The TB REACH Initiative: Supporting TB Elimination Efforts in the Asia-Pacific
Abstract
1. Introduction
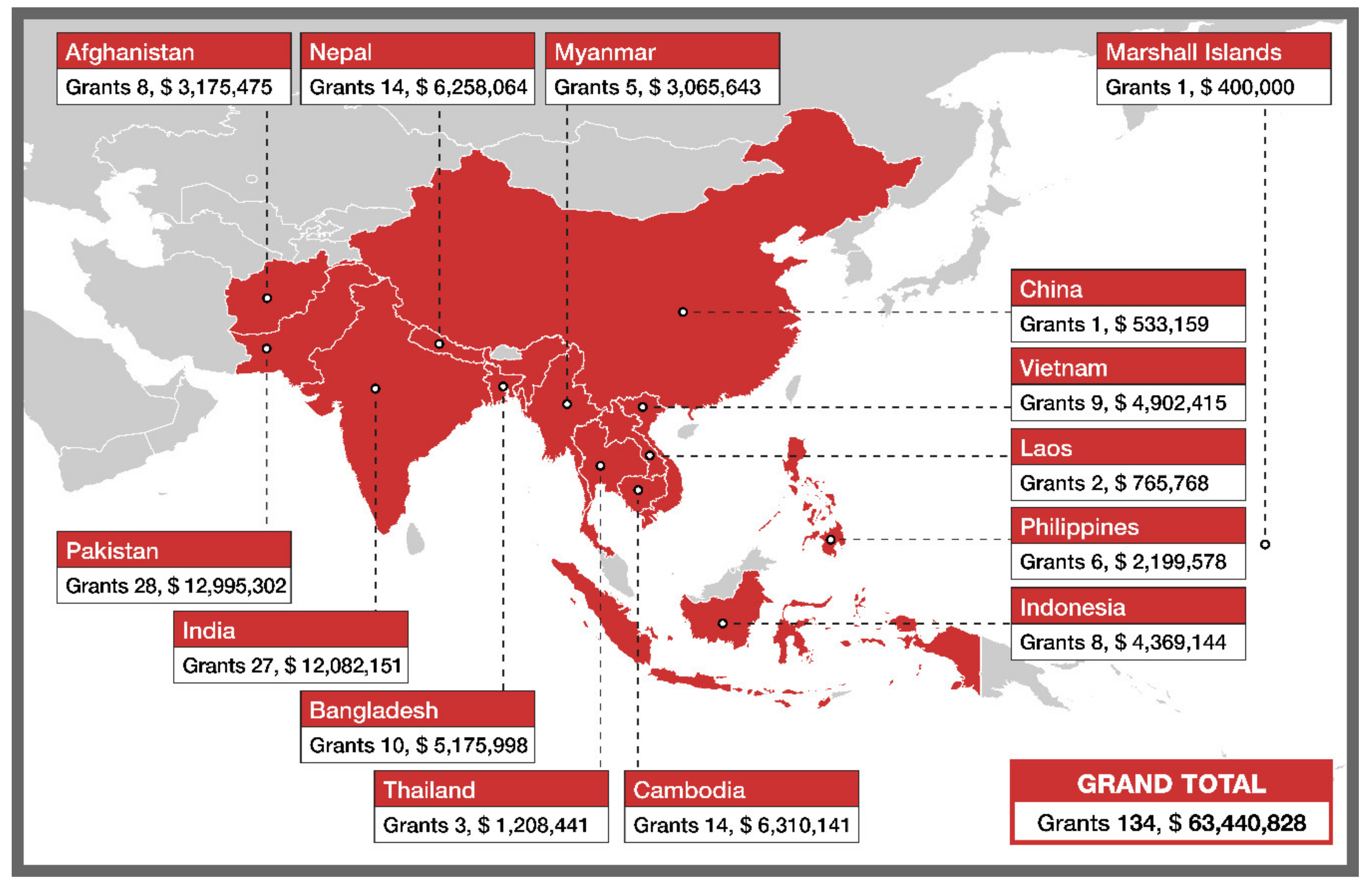
2. The TB REACH Initiative
3. Data and Hotspots
4. Active Case Finding
5. Treating TB Infection
6. Biosocial Approaches
7. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- World Health Organization. What is DOTS? A guide to Understanding the WHO-Recommended TB Control Strategy Known as DOTS; WHO: Geneva, Switzerland, 1999. [Google Scholar]
- World Health Organization. The Stop TB Strategy: Building on and Enhancing DOTS to Meet the TB-Related Millennium Development Goals; WHO: Geneva, Switzerland, 2006. [Google Scholar]
- World Health Organization. The End TB Strategy: Global Strategy and Targets for Tuberculosis Prevention, Care and Control after 2015; WHO: Geneva, Switzerland, 2015. [Google Scholar]
- Dowdy, D.W.; Basu, S.; Andrews, J.R. Is Passive Diagnosis Enough? Am. J. Respir. Crit. Care Med. 2013, 187, 543–551. [Google Scholar] [CrossRef] [PubMed]
- Ho, J.; Fox, G.J.; Marais, B.J. Passive case finding for tuberculosis is not enough. Int. J. Mycobacteriol. 2016, 5, 374–378. [Google Scholar] [CrossRef] [PubMed]
- Abebe, M.; Doherty, M.; Wassie, L.; Demissie, A.; Mihret, A.; Engers, H.; Aseffa, A. TB case detection: Can we remain passive while the process is active? Pan. Afr. Med. J. 2012, 11, 50. [Google Scholar]
- Stop TB Partnership. The Global Plan to End TB 2018—2022: The Paradigm Shift; Stop TB Partnership: Geneva, Switzerland, 2019. [Google Scholar]
- United Nations General Assembly. Political Declaration of the High-Level Meeting of the General Assembly on the Fight against Tuberculosis; United Nations General Assembly: New York, NY, USA, 2018. [Google Scholar]
- Madhukar, P. To Eliminate TB We Need Imagination and Ambition. Available online: https://theconversation.com/to-eliminate-tb-we-need-imagination-and-ambition-103083 (accessed on 24 September 2020).
- Cohen, J. Campaign against TB steps up its ambitions. Science 2015, 350, 1455. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Global Tuberculosis Report 2019; WHO: Geneva, Switzerland, 2019. [Google Scholar]
- United Nations, Department of Economic and Social Affairs, Statistics Division. Standard Country or Area Codes for Statistical Use. Available online: https://unstats.un.org/unsd/methodology/m49/ (accessed on 24 September 2020).
- Arinaminpathy, N.; Batra, D.; Khaparde, S.; Vualnam, T.; Maheshwari, N.; Sharma, L.; Nair, S.A.; Dewan, P. The number of privately treated tuberculosis cases in India: An estimation from drug sales data. Lancet Infect Dis. 2016, 16, 1255–1260. [Google Scholar] [CrossRef]
- Marks, G.B.; Nguyen, N.V.; Nguyen, P.T.B.; Nguyen, T.A.; Nguyen, H.B.; Tran, K.H.; Nguyen, S.V.; Luu, K.B.; Tran, D.T.T.; Vo, Q.T.N.; et al. Community-wide Screening for Tuberculosis in a High-Prevalence Setting. N. Engl. J. Med. 2019, 381, 1347–1357. [Google Scholar] [CrossRef]
- World Bank Open Data. Available online: https://data.worldbank.org/ (accessed on 24 September 2020).
- Keshavjee, S.; Dowdy, D.; Swaminathan, S. Stopping the body count: A comprehensive approach to move towards zero tuberculosis deaths. Lancet 2015, 386, e46–e47. [Google Scholar] [CrossRef]
- Stop TB Partnership. The TB REACH Initiative. Available online: http://www.stoptb.org/global/awards/tbreach/ (accessed on 24 September 2020).
- Qin, Z.Z.; Pai, M.; Van Gemert, W.; Sahu, S.; Ghiasi, M.; Creswell, J. How is Xpert MTB/RIF being implemented in 22 high tuberculosis burden countries? Eur. Respir. J. 2015, 45, 549–554. [Google Scholar] [CrossRef]
- Creswell, J.; Codlin, A.J.; Andre, E.; Micek, M.A.; Bedru, A.; Carter, E.J.; Yadav, R.P.; Mosneaga, A.; Rai, B.; Banu, S.; et al. Results from early programmatic implementation of Xpert MTB/RIF testing in nine countries. BMC Infect Dis. 2014, 14, 2. [Google Scholar] [CrossRef]
- Chry, M.; Smelyanskaya, M.; Ky, M.; Codlin, A.J.; Cazabon, D.; Tan Eang, M.; Creswell, J. Can the High Sensitivity of Xpert MTB/RIF Ultra Be Harnessed to Save Cartridge Costs? Results from a Pooled Sputum Evaluation in Cambodia. Trop. Med. Infect Dis. 2020, 5, 27. [Google Scholar] [CrossRef]
- Abdurrahman, S.T.; Mbanaso, O.; Lawson, L.; Oladimeji, O.; Blakiston, M.; Obasanya, J.; Dacombe, R.; Adams, E.R.; Emenyonu, N.; Sahu, S.; et al. Testing Pooled Sputum with Xpert MTB/RIF for Diagnosis of Pulmonary Tuberculosis To Increase Affordability in Low-Income Countries. J. Clin. Microbiol. 2015, 53, 2502–2508. [Google Scholar] [CrossRef] [PubMed]
- Rahman, M.T.; Codlin, A.J.; Rahman, M.M.; Nahar, A.; Reja, M.; Islam, T.; Qin, Z.Z.; Khan, M.A.S.; Banu, S.; Creswell, J. An evaluation of automated chest radiography reading software for tuberculosis screening among public- and private-sector patients. Eur. Respir. J. 2017, 49, 1602159. [Google Scholar] [CrossRef] [PubMed]
- Qin, Z.Z.; Sander, M.S.; Rai, B.; Titahong, C.N.; Sudrungrot, S.; Laah, S.N.; Adhikari, L.M.; Carter, E.J.; Puri, L.; Codlin, A.J.; et al. Using artificial intelligence to read chest radiographs for tuberculosis detection: A multi-site evaluation of the diagnostic accuracy of three deep learning systems. Sci. Rep. 2019, 18, 15000. [Google Scholar] [CrossRef]
- Muyoyeta, M.; Maduskar, P.; Moyo, M.; Kasese, N.; Milimo, D.; Spooner, R.; Kapata, N.; Hogeweg, L.; van Ginneken, B.; Ayles, H.; et al. The Sensitivity and Specificity of Using a Computer Aided Diagnosis Program for Automatically Scoring Chest X-Rays of Presumptive TB Patients Compared with Xpert MTB/RIF in Lusaka Zambia. PLoS ONE 2014, 9, e93757. [Google Scholar] [CrossRef] [PubMed]
- Wandwalo, E.; Creswell, J. TB REACH and the Global Fund: A Partnership to Find and Treat the “Missing Cases”. Available online: https://www.theglobalfund.org/en/blog/2017-06-02-tb-reach-and-the-global-fund-a-partnership-to-find-and-treat-the-missing-cases/ (accessed on 4 October 2020).
- Stop TB Partnership. TB REACH Presentation to the 32nd Board, Jakarta, Indonesia. 2019. Available online: http://www.stoptb.org/assets/documents/about/cb/meetings/32/32-08%20TB%20REACH%203.0/32-8.8%20TB%20REACH%203.0_Presentation.pdf (accessed on 30 September 2020).
- Theron, G.; Jenkins, H.E.; Cobelens, F.; Abubakar, I.; Khan, A.J.; Cohen, T.; Dowdy, D.W. Data for action: Collection and use of local data to end tuberculosis. Lancet 2015, 386, 2324–2333. [Google Scholar] [CrossRef]
- Rood, E.; Khan, A.H.; Modak, P.K.; Mergenthaler, C.; van Gurp, M.; Blok, L.; Bakker, M. A Spatial Analysis Framework to Monitor and Accelerate Progress towards SDG 3 to End TB in Bangladesh. Int. J. Geo-Inf. 2019, 8, 14. [Google Scholar] [CrossRef]
- Vo, L.N.Q.; Vu, T.N.; Nguyen, H.T.; Truong, T.T.; Khuu, C.M.; Pham, P.Q.; Nguyen, L.H.; Le, G.T.; Creswell, J. Optimizing community screening for tuberculosis: Spatial analysis of localized case finding from door-to-door screening for TB in an urban district of Ho Chi Minh City, Vietnam. PLoS ONE 2018, 13, e0209290. [Google Scholar] [CrossRef]
- Blok, L.; Creswell, J.; Stevens, R.; Brouwer, M.; Ramis, O.; Weil, O.; Klatser, P.; Sahu, S.; Bakker, M.I. A pragmatic approach to measuring, monitoring and evaluating interventions for improved tuberculosis case detection. Int. Health 2014, 6, 181–188. [Google Scholar] [CrossRef]
- Cowan, J.; Michel, C.; Manhiça, I.; Mutaquiha, C.; Monivo, C.; Saize, D.; Beste, J.; Creswell, J.; Codlin, A.J.; Gloyd, S. Remote monitoring of Xpert® MTB/RIF testing in Mozambique: Results of programmatic implementation of GxAlert. Int. J. Tuberc. Lung Dis. 2016, 20, 335–341. [Google Scholar] [CrossRef]
- Vo, L.N.Q.; Forse, R.J.; Codlin, A.J.; Vu, T.N.; Le, G.T.; Do, G.C.; Truong, V.V.; Dang, H.M.; Squire, B.; Levy, J.; et al. A comparative impact evaluation of two human resource models for community-based active tuberculosis case finding in Ho Chi Minh City, Vietnam. BMC Public Health 2020, 20, 934. [Google Scholar] [CrossRef]
- Uplekar, M.; Creswell, J.; Ottmani, S.E.; Weil, D.; Sahu, S.; Lönnroth, K. Programmatic approaches to screening for active tuberculosis. Int. J. Tuberc. Lung Dis. 2013, 17, 1248–1256. [Google Scholar] [CrossRef] [PubMed]
- National Center for Tuberculosis and Leprosy Control (CENAT). National Strategic Plan for Control of Tuberculosis 2014–2020; National Center for Tuberculosis and Leprosy Control: Phnom Penh, Cambodgia, 2014. [Google Scholar]
- National Tuberculosis Programme, Myanmar. National Strategic Plan for Tuberculosis 2016–2020. Available online: https://www.aidsdatahub.org/sites/default/files/resource/myanmar-national-strategic-plan-tuberculosis-2016-2020.pdf (accessed on 19 September 2020).
- Revised National Tuberculosis Control Programme. National Strategic Plan for Tuberculosis Elimination 2017–2025. Available online: https://tbcindia.gov.in/WriteReadData/NSP%20Draft%2020.02.2017%201.pdf (accessed on 19 September 2020).
- Siahaan, E.S.; Bakker, M.I.; Pasaribu, R.; Khan, A.; Pande, T.; Hasibuan, A.M.; Creswell, J. Islands of tuberculosis elimination: An evaluation of community-based active case finding in North Sumatra, Indonesia. Trop. Med. Infect. Dis 2020, 5, 163. [Google Scholar] [CrossRef]
- Malik, A.A.; Hussain, H.; Creswell, J.; Siddiqui, S.; FAhmed, J.; Madhani, F.; Habib, A.; Khan, A.J.; Amanullah, F. The Impact of Funding on Childhood TB Case Detection in Pakistan. Trop. Med. Infect. Dis. 2019, 4, 146. [Google Scholar] [CrossRef]
- Malik, A.A.; Amanullah, F.; Codlin, A.J.; Siddiqui, S.; Jaswal, M.; Ahmed, J.F.; Saleem, S.; Khurshid, A.; Hussain, H. Improving childhood tuberculosis detection and treatment through facility-based screening in rural Pakistan. Int. J. Tuberc. Lung Dis. 2018, 22, 851–857. [Google Scholar] [CrossRef]
- Codlin, A.J.; Monyrath, C.; Ky, M.; Gerstel, L.; Creswell, J.; Eang, M.T. Results from a roving, active case finding initiative to improve tuberculosis detection among older people in rural Cambodia using the Xpert MTB/RIF assay and chest X-ray. J. Clin. Tuberc. Other Mycobact. Dis. 2018, 13, 22–27. [Google Scholar] [CrossRef]
- Blok, L.; Sahu, S.; Creswell, J.; Alba, S.; Stevens, R.; Bakker, M.I. Comparative Meta-Analysis of Tuberculosis Contact Investigation Interventions in Eleven High Burden Countries. PLoS ONE 2015, 10, e0119822. [Google Scholar] [CrossRef]
- Shah, S.A.; Qayyum, S.; Abro, R.; Baig, S.; Creswell, J. Active contact investigation and treatment support: An integrated approach in rural and urban Sindh, Pakistan. Int. J. Tuberc. Lung Dis. 2013, 17, 1569–1574. [Google Scholar] [CrossRef]
- Kranzer, K.; Afnan-Holmes, H.; Tomlin, K.; Golub, J.E.; Shapiro, A.E.; Schaap, A.; Corbett, E.L.; Lönnroth, K.; Glynn, J.R. The benefits to communities and individuals of screening for active tuberculosis disease: A systematic review. Int. J. Tuberc. Lung Dis. 2013, 17, 432–446. [Google Scholar] [CrossRef]
- Vyas, A.; Creswell, J.; Codlin, A.J.; Stevens, R.; Rao, V.G.; Kumar, B.; Khaparde, S.; Sahu, S. Community-based active case-finding to reach the most vulnerable: Tuberculosis in tribal areas of India. Int. J. Tuberc. Lung Dis. 2019, 23, 750–755. [Google Scholar] [CrossRef]
- Eang, M.T.; Satha, P.; Yadav, R.P.; Morishita, F.; Nishikiori, N.; van-Maaren, P.; Lambregts-van Weezenbeek, C. Early detection of tuberculosis through community-based active case finding in Cambodia. BMC Public Health 2012, 12, 469. [Google Scholar] [CrossRef]
- Morishita, F.; Eang, M.T.; Nishikiori, N.; Yadav, R.-P. Increased Case Notification through Active Case Finding of Tuberculosis among Household and Neighbourhood Contacts in Cambodia. PLoS ONE 2016, 11, e0150405. [Google Scholar] [CrossRef] [PubMed]
- Creswell, J.; Sahu, S.; Blok, L.; Bakker, M.I.; Stevens, R.; Ditiu, L. A Multi-Site Evaluation of Innovative Approaches to Increase Tuberculosis Case Notification: Summary Results. PLoS ONE 2014, 9, e94465. [Google Scholar] [CrossRef] [PubMed]
- Mhimbira, F.A.; Cuevas, L.E.; Dacombe, R.; Mkopi, A.; Sinclair, D. Interventions to increase tuberculosis case detection at primary healthcare or community-level services. Cochrane Datab. Syst. Rev. 2017, CD011432. [Google Scholar] [CrossRef] [PubMed]
- Harries, A.D.; Kumar, A.M.V.; Satyanarayana, S.; Thekkur, P.; Lin, Y.; Dlodlo, R.A.; Khogali, M.; Zachariah, R. The Growing Importance of Tuberculosis Preventive Therapy and How Research and Innovation Can Enhance Its Implementation on the Ground. Trop. Med. Infect. Dis. 2020, 5, 61. [Google Scholar] [CrossRef] [PubMed]
- Zero TB Initiative. Available online: https://www.zerotbinitiative.org/ (accessed on 24 September 2020).
- Brostrom, R.; Largen, A.; Konelios-Langinlur, M.; Zachraias, Z.; Yadav, S.; Ko, E.; Dugan, G.; Hill, J.; Finlay, A.; Mermin, J. Voyage to TB elimination: A mass TB treatment and prevention campaign in the Marshall Islands. In Proceedings of the Union World Lung Conference, Hyderabad, India, 30 October–2 November 2019; 2019. Available online: https://hyderabad.worldlunghealth.org/wp-content/uploads/2019/11/20191101_UNION2019_Abstracts_Final.pdf (accessed on 24 September 2020).
- Hargreaves, J.R.; Boccia, D.; Evans, C.A.; Adato, M.; Petticrew, M.; Porter, J.D. The social determinants of tuberculosis: From evidence to action. Am. J. Public Health 2011, 101, 654–662. [Google Scholar] [CrossRef]
- Erlinger, S.; Stracker, N.; Hanrahan, C.; Nonyane, S.; Mmolawa, L.; Tampi, R.; Tucker, A.; West, N.; Lebina, L.; Martinson, N.A.; et al. Tuberculosis patients with higher levels of poverty face equal or greater costs of illness. Int. J. Tuberc. Lung Dis. 2019, 23, 1205–1212. [Google Scholar] [CrossRef] [PubMed]
- Yellapa, V.; Devadasan, N.; Krumeich, A.; Pai, N.P.; Vadnais, C.; Pai, M.; Engel, N. How patients navigate the diagnostic ecosystem in a fragmented health system: A qualitative study from India. Global Health Action 2017, 10, 1350452. [Google Scholar] [CrossRef] [PubMed]
- Garg, T.; Gupta, V.; Sen, D.; Verma, M.; Brouwer, M.; Mishra, R.; Bhardwaj, M. Prediagnostic loss to follow-up in an active case finding tuberculosis programme: A mixed-methods study from rural Bihar, India. BMJ Open 2020, 10, e033706. [Google Scholar] [CrossRef]
- Garg, T.; Panibatla, V.; Bhardwaj, M.; Brouwer, M. Can Patient Navigators Help Potential TB Patients Navigate the Diagnostic and Treatment Pathway? In Proceedings of the Union World Lung Conference, Sevilla, Spain, 21–24 October 2020. (E-poster). [Google Scholar]
- Gurung, S.C.; Dixit, K.; Rai, B.; Levy, J.W.; Malla, D.; Budhathoki, G.; Acharya, S.; Dhital, R.; Paudel, P.R.; Caws, M.; et al. The role of active case finding in reducing patient incurred catastrophic costs for tuberculosis in Nepal. Infect. Dis. Poverty 2019, 8, 99. [Google Scholar] [CrossRef]
- Sagbakken, M.; Frich, J.C.; Bjune, G.A.; Porter, J.D. Ethical aspects of directly observed treatment for tuberculosis: A crosscultural comparison. BMC Med. Ethics 2013, 14, 25. [Google Scholar] [CrossRef]
- Villanueva, A.; de Morales, M.; Alacapa, J.; Powers, R. Digital Adherence Technology in Action: 99DOTS as a Platform for quality TB Treatment by Private Providers in the Philippines. Available online: https://tbhealthtech.org/wp-content/uploads/2020/08/KNCV_99DOTS-in-the-Philippines_poster.jpg (accessed on 24 September 2020).
- Gler, M.; Casalme, D.; Marcelo, D.; Frias, M. Feasibility and Acceptability of Video Observed Therapy Among Multi-Drug Resistant Tuberculosis Patients in Cavite, Philippines. Available online: https://tbhealthtech.org/wp-content/uploads/2020/08/DLSHSI_VOT_FINAL-POSTER.jpg (accessed on 24 September 2020).
- Litner, R. The Potential of Near Field Communication Technology; Results and Lessons from a Digital Tuberculosis Management Program on the Thai-Myanmar Border. Available online: https://tbhealthtech.org/wp-content/uploads/2020/08/TB-Poster_D-treeInternational.jpg (accessed on 24 September 2020).
- Shah, S. Active TB Case Finding Among Transgender and Male Sex Workers in Pakistan. In Oral Presentation in Reaching the Unreached to Find the Missing millions. In Proceedings of the World Lung Conference, The Hague, The Netherlands, 24–27 October 2018. SP46. [Google Scholar]
- Abdurrahman, S.T.; Emenyonu, N.; Obasanya, O.J.; Lawson, L.; Dacombe, R.; Muhammad, M.; Oladimeji, O.; Cuevas, L.E. The hidden costs of installing Xpert machines in a tuberculosis high-burden country: Experiences from Nigeria. Pan Afr. Med. J. 2014, 18, 277. [Google Scholar] [CrossRef] [PubMed]
- Creswell, J.; Rai, B.; Wali, R.; Sudrungrot, S.; Adhikari, L.M.; Pant, R.; Pyakurel, S.; Uranw, D.; Codlin, A.J. Introducing new tuberculosis diagnostics: The impact of Xpert® MTB/RIF testing on case notifications in Nepal. Int. J. Tuberc. Lung Dis. 2015, 19, 545–551. [Google Scholar] [CrossRef] [PubMed]
- Lorent, N.; Choun, K.; Malhotra, S.; Koeut, P.; Thai, S.; Khun, K.E.; Colebunders, R.; Lynen, L. Challenges from Tuberculosis Diagnosis to Care in Community-Based Active Case Finding among the Urban Poor in Cambodia: A Mixed-Methods Study. PLoS ONE 2015, 10, e0130179. [Google Scholar] [CrossRef] [PubMed]
- Morishita, F.; Garfin, A.M.C.G.; Lew, W.; Oh, K.H.; Yadav, R.-P.; Reston, J.C.; Infante, L.L.; Acala, M.R.C.; Palanca, D.L.; Kim, H.J. Bringing state-of-the-art diagnostics to vulnerable populations: The use of a mobile screening unit in active case finding for tuberculosis in Palawan, the Philippines. PLoS ONE 2017, 12, e0171310. [Google Scholar] [CrossRef] [PubMed]
- Onozaki, I.; Law, I.; Sismanidis, C.; Zignol, M.; Glaziou, P.; Floyd, K. National tuberculosis prevalence surveys in Asia, 1990–2012: An overview of results and lessons learned. Trop. Med. Int. Health 2015, 20, 1128–1145. [Google Scholar] [CrossRef]
- Daftary, A.; Furin, J.; Zelnick, J.; Venkatesan, N.; Steingart, K.; Smelyanskaya, M.; Seepamore, B.; Schoeman, I.; Reid, M.; Padayatchi, N.; et al. Tuberculosis and Women: A Call to Action. Int. J. Tuberc. Lung Dis. 2020. In Press. [Google Scholar] [CrossRef]
- Devries, K.M.; Mak, J.Y.; Garcia-Moreno, C.; Petzold, M.; Child, J.C.; Falder, G.; Lim, S.; Bacchus, L.J.; Engell, R.E.; Rosenfeld, L. The global prevalence of intimate partner violence against women. Science 2013, 340, 1527–1528. [Google Scholar] [CrossRef]
- Singer, M.; Bulled, N.; Ostrach, B.; Mendenhall, E. Syndemics and the biosocial conception of health. Lancet 2017, 389, 941–950. [Google Scholar] [CrossRef]
- Global Affairs Canada. Canada’s Feminist International Assistance Policy; Ottawa, ON, Canada. 2017. Available online: https://www.international.gc.ca/world-monde/issues_development-enjeux_developpement/priorities-priorites/policy-politique.aspx?lang=eng (accessed on 24 September 2020).
- Manandhar, M.; Hawkes, S.; Buse, K.; Nosrati, E.; Magar, V. Gender, health and the 2030 agenda for sustainable development. Bull World Health Organ. 2018, 96, 644–653. [Google Scholar] [CrossRef]
- Stop TB Partnership. Framework for the Empowerment of Women and Girls in TB REACH Grants. 2019. Available online: http://stoptb.org/assets/documents/global/awards/tbreach/W7_WEmpowerment_TBREACHGrants.pdf (accessed on 24 September 2020).
- Akundi, S. One Woman’s Decade-Long Fight Against TB Stigma. The Hindu, 25 March 2019. Available online: https://www.thehindu.com/life-and-style/fitness/pharmacist-manjula-devi-has-been-raising-awareness-against-tuberculosis-for-the-last-one-decade/article26631725.ece (accessed on 24 September 2020).
- Interactive Research and Development. The Kiran Sitara Leadership Program. Available online: http://ird.global/program/yepgap/projects/kiran-sitaraadolescent-girls-leadership-health-program/#detail (accessed on 19 September 2020).
- Address by Prime Minister Narendra Modi, Vigyan Bhawan, New Delhi, The Delhi End TB Summit; Ministry of Health and Family Welfare, Government of India, Stop TB Partnership and WHO South-East Asia. 2018. Available online: http://www.stoptb.org/assets/documents/news/PM%20Modi%20speech%20on%2013%20March%202018%20in%20Vigyan%20Bhawan%20in%20Delhi.pdf (accessed on 24 September 2020).
- Office of Assistant to Deputy Cabinet Secretary for State Documents & Translation. Introductory Remarks of the President of the Republic of Indonesia in the Limited Cabinet Meeting on the Acceleration of Tuberculosis Elimination; Jakarta, Indonesia. 2020. Available online: https://setkab.go.id/en/introductory-remarks-of-the-president-of-the-republic-of-indonesia-in-the-limited-cabinet-meeting-on-the-acceleration-of-tuberculosis-elimination-tuesday-21-july-2020-at-the-merdeka-palace-jakarta/ (accessed on 24 September 2020).
| Passive Case Finding | Active Case Finding |
|---|---|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Creswell, J.; Khan, A.; Bakker, M.I.; Brouwer, M.; Kamineni, V.V.; Mergenthaler, C.; Smelyanskaya, M.; Qin, Z.Z.; Ramis, O.; Stevens, R.; et al. The TB REACH Initiative: Supporting TB Elimination Efforts in the Asia-Pacific. Trop. Med. Infect. Dis. 2020, 5, 164. https://doi.org/10.3390/tropicalmed5040164
Creswell J, Khan A, Bakker MI, Brouwer M, Kamineni VV, Mergenthaler C, Smelyanskaya M, Qin ZZ, Ramis O, Stevens R, et al. The TB REACH Initiative: Supporting TB Elimination Efforts in the Asia-Pacific. Tropical Medicine and Infectious Disease. 2020; 5(4):164. https://doi.org/10.3390/tropicalmed5040164
Chicago/Turabian StyleCreswell, Jacob, Amera Khan, Mirjam I Bakker, Miranda Brouwer, Vishnu Vardhan Kamineni, Christina Mergenthaler, Marina Smelyanskaya, Zhi Zhen Qin, Oriol Ramis, Robert Stevens, and et al. 2020. "The TB REACH Initiative: Supporting TB Elimination Efforts in the Asia-Pacific" Tropical Medicine and Infectious Disease 5, no. 4: 164. https://doi.org/10.3390/tropicalmed5040164
APA StyleCreswell, J., Khan, A., Bakker, M. I., Brouwer, M., Kamineni, V. V., Mergenthaler, C., Smelyanskaya, M., Qin, Z. Z., Ramis, O., Stevens, R., Reddy, K. S., & Blok, L. (2020). The TB REACH Initiative: Supporting TB Elimination Efforts in the Asia-Pacific. Tropical Medicine and Infectious Disease, 5(4), 164. https://doi.org/10.3390/tropicalmed5040164






