RETRACTED: Influences of Cr(VI) on SOD Activity, MDA, and MT Content in the Hepatopancreas and Gill of Portunus trituberculatus
Abstract
1. Introduction
2. Materials and Methods
2.1. Test Materials
2.2. Enrichment and Release Tests
2.3. Sample Preparation and Test of Toxicological Indexes
2.3.1. SOD Activity and MDA Content Test
2.3.2. MT Content Test
2.4. Data Processing and Analysis
3. Results
3.1. Effect of Cr(VI) on SOD Activity Induction in the Hepatopancreas and Gill of P. trituberculatus
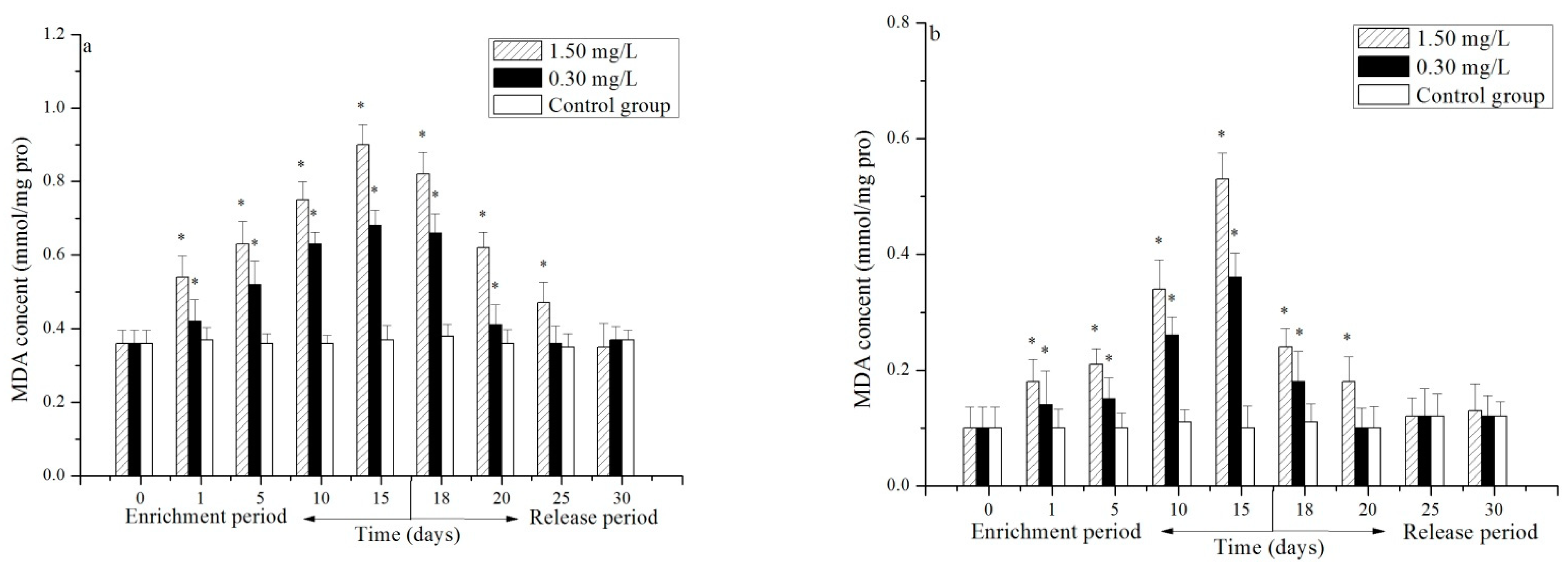
3.2. Effect of Cr(VI) on the MDA Content in the Hepatopancreas and Gill of P. trituberculatus
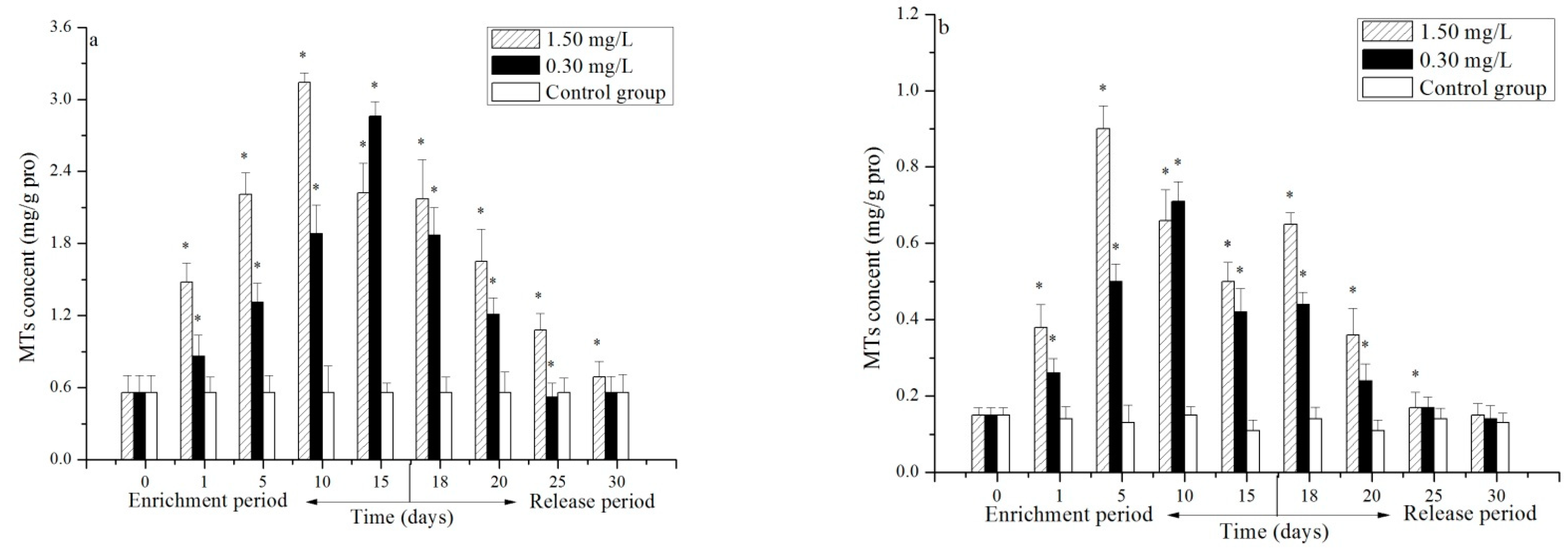
3.3. Effect of Cr(VI) on the MT Content in the Hepatopancreas and Gill of P. trituberculatus
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Costa, M. Potential hazards of hexavalent chromate in our drinking water. Toxicol. Appl. Pharmacol. 2003, 188, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Jeyasingh, J.; Philip, L. Bioremediation of chromium contaminated soil: Optimization of operating parameters under laboratory conditions. J. Hazard. Mater. 2005, 118, 113–120. [Google Scholar] [CrossRef] [PubMed]
- Park, D.; Yun, Y.-S.; Park, J.M. Use of dead fungal biomass for the detoxification of hexavalent chromium: Screening and kinetics. Process Biochem. 2005, 40, 2559–2565. [Google Scholar] [CrossRef]
- Bhattacharya, A.; Mandal, S.; Das, S. Bioaccumulation of chromium and cadmium in commercially edible fishes of gangetic West Bengal. Trends Appl. Sci. Res. 2006, 1, 511–517. [Google Scholar]
- Oshida, P.S.; Word, L.S. Bioaccumulation of chromium and its effects on reproduction in Neanthes arenaceodentata (Polychaeta). Mar. Environ. Res. 1982, 7, 167–174. [Google Scholar] [CrossRef]
- O’Brien, T.J.; Ceryak, S.; Patierno, S.R. Complexities of chromium carcinogenesis: Role of cellular response, repair and recovery mechanisms. Mutat. Res. 2003, 533, 3–36. [Google Scholar] [CrossRef]
- Ding, M.; Shi, X. Molecular mechanisms of Cr (VI)-induced carcinogenesis. Mol. Cell. Biochem. 2002, 234, 293–300. [Google Scholar] [CrossRef]
- Pandey, V.; Dixit, V.; Shyam, R. Antioxidative responses in relation to growth of mustard (Brassica juncea cv. Pusa Jaikisan) plants exposed to hexavalent chromium. Chemosphere 2005, 61, 40–47. [Google Scholar] [CrossRef] [PubMed]
- Ridlington, J.W.; Fowler, B.A. Isolation and partial characterization of a cadmium-binding protein from the American oyster (Crassostrea virginica). Chem.-Biol. Interact. 1979, 25, 127–138. [Google Scholar] [CrossRef]
- Thirumoorthy, N.; Kumar, K.M.; Sundar, A.S.; Panayappan, L.; Chatterjee, M. Metallothionein: An overview. World J. Gastro. 2007, 13, 993. [Google Scholar] [CrossRef]
- Kubrak, O.I.; Lushchak, V.; Lushchak, J.V.; Torous, I.M.; Storey, J.M.; Storey, K.B.; Lushchak, V.I. Chromium effects on free radical processes in goldfish tissues: Comparison of Cr (III) and Cr (VI) exposures on oxidative stress markers, glutathione status and antioxidant enzymes. Comp. Biochem. Physiol. 2010, 152, 360–370. [Google Scholar] [CrossRef]
- Farombi, E.; Adelowo, O.; Ajimoko, Y.R. Biomarkers of oxidative stress and heavy metal levels as indicators of environmental pollution in African cat fish (Clarias gariepinus) from Nigeria Ogun River. Int. J. Environ. Res. Public Health 2007, 4, 158–165. [Google Scholar] [CrossRef] [PubMed]
- Samrana, S.; Ali, A.; Muhammad, U.; Azizullah, A.; Ali, H.; Khan, M.; Naz, S.; Khan, M.D.; Zhu, S.; Chen, J. Physiological, ultrastructural, biochemical, and molecular responses of glandless cotton to hexavalent chromium (Cr6+) exposure. Environ. Pollut. 2020, 266, 115394. [Google Scholar] [CrossRef] [PubMed]
- Sridevi, B.; Reddy, K.V.; Reddy, S.L. Effect of trivalent and hexavalent chromium on antioxidant enzyme activities and lipid peroxidation in a freshwater field crab, Barytelphusa guerini. Bull. Environ. Contam. Toxicol. 1998, 61, 384–390. [Google Scholar] [CrossRef] [PubMed]
- Ahearn, G.A.; Mandal, P.K.; Mandal, A. Mechanisms of heavy-metal sequestration and detoxification in crustaceans: A review. J. Comp. Physiol. B Biochem. Syst. Environ. Physiol. 2004, 174, 439–452. [Google Scholar] [CrossRef]
- Yu, B.; Wang, X.; Dong, K.F.; Xiao, G.; Ma, D. Heavy metal concentrations in aquatic organisms (fishes, shrimp and crabs) and health risk assessment in China. Mar. Pollut. Bull. 2020, 159, 111505. [Google Scholar] [CrossRef]
- Li, K.; Zhou, Z.; Chen, L.; Meng, Y.; Wu, Y. Assessment of various biomarkers in Carassius auratus exposed to benzo [a] pyrene. Res. Environ. Sci. 2006, 19, 91–95. [Google Scholar]
- Ren, J.Y.; Pan, L.Q.; Miao, J. Effects of benzo (a) pyrene and benzo (k) fluoranthene mixture on the toxicology parameter of scallop Chlamys farrerri. Comp. Biochem. Physiol. Toxicol. Pharmacol. CBP 2006, 26, 1180–1186. [Google Scholar]
- Onosaka, S.; Cherian, M.G. Comparison of metallothionein determination by polarographic and cadmium-saturation methods. Toxicol. Appl. Pharmacol. 1982, 63, 270–274. [Google Scholar] [CrossRef]
- Narula, S.S.; Brouwer, M.; Hua, Y.; Armitage, I.M. Three-dimensional solution structure of Callinectes sapidus metallothionein-1 determined by homonuclear and heteronuclear magnetic resonance spectroscopy. Biochemistry 1995, 34, 620–631. [Google Scholar] [CrossRef]
- Pan, L.; Ren, J.; Wu, Z. Effects of heavy metal ions on SOD, CAT activities of hepatopancreas and gill of the crab Eriocheir sinensis. J. Ocean. Univ. China 2004, 34, 189–194. [Google Scholar]
- Isani, G.; Andreani, G.; Kindt, M.; Carpene, E. Metallothioneins (MTs) in marine molluscs. Cell. Mol. Biol. 2000, 46, 311–330. [Google Scholar] [PubMed]
- Xu, X.; Yan, B.; Xu, J.; Pan, N.; Tang, Y.; Du, D.; Zhang, Y. Effects of cadmium stress on activities of antioxidant enzymes, malonaldehyde content and cadmium accumulation in Asian swimming crab Charybdis japonica. Fish. Sci. 2014, 33, 551–555. [Google Scholar]
- Rani, A.U.; Ramamurthi, R. Histopathological alterations in the liver of freshwater teleost Tilapia mossambica in response to cadmium toxicity. Ecotoxicol. Environ. Saf. 1989, 17, 221–226. [Google Scholar] [CrossRef]
- Mouneyrac, C.; Amiard-Triquet, C.; Amiard, J.C.; Rainbow, P.S. Comparison of metallothionein concentrations and tissue distribution of trace metals in crabs (Pachygrapsus marmoratus) from a metal-rich estuary, in and out of the reproductive season. Comparative biochemistry and physiology. Toxicol. Pharmacol. CBP 2001, 129, 193–209. [Google Scholar] [CrossRef]
- Wu, J.-P.; Chen, H.-C. Metallothionein induction and heavy metal accumulation in white shrimp Litopenaeus vannamei exposed to cadmium and zinc. Comp. Biochem. Physiology. Toxicol. Pharmacol. CBP 2005, 140, 383–394. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, L.; Shao, C.; Xv, G.; Lv, L.; Jiang, J.; Zou, W.; Su, W.; Sui, Y.; Jiang, M. RETRACTED: Influences of Cr(VI) on SOD Activity, MDA, and MT Content in the Hepatopancreas and Gill of Portunus trituberculatus. Fishes 2024, 9, 407. https://doi.org/10.3390/fishes9100407
Li L, Shao C, Xv G, Lv L, Jiang J, Zou W, Su W, Sui Y, Jiang M. RETRACTED: Influences of Cr(VI) on SOD Activity, MDA, and MT Content in the Hepatopancreas and Gill of Portunus trituberculatus. Fishes. 2024; 9(10):407. https://doi.org/10.3390/fishes9100407
Chicago/Turabian StyleLi, Lei, Chenshan Shao, Guodong Xv, Linlan Lv, Jiacheng Jiang, Weiyi Zou, Weiwei Su, Yanming Sui, and Mei Jiang. 2024. "RETRACTED: Influences of Cr(VI) on SOD Activity, MDA, and MT Content in the Hepatopancreas and Gill of Portunus trituberculatus" Fishes 9, no. 10: 407. https://doi.org/10.3390/fishes9100407
APA StyleLi, L., Shao, C., Xv, G., Lv, L., Jiang, J., Zou, W., Su, W., Sui, Y., & Jiang, M. (2024). RETRACTED: Influences of Cr(VI) on SOD Activity, MDA, and MT Content in the Hepatopancreas and Gill of Portunus trituberculatus. Fishes, 9(10), 407. https://doi.org/10.3390/fishes9100407






