Hypergolic Synthesis of Inorganic Materials by the Reaction of Metallocene Dichlorides with Fuming Nitric Acid at Ambient Conditions: The Case of Photocatalytic Titania
Abstract
:1. Introduction
2. Materials and Methods
3. Results and Discussion
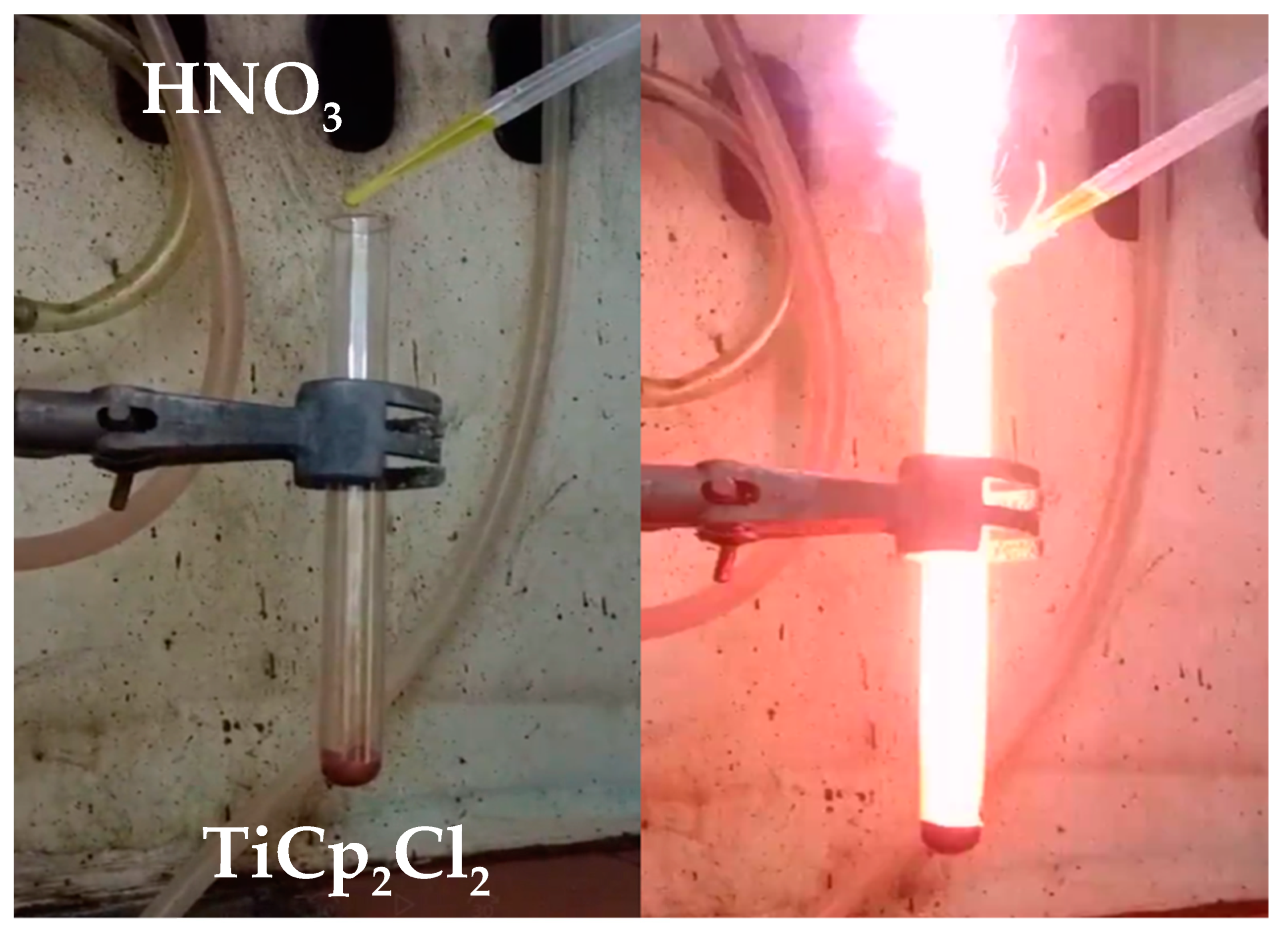
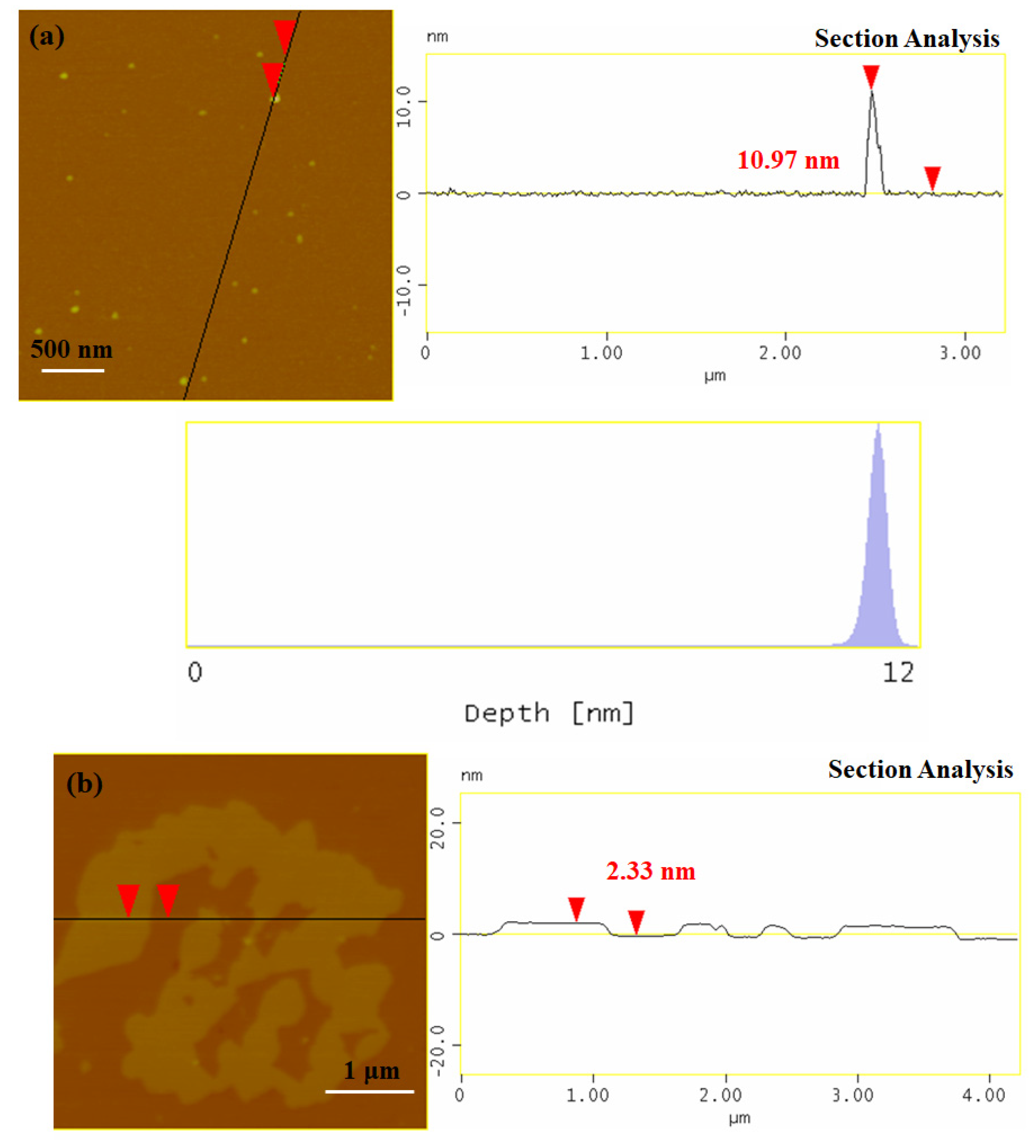
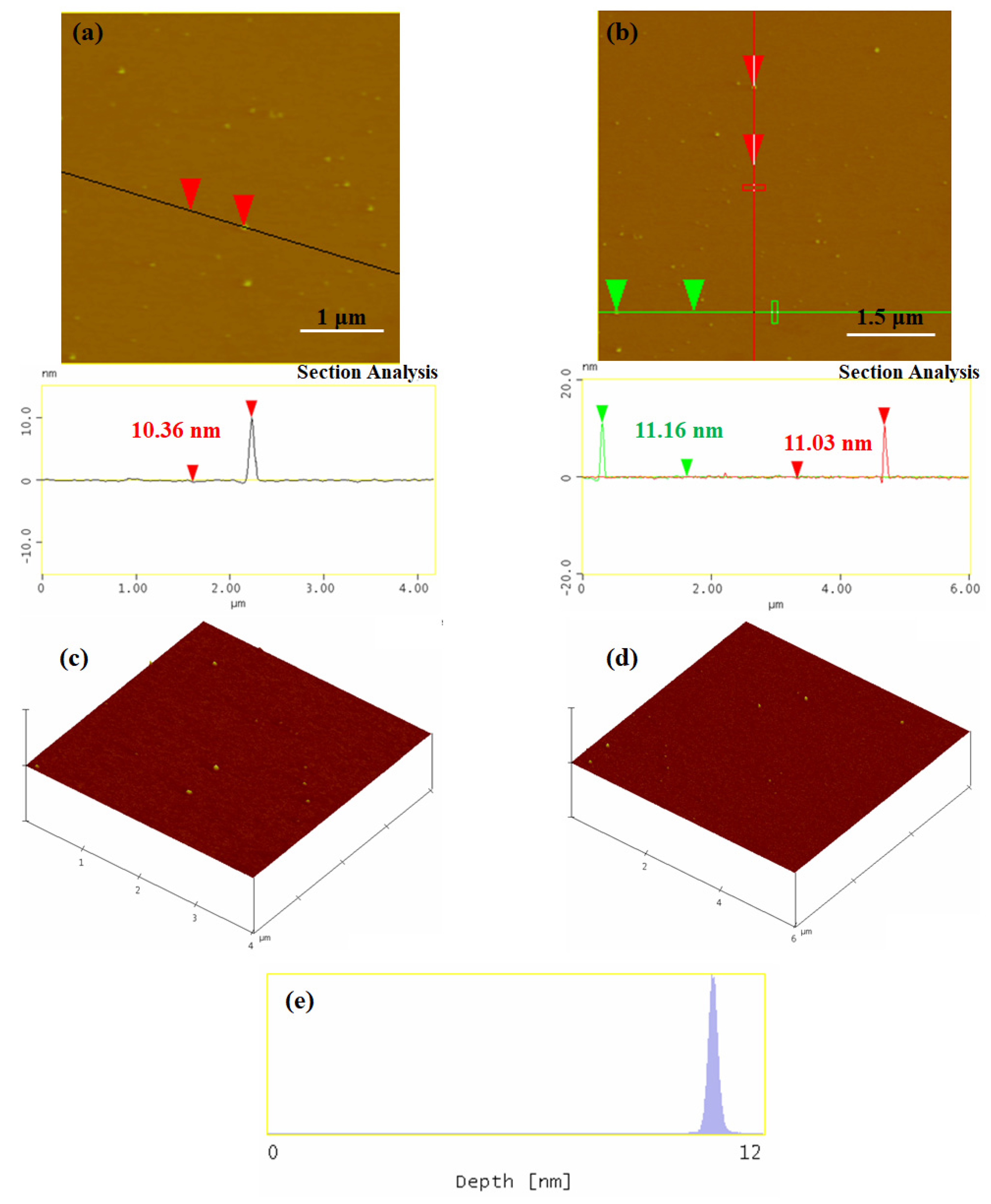
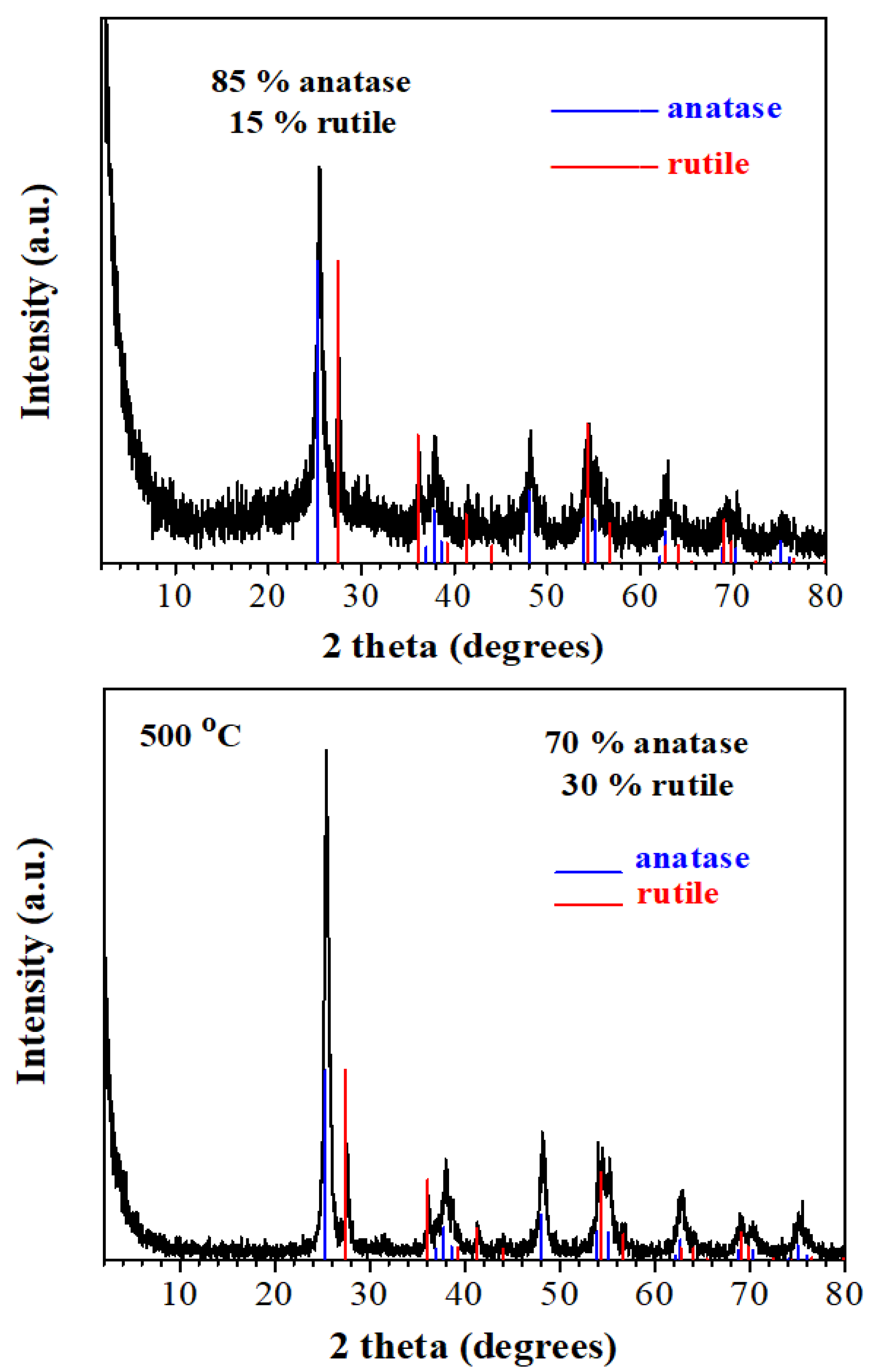
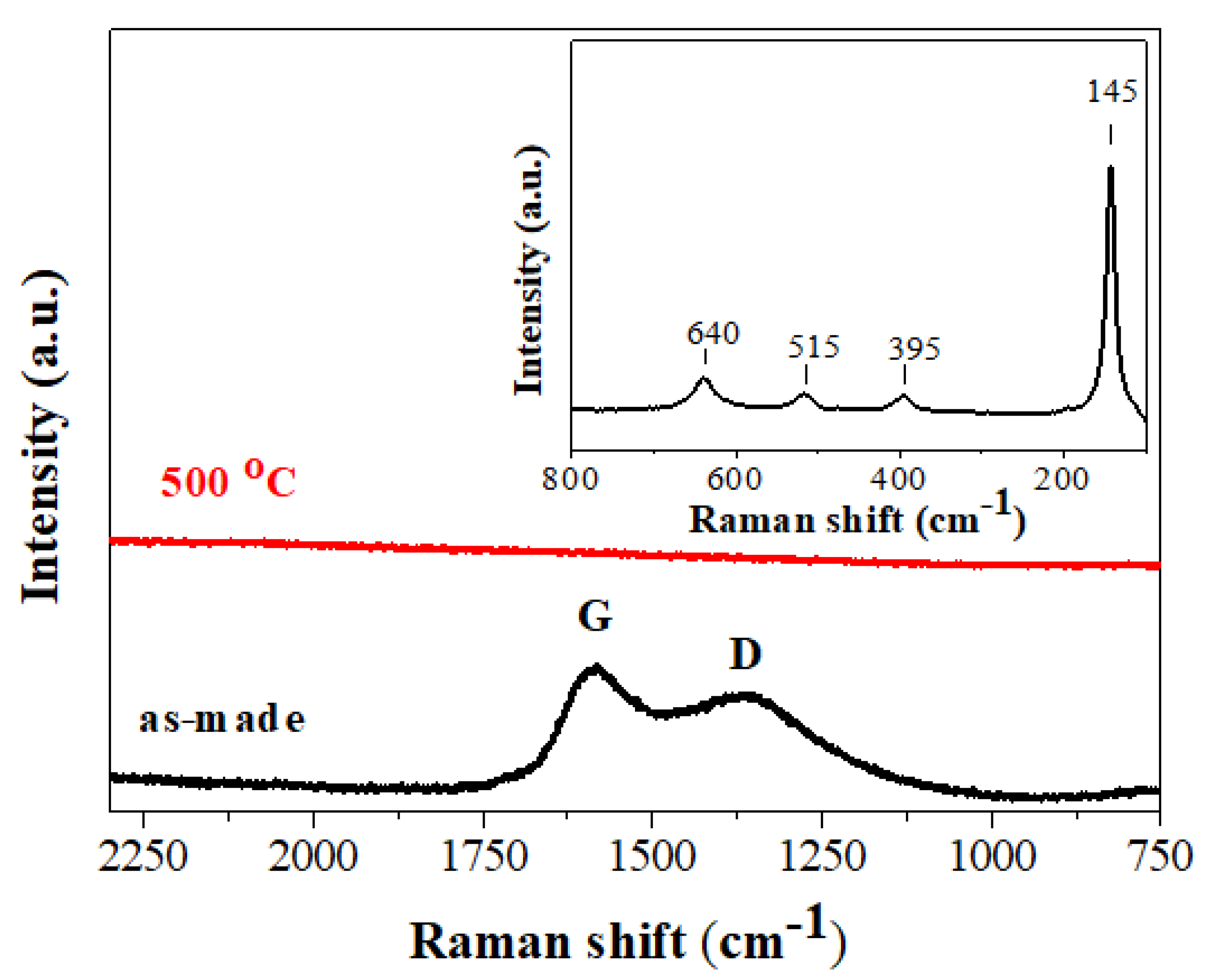
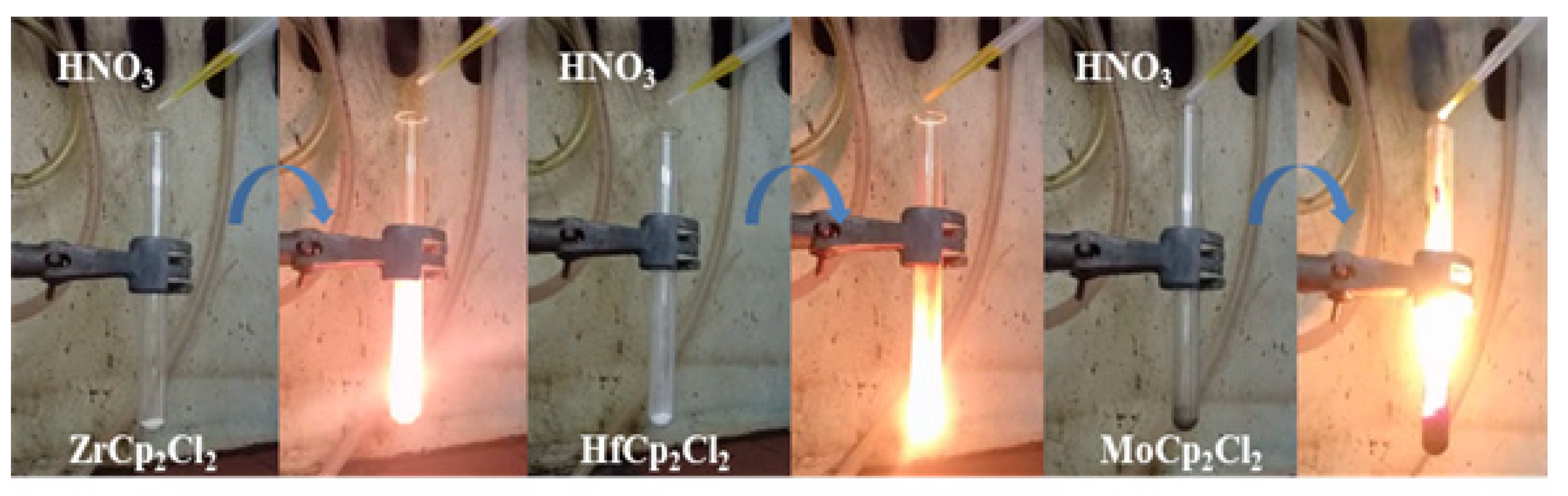
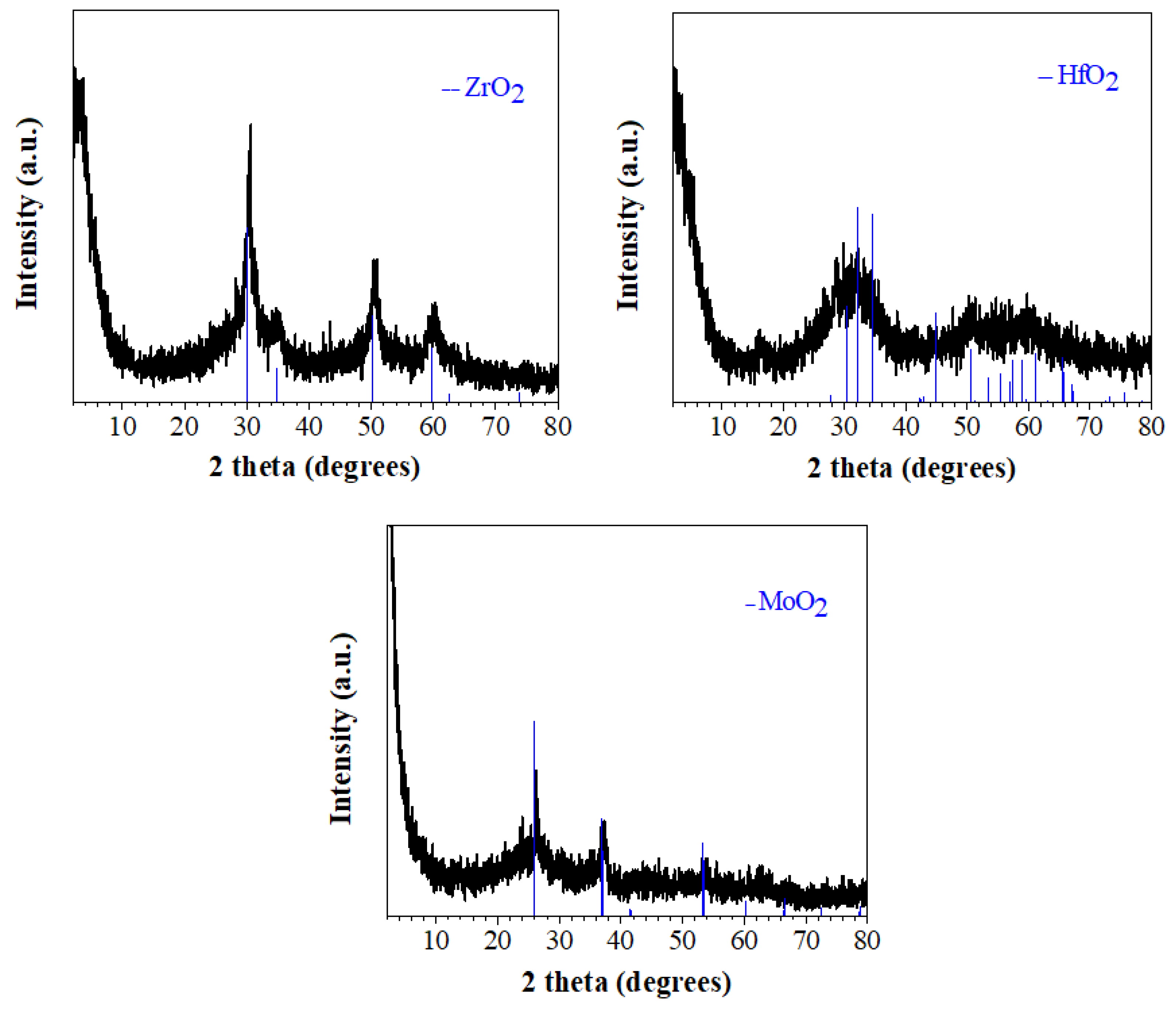
3.1. Synthesis and Characterization
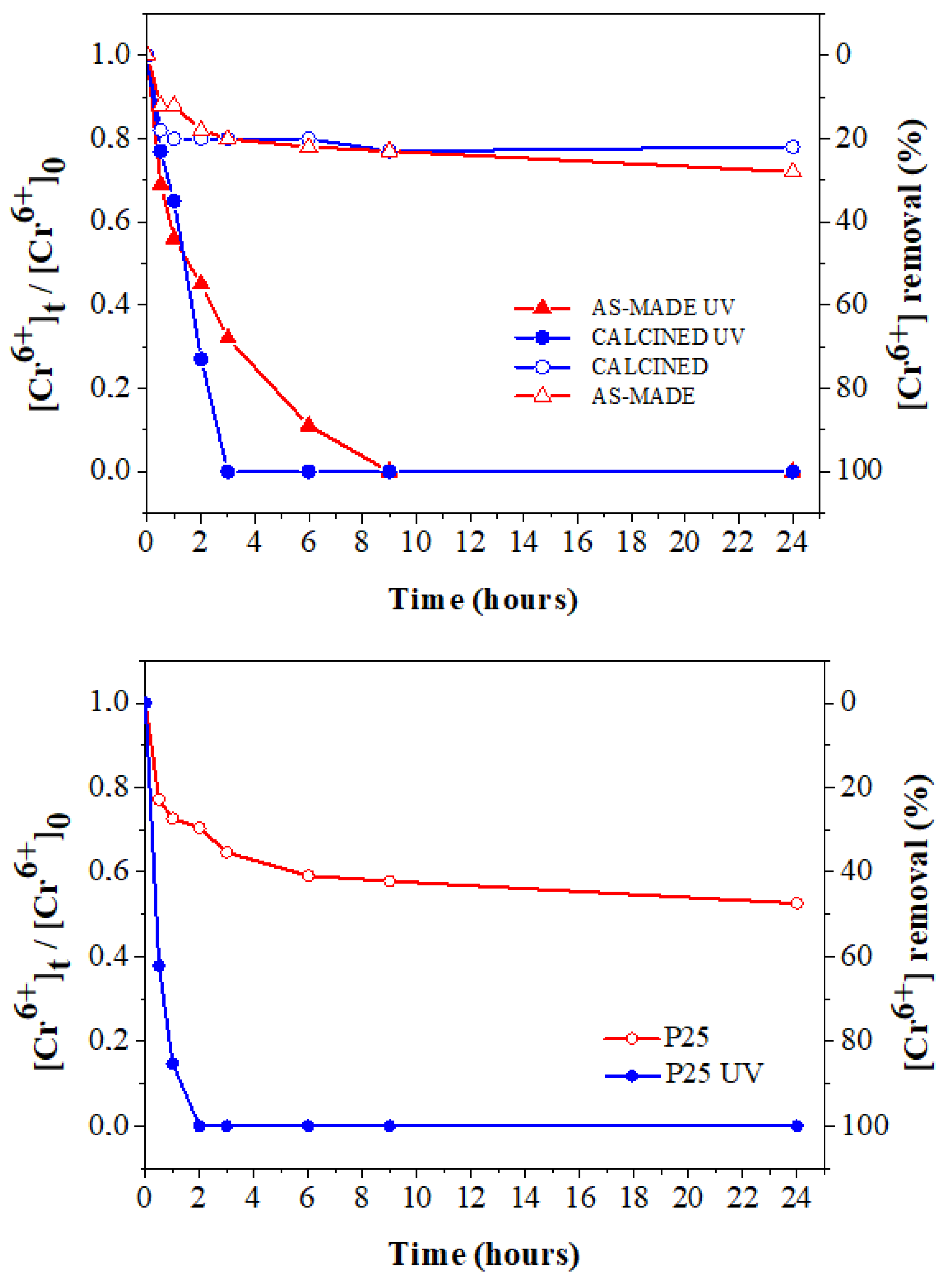
3.2. Photocatalytic Activity of Titania towards Cr(VI) Removal
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tavakoli, A.; Sohrabi, M.; Kargari, A. A review of methods for synthesis of nanostructured metals with emphasis on iron compounds. Chem. Pap. 2007, 61, 151–170. [Google Scholar] [CrossRef]
- Ganachari, S.V.; Banapurmath, N.R.; Salimath, B.; Yaradoddi, J.S.; Shettar, A.S.; Hunashyal, A.M.; Venkataraman, A.; Patil, P.; Shoba, H.; Hiremath, G.B. Synthesis techniques for preparation of nanomaterials. In Handbook of Ecomaterials; Martínez, L.M.T., Kharissova, O.V., Kharisov, B.I., Eds.; Springer International Publishing: Cham, Switzerland, 2017; pp. 1–21. [Google Scholar]
- Ayuk, E.; Mariagoretti, U.; Samuel, A. A review on synthetic methods of nanostructured materials. Chem. Res. J. 2017, 2, 97–123. [Google Scholar]
- Baikousi, M.; Chalmpes, N.; Spyrou, K.; Bourlinos, A.B.; Avgeropoulos, A.; Gournis, D.; Karakassides, M.A. Direct production of carbon nanosheets by self-ignition of pyrophoric lithium dialkylamides in air. Mater. Lett. 2019, 254, 58–61. [Google Scholar] [CrossRef]
- Chalmpes, N.; Spyrou, K.; Bourlinos, A.B.; Moschovas, D.; Avgeropoulos, A.; Karakassides, M.A.; Gournis, D. Synthesis of highly crystalline graphite from spontaneous ignition of in situ derived acetylene and chlorine at ambient conditions. Molecules 2020, 25, 297. [Google Scholar] [CrossRef] [Green Version]
- Chalmpes, N.; Asimakopoulos, G.; Spyrou, K.; Vasilopoulos, K.C.; Bourlinos, A.B.; Moschovas, D.; Avgeropoulos, A.; Karakassides, M.A.; Gournis, D. Functional carbon materials derived through hypergolic reactions at ambient conditions. Nanomaterials 2020, 10, 566. [Google Scholar] [CrossRef] [Green Version]
- Chalmpes, N.; Spyrou, K.; Vasilopoulos, K.C.; Bourlinos, A.B.; Moschovas, D.; Avgeropoulos, A.; Gioti, C.; Karakassides, M.A.; Gournis, D. Hypergolics in carbon nanomaterials synthesis: New paradigms and perspectives. Molecules 2020, 25, 2207. [Google Scholar] [CrossRef]
- Chalmpes, N.; Tantis, I.; Bakandritsos, A.; Bourlinos, A.B.; Karakassides, M.A.; Gournis, D. Rapid carbon formation from spontaneous reaction of ferrocene and liquid bromine at ambient conditions. Nanomaterials 2020, 10, 1564. [Google Scholar] [CrossRef]
- Chalmpes, N.; Bourlinos, A.B.; Šedajová, V.; Kupka, V.; Moschovas, D.; Avgeropoulos, A.; Karakassides, M.A.; Gournis, D. Hypergolic materials synthesis through reaction of fuming nitric acid with certain cyclopentadienyl compounds. J. Carbon Res. 2020, 6, 61. [Google Scholar] [CrossRef]
- Chalmpes, N.; Bourlinos, A.B.; Talande, S.; Bakandritsos, A.; Moschovas, D.; Avgeropoulos, A.; Karakassides, M.A.; Gournis, D. Nanocarbon from rocket fuel waste: The case of furfuryl alcohol-fuming nitric acid hypergolic pair. Nanomaterials 2021, 11, 1. [Google Scholar] [CrossRef]
- Chalmpes, N.; Moschovas, D.; Tantis, I.; Bourlinos, A.B.; Bakandritsos, A.; Fotiadou, R.; Patila, M.; Stamatis, H.; Avgeropoulos, A.; Karakassides, M.A.; et al. Carbon nanostructures derived through hypergolic reaction of conductive polymers with fuming nitric acid at ambient conditions. Molecules 2021, 26, 1595. [Google Scholar] [CrossRef]
- Chalmpes, N.; Moschovas, D.; Bourlinos, A.B.; Spyrou, K.; Vasilopoulos, K.C.; Avgeropoulos, A.; Karakassides, M.A.; Gournis, D. Hypergolic ignition of 1,3-cyclodienes by fuming nitric acid toward the fast and spontaneous formation of carbon nanosheets at ambient conditions. Micro 2021, 1, 15–27. [Google Scholar] [CrossRef]
- El-Sayed, A. Fundamentals of Aircraft and Rocket Propulsion (Chapter 11th). In Fundamentals of Aircraft and Rocket Propulsion; Springer: London, UK, 2016. [Google Scholar]
- Pratsinis, S.E. Flame aerosol synthesis of ceramic powders. Prog. Energy Combust. Sci. 1998, 24, 197–219. [Google Scholar] [CrossRef]
- Lan, Y.; Lu, Y.; Ren, Z. Mini review on photocatalysis of titanium dioxide nanoparticles and their solar applications. Nano Energy 2013, 2, 1031–1045. [Google Scholar] [CrossRef]
- Schneider, J.; Matsuoka, M.; Takeuchi, M.; Zhang, J.; Horiuchi, Y.; Anpo, M.; Bahnemann, D.W. Understanding TiO2 photocatalysis: Mechanisms and materials. Chem. Rev. 2014, 114, 9919–9986. [Google Scholar] [CrossRef] [PubMed]
- Hanaor, D.A.H.; Sorrell, C.C. Review of the anatase to rutile phase transformation. J. Mater. Sci. 2011, 46, 855–874. [Google Scholar] [CrossRef] [Green Version]
- Ohno, T.; Sarukawa, K.; Tokieda, K.; Matsumura, M. Morphology of a TiO2 Photocatalyst (Degussa, P-25) consisting of anatase and rutile crystalline phases. J. Catal. 2001, 203, 82–86. [Google Scholar] [CrossRef]
- Su, R.; Bechstein, R.; Sø, L.; Vang, R.T.; Sillassen, M.; Esbjörnsson, B.; Palmqvist, A.; Besenbacher, F. How the anatase-to-rutile ratio influences the photoreactivity of TiO2. J. Phys. Chem. C 2011, 115, 24287–24292. [Google Scholar] [CrossRef]
- He, J.; Du, Y.-E.; Bai, Y.; An, J.; Cai, X.; Chen, Y.; Wang, P.; Yang, X.; Feng, Q. Facile formation of anatase/rutile TiO2 nanocomposites with enhanced photocatalytic activity. Molecules 2019, 24, 2996. [Google Scholar] [CrossRef] [Green Version]
- Estrada Flores, M.; Reza, C.; Salmones, J.; Wang, J.A.; Manríquez, M.E.; Mora-Hernandez, J.; Hernández, M.; Zúñiga, A.; Contreras, J. Synthesis of nanoporous TiO2 thin films for photocatalytic degradation of methylene blue. J. New Mater. Electrochem. Syst. 2014, 17, 1–48. [Google Scholar] [CrossRef]
- Xing, Z.; Zhou, W.; Du, F.; Zhang, L.; Li, Z.; Zhang, H.; Li, W. Facile synthesis of hierarchical porous TiO2 ceramics with enhanced photocatalytic performance for micropolluted pesticide degradation. ACS Appl. Mater. Interfaces 2014, 6, 16653–16660. [Google Scholar] [CrossRef]
- Tsirka, K.; Katsiki, A.; Chalmpes, N.; Gournis, D.; Paipetis, A.S. Mapping of graphene oxide and single layer graphene flakes—defects annealing and healing. Front. Mater. 2018, 5, 37. [Google Scholar] [CrossRef] [Green Version]
- Pakade, V.E.; Tavengwa, N.T.; Madikizela, L.M. Recent advances in hexavalent chromium removal from aqueous solutions by adsorptive methods. RSC Adv. 2019, 9, 26142–26164. [Google Scholar] [CrossRef] [Green Version]
- Shaban, Y. Effective photocatalytic reduction of Cr(VI) by carbon modified (CM)-n-TiO2 nanoparticles under solar irradiation. World J. Nano Sci. Eng. 2013, 3, 154–160. [Google Scholar] [CrossRef] [Green Version]
- Ali, M.E.M.; Assirey, E.A.; Abdel-Moniem, S.M.; Ibrahim, H.S. Low temperature-calcined TiO2 for visible light assisted decontamination of 4-nitrophenol and hexavalent chromium from wastewater. Sci. Rep. 2019, 9, 19354. [Google Scholar] [CrossRef] [PubMed]
- Asimakopoulos, G.; Baikousi, M.; Salmas, C.; Bourlinos, A.B.; Zboril, R.; Karakassides, M.A. Advanced Cr(VI) sorption properties of activated carbon produced via pyrolysis of the “Posidonia oceanica” seagrass. J. Hazard. Mater. 2021, 405, 124274. [Google Scholar] [CrossRef]
- Asimakopoulos, G.; Baikousi, M.; Kostas, V.; Papantoniou, M.; Bourlinos, A.B.; Zbořil, R.; Karakassides, M.A.; Salmas, C.E. Nanoporous activated carbon derived via pyrolysis process of spent coffee: Structural characterization. Investigation of its use for hexavalent chromium removal. Appl. Sci. 2020, 10, 8812. [Google Scholar] [CrossRef]
- Chi, Y.; Tian, C.; Li, H.; Zhao, Y. Polymerized titanium salts for algae-laden surface water treatment and the algae-rich sludge recycle toward chromium and phenol degradation from aqueous solution. ACS Sustain. Chem. Eng. 2019, 7, 12964–12972. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chalmpes, N.; Asimakopoulos, G.; Baikousi, M.; Bourlinos, A.B.; Karakassides, M.A.; Gournis, D. Hypergolic Synthesis of Inorganic Materials by the Reaction of Metallocene Dichlorides with Fuming Nitric Acid at Ambient Conditions: The Case of Photocatalytic Titania. Sci 2021, 3, 46. https://doi.org/10.3390/sci3040046
Chalmpes N, Asimakopoulos G, Baikousi M, Bourlinos AB, Karakassides MA, Gournis D. Hypergolic Synthesis of Inorganic Materials by the Reaction of Metallocene Dichlorides with Fuming Nitric Acid at Ambient Conditions: The Case of Photocatalytic Titania. Sci. 2021; 3(4):46. https://doi.org/10.3390/sci3040046
Chicago/Turabian StyleChalmpes, Nikolaos, Georgios Asimakopoulos, Maria Baikousi, Athanasios B. Bourlinos, Michael A. Karakassides, and Dimitrios Gournis. 2021. "Hypergolic Synthesis of Inorganic Materials by the Reaction of Metallocene Dichlorides with Fuming Nitric Acid at Ambient Conditions: The Case of Photocatalytic Titania" Sci 3, no. 4: 46. https://doi.org/10.3390/sci3040046
APA StyleChalmpes, N., Asimakopoulos, G., Baikousi, M., Bourlinos, A. B., Karakassides, M. A., & Gournis, D. (2021). Hypergolic Synthesis of Inorganic Materials by the Reaction of Metallocene Dichlorides with Fuming Nitric Acid at Ambient Conditions: The Case of Photocatalytic Titania. Sci, 3(4), 46. https://doi.org/10.3390/sci3040046








