Beneficial Effects of Physical Activity in Diabetic Patients
Abstract
1. Introduction
2. Type of Physical Activity in Type 1 Diabetes and Type 2 Diabetes Subjects
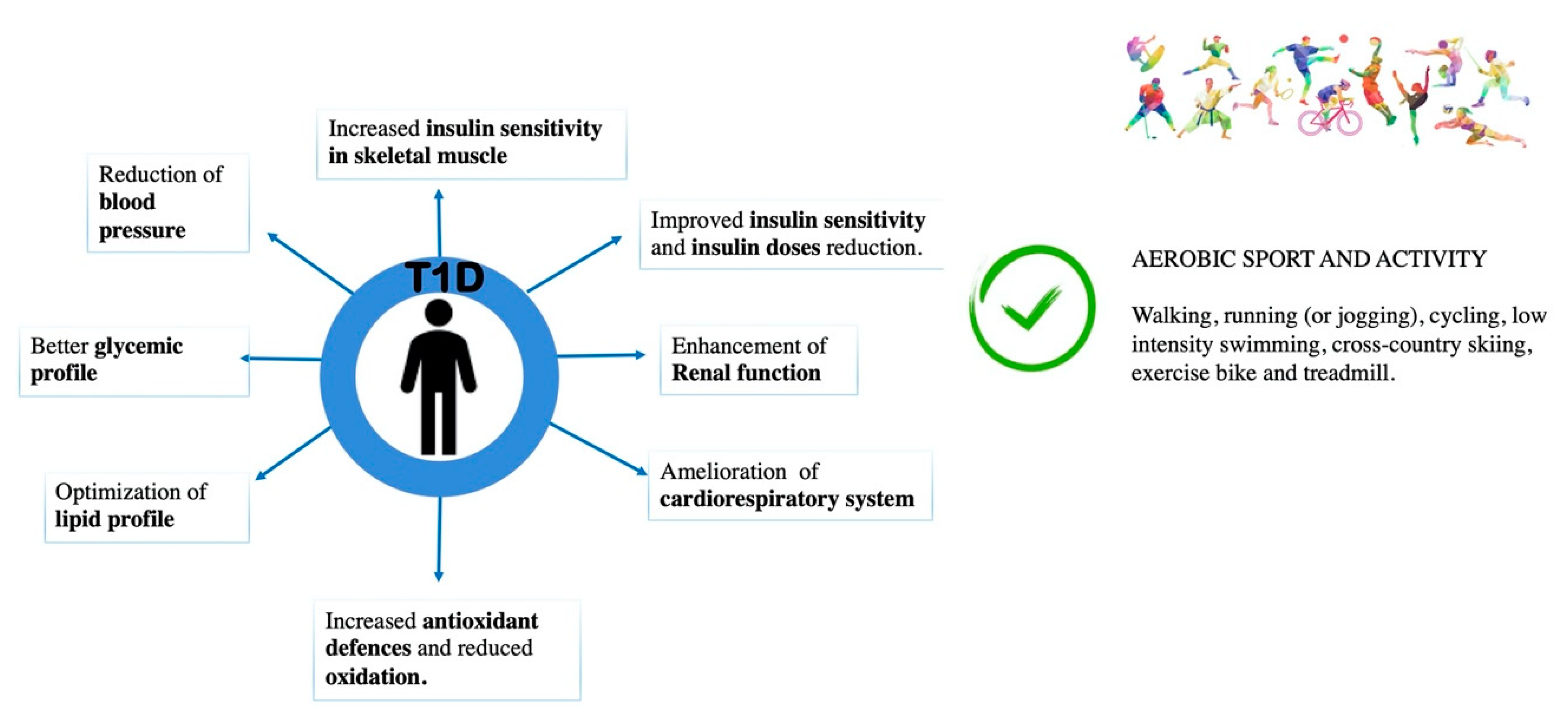
3. Beneficial Effects of Physical Activity in Type 1 Diabetes
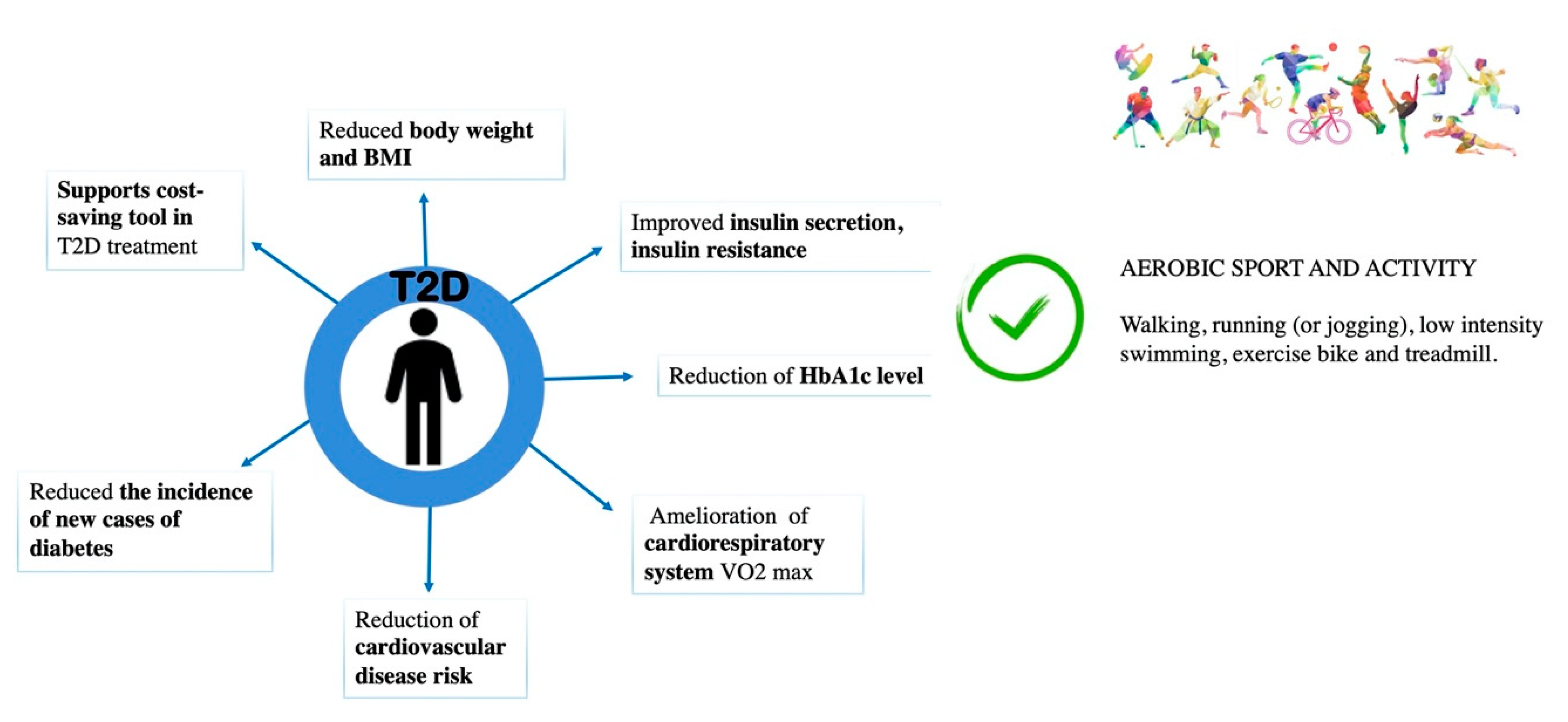
4. Beneficial Effect of Physical Activity in Type 2 Diabetes
5. Discussion
6. Conclusions
Funding
Conflicts of Interest
References
- American Diabetes, A. 2. Classification and Diagnosis of Diabetes: Standards of Medical Care in Diabetes-2018. Diabetes Care 2018, 41, S13–S27. [Google Scholar] [CrossRef] [PubMed]
- Harreiter, J.; Roden, M. Diabetes mellitus-Definition, classification, diagnosis, screening and prevention (Update 2019). Wien. Klin. Wochenschr. 2019, 131, 6–15. [Google Scholar] [CrossRef] [PubMed]
- Petersmann, A.; Muller-Wieland, D.; Muller, U.A.; Landgraf, R.; Nauck, M.; Freckmann, G.; Heinemann, L.; Schleicher, E. Definition, Classification and Diagnosis of Diabetes Mellitus. Exp. Clin. Endocrinol. Diabetes 2019, 127, S1–S7. [Google Scholar] [CrossRef] [PubMed]
- Khursheed, R.; Singh, S.K.; Wadhwa, S.; Kapoor, B.; Gulati, M.; Kumar, R.; Ramanunny, A.K.; Awasthi, A.; Dua, K. Treatment strategies against diabetes: Success so far and challenges ahead. Eur. J. Pharmacol. 2019, 862, 172625. [Google Scholar] [CrossRef] [PubMed]
- Balducci, S.; D’Errico, V.; Haxhi, J.; Sacchetti, M.; Orlando, G.; Cardelli, P.; Vitale, M.; Bollanti, L.; Conti, F.; Zanuso, S.; et al. Effect of a Behavioral Intervention Strategy on Sustained Change in Physical Activity and Sedentary Behavior in Patients With Type 2 Diabetes: The IDES_2 Randomized Clinical Trial. JAMA 2019, 321, 880–890. [Google Scholar] [CrossRef] [PubMed]
- Teich, T.; Zaharieva, D.P.; Riddell, M.C. Advances in Exercise, Physical Activity, and Diabetes Mellitus. Diabetes Technol. Ther. 2019, 21, S112–S122. [Google Scholar] [CrossRef]
- Huys, N.; Van Stappen, V.; Shadid, S.; De Craemer, M.; Androutsos, O.; Lindstrom, J.; Makrilakis, K.; de Sabata, M.S.; Moreno, L.; De Miguel-Etayo, P.; et al. Influence of Educational Level on Psychosocial Correlates and Perceived Environmental Correlates of Physical Activity in Adults at Risk for Type 2 Diabetes: The Feel4Diabetes-Study. J. Phys. Act. Health 2019, 16, 1105–1112. [Google Scholar] [CrossRef]
- Johnson, N.A.; Barwick, A.L.; Searle, A.; Spink, M.J.; Twigg, S.M.; Chuter, V.H. Self-reported physical activity in community-dwelling adults with diabetes and its association with diabetes complications. J. Diabetes Complicat. 2019, 33, 33–38. [Google Scholar] [CrossRef]
- Francesconi, C.; Niebauer, J.; Haber, P.; Weitgasser, R.; Lackinger, C. [Lifestyle: Physical activity and training as prevetion and therapy of type 2 diabetes mellitus (Update 2019)]. Wien. Klin. Wochenschr. 2019, 131, 61–66. [Google Scholar] [CrossRef]
- Cannata, F.; Vadala, G.; Ambrosio, L.; Napoli, N.; Papalia, R.; Denaro, V.; Pozzilli, P. Osteoarthritis and type 2 diabetes: From pathogenetic factors to therapeutic intervention. Diabetes Metab. Res. Rev. 2020, 36, e3254. [Google Scholar] [CrossRef]
- Cannata, F.; Vadala, G.; Ambrosio, L.; Fallucca, S.; Napoli, N.; Papalia, R.; Pozzilli, P.; Denaro, V. Intervertebral disc degeneration: A focus on obesity and type 2 diabetes. Diabetes Metab. Res. Rev. 2020, 36, e3224. [Google Scholar] [CrossRef] [PubMed]
- Napoli, N.; Conte, C.; Pedone, C.; Strotmeyer, E.S.; Barbour, K.E.; Black, D.M.; Samelson, E.J.; Schwartz, A.V. Effect of Insulin Resistance on BMD and Fracture Risk in Older Adults. J. Clin. Endocrinol. Metab. 2019, 104, 3303–3310. [Google Scholar] [CrossRef] [PubMed]
- Russo, F.; Ambrosio, L.; Ngo, K.; Vadala, G.; Denaro, V.; Fan, Y.; Sowa, G.; Kang, J.D.; Vo, N. The Role of Type I Diabetes in Intervertebral Disc Degeneration. Spine (Phila Pa 1976) 2019, 44, 1177–1185. [Google Scholar] [CrossRef] [PubMed]
- Garber, C.E.; Blissmer, B.; Deschenes, M.R.; Franklin, B.A.; Lamonte, M.J.; Lee, I.M.; Nieman, D.C.; Swain, D.P.; American College of Sports Medicine. American College of Sports Medicine position stand. Quantity and quality of exercise for developing and maintaining cardiorespiratory, musculoskeletal, and neuromotor fitness in apparently healthy adults: Guidance for prescribing exercise. Med. Sci. Sports Exerc. 2011, 43, 1334–1359. [Google Scholar] [CrossRef]
- Sluik, D.; Buijsse, B.; Muckelbauer, R.; Kaaks, R.; Teucher, B.; Johnsen, N.F.; Tjonneland, A.; Overvad, K.; Ostergaard, J.N.; Amiano, P.; et al. Physical Activity and Mortality in Individuals With Diabetes Mellitus: A Prospective Study and Meta-analysis. Arch. Intern. Med. 2012, 172, 1285–1295. [Google Scholar] [CrossRef]
- Chimen, M.; Kennedy, A.; Nirantharakumar, K.; Pang, T.T.; Andrews, R.; Narendran, P. What are the health benefits of physical activity in type 1 diabetes mellitus? A literature review. Diabetologia 2012, 55, 542–551. [Google Scholar] [CrossRef]
- Colberg, S.R.; Sigal, R.J.; Yardley, J.E.; Riddell, M.C.; Dunstan, D.W.; Dempsey, P.C.; Horton, E.S.; Castorino, K.; Tate, D.F. Physical Activity/Exercise and Diabetes: A Position Statement of the American Diabetes Association. Diabetes Care 2016, 39, 2065–2079. [Google Scholar] [CrossRef]
- American Diabetes, A. 3. Prevention or Delay of Type 2 Diabetes: Standards of Medical Care in Diabetes-2019. Diabetes Care 2019, 42, S29–S33. [Google Scholar] [CrossRef]
- Levinger, I.; Shaw, C.S.; Stepto, N.K.; Cassar, S.; McAinch, A.J.; Cheetham, C.; Maiorana, A.J. What Doesn’t Kill You Makes You Fitter: A Systematic Review of High-Intensity Interval Exercise for Patients with Cardiovascular and Metabolic Diseases. Clin. Med. Insights Cardiol. 2015, 9, 53–63. [Google Scholar] [CrossRef]
- Ramalho, A.C.; de Lourdes Lima, M.; Nunes, F.; Cambui, Z.; Barbosa, C.; Andrade, A.; Viana, A.; Martins, M.; Abrantes, V.; Aragao, C.; et al. The effect of resistance versus aerobic training on metabolic control in patients with type-1 diabetes mellitus. Diabetes Res. Clin. Pract. 2006, 72, 271–276. [Google Scholar] [CrossRef]
- Gordon, B.A.; Benson, A.C.; Bird, S.R.; Fraser, S.F. Resistance training improves metabolic health in type 2 diabetes: A systematic review. Diabetes Res. Clin. Pract. 2009, 83, 157–175. [Google Scholar] [CrossRef] [PubMed]
- Strasser, B.; Siebert, U.; Schobersberger, W. Resistance training in the treatment of the metabolic syndrome: A systematic review and meta-analysis of the effect of resistance training on metabolic clustering in patients with abnormal glucose metabolism. Sports Med. 2010, 40, 397–415. [Google Scholar] [CrossRef] [PubMed]
- Morrison, S.; Colberg, S.R.; Mariano, M.; Parson, H.K.; Vinik, A.I. Balance training reduces falls risk in older individuals with type 2 diabetes. Diabetes Care 2010, 33, 748–750. [Google Scholar] [CrossRef] [PubMed]
- Abate, M.; Schiavone, C.; Pelotti, P.; Salini, V. Limited joint mobility in diabetes and ageing: Recent advances in pathogenesis and therapy. Int. J. Immunopathol. Pharmacol. 2010, 23, 997–1003. [Google Scholar] [CrossRef] [PubMed]
- Colberg, S.R.; Sigal, R.J.; Fernhall, B.; Regensteiner, J.G.; Blissmer, B.J.; Rubin, R.R.; Chasan-Taber, L.; Albright, A.L.; Braun, B.; American College of Sports Medicine; et al. Exercise and type 2 diabetes: The American College of Sports Medicine and the American Diabetes Association: Joint position statement. Diabetes Care 2010, 33, e147–e167. [Google Scholar] [CrossRef]
- Innes, K.E.; Selfe, T.K. Yoga for Adults with Type 2 Diabetes: A Systematic Review of Controlled Trials. J. Diabetes Res. 2016, 2016, 6979370. [Google Scholar] [CrossRef]
- Ahn, S.; Song, R. Effects of Tai Chi Exercise on glucose control, neuropathy scores, balance, and quality of life in patients with type 2 diabetes and neuropathy. J. Altern. Complement. Med. 2012, 18, 1172–1178. [Google Scholar] [CrossRef]
- Barnett, R. Type 1 diabetes. Lancet 2018, 391, 195. [Google Scholar] [CrossRef]
- Cnop, M.; Welsh, N.; Jonas, J.C.; Jörns, A.; Lenzen, S.; Eizirik, D.L. Mechanisms of pancreatic beta-cell death in type 1 and type 2 diabetes: Many differences, few similarities. Diabetes 2005, 54 (Suppl. 2), S97–S107. [Google Scholar] [CrossRef]
- Riddell, M.C.; Gallen, I.W.; Smart, C.E.; Taplin, C.E.; Adolfsson, P.; Lumb, A.N.; Kowalski, A.; Rabasa-Lhoret, R.; McCrimmon, R.J.; Hume, C.; et al. Exercise management in type 1 diabetes: A consensus statement. Lancet Diabetes Endocrinol. 2017, 5, 377–390. [Google Scholar] [CrossRef]
- Ebeling, P.; Bourey, R.; Koranyi, L.; Tuominen, J.A.; Groop, L.C.; Henriksson, J.; Mueckler, M.; Sovijarvi, A.; Koivisto, V.A. Mechanism of enhanced insulin sensitivity in athletes. Increased blood flow, muscle glucose transport protein (GLUT-4) concentration, and glycogen synthase activity. J. Clin. Investig. 1993, 92, 1623–1631. [Google Scholar] [CrossRef] [PubMed]
- Ebeling, P.; Tuominen, J.A.; Bourey, R.; Koranyi, L.; Koivisto, V.A. Athletes with IDDM exhibit impaired metabolic control and increased lipid utilization with no increase in insulin sensitivity. Diabetes 1995, 44, 471–477. [Google Scholar] [CrossRef] [PubMed]
- Jimenez, C.; Santiago, M.; Sitler, M.; Boden, G.; Homko, C. Insulin-sensitivity response to a single bout of resistive exercise in type 1 diabetes mellitus. J. Sport Rehabil. 2009, 18, 564–571. [Google Scholar] [CrossRef] [PubMed]
- Tikkanen-Dolenc, H.; Waden, J.; Forsblom, C.; Harjutsalo, V.; Thorn, L.M.; Saraheimo, M.; Elonen, N.; Rosengard-Barlund, M.; Gordin, D.; Tikkanen, H.O.; et al. Frequent and intensive physical activity reduces risk of cardiovascular events in type 1 diabetes. Diabetologia 2017, 60, 574–580. [Google Scholar] [CrossRef] [PubMed]
- Austin, A.; Warty, V.; Janosky, J.; Arslanian, S. The relationship of physical fitness to lipid and lipoprotein(a) levels in adolescents with IDDM. Diabetes Care 1993, 16, 421–425. [Google Scholar] [CrossRef] [PubMed]
- Lehmann, R.; Kaplan, V.; Bingisser, R.; Bloch, K.E.; Spinas, G.A. Impact of physical activity on cardiovascular risk factors in IDDM. Diabetes Care 1997, 20, 1603–1611. [Google Scholar] [CrossRef] [PubMed]
- Torres-Tamayo, M.; Perez-Pasten, L.E.; Barron-Uribe, C.; Hermida-Gutierrez, I.; Zamora-Gonzalez, J.; Cardoso-Saldana, G.; Posadas-Romero, C. Improved metabolic control does not change plasma lipoprotein(a) levels in adolescents with type 1 diabetes mellitus. Arch. Med. Res. 1998, 29, 307–312. [Google Scholar]
- Katz, M.L.; Guo, Z.; Laffel, L.M. Management of Hypertension and High Low-Density Lipoprotein in Pediatric Type 1 Diabetes. J. Pediatr. 2018, 197, 140–146.e112. [Google Scholar] [CrossRef]
- Lehmann, R.; Spinas, G.A. Diabetic nephropathy: Significance of microalbuminuria and proteinuria in Type I and Type II diabetes mellitus. Praxis 1995, 84, 1265–1271. [Google Scholar]
- Mosher, P.E.; Nash, M.S.; Perry, A.C.; LaPerriere, A.R.; Goldberg, R.B. Aerobic circuit exercise training: Effect on adolescents with well-controlled insulin-dependent diabetes mellitus. Arch. Phys. Med. Rehabil. 1998, 79, 652–657. [Google Scholar] [CrossRef]
- Miculis, C.P.; Mascarenhas, L.P.; Boguszewski, M.C.; Campos, W. Physical activity in children with type 1 diabetes. J. Pediatr. 2010, 86, 271–278. [Google Scholar] [CrossRef] [PubMed]
- Khawali, C.; Andriolo, A.; Ferreira, S.R. Comparison of methods for urinary albumin determination in patients with type 1 diabetes. Braz. J. Med. Biol. Res. 2002, 35, 337–343. [Google Scholar] [CrossRef]
- O’Donnell, M.J.; Martin, P.; Cavan, D.; Parkes, A.; Chapman, J.; Chapman, C.; Barnett, A.H. Increased urinary transferrin excretion in exercising normoalbuminuric insulin-dependent diabetic patients. Ann. Clin. Biochem. 1991, 28 Pt 5, 456–460. [Google Scholar] [CrossRef]
- Gross, J.L.; de Azevedo, M.J.; Silveiro, S.P.; Canani, L.H.; Caramori, M.L.; Zelmanovitz, T. Diabetic nephropathy: Diagnosis, prevention, and treatment. Diabetes Care 2005, 28, 164–176. [Google Scholar] [CrossRef] [PubMed]
- Jun, M.; Ohkuma, T.; Zoungas, S.; Colagiuri, S.; Mancia, G.; Marre, M.; Matthews, D.; Poulter, N.; Williams, B.; Rodgers, A.; et al. Changes in Albuminuria and the Risk of Major Clinical Outcomes in Diabetes: Results From ADVANCE-ON. Diabetes Care 2018, 41, 163–170. [Google Scholar] [CrossRef] [PubMed]
- Chobanian, A.V.; Bakris, G.L.; Black, H.R.; Cushman, W.C.; Green, L.A.; Izzo, J.L., Jr.; Jones, D.W.; Materson, B.J.; Oparil, S.; Wright, J.T., Jr.; et al. The Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure: The JNC 7 report. JAMA 2003, 289, 2560–2572. [Google Scholar] [CrossRef] [PubMed]
- Harmer, A.R.; Ruell, P.A.; McKenna, M.J.; Chisholm, D.J.; Hunter, S.K.; Thom, J.M.; Morris, N.R.; Flack, J.R. Effects of sprint training on extrarenal potassium regulation with intense exercise in Type 1 diabetes. J. Appl. Physiol. 2006, 100, 26–34. [Google Scholar] [CrossRef][Green Version]
- Woo, J.; Yeo, N.H.; Shin, K.O.; Lee, H.J.; Yoo, J.; Kang, S. Antioxidant enzyme activities and DNA damage in children with type 1 diabetes mellitus after 12 weeks of exercise. Acta Paediatr. 2010, 99, 1263–1268. [Google Scholar] [CrossRef]
- Hoier, B.; Passos, M.; Bangsbo, J.; Hellsten, Y. Intense intermittent exercise provides weak stimulus for vascular endothelial growth factor secretion and capillary growth in skeletal muscle. Exp. Physiol. 2013, 98, 585–597. [Google Scholar] [CrossRef]
- Wei, M.; Gibbons, L.W.; Kampert, J.B.; Nichaman, M.Z.; Blair, S.N. Low cardiorespiratory fitness and physical inactivity as predictors of mortality in men with type 2 diabetes. Ann. Intern. Med. 2000, 132, 605–611. [Google Scholar] [CrossRef]
- Boule, N.G.; Kenny, G.P.; Haddad, E.; Wells, G.A.; Sigal, R.J. Meta-analysis of the effect of structured exercise training on cardiorespiratory fitness in Type 2 diabetes mellitus. Diabetologia 2003, 46, 1071–1081. [Google Scholar] [CrossRef] [PubMed]
- Church, T.S.; Cheng, Y.J.; Earnest, C.P.; Barlow, C.E.; Gibbons, L.W.; Priest, E.L.; Blair, S.N. Exercise capacity and body composition as predictors of mortality among men with diabetes. Diabetes Care 2004, 27, 83–88. [Google Scholar] [CrossRef] [PubMed]
- Kriska, A.M.; LaPorte, R.E.; Pettitt, D.J.; Charles, M.A.; Nelson, R.G.; Kuller, L.H.; Bennett, P.H.; Knowler, W.C. The association of physical activity with obesity, fat distribution and glucose intolerance in Pima Indians. Diabetologia 1993, 36, 863–869. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Rogers, M.A.; Yamamoto, C.; King, D.S.; Hagberg, J.M.; Ehsani, A.A.; Holloszy, J.O. Improvement in glucose tolerance after 1 wk of exercise in patients with mild NIDDM. Diabetes Care 1988, 11, 613–618. [Google Scholar] [CrossRef]
- Prior, S.J.; Blumenthal, J.B.; Katzel, L.I.; Goldberg, A.P.; Ryan, A.S. Increased skeletal muscle capillarization after aerobic exercise training and weight loss improves insulin sensitivity in adults with IGT. Diabetes Care 2014, 37, 1469–1475. [Google Scholar] [CrossRef]
- Prior, S.J.; McKenzie, M.J.; Joseph, L.J.; Ivey, F.M.; Macko, R.F.; Hafer-Macko, C.E.; Ryan, A.S. Reduced skeletal muscle capillarization and glucose intolerance. Microcirculation 2009, 16, 203–212. [Google Scholar] [CrossRef]
- Boule, N.G.; Haddad, E.; Kenny, G.P.; Wells, G.A.; Sigal, R.J. Effects of exercise on glycemic control and body mass in type 2 diabetes mellitus: A meta-analysis of controlled clinical trials. JAMA 2001, 286, 1218–1227. [Google Scholar] [CrossRef]
- Najafipour, F.; Mobasseri, M.; Yavari, A.; Nadrian, H.; Aliasgarzadeh, A.; Mashinchi Abbasi, N.; Niafar, M.; Houshyar Gharamaleki, J.; Sadra, V. Effect of regular exercise training on changes in HbA1c, BMI and VO2max among patients with type 2 diabetes mellitus: An 8-year trial. BMJ Open Diabetes Res. Care 2017, 5, e000414. [Google Scholar] [CrossRef]
- Caspersen, C.J.; Fulton, J.E. Epidemiology of walking and type 2 diabetes. Med. Sci. Sports Exerc. 2008, 40, S519–S528. [Google Scholar] [CrossRef]
- Hu, F.B.; Sigal, R.J.; Rich-Edwards, J.W.; Colditz, G.A.; Solomon, C.G.; Willett, W.C.; Speizer, F.E.; Manson, J.E. Walking compared with vigorous physical activity and risk of type 2 diabetes in women: A prospective study. JAMA 1999, 282, 1433–1439. [Google Scholar] [CrossRef]
- Di Loreto, C.; Fanelli, C.; Lucidi, P.; Murdolo, G.; De Cicco, A.; Parlanti, N.; Ranchelli, A.; Fatone, C.; Taglioni, C.; Santeusanio, F.; et al. Make your diabetic patients walk: Long-term impact of different amounts of physical activity on type 2 diabetes. Diabetes Care 2005, 28, 1295–1302. [Google Scholar] [CrossRef] [PubMed]
- Biddle, S. Physical activity and mental health: Evidence is growing. World Psychiatry 2016, 15, 176–177. [Google Scholar] [CrossRef] [PubMed]
- Colcombe, S.; Kramer, A.F. Fitness effects on the cognitive function of older adults: A meta-analytic study. Psychol. Sci. 2003, 14, 125–130. [Google Scholar] [CrossRef] [PubMed]
| Subjects | Aerobic | Resistance | HIIT |
|---|---|---|---|
| T1D | ↑ lipid metabolism ↓ insulin resistance | ↑ muscular strength ↑ lipid profile ↑ better control of blood glucose levels ↓ dose of insulin | ↑ cardioprotective ↑ metabolic benefits |
| Recommendations | 150 min/week at moderate to vigorous intensity | Engaging in 2–3 non-consecutive days/week | In younger when vigorously performed for 75 min/week. |
| T2D | ↓ blood pressure ↓ triglycerides ↓ insulin resistance ↓ A1C | ↑ blood pressure ↑ muscle mass and strength ↑ insulin responsiveness ↑ metabolic control ↑ lipid profile ↑ cardiovascular disease | ↑ insulin sensitivity ↑ metabolic control |
| Recommendations | 150 min/week at moderate to vigorous intensity | Perform both flexibility and balance activities for 2 or more sessions/week. | In physically fit patients when vigorously performed for 75 min/week. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cannata, F.; Vadalà, G.; Russo, F.; Papalia, R.; Napoli, N.; Pozzilli, P. Beneficial Effects of Physical Activity in Diabetic Patients. J. Funct. Morphol. Kinesiol. 2020, 5, 70. https://doi.org/10.3390/jfmk5030070
Cannata F, Vadalà G, Russo F, Papalia R, Napoli N, Pozzilli P. Beneficial Effects of Physical Activity in Diabetic Patients. Journal of Functional Morphology and Kinesiology. 2020; 5(3):70. https://doi.org/10.3390/jfmk5030070
Chicago/Turabian StyleCannata, Francesca, Gianluca Vadalà, Fabrizio Russo, Rocco Papalia, Nicola Napoli, and Paolo Pozzilli. 2020. "Beneficial Effects of Physical Activity in Diabetic Patients" Journal of Functional Morphology and Kinesiology 5, no. 3: 70. https://doi.org/10.3390/jfmk5030070
APA StyleCannata, F., Vadalà, G., Russo, F., Papalia, R., Napoli, N., & Pozzilli, P. (2020). Beneficial Effects of Physical Activity in Diabetic Patients. Journal of Functional Morphology and Kinesiology, 5(3), 70. https://doi.org/10.3390/jfmk5030070







