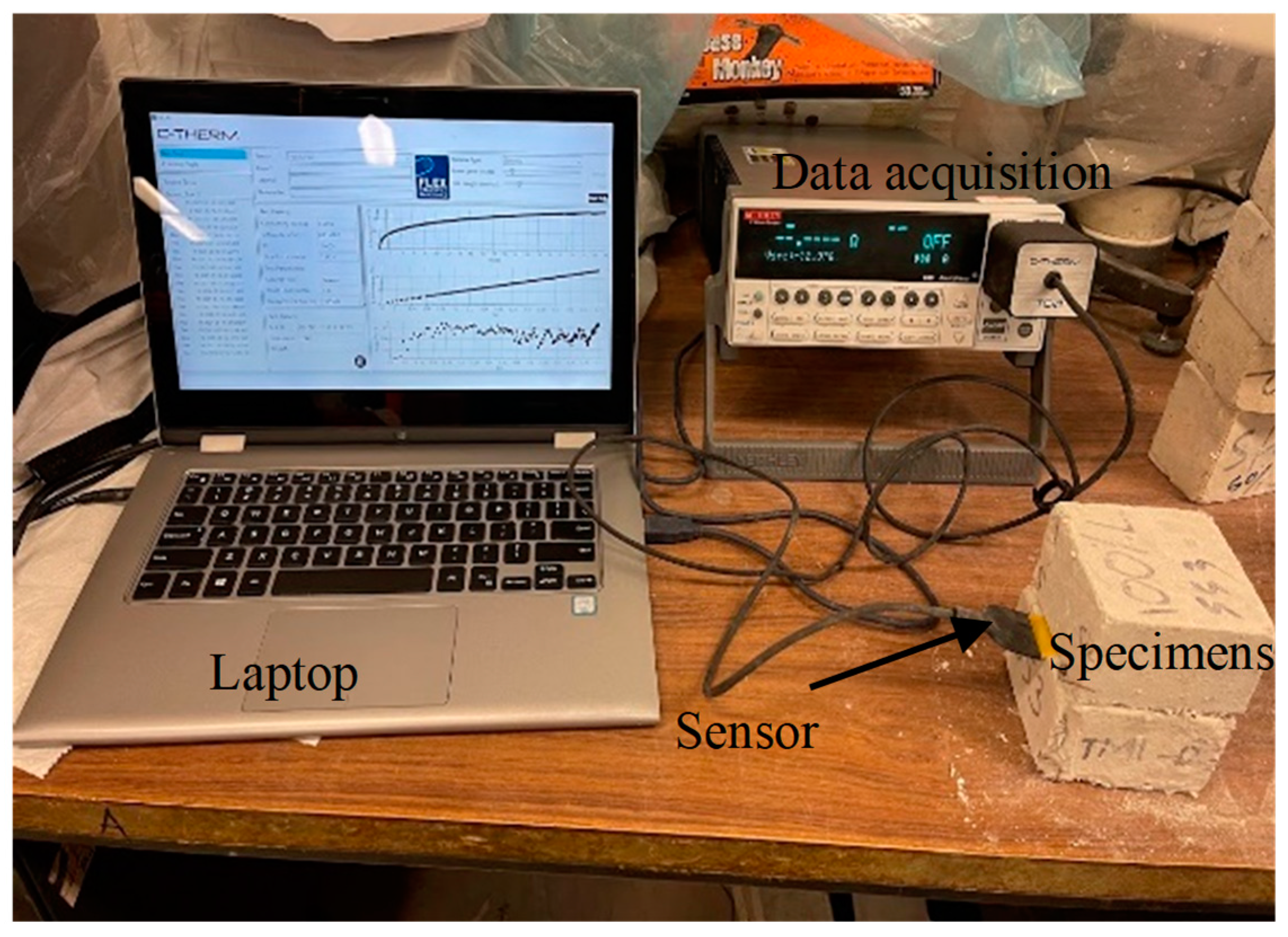







| Chemical Composition (%) | FA-C | FA-F | GGBFS | Silica Sand | HRWRA | MgO |
|---|---|---|---|---|---|---|
| SiO2 | 36.53 | 55.66 | 35.97 | 99.70 | 2.02 | |
| Al2O3 | 18.26 | 22.09 | 9.18 | 0.14 | 6.124 | |
| Fe2O3 | 5.66 | 4.26 | 0.50 | 0.016 | 0.94 | |
| CaO | 20.97 | 7.97 | 38.61 | 0.01 | 2.40 | |
| MgO | 5.08 | 1.16 | 10.99 | 0.01 | 92.26 | |
| K2O | 0.68 | 1.49 | 0.36 | 0.04 | - | |
| Na2O | 4.04 | 4.10 | 0.28 | 0.01 | - | |
| MnO | 0.03 | 0.03 | 0.25 | 0.00 | - | |
| TiO2 | 1.26 | 0.61 | 0.39 | 0.00 | - | |
| P2O5 | 0.96 | 0.43 | 0.01 | 0.00 | - | |
| L.O.I. | 2.18 | 1.05 | 0.74 | 0.00 | 1.14 | |
| pH | 6.00 | |||||
| Density (g/cm3) | 2.61 | 2.02 | 2.87 | 2.65 | 1.06 | 3.58 |
| Retained on 45 µ, % | - | 18.00 | - | 3.00 | ||
| Blaine fineness (m2/kg) | 315.00 | 306.00 | 489.30 | - |
| AAECs Mix ID. | Total SCMs (Binder *) | MgO/MWCNTs/rGO | SCMs | Reagent Component Ratio | R./B | Chemical Ratios (SCMs + Reagent) | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| FA-C | FA-F | GGBFS | SiO2/ Al2O3 | Na2O/ SiO2 | CaO/ SiO2 | Na2O/ Al2O3 | |||||
| Four basic AAEC mixes (with 0% MgO/MWCNTs/rGO) | |||||||||||
| B1 | 1 | 0 | 0.55 | 0 | 0.45 | 1:2.5 | 0.09 | 2.62 | 0.09 | 0.84 | 0.23 |
| B2 | 0.55 | 0 | 0.45 | 2.5:1 | 0.12 | 2.56 | 0.14 | 1.02 | 0.35 | ||
| T1 | 0.25 | 0.35 | 0.40 | 1:2.5 | 0.09 | 2.75 | 0.08 | 0.59 | 0.22 | ||
| T2 | 0.25 | 0.35 | 0.40 | 2.5:1 | 0.12 | 2.69 | 0.12 | 0.73 | 0.32 | ||
| Four AAEC mixes with 5% MgO | |||||||||||
| B1M5 | 1 | 0.05 | 0.52 | 0 | 0.43 | 1:2.5 | 0.09 | 2.58 | 0.09 | 0.85 | 0.23 |
| B2M5 | 0.52 | 0 | 0.43 | 2.5:1 | 0.12 | 2.51 | 0.14 | 1.03 | 0.35 | ||
| T1M5 | 0.24 | 0.33 | 0.38 | 1:2.5 | 0.09 | 2.97 | 0.05 | 0.54 | 0.14 | ||
| T2M5 | 0.24 | 0.33 | 0.38 | 2.5:1 | 0.12 | 2.97 | 0.05 | 0.54 | 0.14 | ||
| Eight AAEC mixes with 0.3% and 0.6% MWCNTs | |||||||||||
| B1C3 | 1 | 0.003 | 0.55 | 0 | 0.45 | 1:2.5 | 0.09 | 2.62 | 0.09 | 0.84 | 0.23 |
| B2C3 | 0.55 | 0 | 0.45 | 2.5:1 | 0.12 | 2.56 | 0.14 | 1.02 | 0.35 | ||
| T1C3 | 0.25 | 0.35 | 0.40 | 1:2.5 | 0.09 | 2.75 | 0.08 | 0.59 | 0.22 | ||
| T2C3 | 0.25 | 0.35 | 0.40 | 2.5:1 | 0.12 | 2.69 | 0.12 | 0.73 | 0.32 | ||
| B1C6 | 1 | 0.006 | 0.55 | 0 | 0.45 | 1:2.5 | 0.09 | 2.62 | 0.09 | 0.84 | 0.23 |
| B2C6 | 0.55 | 0 | 0.45 | 2.5:1 | 0.12 | 2.56 | 0.14 | 1.02 | 0.35 | ||
| T1C6 | 0.25 | 0.35 | 0.40 | 1:2.5 | 0.09 | 2.75 | 0.08 | 0.59 | 0.22 | ||
| T2C6 | 0.25 | 0.35 | 0.40 | 2.5:1 | 0.12 | 2.69 | 0.12 | 0.73 | 0.32 | ||
| Eight AAEC mixes with 0.3% and 0.6% rGO | |||||||||||
| B1R3 | 1 | 0.003 | 0.55 | 0 | 0.45 | 1:2.5 | 0.09 | 2.62 | 0.09 | 0.84 | 0.23 |
| B2R3 | 0.55 | 0 | 0.45 | 2.5:1 | 0.12 | 2.56 | 0.14 | 1.02 | 0.35 | ||
| T1R3 | 0.25 | 0.35 | 0.40 | 1:2.5 | 0.09 | 2.75 | 0.08 | 0.59 | 0.22 | ||
| T2R3 | 0.25 | 0.35 | 0.40 | 2.5:1 | 0.12 | 2.69 | 0.12 | 0.73 | 0.32 | ||
| B1R6 | 1 | 0.006 | 0.55 | 0 | 0.45 | 1:2.5 | 0.09 | 2.62 | 0.09 | 0.84 | 0.23 |
| B2R6 | 0.55 | 0 | 0.45 | 2.5:1 | 0.12 | 2.56 | 0.14 | 1.02 | 0.35 | ||
| T1R6 | 0.25 | 0.35 | 0.40 | 1:2.5 | 0.09 | 2.75 | 0.08 | 0.59 | 0.22 | ||
| T2R6 | 0.25 | 0.35 | 0.40 | 2.5:1 | 0.12 | 2.69 | 0.12 | 0.73 | 0.32 | ||
| Eight AAEC mixes with 5%MgO and MWCNTs or rGO (0.3% or 0.6%) | |||||||||||
| B2M5C3 | 1 | 0.05/0.003 | 0.52 | 0 | 0.43 | 2.5:1 | 0.12 | 2.56 | 0.14 | 1.02 | 0.35 |
| T2M5C3 | 0.24 | 0.33 | 0.38 | 2.5:1 | 0.12 | 2.97 | 0.05 | 0.54 | 0.14 | ||
| B2M5C6 | 0.52 | 0 | 0.43 | 2.5:1 | 0.12 | 2.56 | 0.14 | 1.02 | 0.35 | ||
| T2M5C6 | 0.24 | 0.33 | 0.38 | 2.5:1 | 0.12 | 2.97 | 0.05 | 0.54 | 0.14 | ||
| B2M5R3 | 1 | 0.05/0.006 | 0.52 | 0 | 0.43 | 2.5:1 | 0.12 | 2.56 | 0.14 | 1.02 | 0.35 |
| T2M5R3 | 0.24 | 0.33 | 0.38 | 2.5:1 | 0.12 | 2.97 | 0.05 | 0.54 | 0.14 | ||
| B2M5R6 | 0.52 | 0 | 0.43 | 2.5:1 | 0.12 | 2.56 | 0.14 | 1.02 | 0.35 | ||
| T2M5R6 | 0.24 | 0.33 | 0.38 | 2.5:1 | 0.12 | 2.97 | 0.05 | 0.54 | 0.14 | ||
| AAEC Types | AAEC Mix ID | 28-Day Dry Density (kg/m3) | 28-Day Thermal Conductivity (λ) (W/mK) | 28-Day Thermal Diffusivity (α) (×10−6 m2/s) | Change in Thermal Conductivity as Compared to Control Specimens (%) |
|---|---|---|---|---|---|
| Control | B1 | 2000 | 0.76 | 1.04 | 0 |
| B2 | 2032 | 0.84 | 1.57 | 0 | |
| T1 | 2120 | 0.76 | 1.42 | 0 | |
| T2 | 2064 | 0.74 | 0.70 | 0 | |
| MgO 5% | B1M5 | 2016 | 0.83 | 0.87 | 10.10 |
| B2M5 | 2072 | 0.85 | 0.54 | 0.44 | |
| T1M5 | 1984 | 0.79 | 1.65 | 3.18 | |
| T2M5 | 2056 | 0.75 | 0.70 | 1.68 | |
| MWCNT 0.3% | B1C3 | 2008 | 0.91 | 2.15 | 20.62 |
| B2C3 | 2080 | 0.98 | 2.28 | 16.62 | |
| T1C3 | 2016 | 0.97 | 1.87 | 27.46 | |
| T2C3 | 2096 | 0.97 | 0.74 | 31.60 | |
| MWCNT 0.6% | B1C6 | 2044 | 1.07 | 2.28 | 41.65 |
| B2C6 | 2036 | 1.26 | 2.24 | 49.44 | |
| T1C6 | 1960 | 0.94 | 1.68 | 23.16 | |
| T2C6 | 2168 | 0.92 | 0.81 | 24.25 | |
| rGO 0.3% | B1R3 | 2032 | 0.87 | 1.20 | 15.32 |
| B2R3 | 2152 | 1.02 | 1.44 | 20.92 | |
| T1R3 | 2060 | 0.88 | 1.73 | 14.65 | |
| T2R3 | 2096 | 0.90 | 0.84 | 21.50 | |
| rGO 0.6% | B1R6 | 2060 | 0.90 | 1.16 | 18.73 |
| B2R6 | 2060 | 1.05 | 1.69 | 24.60 | |
| T1R6 | 2068 | 0.88 | 1.84 | 15.33 | |
| T2R6 | 2176 | 0.92 | 0.89 | 24.42 | |
| Combination of MWCNTs, rGO, and MgO | B2M5C3 | 2073 | 0.88 | 0.65 | 3.55 |
| T2M5C3 | 2058 | 0.95 | 0.95 | 26.80 | |
| B2M5C6 | 2074 | 0.88 | 0.74 | 4.49 | |
| T2M5C6 | 2059 | 0.96 | 0.88 | 27.69 | |
| B2M5R3 | 2081 | 0.86 | 0.72 | 2.06 | |
| T2M5R3 | 2098 | 0.84 | 1.24 | 11.75 | |
| B2M5R6 | 2082 | 0.87 | 0.78 | 3.70 | |
| T2M5R6 | 2099 | 0.88 | 1.15 | 18.61 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hossain, M.A.; Hossain, K.M.A. Thermal Properties of MWCNT-rGO-MgO-Incorporated Alkali-Activated Engineered Composites. J. Compos. Sci. 2025, 9, 117. https://doi.org/10.3390/jcs9030117
Hossain MA, Hossain KMA. Thermal Properties of MWCNT-rGO-MgO-Incorporated Alkali-Activated Engineered Composites. Journal of Composites Science. 2025; 9(3):117. https://doi.org/10.3390/jcs9030117
Chicago/Turabian StyleHossain, Mohammad A., and Khandaker M. A. Hossain. 2025. "Thermal Properties of MWCNT-rGO-MgO-Incorporated Alkali-Activated Engineered Composites" Journal of Composites Science 9, no. 3: 117. https://doi.org/10.3390/jcs9030117
APA StyleHossain, M. A., & Hossain, K. M. A. (2025). Thermal Properties of MWCNT-rGO-MgO-Incorporated Alkali-Activated Engineered Composites. Journal of Composites Science, 9(3), 117. https://doi.org/10.3390/jcs9030117





