Potential Effect of Intravascular Laser Irradiation of Blood (ILIB) in Improving Physical Performance: A Narrative Review
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Search Strategy
3. Results and Discussion
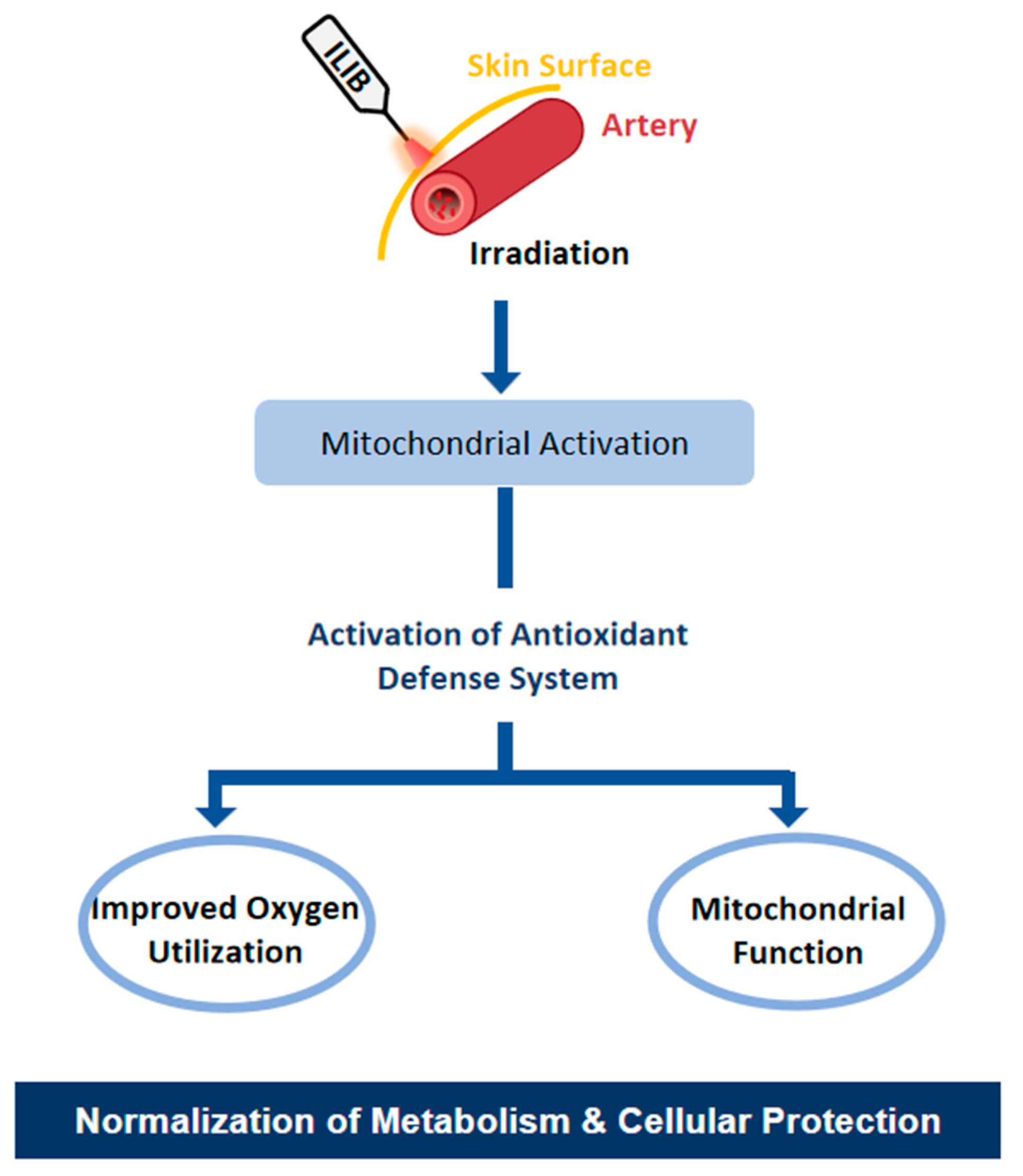
3.1. Activation of the Antioxidant System: Enzymatic and Oxidative Effects of ILIB
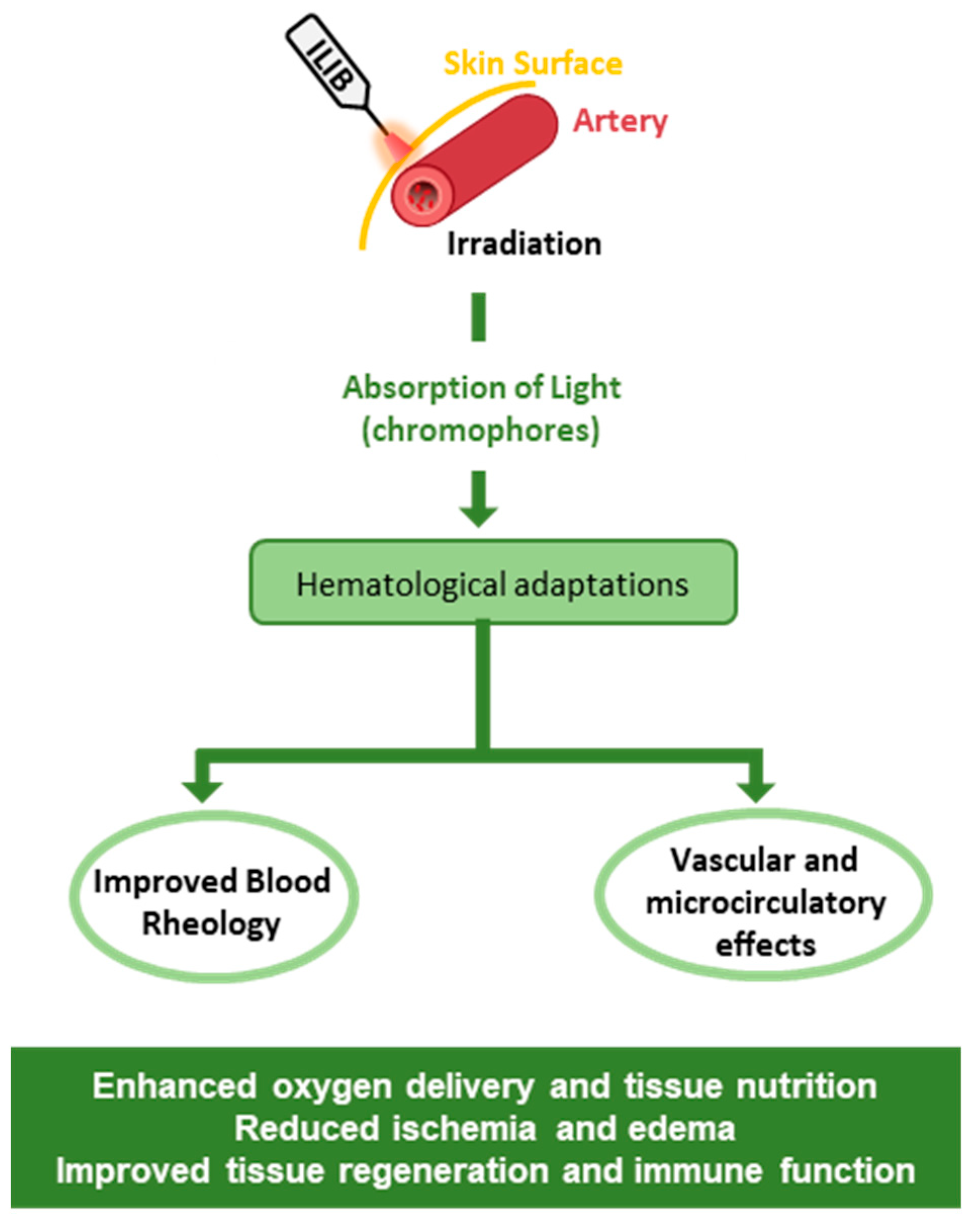
3.2. Effects on Blood Cells and Vascular Structures
3.3. ILIB in Sports and Potential Applications
4. Conclusions and Future Directions
- -
- Studies in Active Athletes: To conduct specific studies in athletes from different sports to assess the effects of ILIB on physically active individuals, including analyses of physical performance, recovery capacity, fatigue resistance, and improvements in muscle function.
- -
- Post-Training Recovery: To investigate how ILIB can accelerate the post-exercise recovery process in athletes, including reducing delayed onset muscle soreness, restoring energy levels, and restoring metabolic balance.
- -
- Impact on Sports Injuries: To exploit the potential of ILIB in the prevention and rehabilitation of sports injuries, including muscle, tendon, and articulation injuries. Longitudinal studies can help us understand whether ILIB therapy can reduce recovery time and promote faster healing.
- -
- Analysis of Physiological Mechanisms: To investigate the physiological mechanisms underlying the effects of ILIB on sports performance, including its impact on muscle metabolism, inflammatory response, and antioxidant system.
- -
- -
- Interaction with Other Interventions: To investigate the synergistic effects of ILIB when combined with other interventions, such as resistance training, sports nutrition, and recovery techniques, aiming to optimize athletic performance.
- -
- Long-Term Studies: To assess the long-term effects of ILIB on sports performance, as well as its long-term safety and tolerability in elite and recreational athletes.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Díaz, L.; Gil, A.C.; Von Marttens, A.; Basualdo, J.; Sotomayor, C.; Becerra, A.V.; Beltrán, V.; Jorquera, G.; Caviedes, R.; Fernández, E. The clinical efficacy of intravascular laser irradiation of blood (ILIB): A narrative review of randomized controlled trial. Photodiagnosis Photodyn. Ther. 2025, 53, 104618. [Google Scholar] [CrossRef]
- Baker, T.L. Laser phototherapy: The future of medicine. Micro- Nanotechnol. Sens. Syst. Appl. VII 2015, 9467, 94670J. [Google Scholar] [CrossRef]
- Abijo, A.; Lee, C.Y.; Huang, C.Y.; Ho, P.C.; Tsai, K.J. The Beneficial Role of Photobiomodulation in Neurodegenerative Diseases. Biomedicines 2023, 11, 1828. [Google Scholar] [CrossRef] [PubMed]
- Lopez, T.C.C.; Malavazzi, T.C.d.S.; Rodrigues, M.F.S.D.; Bach, E.E.; Silva, D.T.; Hi, E.M.B.; França, C.M.; Bussadori, S.K.; Mesquita-Ferrari, R.A.; Fernandes, K.P.S. Histological and biochemical effects of preventive and therapeutic vascular photobiomodulation on rat muscle injury. J. Biophotonics 2022, 15, e202100271. [Google Scholar] [CrossRef] [PubMed]
- Chiari, S. Photobiomodulation and Lasers. In Tooth Movement; Kantarci, A., Will, L., Yen, S., Eds.; S.Karger AG: Basel, Switzerland, 2016; pp. 118–123. [Google Scholar]
- Tomazoni, S.S.; Machado, C.d.S.M.; De Marchi, T.; Casalechi, H.L.; Bjordal, J.M.; de Carvalho, P.d.T.C.; Leal-Junior, E.C.P. Infrared Low-Level Laser Therapy (Photobiomodulation Therapy) before Intense Progressive Running Test of High-Level Soccer Players: Effects on Functional, Muscle Damage, Inflammatory, and Oxidative Stress Markers—A Randomized Controlled Trial. Oxid. Med. Cell. Longev. 2019, 2019, 6239058. [Google Scholar] [CrossRef] [PubMed]
- Hamblin, M.R.; Agrawal, T.; de Sousa, M. Handbook of Low-Level Laser Therapy; Hamblin, M.R., Agrawal, T., de Sousa, M., Eds.; Jenny Stanford Publishing: Singapore, 2016. [Google Scholar]
- Moskvin, S.V. Low-Level Laser Therapy in Russia: History, Science and Practice. J. Lasers Med. Sci. 2017, 8, 56–65. [Google Scholar] [CrossRef]
- Silvério-Lopes, S.; da Mota, M.P.G. Influence of acupuncture on the pain perception threshold of muscles submitted to repetitive strain. Braz. J. Pain 2018, 1, 207–211. [Google Scholar] [CrossRef]
- Moskvin, S.; Askhadulin, E.; Kochetkov, A. Low-Level Laser Therapy in Prevention of the Development of Endothelial Dysfunction and Clinical Experience of Treatment and Rehabilitation of COVID-19 Patients. Rehabil. Res. Pract. 2021, 2021, 6626932. [Google Scholar] [CrossRef]
- Weber, M.H.; Fußgänger-May, T.; Wolf, T. Die intravasale Laserblutbestrahlung–Vorstellung einer neuen Therapiemethode. Dtsch. Z. Akupunkt. 2007, 50, 12–23. [Google Scholar] [CrossRef]
- Lopes-Martins, R.A.B.; Bueno, F.; Ferreira, H.O.d.C.; Faria, L.A.; Sousa, M.M.B.; Lobo, A.B.; Freitas, V.F.d.S.; Lopes-Martins, P.S.L.; Aimbire, F.; Leonardo, P.S. Local and systemic photobiomodulation using a 650 nm LED on skin temperature and hyperalgesia in cellulite: A randomized, placebo-controlled and double-blinded clinical trial. Lasers Med. Sci. 2024, 39, 275. [Google Scholar] [CrossRef]
- Su, Y.C.; Shen, Y.P.; Chang, C.Y.; Pan, K.T.; Huang, S.M.; Chen, L.C. The Effect of Intravascular Laser Irradiation of Blood on Serum Biomarkers and Clinical Outcome in Knee Osteoarthritis Patients: A Double-Blind Randomized Control Trial. Int. J. Mol. Sci. 2024, 25, 13608. [Google Scholar] [CrossRef]
- Fu, C.M.; Wang, N.K.; Cheng, Y.Y.; Chang, S.T. The Adjuvant Therapy of Intravenous Laser Irradiation of Blood (ILIB) on Pain and Sleep Disturbance of Musculoskeletal Disorders. J. Pers. Med. 2022, 12, 1333. [Google Scholar] [CrossRef]
- Tomé, R.F.F.; Silva, D.F.B.; dos Santos, C.A.O.; de Vasconcelos Neves, G.; Rolim, A.K.A.; de Castro Gomes, D.Q. ILIB (intravascular laser irradiation of blood) as an adjuvant therapy in the treatment of patients with chronic systemic diseases—An integrative literature review. Lasers Med. Sci. 2020, 35, 1899–1907. [Google Scholar] [CrossRef]
- Brassolatti, P.; Parizotto, N.A.; Guirro, E.C.d.O.; de Almeida, L.A.; Tim, C.R.; Nishioka, M.A.; de Souza, J.R.; de Andrade, A.L.M. Systemic photobiomodulation: An integrative review of evidence for intravascular laser irradiation of blood and vascular photobiomodulation. Lasers Med. Sci. 2025, 40, 35. [Google Scholar] [CrossRef]
- Derkacz, A.; Protasiewicz, M.; Rola, P.; Podgorska, K.; Szymczyszyn, A.; Gutherc, R.; Poręba, R.; Doroszko, A. Effects of Intravascular Low-Level Laser Therapy During Coronary Intervention on Selected Growth Factors Levels. Photomed. Laser Surg. 2014, 32, 582–587. [Google Scholar] [CrossRef] [PubMed]
- Manteifel, V.; Bakeeva, L.; Karu, T. Ultrastructural changes in chondriome of human lymphocytes after irradiation with He-Ne laser: Appearance of giant mitochondria. J. Photochem. Photobiol. B Biol. 1997, 38, 25–30. [Google Scholar] [CrossRef]
- Huang, S.-F.; Tsai, Y.-A.; Wu, S.-B.; Wei, Y.-H.; Tsai, P.-Y.; Chuang, T.-Y. Effects of Intravascular Laser Irradiation of Blood in Mitochondria Dysfunction and Oxidative Stress in Adults with Chronic Spinal Cord Injury. Photomed. Laser Surg. 2012, 30, 579–586. [Google Scholar] [CrossRef] [PubMed]
- Hamblin, M.R. Mechanisms and Mitochondrial Redox Signaling in Photobiomodulation. Photochem. Photobiol. 2018, 94, 199–212. [Google Scholar] [CrossRef] [PubMed]
- Isabella, A.P.J.; Silva, J.T.C.; da Silva, T.; Rodrigues, M.F.S.D.; Horliana, A.C.R.T.; Motta, L.J.; Bussadori, S.K.; Pavani, C.; Silva, D.d.F.T.d. Effect of irradiation with intravascular laser on the hemodynamic variables of hypertensive patients. Medicine 2019, 98, e15111. [Google Scholar] [CrossRef]
- Green, B.N.; Johnson, C.D.; Adams, A. Writing narrative literature reviews for peer-reviewed journals: Secrets of the trade. J. Chiropr. Med. 2006, 5, 101–117. [Google Scholar] [CrossRef]
- Rother, E.T. Revisão sistemática X revisão narrativa. Acta Paul. Enferm. 2007, 20, v–vi. [Google Scholar] [CrossRef]
- Mikhaylov, V. The use of Intravenous Laser Blood Irradiation (ILBI) at 630-640 nm to prevent vascular diseases and to increase life expectancy. LASER Ther. 2015, 24, 15–26. [Google Scholar] [CrossRef]
- Choi, H.; Choi, M.; Choi, K.; Choi, C. Blockade of vascular endothelial growth factor sensitizes tumor-associated vasculatures to angiolytic therapy with a high-frequency ultrashort pulsed laser. Microvasc. Res. 2011, 82, 141–146. [Google Scholar] [CrossRef]
- Yi Wu, P.; Penn, I.W.; Lin, P.H.; Wang, J.C.; Chuang, E.; Wu, S.H.; Chuang, T.Y. Effects of Intravenous Laser Irradiation of Blood on Pain, Function and Depression of Fibromyalgia Patients. Gen. Med. Open Access 2018, 6, 2. [Google Scholar] [CrossRef]
- Lin, L.T.; Li, C.J.; Chern, C.U.; Lin, P.H.; Lin, P.W.; Chen, Y.C.; Tsai, H.W.; Tsui, K.H. Intravascular Laser Blood Irradiation (ILIB) Enhances Antioxidant Activity and Energy Metabolism in Aging Ovaries. J. Pers. Med. 2024, 14, 551. [Google Scholar] [CrossRef]
- dos Santos Malavazzi, T.C.; Fernandes, K.P.S.; Lopez, T.C.C.; Rodrigues, M.F.S.D.; Horliana, A.C.R.T.; Bussadori, S.K.; Mesquita-Ferrari, R.A. Effects of the invasive and non-invasive systemic photobiomodulation using low-level laser in experimental models: A systematic review. Lasers Med. Sci. 2023, 38, 137. [Google Scholar] [CrossRef]
- Sarycheva, T.G.; Tsybzhitova, E.B.; Popova, O.V.; Aleksandrov, O.V. [Morphometry and electrophoretic mobility of red blood cells from patients with asthma in the intravenous blood laser irradiation]. Klin. Lab. Diagn. 2009, 3, 13–14. Available online: http://www.ncbi.nlm.nih.gov/pubmed/19391239 (accessed on 1 September 2023).
- Iaitskiĭ, N.A.; Ageenko, E.M.; Davydenko, T.E.; Volchkov, V.A.; Churzin, O.A.; Zharskaia, V.D. [Intravascular laser irradiation of blood in complex treatment of obliterating atherosclerosis of the lower extremity vessels in elderly and senile patients]. Vestn. Khirurgii Im. II Grek. 2006, 165, 34–37. Available online: http://www.ncbi.nlm.nih.gov/pubmed/17120419 (accessed on 1 September 2023).
- Yoon, J.; Park, J.H.; Choi, J.W.; Kim, Y.C. Optimal fluence and duration of low-level laser therapy for efficient wound healing in mice. Ann. Dermatol. 2021, 33, 318–323. [Google Scholar] [CrossRef] [PubMed]
- Tomimura, S.; Silva, B.P.A.; Sanches, I.C.; Canal, M.; Consolim-Colombo, F.; Conti, F.F.; De Angelis, K.; Chavantes, M.C. Hemodynamic Effect of Laser Therapy in Spontaneously Hypertensive Rats. Arq. Bras. Cardiol. 2014, 103, 161–164. [Google Scholar] [CrossRef] [PubMed]
- Kulova, L.A.; Burduli, N.M. [The influence of intravenous laser therapy on the endothelial function and the state of microcirculation in the patients presenting with rheumatoid arthritis]. Vopr. Kurortol. Fizioter. Lech. Fiz. Kult. 2014, 9–12. Available online: http://www.ncbi.nlm.nih.gov/pubmed/25087414 (accessed on 1 May 2025).
- Benevento, E.M.; Lisboa, F.S.S.; Kaneko, L.d.O.; Bertolucci, V.; Silva, Á.A.R.; de Oliveira, D.C.; Sardim, A.C.; dos Reis, I.G.M.; Porcari, A.M.; Messias, L.H.D. Transcutaneous intravascular laser irradiation of blood affects plasma metabolites of women. Sci. Rep. 2024, 14, 29839. [Google Scholar] [CrossRef]
- Ferreira, S.L.d.S.; da Cunha, D.A.; de Almeida, A.N.S.; da Cunha, M.D.; Bastos, R.S.d.A.; da Silva, H.J. The use of photobiomodulation for the muscles of head and neck: An integrative review. Audiol.-Commun. Res. 2021, 26, e2552. [Google Scholar] [CrossRef]
- Lawrence, J.; Sorra, K. Photobiomodulation as Medicine: Low-Level Laser Therapy (LLLT) for Acute Tissue Injury or Sport Performance Recovery. J. Funct. Morphol. Kinesiol. 2024, 9, 181. [Google Scholar] [CrossRef] [PubMed]
- Pandita, S.; Zaki, S.; Sharma, S.; Alam, M.F.; Nuhmani, S. Effect of low-level laser therapy on muscle activation and fatigue in university-level overhead athletes: A randomized crossover trial. Sport. Sci. Health 2025, 21, 1–8. [Google Scholar] [CrossRef]
- Ferraresi, C.; Huang, Y.; Hamblin, M.R. Photobiomodulation in human muscle tissue: An advantage in sports performance? J. Biophotonics 2016, 9, 1273–1299. [Google Scholar] [CrossRef] [PubMed]
- dos Reis, F.A.; da Silva, B.A.K.; Laraia, E.M.S.; de Melo, R.M.; Silva, P.H.; Leal-Junior, E.C.P.; de Carvalho, P.d.T.C. Effects of Pre- or Post-Exercise Low-Level Laser Therapy (830 nm) on Skeletal Muscle Fatigue and Biochemical Markers of Recovery in Humans: Double-Blind Placebo-Controlled Trial. Photomed. Laser Surg. 2014, 32, 106–112. [Google Scholar] [CrossRef]
- Aver Vanin, A.; De Marchi, T.; Silva Tomazoni, S.; Tairova, O.; Leão Casalechi, H.; de Tarso Camillo de Carvalho, P.; Bjordal, J.M.; Leal-Junior, E.C. Pre-Exercise Infrared Low-Level Laser Therapy (810 nm) in Skeletal Muscle Performance and Postexercise Recovery in Humans, What Is the Optimal Dose? A Randomized, Double-Blind, Placebo-Controlled Clinical Trial. Photomed. Laser Surg. 2016, 34, 473–482. [Google Scholar] [CrossRef]
- Laakso, E.L.; Ewais, T. A Holistic Perspective on How Photobiomodulation May Influence Fatigue, Pain, and Depression in Inflammatory Bowel Disease: Beyond Molecular Mechanisms. Biomedicines 2023, 11, 1497. [Google Scholar] [CrossRef]
- Raggi, F.; Vallesi, G. Intravenous Laser Blood Irradiation In Sports Medicine. Schmerz Akupunkt. 2007, 3, 129. Available online: https://www.isla-laser.org/wp-content/uploads/Intravenous-Laser-Blood-Irradiation-in-Sports-Medicine.pdf (accessed on 1 September 2023).
- Ferraresi, C.; Parizotto, N.A.; Pires de Sousa, M.V.; Kaippert, B.; Huang, Y.; Koiso, T.; Bagnato, V.S.; Hamblin, M.R. Light-emitting diode therapy in exercise-trained mice increases muscle performance, cytochrome c oxidase activity, ATP and cell proliferation. J. Biophotonics 2015, 8, 740–754, Erratum in J. Biophotonics 2016, 9, 976. [Google Scholar] [CrossRef]
- Hohenauer, E.; Taeymans, J.; Baeyens, J.P.; Clarys, P.; Clijsen, R. The effect of post-exercise cryotherapy on recovery characteristics: A systematic review and meta-analysis. PLoS ONE 2015, 10, e0139028. [Google Scholar] [CrossRef]
- Hohenauer, E.; Costello, J.T.; Deliens, T.; Clarys, P.; Stoop, R.; Clijsen, R. Partial-body cryotherapy (−135°C) and cold-water immersion (10°C) after muscle damage in females. Scand. J. Med. Sci. Sport. 2020, 30, 485–495. [Google Scholar] [CrossRef]
- Chen, R.; Ma, X.; Ma, X.; Cui, C. The effects of hydrotherapy and cryotherapy on recovery from acute post-exercise induced muscle damage—A network meta-analysis. BMC Musculoskelet. Disord. 2024, 25, 749. [Google Scholar] [CrossRef]
- Gaspar-Junior, J.J.; Dellagrana, R.A.; Barbosa, F.S.S.; Anghinoni, A.P.; Taciro, C.; Carregaro, R.L.; Martinez, P.F.; Oliveira-Junior, S.A. Efficacy of Different Cold-Water Immersion Temperatures on Neuromotor Performance in Young Athletes. Life 2022, 12, 683. [Google Scholar] [CrossRef]
- Naderi, A.; Aminian-Far, A.; Gholami, F.; Mousavi, S.H.; Saghari, M.; Howatson, G. Massage enhances recovery following exercise-induced muscle damage in older adults. Scand. J. Med. Sci. Sport. 2021, 31, 623–632. [Google Scholar] [CrossRef]
- Dupuy, O.; Douzi, W.; Theurot, D.; Bosquet, L.; Dugué, B. An evidence-based approach for choosing post-exercise recovery techniques to reduce markers of muscle damage, Soreness, fatigue, and inflammation: A systematic review with meta-analysis. Front. Physiol. 2018, 9, 312968. [Google Scholar] [CrossRef]
- Ferreira, R.M.; Silva, R.; Vigário, P.; Martins, P.N.; Casanova, F.; Fernandes, R.J.; Sampaio, A.R. The Effects of Massage Guns on Performance and Recovery: A Systematic Review. J. Funct. Morphol. Kinesiol. 2023, 8, 138. [Google Scholar] [CrossRef]
- López-Laval, I.; Mielgo-Ayuso, J.; Terrados, N.; Calleja-González, J. Evidence-based post exercise recovery in combat sports: A narrative review. J. Sports Med. Phys. Fit. 2021, 61, 386–400. [Google Scholar] [CrossRef]
- Calleja-González, J.; Mielgo-Ayuso, J.; Ostojic, S.M.; Jones, M.T.; Marques-Jiménez, D.; Caparros, T.; Terrados, N. Evidence-based post-exercise recovery strategies in rugby: A narrative review. Phys. Sportsmed. 2019, 47, 137–147. [Google Scholar] [CrossRef]
- Pastre, C.M.; Bastos, F.d.N.; Netto Júnior, J.; Vanderlei, L.C.M.; Hoshi, R.A. Post-exercise recovery methods: A systematic review. Rev. Bras. Med. Esporte 2009, 15, 138–144. [Google Scholar] [CrossRef]
- Calleja-González, J.; Terrados, N.; Mielgo-Ayuso, J.; Delextrat, A.; Jukic, I.; Vaquera, A.; Torres, L.; Schelling, X.; Stojanovic, M.; Ostojic, S.M. Evidence-based post-exercise recovery strategies in basketball. Phys. Sportsmed. 2016, 44, 74–78. [Google Scholar] [CrossRef] [PubMed]
- Calleja-González, J.; Mielgo-Ayuso, J.; Miguel-Ortega, Á.; Marqués-Jiménez, D.; Del Valle, M.; Ostojic, S.M.; Sampaio, J.; Terrados, N.; Refoyo, I. Post-exercise Recovery Methods Focus on Young Soccer Players: A Systematic Review. Front. Physiol. 2021, 12, 505149. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rotta-Ribas, M.C.B.; Zurutuza, Y.; Castoldi, R.C.; Martinez, P.F.; Oliveira-Junior, S.A.d. Potential Effect of Intravascular Laser Irradiation of Blood (ILIB) in Improving Physical Performance: A Narrative Review. J. Funct. Morphol. Kinesiol. 2025, 10, 466. https://doi.org/10.3390/jfmk10040466
Rotta-Ribas MCB, Zurutuza Y, Castoldi RC, Martinez PF, Oliveira-Junior SAd. Potential Effect of Intravascular Laser Irradiation of Blood (ILIB) in Improving Physical Performance: A Narrative Review. Journal of Functional Morphology and Kinesiology. 2025; 10(4):466. https://doi.org/10.3390/jfmk10040466
Chicago/Turabian StyleRotta-Ribas, Marcia Cristina Bortoleto, Yann Zurutuza, Robson Chacon Castoldi, Paula Felippe Martinez, and Silvio Assis de Oliveira-Junior. 2025. "Potential Effect of Intravascular Laser Irradiation of Blood (ILIB) in Improving Physical Performance: A Narrative Review" Journal of Functional Morphology and Kinesiology 10, no. 4: 466. https://doi.org/10.3390/jfmk10040466
APA StyleRotta-Ribas, M. C. B., Zurutuza, Y., Castoldi, R. C., Martinez, P. F., & Oliveira-Junior, S. A. d. (2025). Potential Effect of Intravascular Laser Irradiation of Blood (ILIB) in Improving Physical Performance: A Narrative Review. Journal of Functional Morphology and Kinesiology, 10(4), 466. https://doi.org/10.3390/jfmk10040466







