Abstract
Animal testing is mandatory in drug testing and is the gold standard for toxicity and efficacy evaluations. This situation is expected to change in the future as the 3Rs principle, which stands for the replacement, reduction, and refinement of the use of animals in science, is reinforced by many countries. On the other hand, technologies for alternatives to animal testing have increased. The need to develop and use alternatives depends on the complexity of the research topic and also on the extent to which the currently used animal models can mimic human physiology and/or exposure. The lung morphology and physiology of commonly used animal species differs from that of human lungs, and the realistic inhalation exposure of animals is challenging. In vitro and in silico methods can assess important aspects of the in vivo effects, namely particle deposition, dissolution, action at, and permeation through, the respiratory barrier, and pharmacokinetics. This review discusses the limitations of animal models and exposure systems and proposes in vitro and in silico techniques that could, when used together, reduce or even replace animal testing in inhalation testing in the future.
1. Introduction
Animals are used in science worldwide, but the actual numbers are often unknown and difficult to compare between countries because reporting varies considerably (https://speakingofresearch.com/facts/animal-research-statistics/ (accessed on 2 April 2021)). According to the most recent report of the European Commission, more than 60% of the animals used in 2017 were mice, 12% were rats, 13% were fish, and 6% were birds. Dogs, cats, and non-human primates accounted for 0.3% of the total. Animals are mainly used in basic science (45%), translational research (23%), and regulatory testing (23%) (https://www.nature.com/articles/d41586-020-00352-6, assessed on 15 April 2021). Many studies are linked to the development of medical products. The most recent analysis indicated costs ranging from USD 765.9 million for nervous-system-related therapies to USD 2.7 billion for antineoplastic and immunomodulating agents until the products were on the market [1]. A study published a decade ago, reported average costs of 1.5 billion for a marketed drug [2]. Drug development to approval has been estimated to take 10–15 years, with pre-clinical testing taking about 5 years. The most expensive and longest part in the development is the clinical phase of the testing and it is, therefore, important that the development of non-promising drugs is stopped in the preclinical phase. This phase includes target identification and dose finding in cellular screening, the pharmacokinetic profile, the pharmacodynamic profile, the bioavailability, and safety studies (acute and chronic toxicity testing, reproductive toxicity and teratogenicity, mutagenicity and carcinogenicity, immunotoxicity, and local tolerance) of animals.
2. Animal Testing and 3Rs
Opinion on animal testing in science and research has varied in history [3]. Safety tests of drugs were required by the Federal Food, Drug, and Cosmetic Act in 1938 after the tragic incident caused by ‘Elixir Sulfanilamide’. The raspberry flavouring dissolved in diethylene glycol (DEG) was intended to make the drug more attractive to consumers and had not been tested on animals. The product caused the death of more than a hundred people. Another drug fiasco, the “thalidomide scandal”, supported the request for more extensive animal testing in the production of safe drugs [4]. Thalidomide was marketed under the name Contergan in the late 1950s and early 1960s and prescribed as a medication for anxiety, sleep problems, and morning sickness. The poorly water-soluble compound showed poor oral absorption in animal studies and low acute toxicity. When taken by pregnant women between the 20th and 40th day after conception, severe malformations of the newborns were observed. As a reaction to this tragedy, reproductive toxicity testing and teratogenicity testing were made mandatory for systemically acting drugs.
At about the same time, the scientists Russell and Burk introduced in 1959 the concept of the 3Rs, which stand for the replacement, refinement, and reduction in the use of animals in science. The two researchers did not find the use of animals in research problematic but wanted to avoid the infliction of unnecessary or avoidable pain, fear, stress, anxiety, bodily discomfort, and other significantly unpleasant feelings [5]. Marshal Hall promoted this idea many years earlier in his essay “on experiments in physiology as a question of medical ethics”, published in 1847. These principles were not completely clear to all researchers, and over many years, no fundamental change in the use of animals in science was noted. Important milestones for a better implementation of the 3Rs came from the regulatory side by the provision of guidelines for in vitro characterization, mandatory approval of animal studies, the implementation of ethics committees, the European Centre for the Validation of Alternative Methods (ECVAM; Europe), and the Interagency Coordinating Committee on the Validation of Alternative Methods (ICCVAM; USA). From the scientific side, the availability of more representative cell-based and computer models, disease-specific animal models, new imaging modalities, better experimental designs, improved statistical programs, data and tissues sharing, new biomarkers, anesthesia and analgesia management, and better analytical methods supported the 3Rs principle [6]. Cellular studies to evaluate the potency and mechanism of action and in silico models to predict the effects in vivo can replace animal studies. New imaging and analysis methods, disease-specific animal models, and better anesthesia and analgesia management are examples for the refinement of animal studies. Better experimental designs and statistical programs, as well as data and tissue sharing, are strategies to reduce the number of animals in studies.
The use of animals was reduced in the safety testing of cosmetics because the Cosmetics Regulation released by the European Commission in 2009 prohibited the marketing of products and ingredients in the European Union that had been tested on animals. However, a comparison of the use of alternative methods in the dossiers submitted to the Scientific Committee on Consumer Safety (SCCS) between the periods of 2008–2013 and 2013–2016 showed that the increase in in vitro testing was not as pronounced as expected [7]. The authors hypothesized that this was due to the fact that several ingredients were developed prior to the ban. In contrast to the legally required tests for cosmetics, research into various systemic diseases and their treatment will not be possible without animal testing.
Testing of inhaled substances, toxins, or drugs is an area of research where alternative techniques may be more predictive than the data generated from rodents. Acute inhalation toxicity testing in rats was not relevant for humans according to an assessment of 52 studies, indicating that the currently used tests for inhaled toxicants may not be optimal [8]. The high failure rate of 60% in clinical phases between 2011–2012 is indicative of the fact that animal models, particularly in respiratory research, are poorly predictive of the human condition [9]. Furthermore, there are only a few animal models that show the relevant aspects of human respiratory diseases. Another limitation is the fact that animal procedures often involve sedation of the animal. The depression of respiration can lead to hypercapnia, hypoxia, and acidosis and may impact cytokine secretion [10]. Such effects could be misinterpreted as drug-induced effects.
This review highlights the limitations of animal testing with respect to the specific differences in the anatomy, physiology, and pathology of the respiratory systems of laboratory animals commonly used in pulmonary research and discusses the status of in vitro and in silico techniques as alternatives to the efficacy and toxicity testing of drugs for oral inhalation and inhaled toxicants.
3. Respiratory Diseases
Chronic respiratory diseases are relatively common and have been identified as the third leading cause of death worldwide in 2017 [11]. Their incidence increased by 39.8% from 1990 to 2017, with chronic obstructive pulmonary disease (COPD) (3.9% global prevalence) and asthma (3.6%) as the most prevalent diseases. In addition to chronic respiratory diseases, acute lower respiratory infections not only predispose to chronic respiratory disease later in life but are also responsible for millions of deaths each year (https://www.who.int/gard/publications/The_Global_Impact_of_Respiratory_Disease.pdf, assessed on 15 April 2021). The pandemics caused by Severe Acute Respiratory Syndrome Coronary Virus 2 (SARS-CoV-2) have raised the awareness of the dramatic consequences of respiratory infections. COPD, asthma, cystic fibrosis (CF), pulmonary infections, and pulmonary fibrosis, as the most prevalent respiratory diseases, are addressed.
3.1. Cellular Screening
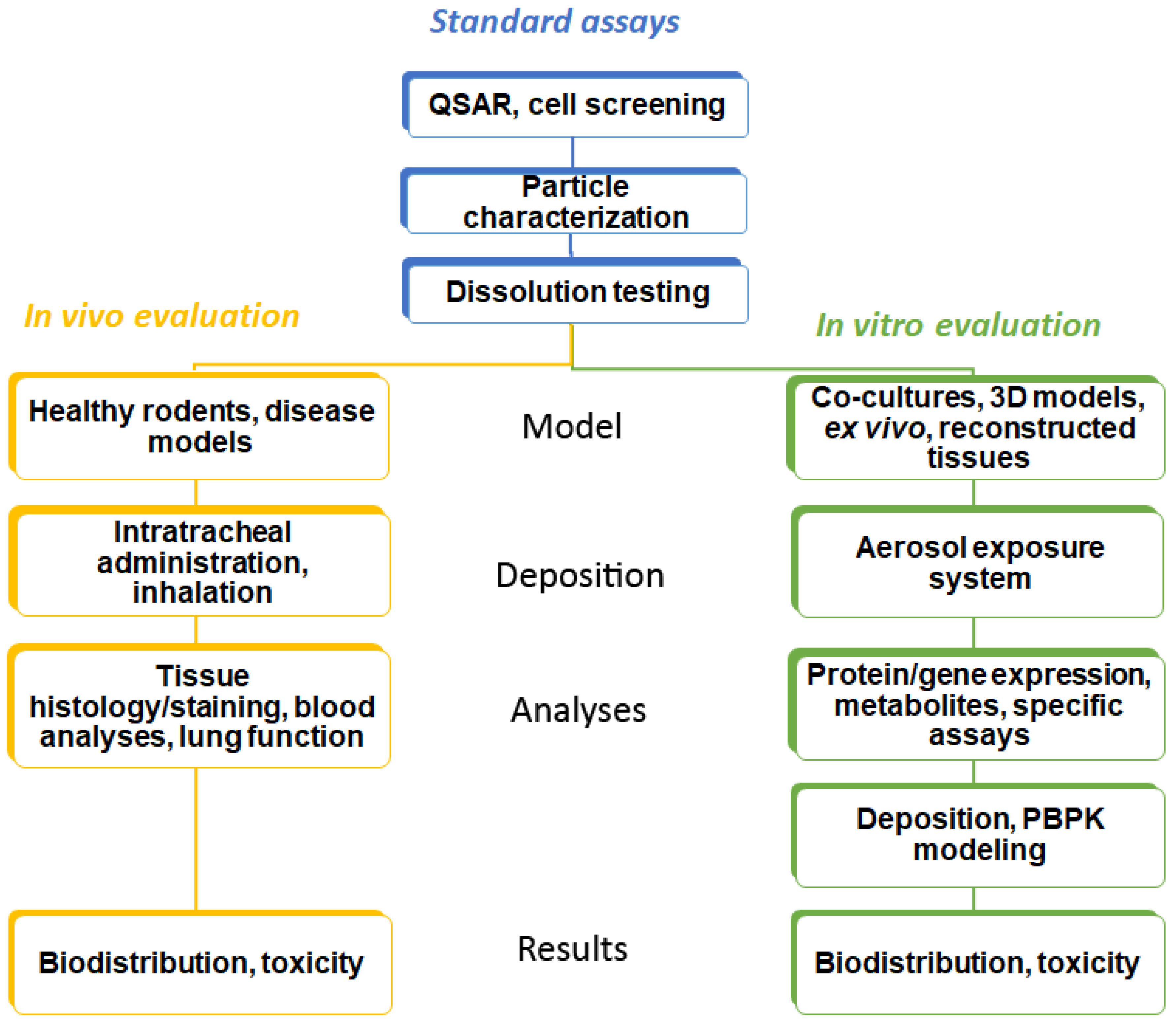
Identification of lead compounds in respiratory research starts with defining a series of attributes (potency, low oral bioavailability, rapid onset of action, low dose, high plasma protein binding, and metabolic vulnerability) in order to design specific drug molecules [12]. In this process, a quantitative structure–activity relationship (QSAR) is often applied. Cell lines and specific (e.g., genetically modified or infected with the pathogen of interest) cells cultured on plastic surfaces and exposed to the compound dissolved in a medium (so-called conventional culture) are used to assess efficacy in target cells [13]. Basal cytotoxicity can be performed in the same way. This strategy is common for all administration routes. Drugs for inhalation have to be provided as aerosols with an appropriate droplet or particle size. For drug particles, fine particle fraction (FPF) and dissolution are determined by in vitro techniques (Figure 1).
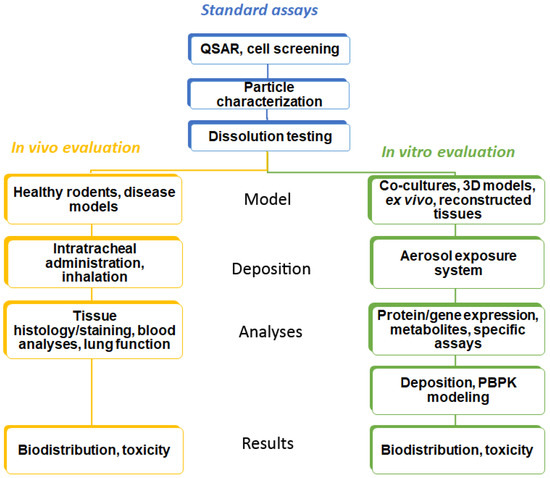
Figure 1.
Workflow in the testing of inhaled substances. Common techniques in the testing are shown in blue, in vivo testing methods in yellow, and alternative testing methods (in silico and cellular models) in green boxes.
3.2. Characterization of Aerosols
The deposition is a highly relevant parameter in the study of inhaled substances as it correlates directly with the efficacy of the treatment. The data are therefore used to show bioequivalence between different inhalers or formulations. Deposition patterns have been studied over the past 50 years, and the particle properties (size, shape, density charge, and hygroscopy of the particles) as well as the airway geometry (gender, age, and disease status) and breathing patterns (frequency, tidal volume, and breath-holding) have been identified as the most relevant parameters [14]. Mechanisms of particle deposition include sedimentation, impaction, diffusion, electrostatic effects, and interception and are described in several reviews (e.g., [15]).
Established techniques for the characterization of aerosols are described in detail by, e.g., [16]. The FPF, representing particles with an aerodynamic size of <5 µm, is regarded as the most important parameter for the deposition of orally inhaled drugs in the deep lung. It is correlated to lung deposition in vivo according to gamma scintigraphy. If different instruments (e.g., the Anderson Cascade Impactor, Marple-Miller Impactor, Next Generation Impactor, Twin-stage Impinger, or Multistage Liquid Impinger) are used, the FPF differs because the instruments work at different conditions and airflows [14].
3.3. Dissolution
Another relevant parameter for action in the lung is the degree of dissolution. Dissolution testing is well standardized for oral formulations according to the relevant guidelines (Guidance for Industry: Dissolution Testing of Immediate Release Solid Oral Dosage Form, August 1997). For metal nanoparticles, dissolution in acidic media is recommended in order to mimic the environment of lysosomes, as they may act toxically to the release of ions after dissolution in lysosomes [17]. For inhaled drugs, there is a variety of different compositions, pH levels, and volumes of the dissolution fluid in the literature. The different protocols are not discussed in this review, and the reader is referred to reviews dedicated to dealing with lung-specific dissolution [18,19].
4. In Vivo Testing in Pulmonary Research
These tests require relevant animal models and aerosol exposure, as well as a broad spectrum of analyses to determine biodistribution, efficacy, and toxicity. In general, no animal model can mimic the entire clinical disease, and only models for some aspects are available.
4.1. Differences between Human Respiratory System and Animals Used in Pulmonary Research
Rats and mice, followed by rabbits, guinea pigs, and hamsters are the most common species used for respiratory research, while larger species such as pigs are rarely used in respiratory research [20]. The use of rodents is favoured by the low cost and the ease of the handling of these animals.
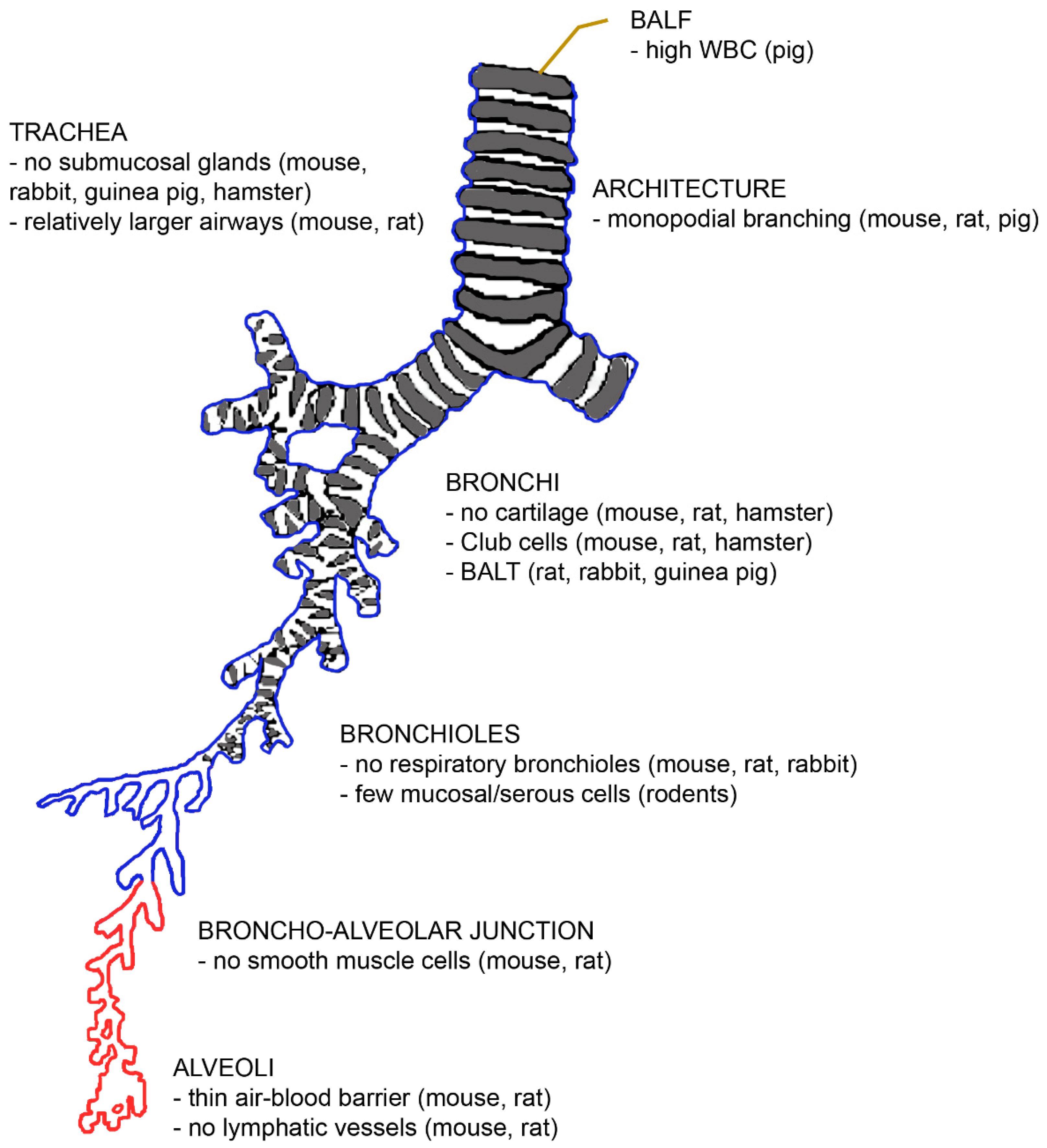
The architecture and function of mammalian lungs are similar in principle. The diameter of the ventilatory unit is constant at 17.5 times the alveolar diameter [21]. Furthermore, the volume fraction of the alveolar parenchyma (alveolar air + alveolar septa + capillaries) varies little between rats, dogs, pigs, and humans (85–90%) [22]. The lung parenchyma contributes to 18% in mice, to 24% in rats, and to 12% in humans of the entire lung volume [23]. However, there are several morphological differences between human and animal lungs, which are summarized in Figure 2.
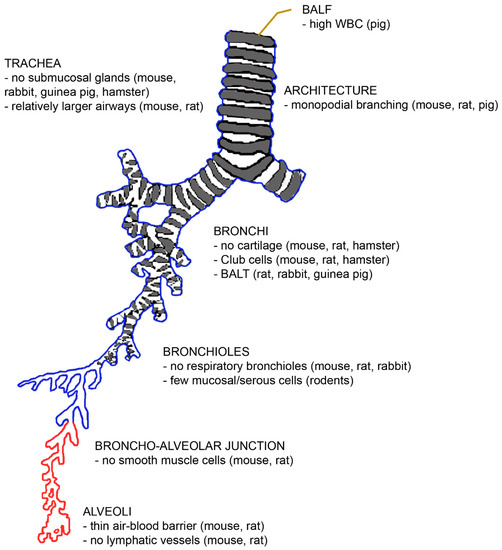
Figure 2.
The architecture of the bronchial tree with an indication of differences with the human situation. Abbreviations: BALF, bronchoalveolar lavage fluid; BALT, bronchus-associated lymphatic tissue; WBC, white blood count.
First, the number of lobes differs between laboratory species. Rodents and hamsters have five lobes like humans, but the distribution between the left and right lungs is different [24]. While rodents have one lobe in the left lung and four in the right lung, humans have two lobes in the left and three in the right lung. Pigs, sheep, and dogs have six lobes (two in the left and four in the right lung) [25,26,27]. Seven lobes (three in the left and four in the right lung) have been reported for rabbits and guinea pigs [28].
The branching pattern of the airways differs in the way that rodents and pigs have monopodial branching, while humans have symmetric dichotomous branching patterns. A monopodial branching pattern means that each larger “parent” bronchus gives rise to smaller side-branches (bronchi) that branch off at obtuse angles. It has been hypothesized that the monopodial branching is linked to the quadruped posture [29], but several quadrupeds (rabbits, guinea pigs, and hamsters) also have irregular dichotomous branching. The symmetrical branching results in the deposition of particles mainly at the branching points.
Due to the small size and the length of the bronchial tree, the diameters of the airways and of the alveoli are much smaller in rodents. However, the relative lumen of the airways is larger in rodents than in humans, resulting in lower flow resistance and providing the physiological basis for the high respiration rate (250–350 bpm) of these animals. Inflammatory processes compromise lung function in rodents less than in the human because the airways are larger and lack mucus glands. However, due to the smaller absolute airway diameter, the administration of larger amounts of particles may cause airway obstruction and induce death after instillation in rodents. Cartilage is present only in the extrapulmonary airways of rodents [30]. The architecture of the junctions between the distal conducting airways and the alveolar parenchyma is also different [31]. While terminal bronchioles and alveoli are connected by respiratory bronchioles in primates and carnivore lungs, rodents, equids, pigs, and ruminants have (the thinner) alveolar ducts between these structures. Respiratory bronchioles are alveolarised airways and their presence or absence appears to play an important role in the action of insoluble particles [32]. The absence of respiratory bronchioles favours the clearance of insoluble particles via the terminal bronchioles [22]. The lower clearance in humans may also explain why the proximal acinus in humans is more sensitive to smoke than the acinus of rodents.
Differences on the cellular level were also identified. The rodent intralobular airways consist of simple epithelium without basal cells, and the non-ciliated cells serve as progenitor cells, while humans have basal cells for regeneration [10]. The air–blood barrier is thinner in mice (0.32 µm) and rats (0.38 µm) than in humans (0.62 µm), which allows a more rapid diffusion of gases and hydrophobic molecules in rodents. The slower gas exchange in human lungs is also due to the fact that 50% of the human air–blood barrier surface is insufficient for diffusion because the barrier is too thick [33]. Collagen type I and occasional fibroblasts hinder the exchange but are, on the other hand, necessary for the mechanical stability of the delicate alveolar walls. Despite the mechanical support, intercellular epithelial junctions may break under physiological stress (exercise close to the maximal oxygen consumption in cyclists or the mechanical ventilation of patients in the intensive care unit or living at a high altitude) and lead to bleeding into the alveoli. Further differences exist with regard to the absence of smooth muscle cells beyond the bronchoalveolar duct junction, the smaller size of alveolar macrophages with higher nitric oxide generation [34], and the presence of secretory Club cells with a high content of metabolizing enzymes in bronchi down to the terminal bronchioles of rodents [35]. Differences in the immune response to injury exist between human and murine eosinophil and neutrophil granulocytes and M1 and M2 macrophages [10]. Mucociliary clearance is faster in rodents, whereas supply with the lymphatic vessels of the pleura is higher in humans [35]. Cytochrome P450 enzymes are poorly active in humans, unlike in rodents, and carboxylesterase is particularly inefficient in humans but not in rodents. Phase III enzymes, on the other hand, are more efficient in humans than in rodents, which means that this particular clearance mechanism cannot be mimicked in animal studies.
Murine and rat lungs differ from human lungs in several ways that are species-specific. There are no anastomoses between the bronchial artery and pulmonary artery in murine lungs [30]. Rats are characterized by a higher lung surface area than the other rodents [20,30], and the ratio of trachea to body mass in rats is different from that of humans [29]. Rats possess bronchus-associated lymphatic tissue (BALT) [20,30,36]. However, they have no lymphatic vessels at the alveolar level [22]. Nevertheless, the rat lung is more prone to particle overload with subsequent inflammation and fibroproliferative alterations. These alterations are more prominent in rat lungs than in hamster and human lungs [37]. Serous cells are present in the respiratory epithelium, but fewer mucosal glands are seen. Rat surfactant is rich not only in monounsaturated but also in polyunsaturated phospholipids [30].
Rabbit lungs are more similar to human lungs because they possess irregular dichotomous branching [20]. However, they differ from human lungs by the presence of BALT and the absence of the cough reflex [36].
The lungs of guinea pigs are smaller than those of all other rodents [20]. There is a very low amount of connective tissue and no lobulization, which makes guinea pig lungs very fragile [38]. The bronchial tree has a dichotomous branching pattern. Due to the strong bronchial muscle layer, the bronchial tract is a good model for human airway hyper-responsiveness. There are numerous goblet cells in the epithelium but no submucosal glands. Similar to rats and rabbits, guinea pigs have constitutive BALT, while humans have inducible BALT [36].
Hamster lungs have cartilage only in the extrapulmonary part of the airways [30]. There is no constitutive BALT, and this species is resistant to pulmonary infections and able to decompose nicotine. Their respiratory bronchioles are short but present, and the branching pattern is dichotomous [24]. The surface decline in the peripheral airways is not seen in most species. Club cells in bronchi and the absence of submucosal glands determine the mucus composition, which is different to that of humans.
The lungs of pigs have high lobularization with no interdependence between lobuli [39]. The ratio between the trachea and the body mass differs from that of humans [40]. Porcine lungs have intravascular macrophages and are characterized by much higher white blood counts (163 ± 73 × 104/mL) in the bronchoalveolar lavage fluid (BALF) than humans with 12 ± 2 × 104/mL) [25,34]. A broad range of white blood cell numbers in BALF has been reported for rodents (2.5–50 × 104/mL) [41,42].
In addition to morphological differences, respiratory physiology differs between the species. It is important to realize that rodents are obligatory nose breathers and humans are oro-nasal breathers, which leads to less particle filtering and higher delivered particle amounts to the human lungs [43]. The burrowing of the nose in the fur may further decrease exposure in the rodents, but the licking of aerosol deposited on the fur may increase exposure by oral uptake. The reflexes induced by the inhalation manoeuvre may mimic toxic action by a decrease in the ventilation rate and/or blood pressure upon stimulation of the parasympathetic system [8]. Mice lack a cough reflex, which might induce toxicity and death by choking.
4.2. Animal Models for Lung Diseases
The identification of the pathways and genes in the pathology of respiratory diseases and the efficacy testing of drugs require representative animal models.
Lung cancer does not only affect the lung but is a systemic disease and, similar to other cancers, the screening of candidates for lung cancer treatment is performed in rodent xenografts (implantation of human tumour cells into immunocompromised animals). Furthermore, inhalation treatment is not the typical way of drug administration. The general limitations of cancer models apply also to lung cancer. The most relevant include the lack of assessment of immunological aspects, the presence of the enhanced permeability and retention (EPR) effect in the xenografts but not in human tumours, and differences in the ratio of tumour size to bodyweight and age of treatment between rodent models and humans. Numerous reviews are available on this topic (e.g., [44,45]).
Animal models of respiratory diseases are generally induced rodent models. This means that animals do not develop the disease naturally but require specific inducers to develop a phenotype with symptoms similar to the human disease. Natural models would be available through genetic variants in larger animals but are rare in rodents [31]. The best known model is the viable motheaten mouse for lung fibrosis, which is characterized by elevated tumor necrosis factor-alpha (TNF-α) levels [46].
4.2.1. Animal Models for CF
Pig and ferret CF are transgenics that mimic quite well the manifestations of human CF. The models were generated by disruption of the cystic fibrosis transmembrane conductance regulator (CFTR) gene or the introduction of the ΔF508 CFTR mutation. Both species develop the typical phenotype in the lung, pancreas, gallbladder, and intestinal tissue. The transgenic mice are less suitable for studying human CF because the animals lack spontaneous lung infections and manifestation in the pancreas. The presence of submucosal glands only in the proximal trachea of mice and the absence of cartilage in the intrapulmonary bronchial airways is regarded as a major reason for the different presentation in the lung [47].
4.2.2. Animal Models for Asthma
The ovalbumin sensitization of mice for asthma is a typical example of an induced disease model. It comprises a sensitization step in the presence of aluminum hydroxide as an adjuvant, and a second step where mice are challenged with the allergen introduced directly into the airways to induce asthma features [48]. The main difference between mouse models and human asthma is the fact that airway inflammation and airway hyperreactivity seem to resolve within a few weeks in the mice, while they persist in humans. House dust mites should in the future replace the ovalbumin because this allergen is more relevant for human asthma. Hyperreactive airways are seen in the naturally occurring non-allergic asthma of horses and the exercise-induced asthma of dogs. Cats, dogs, and sheep also develop allergic asthma [10].
4.2.3. Animal Models for COPD
In contrast to rats, which do not develop COPD with exposure to cigarette smoke, guinea pigs show the pathological changes of human COPD [31]. The model has the disadvantage that guinea pigs are more expensive than rats and that the antibodies needed to study the physiological changes are less available for this species. Murine models show considerable variation in expression of the COPD phenotype and considerable inter-strain differences. Although mice may respond differently to humans because mucus is not produced in the murine bronchial tract of mice [49], the mouse model shows several similarities to human COPD, including the more pronounced manifestation of the disease in females. Spontaneous COPD is observed in dogs and horses.
4.2.4. Animal Models for Lung Infection and Acute Respiratory Stress Syndrome (ARDS, Acute Lung Injury)
Pneumonia is very difficult to study in animal models due to the species-specific pathogenicity of the microbes and the differences in lung microbiota caused by the specific pathogen-free (SPF) conditions of the animals. SPF-housed animals do not have contact with the disease-causing pathogens that can affect mouse health and research outcomes or to the opportunistic and commensal organisms that typically do not cause illness in normal, healthy mice. The lack of contact with the variety of microbes present in the normal environment has a marked influence on biological reactions, especially on the immune systems of these animals [50]. Compared to wild animals, they have a less activated immune system (antibody levels and circulating myeloid cells), which makes them more vulnerable to pathogens. The phenotype of T cells subsets in SPF mice resembles human neonatal blood, while the T cells of mice from the pet store were similar to adult T cell populations [51]. Larger animal models are used for the studying of infections with Mycobacterium tuberculosis (calves), Chlamydia psittaci (calves), Pseudomonas aeruginosa (sheep), and Staphylococcus aureus (sheep, pig). Spontaneous lung inflammations are observed in dogs, cats, and horses.
The instillation of lipopolysaccharide (LPS) into rodents is a rather common model to cause ARDS. The model does not reflect the human pathology because animals either die within 72 h after the LPS administration, or they recover completely. This is in marked contrast to the human condition, where the changes are progressive, and mortality is 20% in the first week and 40% after 4 weeks. In contrast to the small animals, pigs and sheep can spontaneously develop ARDS [10].
4.2.5. Animal Models for Viral Infections
The most important viral infections include respiratory syncytial virus (RSV), influenza, and, especially most recently, coronavirus infections. RSV infections affect young children and are associated with significant mortality. Mice are not susceptible and also other species require high doses of the virus to obtain relatively few clinical signs [52]. The lack of clinical disease in virus-susceptible species, such as cotton rats and ferrets, represents a major limitation for their use as an animal model. Nevertheless, both species are suitable for the screening of antiviral agents [53].
Other viruses, such as influenza A and coronavirus, have similar preferences. The highly pathogenic influenza A viral strains H1N1 2009, H5N1, and H7N7 can naturally infect mice, whereas the other strains need repeated passages. Ferrets, guinea pigs, cotton rats, and hamsters are susceptible to influenza, but the clinical signs are missing in guinea pigs and hamsters [54]. Mice and cotton rats show hypothermia and weight loss as a reaction to influenza infection. Ferrets recapitulate the illness and animals can be infected with a broad range of human, avian, and porcine strains. Ferrets, guinea pigs and hamsters are suitable for vaccine testing [55]. Pigs can naturally be infected and are, therefore, the model of choice.
Rats and normal mice do not mimic the disease, and hamsters were identified as the best model to study the pulmonary changes caused by the SARS-CoV-2 virus [56]. This presents some problems because the availability of analytic tools (antibodies, cytokines, etc.) and the handling are more complicated than for rodents [57]. Ferrets have similar lung physiology to humans, although they have not the five lung lobes like humans but six (two left, four right) [58].
4.2.6. Animal Models for Lung Fibrosis
The phenotype in rodents is most commonly induced by the instillation of bleomycin, although other agents, such as hypoxic chloride, asbestos, silica, vanadium pentoxide, fluorescein isothiocyanate, lung irradiation, and species-specific viruses can also be used. Strain specificity in the reaction to these toxicants, variability in fibrosis, and high mortality represent the major limitations of the model [31]. Naturally occurring progressive lung fibrosis is seen in dogs, horses, cats, and donkeys.
4.3. Animal Exposure to Aerosols
Not only should the anatomy, physiology, and disease phenotype resemble the human situation, but also similar amounts of drug or toxicants should be distributed in the relevant regions of the bronchial tree. In general, animals should receive the aerosol not by local instillation but by inhalation, for the following reasons: (i) this is the physiological route of exposure; (ii) inhalation considers all effects (e.g., defense mechanisms) in the upper respiratory tract; (iii) it leads to a more even distribution in the lungs; (iv) inhalation delivers continuous low deposition; and (v) it does not produce any tissue inflammation per se [59]. The biological effect of inhaled particles is greater than that of instilled ones [60]. This could be due to the more even distribution in the lungs during inhalation and the higher delivery in the peripheral regions of the bronchial tree. The thin alveoli in the lung periphery have a greater capacity for absorption than the thicker and mucus-covered bronchial epithelium of the upper airways. The bypassing of the filtering by the nose through intratracheal administration, on the other hand, results in high concentrations of the materials at the administration site, which may cause a local inflammatory response and a delay of lung clearance by overload. The recommendation to use instillation only for dose-finding and to back it up with nose-only inhalation is published in the OECD guidelines [61]. Of the two most commonly used inhalation methods, the nose-only exposure is considered a better exposure method for rodents than whole-body exposure because exposure occurs only by the pulmonary route, whereas in whole-body exposure, oral exposure may occur because animals may lick the aerosol that deposited on their pelts [62]. The airflow to each port of the nose-only exposure unit must exceed the minute ventilation rate of the animal in order to remove the exhaled air. Otherwise, if the animal re-breathed exhaled air, the oxygen concentration and exposure dose would decrease and the carbon dioxide concentration would increase. Changes in the ventilation rate of the animal either by stress or by sedation will influence the inhaled dose. Whole-body exposure induces less stress because the animals are not restrained and are suitable for chronic inhalation studies. The disadvantage of this exposure is that the dose is difficult to determine. The aerosol may stick to the exposure chambers, the animals may prevent exposure by covering their noses and aerosol will deposit on their pelts.
4.4. Dose Selection for Animal Experiments
Calculation of the drug dose to be used in clinical trials and the determination of the therapeutic window (the difference between effective and toxic dose) require allometric scaling. Allometric scaling is an accepted method because body mass to the surface area, lung mass to body mass, and lung surface to body mass correlate in a linear way for mice, rats, guinea pigs, monkeys, dogs, and humans [63]. Deposition fractions not taking particle size into account are assumed to be 10% for rodents, 25% for canines, 30% for non-human primates, and 40% for humans [64]. As larger animals generally have a slower metabolism than smaller mammals, systemically acting drugs are scaled with a fixed exponent of 0.67 from rodents to humans. In one study, the deposited and effectively deposited doses of orally inhaled salbutamol, budesonide, ipratropium, and mometasone in mice and rats were compared with those in humans, and exponents of 0.44, 0.60, 0.95, and 0.78, respectively [60], were obtained. The average of 0.69 showed that also for the inhaled drugs the effective doses are lower in larger species than in smaller species. For the calculation of toxicity, the effective deposited dose in animals is used as a starting point. Allometric scaling is used to determine the efficient dose in humans, with a multiplication by 2.5 to account for the 40% deposition rate in humans (=projected effective human delivered dose). The safety margin is usually a factor of 10; so, this dose must be multiplied by 10. For the safety testing in animals, allometric scaling has to be applied again, and finally, the deposition fraction has to be considered by multiplying by 10 for rodents and 4 for dogs (rodents have a 10% deposition fraction and dogs 25%).
4.5. Analyses of Animal Experiments
In addition to efficacy, animal experiments provide data on absorption, distribution, metabolism, excretion, and toxicity (ADMET). The effects on organs (histopathology, histological stains, immunohistochemical staining, in-situ hybridization, Western blot, and PCR); changes in the blood (whole blood count, serum chemistry, and cytokine levels); and biodistribution (tissue, plasma, urine analysis, scintigraphy, magnetic resonance imaging, computer tomography, ultrasound, positron emission tomography, and single-photon emission computed tomography), including excretion and changes in body weight and in behaviour can be determined.
5. In Vitro Techniques in Pulmonary Research
In silico modelling by a combination of lung deposition and physiologically based pharmacokinetic (PBPK) models are a good complementation of the cellular studies. Experimental data can be used as input parameters for the calculation of deposition, absorption, metabolism, and excretion.
5.1. In Vitro Models for the Healthy Lung
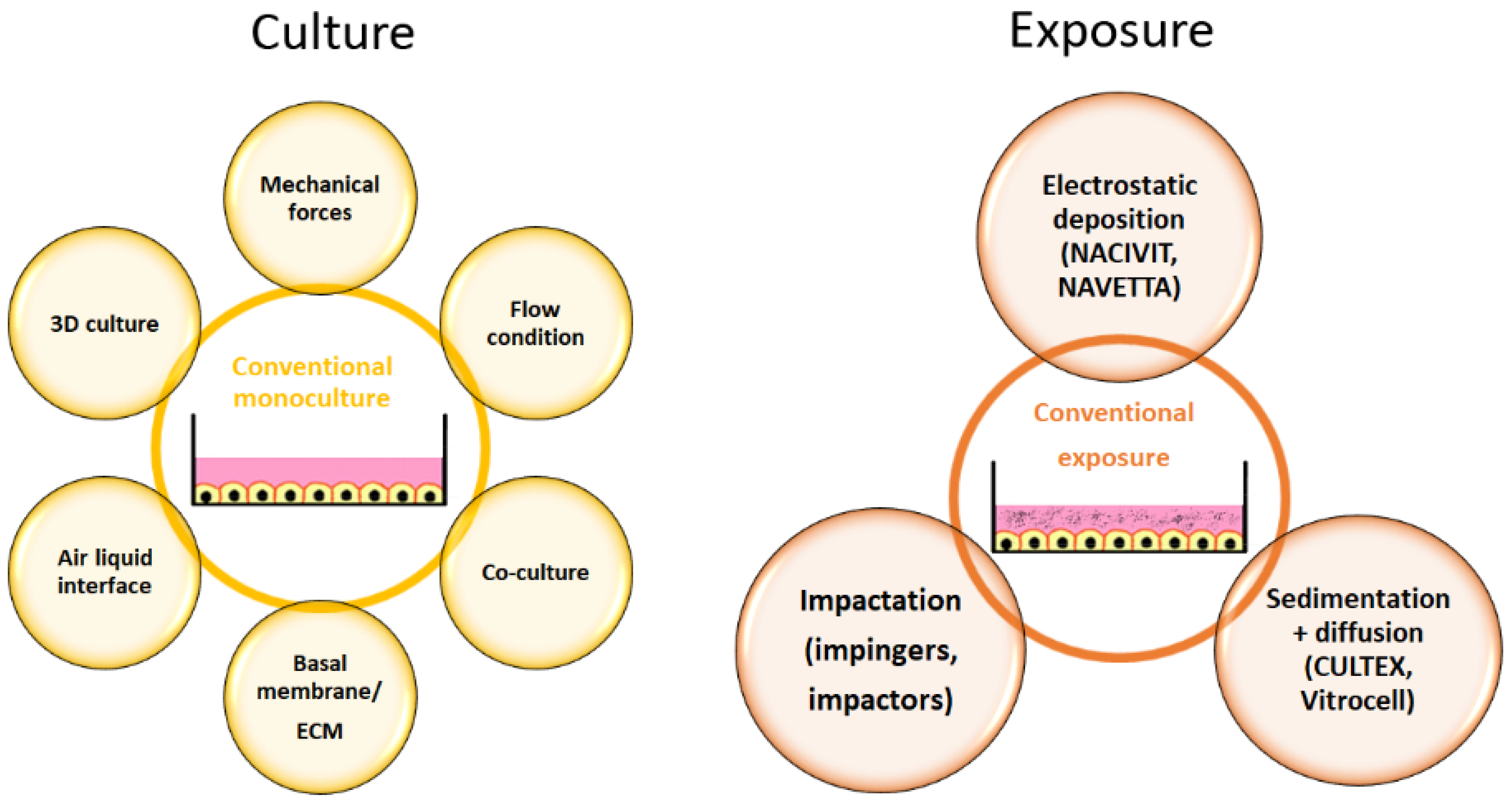
Cellular models for respiratory cells have improved over the years compared to the conventional cultures in the past (Figure 3).
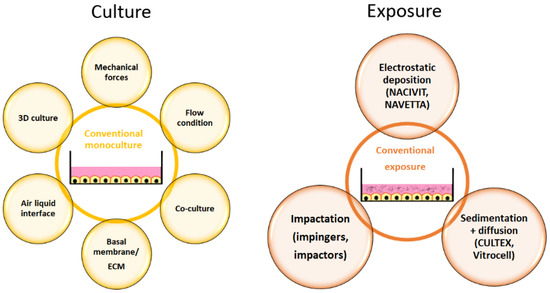
Figure 3.
Adaptations in cell culture (yellow) and exposure (brown) that result in physiologically more relevant in vitro models. Abbreviation: ECM, extracellular matrix.
The supply of nutrients from the apical side is not physiological for most cells of the human body. Rare examples are endocardial endothelial cells, which receive O2 and nutrients by diffusion from the circulating blood at their apical surface. Respiratory cells are special because drug/toxicant exposure and supply with nutrients are at opposite sites of the cells. This situation can be realized in vitro by air-liquid interface (ALI) culture. ALI culture is characterized by cell growth on membranes and a supply of medium only at the basal side. Respiratory cells are one of the few cells that, physiologically, are exposed to high O2 concentrations (104–108 mm Hg), while the cells of the inner organs experience concentrations of 28.9–88 mm Hg [65]. The polar environment increases cell differentiation and induces cilia formation in bronchial epithelial cells, the expression of lung-specific metabolizing enzymes, the cytochrome isoenzymes CYP2A13, CYP2F1, and CYP4B1, correct localization of the tight junction complex and function of the sodium channel, and CFTR [66]. The inclusion of more than one cell type (co-culture) is an important feature of the advanced lung models, although not all of the 40 cell types presented in the lung can be included in the culture [67]. Researchers mostly co-culture in addition to epithelial cells, endothelial cells, and fibroblasts and also the cells of the immune system, such as macrophages and dendritic cells [68]. If the entire tissue complexity is relevant, the use of precision-cut lung slices as an ex vivo model represents the best option. However, these samples are stable only for a short period of time.
The choice of the model should be adapted to the specific research question. For cancer-related questions, the use of 3D culture to mimic the different zones of the tumour is essential; for the assessment of drug absorption or studies of local effects at the pulmonary barrier, the presence of immune cells, perfusion, and mechanical forces may be the most relevant.
Due to the specific requirements, it is not surprising that researchers established or adapted existing models and/or read-out parameters in various ways. The use of primary cells, extracellular matrix (ECM), and induced pluripotent stem cells are options to increase physiological relevance. Airway mechanics can be assessed by cell-stretch devices; cell heterogeneity by precision-cut lung slices; hemodynamics by using perfused lungs, shear stress models, and isolated vessel segments; and barrier function by permeation assays, lung-on-a-chip models, and perfused lungs. It should be taken into account that the inclusion of mechanical forces is only relevant when the respiratory part of the airways (e.g., alveoli) is being examined [49]. Co-cultures create specific problems. Basic ALI airway models, consisting of respiratory cells only, are stable for prolonged times. If, however, immune cells are included, particularly when cell lines are used, the model becomes unstable [69]. This is due to the fact that cell types have different requirements for survival and growth. Co-culture on organ chips for prolonged times also appears problematic because epithelial cells prefer laminin-rich matrices, whereas endothelial cells and mesenchymal cells prefer collagen-rich surfaces [49].
There are numerous homemade models that combine microfluidics, primary cells, and 3D culture, for lung cancer for instance [70,71], but this review will focus only on the commercial systems because they are more suitable for standardized testing. Ready-to-use reconstructed tissues are available from MatTek Corporation (EpiAirway™ and EpiAirway-FT™) and Epithelix Sarl (MucilAir™, SmallAir™). The generation of homemade systems can be standardized by using PneumaCult™ Expansion and Differentiation medium kits from STEMCELL Technologies [66]. There is also much activity in the design of commercially available lung-on-a-chip products [72]. The well-known companies are Emulate Inc. and Alveolix AG, but also other companies, such as NORTIS, Quorum Technologies (Artery-on-a-chip Vessel), MIMETAS, SYnVIVO, 4DESIGN BIOSCIENCES, and AIM BIOTECH, develop chip solutions for human tissues. The ideal alveolar model would be a chip based on a mechanically active membrane and consisting of human cells cultured in ALI on ECM on one side and endothelial cells on the other. The structural and mechanical functions of the ECM are pivotal for normal cell function and differentiation [73]. The ECM regulates the passage of molecules, acts as a local reservoir of growth factors and bioactive molecules, and plays a key role in the development of respiratory diseases. However, the commonly used polydimethylsiloxane (PDMS) membranes cannot act as a reservoir but adsorb specific small molecules, which prevents the assessment of permeation. Furthermore, the fabrication of ultrathin and porous membranes to allow mechanical action and the passage of compounds is challenging [74]. All these issues probably contribute to the fact that no ready-to-use product is currently commercially available. Recently, Zamprogno et al. developed a material that may be suitable for the commercialization of such devices [75].
5.2. Cellular Models for Lung Diseases
Commonly used lung cancer models consist of lung carcinoma cells co-cultured with fibroblasts and immune cells. They are prepared by cell aggregation, with or without scaffold, in static or dynamic conditions [76]. Different culture conditions (hypoxia, microfluidics, ALI, and scaffolds) can be used. If the organoids are embedded in ECM, they form a lumen, and drug candidates can be microinjected into the construct [77]. Organoids, either generated from induced pluripotent stem cells or from primary cells isolated from patients, are an important tool for drug screening. OncoCilAir™ tissues from OncoTheis, which are commercially available constructs on membranes, consist of bronchial epithelial cells, fibroblasts, and non-small cell lung cancer cells (NSCLC) and provide a greater level of standardization.
There are no standardized protocols, ready-to-use models, or accepted alternatives to in vivo tests for obstructive lung diseases, inflammation, and pulmonary fibrosis. Examples for published setups to assess disease-relevant parameters are listed below. Mucus flow, as an indication for mucociliary clearance, can be determined using the transport of fluorescent spheres in CF patient samples [78] or in reconstructed tissues (e.g., MucilAir™). CFTR activity can be determined in CF patient-derived nasospheroids in addition to mucus secretion and fluid secretion [79]. Alterations in asthma can be mimicked by the wounding of primary bronchial epithelial cells from asthma patients compared to healthy controls [80]. Cytokines, particularly transforming growth factor-beta (TGF-β) 1 level, are measured as marker for tissue remodeling in asthma. A chip mimicking bronchial constriction has been developed for better mimicking of the in vivo situation. COPD can be studied by the re-popularization of cadaveric scaffolds with normal or COPD patient-derived cells [81]. The process of EMT can be followed by exposure of Matrigel-embedded healthy bronchial tissue in ALI to cigarette smoke [82].
The use of in vitro models could represent advantages over animal testing for infections because the species-specificity could be excluded [49]. For these studies, the co-cultures of bacteria and lung models and the co-culture of ex vivo lung tissue with Pseudomonas aeruginosa biofilms to assess the impact of infection and the efficacy of antimicrobial agents could be used [83,84].
Pulmonary fibrosis was studied in the co-cultures of A549 alveolar epithelial cells, MRC-5 fibroblasts, and THP-1 macrophages treated with bleomycin in a microfluidic system [85] and in pulmospheres generated from pulmonary fibrosis patients [86]. The pulmospheres can, for instance, be used as a screening system for anti-fibrotic drugs. Fibroblasts cultured on an extracellular matrix and damaged by mechanical or oxidative stress serve as a model for lung fibrosis and ageing [87]. Gel contraction induced by TGF-β stimulated A549 cells mimics the process of epithelial-mesenchymal transition(EMT) [88].
5.3. Cell Exposure to Aerosols
Exposure of cells to aerosols is not straightforward, as damage to cells by the airflow must be prevented. In contrast to the large variety of homemade models, commercially available exposure systems allow better standardization [89]. The available instruments mimic the mechanisms that also occur in the body, namely primary deposition by impaction, sedimentation, and Brownian diffusion and secondary deposition in the turbulent flow of the upper airways by interception and electrostatic precipitation [15]. Impingers and impactors used in particle characterization separate particles based on impaction but cannot be used for cell exposures because they cause cell damage by airflow. This deposition is typical for the large (conducting) airways. Devices based on electrostatic impaction include the NanoAerosol Chamber for in Vitro Toxicity (NACIVIT), the Electrostatic Particulate Dosage and Exposure System (EPDExS), and the Novel ALI Exposure System (NAVETTA). These devices allow faster and higher (up to 100%) deposition efficacy compared to the 2% in CULTEX systems [90,91]. However, they are not suitable for all particles as a charge is required. Sedimentation and diffusion, as seen in the alveolar region of the lung, are used in CULTEX CG and RFS, VITROCELL, Pharmaceutical Aerosol Deposition Device on Cell Cultures (PADDOCC), and Precise Inhale Xpose® ALI. A detailed description and illustration of the available exposure systems is provided by Karra et al. [15]. The most commonly used systems are commercialized by Cultex® Technology GmbH and by VITROCELL Systems GmbH. CULTEX® RFS, CULTEX® RFS compact, and CULTEX® LongTermCultivation-continuous use radial flow instead of the vertical flow used in the Vitrocell® systems. The Vitrocell® aerosol exposure system consists of the vibrating mesh nebulizer Aeroneb® Pro, exposure chamber, cultivation module, quartz microbalance, and heating [66]. The Vitrocell® Spiking System applies vapour mixed with air. Vitrocell® VC1 and VC10 and Borgwaldt® RM20S are smoking robots.
5.4. Readout Parameters of In Vitro Studies
Protein expression, cytokine release, gene expression, viability, metabolism, proliferation, migration, apoptosis, mitochondrial membrane potential, DNA damage, intracellular calcium changes, and the generation of reactive oxygen species are routinely used as read-out parameters for in vitro/ex vivo experiments and inform about changes in cell physiology and intercellular interaction. In membrane-cultured cells and reconstructed tissues, measurements of transepithelial electrical resistance and permeation can be analysed [92]. Reconstructed tissues/ex vivo samples are used to assess cilia beating frequency, mucus production, airway surface liquid volume, and mucociliary clearance [93]. The effects on alveolar macrophages can be assessed by measuring chemotaxis, phagocytosis, and phospholipidosis [94]. Additional analyses may be required to study the effects linked to a specific respiratory disease. In spheroids, vascularization and cellular crosstalk are particularly relevant [95]. TGF-β pre-treated cells can be evaluated for cell contraction, EMT, airway remodelling, and elastin levels [88]. For the study of tissue remodelling, specific read-out parameters, e.g., proliferation, differentiation, transdifferentiation, matrix deposition, and juxta/para/endocrine signalling, should be determined, and for immunity and inflammation adhesion, migration, juxta/para/endocrine signalling, and phagocytosis should be determined [10].
6. Lung Deposition Models and PBPK Models
In silico models can support studies with analysis of deposition and ADME. Deposition models do not consider the absolute deposited dose but the fraction of particles of a given size, shape, and density that is deposited at a given region of the respiratory tract. Hofmann [96] classified the models into five groups and regarded lung morphology as the most important factor in the calculations. This classification differentiates between (1) semi-empiric models, (2) continuous or trumpet models, and (3–5) truly mechanistic models. (Semi)-empiric models combine first principle mechanistic models with experimental data [97]. In the following sections, only the programs that are publicly available are mentioned.
The International Commission of Radioactive Protection (ICRP) has published a number of models, of which ICRP66 is probably the best known [98]. It is an empirical regional compartment model and corresponds to a series of filters. The model indicates deposition resulting from inhalation and exhalation. The particle parameters, size, density, and hygroscopicity, can be adjusted. For the biological parameters, the gender, ethnicity, physical activity, nose/mouth breathing, and body weight can be adjusted. The most recent ICRP130 model also includes clearance from the alveoli to the blood [99]. The National Council on Radiation Protection and Measurement (NCRP) published a model very similar to the ICRP66 model in which a different, and presumably more correct, description of nanoparticle deposition is implemented. The NCRP may better predict nanoparticle deposition pattern but clearance mechanisms may be better reflected by the ICRP130 model [100]. Another similar model to the IRCP66 model is the RADEP (Radon Dose Evaluation Program). The freely available Lung Dose Evaluation Program (LUDEP) is based on the ICRP130 regional compartment model. It allows modification of particle properties and of physiological parameters, such as tidal volume, FRC, breathing pattern, and symmetric or asymmetric lung structure, but not airway diameter and alveolar volume. Mucus transport of 20 mm/min in the trachea to 2 mm/min in the small airways is assumed. Translocation from the alveoli in other tissues is estimated as 0.1% of the deposited dose, and a calculation of bone marrow, bone, lung, liver, and gonad dose is possible [101].
Deterministic simple path models use symmetric branching and suffer from the lack of geometrical data in alveolar ducts and sacks. Typical paths for all five lobes have been implemented, which improved the performance of these models [102]. The deterministic multiple paths models use typical paths and asymmetric branching patterns. The deposition is calculated for every single airway, and some alveoli receive deposited particles from different paths. This may explain the preferential localization of lung cancer in specific parts of the lungs. In deterministic models, simplified assumptions about airway geometry and airflow conditions are used to derive analytical solutions of air and particle motion. The model tracks the path of a population of particles within a bronchial tree. These programs are freely available and have user-friendly software. The most commonly used, the Multiple Path Particle Dosimetry (MPPD) and the Hygroscopic Particle Lung Deposition Model B (HPLDB) provided by Helmholtzzentrum München (German Research Center for Environmental Health), are deterministic models. Morphologies of the mouse, rat, rhesus monkey, sheep, pig, and human lungs are available.
Mechanistic models determine particle transport and deposition by computational fluid and particle dynamics (CFPD). From an elementary viewpoint, CFPD can be seen as an extension of the well-known and established Computational Fluid Dynamics (CFD) knowledge, with additional modelling requirements to reflect the particle dynamics within the fluid flow [103]. Stochastic multiple path models allow randomization of tube lengths, angles, and diameters and perform runs for single particles. These routes differ for individual particles between the simulations but after the simulations of hundreds of particles, an average pattern is obtained. In stochastic models, the morphology of the lung is considered to vary within certain limits in a random manner. Deposition fractions are derived from classical flow equations in the respiratory tract model, followed by the particle behaviour in that flow. The anatomical regions are seen as compartments with the connecting flow, concentration, and time properties and the airways are tubes that branch into finer airways, and the way of splitting affects the model characteristics. There are basically two ways of particle tracking, Lagrangian and Eulerian. In the former, single particles are tracked, which is often compared to a person travelling on a racket. In the Eulerian model, an ensemble or a concentration of particles is tracked, and this is described as a person standing on the ground and watching a group of rackets flying by. In these models, the outputs are solved mathematically and are not based on assumptions from empirical models and fitting parameters. They are able to predict deposition at a localized level [104]. Some CFPD models include bronchoconstriction (airway diameter), emphysema (alveolar volume), elastic recoil, breathing conditions, lung clearance, and mucus clearance. Despite the advances in this field due to increasing computing processes and imaging capabilities, most CFPD-based lung models address deposition in the upper airway regions only because of the limited availability of high-quality in vivo data of the lower respiratory tract. Furthermore, CFD simulations are computer-intensive and need skilled users [105]. There are whole lung and site-specific models [101].
Trends in the deposition as a function of particle diameter and breathing conditions were similar in the comparison of five stochastic models, but variation arose depending on the choice of central bifurcation geometry (branching angle and bifurcation shape), flow profiles, and methods used in the derivation of the equations. Deposition fraction by diffusion and impaction were much more affected than sedimentation [106]. Multiple-path models are more realistic than semi-empirical models, deterministic single-path models, and trumpet models because they are based on actual airway measurements rather than on average values. The current lack of a complete, deterministic, asymmetric description of the lower airways presents the main limitation. In a more recent publication, one dimensional cross-section (trumpet), deterministic symmetric generation (single-path), deterministic asymmetric generation (multi-path), stochastic asymmetric generation (multi-path) models, and single-path CFPD were compared [107]. The same trends for particle diameters and breathing regarding regional bronchial and alveolar deposition, general lung deposition, lobar deposition, generational lobar deposition, and generational surface deposition were identified. The author concluded that current deposition models correctly predict regional and generational deposition. The deposition fraction calculated by semi-empirical (IRCP66), trumpet, single-path, multiple-path, and stochastic models varied only by 10% and showed a typical U-shape curve for all the models. Regional differences in the deposition in the alveoli showed a variation of 15% [108]. All models suffer from the lack of complete lung structure measurements, and alveolar structures are extrapolated. The prominent inter-individual variability of 30–50% confirmed in experimental studies is realized in the models by scaling the linear airway dimensions according to body weight and height. Breathing patterns and ventilation rate are anticipated to be major sources of error. From the particle side, not only size but also hygroscopicity has a prominent effect. Hygroscopicity is accounted for in the MPPD model [97].
The majority of models (IRCP, LUDEP, RADEP) were designed for environmental exposure. They underestimate the oropharyngeal deposition of pharmaceutical aerosols due to the lower filtering in mouth breathing compared to nose breathing. The underestimation of oropharyngeal deposition by environmental deposition software programs can be improved by the use of mouth–throat replicas in combination with CFPD simulations. In general, MPPD appears to be well suited for toxicity studies and NCRP better suited for determining nanoparticle deposition.
Data for ADMET are usually obtained from animal studies, but in vitro data can also be used as input. The process of absorption can be mimicked by permeation across cell monolayers because apparent permeability (Papp) values obtained in Calu-3 monolayers correspond well to the permeability of the lung [109]. Other physiological parameters are available in data banks for the known compounds or molecules. With further improvements in the organ-on-a-chip technology, it appears possible that data on metabolism and excretion can also be obtained from these systems rather than relying on the data obtained from animals.
Mimetikos™ Preludium (Emmace Consulting) contains calculations for regional distribution, dissolution, barrier permeation, and mucociliary clearance. The program allows the adjusting of default settings based on the data obtained from other deposition programs or from experimental data (e.g., precision-cut lung slices) [110]. The only commercially available program that combines mechanistic models for deposition, absorptive, and non-absorptive clearance in an anatomical representation of the lung and PBPK modelling is the GastroPlus™ Nasal-Pulmonary Compartmental Absorption and Transit Models from SimulationsPlus Inc. [111]. The model consists of three lung compartments and one extrathoracic compartment, each of them with an epithelial/tissue and airway liquid compartment. Deposition is calculated by the ICRP66 model and dissolution by the Noyes–Whitney principle. The particles deposited in the extrathoracic compartment are cleared to the gastrointestinal tract and oral absorption is assumed. Mucociliary clearance, lung metabolism, and mucus binding are integrated into the software. PBPK description is missing for the other models, the Simcyp™ Simulator and PK-Sim. PulmoSim™, developed from SimCyp® by Pfizer, divides the dose into two fractions, one to the lung, the other to the gastrointestinal tract. Mucociliary clearance, dissolution, absorption, tissue binding, systemic distribution, and clearances are included, but no data are available for pulmonary and systemic exposures. The SimCyp® Simulator (Certara) is based on a first-order non-mechanistic inhalation model, where deposition is not calculated. The alveolar dose is treated like an intravenous dose and the dose to the conducting airways is like a delayed oral dose. PK-Sim (Bayer AG) is used to generate systemic profiles.
The various alternative models (Table 1) can be combined to improve the outcome.

Table 1.
Overview of advantages and disadvantages of different models (in vivo, ex vivo, in vitro, in silico). Abbreviations: HPLDB, Hygroscopic Particle Lung Deposition model B; ICRP, international conference on radiation protection; MPPD, multiple path particle dosimetry; PCLS, precision-cut lung slices.
7. Conclusions
The screening of inhaled compounds for the treatment of lung diseases, safety testing, and the assessment of environmental toxicants suffer from the lack of suitable animal models and the limitations of inhalation exposure in animals. On the one hand, there are good in silico models for aerosol characterization and deposition, as well as cellular/tissue models that could be used to predict effects in humans. Due to differences in the anatomy and physiology of the upper and lower airways, models representing both regions are needed to fully assess the effects on the lung. A major obstacle to the widespread acceptance of these systems is the fact that they must be constructed using rodent cells in order to validate them with animal models [66]. Alternatively, human data could be used to validate the results of experiments with human cells. Human data of environmental pollutants and particles are available. Assessment of pharmacological aerosols has the methodological limitation that commonly measured plasma levels cannot describe drug levels in lung tissue. This is because the blood containing the compound after inhalation passes through the heart and upper extremity before being collected. The compound can be extracted from the blood and metabolized during the passage.
Pulmonary exposure presents more differences between animals and humans regarding lung morphology and physiology and administration than, for instance, parenteral administration. Therefore, the combined use of in vitro techniques for the characterization of the aerosols, studies using advanced cell and tissue models, and prediction by in silico modelling may have a good chance to replace animal studies in the screening for inhaled drugs for the local treatment of pulmonary diseases. For pulmonary diseases causing systemic effects, which are not treated by oral inhalation (viral infections, lung cancer, etc.), it is more likely that animal studies will still be needed. Nevertheless, the information obtained by the advanced cellular models and in silico techniques will help to reduce the extent of animal testing.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Conflicts of Interest
The author declares no conflict of interest.
References
- Wouters, O.J.; McKee, M.; Luyten, J. Research and Development Costs of New Drugs-Reply. JAMA 2020, 324, 518. [Google Scholar] [CrossRef] [PubMed]
- Tamimi, N.A.; Ellis, P. Drug development: From concept to marketing! Nephron Clin. Pract. 2009, 113, c125–c131. [Google Scholar] [CrossRef] [PubMed]
- Hajar, R. Animal testing and medicine. Heart Views 2011, 12, 42. [Google Scholar] [CrossRef] [PubMed]
- Bottin, J. The History of Thalidomide. In Animals and Medicine, The Contribution of Animal Experiments to the Control of Disease, III. Drugs for Organic Diseases; Bottin, J., Ed.; Open Book Publishers: Cambridge, UK, 2015; pp. 183–198. [Google Scholar]
- Tannenbaum, J.; Bennett, B.T. Response to Dr. Carbone’s Letter to the Editor. J. Am. Assoc. Lab. Anim. Sci. 2015, 54, 351–352. [Google Scholar]
- Hubrecht, R.C.; Carter, E. The 3Rs and Humane Experimental Technique: Implementing Change. Animals 2019, 9, 754. [Google Scholar] [CrossRef] [PubMed]
- Vinardell, M.P.; Mitjans, M. Lignins and Their Derivatives with Beneficial Effects on Human Health. Int. J. Mol. Sci. 2017, 18, 1219. [Google Scholar] [CrossRef] [PubMed]
- Movia, D.; Bruni-Favier, S.; Prina-Mello, A. In Vitro Alternatives to Acute Inhalation Toxicity Studies in Animal Models—A Perspective. Front. Bioeng. Biotechnol. 2020, 8, 549. [Google Scholar] [CrossRef] [PubMed]
- Barnes, P.J.; Bonini, S.; Seeger, W.; Belvisi, M.G.; Ward, B.; Holmes, A. Barriers to new drug development in respiratory disease. Eur. Respir. J. 2015, 45, 1197–1207. [Google Scholar] [CrossRef] [PubMed]
- Bonniaud, P.; Fabre, A.; Frossard, N.; Guignabert, C.; Inman, M.; Kuebler, W.M.; Maes, T.; Shi, W.; Stampfli, M.; Uhlig, S.; et al. Optimising experimental research in respiratory diseases: An ERS statement. Eur. Respir. J. 2018, 51, 1702133. [Google Scholar] [CrossRef] [PubMed]
- GBD Chronic Respiratory Disease Collaborators. Prevalence and attributable health burden of chronic respiratory diseases, 1990–2017: A systematic analysis for the Global Burden of Disease Study 2017. Lancet Respir. Med. 2020, 8, 585–596. [Google Scholar] [CrossRef]
- Strong, P.; Ito, K.; Murray, J.; Rapeport, G. Current approaches to the discovery of novel inhaled medicines. Drug Discov. Today 2018, 23, 1705–1717. [Google Scholar] [CrossRef]
- Merkert, S.; Schubert, M.; Olmer, R.; Engels, L.; Radetzki, S.; Veltman, M.; Scholte, B.J.; Zöllner, J.; Pedemonte, N.; Galietta, L.J.V.; et al. High-Throughput Screening for Modulators of CFTR Activity Based on Genetically Engineered Cystic Fibrosis Disease-Specific iPSCs. Stem Cell Rep. 2019, 12, 1389–1403. [Google Scholar] [CrossRef]
- Cheng, Y.S. Mechanisms of pharmaceutical aerosol deposition in the respiratory tract. AAPS PharmSciTech 2014, 15, 630–640. [Google Scholar] [CrossRef] [PubMed]
- Karra, N.; Swindle, E.; Morgan, H. Drug delivery for traditional and emerging airway models. Organs-on-a-Chip 2019, 1, 100002. [Google Scholar] [CrossRef]
- Hickey, A. Complexity in Pharmaceutical Powders for Inhalation: A perspective. KONA Powder Part. J. 2018, 35, 3–13. [Google Scholar] [CrossRef]
- Sabella, S.; Carney, R.P.; Brunetti, V.; Malvindi, M.A.; Al-Juffali, N.; Vecchio, G.; Janes, S.M.; Bakr, O.M.; Cingolani, R.; Stellacci, F.; et al. A general mechanism for intracellular toxicity of metal-containing nanoparticles. Nanoscale 2014, 6, 7052–7061. [Google Scholar] [CrossRef] [PubMed]
- Radivojev, S.; Zellnitz, S.; Paudel, A.; Fröhlich, E. Searching for physiologically relevant in vitro dissolution techniques for orally inhaled drugs. Int. J. Pharm. 2019, 556, 45–56. [Google Scholar] [CrossRef] [PubMed]
- Innes, E.; Yiu, H.H.P.; McLean, P.; Brown, W.; Boyles, M. Simulated biological fluids—A systematic review of their biological relevance and use in relation to inhalation toxicology of particles and fibres. Crit. Rev. Toxicol. 2021, 51, 217–248. [Google Scholar] [CrossRef] [PubMed]
- Tanner, L.; Single, A.B. Animal Models Reflecting Chronic Obstructive Pulmonary Disease and Related Respiratory Disorders: Translating Pre-Clinical Data into Clinical Relevance. J. Innate Immun. 2020, 12, 203–225. [Google Scholar] [CrossRef] [PubMed]
- Miller, F.; Mercer, R.; Crapo, D. Lower Respiratory Tract Structure of Laboratory Animals and Humans: Dosimetry Implications. Aerosol Sci. Technol. 1993, 18, 257–271. [Google Scholar] [CrossRef]
- Nikula, K.J.; Avila, K.J.; Griffith, W.C.; Mauderly, J.L. Sites of particle retention and lung tissue responses to chronically inhaled diesel exhaust and coal dust in rats and cynomolgus monkeys. Environ. Health Perspect. 1997, 105 (Suppl. S5), 1231–1234. [Google Scholar] [CrossRef] [PubMed]
- Irvin, C.G.; Bates, J.H. Measuring the lung function in the mouse: The challenge of size. Respir. Res. 2003, 4, 1. [Google Scholar] [CrossRef]
- Kennedy, A.R.; Desrosiers, A.; Terzaghi, M.; Little, J.B. Morphometric and histological analysis of the lungs of Syrian golden hamsters. J. Anat. 1978, 125, 527–553. [Google Scholar]
- Judge, E.P.; Hughes, J.M.; Egan, J.J.; Maguire, M.; Molloy, E.L.; O’Dea, S. Anatomy and bronchoscopy of the porcine lung. A model for translational respiratory medicine. Am. J. Respir. Cell Mol. Biol. 2014, 51, 334–343. [Google Scholar] [CrossRef] [PubMed]
- Meeusen, E.N.; Snibson, K.J.; Hirst, S.J.; Bischof, R.J. Sheep as a model species for the study and treatment of human asthma and other respiratory diseases. Drug Discov. Today Dis. Models 2009, 6, 101–106. [Google Scholar] [CrossRef]
- Nakakuki, S. The bronchial tree and lobular division of the dog lung. J. Vet. Med. Sci. 1994, 56, 455–458. [Google Scholar] [CrossRef] [PubMed]
- Stan, F. Comparative anatomical study of lungs in domestic rabbits (Oryctolagus cuniculus) and guinea pigs (Cavia porcellus). Bull. Univ. Agric. Sci. Vet. Med. 2015, 72, 195–196. [Google Scholar] [CrossRef][Green Version]
- Monteiro, A.; Smith, R. Bronchial Tree Architecture in Mammals of Diverse Body Mass. Int. J. Morphol. 2014, 32, 312–316. [Google Scholar] [CrossRef]
- Kling, M.A. A review of respiratory system anatomy, physiology, and disease in the mouse, rat, hamster, and gerbil. Vet. Clin. N. Am. Exot. Anim. Pract. 2011, 14, 287–337. [Google Scholar] [CrossRef] [PubMed]
- Williams, K.; Roman, J. Studying human respiratory disease in animals—Role of induced and naturally occurring models. J. Pathol. 2016, 238, 220–232. [Google Scholar] [CrossRef]
- Kamaruzaman, N.A.; Kardia, E.; Kamaldin, N.; Latahir, A.Z.; Yahaya, B.H. The rabbit as a model for studying lung disease and stem cell therapy. BioMed Res. Int. 2013, 2013, 691830. [Google Scholar] [CrossRef] [PubMed]
- West, J.B. Comparative physiology of the pulmonary blood-gas barrier: The unique avian solution. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2009, 297, R1625–R1634. [Google Scholar] [CrossRef] [PubMed]
- Matute-Bello, G.; Frevert, C.W.; Martin, T.R. Animal models of acute lung injury. Am. J. Physiol. Lung Cell Mol. Physiol. 2008, 295, L379–L399. [Google Scholar] [CrossRef] [PubMed]
- Fröhlich, E. Toxicity of orally inhaled drug formulations at the alveolar barrier: Parameters for initial biological screening. Drug Deliv. 2017, 24, 891–905. [Google Scholar] [CrossRef] [PubMed]
- Randall, T.D. Bronchus-associated lymphoid tissue (BALT) structure and function. Adv. Immunol. 2010, 107, 187–241. [Google Scholar] [CrossRef] [PubMed]
- Warheit, D.B.; Kreiling, R.; Levy, L.S. Relevance of the rat lung tumor response to particle overload for human risk assessment-Update and interpretation of new data since ILSI 2000. Toxicology 2016, 374, 42–59. [Google Scholar] [CrossRef] [PubMed]
- Brewer, N.R.; Cruise, L.J. The Respiratory System of the Guinea Pig: Emphasis on Species Differences. Contemp. Top. Lab. Anim. Sci. 1997, 36, 100–108. [Google Scholar]
- Kirschvink, N.; Reinhold, P. Use of alternative animals as asthma models. Curr. Drug Targets 2008, 9, 470–484. [Google Scholar] [CrossRef] [PubMed]
- Deng, Z.J.; Butcher, N.J.; Mortimer, G.M.; Jia, Z.; Monteiro, M.J.; Martin, D.J.; Minchin, R.F. Interaction of human arylamine N-acetyltransferase 1 with different nanomaterials. Drug Metab. Dispos. 2014, 42, 377–383. [Google Scholar] [CrossRef]
- Busch, C.J.; Favret, J.; Geirsdóttir, L.; Molawi, K.; Sieweke, M.H. Isolation and Long-term Cultivation of Mouse Alveolar Macrophages. Bio-Protocol 2019, 9, e3302. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Kusaka, Y.; Sato, K.; Wang, D.; Donaldson, K. Tumor necrosis factor-alpha release from rat pulmonary leukocytes exposed to ultrafine cobalt:in vivo andin vitro studies. Environ. Health Prev. Med. 1999, 4, 87–91. [Google Scholar] [CrossRef][Green Version]
- Fröhlich, E.; Salar-Behzadi, S. Toxicological assessment of inhaled nanoparticles: Role of in vivo, ex vivo, in vitro, and in silico studies. Int. J. Mol. Sci. 2014, 15, 4795–4822. [Google Scholar] [CrossRef] [PubMed]
- Cekanova, M.; Rathore, K. Animal models and therapeutic molecular targets of cancer: Utility and limitations. Drug Des. Dev. Ther. 2014, 8, 1911–1921. [Google Scholar] [CrossRef] [PubMed]
- Kellar, A.; Egan, C.; Morris, D. Preclinical Murine Models for Lung Cancer: Clinical Trial Applications. BioMed Res. Int. 2015, 2015, 621324. [Google Scholar] [CrossRef]
- Rossi, G.A.; Hunninghake, G.W.; Kawanami, O.; Ferrans, V.J.; Hansen, C.T.; Crystal, R.G. Motheaten mice--an animal model with an inherited form of interstitial lung disease. Am. Rev. Respir. Dis. 1985, 131, 150–158. [Google Scholar] [CrossRef]
- Yan, Z.; Stewart, Z.A.; Sinn, P.L.; Olsen, J.C.; Hu, J.; McCray, P.B., Jr.; Engelhardt, J.F. Ferret and pig models of cystic fibrosis: Prospects and promise for gene therapy. Hum. Gene Ther. Clin. Dev. 2015, 26, 38–49. [Google Scholar] [CrossRef]
- Daubeuf, F.; Frossard, N. Acute Asthma Models to Ovalbumin in the Mouse. Curr. Protoc. Mouse Biol. 2013, 3, 31–37. [Google Scholar] [CrossRef]
- Hynes, J.; Marshall, L.; Adcock, I.; Novotny, T.; Nic, M.; Dibusz, K.; Gribaldo, L.; Whelan, M. Advanced Non-Animal Models in Biomedical Research; Publications Office of the European Union: Luxembourg, 2020. [Google Scholar] [CrossRef]
- Dobson, G.P.; Letson, H.L.; Biros, E.; Morris, J. Specific pathogen-free (SPF) animal status as a variable in biomedical research: Have we come full circle? EBioMedicine 2019, 41, 42–43. [Google Scholar] [CrossRef] [PubMed]
- Huggins, M.A.; Jameson, S.C.; Hamilton, S.E. Embracing microbial exposure in mouse research. J. Leukoc. Biol. 2019, 105, 73–79. [Google Scholar] [CrossRef] [PubMed]
- Taylor, G. Animal models of respiratory syncytial virus infection. Vaccine 2017, 35, 469–480. [Google Scholar] [CrossRef] [PubMed]
- Altamirano-Lagos, M.J.; Díaz, F.E.; Mansilla, M.A.; Rivera-Pérez, D.; Soto, D.; McGill, J.L.; Vasquez, A.E.; Kalergis, A.M. Current Animal Models for Understanding the Pathology Caused by the Respiratory Syncytial Virus. Front. Microbiol. 2019, 10, 873. [Google Scholar] [CrossRef] [PubMed]
- Bouvier, N.M.; Lowen, A.C. Animal Models for Influenza Virus Pathogenesis and Transmission. Viruses 2010, 2, 1530–1563. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, T.Q.; Rollon, R.; Choi, Y.K. Animal Models for Influenza Research: Strengths and Weaknesses. Viruses 2021, 13, 1011. [Google Scholar] [CrossRef] [PubMed]
- Muñoz-Fontela, C.; Dowling, W.E.; Funnell, S.G.P.; Gsell, P.S.; Riveros-Balta, A.X.; Albrecht, R.A.; Andersen, H.; Baric, R.S.; Carroll, M.W.; Cavaleri, M.; et al. Animal models for COVID-19. Nature 2020, 586, 509–515. [Google Scholar] [CrossRef] [PubMed]
- Imai, M.; Iwatsuki-Horimoto, K.; Hatta, M.; Loeber, S.; Halfmann, P.J.; Nakajima, N.; Watanabe, T.; Ujie, M.; Takahashi, K.; Ito, M.; et al. Syrian hamsters as a small animal model for SARS-CoV-2 infection and countermeasure development. Proc. Natl. Acad. Sci. USA 2020, 117, 16587–16595. [Google Scholar] [CrossRef] [PubMed]
- Rosa, R.B.; Dantas, W.M.; do Nascimento, J.C.F.; da Silva, M.V.; de Oliveira, R.N.; Pena, L.J. In Vitro and In Vivo Models for Studying SARS-CoV-2, the Etiological Agent Responsible for COVID-19 Pandemic. Viruses 2021, 13, 379. [Google Scholar] [CrossRef] [PubMed]
- ECETOC. TR 122–Poorly Soluble Particles/Lung Overload; European Centre for Ecotoxicology and Toxicology of Chemicals: Brussels, Belgium, 2014. [Google Scholar]
- Phillips, J.E. Inhaled efficacious dose translation from rodent to human: A retrospective analysis of clinical standards for respiratory diseases. Pharmacol. Ther. 2017, 178, 141–147. [Google Scholar] [CrossRef]
- Joint Meeting of the Chemicals Committee and the Working Party on Chemicals, Pesticides and Biotechnology. Guidance Document on Inhalation Toxicity Studies; Environment Directorate Organisation for Economic Co-Operation and Development: Paris, France, 2018; Volume 39. [Google Scholar]
- Wong, B.A. Inhalation exposure systems: Design, methods and operation. Toxicol. Pathol. 2007, 35, 3–14. [Google Scholar] [CrossRef] [PubMed]
- Chappell, W.; Mordenti, J. Extrapolation of Toxicological and Pharmacological Data from Animals to Humans. In Advances in Drug Research; Testa, B., Ed.; Academic Press Limited: London, UK, 1991; Volume 20, pp. 1–116. [Google Scholar]
- Snipes, M.B.; McClellan, R.O.; Mauderly, J.L.; Wolff, R.K. Retention patterns for inhaled particles in the lung: Comparisons between laboratory animals and humans for chronic exposures. Health Phys. 1989, 57 (Suppl. S1), 69–77, discussion 77–68. [Google Scholar] [CrossRef]
- Ortiz-Prado, E.; Dunn, J.F.; Vasconez, J.; Castillo, D.; Viscor, G. Partial pressure of oxygen in the human body: A general review. Am. J. Blood Res. 2019, 9, 1–14. [Google Scholar]
- Cao, X.; Coyle, J.P.; Xiong, R.; Wang, Y.; Heflich, R.H.; Ren, B.; Gwinn, W.M.; Hayden, P.; Rojanasakul, L. Invited review: Human air-liquid-interface organotypic airway tissue models derived from primary tracheobronchial epithelial cells-overview and perspectives. In Vitro Cell. Dev. Biol. Anim. 2021, 57, 104–132. [Google Scholar] [CrossRef] [PubMed]
- Franks, T.J.; Colby, T.V.; Travis, W.D.; Tuder, R.M.; Reynolds, H.Y.; Brody, A.R.; Cardoso, W.V.; Crystal, R.G.; Drake, C.J.; Engelhardt, J.; et al. Resident cellular components of the human lung: Current knowledge and goals for research on cell phenotyping and function. Proc. Am. Thorac. Soc. 2008, 5, 763–766. [Google Scholar] [CrossRef]
- Fröhlich, E. Comparison of conventional and advanced in vitro models in the toxicity testing of nanoparticles. Artif. Cells Nanomed. Biotechnol. 2018, 46, 1091–1107. [Google Scholar] [CrossRef] [PubMed]
- Meindl, C.; Öhlinger, K.; Zrim, V.; Steinkogler, T.; Fröhlich, E. Screening for Effects of Inhaled Nanoparticles in Cell Culture Models for Prolonged Exposure. Nanomaterials 2021, 11, 606. [Google Scholar] [CrossRef] [PubMed]
- Artzy-Schnirman, A.; Hobi, N.; Schneider-Daum, N.; Guenat, O.T.; Lehr, C.M.; Sznitman, J. Advanced in vitro lung-on-chip platforms for inhalation assays: From prospect to pipeline. Eur. J. Pharm. Biopharm. 2019, 144, 11–17. [Google Scholar] [CrossRef]
- Ziółkowska-Suchanek, I. Mimicking Tumor Hypoxia in Non-Small Cell Lung Cancer Employing Three-Dimensional In Vitro Models. Cells 2021, 10, 141. [Google Scholar] [CrossRef] [PubMed]
- Allwardt, V.; Ainscough, A.J.; Viswanathan, P.; Sherrod, S.D.; McLean, J.A.; Haddrick, M.; Pensabene, V. Translational Roadmap for the Organs-on-a-Chip Industry toward Broad Adoption. Bioengineering 2020, 7, 112. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Horowitz, J.C.; Naba, A.; Ambalavanan, N.; Atabai, K.; Balestrini, J.; Bitterman, P.B.; Corley, R.A.; Ding, B.S.; Engler, A.J.; et al. Extracellular matrix in lung development, homeostasis and disease. Matrix Biol. 2018, 73, 77–104. [Google Scholar] [CrossRef] [PubMed]
- Huh, D.; Kim, H.J.; Fraser, J.P.; Shea, D.E.; Khan, M.; Bahinski, A.; Hamilton, G.A.; Ingber, D.E. Microfabrication of human organs-on-chips. Nat. Protoc. 2013, 8, 2135–2157. [Google Scholar] [CrossRef] [PubMed]
- Zamprogno, P.; Wüthrich, S.; Achenbach, S.; Thoma, G.; Stucki, J.D.; Hobi, N.; Schneider-Daum, N.; Lehr, C.M.; Huwer, H.; Geiser, T.; et al. Second-generation lung-on-a-chip with an array of stretchable alveoli made with a biological membrane. Commun. Biol. 2021, 4, 168. [Google Scholar] [CrossRef]
- Fröhlich, E. Issues with Cancer Spheroid Models in Therapeutic Drug Screening. Curr. Pharm. Des. 2020, 26, 2137–2148. [Google Scholar] [CrossRef]
- Cidem, A.; Bradbury, P.; Traini, D.; Ong, H.X. Modifying and Integrating in vitro and ex vivo Respiratory Models for Inhalation Drug Screening. Front. Bioeng. Biotechnol. 2020, 8, 581995. [Google Scholar] [CrossRef] [PubMed]
- Sears, P.R.; Davis, C.W.; Chua, M.; Sheehan, J.K. Mucociliary interactions and mucus dynamics in ciliated human bronchial epithelial cell cultures. Am. J. Physiol. Lung Cell Mol. Physiol. 2011, 301, L181–L186. [Google Scholar] [CrossRef][Green Version]
- Pranke, I.M.; Hatton, A.; Simonin, J.; Jais, J.P.; Le Pimpec-Barthes, F.; Carsin, A.; Bonnette, P.; Fayon, M.; Stremler-Le Bel, N.; Grenet, D.; et al. Correction of CFTR function in nasal epithelial cells from cystic fibrosis patients predicts improvement of respiratory function by CFTR modulators. Sci. Rep. 2017, 7, 7375. [Google Scholar] [CrossRef]
- Freishtat, R.J.; Watson, A.M.; Benton, A.S.; Iqbal, S.F.; Pillai, D.K.; Rose, M.C.; Hoffman, E.P. Asthmatic airway epithelium is intrinsically inflammatory and mitotically dyssynchronous. Am. J. Respir. Cell Mol. Biol. 2011, 44, 863–869. [Google Scholar] [CrossRef]
- Wagner, D.E.; Bonenfant, N.R.; Parsons, C.S.; Sokocevic, D.; Brooks, E.M.; Borg, Z.D.; Lathrop, M.J.; Wallis, J.D.; Daly, A.B.; Lam, Y.W.; et al. Comparative decellularization and recellularization of normal versus emphysematous human lungs. Biomaterials 2014, 35, 3281–3297. [Google Scholar] [CrossRef] [PubMed]
- Bucchieri, F.; Pitruzzella, A.; Fucarino, A.; Gammazza, A.M.; Bavisotto, C.C.; Marcianò, V.; Cajozzo, M.; Lo Iacono, G.; Marchese, R.; Zummo, G.; et al. Functional characterization of a novel 3D model of the epithelial-mesenchymal trophic unit. Exp. Lung Res. 2017, 43, 82–92. [Google Scholar] [CrossRef]
- Montefusco-Pereira, C.V.; Horstmann, J.C.; Ebensen, T.; Beisswenger, C.; Bals, R.; Guzmán, C.A.; Schneider-Daum, N.; Carvalho-Wodarz, C.S.; Lehr, C.M. P. aeruginosa Infected 3D Co-Culture of Bronchial Epithelial Cells and Macrophages at Air-Liquid Interface for Preclinical Evaluation of Anti-Infectives. J. Vis. Exp. 2020, 160, 61069. [Google Scholar] [CrossRef] [PubMed]
- Harrington, N.E.; Sweeney, E.; Alav, I.; Allen, F.; Moat, J.; Harrison, F. Antibiotic Efficacy Testing in an Ex vivo Model of Pseudomonas aeruginosa and Staphylococcus aureus Biofilms in the Cystic Fibrosis Lung. J. Vis. Exp. 2021, 167, 62187. [Google Scholar] [CrossRef] [PubMed]
- He, J.; Chen, W.; Deng, S.; Xie, L.; Feng, J.; Geng, J.; Jiang, D.; Dai, H.; Wang, C. Modeling alveolar injury using microfluidic co-cultures for monitoring bleomycin-induced epithelial/fibroblastic cross-talk disorder. RSC Adv. 2017, 7, 42738–42749. [Google Scholar] [CrossRef]
- Surolia, R.; Li, F.J.; Wang, Z.; Li, H.; Liu, G.; Zhou, Y.; Luckhardt, T.; Bae, S.; Liu, R.M.; Rangarajan, S.; et al. 3D pulmospheres serve as a personalized and predictive multicellular model for assessment of antifibrotic drugs. JCI Insight 2017, 2, e91377. [Google Scholar] [CrossRef] [PubMed]
- Vicens-Zygmunt, V.; Estany, S.; Colom, A.; Montes-Worboys, A.; Machahua, C.; Sanabria, A.J.; Llatjos, R.; Escobar, I.; Manresa, F.; Dorca, J.; et al. Fibroblast viability and phenotypic changes within glycated stiffened three-dimensional collagen matrices. Respir. Res. 2015, 16, 82. [Google Scholar] [CrossRef]
- Mikami, Y.; Matsuzaki, H.; Takeshima, H.; Makita, K.; Yamauchi, Y.; Nagase, T. Development of an In Vitro Assay to Evaluate Contractile Function of Mesenchymal Cells that Underwent Epithelial-Mesenchymal Transition. J. Vis. Exp. 2016, 112, 53974. [Google Scholar] [CrossRef]
- Nossa, R.; Costa, J.; Cacopardo, L.; Ahluwalia, A. Breathing in vitro: Designs and applications of engineered lung models. J. Tissue Eng. 2021, 12, 1–28. [Google Scholar] [CrossRef]
- Stevens, J.P.; Zahardis, J.; MacPherson, M.; Mossman, B.T.; Petrucci, G.A. A new method for quantifiable and controlled dosage of particulate matter for in vitro studies: The electrostatic particulate dosage and exposure system (EPDExS). Toxicol. In Vitro 2008, 22, 1768–1774. [Google Scholar] [CrossRef]
- Mülhopt, S.; Dilger, M.; Diabaté, S.; Schlager, C.; Krebs, T.; Zimmermann, R.; Buters, J.; Oeder, S.; Wäscher, T.; Weiss, C.; et al. Toxicity testing of combustion aerosols at the air–liquid interface with a self-contained and easy-to-use exposure system. J. Aerosol Sci. 2016, 96, 38–55. [Google Scholar] [CrossRef]
- Meindl, C.; Stranzinger, S.; Dzidic, N.; Salar-Behzadi, S.; Mohr, S.; Zimmer, A.; Fröhlich, E. Permeation of Therapeutic Drugs in Different Formulations across the Airway Epithelium In Vitro. PLoS ONE 2015, 10, e0135690. [Google Scholar] [CrossRef]
- Piqué, N.; De Servi, B. Rhinosectan® spray (containing xyloglucan) on the ciliary function of the nasal respiratory epithelium; results of an in vitro study. Allergy Asthma Clin. Immunol. 2018, 14, 41. [Google Scholar] [CrossRef]
- Öhlinger, K.; Absenger-Novak, M.; Meindl, C.; Ober, J.; Fröhlich, E. Different Sensitivity of Macrophages to Phospholipidosis Induction by Amphiphilic Cationic Drugs. Int. J. Mol. Sci. 2020, 21, 8391. [Google Scholar] [CrossRef]
- Amann, A.; Zwierzina, M.; Koeck, S.; Gamerith, G.; Pechriggl, E.; Huber, J.M.; Lorenz, E.; Kelm, J.M.; Hilbe, W.; Zwierzina, H.; et al. Development of a 3D angiogenesis model to study tumour—Endothelial cell interactions and the effects of anti-angiogenic drugs. Sci. Rep. 2017, 7, 2963. [Google Scholar] [CrossRef]
- Hofmann, W. Modelling particle deposition in human lungs: Modelling concepts and comparison with experimental data. Biomarkers 2009, 14 (Suppl. S1), 59–62. [Google Scholar] [CrossRef] [PubMed]
- Lejon, C. Lung Deposition Models for Exposure and Risk assessment; FOI Defence Research Agency: Stockholm, Sweden, 2019; Available online: https://www.foi.se/rest-api/report/FOI-R--4753--SE (accessed on 2 May 2021).
- ICRP. Human Respiratory Tract Model for Radiological Protection. Ann. ICRP 1994, 24, 1–3. [Google Scholar] [CrossRef]
- ICRP. Occupational Intakes of Radionuclides: Part 1. (ICRP Publication 130). Ann. ICRP 2015, 44, 5–188. [Google Scholar] [CrossRef]
- Yeh, H.C.; Cuddihy, R.G.; Phalen, R.F.; Chang, I. Comparisons of Calculated Respiratory Tract Deposition of Particles Based on the Proposed NCRP Model and the New ICRP66 Model. Aerosol Sci. Technol. 1996, 25, 134–140. [Google Scholar] [CrossRef]
- Ganguly, K.; Carlander, U.; Garessus, E.D.; Fridén, M.; Eriksson, U.G.; Tehler, U.; Johanson, G. Computational modeling of lung deposition of inhaled particles in chronic obstructive pulmonary disease (COPD) patients: Identification of gaps in knowledge and data. Crit. Rev. Toxicol. 2019, 49, 160–173. [Google Scholar] [CrossRef]
- Verma, R.; Ibrahim, M.; Garcia-Contreras, L. Lung Anatomy and Physiology and Their Implications for Pulmonary Drug Delivery. In Advances in Phamaceutical Technology; Nokhodchi, A., Martin, G.P., Eds.; John Wiley & Sonds, Ltd.: Chichester, UK, 2015; pp. 1–18. [Google Scholar]
- Feng, Y.; Xu, Z.; Haghnegahdar, A. Computational Fluid-Particle Dynamics Modeling for Unconvetional Inhaled Aerosols in Human Respiratory Systems. In Aerosols—Science and Case Studies; Volkov, K., Ed.; IntechOpen: London, UK, 2016. [Google Scholar] [CrossRef]
- Longest, P.W.; Holbrook, L.D. In silico models of aerosol delivery to the respiratory tract—Development and applications. Adv. Drug Deliv. Rev. 2012, 64, 296–311. [Google Scholar] [CrossRef]
- Bui, V.; Moon, J.; Chae, M.; Park, D.; Lee, Y. Prediction of Aerosol Deposition in the Human Respiratory Tract via Computational Models: A Review with Recent Updates. Atmosphere 2020, 11, 137. [Google Scholar] [CrossRef]
- Majid, H.; Hofmann, W.; Winkler-Heil, R. Comparison of stochastic lung deposition fractions with experimental data. Ann. Occup. Hyg. 2012, 56, 278–291. [Google Scholar] [CrossRef]
- Hofmann, W. Regional Deposition: Deposition Models. J. Aerosol Med. Pulm. Drug Deliv. 2020, 33, 239–248. [Google Scholar] [CrossRef] [PubMed]
- Hofmann, W. Modelling inhaled particle deposition in the human lung—A review. J. Aerosol Sci. 2011, 42, 693–724. [Google Scholar] [CrossRef]
- Mathias, N.R.; Timoszyk, J.; Stetsko, P.I.; Megill, J.R.; Smith, R.L.; Wall, D.A. Permeability characteristics of calu-3 human bronchial epithelial cells: In vitro-in vivo correlation to predict lung absorption in rats. J. Drug Targets 2002, 10, 31–40. [Google Scholar] [CrossRef]
- Eriksson, J.; Thörn, H.; Lennernäs, H.; Sjögren, E. Pulmonary drug absorption and systemic exposure in human: Predictions using physiologically based biopharmaceutics modeling. Eur. J. Pharm. Biopharm. 2020, 156, 191–202. [Google Scholar] [CrossRef] [PubMed]
- Kolli, A.R.; Kuczaj, A.K.; Martin, F.; Hayes, A.W.; Peitsch, M.C.; Hoeng, J. Bridging inhaled aerosol dosimetry to physiologically based pharmacokinetic modeling for toxicological assessment: Nicotine delivery systems and beyond. Crit. Rev. Toxicol. 2019, 49, 725–741. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).