Plant-Derived Saponins: A Review of Their Surfactant Properties and Applications
Abstract
1. Introduction
2. Molecular Structures of Saponins
3. Sources of Saponins
4. Extraction of Saponins
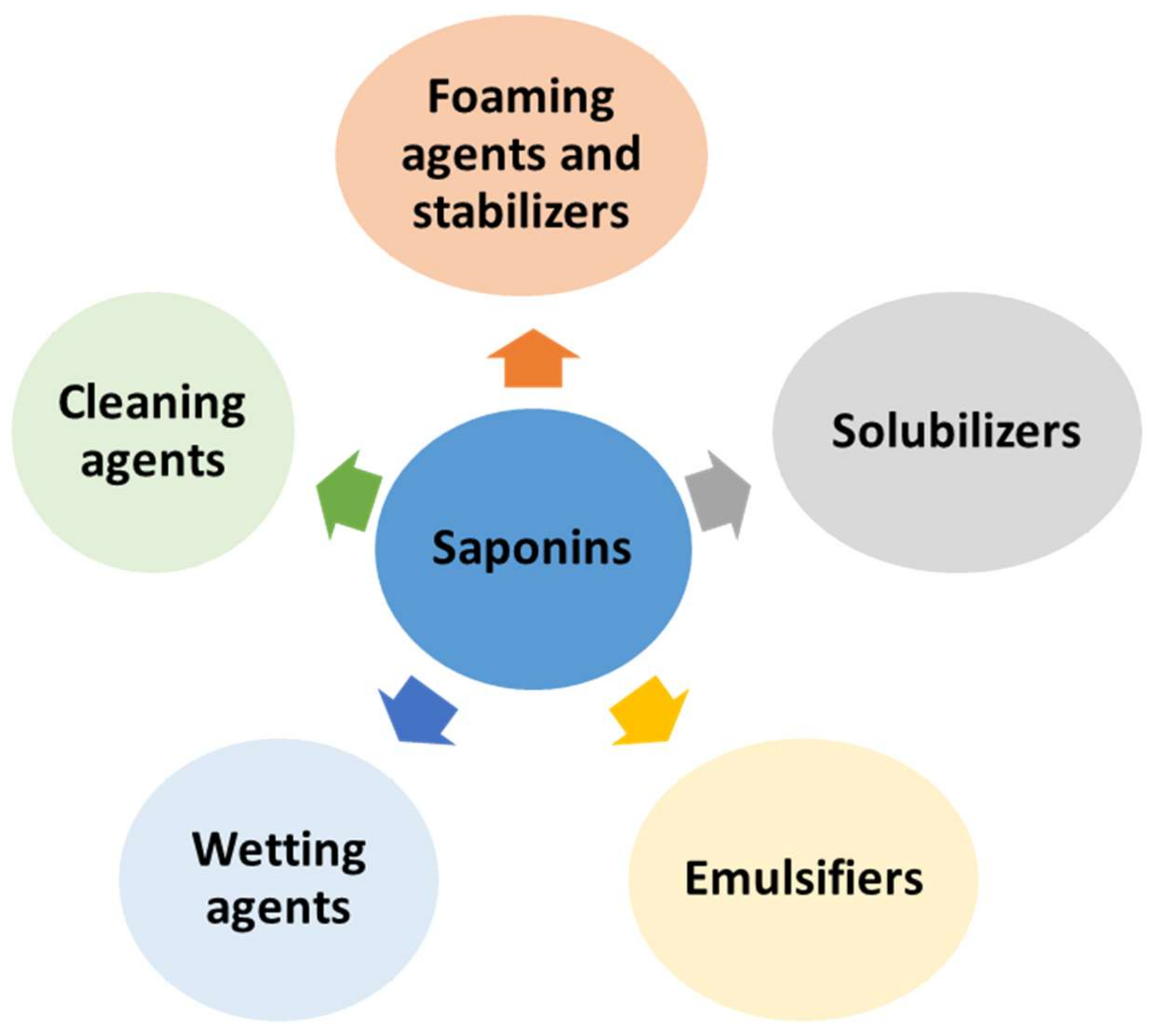
5. Surfactant Properties of Saponins and Their Potential Applications
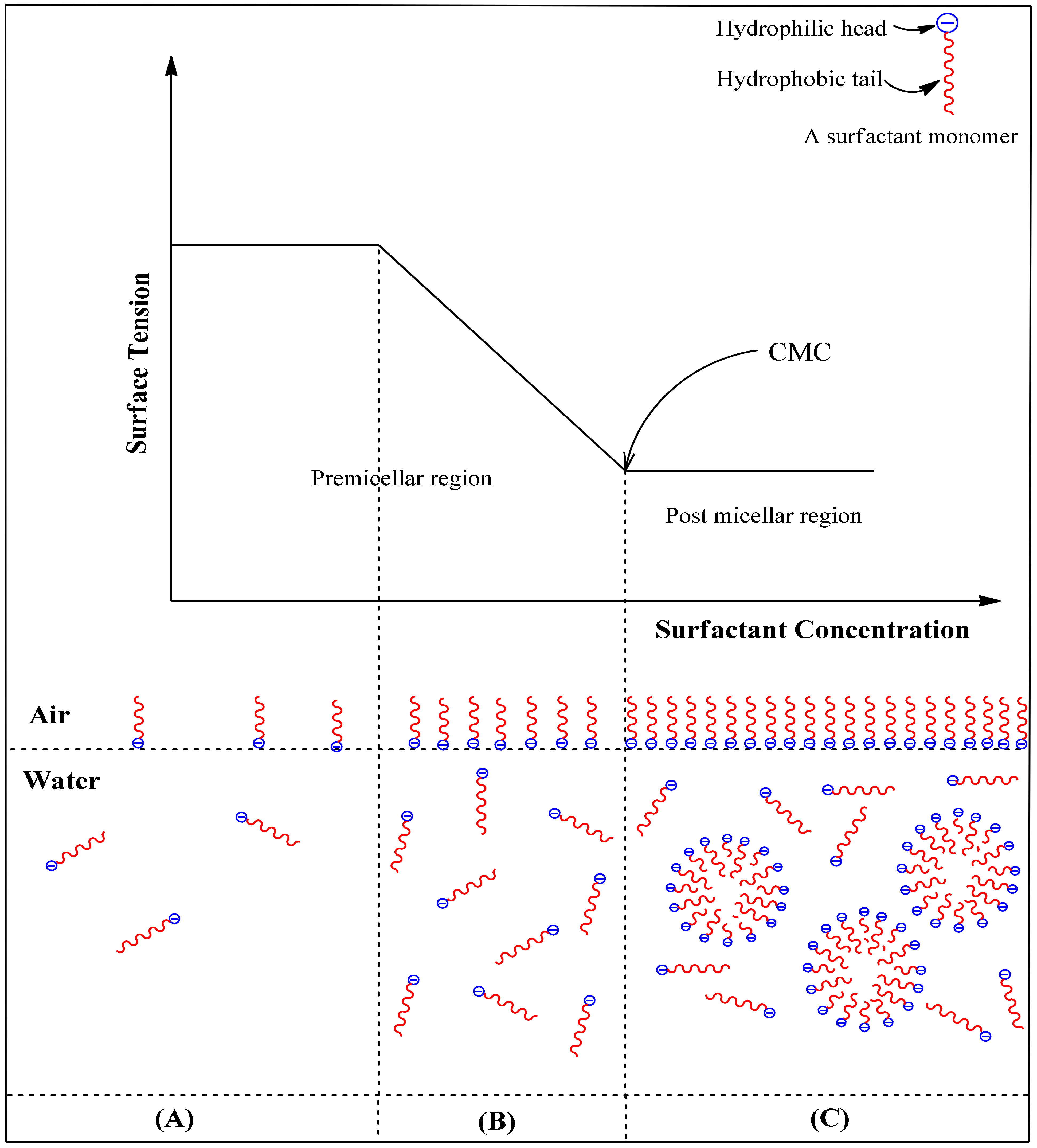
5.1. Micellization Behavior and Reduction of Surface Tension
5.2. Saponins as Cleaning and Wetting Agents
5.3. Saponins as Foaming Agents and Stabilizers
5.4. Saponins as Emulsifiers
5.5. Saponins as Solubilizers
5.6. Saponins in Commercial Formulations
5.7. Other Applications of Saponins
6. Conclusions, Challenges and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Landrigan, P.J.; Fuller, R.; Acosta, N.J.; Adeyi, O.; Arnold, R.; Baldé, A.B.; Bertollini, R.; Bose-O’Reilly, S.; Boufford, J.I.; Breysse, P.N.; et al. The Lancet Commission on pollution and health. Lancet 2018, 391, 462–512. [Google Scholar] [CrossRef]
- Kregiel, D.; Berlowska, J.; Witonska, I.; Antolak, H.; Proestos, C.; Babic, M.; Babic, L.; Zhang, B. Saponin-Based, Biological-Active Surfactants from Plants. In Application and Characterization of Surfactants; InTech: London, UK, 2017. [Google Scholar]
- Wisetkomolmat, J.; Suksathan, R.; Puangpradab, R.; Kunasakdakul, K.; Jantanasakulwong, K.; Rachtanapun, P.; Sommano, S.R. Natural Surfactant Saponin from Tissue of Litsea glutinosa and Its Alternative Sustainable Production. Plants 2020, 9, 1521. [Google Scholar] [CrossRef]
- Pradhan, A.; Bhattacharyya, A. Quest for an eco-friendly alternative surfactant: Surface and foam characteristics of natural surfactants. J. Clean. Prod. 2017, 150, 127–134. [Google Scholar] [CrossRef]
- Holmberg, K. Natural surfactants. Curr. Opin. Colloid Interface Sci. 2001, 6, 148–159. [Google Scholar] [CrossRef]
- García-Becerra, F.Y.; Allen, D.G.; Acosta, E.J. Surfactants from Waste Biomass. In Surfactants from Renewable Resources; John Wiley & Sons, Ltd.: West Sussex, UK, 2010. [Google Scholar]
- Sparg, S.G.; Light, M.E.; Van Staden, J. Biological activities and distribution of plant saponins. J. Ethnopharmacol. 2004, 94, 219–243. [Google Scholar] [CrossRef]
- Vincken, J.P.; Heng, L.; de Groot, A.; Gruppen, H. Saponins, classification and occurrence in the plant kingdom. Phytochemistry 2007, 68, 275–297. [Google Scholar] [CrossRef] [PubMed]
- Böttcher, S.; Drusch, S. Saponins—Self-assembly and behavior at aqueous interfaces. Adv. Colloid Interface Sci. 2017, 243, 105–113. [Google Scholar] [CrossRef]
- Farias, C.B.B.; Almeida, F.C.; Silva, I.A.; Souza, T.C.; Meira, H.M.; Rita de Cássia, F.; Luna, J.M.; Santos, V.A.; Converti, A.; Banat, I.M.; et al. Production of green surfactants: Market prospects. Electron. J. Biotechnol. 2021, 51, 28–39. [Google Scholar] [CrossRef]
- Chhetri, A.B.; Watts, K.C.; Rahman, M.S.; Islam, M.R. Soapnut extract as a natural surfactant for enhanced oil recovery. Energy Sources Part A Recovery Util. Environ. Eff. 2009, 31, 1893–1903. [Google Scholar] [CrossRef]
- Wisetkomolmat, J.; Suppakittpaisarn, P.; Sommano, S.R. Detergent Plants of Northern Thailand: Potential Sources of Natural Saponins. Resources 2019, 8, 10. [Google Scholar] [CrossRef]
- Moghimipour, E.; Jasemnezhad, M.; Mohammad Soleymani, S.; Salimi, A. Preparation and evaluation of a free surfactant herbal shampoo with Acanthophyllum squarrosum Saponins. J. Cosmet. Dermatol. 2021, 20, 181–187. [Google Scholar] [CrossRef]
- Aghel, N.; Moghimipour, E.; Raies, A. Formulation of a Herbal Shampoo using Total Saponins of Acanthophyllum squarrosum. Iran. J. Pharm. Res. 2007, 6, 167–172. [Google Scholar]
- Oleszek, W.; Hamed, A. Saponin-Based Surfactants. In Surfactants from Renewable Resources; John Wiley & Sons, Ltd.: West Sussex, UK, 2010. [Google Scholar]
- Oleszek, W.; Bialy, Z. Chromatographic determination of plant saponins—An update (2002–2005). J. Chromatogr. A 2006, 1112, 78–91. [Google Scholar] [CrossRef]
- Moghimipour, E.; Handali, S. Saponin: Properties, Methods of Evaluation and Applications. Annu. Res. Rev. Biol. 2015, 5, 207–220. [Google Scholar] [CrossRef]
- Sharma, O.P.; Kumar, N.; Singh, B.; Bhat, T.K. An improved method for thin layer chromatographic analysis of saponins. Food Chem. 2012, 132, 671–674. [Google Scholar] [CrossRef]
- Yu, X.L.; He, Y. Tea saponins: Effective natural surfactants beneficial for soil remediation, from preparation to application. RSC Adv. 2018, 8, 24312–24321. [Google Scholar] [CrossRef]
- Savage, G.P. Saponins. In Encyclopedia of Food and Health; Academic Press: Cambridge, MA, USA, 2016. [Google Scholar]
- Canto, G.S.D.; Treter, J.; Yang, S.; Borré, G.L.; Peixoto, M.P.G.; Ortega, G.G. Evaluation of foam properties of saponin from Ilex paraguariensis A. St. Hil. (Aquifoliaceae) fruits. Braz. J. Pharm. Sci. 2010, 46, 237–243. [Google Scholar] [CrossRef]
- Wojtoń, P.; Szaniawska, M.; Hołysz, L.; Miller, R.; Szcześ, A. Surface activity of natural surfactants extracted from Sapindus mukorossi and Sapindus trifoliatus soapnuts. Colloids Interfaces 2021, 5, 7. [Google Scholar] [CrossRef]
- Guclu-Ustundag, Ö.; Mazza, G. Saponins: Properties, applications and processing. Crit. Rev. Food Sci. Nutr. 2007, 47, 231–258. [Google Scholar] [CrossRef] [PubMed]
- Gafur, M.A.; Obata, T.; Kiuchi, F.; Tsuda, Y. Acacia concinna Saponins. I. Structures of Prosapogenols, Concinnosides A-F, Isolated from the Alkaline Hydrolysate of the Highly Polar Saponin Fraction. Chem. Pharm. Bull. 1997, 45, 620–625. [Google Scholar] [CrossRef] [PubMed]
- Tezuka, Y.; Honda, K.; Banskota, A.H.; Thet, M.M.; Kadota, S. Kinmoonosides A-C, three new cytotoxic saponins from the fruits of Acacia concinna, a medicinal plant collected in Myanmar. J. Nat. Prod. 2000, 63, 1658–1664. [Google Scholar] [CrossRef] [PubMed]
- Yoshikawa, K.; Satou, Y.; Tokunaga, Y.; Tanaka, M.; Arihara, S.; Nigam, S.K. Four Acylated Triterpenoid Saponins from Albizia procera. J. Nat. Prod. 1998, 61, 440–445. [Google Scholar] [CrossRef]
- Chen, C.; Li, R.; Li, D.; Shen, F.; Xiao, G.; Zhou, J. Extraction and purification of saponins from Sapindus mukorossi. New J. Chem. 2021, 45, 952–960. [Google Scholar] [CrossRef]
- Kimata, H.; Nakashima, T.; Kokubun, S.; Nakayamw, K.; Mitoma, Y.; Kitahara, T.; Yata, N.; Tanaka, O. Saponins of Pericarps of Sapindus mukorossi Gaertn and Solubilization of Monodesmosides by Bisdesmosides. Chem. Pharm. Bull. 1983, 31, 1998–2005. [Google Scholar] [CrossRef]
- Bhaskar, K. Potential Soap, Shampoo and Detergent Plant Resources of India and Their Associated Traditional Knowledge. Plant Arch. 2018, 18, 301–319. [Google Scholar]
- Pérez, A.J.; Calle, J.M.; Simonet, A.M.; Guerra, J.O.; Stochmal, A.; Macías, F.A. Bioactive steroidal saponins from Agave offoyana flowers. Phytochemistry 2013, 95, 298–307. [Google Scholar] [CrossRef]
- Pérez, A.J.; Simonet, A.M.; Calle, J.M.; Pecio, Ł.; Guerra, J.O.; Stochmal, A.; Macías, F.A. Phytotoxic steroidal saponins from Agave offoyana leaves. Phytochemistry 2014, 105, 92–100. [Google Scholar] [CrossRef]
- Ribeiro, B.D.; Alviano, D.S.; Barreto, D.W.; Coelho, M.A.Z. Functional properties of saponins from sisal (Agave sisalana) and juá (Ziziphus joazeiro): Critical micellar concentration, antioxidant and antimicrobial activities. Colloids Surf. A Physicochem. Eng. Asp. 2013, 436, 736–743. [Google Scholar] [CrossRef]
- Sharma, P.; Saini, M.K.; Prasad, J.; Gour, V.S. Evaluation of Robustness of the Biosurfactant Derived from Balanites aegyptiaca (L.) Del. J. Surfactants Deterg. 2019, 22, 403–408. [Google Scholar] [CrossRef]
- Chen, Y.F.; Yang, C.H.; Chang, M.S.; Ciou, Y.P.; Huang, Y.C. Foam properties and detergent abilities of the saponins from Camellia oleifera. Int. J. Mol. Sci. 2010, 11, 4417–4425. [Google Scholar] [CrossRef]
- Chindo, B.A.; Anuka, J.A.; McNeil, L.; Yaro, A.H.; Adamu, S.S.; Amos, S.; Connelly, W.K.; Lees, G.; Gamaniel, K.S. Anticonvulsant properties of saponins from Ficus platyphylla stem bark. Brain Res. Bull. 2009, 78, 276–282. [Google Scholar] [CrossRef]
- Do, D.N.; Dang, T.T.; Le, Q.T.; Lam, T.D.; Bach, L.G.; Nguyen, D.C.; Toan, T.Q. Extraction of saponin from gleditsia peel and applications on natural dishwashing liquid detergent. Mater. Today Proc. 2019, 18, 5219–5230. [Google Scholar]
- Yoshizaki, K.; Devkota, H.P.; Fujino, H.; Yahara, S. Saponins composition of rhizomes, taproots, and lateral roots of satsuma-ninjin (Panax japonicus). Chem. Pharm. Bull. 2013, 61, 344–350. [Google Scholar] [CrossRef] [PubMed]
- Majinda, R.R.T. Extraction and isolation of saponins. Methods Mol. Biol. 2012, 864, 415–426. [Google Scholar]
- Deore, S.L.; Baviskar, B.A.; Rangari, A.S. Rapid and high yield extraction method for saponins from safed musli. Pharmacogn. J. 2015, 7, 210–214. [Google Scholar] [CrossRef]
- Ashour, A.S.; El Aziz, M.M.A.; Gomha Melad, A.S. A review on saponins from medicinal plants: Chemistry, isolation, and determination. J. Nanomed. Res. 2019, 7, 282–288. [Google Scholar] [CrossRef]
- Ballesteros, O.J.V.; Perea, E.M.; Méndez, J.J.; Arango, W.M.; Noreña, D.A. Quantification, chemical and biological characterization of the saponosides material from Sida cordifolia L. Rev. Cuba. Plantas Med. 2013, 18, 298–314. [Google Scholar]
- Müller, L.E.; Schiedeck, G. Physical properties of botanical surfactants. Sci. Total Environ. 2018, 610–611, 1133–1137. [Google Scholar] [CrossRef]
- Koczo, K.; Racz, G. Foaming properties of surfactant solutions. Colloids Surf. 1991, 56, 59–82. [Google Scholar] [CrossRef]
- Negi, J.S.; Negi, P.S.; Pant, G.J.; Rawat, M.; Negi, S.K. Naturally occurring saponins: Chemistry and biology. J. Poisonous Med. Plant Res. 2013, 1, 1–6. [Google Scholar]
- Pradhan, A.; Bhattacharyya, A. An Alternative Approach for Determining Critical Micelle Concentration: Dispersion of Ink in Foam. J. Surfactants Deterg. 2018, 21, 745–750. [Google Scholar] [CrossRef]
- Muhammad, M.T.; Khan, M.N. Eco-friendly, biodegradable natural surfactant (Acacia concinna): An alternative to the synthetic surfactants. J. Clean. Prod. 2018, 188, 678–685. [Google Scholar] [CrossRef]
- Belwal, T.; Chemat, F.; Venskutonis, P.R.; Cravotto, G.; Jaiswal, D.K.; Bhatt, I.D.; Devkota, H.P.; Luo, Z. Recent advances in scaling-up of non-conventional extraction techniques: Learning from successes and failures. TrAC Trends Anal. Chem. 2020, 127, 115895. [Google Scholar] [CrossRef]
- Belwal, T.; Ezzat, S.M.; Rastrelli, L.; Bhatt, I.D.; Daglia, M.; Baldi, A.; Devkota, H.P.; Orhan, I.E.; Patra, J.K.; Das, G.; et al. A critical analysis of extraction techniques used for botanicals: Trends, priorities, industrial uses and optimization strategies. TrAC Trends Anal. Chem. 2018, 100, 82–102. [Google Scholar] [CrossRef]
- Cheok, C.Y.; Abdel, H.; Salman, K.; Sulaiman, R. Extraction and quantification of saponins: A review. Food Res. Int. 2014, 59, 16–40. [Google Scholar] [CrossRef]
- Rosen, M.J.; Kunjappu, J.T. Surfactants and Interfacial Phenomena, 4th ed.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2012. [Google Scholar]
- Schramm, L.L.; Stasiuk, E.N.; Marangoni, D.G. Surfactants and their applications. Annu. Rep. Prog. Chem. Sect. C 2003, 99, 3–48. [Google Scholar] [CrossRef]
- Böttger, S.; Hofmann, K.; Melzig, M.F. Bioorganic & Medicinal Chemistry Saponins can perturb biologic membranes and reduce the surface tension of aqueous solutions: A correlation? Bioorg. Med. Chem. 2012, 20, 2822–2828. [Google Scholar]
- Tmáková, L.; Sekretár, S.; Schmidt, Š. Plant-derived surfactants as an alternative to synthetic surfactants: Surface and antioxidant activities. Chem. Pap. 2015, 70, 188–196. [Google Scholar] [CrossRef]
- Wu, S.; Liang, F.; Hu, D.; Li, H.; Yang, W.; Zhu, Q. Determining the Critical Micelle Concentration of Surfactants by a Simple and Fast Titration Method. Anal. Chem. 2020, 92, 4259–4265. [Google Scholar] [CrossRef] [PubMed]
- Pradhan, A.; Bhattacharyya, A. Shampoos Then and Now: Synthetic versus Natural. J. Surf. Sci. Technol. 2014, 30, 59–76. [Google Scholar]
- Almeida, F.C.; Silva, N.R.E.; Meira, H.; Jara, A.M.; Luna, J.M.; Sarubbo, L.A. Physico-chemical properties of the biosurfactant obtained from fruit extract of Genipa americana L. and Tamarindus indica L. and its application in oil removal. Chem. Eng. Trans. 2017, 57, 1549–1554. [Google Scholar]
- Sabri, N.; Moulai-Mostefa, N. Formulation and characterization of oil-in-water emulsions stabilized by saponins extracted from Hedera Helix Algeriensis using response surface method. Biointerface Res. Appl. Chem. 2020, 10, 6282–6292. [Google Scholar]
- Anuragi, J.L.; Pradesh, R.M.; Pradesh, M.; Pradesh, M. Separation of Saponins from Sapindus laurifolia (L.). Int. J. Bot. Stud. 2017, 2, 21–24. [Google Scholar]
- Balakrishnan, S.; Varughese, S.; Deshpande, A.P. Micellar characterisation of saponin from Sopindus mukorossi. Tenside Surfactants Deterg. 2006, 43, 262–268. [Google Scholar] [CrossRef]
- Shah, S.K.; Chatterjee, S.K.; Bhattarai, A. Micellization of cationic surfactants in alcohol—Water mixed solvent media. J. Mol. Liq. 2016, 222, 906–914. [Google Scholar] [CrossRef]
- Zhang, S.; Gao, Y.; Dong, B.; Zheng, L. Colloids and Surfaces A: Physicochemical and Engineering Aspects Interaction between the added long-chain ionic liquid 1-dodecyl-3-methylimidazolium tetrafluoroborate and Triton X-100 in aqueous solutions. Colloids Surf. A Physicochem. Eng. Asp. 2010, 372, 182–189. [Google Scholar] [CrossRef]
- Rakowska, J.; Radwan, K.; Porycka, B.; Prochaska, K. Experimental study on surface activity of surfactants on their ability to cleaning oil contaminations. J. Clean. Prod. 2017, 144, 437–447. [Google Scholar] [CrossRef]
- Yakimchuk, O.D.; Kotomin, A.A.; Petel’Skii, M.B.; Naumov, V.N. Cleaning action and surfactant properties of alkyl glucosides. Russ. J. Appl. Chem. 2004, 77, 2001–2005. [Google Scholar] [CrossRef]
- Lee, K.S.; Ivanova, N.; Starov, V.M.; Hilal, N.; Dutschk, V. Kinetics of wetting and spreading by aqueous surfactant solutions. Adv. Colloid Interface Sci. 2008, 144, 54–65. [Google Scholar] [CrossRef] [PubMed]
- Jurado Gonzalez, P.; Sörensen, P.M. Characterization of saponin foam from Saponaria officinalis for food applications. Food Hydrocoll. 2020, 101, 105541. [Google Scholar] [CrossRef]
- Rekiel, E.; Smułek, W.; Zdziennicka, A.; Kaczorek, E.; Jańczuk, B. Wetting properties of Saponaria officinalis saponins. Colloids Surf. A Physicochem. Eng. Asp. 2020, 584, 123980. [Google Scholar] [CrossRef]
- Vinarov, Z.; Radeva, D.; Katev, V.; Tcholakova, S.; Denkov, N. Solubilisation of hydrophobic drugs by saponins. Indian J. Pharm. Sci. 2018, 80, 709–718. [Google Scholar] [CrossRef]
- Wang, H.; Guo, W.; Zheng, C.; Wang, D.; Zhan, H. Effect of Temperature on Foaming Ability and Foam Stability of Typical Surfactants Used for Foaming Agent. J. Surfactants Deterg. 2017, 20, 615–622. [Google Scholar] [CrossRef]
- Saxena, N.; Pal, N.; Ojha, K.; Dey, S.; Mandal, A. Synthesis, characterization, physical and thermodynamic properties of a novel anionic surfactant derived from: Sapindus laurifolius. RSC Adv. 2018, 8, 24485–24499. [Google Scholar] [CrossRef]
- Tao, W.; Duan, J.; Zhao, R.; Li, X.; Yan, H.; Li, J.; Guo, S. Comparison of three officinal Chinese pharmacopoeia species of Glycyrrhiza based on separation and quantification of triterpene saponins and chemometrics analysis. Food Chem. 2013, 141, 1681–1689. [Google Scholar] [CrossRef]
- Das, C.; DasGupta, S.; De, S. Prediction of permeate flux and counterion binding during cross-flow micellar-enhanced ultrafiltration. Colloids Surf. A Physicochem. Eng. Asp. 2008, 318, 125–133. [Google Scholar] [CrossRef]
- Purkait, M.K.; DasGupta, S.; De, S. Removal of dye from wastewater using micellar-enhanced ultrafiltration and recovery of surfactant. Sep. Purif. Technol. 2004, 37, 81–92. [Google Scholar] [CrossRef]
- Gzara, L.; Dhahbi, M. Removal of chromate anions by micellar-enhanced ultrafiltration using cationic surfactants. Desalination 2001, 137, 241–250. [Google Scholar] [CrossRef]
- Pennell, K.D.; Jin, M.; Abriola, L.M.; Pope, G.A. Surfactant enhanced remediation of soil columns contaminated by residual tetrachloroethylene. J. Contam. Hydrol. 1994, 16, 35–53. [Google Scholar] [CrossRef]
- Mulligan, C.N.; Yong, R.N.; Gibbs, B.F. Surfactant-enhanced remediation of contaminated soil: A review. Eng. Geol. 2001, 60, 371–380. [Google Scholar] [CrossRef]
- Bordoloi, N.K.; Konwar, B.K. Microbial surfactant-enhanced mineral oil recovery under laboratory conditions. Colloids Surf. B Biointerfaces 2008, 63, 73–82. [Google Scholar] [CrossRef] [PubMed]
- Pandit, P.; Basu, S. Removal of Ionic Dyes from Water by Solvent Extraction Using Reverse Micelles. Environ. Sci. Technol. 2004, 38, 2435–2442. [Google Scholar] [CrossRef] [PubMed]
- Tehrani-Bagha, A.R.; Holmberg, K. Solubilization of hydrophobic dyes in surfactant solutions. Materials 2013, 6, 580–608. [Google Scholar] [CrossRef] [PubMed]
- Roy, D.; Kommalapati, R.R.; Mandava, S.S.; Valsaraj, K.T.; Constant, W.D. Sail washing potential of a natural surfactant. Environ. Sci. Technol. 1997, 31, 670–675. [Google Scholar] [CrossRef]
- Samal, K.; Das, C.; Mohanty, K. Eco-friendly biosurfactant saponin for the solubilization of cationic and anionic dyes in aqueous system. Dyes Pigments 2017, 140, 100–108. [Google Scholar] [CrossRef]
- Mainkar, A.R.; Jolly, C.I. Evaluation of commercial herbal shampoos. Int. J. Cosmet. Sci. 2000, 22, 385–391. [Google Scholar] [CrossRef] [PubMed]
- Mainkar, A.R.; Jolly, C.I. Formulation of natural shampoos. Int. J. Cosmet. Sci. 2001, 23, 59–62. [Google Scholar] [CrossRef]
- Nizioł-Łukaszewska, Z.; Bujak, T. Saponins as Natural Raw Materials for Increasing the Safety of Bodywash Cosmetic Use. J. Surfactants Deterg. 2018, 21, 767–776. [Google Scholar] [CrossRef]
- Sur, P.; Chaudhuri, T.; Vedasiromoni, J.R.; Gomes, A.; Ganguly, D.K.; Wiley, J. Antiinflammatory and antioxidant property of saponins of tea [Camellia sinensis (L) O. Kuntze] root extract. Phytother. Res. 2001, 176, 174–176. [Google Scholar] [CrossRef]
- Hu, J.; Nie, S.; Huang, D.; Li, C.; Xie, M. Extraction of saponin from Camellia oleifera cake and evaluation of its antioxidant activity. Int. J. Food Sci. Technol. 2012, 47, 1676–1687. [Google Scholar] [CrossRef]
- Dong, S.; Yang, X.; Zhao, L.; Zhang, F.; Hou, Z.; Xue, P. Antibacterial activity and mechanism of action saponins from Chenopodium quinoa Willd. husks against foodborne pathogenic bacteria. Ind. Crops Prod. 2020, 149, 112350. [Google Scholar] [CrossRef]
- Chen, Z.; Li, J.; Liu, J.; Zhao, Y.; Zhang, P.; Zhang, M.; Zhang, L. Saponins Isolated from the Root of Panax notoginseng Showed Significant Anti-Diabetic Effects in KK-Ay Mice. Am. J. Chin. Med. 2008, 36, 939–951. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Wang, J.; Zhao, Y.; Shen, L.; Jiang, X.; Xie, Z. Anti-diabetic effects of Panax notoginseng saponins and its major anti-hyperglycemic components. J. Ethnopharmacol. 2010, 130, 231–236. [Google Scholar] [CrossRef] [PubMed]
- Zheng, T.; Shu, G.; Yang, Z.; Mo, S.; Zhao, Y.; Mei, Z. Antidiabetic effect of total saponins from Entada phaseoloides (L.) Merr. in type 2 diabetic rats. J. Ethnopharmacol. 2012, 139, 814–821. [Google Scholar] [CrossRef]
- Xu, J.; Wang, S.; Feng, T.; Chen, Y.; Yang, G. Hypoglycemic and hypolipidemic effects of total saponins from Stauntonia chinensis in diabetic db/db mice. J. Cell. Mol. Med. 2018, 22, 6026–6038. [Google Scholar] [CrossRef]
- Verza, S.G.; Silveira, F.; Cibulski, S.; Kaiser, S.; Ferreira, F.; Gosmann, G.; Roehe, P.M.; Ortega, G.G. Immunoadjuvant Activity, Toxicity Assays, and Determination by UPLC/Q-TOF-MS of Triterpenic Saponins from Chenopodium quinoa Seeds. J. Agric. Food. Chem. 2012, 60, 3113–3118. [Google Scholar] [CrossRef]
- Sun, H. Haemolytic activities and adjuvant effect of Bupleurum chinense saponins on the immune responses to ovalbumin in mice. Vaccine 2006, 24, 1324–1331. [Google Scholar] [CrossRef]
- Fleck, J.D.; Kauffmann, C.; Spilki, F.; Lencina, C.L.; Roehe, P.M.; Gosmann, G. Adjuvant activity of Quillaja brasiliensis saponins on the immune responses to bovine herpesvirus type 1 in mice. Vaccine 2006, 24, 7129–7134. [Google Scholar] [CrossRef]
- Qiao, N.; Liu, Q.; Meng, H.; Zhao, D. International Immunopharmacology Haemolytic activity and adjuvant effect of soyasaponins and some of their derivatives on the immune responses to ovalbumin in mice. Int. Immunopharmacol. 2014, 18, 333–339. [Google Scholar] [CrossRef]
- Sun, Y.; Tong, H.; Li, M.; Li, Y.; Guan, S.; Liu, J. Immunological adjuvant effect of Japanese ginseng saponins (JGS) on specific antibody and cellular response to ovalbumin and its haemolytic activities. Vaccine 2008, 26, 5911–5917. [Google Scholar] [CrossRef]
- Singh, R.; Sharma, R.; Mal, G.; Varshney, R. A comparative analysis of saponin-enriched fraction from Silene vulgaris (Moench) Garcke, Sapindus mukorossi (Gaertn) and Chlorophytum borivilianum (Santapau and Fernandes): An in vitro hemolytic and cytotoxicity evaluation. Anim. Biotechnol. 2020, 1–7. [Google Scholar] [CrossRef]
- Hsu, Y.; Kuo, P.; Lin, C. The proliferative inhibition and apoptotic mechanism of Saikosaponin D in human non-small cell lung cancer A549 cells. Life Sci. 2004, 75, 1231–1242. [Google Scholar] [CrossRef]
- Yao, M.; Yang, J.; Cao, L.; Zhang, L.; Qu, S.; Gao, H. Saikosaponin-d inhibits proliferation of DU145 human prostate cancer cells by inducing apoptosis and arresting the cell cycle at G0/G1 phase. Mol. Med. Rep. 2014, 10, 365–372. [Google Scholar] [CrossRef]
- Kim, E.A.; Jang, J.H.; Lee, Y.H.; Sung, E.G.; Song, I.H.; Kim, J.Y.; Kim, S.; Sohn, H.Y.; Lee, T.J. Dioscin induces caspase-independent apoptosis through activation of apoptosis-inducing factor in breast cancer cells. Apoptosis 2014, 19, 1165–1175. [Google Scholar]
- Liu, M.J.; Wang, Z.; Ju, Y.; Zhou, J.B.; Wang, Y.; Wong, R.N. The Mitotic-Arresting and Apoptosis-Inducing Effects of Diosgenyl Saponins on Human Leukemia Cell Lines. Biol. Pharm. Bull. 2004, 27, 1059–1065. [Google Scholar]
- Siu, F.M.; Ma, D.L.; Cheung, Y.W.; Lok, C.N.; Yan, K.; Yang, Z.; Yang, M.; Xu, S.; Ko, B.C.; He, Q.Y.; et al. Proteomic and transcriptomic study on the action of a cytotoxic saponin (Polyphyllin D): Induction of endoplasmic reticulum stress and mitochondria- mediated apoptotic pathways. Proteomics 2008, 8, 3105–3117. [Google Scholar] [CrossRef] [PubMed]
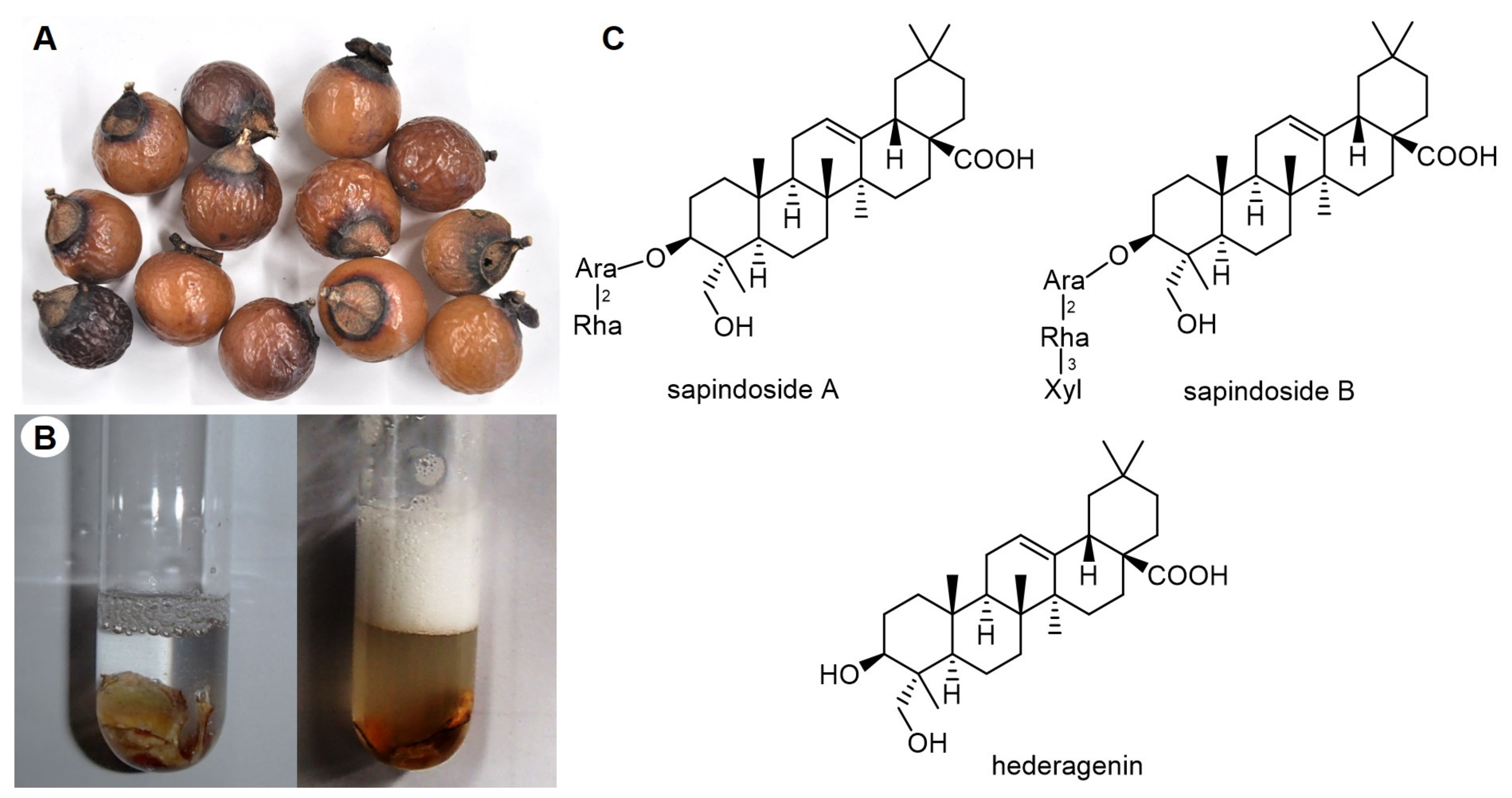
| Scientific Name | Common Name | Saponin Type | Parts Used | References |
|---|---|---|---|---|
| Acacia concinna | Shikakai | Triterpene | Pods and bark | [24,25] |
| Acanthophyllum squarrosum | Triterpene | Roots, grooves, shell and white interior. | [13,14] | |
| Albizia procera | Seto Siris | Triterpene | Leaves | [26] |
| Chlorogalum pomeridia | Soap root | Triterpene | Root | [27,28,29] |
| Quillaja saponaria | Soap bark | Triterpene | Inner bark | |
| Sapindus mukorossi | Soap nut, Indian Soapberry, Ritha, Washnut | Triterpene | Fruit pericarp | |
| Saponaria officinalis | Soapwort | Triterpene | Roots and Leaves | |
| Sapindus saponaria | Soap berry | Triterpene | Seed |
| Scientific Name | Common Name | Parts Used | References |
|---|---|---|---|
| Acorus gramineus | Grass-leaved Sweet Rush, Japanese Sweet Flag, Dwarf Sweet Flag | Leaves | [12] |
| Aesculus assamica | Horse Chestnut | Leaves | [12] |
| Aesculus indica | Kanor, Indian horse chestnut, Barkhor | Fruits | [18] |
| Agave americana | Ran Ban, Kantala, Bara Kunwar, Agave | Leaves | [18] |
| Agave offoyana | Maguey | Flowers and leaves | [30,31] |
| Agave sislana | Sisal | Leaves | [32] |
| Allium nigrum | Ornamental onion | Roots and Leaves | [17] |
| Asparagus adscendens | Saunspali, Sansban | Fruits and roots | [18] |
| Asparagus racemosus | Shatavari | Roots | [18] |
| Balanites aegyptiaca | Heglig | Fruits, seeds and bark | [33] |
| Beaucarnea recurvata | Ponytail palm | Leaves | [17] |
| Bupleurum chinense | Bei Chai Hu | Roots | [17] |
| Camellia chekiangoleosa | Seed | [19] | |
| Camellia japonica | Leaf and stem | [19] | |
| Camellia oleifera | Tea | Seeds | [34] |
| Camellia reticulata | Seeds | [19] | |
| Camellia sinensis | Tea | Root, seeds, leaves and flowers | [18,19] |
| Caryocar villosum | Piquia | Stems | [17] |
| Chiococca alba | West Indian milkberry | Roots | [17] |
| Chlorogalum pomeridianum | Soap plant | Bulbs | [15] |
| Chlorophytum borivilianum | Safed musli | Leaves | [18] |
| Cicer arietinum | Chickpeas | Seeds | [20] |
| Cissus modeccoides | Leaves and stems | [12] | |
| Cissus repen | Stems | [12] | |
| Digitalis lanata | Woolly foxglove | Leaves | [15] |
| Digitalis purpurea | Purple foxglove | Leaves and seeds | [15] |
| Dillenia parviflor | Fruits | [12] | |
| Discorea composite | Yams | Rhizomes and roots | [15] |
| Garcinia | Yellow Mangosteen | Fruits | [12] |
| Garuga pinnata | Garuga | Leaves | [12] |
| Glinus lotoides | Soap Jacob | Roots, leaves and seeds | [15] |
| Glycine max | Soya bean | Sprouts and seeds | [16] |
| Gypsophilla paniculata | Baby’s breath | Roots | [15] |
| Harpullia austrocaledonica | Bark | [17] | |
| Ilex paraguariensis | Mate | Fruits | [21] |
| Lens culinaris | Lentils | Seeds | [20] |
| Lonicera japonica | Honeysuckle | Leaves | [18] |
| Luffa cylindrica | Sponge Gourd | Fruits | [12] |
| Microcos tomentosa | Leaves | [12] | |
| Momordica charantia | Bitter melon | Fruits and stems | [17] |
| Oryza sativa | Asian rice | Peels | [12] |
| Oxalis corniculata | Creeping wood sorrel | Leaves and stems | [12] |
| Phaseolus vulgaris | Haricot bean | Seeds | [20] |
| Phaseolus vulgaris | Kidney beans | Seeds | [20] |
| Pisum sativum | Green pea | Seeds | [20] |
| Sapindus rarak | Fruits | [12] | |
| Sapindus trifoliatus | Pericarp | [22] | |
| Sesamun orientale | Beniseed | Leaves | [12] |
| Silene inflata. | Bigru | Roots | [18] |
| Silphium asteriscus | Starry rosinweed | Leaves and stems | [17] |
| Solanum xanthocarpum | Yellow-berried Nightshade | Fruits and stems | [17] |
| Tribulus terrestris | Puncture vine | Fruits | [17] |
| Trigonella faenum graecum | Fenugreek | Seeds and leaves | [15] |
| Vigna radiata | Mung bean | Seeds | [20] |
| Yucca schidigera | Yucca | Bark | [17] |
| Zephyranthes carinata | Pink rain lily | Bulb | [4] |
| Ziziphus joazeiro | Juá | Bark | [32] |
| Extraction Method | Extraction Principle | Extraction Solvent | Extraction Temperature | Extraction Time | Extraction Procedure | Advantage/Disadvnatages | |
|---|---|---|---|---|---|---|---|
| Conventional Methods | Maceration | Solid-liquid interface extraction based on the solubility and effective diffusion of desired solute into the solvent. | Water or 50–98% aqueous alcohol (methanol and ethanol) | Varies from room temperature to the boiling point of the solvent used | Varies from few hours to days to weeks | Defatted plant material is soaked in the suitable solvent for desirable period of time. It is sometimes assisted by periodic mechanical stirring. | Longer extraction time. High amount of extraction solvent.Low saponin yield |
| Soxhlet Extraction and Reflux Extraction | Extraction by continuous distillation process. | Mostly 50–98% aqueous ethanol. | Heated up to the boiling point of the extracting solvent. | 24–72 h for Soxhlet extraction. 1–4 h for reflux extraction. | Extraction process involves heating a solvent to boiling and then returning the condensed vapors to the flask containing plant material resulting in subsequent dissolution of active components. In case of soxhlet extraction, plant material is separately placed in thimble. | Degradation of thermally labile components. | |
| Green Technologies | Ultrasound assisted extraction | Ultrasound radiation disrupts the cell structure and facilitates release of intracellular contents due to mechanical effects of acoustic cavitation in solvents. | Pure or aqueous solvents of ethanol and methanol | 10–20 min | Plant material dissolved in the suitable solvent is irradiated through ultrasound radiation. | Higher saponin yield. Relatively short extraction time. Minimum extraction solvent. | |
| Microwave assisted extraction | Absorption of microwave radiation by the water molecules in the plant material disrupts the cell structure which facilitates the release of desired component into solvents | Pure or aqueous solvents of ethanol and methanol | 10–20 min | Plant material dissolved in the suitable solvent is irradiated through the microwave radiation (0.3–300 GHz). | Higher saponin yield. Relatively short extraction time. Minimum extraction solvent | ||
| Pressurized solvent extraction | Automated Pressurized solvent extraction. | Water or methanol | Most common operating temperature is 100 °C at 1500 psi | 15–25 min | Extraction solvent is pumped through the sample vessel continuously by applying high pressure. | Higher saponin yield. Relatively short extraction time. Minimum extraction solvent. | |
| Saponin Sources | Parts Used | CMC (g/cc) | Reduced Surface Tension (mN/m)/[Saponin](g/cc) | Temperature (°C) | References |
|---|---|---|---|---|---|
| Acacia concinna | Pods | 7.00 × 10−2 | ≈32.5/1.00 × 10−2 | 20 ± 2 | [45] |
| Pods | NA | 35.6 ± 0.2/1.00 × 10−2 | 25 | [55] | |
| Pericarp | 4.6552 × 10−2 | NA | NA | [46] | |
| Agave sislana | 6.84 × 10−4 | (33.57–45.13)/(1.5–4.5) × 10−4 | 25 | [32] | |
| Albizia procera | Leaves | 7.00 × 10−3 | ≈43.75/1.00 × 10−2 | 20 ± 2 | [45] |
| NA | 46.6 ± 0.2/6.0 × 10–3 | 25 | [55] | ||
| Bellis perennis | Flowers | 7.60 × 10−5 | 36.8/7.60 × 10−5 | 20 | [53] |
| Betula pendula | Leaves | 2.4 × 10−4 | 45.7/2.4 × 10−4 | 20 | [53] |
| Camellia oleifera | Seeds | NA | 50/5 × 10–3 | NA | [3] |
| Equisetum arvense | Haulm | 3.30 × 10−5 | 37.9/3.30 × 10−5 | 20 | [53] |
| Genipa americana | Fruits | 6.50 × 10−4 | 31.39 ± 0.15 | 25 ± 1 | [56] |
| Hedera algeriensis | Leaves | 5.00 × 10−4 | 40 | 20 | [57] |
| Ilex paraguariensis | Fruits | 1.4946 × 10−1 | 52.8 | 20 ± 2 | [21] |
| Juglans regia | Bark | 8.80 × 10−3 | ≈45.00/1.00 × 10−2 | 20 ± 2 | [45] |
| Panax ginseng | Roots | 6.27 × 10−4 | NA | 25 | [32] |
| Quillaja saponaria | 2.84 × 10−4 | NA | 25 | [32] | |
| Tamarindus indica | Fruits | 8.70 × 10−4 | 30.02 ± 0.17 | 25 ± 1 | [56] |
| Sapindus laurifolia | Fruits | 1.70 × 10−2 | 38.00 | NA | [58] |
| Sapindus mukorossi | Pods | 7.50 × 10−3 | ≈35.00/1.00 × 10−2 | 20 ± 2 | [45] |
| 7.50 × 10−3 | 35.30/9.50 × 10−2 | NA | [4] | ||
| Pericarp | 4.50 × 10−3 | ≈39/4.50 × 10−3 | 25 | [59] | |
| Verbascum densiflorum | Flowers | 3.55 × 10−4 | 41.5/3.55 × 10−4 | 20 | [53] |
| Zephyranthes carinata | Bulbs | 6.40 × 10−4 | ≈41.25/1.00 × 10−2 | 20 ± 2 | [45] |
| 6.40 × 10−4 | 40.76/2.05 × 10−2 | NA | [4] | ||
| Ziziphus joazeiro | Barks | 1.064 × 10−3 | (33.94–46.52)/(0.8–5.5) × 10−4 | 25 | [32] |
| Surfactant/Nature | CMC (g/cc) | Surface Tension at CMC (mN/m) | Temperature (°C) | References |
|---|---|---|---|---|
| Cetyl trimethyl ammonium bromide [CTAB]/Cationic | 1.131 × 10−3 | NA | 25 | [59] |
| 3.53 × 10−1 | 33.4 | 25 | [60] | |
| Sodium Lauryl Sulphate/Anionic | 2.004 × 10−3 | 39.2 | 20 | [53] |
| Tween 80/Non-ionic | 4.42 × 10−5 | 44.4 | 20 | [53] |
| Triton X100/Non-ionic | 1.30 × 10−3 | NA | 25 | [59] |
| 1.8763 × 10−1 | 34.6 | 25 | [61] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rai, S.; Acharya-Siwakoti, E.; Kafle, A.; Devkota, H.P.; Bhattarai, A. Plant-Derived Saponins: A Review of Their Surfactant Properties and Applications. Sci 2021, 3, 44. https://doi.org/10.3390/sci3040044
Rai S, Acharya-Siwakoti E, Kafle A, Devkota HP, Bhattarai A. Plant-Derived Saponins: A Review of Their Surfactant Properties and Applications. Sci. 2021; 3(4):44. https://doi.org/10.3390/sci3040044
Chicago/Turabian StyleRai, Summi, Eliza Acharya-Siwakoti, Ananda Kafle, Hari Prasad Devkota, and Ajaya Bhattarai. 2021. "Plant-Derived Saponins: A Review of Their Surfactant Properties and Applications" Sci 3, no. 4: 44. https://doi.org/10.3390/sci3040044
APA StyleRai, S., Acharya-Siwakoti, E., Kafle, A., Devkota, H. P., & Bhattarai, A. (2021). Plant-Derived Saponins: A Review of Their Surfactant Properties and Applications. Sci, 3(4), 44. https://doi.org/10.3390/sci3040044









