Organic Solvent Free Process to Fabricate High Performance Silicon/Graphite Composite Anode
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Composite Electrode Preparation
2.3. Coin Cell Fabrication and Testing
2.4. Characterization
3. Results
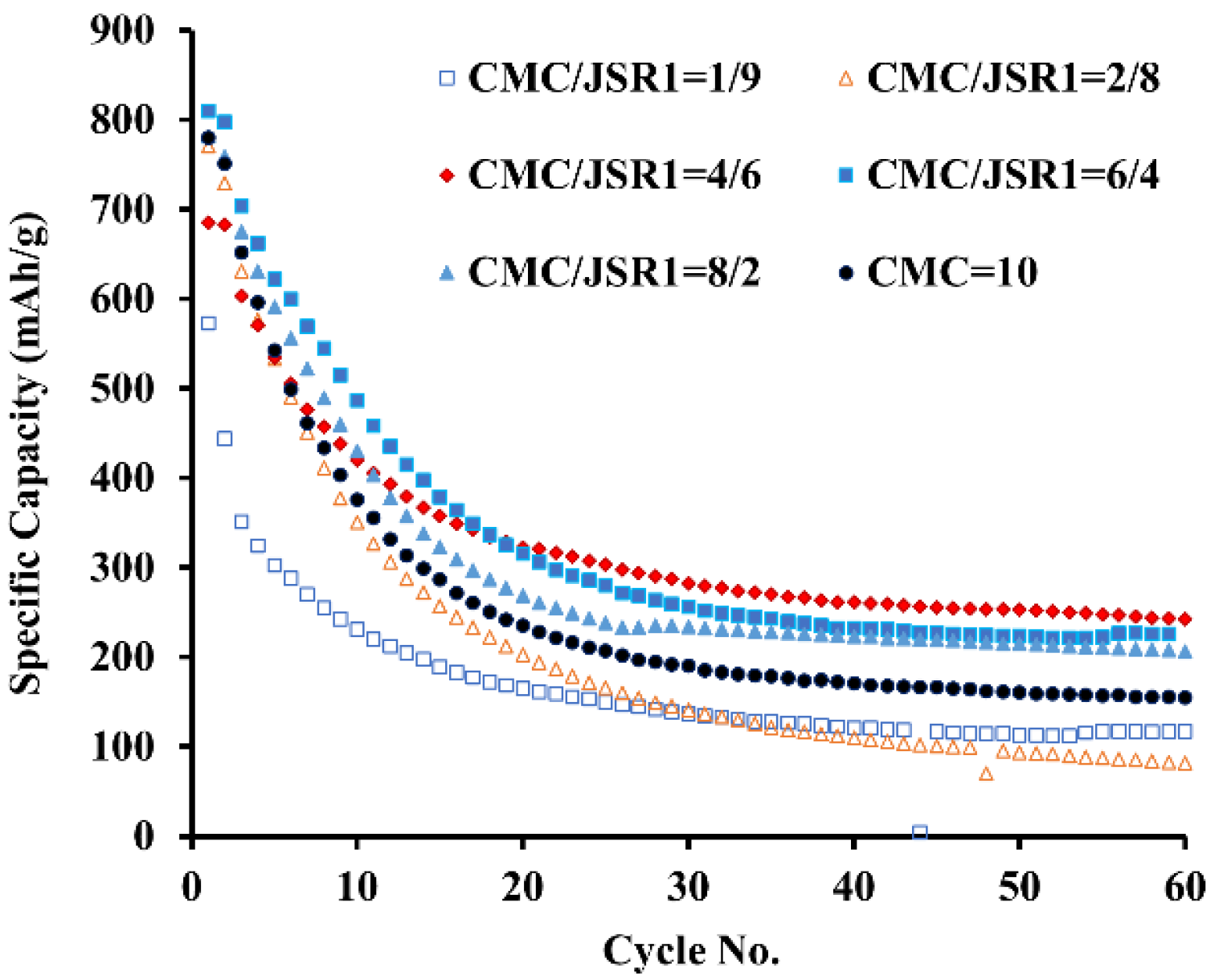
3.1. The Effect of CMC and SBR Ratio
3.2. The Effect of Silicon Particle Size
3.3. The Selection of Graphite Materials
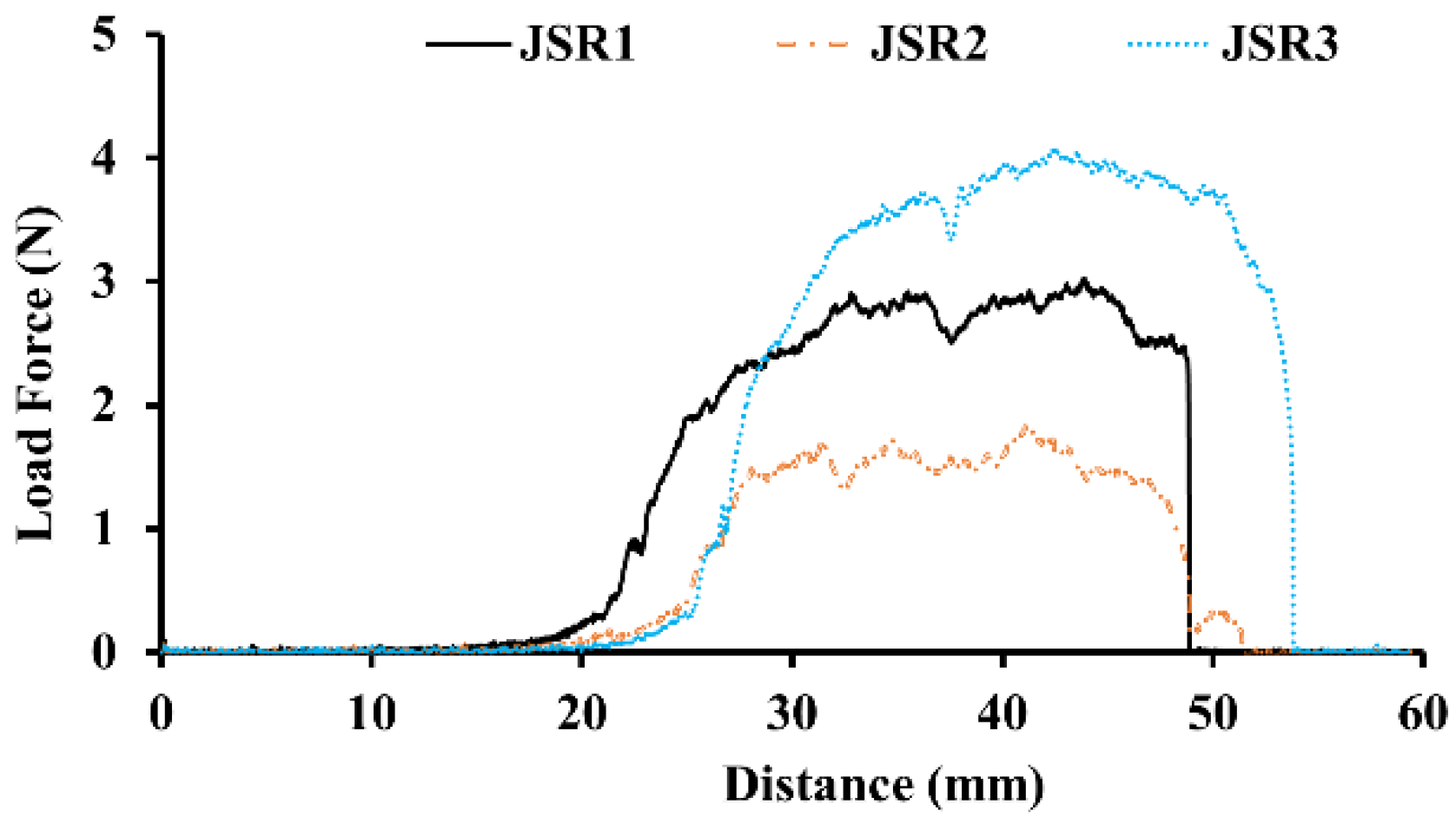
3.4. The Selection of SBR Binders
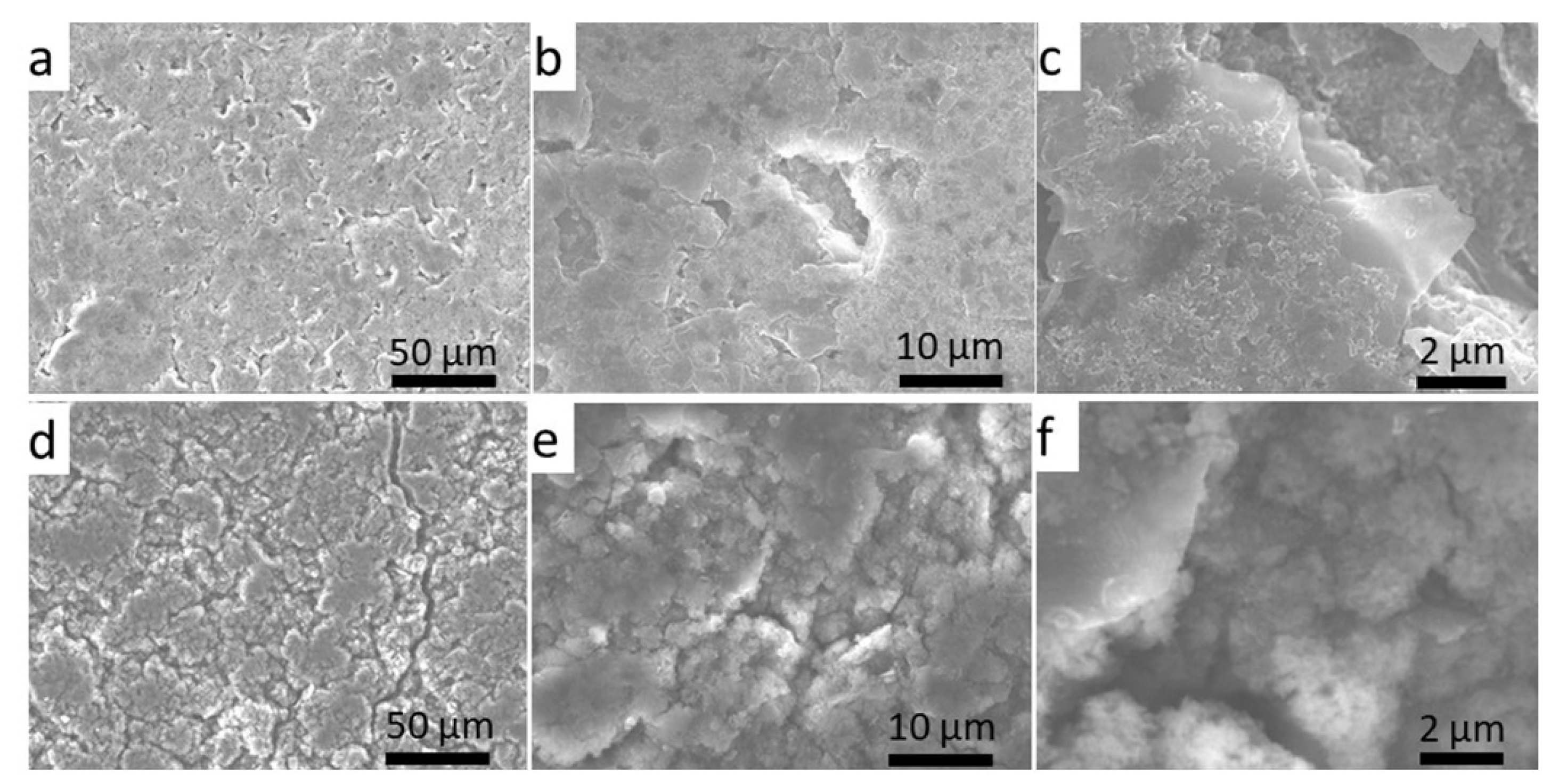
3.5. Morphology Changes of the Si/C Composite Electrode after Cycling
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Armand, M.; Tarascon, J.M. Building better batteries. Nature 2008, 451, 652–657. [Google Scholar] [CrossRef]
- Fang, C.; Zhang, G.; Lau, J.; Liu, G. Recent advances in polysulfide mediation of lithium-sulfur batteries via facile cathode and electrolyte modification. APL Mater. 2019, 7, 080902. [Google Scholar] [CrossRef] [Green Version]
- Zhao, Y.; Fang, C.; Zhang, G.; Hubble, D.; Nallapaneni, A.; Zhu, C.; Zhao, Z.; Liu, Z.; Lau, J.; Fu, Y.; et al. A Micelle Electrolyte Enabled by Fluorinated Ether Additives for Polysulfide Suppression and Li Metal Stabilization in Li-S Battery. Front. Chem. 2020, 8, 484. [Google Scholar] [CrossRef]
- Hopkins, E.J.; Frisco, S.; Pekarek, R.T.; Stetson, C.; Huey, Z.; Harvey, S.; Li, X.; Key, B.; Fang, C.; Liu, G.; et al. Examining CO2 as an Additive for Solid Electrolyte Interphase Formation on Silicon Anodes. J. Electrochem. Soc. 2021, 168, 030534. [Google Scholar] [CrossRef]
- Fang, C.; Lau, J.; Hubble, D.; Khomein, P.; Dailing, E.A.; Liu, Y.; Liu, G. Large-Molecule Decomposition Products of Electrolytes and Additives Revealed by On-Electrode Chromatography and MALDI. Joule 2021, 5, 415–428. [Google Scholar] [CrossRef]
- Chen, H.; Yang, Y.; Boyle, D.T.; Jeong, Y.K.; Xu, R.; de Vasconcelos, L.S.; Huang, Z.; Wang, H.; Wang, H.; Huang, W.; et al. Free-standing ultrathin lithium metal–graphene oxide host foils with controllable thickness for lithium batteries. Nat. Energy 2021, 1–9. [Google Scholar] [CrossRef]
- Zhou, S.; Fang, C.; Song, X.; Liu, G. Highly Ordered Carbon Coating Prepared with Polyvinylidene Chloride Precursor for High-Performance Silicon Anodes in Lithium-Ion Batteries. Batter. Supercaps 2021, 4, 240–247. [Google Scholar] [CrossRef]
- Wang, H.; Fu, J.; Wang, C.; Wang, J.; Yang, A.; Li, C.; Sun, Q.; Cui, Y.; Li, H. A binder-free high silicon content flexible anode for Li-ion batteries. Energy Environ. Sci. 2020, 13, 848–858. [Google Scholar] [CrossRef]
- Asenbauer, J.; Eisenmann, T.; Kuenzel, M.; Kazzazi, A.; Chen, Z.; Bresser, D. The success story of graphite as a lithium-ion anode material—Fundamentals, remaining challenges, and recent developments including silicon (oxide) composites. Sustain. Energy Fuels 2020, 4, 5387–5416. [Google Scholar] [CrossRef]
- Fang, C.; Liu, Z.; Lau, J.; Elzouka, M.; Zhang, G.; Khomein, P.; Lubner, S.; Ross, P.N.; Liu, G. Gradient Polarity Solvent Wash for Separation and Analysis of Electrolyte Decomposition Products on Electrode Surfaces. J. Electrochem. Soc. 2020, 167, 020506. [Google Scholar] [CrossRef]
- Zhou, Y.; Yang, Y.; Hou, G.; Yi, D.; Zhou, B.; Chen, S.; Lam, T.D.; Yuan, F.; Golberg, D.; Wang, X. Stress-relieving defects enable ultra-stable silicon anode for Li-ion storage. Nano Energy 2020, 70, 104568. [Google Scholar] [CrossRef]
- Zhou, S.; Fang, C.; Song, X.; Liu, G. The influence of compact and ordered carbon coating on solid-state behaviors of silicon during electrochemical processes. Carbon Energy 2020, 2, 143–150. [Google Scholar] [CrossRef] [Green Version]
- Jung, D.S.; Hwang, T.H.; Park, S.B.; Choi, J.W. Spray Drying Method for Large-Scale and High-Performance Silicon Negative Electrodes in Li-Ion Batteries. Nano Lett. 2013, 13, 2092–2097. [Google Scholar] [CrossRef]
- Feng, K.; Li, M.; Liu, W.; Kashkooli, A.G.; Xiao, X.; Cai, M.; Chen, Z. Silicon-Based Anodes for Lithium-Ion Batteries: From Fundamentals to Practical Applications. Small 2018, 14, 1702737. [Google Scholar] [CrossRef]
- Chou, S.-L.; Pan, Y.; Wang, J.-Z.; Liu, H.-K.; Dou, S.-X. Small things make a big difference: Binder effects on the performance of Li and Na batteries. Phys. Chem. Chem. Phys. 2014, 16, 20347–20359. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; He, X.; Fang, C.; Camacho-Forero, L.E.; Zhao, Y.; Fu, Y.; Feng, J.; Kostecki, R.; Balbuena, P.B.; Zhang, J.; et al. Reversible Crosslinked Polymer Binder for Recyclable Lithium Sulfur Batteries with High Performance. Adv. Funct. Mater. 2020, 30, 2003605. [Google Scholar] [CrossRef]
- Li, Z.; Fang, C.; Qian, C.; Zhou, S.; Song, X.; Ling, M.; Liang, C.; Liu, G. Polyisoprene Captured Sulfur Nanocomposite Materials for High-Areal-Capacity Lithium Sulfur Battery. ACS Appl. Polym. Mater. 2019, 1, 1965–1970. [Google Scholar] [CrossRef]
- Zhu, T.; Liu, G. Communication—Functional Conductive Polymer Binder for Practical Si-Based Electrodes. J. Electrochem. Soc. 2021, 168, 050533. [Google Scholar] [CrossRef]
- Chen, H.; Wu, Z.; Su, Z.; Chen, S.; Yan, C.; Al-Mamun, M.; Tang, Y.; Zhang, S. A mechanically robust self-healing binder for silicon anode in lithium ion batteries. Nano Energy 2021, 81, 105654. [Google Scholar] [CrossRef]
- Yen, J.-P.; Chang, C.-C.; Lin, Y.-R.; Shen, S.-T.; Hong, J.-L. Effects of Styrene-Butadiene Rubber/Carboxymethylcellulose (SBR/CMC) and Polyvinylidene Difluoride (PVDF) Binders on Low Temperature Lithium Ion Batteries. J. Electrochem. Soc. 2013, 160, A1811–A1818. [Google Scholar] [CrossRef]
- Buqa, H.; Holzapfel, M.; Krumeich, F.; Veit, C.; Novák, P. Study of styrene butadiene rubber and sodium methyl cellulose as binder for negative electrodes in lithium-ion batteries. J. Power Sources 2006, 161, 617–622. [Google Scholar] [CrossRef]
- Lestriez, B.; Bahri, S.; Sandu, I.; Roué, L.; Guyomard, D. On the binding mechanism of CMC in Si negative electrodes for Li-ion batteries. Electrochem. Commun. 2007, 9, 2801–2806. [Google Scholar] [CrossRef]
- Erk, C.; Brezesinski, T.; Sommer, H.; Schneider, R.; Janek, J. Toward Silicon Anodes for Next-Generation Lithium Ion Batteries: A Comparative Performance Study of Various Polymer Binders and Silicon Nanopowders. ACS Appl. Mater. Interfaces 2013, 5, 7299–7307. [Google Scholar] [CrossRef]
- Hochgatterer, N.S.; Schweiger, M.R.; Koller, S.; Raimann, P.R.; Wöhrle, T.; Wurm, C.; Winter, M. Silicon/Graphite Composite Electrodes for High-Capacity Anodes: Influence of Binder Chemistry on Cycling Stability. Electrochem. Solid-State Lett. 2008, 11, A76. [Google Scholar] [CrossRef]
- Liu, W.-R.; Yang, M.-H.; Wu, H.-C.; Chiao, S.M.; Wu, N.-L. Enhanced Cycle Life of Si Anode for Li-Ion Batteries by Using Modified Elastomeric Binder. Electrochem. Solid-State Lett. 2005, 8, A100. [Google Scholar] [CrossRef]
- Li, J.; Lewis, R.B.; Dahn, J.R. Sodium Carboxymethyl Cellulose: A Potential Binder for Si Negative Electrodes for Li-Ion Batteries. Electrochem. Solid-State Lett. 2007, 10, A17. [Google Scholar] [CrossRef]
- Dimov, N.; Noguchi, H.; Yoshio, M. A chemometric investigation of the effect of the process parameters on the performance of mixed Si/C electrodes. J. Power Sources 2006, 156, 567–573. [Google Scholar] [CrossRef]
- Ding, N.; Xu, J.; Yao, Y.; Wegner, G.; Lieberwirth, I.; Chen, C. Improvement of cyclability of Si as anode for Li-ion batteries. J. Power Sources 2009, 192, 644–651. [Google Scholar] [CrossRef]
- Bridel, J.S.; Azaïs, T.; Morcrette, M.; Tarascon, J.M.; Larcher, D. Key Parameters Governing the Reversibility of Si/Carbon/CMC Electrodes for Li-Ion Batteries. Chem. Mater. 2010, 22, 1229–1241. [Google Scholar] [CrossRef]
- Zuo, P.; Yang, W.; Cheng, X.; Yin, G. Enhancement of the electrochemical performance of silicon/carbon composite material for lithium ion batteries. Ionics 2011, 17, 87–90. [Google Scholar] [CrossRef]
- Kim, H.; Seo, M.; Park, M.-H.; Cho, J. A Critical Size of Silicon Nano-Anodes for Lithium Rechargeable Batteries. Angew. Chem. Int. Ed. 2010, 49, 2146–2149. [Google Scholar] [CrossRef]
- Zheng, H.; Ridgway, P.; Song, X.; Xun, S.; Chong, J.; Liu, G.; Battaglia, V. Comparison of Cycling Performance of Lithium Ion Cell Anode Graphites. ECS Trans. 2011, 33, 91–100. [Google Scholar] [CrossRef]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fang, C.; Xiao, H.; Zheng, T.; Bai, H.; Liu, G. Organic Solvent Free Process to Fabricate High Performance Silicon/Graphite Composite Anode. J. Compos. Sci. 2021, 5, 188. https://doi.org/10.3390/jcs5070188
Fang C, Xiao H, Zheng T, Bai H, Liu G. Organic Solvent Free Process to Fabricate High Performance Silicon/Graphite Composite Anode. Journal of Composites Science. 2021; 5(7):188. https://doi.org/10.3390/jcs5070188
Chicago/Turabian StyleFang, Chen, Haiqing Xiao, Tianyue Zheng, Hua Bai, and Gao Liu. 2021. "Organic Solvent Free Process to Fabricate High Performance Silicon/Graphite Composite Anode" Journal of Composites Science 5, no. 7: 188. https://doi.org/10.3390/jcs5070188
APA StyleFang, C., Xiao, H., Zheng, T., Bai, H., & Liu, G. (2021). Organic Solvent Free Process to Fabricate High Performance Silicon/Graphite Composite Anode. Journal of Composites Science, 5(7), 188. https://doi.org/10.3390/jcs5070188






