Thermodynamics of Manufacturing Processes—The Workpiece and the Machinery
Abstract
:1. Introduction
1.1. Least Dissipation Energy and Minimum Entropy Generation
1.2. Exergy and System Irreversibilities
1.3. Local Equilibrium
2. Product in Formation—The Workpiece
3. Manufacturing Equipment and Process—The Machinery
4. Entropy Content S and Internal Free Energy Dissipation–SdT
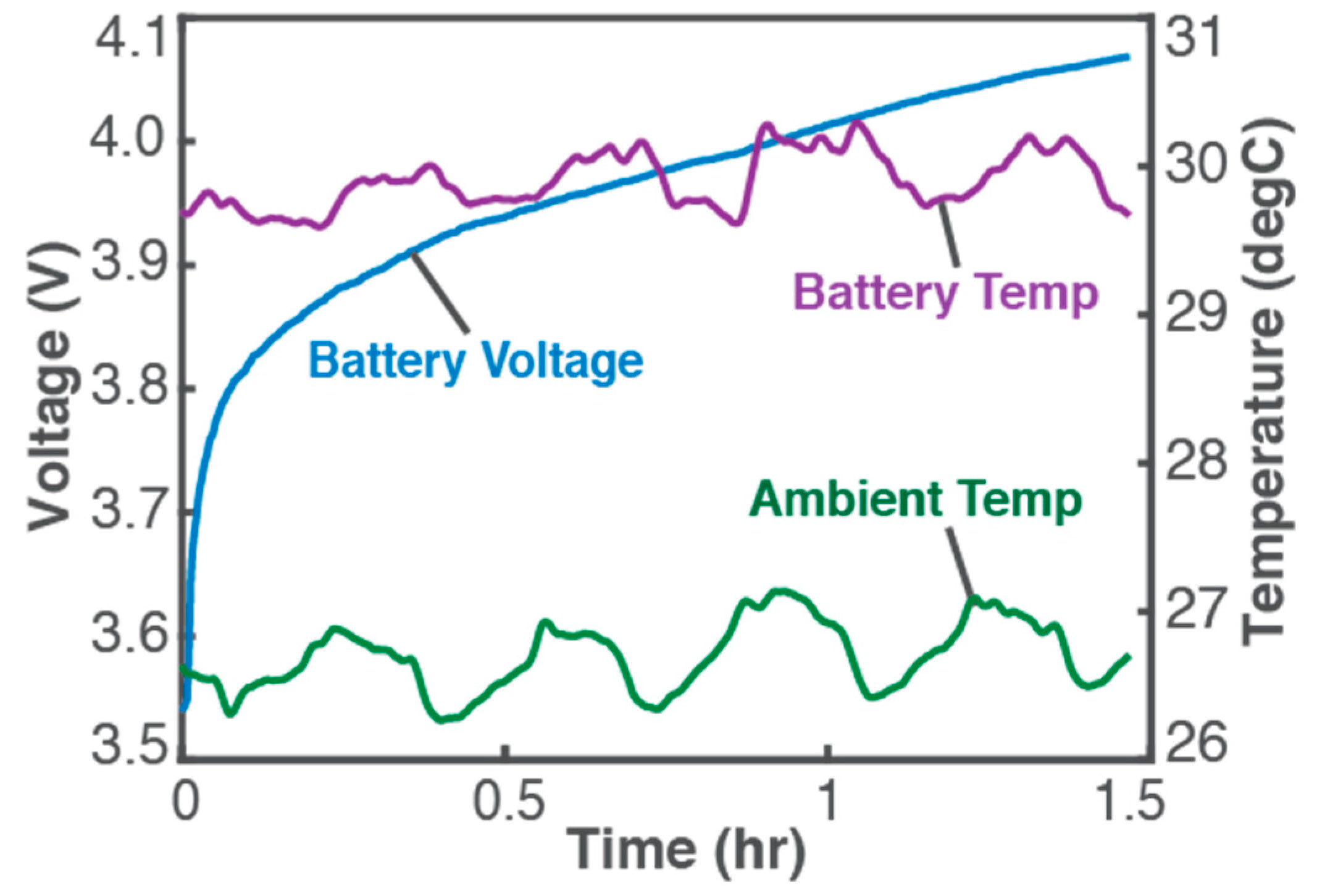
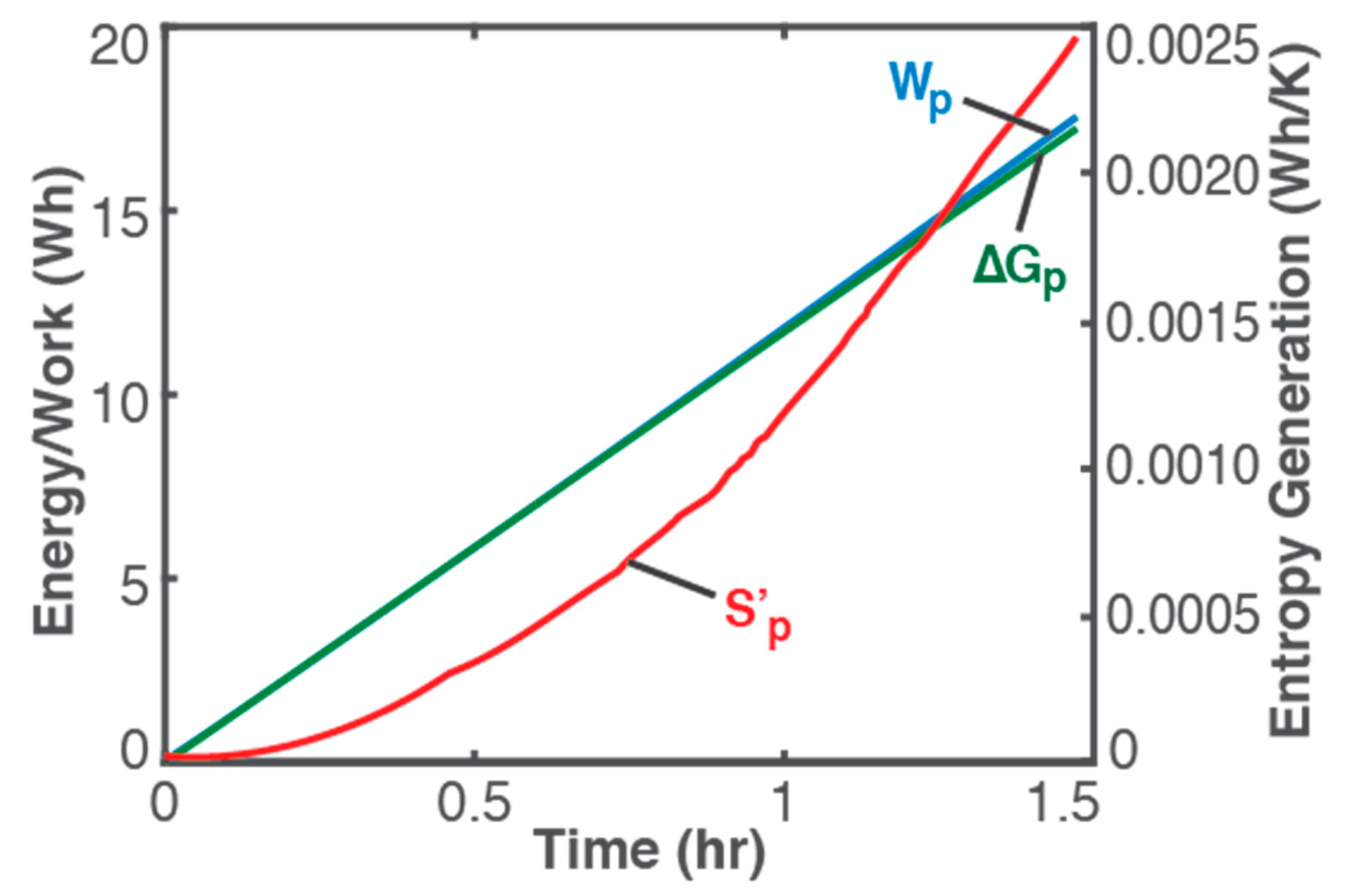
5. Battery Charging and Other Example Processes
- Wp ≥ ∆Gp and S’p ≥ 0 for a product-forming or energy-adding process; and
- as Wp → ∆Gp, S’p → 0 and ∆T → 0, limit of which is the reversible transformation, i.e., Wp = ∆Gp, S’p = 0 and ∆T = 0.
6. Product in Use
7. Entropy: Generation or Change
8. Utility vs. Availability Analysis—Local Equilibrium vs. Thermodynamic Dead State
9. Fluctuations and Instabilities
10. Conclusions
Funding
Conflicts of Interest
Abbreviations
| Nomenclature | Name | Unit |
| A | Helmholtz free energy | J |
| G | Gibbs energy | J, Wh |
| I | discharge/charge current or rate | A |
| Q | heat | J |
| S | entropy or entropy content | J/K, Wh/K |
| S’ | entropy generation or production | J/K Wh/K |
| t | time | sec |
| T | temperature | degC or K |
| U | internal energy | J |
| V | voltage | V |
| volume | m3 | |
| W | work | J |
| Subscripts & acronyms | ||
| 0 | constant or initial reference | |
| p | product, workpiece, component | |
| m/c | machine, machinery | |
| total | total | |
| in | input | |
| out | output | |
| rev | reversible |
References
- Osara, J.A. The Thermodynamics of Degradation. Ph.D. Thesis, The University of Texas at Austin, Austin, TX, USA, May 2017. [Google Scholar] [CrossRef]
- Doelling, K.L.; Ling, F.F.; Bryant, M.D.; Heilman, B.P. An experimental study of the correlation between wear and entropy flow in machinery components. J. Appl. Phys. 2000, 88, 2999–3003. [Google Scholar] [CrossRef]
- Onsager, L. Reciprocal Relations in Irreversible processes 1. Am. Phys. Soc. 1931. [Google Scholar] [CrossRef]
- Kondepudi, D.; Prigogine, I. Modern Thermodynamics: From Heat Engines to Dissipative Structures; John Wiley & Sons Ltd.: Hoboken, NJ, USA, 1998. [Google Scholar]
- Prigogine, I. Introduction to Thermodynamics of Irreversible Processes; Charles C. Thomas Pulisher: Springfield, IL, USA, 1955. [Google Scholar]
- Nicolis, G.; Wiley, J. Self-Organization in Nonequilibrium Systems: From Dissipative Structures to Order through Fluctuations; Wiley: New York, NY, USA, 1977; pp. 339–426. [Google Scholar]
- Glansdorff, P.; Prigogine, I. Thermodynamic Theory of Structure, Stability and Fluctuations; Wiley-Interscience: Hoboken, NJ, USA, 1971. [Google Scholar] [CrossRef]
- Moran, M.J.; Shapiro, H.N. Fundamentals of Engineering Thermodynamics, 5th ed.; Wiley: Hoboken, NJ, USA, 2004. [Google Scholar]
- Bejan, A. Advanced Engineering Thermodynamics, 3rd ed.; John Wiley & Sons Inc.: Hoboken, NJ, USA, 1997. [Google Scholar]
- Burghardt, M.D.; Harbach, J.A. Engineering Thermodynamics, 4th ed.; HarperCollinsCollege Publishers: New York, NY, USA, 1993. [Google Scholar]
- Pal, R. Demystification of the Gouy-Stodola theorem of thermodynamics for closed systems. Int. J. Mech. Eng. Educ. 2017, 45, 142–153. [Google Scholar] [CrossRef]
- Bryant, M.D. Entropy and dissipative processes of friction and wear. FME Trans. 2009, 37, 55–60. [Google Scholar]
- Ling, F.F.; Bryant, M.D.; Doelling, K.L. On irreversible thermodynamics for wear prediction. Wear 2002, 253, 1165–1172. [Google Scholar] [CrossRef]
- Bryant, M.D.; Khonsari, M.M.; Ling, F.F. On the thermodynamics of degradation. Proc. R. Soc. A Math. Phys. Eng. Sci. 2008, 464. [Google Scholar] [CrossRef]
- Callen, H.B. Thermodynamics and an Introduction to Thermostatistics; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 1985. [Google Scholar]
- De Groot, S.R. Thermodynamics of irreversible processes. J. Phys. Chem. 1951, 55, 1577–1578. [Google Scholar] [CrossRef]
- De Groot, S.R.; Mazur, P. Non-Equilibrium Thermodynamics; Dover Publications: New York, NY, USA, 2011. [Google Scholar]
- Osara, J.A.; Bryant, M.D. Thermodynamics of fatigue: degradation-entropy generation methodology for system and process characterization and failure analysis. Entropy 2019. under review. [Google Scholar]
- Osara, J.A.; Bryant, M.D. Thermodynamics of grease degradation. Tribol. Int. 2019. in print. [Google Scholar]
- Osara, J.A.; Bryant, M.D. A thermodynamic model for lithium-ion battery degradation: application of the degradation-entropy generation theorem. Inventions 2019, 4, 23. [Google Scholar] [CrossRef]
- Morrow, J. Cyclic Plastic Strain Energy and Fatigue of Metals. In Internal Friction, Damping, and Cyclic Plasticity; ASTM International: West Conshohocken, PA, USA, 1965. [Google Scholar]
- Bryant, M. Unification of friction and wear. Recent Dev. Wear Prev. Frict. Lubr. 2010, 248, 159–196. [Google Scholar]
- Kalpakjian, S. Manufacturing Processes for Engineering Materials, 2nd ed.; Addision-Wesley: Boston, MA, USA, 1991. [Google Scholar]
- Naderi, M.; Amiri, M.; Khonsari, M.M. On the thermodynamic entropy of fatigue fracture. Proc. R. Soc. A Math. Phys. Eng. Sci. 2010, 466. [Google Scholar] [CrossRef]
- Bejan, A. The method of entropy generation minimization. In Energy and the Environment; Springer: Dordrecht, The Netherlands, 1990; pp. 11–22. [Google Scholar]
- Bejan, A.; Tsatsaronis, G.; Moran, M. Thermal Design and Optimization; Wiley: Hoboken, NJ, USA, 1999. [Google Scholar] [CrossRef]
- EnHeGi. Available online: https://www.enhegi.com (accessed on 3 March 2019).
© 2019 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Osara, J.A. Thermodynamics of Manufacturing Processes—The Workpiece and the Machinery. Inventions 2019, 4, 28. https://doi.org/10.3390/inventions4020028
Osara JA. Thermodynamics of Manufacturing Processes—The Workpiece and the Machinery. Inventions. 2019; 4(2):28. https://doi.org/10.3390/inventions4020028
Chicago/Turabian StyleOsara, Jude A. 2019. "Thermodynamics of Manufacturing Processes—The Workpiece and the Machinery" Inventions 4, no. 2: 28. https://doi.org/10.3390/inventions4020028
APA StyleOsara, J. A. (2019). Thermodynamics of Manufacturing Processes—The Workpiece and the Machinery. Inventions, 4(2), 28. https://doi.org/10.3390/inventions4020028




