Optimizing Active Tuberculosis Case Finding: Evaluating the Impact of Community Referral for Chest X-ray Screening and Xpert Testing on Case Notifications in Two Cities in Viet Nam
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
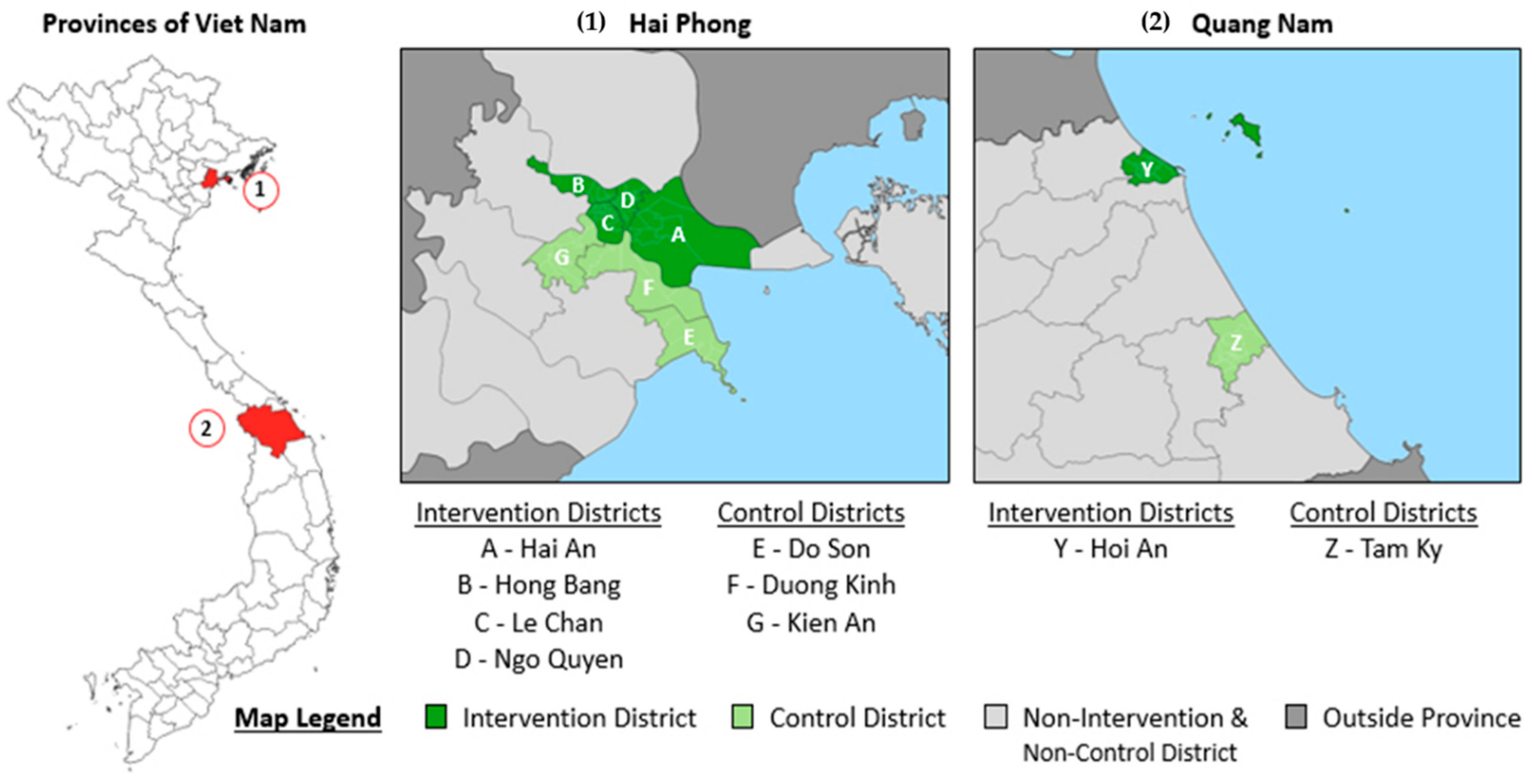
2.2. Study Setting
2.3. Community Health Workers
2.4. Target Populations
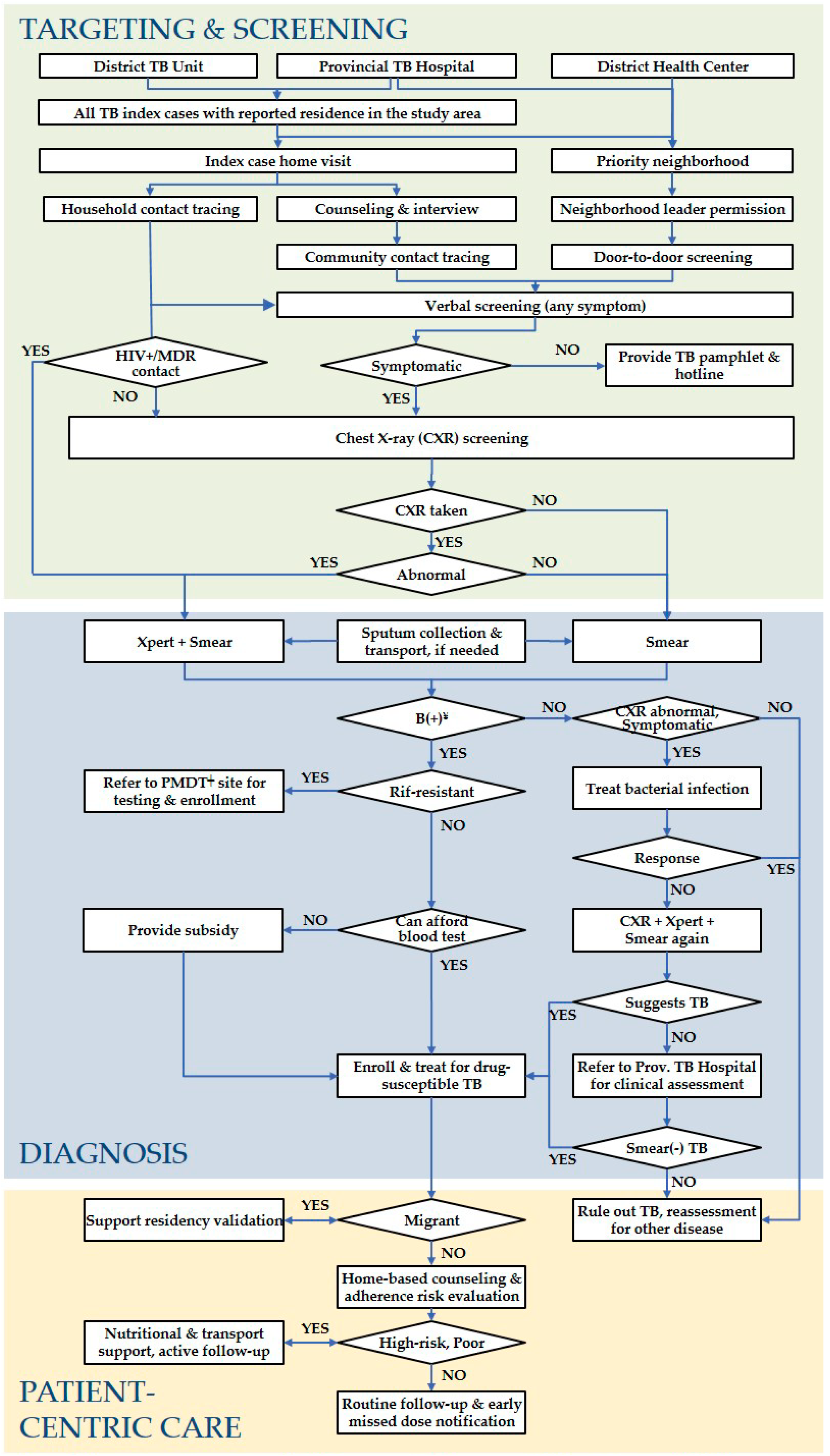
2.5. ACF Activities
2.6. Data Analysis
2.7. Ethical Considerations
3. Results
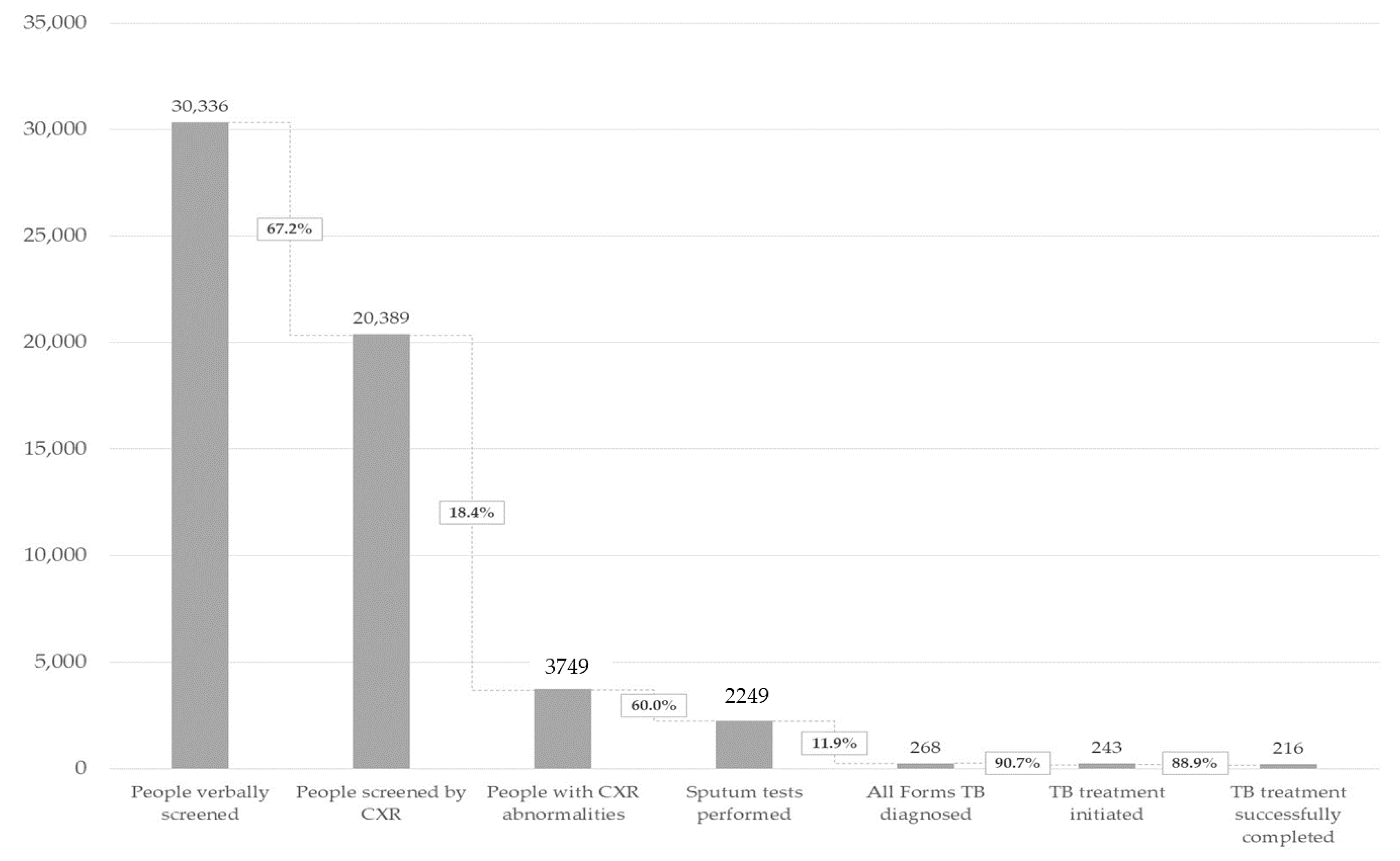
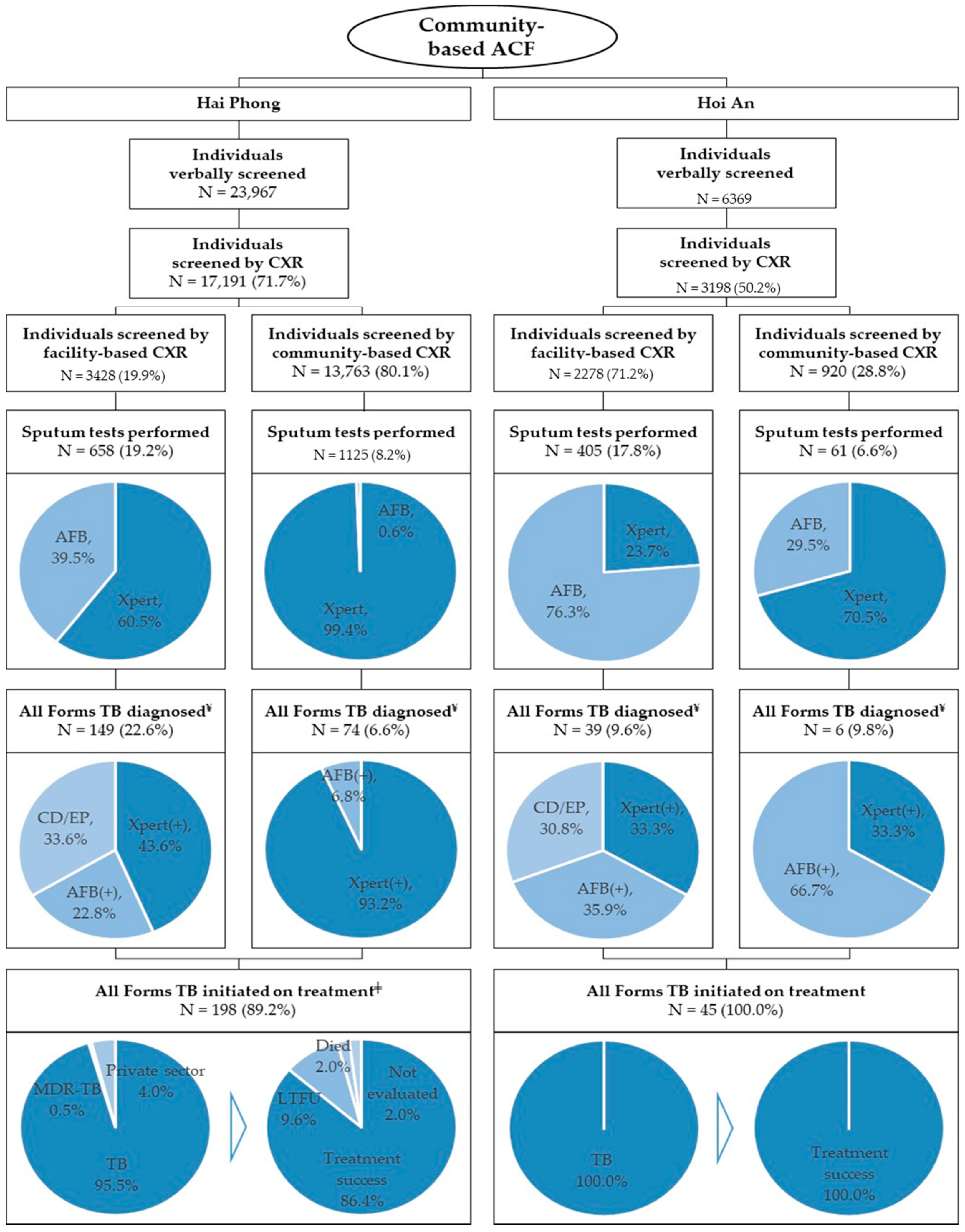
3.1. ACF Outputs
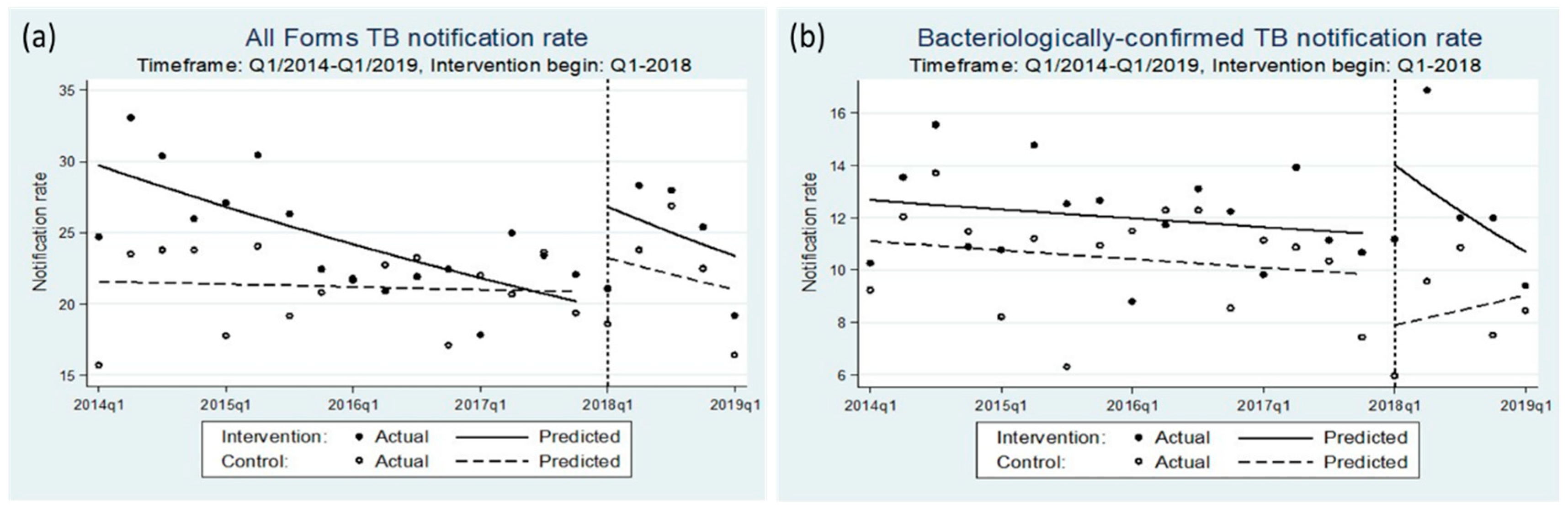
3.2. Impact on Notifications
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Office of the Prime Minister. Approval of the National Strategy for TB Prevention and Control Until 2020 with Vision to 2030 [Vietnamese]; Government of Viet Nam: Ha Noi, Vietnam, 2014.
- World Health Organization. Global Tuberculosis Report 2018; World Health Organization: Geneva, Switzerland, 2018. [Google Scholar]
- World Health Organization. Global Tuberculosis Report 2019; World Health Organization: Geneva, Switzerland, 2019. [Google Scholar]
- Marks, G.B.; Nguyen, N.V.; Nguyen, P.T.B.; Nguyen, T.A.; Nguyen, H.B.; Tran, K.H.; Nguyen, S.V.; Luu, K.B.; Tran, D.T.T.; Vo, Q.T.N.; et al. Community-wide screening for tuberculosis in a high-prevalence setting. N. Engl. J. Med. 2019, 381, 1347–1357. [Google Scholar] [CrossRef] [PubMed]
- Fajans, P.; Simmons, R.; Ghiron, L. Helping public sector health systems innovate: The strategic approach to strengthening reproductive health policies and programs. Am. J. Public Health 2006, 96, 435–440. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Guidelines for Intensified Tuberculosis Case-Finding and Isoniazid Preventive Therapy for People Living with HIV in Resource-Constrained Settings; World Health Organization: Geneva, Switzerland, 2011. [Google Scholar]
- Thanh, T.H.T.; Ngoc, S.D.; Viet, N.N.; Van, H.N.; Horby, P.; Cobelens, F.G.; Wertheim, H.F. A household survey on screening practices of household contacts of smear positive tuberculosis patients in Vietnam. BMC Public Health 2014, 14, 713. [Google Scholar] [CrossRef] [PubMed]
- ExpandNet. Beginning with the End in Mind: Planning Pilot Projects and Other Programmatic Research for Successful Scaling Up; World Health Organization: Geneva, Switzerland, 2011. [Google Scholar]
- Viet Nam National TB Control Programme. NTP Year-End Report 2019 [Vietnamese]; National Lung Hospital: Ha Noi, Vietnam, 2020. [Google Scholar]
- World Health Organization. Systematic Screening for Active Tuberculosis: Principles and Recommendations; World Health Organization: Geneva, Switzerland, 2013. [Google Scholar]
- World Health Organization. Tuberculosis Prevalence Surveys: A Handbook; World Health Organization: Geneva, Switzerland, 2007; ISBN 978 92 4 154816 8. [Google Scholar]
- World Health Organization. Chest Radiography in Tuberculosis Detection—Summary of Current WHO Recommendations and Guidance on Programmatic Approaches; World Health Organization: Geneva, Switzerland, 2016. [Google Scholar]
- World Health Organisation. Chest Radiography in Tuberculosis; WHO Libr. Cat. Data: Geneva, Switzerland, 2016. [Google Scholar]
- Van’t Hoog, A.H.; Cobelens, F.; Vassall, A.; Van Kampen, S.; Dorman, S.E.; Alland, D.; Ellner, J. Optimal triage test characteristics to improve the cost-effectiveness of the Xpert MTB/RIF assay for TB diagnosis: A decision analysis. PLoS ONE 2013, 8, e82786. [Google Scholar] [CrossRef] [PubMed]
- Shazzadur Rahman, A.A.M.; Langley, I.; Galliez, R.; Kritski, A.; Tomeny, E.; Squire, S.B. Modelling the impact of chest X-ray and alternative triage approaches prior to seeking a tuberculosis diagnosis. BMC Infect. Dis. 2019, 19, 93. [Google Scholar] [CrossRef]
- Creswell, J.; Qin, Z.Z.; Gurung, R.; Lamichhane, B.; Yadav, D.K.; Prasai, M.K.; Bista, N.; Adhikari, L.M.; Rai, B.; Sudrungrot, S. The performance and yield of tuberculosis testing algorithms using microscopy, chest x-ray, and Xpert MTB/RIF. J. Clin. Tuberc. Other Mycobact. Dis. 2019, 14, 1–6. [Google Scholar] [CrossRef]
- Keshavjee, S.; Dowdy, D.; Swaminathan, S. Stopping the body count: A comprehensive approach to move towards zero tuberculosis deaths. Lancet 2015, 386, e46–e47. [Google Scholar] [CrossRef]
- Yuen, C.M.; Amanullah, F.; Dharmadhikari, A.; Nardell, E.A.; Seddon, J.A.; Vasilyeva, I.; Zhao, Y.; Keshavjee, S.; Becerra, M.C. Turning off the tap: Stopping tuberculosis transmission through active case-finding and prompt effective treatment. Lancet 2015, 386, 2334–2343. [Google Scholar] [CrossRef]
- Rangaka, M.X.; Cavalcante, S.C.; Marais, B.J.; Thim, S.; Martinson, N.A.; Swaminathan, S.; Chaisson, R.E. Controlling the seedbeds of tuberculosis: Diagnosis and treatment of tuberculosis infection. Lancet 2015, 386, 2344–2353. [Google Scholar] [CrossRef]
- Ortblad, K.F.; Salomon, J.A.; Bärnighausen, T.; Atun, R. Stopping tuberculosis: A biosocial model for sustainable development. Lancet 2015, 386, 2354–2362. [Google Scholar] [CrossRef]
- Nguyen, T.H.; Vo Nguyen Quang, L.; Le, T.G.; Vu, N.T.; Nguyen, H.D. Results of the community—Based intervention for the prevention and control of TB in Go Vap district, Ho Chi Minh city, 2014 [vietnamese]. Viet Nam J. Public Heal. 2015, 38, 6–12. [Google Scholar]
- General Department of Population and Family Planning. End-Year Report 2019; Ministry of Health: Ha Noi, Vietnam, 2019.
- MacPherson, P.; Houben, R.M.; Glynn, J.R.; Corbett, E.L.; Kranzer, K. Pre-treatment loss to follow-up in tuberculosis patients in low- and lower-middle-income countries and high-burden countries: A systematic review and meta-analysis. Bull. World Health Organ. 2014, 92, 126–138. [Google Scholar] [CrossRef]
- Blok, L.; Creswell, J.; Stevens, R.; Brouwer, M.; Ramis, O.; Weil, O.; Klatser, P.; Sahu, S.; Bakker, M.I. A pragmatic approach to measuring monitoring and evaluating interventions for improved tuberculosis case detection. Int. Health 2014, 6, 181–188. [Google Scholar] [CrossRef]
- Bernal, J.L.; Cummins, S.; Gasparrini, A. Interrupted time series regression for the evaluation of public health interventions: A tutorial. Int. J. Epidemiol. 2017, 46, 348–355. [Google Scholar] [CrossRef]
- Mhimbira, F.A.; Cuevas, L.E.; Dacombe, R.; Mkopi, A.; Sinclair, D. Interventions to increase tuberculosis case detection at primary healthcare or community-level services. Cochrane Database Syst. Rev. 2017. [Google Scholar] [CrossRef]
- Shewade, H.D.; Gupta, V.; Ghule, V.H.; Nayak, S.; Satyanarayana, S.; Dayal, R.; Mohanty, S.; Singh, S.; Biswas, M.; Kumar Reddy, K.; et al. Impact of advocacy, communication, social mobilization and active case finding on TB notification in Jharkhand, India. J. Epidemiol. Glob. Health 2019, 9, 233–242. [Google Scholar] [CrossRef]
- Calligaro, G.L.; Zijenah, L.S.; Peter, J.G.; Theron, G.; Buser, V.; Mcnerney, R.; Bara, W.; Bandason, T.; Govender, U.; Tomasicchio, M.; et al. Effect of new tuberculosis diagnostic technologies on community-based intensified case finding: A multicentre randomised controlled trial. Lancet 2017, 3099, 441–450. [Google Scholar] [CrossRef]
- Ho, J.; Nguyen, P.T.B.; Nguyen, T.A.; Tran, K.H.; Van Nguyen, S.; Nguyen, N.V.; Nguyen, H.B.; Luu, K.B.; Fox, G.J.; Marks, G.B. Reassessment of the positive predictive value and specificity of Xpert MTB/RIF: A diagnostic accuracy study in the context of community-wide screening for tuberculosis. Lancet Infect. Dis. 2016, 16, 1045–1051. [Google Scholar] [CrossRef]
- Creswell, J.; Codlin, A.J.; Andre, E.; Micek, M.A.; Bedru, A.; Carter, E.J.; Yadav, R.P.; Mosneaga, A.; Rai, B.; Banu, S.; et al. Results from early programmatic implementation of Xpert MTB/RIF testing in nine countries. BMC Infect. Dis. 2014, 14. [Google Scholar] [CrossRef]
- Theron, G.; Peter, J.; Dowdy, D.; Langley, I.; Squire, S.B.; Dheda, K. Do high rates of empirical treatment undermine the potential effect of new diagnostic tests for tuberculosis in high-burden settings? Lancet Infect. Dis. 2014, 14, 527–532. [Google Scholar] [CrossRef]
- Theron, G.; Zijenah, L.; Chanda, D.; Clowes, P.; Rachow, A.; Lesosky, M.; Bara, W.; Mungofa, S.; Pai, M.; Hoelscher, M.; et al. Feasibility, accuracy, and clinical effect of point-of-care Xpert MTB/RIF testing for tuberculosis in primary-care settings in Africa: A multicentre, randomised, controlled trial. Lancet 2014, 383, 424–435. [Google Scholar] [CrossRef]
- Creswell, J.; Rai, B.; Wali, R.; Sudrungrot, S.; Adhikari, L.M.; Pant, R.; Pyakurel, S.; Uranw, D.; Codlin, A.J. Introducing new tuberculosis diagnostics: The impact of Xpert MTB/RIF testing on case notifications in Nepal. Int. J. Tuberc. Lung Dis. 2015, 19, 545–551. [Google Scholar] [CrossRef]
- Wells, W.A. Onions and prevalence surveys: How to analyze and quantify tuberculosis case-finding gaps. Int. J. Tuberc. Lung Dis. 2017, 21, 1101–1113. [Google Scholar] [CrossRef]
- Mason, P.H.; Roy, A.; Spillane, J.; Singh, P. Social, historical and cultural dimensions of tuberculosis. J. Biosoc. Sci. 2015, 48, 206–232. [Google Scholar] [CrossRef]
- Stop TB Partnership. Stop TB Field Guide 3: Finding Missing People with TB in Communities; Stop TB Partnership: Geneva, Switzerland, 2018. [Google Scholar]
- Long, N.H.; Johansson, E.; Lönnroth, K.; Eriksson, B.; Winkvist, A.; Diwan, V.K. Longer delays in tuberculosis diagnosis among women in Vietnam. Int. J. Tuberc. Lung Dis. 1999, 3, 388–393. [Google Scholar]
- Lönnroth, K.; Jaramillo, E.; Williams, B.G.; Dye, C.; Raviglione, M. Drivers of tuberculosis epidemics: The role of risk factors and social determinants. Soc. Sci. Med. 2009, 68, 2240–2246. [Google Scholar] [CrossRef]
- Datiko, D.G.; Yassin, M.A.; Theobald, S.J.; Blok, L.; Suvanand, S.; Creswell, J.; Cuevas, L.E. Health extension workers improve tuberculosis case finding and treatment outcome in Ethiopia: A large-scale implementation study. BMJ Glob. Heal. 2017, 2, e000390. [Google Scholar] [CrossRef]
- World Health Organization. ENGAGE-TB: Integrating Community-Based Tuberculosis Activities into the Work of Nongovernmental and Other Civil Society Organizations: Training of Community Health Workers and Community Volunteers: Facilitators’ Guide; World Health Organization: Geneva, Switzerland, 2015. [Google Scholar]
- Agarwal, S.; Kirk, K.; Sripad, P.; Bellows, B.; Abuya, T.; Warren, C. Setting the global research agenda for community health systems: Literature and consultative review. Hum. Resour. Health 2019, 17, 22. [Google Scholar] [CrossRef] [PubMed]
- Scott, K.; Beckham, S.W.; Gross, M.; Pariyo, G.; Rao, K.D.; Cometto, G.; Perry, H.B. What do we know about community-based health worker programs? A systematic review of existing reviews on community health workers. Hum. Resour. Health 2018, 16, 39. [Google Scholar] [CrossRef]
- Shapiro, A.; Akande, T.; Lonnroth, K.; Golub, J.; Chakravorty, R. A Systematic Review of the Number Needed to Screen to Detect a Case of Active Tuberculosis in Different Risk Groups; World Health Organization: Geneva, Switzerland, 2013. [Google Scholar]
- Getahun, H.; Raviglione, M. Transforming the global tuberculosis response through effective engagement of civil society organizations: The role of the World Health Organization. Bull. World Health Organ. 2011, 89, 616–618. [Google Scholar] [CrossRef] [PubMed]
- Shelley, K.D.; Frumence, G.; Mpembeni, R.; George, A.S.; Stuart, E.A.; Killewo, J.; Baqui, A.H.; Peters, D.H. Can volunteer community health workers manage multiple roles? An interrupted time-series analysis of combined HIV and maternal and child health promotion in Iringa, Tanzania. Health Policy Plan. 2018, 33, 1096–1106. [Google Scholar] [CrossRef]
- Datiko, D.G.; Lindtjørn, B. Health extension workers improve tuberculosis case detection and treatment success in southern Ethiopia: A community randomized trial. PLoS ONE 2009, 4, e5443. [Google Scholar] [CrossRef]
- Sinha, P.; Shenoi, S.V.; Friedland, G.H. Opportunities for community health workers to contribute to global efforts to end tuberculosis. Glob. Public Health 2019, 474–484. [Google Scholar] [CrossRef]
- Datiko, D.G.; Yassin, M.A.; Tulloch, O.; Asnake, G.; Tesema, T.; Jamal, H.; Markos, P.; Cuevas, L.E.; Theobald, S. Exploring providers’ perspectives of a community based TB approach in Southern Ethiopia: Implication for community based approaches. BMC Health Serv. Res. 2015, 15, 501. [Google Scholar] [CrossRef]
- Nhung, N.V.; Hoa, N.B.; Anh, N.T.; Anh, L.T.N.; Siroka, A.; Lönnroth, K.; Garcia Baena, I. Measuring catastrophic costs due to tuberculosis in Viet Nam. Int. J. Tuberc. Lung Dis. 2018, 22, 983–990. [Google Scholar] [CrossRef] [PubMed]
- Viet Nam National TB Control Programme. Viet Nam Global Fund Funding Request 2018–2020; National Lung Hospital: Ha Noi, Vietnam, 2016. [Google Scholar]
- Morishita, F.; Eang, M.T.; Nishikiori, N.; Yadav, R.P. Increased case notification through active case finding of tuberculosis among household and neighbourhood contacts in Cambodia. PLoS ONE 2016, 11, e150405. [Google Scholar] [CrossRef]
- Morishita, F.; Garfin, A.M.C.G.; Lew, W.; Oh, K.H.; Yadav, R.P.; Reston, J.C.; Infante, L.L.; Acala, M.R.C.; Palanca, D.L.; Kim, H.J.; et al. Bringing state-of-the-art diagnostics to vulnerable populations: The use of a mobile screening unit in active case finding for tuberculosis in Palawan, the Philippines. PLoS ONE 2017, 12, e171310. [Google Scholar] [CrossRef]
- Khan, F.A.; Pande, T.; Tessema, B.; Song, R.; Benedetti, A.; Pai, M.; Lönnroth, K.; Denkinger, C.M. Computer-aided reading of tuberculosis chest radiography: Moving the research agenda forward to inform policy. Eur. Respir. J. 2017, 50. [Google Scholar] [CrossRef]
- Do, T.T.; Kumar, A.M.; Ramaswamy, G.; Htun, T.; Le, V.H.; Vo, L.N.Q.; Dong, T.T.T.; Codlin, A.; Forse, R.; Crewsell, J.; et al. An innovative public—Private mix model for improving tuberculosis care in vietnam: How well are we doing? Trop. Med. Infect. Dis. 2020, 5, 26. [Google Scholar] [CrossRef]
| Total N (%) | Household Contacts N (%) | Social & Close Contacts N (%) | Urban Priority Area Residents N (%) | |
|---|---|---|---|---|
| Individuals verbally screened | 30,336 (100.0%) | 4259 (100.0%) | 1313 (100.0%) | 24,764 (100.0%) |
| Individuals screened by CXR | 20,389 (67.2%) | 2087 (49.0%) | 563 (42.9%) | 17,739 (71.6%) |
| --Individuals with abnormal CXR screen | 3749 (12.4%) | 266 (6.2%) | 101 (7.7%) | 3382 (13.7%) |
| Individuals tested for TB (any sputum test) | 2249 (7.4%) | 184 (4.3%) | 65 (5.0%) | 2000 (8.1%) |
| --Individuals tested for TB with Xpert | 1655 (5.5%) | 120 (2.8%) | 45 (3.4%) | 1490 (6.0%) |
| Individuals diagnosed with All Forms TB | 268 (0.9%) | 44 (1.0%) | 9 (0.7%) | 215 (0.9%) |
| --Individuals diagnosed Xpert(+) | 149 (0.5%) | 14 (0.3%) | 8 (0.6%) | 127 (0.5%) |
| All Forms TB patients started on treatment | 243 (0.8%) | 41 (1.0%) | 8 (0.6%) | 194 (0.8%) |
| --NNS | 125 | 104 | 164 | 128 |
| Total N (%) | Household Contacts N (%) | Social & Close Contacts N (%) | Urban Priority Area Residents N (%) | |
|---|---|---|---|---|
| Treated successfully | 216 (88.9%) | 36 (87.8%) | 8 (100.0%) | 172 (88.7%) |
| Lost to follow-up | 19 (7.8%) | 5 (12.2%) | 0 (0.0%) | 14 (7.2%) |
| Died | 4 (1.6%) | 0 (0.0%) | 0 (0.0%) | 4 (2.1%) |
| Not evaluated/failure | 4 (1.6%) | 0 (0.0%) | 0 (0.0%) | 4 (2.1%) |
| Cumulative Notifications | Trend Differences | |||||
|---|---|---|---|---|---|---|
| Baseline Period † | Intervention Period | # Cases | 95% CI | % Change § | 95% CI | |
| All forms TB | ||||||
| Cumulative additional notifications | 165 | (142,188) | 18.3% | (15.8%, 20.8%) | ||
| Hai Phong | 123 | (102,144) | 11.0% | (9.2%, 12.9%) | ||
| Intervention area | 706 | 850 | 144 | (123,165) | 20.4% | (17.4%, 23.4%) |
| Control area | 224 | 245 | 21 | (12,30) | 9.4% | (5.6%, 13.2%) |
| Hoi An | 42 | (32,52) | 35.4% | (26.8%, 44.1%) | ||
| Intervention area | 112 | 148 | 36 | (26,46) | 32.1% | (23.5%, 40.8%) |
| Control area | 182 | 176 | −6 | (–11,–1) | −3.3% | (−5.9%, −0.7%) |
| Bacteriologically confirmed TB | ||||||
| Cumulative additional notifications | 108 | (91,125) | 32.9% | (27.8%, 37.9%) | ||
| Hai Phong | 76 | (62,90) | 30.6% | (24.9%, 36.3%) | ||
| Intervention area | 354 | 419 | 65 | (51,79) | 18.4% | (14.3%, 22.4%) |
| Control area | 90 | 79 | −11 | (–17,–5) | −12.2% | (−19.0%, −5.5%) |
| Hoi An | 32 | (23,41) | 36.5% | (26.4%, 46.5%) | ||
| Intervention area | 77 | 93 | 16 | (9,23) | 20.8% | (11.7%, 29.8%) |
| Control area | 102 | 86 | −16 | (–23,–9) | −15.7% | (−22.7%, −8.6%) |
| Comparative ITS Analysis Model Parameters | Intervention vs. Control Districts | ||
|---|---|---|---|
| IRR ‡ | 95% CI | p-Value þ | |
| All Forms TB | |||
| Baseline rate (β0) | 21.563 | (20.108, 23.124) | <0.001 |
| Preintervention trend, control (β1) | 0.998 | (0.990, 1.006) | 0.590 |
| Postintervention step change, control (β2) | 1.116 | (0.952, 1.308) | 0.178 |
| Postintervention trend, control (β3) | 0.977 | (0.923, 1.034) | 0.427 |
| Difference in baseline (β4) | 1.378 | (1.270, 1.495) | <0.001 |
| Difference in preintervention trends (β5) | 0.977 | (0.967, 0.986) | <0.001 |
| Difference in postintervention step change (β6) | 1.221 | (1.011, 1.475) | 0.038 |
| Difference in postintervention trends (β7) | 1.015 | (0.948, 1.086) | 0.676 |
| Bacteriologically confirmed TB | |||
| Baseline rate (β0) | 11.107 | (9.562, 12.901) | <0.001 |
| Preintervention trend, control (β1) | 0.992 | (0.975, 1.009) | 0.361 |
| Postintervention step change, control (β2) | 0.807 | (0.587, 1.109) | 0.186 |
| Postintervention trend, control (β3) | 1.043 | (0.935, 1.163) | 0.448 |
| Difference in baseline (β4) | 1.141 | (0.956, 1.362) | 0.144 |
| Difference in preintervention trends (β5) | 1.001 | (0.981, 1.021) | 0.928 |
| Difference in postintervention step change (β6) | 1.535 | (1.067, 2.210) | 0.021 |
| Difference in postintervention trends (β7) | 0.902 | (0.796, 1.023) | 0.108 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mac, T.H.; Phan, T.H.; Nguyen, V.V.; Dong, T.T.T.; Le, H.V.; Nguyen, Q.D.; Nguyen, T.D.; Codlin, A.J.; Mai, T.D.T.; Forse, R.J.; et al. Optimizing Active Tuberculosis Case Finding: Evaluating the Impact of Community Referral for Chest X-ray Screening and Xpert Testing on Case Notifications in Two Cities in Viet Nam. Trop. Med. Infect. Dis. 2020, 5, 181. https://doi.org/10.3390/tropicalmed5040181
Mac TH, Phan TH, Nguyen VV, Dong TTT, Le HV, Nguyen QD, Nguyen TD, Codlin AJ, Mai TDT, Forse RJ, et al. Optimizing Active Tuberculosis Case Finding: Evaluating the Impact of Community Referral for Chest X-ray Screening and Xpert Testing on Case Notifications in Two Cities in Viet Nam. Tropical Medicine and Infectious Disease. 2020; 5(4):181. https://doi.org/10.3390/tropicalmed5040181
Chicago/Turabian StyleMac, Tuan Huy, Thuc Huy Phan, Van Van Nguyen, Thuy Thu Thi Dong, Hoi Van Le, Quan Duc Nguyen, Tho Duc Nguyen, Andrew James Codlin, Thuy Doan To Mai, Rachel Jeanette Forse, and et al. 2020. "Optimizing Active Tuberculosis Case Finding: Evaluating the Impact of Community Referral for Chest X-ray Screening and Xpert Testing on Case Notifications in Two Cities in Viet Nam" Tropical Medicine and Infectious Disease 5, no. 4: 181. https://doi.org/10.3390/tropicalmed5040181
APA StyleMac, T. H., Phan, T. H., Nguyen, V. V., Dong, T. T. T., Le, H. V., Nguyen, Q. D., Nguyen, T. D., Codlin, A. J., Mai, T. D. T., Forse, R. J., Nguyen, L. P., Luu, T. H. T., Nguyen, H. B., Nguyen, N. V., Pham, X. T., Tran, P. N., Khan, A., Vo, L. N. Q., & Creswell, J. (2020). Optimizing Active Tuberculosis Case Finding: Evaluating the Impact of Community Referral for Chest X-ray Screening and Xpert Testing on Case Notifications in Two Cities in Viet Nam. Tropical Medicine and Infectious Disease, 5(4), 181. https://doi.org/10.3390/tropicalmed5040181






