Optimization of Direct Bonding Process for Lotus-Type Porous Copper to Alumina Substrates
Abstract
1. Introduction
2. Materials and Methods
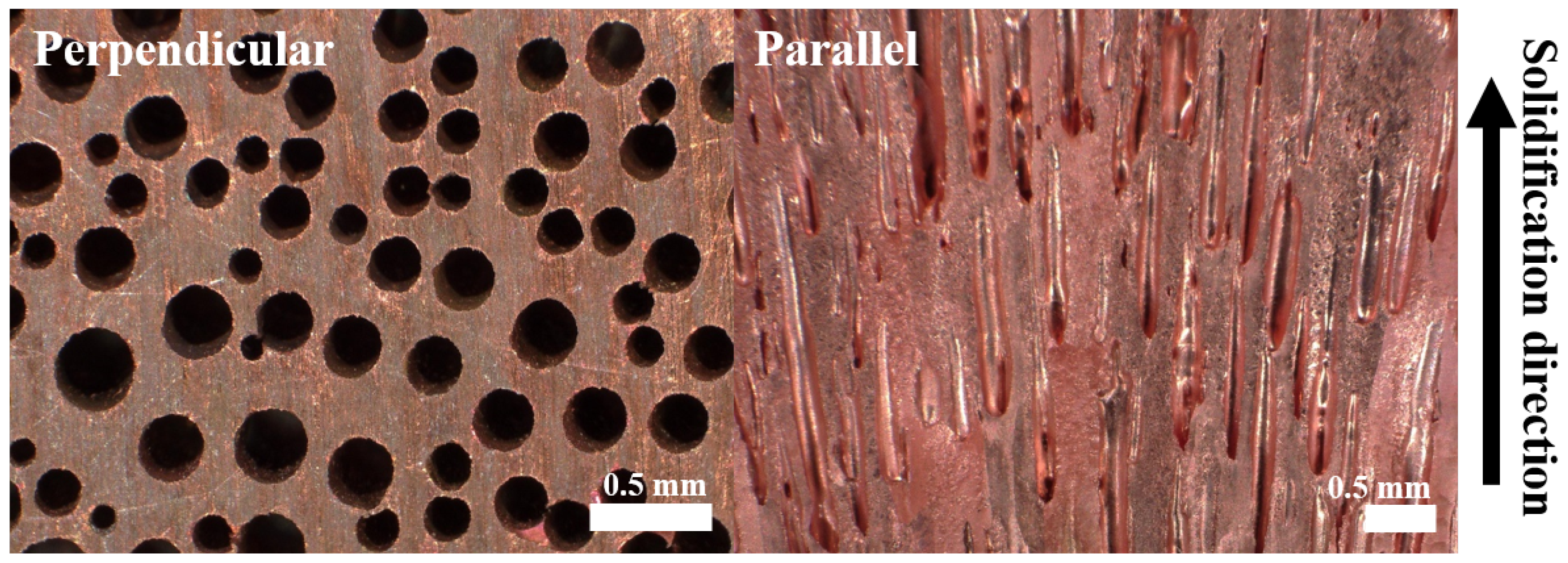
2.1. Preparation of Materials
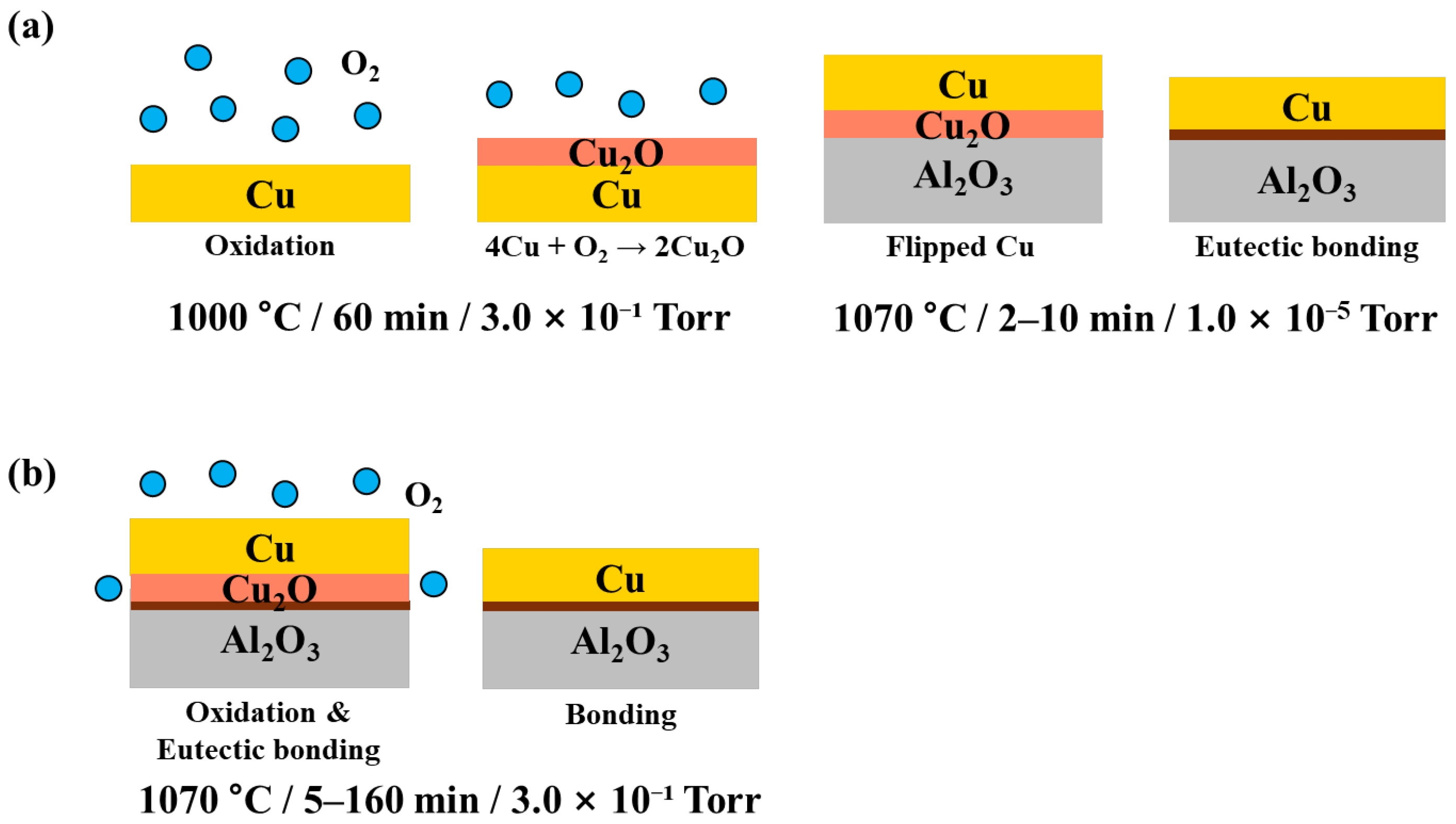
2.2. DBC Joints Process Design
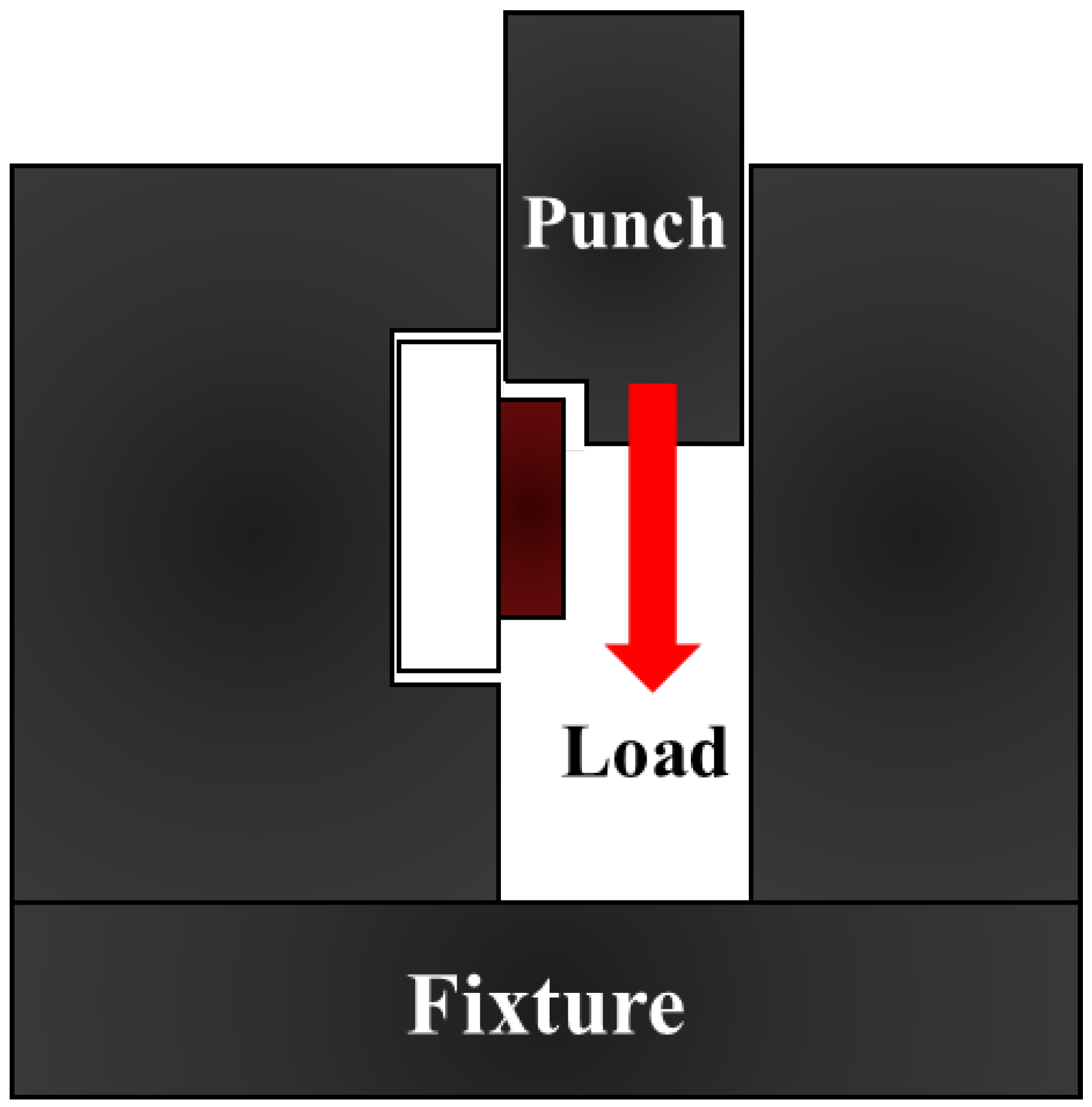
2.3. Shear Strength Test and Characterization Analysis
3. Results and Discussion
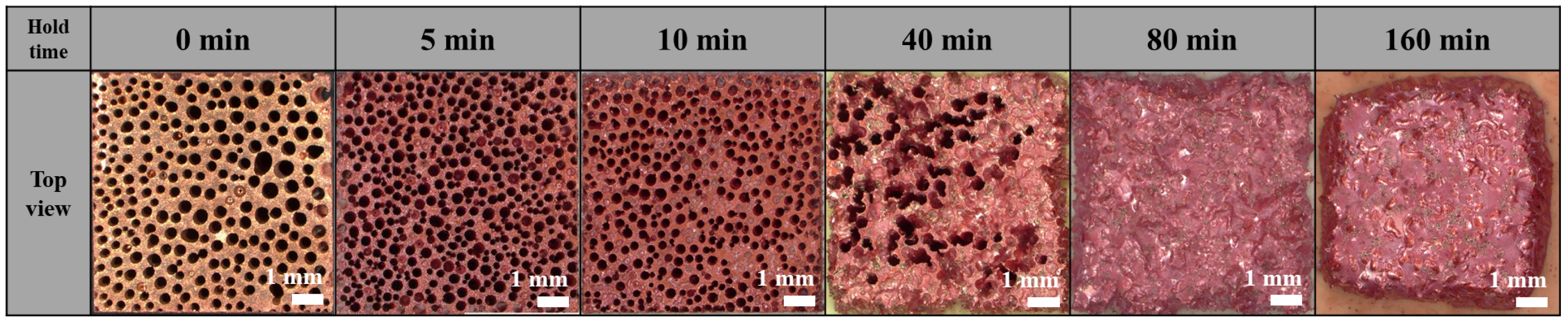
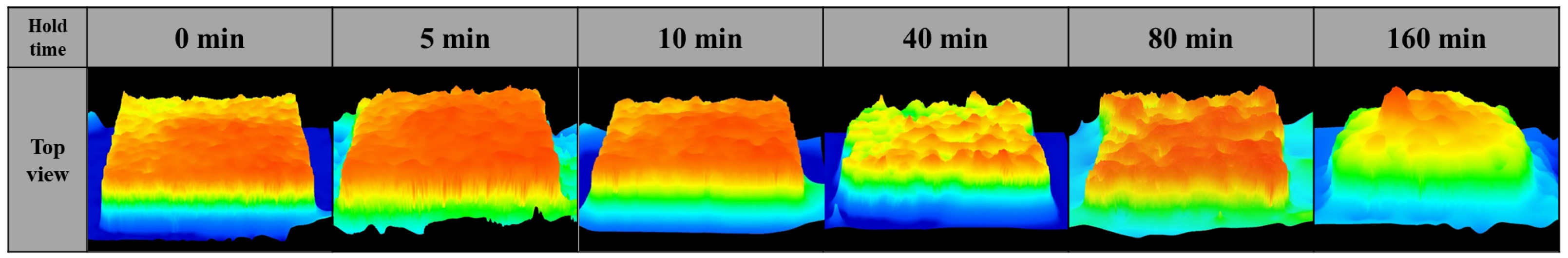
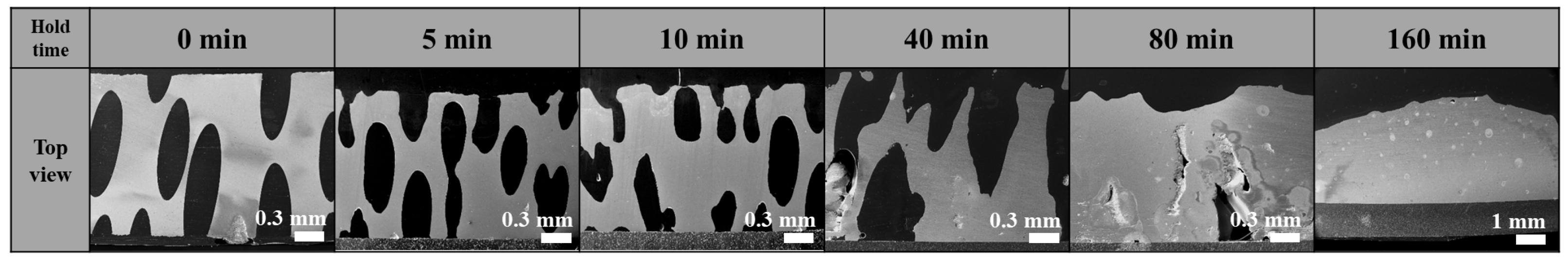
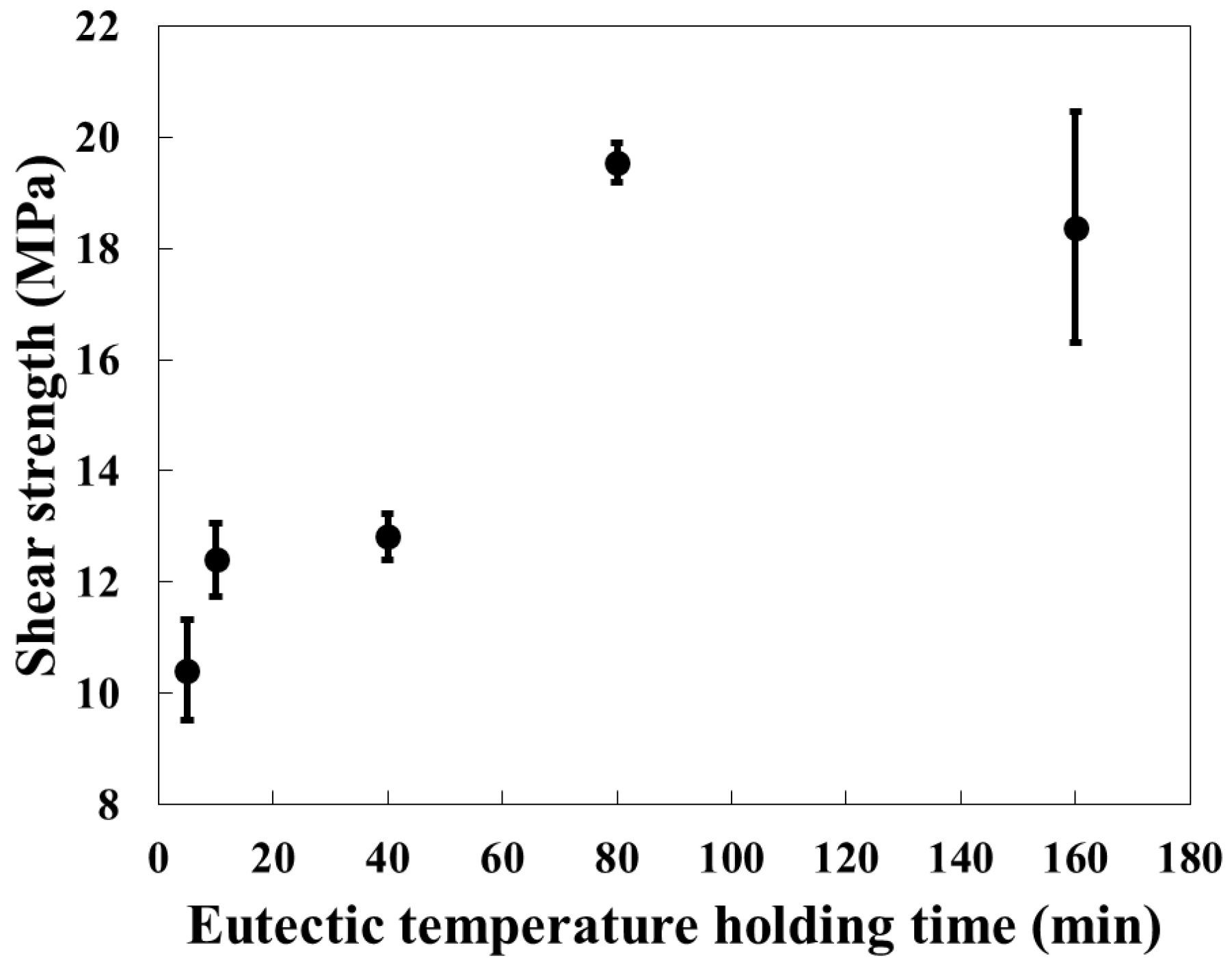
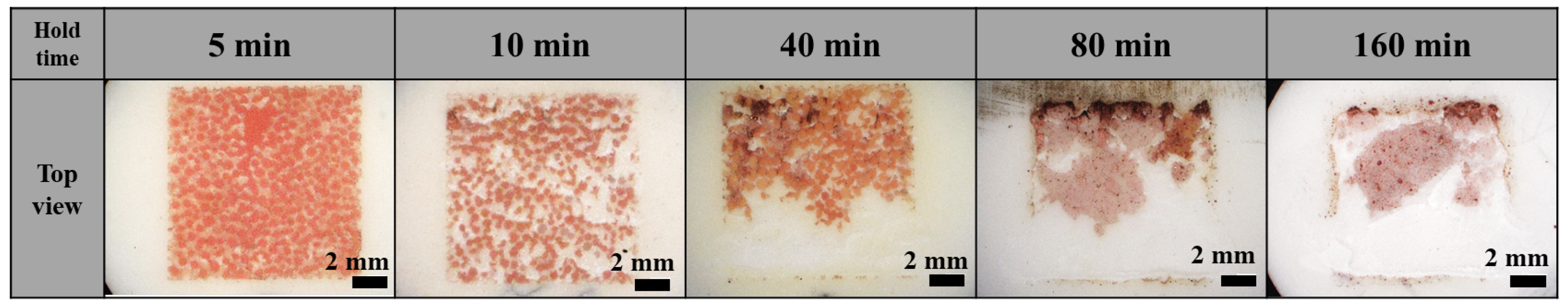
3.1. Effect of Holding Time on Lotus-Type Copper/Alumina Bond Quality
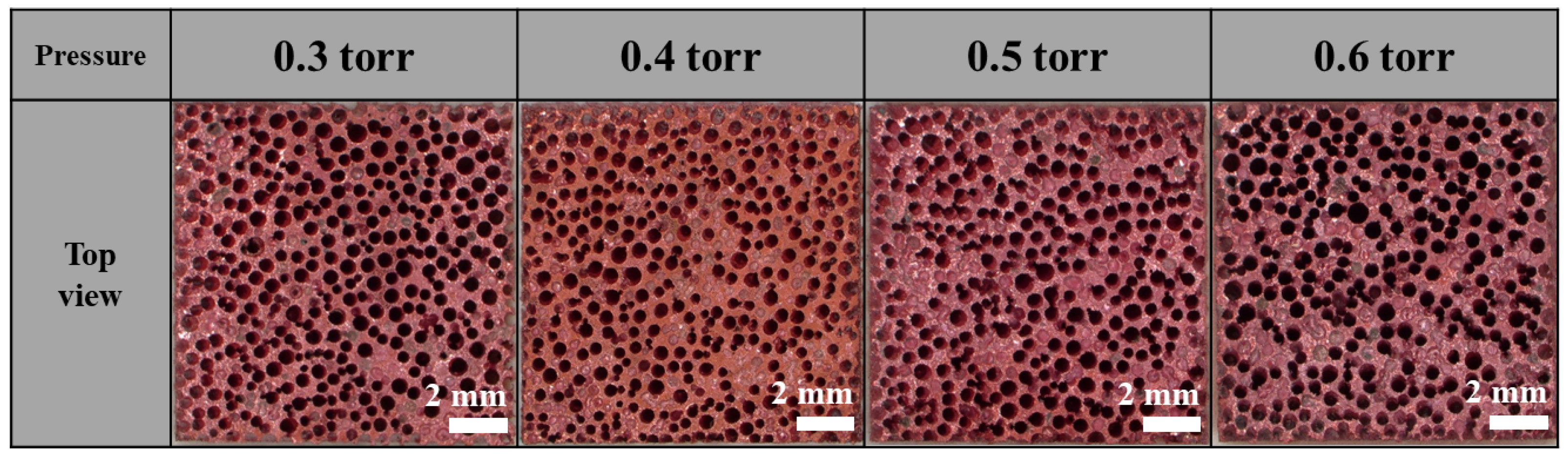
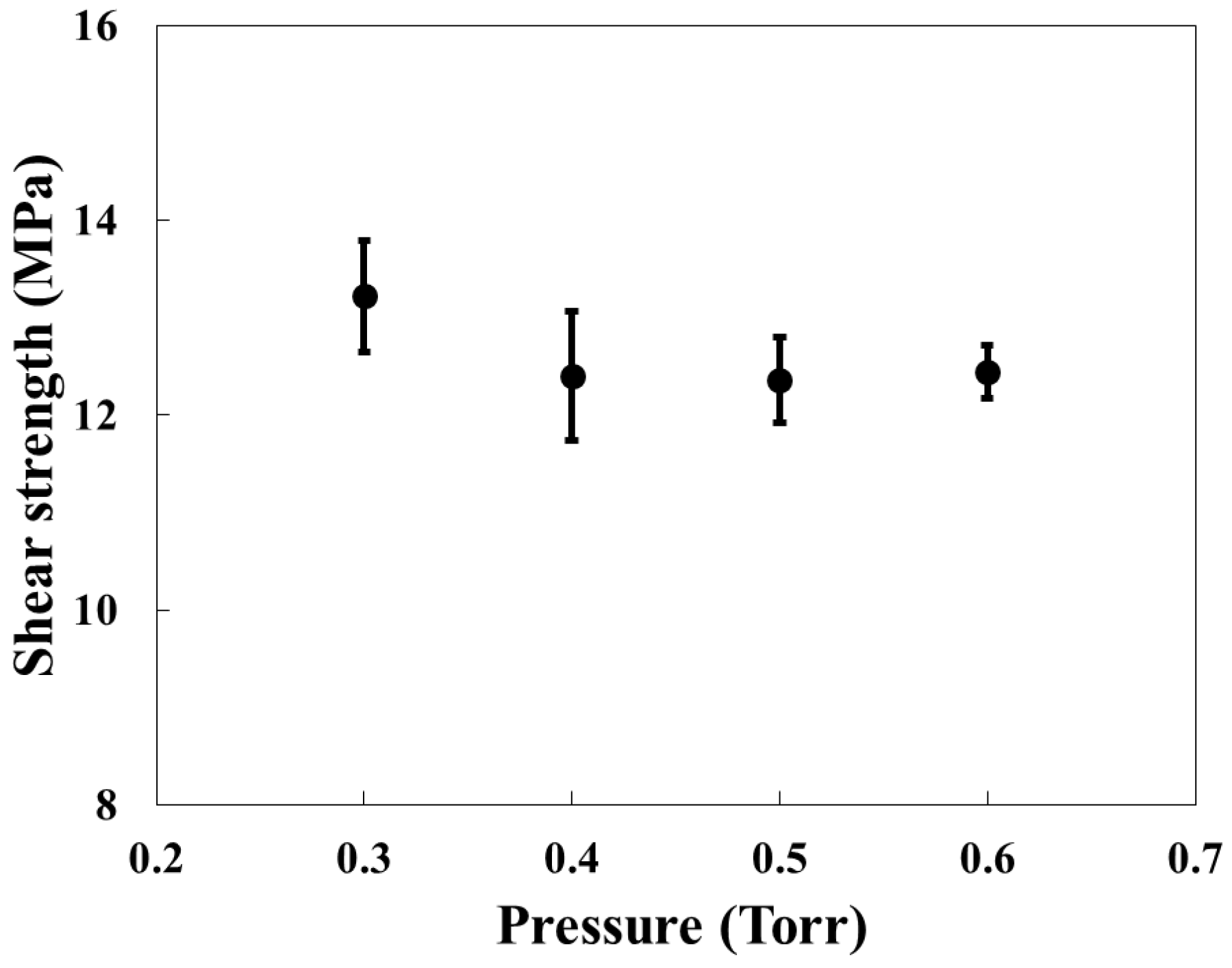
3.2. Effect of Pressure on Lotus-Type Copper/Alumina Bond Quality
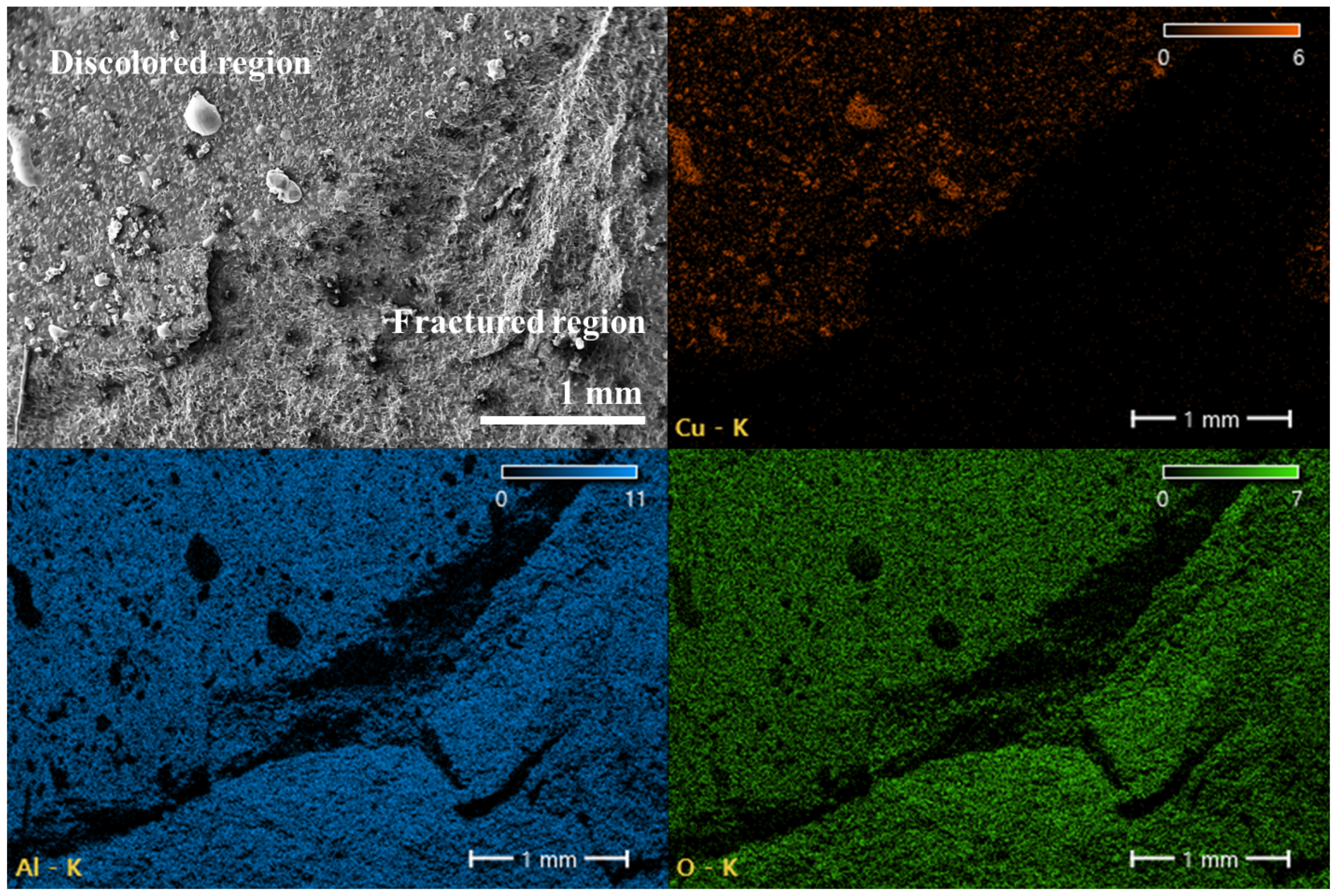
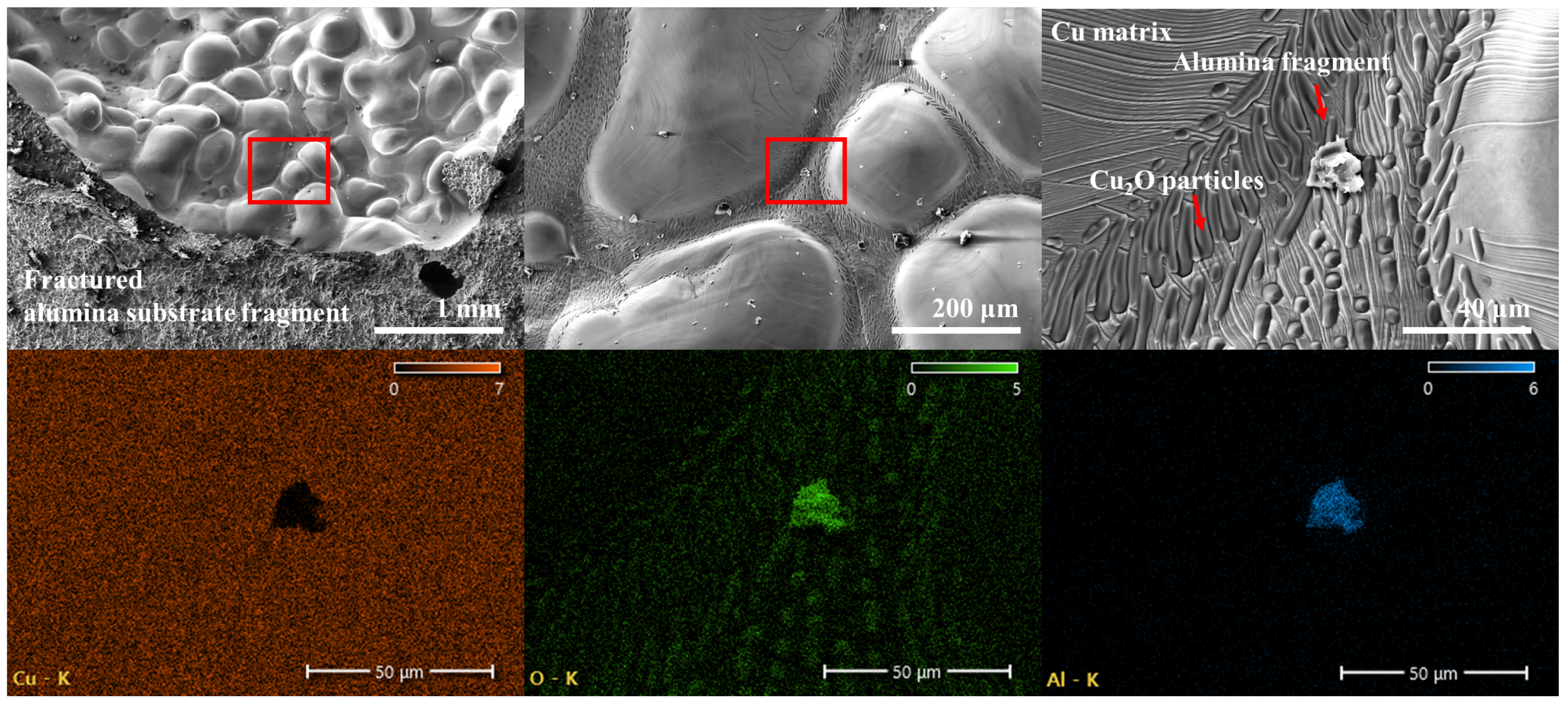
3.3. Morphological Changes with Oxidation Rate
4. Conclusions
- Within the range of 0.3–0.6 Torr, pressure had little effect on bonding quality. Cu2O formation occurred consistently under all tested pressures, and the shear strength exhibited minimal variation.
- Maintaining the specimens at the bonding temperature for 10 min provided the optimal balance between morphology preservation and joint strength. Shorter hold times (5 min) resulted in insufficient bonding, whereas longer hold times (>40 min) caused excessive deformation of the porous copper, leading to unpredictable surface morphology and unreliable shear strength.
- The evolution of copper morphology and bonding is governed by the oxidation kinetics of copper and is influenced by temperature, pressure, and oxidation duration. Specifically, the formation of the Cu2O layer follows parabolic growth behavior, and since the eutectic bonding reaction proceeds simultaneously while controlling the oxidation rate, a bonding duration of 10 min—longer than the 2–3 min typically demanded in conventional DBC processes of sheet type—is required. Excessive hold times accelerate liquid-phase flow, leading to pore closure and aggregation, which degrade the quality of the bond.
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sanchez, J.L. State of the Art and Trends in Power Integration; MSM: San Juan, Puerto Rico, 1999; pp. 20–29. [Google Scholar]
- Sanchez, J.L.; Bourennane, A.; Breil, M.; Austin, P.; Brunet, M.; Laur, J.P. Evolution of the classical functional integration towards a 3D heterogeneous functional integration. In Proceedings of the 2007 14th International Conference on Mixed Design of Integrated Circuits and Systems, Ciechocinek, Poland, 21–23 June 2007; pp. 23–34. [Google Scholar]
- Schulz-Harder, J. Advantages and new development of direct bonded copper substrates. Microelectron. Reliab. 2003, 43, 359–365. [Google Scholar] [CrossRef]
- Liu, Y. Power Electronic Packaging: Design, Assembly Process, Reliability and Modeling; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2012. [Google Scholar]
- Smet, V.; Forest, F.; Huselstein, J.J.; Richardeau, F.; Khatir, Z.; Lefebvre, S.; Berkani, M. Ageing and failure modes of IGBT modules in high-temperature power cycling. IEEE 2011, 58, 4931–4941. [Google Scholar] [CrossRef]
- McCluskey, P. Reliability of power electronics under thermal loading. In Proceedings of the 2012 7th International Conference on Integrated Power Electronics Systems (CIPS), Nuremberg, Germany, 6–8 March 2012; pp. 1–8. [Google Scholar]
- Squiller, D.; Greve, H.; Mengotti, E.; McCluskey, F.P. Physics-of-failure assessment methodology for power electronic systems. Microelectron. Reliab. 2014, 54, 1680–1685. [Google Scholar] [CrossRef]
- Karageorgiou, V.; Kaplan, D. Porosity of 3D biomaterial scaffolds and osteogenesis. Biomaterials 2005, 26, 5474–5491. [Google Scholar] [CrossRef] [PubMed]
- Goodall, R.; Mortensen, A. Porous metals. In Physical Metallurgy; Elsevier: Amsterdam, The Netherlands, 2014; pp. 2399–2595. [Google Scholar]
- Lee, J.W.; Hyun, S.K.; Kim, M.S.; Kim, M.G.; Ide, T.; Nakajima, H. Ductility improvement of intermetallic compound NiAl by unidirectional pores. Mater. Lett. 2012, 74, 213–216. [Google Scholar] [CrossRef]
- Kara-Slimane, A.; Juve, D.; Leblond, E.; Treheux, D. Joining of AlN with metals and alloys. J. Eur. Ceram. Soc. 2000, 20, 1829–1836. [Google Scholar] [CrossRef]
- Honjo, G. Electron Diffraction Studies on Oxide Films formed on Metals and Alloys Part 2 Selective Oxidation of Alloys. J. Phys. Soc. Jpn. 1953, 8, 113–118. [Google Scholar] [CrossRef]
- Shin, B.S.; Hyun, S.K. Optimizing the pore structure of lotus-type porous copper fabricated by continuous casting. Materials 2024, 17, 5015. [Google Scholar] [CrossRef] [PubMed]
- Hansen, M.; Anderko, K. Constitution of Binary Alloys; McGraw Hill: New York, NY, USA, 1958. [Google Scholar]
- Beraud, C.; Courbiere, M.; Esnouf, C.; Juve, D.; Treheux, D. Study of copper-alumina bonding. J. Mater. Sci. 1989, 24, 4545–4554. [Google Scholar] [CrossRef]
- Burgess, J.F.; Neugebauer, C.A.; Flanagan, G. The Direct Bonding of Metals to Ceramics by the Gas-Metal Eutectic Method. J. Electrochem. Soc. 1975, 122, 688. [Google Scholar] [CrossRef]
- Lee, S.K.; Tuan, W.H.; Wu, Y.Y.; Shih, S.J. Microstructure–thermal properties of Cu/Al2O3 bilayer prepared by direct bonding. J. Eur. Ceram. Soc. 2013, 33, 277–285. [Google Scholar] [CrossRef]
- Schmidt-Whitley, R.D.; Martinez-Clemente, M.; Revcolevschi, A. Growth and microstructural control of single crystal cuprous oxide Cu2O. J. Cryst. Growth 1974, 23, 113–120. [Google Scholar] [CrossRef]
- Mimura, K.; Lim, J.W.; Isshiki, M.; Zhu, Y.; Jiang, Q. Brief review of oxidation kinetics of copper at 350 C to 1050 C. Metall. Mater. Trans. A 2006, 37, 1231–1237. [Google Scholar] [CrossRef]
- Belousov, V.V.; Klimashin, A.A. High-temperature oxidation of copper. Russ. Chem. Rev. 2013, 82, 273. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Choi, S.-G.; Kim, S.; Lee, J.; Kim, K.-S.; Hyun, S. Optimization of Direct Bonding Process for Lotus-Type Porous Copper to Alumina Substrates. J. Manuf. Mater. Process. 2025, 9, 352. https://doi.org/10.3390/jmmp9110352
Choi S-G, Kim S, Lee J, Kim K-S, Hyun S. Optimization of Direct Bonding Process for Lotus-Type Porous Copper to Alumina Substrates. Journal of Manufacturing and Materials Processing. 2025; 9(11):352. https://doi.org/10.3390/jmmp9110352
Chicago/Turabian StyleChoi, Sang-Gyu, Sangwook Kim, Jinkwan Lee, Keun-Soo Kim, and Soongkeun Hyun. 2025. "Optimization of Direct Bonding Process for Lotus-Type Porous Copper to Alumina Substrates" Journal of Manufacturing and Materials Processing 9, no. 11: 352. https://doi.org/10.3390/jmmp9110352
APA StyleChoi, S.-G., Kim, S., Lee, J., Kim, K.-S., & Hyun, S. (2025). Optimization of Direct Bonding Process for Lotus-Type Porous Copper to Alumina Substrates. Journal of Manufacturing and Materials Processing, 9(11), 352. https://doi.org/10.3390/jmmp9110352







