Using Muscle Element Fingerprint Analysis (EFA) to Trace and Determine the Source of Hypophthalmichthys nobilis in the Yangtze River Basin
Abstract
1. Introduction
2. Materials and Methods
2.1. Sampling Sites, Sample Collection, and Processing
2.2. Elemental Analysis
2.3. Data Analysis
3. Results
3.1. Elemental Fingerprints Composition
3.2. Principal Component Analysis (PCA)
3.3. Discriminant Element Screening
3.4. Traceability and Verification Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhuang, P.; Zhang, T.; Li, S.F.; Ni, Y.; Wang, Y.H.; Deng, S.M.; Zhang, L.Z.; Ling, J.Z.; Hu, F.; Yang, G.; et al. Fishes of the Yangtze Estuary; China Agriculture Press: Beijing, China, 2018; pp. 124–127. [Google Scholar]
- Wang, D.; Wu, F.X.; Song, D.D.; Gao, H.Q. China Fisheries Statistical Yearbook, Compiled by Fishery and Fisheries Administration of the Ministry of Agriculture and Rural Affairs, National Aquatic Technology Promotion Station and China Fisheries Society Compile; China Agriculture Press: Beijing, China, 2023; p. 30. [Google Scholar]
- Farrington, H.L.; Edwards, C.E.; Guan, X.; Carr, M.R.; Baerwaldt, K.; Lance, R.F. Mitochondrial Genome Sequencing and Development of Genetic Markers for the Detection of DNA of Invasive Bighead and Silver Carp (Hypophthalmichthys nobilis and H. molitrix) in Environmental Water Samples from the United States. PLoS ONE 2015, 10, e0117803. [Google Scholar] [CrossRef] [PubMed]
- Kumar, R.; Jaiswar, A.K.; Sharma, R.; Prasad, L. Quantification of morphological variations among populations of Channa gachua (Hamilton, 1822) from different geographical locations in India. Indian J. Fish. 2020, 67, 114–119. [Google Scholar] [CrossRef]
- Milosevic, D.; Bigovic, M.; Mrdak, D.; Milasevic, I.; Piria, M. Otolith morphology and microchemistry fingerprints of European eel, Anguilla anguilla (Linnaeus, 1758) stocks from the Adriatic Basin in Croatia and Montenegro. Sci. Total Environ. 2021, 786, 147478. [Google Scholar] [CrossRef]
- Astorga, M.P.; Valenzuela, A.; Segovia, N.I.; Poulin, E.; Vargas-Chacoff, L.; Gonzalez-Wevar, C.A. Contrasting Patterns of Genetic Diversity and Divergence Between Landlocked and Migratory Populations of Fish, Evaluated Through Mitochondrial DNA Sequencing and Nuclear DNA Microsatellites. Front. Genet. 2022, 13, 854362. [Google Scholar] [CrossRef]
- Biton-Porsmoguer, S.; Bou, R.; Lloret, E.; Alcaide, M.; Lloret, J. Fatty acid composition and parasitism of European sardine (Sardina pilchardus) and anchovy (Engraulis encrasicolus) populations in the northern Catalan Sea in the context of changing environmental conditions. Conserv. Physiol. 2020, 8, coaa121. [Google Scholar] [CrossRef]
- Xuan, Z.Y.; Wang, W.X. Trace elemental and stable isotopic signatures to reconstruct the large-scale environmental connectivity of fish populations. Mar. Ecol. Prog. Ser. 2024, 730, 95–111. [Google Scholar] [CrossRef]
- Bai, S.Y.; Qin, D.L.; Chen, Z.X.; Wu, S.; Tang, S.Z.; Gao, L.; Wang, P. Geographic origin discrimination of red swamp crayfish Procambarus clarkia from different Chinese regions using mineral element analysis assisted by machine learning techniques. Food Control 2022, 138, 109047. [Google Scholar] [CrossRef]
- Ben Ghorbel, M.; Mejri, M.; Adjibayo, H.M.F.; Chalh, A.; Quignard, J.P.; Trabelsi, M.; Bouriga, N. Use of otolith microchemical and morphological analyses for stock discrimination of Sarpa salpa on two Tunisian islands, Djerba and Kerkennah. J. Mar. Biol. Assoc. UK 2024, 104, e33. [Google Scholar] [CrossRef]
- Ricardo, F.; Lopes, M.L.; Mamede, R.; Domingues, M.R.; da Silva, E.F.; Patinha, C.; Calado, R. Combined Use of Fatty Acid Profiles and Elemental Fingerprints to Trace the Geographic Origin of Live Baits for Sports Fishing: The Solitary Tube Worm (Diopatra neapolitana, Annelida, Onuphidae) as a Case Study. Animals 2024, 14, 1361. [Google Scholar] [CrossRef] [PubMed]
- Duarte, B.; Duarte, I.A.; Caçador, I.; Reis-Santos, P.; Vasconcelos, R.P.; Gameiro, C.; Tanner, S.E.; Fonseca, V.F. Elemental fingerprinting of thornback ray (Raja clavata) muscle tissue as a tracer for provenance and food safety assessment. Food Control 2022, 133, 108592. [Google Scholar] [CrossRef]
- Mamede, R.; Duarte, I.A.; Cacador, I.; Tanner, S.E.; Silva, M.; Jacinto, D.; Fonseca, V.F.; Duarte, B. Elemental fingerprinting of sea urchin (Paracentrotus lividus) gonads to assess food safety and trace its geographic origin. J. Food Compos. Anal. 2022, 114, 104764. [Google Scholar] [CrossRef]
- Ricardo, F.; Mamede, R.; Bruzos, A.L.; Díaz, S.; Thébault, J.; da Silva, E.F.; Patinha, C.; Calado, R. Assessing the elemental fingerprints of cockle shells (Cerastoderma edule) to confirm their geographic origin from regional to international spatial scales. Sci. Total Environ. 2022, 814, 152304. [Google Scholar] [CrossRef] [PubMed]
- Lai, J.; Zhao, L.J.; Fan, Y.C.; Qu, X.C.; Liu, D.Y.; Guo, Z.L.; Wang, Y.H.; Liu, Q.; Chen, Y.S. Using whole body elemental fingerprint analysis to distinguish different populations of Coilia nasus in a large river basin. Biochem. System. Ecol. 2015, 60, 249–257. [Google Scholar] [CrossRef]
- Davis, R.P.; Boyd, C.E.; Godumala, R.; Mohan, A.B.C.; Gonzalez, A.; Duy, N.P.; Sasmita, P.G.; Ahyani, N.; Shatova, O.; Wakefield, J.; et al. Assessing the variability and discriminatory power of elemental fingerprints in whiteleg shrimp Litopenaeus vannamei from major shrimp production countries. Food Control 2022, 133, 108589. [Google Scholar] [CrossRef]
- Campana, S.E.; Chouinard, G.A.; Hanson, J.M.; Fréchet, A.; Brattey, J. Otolith elemental fingerprints as biological tracers of fish stocks. Fish. Res. 2000, 46, 343–357. [Google Scholar] [CrossRef]
- Gillanders, B.M.; Kingsford, M.J. Elemental fingerprints of otoliths of fish may distinguish estuarine ‘nursery’ habitats. Mar. Ecol. Prog. Ser. 2000, 201, 273–286. [Google Scholar] [CrossRef]
- D’Avignon, G.; Rose, G.A. Otolith elemental fingerprints distinguish Atlantic cod spawning areas in Newfoundland and Labrador. Fish. Res. 2013, 147, 1–9. [Google Scholar] [CrossRef]
- Avigliano, E.; Domanico, A.; Sánchez, S.; Volpedo, A.V. Otolith elemental fingerprint and scale and otolith morphometry in Prochilodus lineatus provide identification of natal nurseries. Fish. Res. 2017, 186, 1–10. [Google Scholar] [CrossRef]
- Coussau, L.; Robert, D.; Sirois, P. Spatiotemporal variability in otolith elemental fingerprint and the potential to determine deepwater redfish (Sebastes mentella) origins and migrations in the Estuary and Gulf of St. Lawrence, Canada. Fish. Res. 2023, 265, 106739. [Google Scholar] [CrossRef]
- Bassi, L.; Tremblay, R.; Morissette, O.; Sirois, P. Otolith elemental fingerprints reveal source-sink dynamics between two Greenland halibut nurseries in the St. Lawrence Estuary and Gulf. Mar. Ecol. Prog. Ser. 2024, 731, 217–229. [Google Scholar] [CrossRef]
- Khan, S.; Schilling, H.T.; Khan, M.A.; Patel, D.K.; Maslen, B.; Miyan, K. Stock delineation of striped snakehead, Channa striata using multivariate generalised linear models with otolith shape and chemistry data. Sci. Rep. 2021, 11, 8158. [Google Scholar] [CrossRef]
- Mamede, R.; Santos, A.; Díaz, S.; da Silva, E.F.; Patinha, C.; Calado, R.; Ricardo, F. Elemental fingerprints of bivalve shells (Ruditapes decussatus and R. philippinarum) as natural tags to confirm their geographic origin and expose fraudulent trade practices. Food Control 2022, 135, 108785. [Google Scholar] [CrossRef]
- Mamede, R.; Duarte, I.A.; Caçador, I.; Reis-Santos, P.; Vasconcelos, R.P.; Gameiro, C.; Canada, P.; Ré, P.; Tanner, S.E.; Fonseca, V.F.; et al. Elemental Fingerprinting of Wild and Farmed Fish Muscle to Authenticate and Validate Production Method. Foods 2022, 11, 3081. [Google Scholar] [CrossRef]
- Mamede, R.; Santos, A.; da Silva, E.F.; Patinha, C.; Calado, R.; Ricardo, F. New evidence of fraudulent mislabeling and illegal harvesting of Manila clams (philippinarum) through elemental fingerprints of their shells and chemometric analyses. Food Control 2024, 163, 110501. [Google Scholar] [CrossRef]
- Orlowski, G.; Niedzielski, P.; Karg, J.; Proch, J. Colour-assisted variation in elytral ICP-OES-based ionomics in an aposematic beetle. Sci. Rep. 2020, 10, 22262. [Google Scholar] [CrossRef] [PubMed]
- Di Donato, F.; Gornati, G.; Biancolillo, A.; D’Archivio, A.A. ICP-OES analysis coupled with chemometrics for the characterization and the discrimination of high added value Italian Emmer samples. J. Food Compos. Anal. 2021, 98, 103842. [Google Scholar] [CrossRef]
- Lossow, K.; Schlörmann, W.; Tuchtenhagen, M.; Schwarz, M.; Schwerdtle, T.; Kipp, A.P. Measurement of trace elements in murine liver tissue samples: Comparison between ICP-MS/MS and TXRF. J. Trace Elem. Med. Bio. 2023, 78, 127167. [Google Scholar] [CrossRef]
- Güven, G. Testing the equality of treatment means in one-way ANOVA: Short-tailed symmetric error terms with heterogeneous variances. Hacet. J. Math. Stat. 2022, 51, 1736–1751. [Google Scholar] [CrossRef]
- Zhao, H.Y.; Wang, F.; Yang, Q.L. Origin traceability of peanut kernels based on multi-element fingerprinting combined with multivariate data analysis. J. Sci. Food Agr. 2020, 100, 4040–4048. [Google Scholar] [CrossRef]
- Quinn, G.P.; Keough, M.J. Experimental Design and Data Analysis for Biologists; Cambridge University Press: Cambridge, UK, 2002. [Google Scholar]
- Legendre, P.; Legendre, L. Numerical Ecology; Elsevier: Amsterdam, The Netherlands, 2012. [Google Scholar]
- Anderson, C.L.; Whitledge, G.W.; Rude, N.P.; Lamer, J.T. Using Otolith Chemistry to Determine Early Life Environments and Movement of the Emerging Bigheaded Carp Population in Pools 16–19 of the Upper Mississippi River. N. Am. J. Fish. Manag. 2023, 43, 126–140. [Google Scholar] [CrossRef]
- Whitledge, G.W.; Knights, B.; Vallazza, J.; Larson, J.; Weber, M.J.; Lamer, J.T.; Phelps, Q.E.; Norman, J.D. Identification of Bighead Carp and Silver Carp early-life environments and inferring Lock and Dam 19 passage in the Upper Mississippi River: Insights from otolith chemistry. Biol. Invasions 2019, 21, 1007–1020. [Google Scholar] [CrossRef]
- Domínguez-Contreras, J.F.; Jiménez-Rosenberg, S.P.A.; Reguera-Rouzaud, N.; Sánchez-Velasco, L.; Díaz-Viloria, N.; Domínguez-Contreras, J.F.; Jiménez-Rosenberg, S.P.A.; Reguera-Rouzaud, N.; Sánchez-Velasco, L.; Díaz-Viloria, N. Molecular identification and first morphological description of the Colorado snapper Lutjanus colorado (Perciformes: Lutjanidae) larvae. J. Fish Biol. 2023, 102, 1481–1491. [Google Scholar] [CrossRef] [PubMed]
- Jiang, T.; Liu, H.B.; Hu, Y.H.; Chen, X.B.; Yang, J. Revealing Population Connectivity of the Estuarine Tapertail Anchovy Coilia nasus in the Changjiang River Estuary and Its Adjacent Waters Using Otolith Microchemistry. Fishes 2022, 7, 147. [Google Scholar] [CrossRef]
- Xuan, Z.Y.; Jiang, T.; Liu, H.B.; Yang, J. Otolith microchemistry and microsatellite DNA provide evidence for divergence between estuarine tapertail anchovy (Coilia nasus) populations from the Poyang Lake and the Yangtze River Estuary of China. Reg. Stud. Mar. Sci. 2022, 56, 102649. [Google Scholar] [CrossRef]
- Li, Y.; Chen, J.; Feng, G.; Yang, J.; Zhao, F.; Shen, C.; Song, C.; Jiang, T. Otolith Microchemistry Assessment: Evidence of Migratory Coilia nasus of Yangtze River Living in the Shengsi Sea Area. Fishes 2022, 7, 172. [Google Scholar] [CrossRef]
- Lehel, J.; Plachy, M.; Palotás, P.; Bartha, A.; Budai, P. Possible Metal Burden of Potentially Toxic Elements in Rainbow Trout (Oncorhynchus mykiss) on Aquaculture Farm. Fishes 2024, 9, 252. [Google Scholar] [CrossRef]
- Liu, P.C.; Ma, Z.H.; Wei, P.G.; Zhao, Y.X.; Liu, H.L. Progress of Researches on Heavy Metal Pollution Characteristics and Comprehensive Prevention and Control in the Yangtze River Basin. Ecol. Environ. Monitor. Three Gorge 2018, 3, 33–37. [Google Scholar]
- Guo, J.; Wang, K.; Yu, Q.; Duan, X.; Liu, S.; Chen, D. Pollution characteristics of the heavy metals and their potential ecological risk assessment in nearshore sediments of the middle reaches of the Yangtze River. Acta Sci. Circum. 2021, 41, 4625–4636. [Google Scholar]
- Pan, L.; Zhang, R.B.; Pan, Z.X.; Xi, D.G.; Wang, L.Y. Process for Deep Nitrogen and Phosphorus Removal of Tail water from Sewage Treatment Facilities in the Taihu Lake Basin. China Environ. Prot. Ind. 2020, 2, 50–52. [Google Scholar]
- Wu, K.Q.; Meng, Y.H.; Gong, Y.; Wu, L.L.; Liu, W.W.; Ding, X.L. Drinking water elements constituent profiles ansd health risk assessment in Wuxi, China. Environ. Monit. Assess. 2022, 194, 106. [Google Scholar] [CrossRef]
- Liu, P.; Wu, Q.M.; Hu, W.Y.; Tian, K.; Huang, B.; Zhao, Y.C. Comparison of heavy metals in riverine and estuarine sediments in the lower Yangtze River: Distribution, sources, and ecological risks. Environ. Technol. Innov. 2023, 30, 103076. [Google Scholar] [CrossRef]
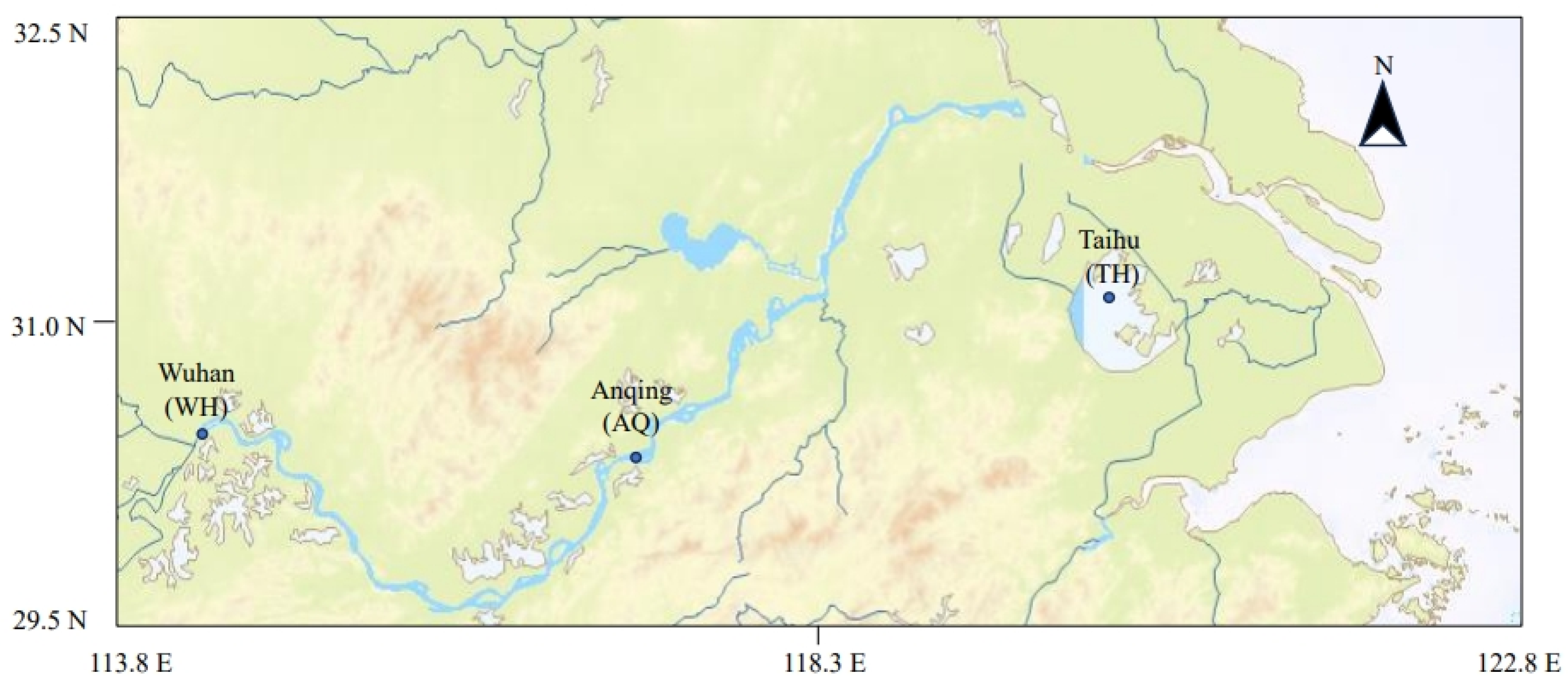
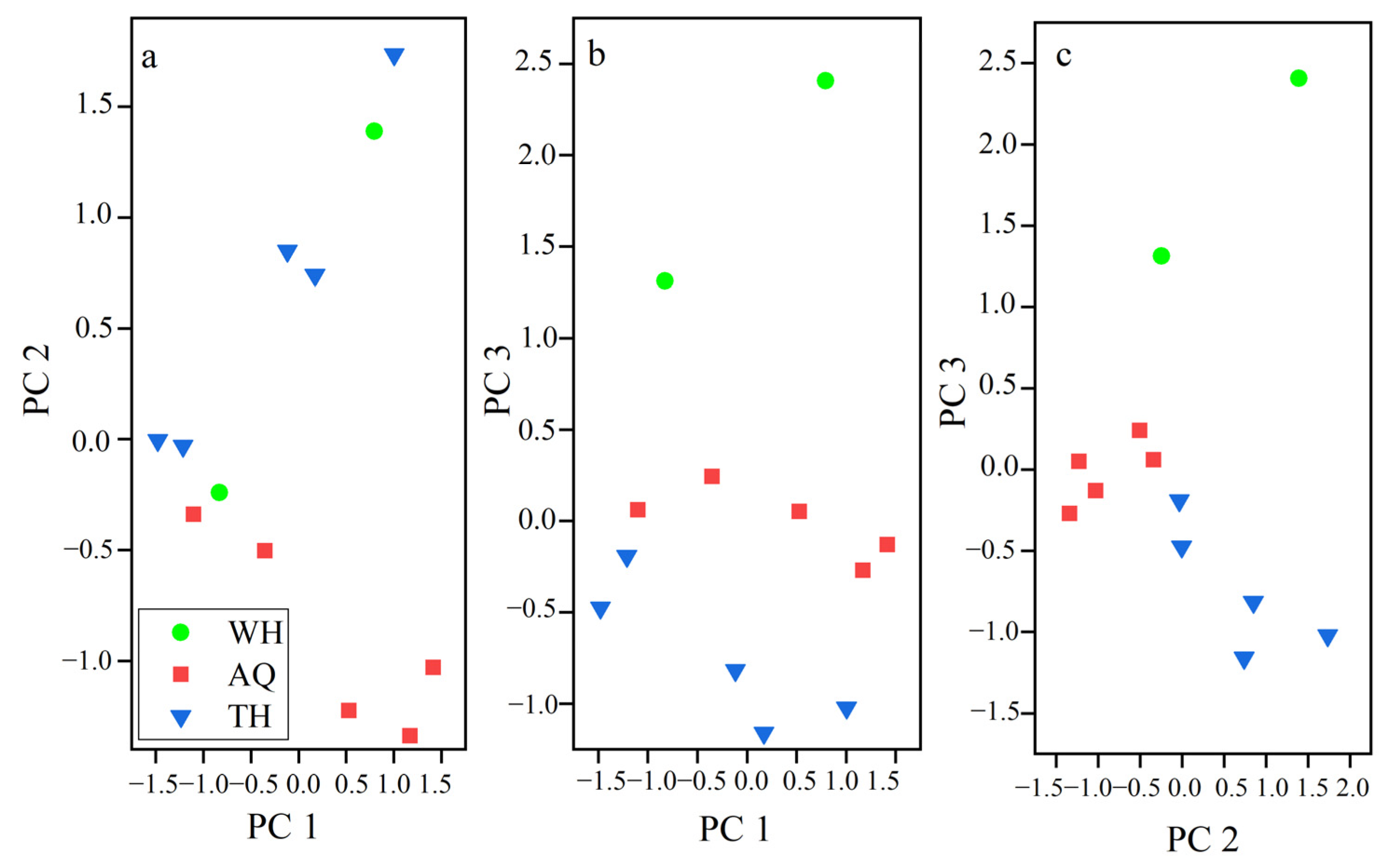
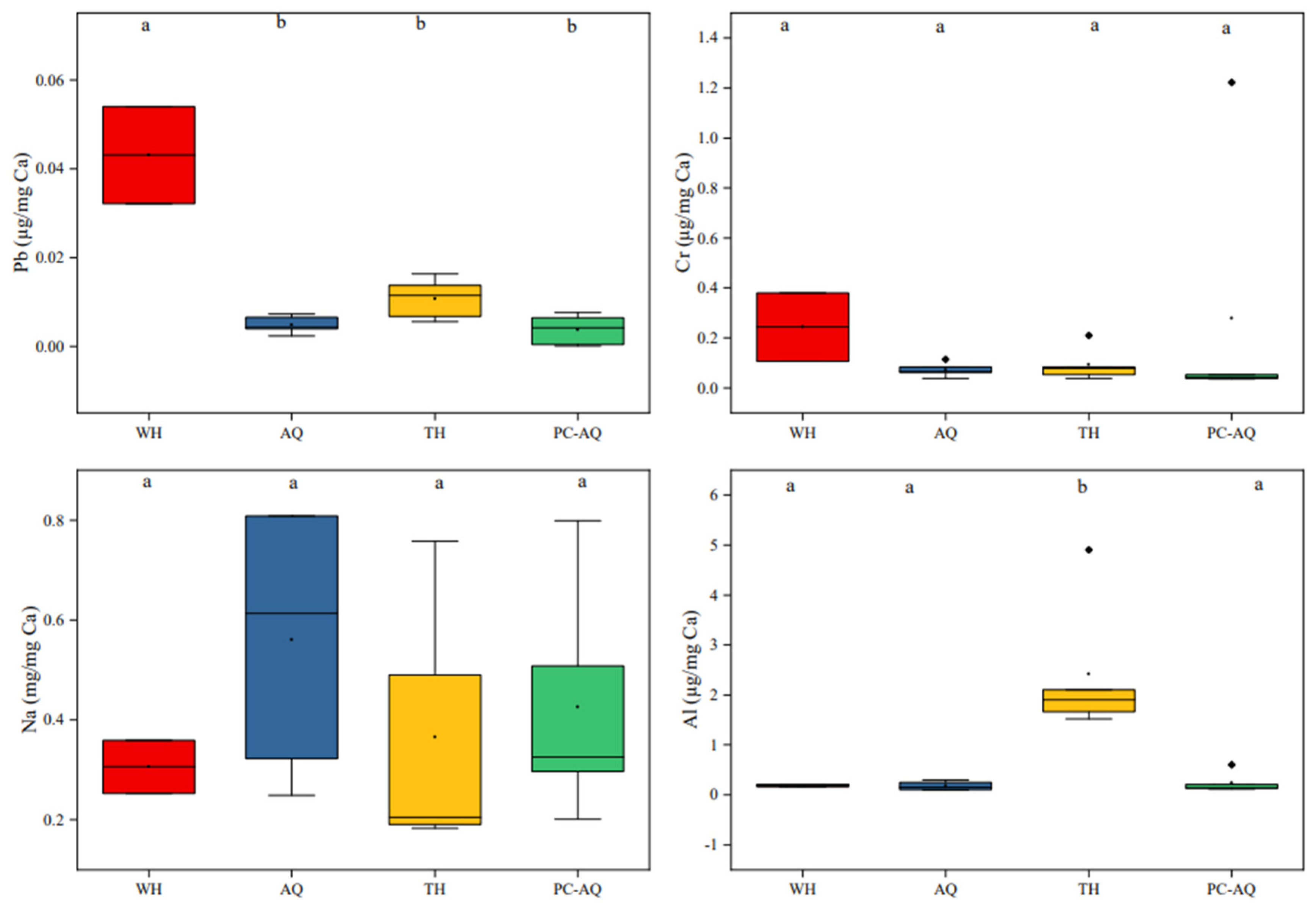
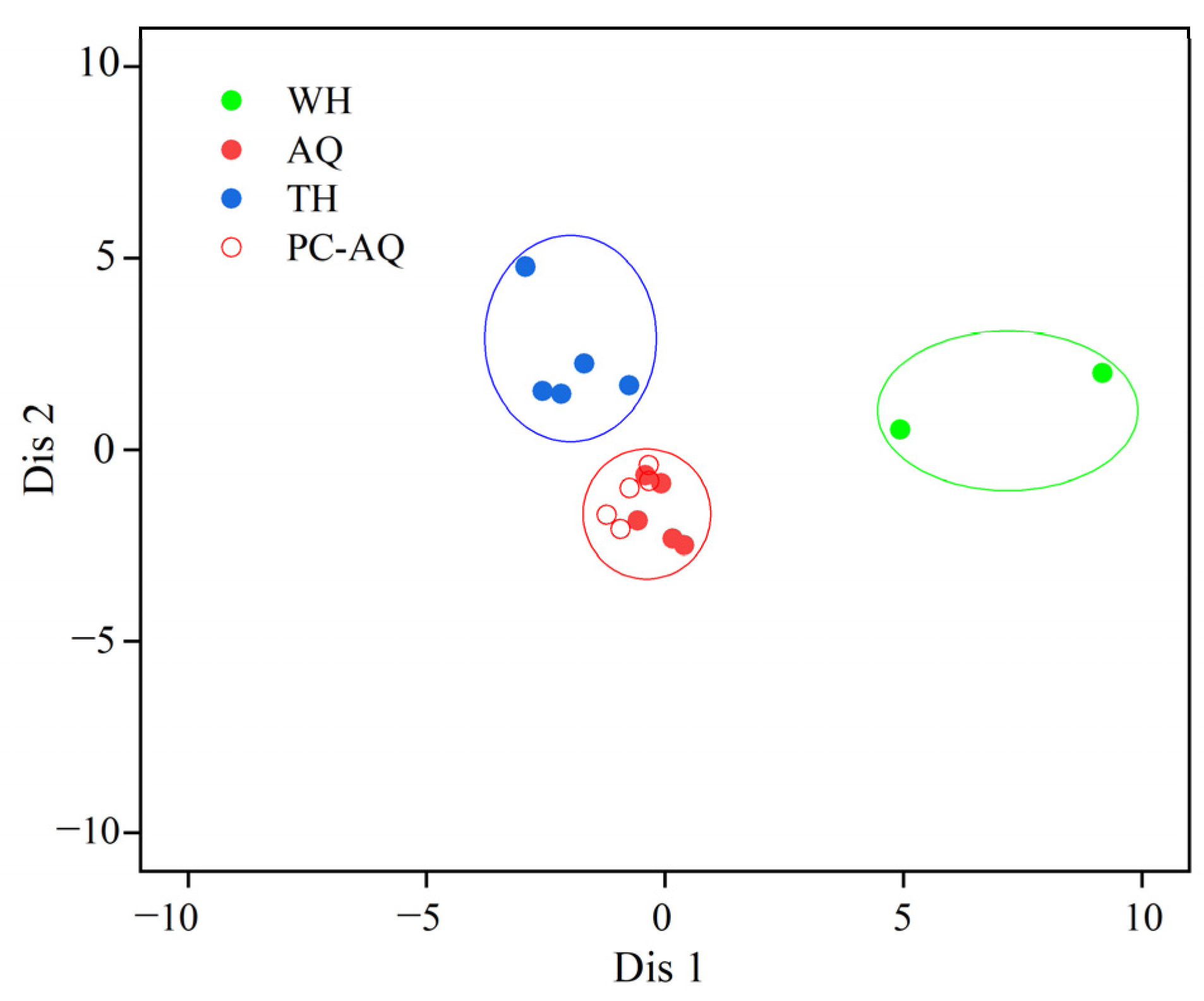
| Groups | Stations | Collection Month | Number | Standard Length (cm) | Wet Weight (g) |
|---|---|---|---|---|---|
| WH | Wuhan | November | 2 | 64.90 ± 4.10 | 4927.0 ± 1105.9 |
| AQ | Anqing | September | 5 | 70.08 ± 2.30 | 5648.0 ± 497.3 |
| TH | Taihu | December | 5 | 65.16 ± 8.20 | 4912.0 ± 1711.1 |
| PC-AQ | Anqing | September | 5 | 59.61 ± 5.53 | 3760.0 ± 1435.6 |
| Index | WH | AQ | TH | 95% Confidence | p |
|---|---|---|---|---|---|
| Hg/Ca | 0.0250 ± 0.0071 a | 0.0860 ± 0.0483 b | 0.0100 ± 0.0071 a | 0.0139~0.0744 | 0.011 |
| Pb/Ca | 0.0400 ± 0.0141 a | 0.0040 ± 0.0055 b | 0.0120 ± 0.0045 b | 0.0041~0.0232 | 0.019 |
| Cr/Ca | 0.2450 ± 0.1909 a | 0.0720 ± 0.0303 a | 0.0920 ± 0.0683 a | 0.0474~0.1710 | 0.218 |
| Cd/Ca | 0.7000 ± 0.7495 a | 0.3380 ± 0.1853 a | 0.9380 ± 0.6492 a | 0.3008~0.9959 | 0.227 |
| Cu/Ca | 0.4600 ± 0.3677 a | 0.2420 ± 0.0746 a | 0.3920 ± 0.1417 a | 0.2311~0.4506 | 0.300 |
| Zn/Ca | 6.7850 ± 0.2758 a | 9.1340 ± 3.0404 a | 6.5100 ± 2.8400 a | 5.8488~9.4495 | 0.304 |
| Ni/Ca | 0.0350 ± 0.0071 a | 0.0280 ± 0.0045 a | 0.0400 ± 0.0187 a | 0.0258~0.0425 | 0.289 |
| As/Ca | 0.200 ± 0.1697 a | 0.0940 ± 0.0241 a | 0.1360 ± 0.0602 a | 0.0814~0.1769 | 0.516 |
| Na/Ca | 0.3050 ± 0.0778 a | 0.5600 ± 0.2651 a | 0.3660 ± 0.2552 a | 0.2783~0.5950 | 0.252 |
| Mg/Ca | 0.2300 ± 0.0424 a | 0.3480 ± 0.1596 a | 0.3040 ± 0.1974 a | 0.2087~0.4113 | 0.755 |
| K/Ca | 3.3750 ± 1.4354 a | 4.3580 ± 2.2346 a | 2.8900 ± 1.6509 a | 2.3950~4.7700 | 0.421 |
| Fe/Ca | 0.0150 ± 0.0071 a | 0.0140 ± 0.0055 a | 0.0160 ± 0.0055 a | 0.0117~0.0183 | 0.832 |
| Al/Ca | 0.1850 ± 0.0354 a | 0.1800 ± 0.0925 a | 2.4180 ± 1.4107 b | 0.2029~0.0238 | 0.017 |
| Mn/Ca | 1.1750 ± 0.0495 ab | 2.8080 ± 1.418 a | 0.5260 ± 0.1673 b | 0.6951~2.4749 | 0.014 |
| Mo/Ca | 0.1250 ± 0.0495 a | 0.1100 ± 0.0557 a | 0.0420 ± 0.0192 a | 0.0500~0.1184 | 0.056 |
| Sr/Ca | 3.4550 ± 1.9445 a | 3.9280 ± 1.5340 a | 2.2260 ± 0.4769 a | 2.2500~4.0300 | 0.167 |
| Ba/Ca | 0.4300 ± 0.2687 a | 1.2600 ± 0.6401 a | 1.1360 ± 0.6241 a | 0.6732~1.4668 | 0.224 |
| Ti/Ca | 5.3250 ± 0.3606 a | 5.2300 ± 0.1037 a | 4.5520 ± 1.0059 a | 4.5066~5.4200 | 0.384 |
| V/Ca | 0.0150 ± 0.0212 a | 0.0120 ± 0.0045 a | 0.0220 ± 0.0130 a | 0.0093~0.0240 | 0.421 |
| Variable | Principal Component | ||||
|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | |
| Hg/Ca | 0.679 | −0.703 | 0.037 | −0.020 | −0.036 |
| Pb/Ca | −0.243 | 0.452 | 0.595 | 0.245 | 0.453 |
| Cr/Ca | 0.501 | 0.601 | 0.598 | 0.063 | −0.130 |
| Cd/Ca | 0.296 | 0.895 | −0.183 | −0.034 | 0.103 |
| Cu/Ca | 0.534 | 0.753 | 0.196 | −0.099 | −0.254 |
| Zn/Ca | 0.876 | −0.233 | −0.120 | 0.083 | 0.205 |
| Ni/Ca | 0.385 | 0.775 | −0.244 | 0.252 | 0.242 |
| As/Ca | 0.522 | 0.739 | 0.360 | −0.098 | −0.168 |
| Na/Ca | 0.826 | −0.227 | −0.209 | 0.399 | −0.053 |
| Mg/Ca | 0.824 | 0.006 | −0.296 | 0.468 | −0.052 |
| K/Ca | 0.921 | −0.152 | 0.004 | 0.260 | −0.105 |
| Fe/Ca | 0.773 | 0.409 | −0.173 | −0.337 | 0.008 |
| Al/Ca | 0.094 | 0.696 | −0.600 | 0.332 | −0.039 |
| Mn/Ca | 0.657 | −0.729 | 0.073 | −0.001 | 0.012 |
| Mo/Ca | 0.779 | −0.294 | 0.500 | 0.069 | −0.070 |
| Sr/Ca | 0.747 | −0.360 | 0.301 | −0.292 | −0.133 |
| Ba/Ca | 0.504 | −0.321 | −0.568 | −0.471 | 0.153 |
| Ti/Ca | 0.533 | −0.028 | 0.151 | −0.201 | 0.767 |
| V/Ca | 0.380 | 0.611 | −0.191 | −0.605 | −0.102 |
| Characteristic Value | 7.426 | 5.581 | 2.237 | 1.543 | 1.091 |
| Contribution Rate | 39.085 | 29.371 | 11.773 | 8.122 | 5.740 |
| Cumulative Contribution | 39.085 | 68.456 | 80.229 | 88.352 | 94.092 |
| Discriminative Elements | WH | AQ | TH |
|---|---|---|---|
| Pb/Ca | 19,468.862 | 5120.551 | 780.090 |
| Cr/Ca | 1369.079 | 348.931 | 47.029 |
| Na/Ca | 293.634 | 91.816 | 10.597 |
| Al/Ca | −174.431 | −47.715 | −3.683 |
| Constant | −586.832 | −45.315 | −5.429 |
| Method | Groups | Prediction Category | Discriminant Accuracy (%) | Comprehensive Discrimination Rate (%) | ||
|---|---|---|---|---|---|---|
| WH | AQ + PC-AQ | TH | ||||
| Stepwise Discrimination | WH | 2 | 0 | 0 | 100.00 | 100.00 |
| AQ + PC-AQ | 0 | 10 | 0 | 100.00 | ||
| TH | 0 | 0 | 5 | 100.00 | ||
| Cross Verification | WH | 2 | 0 | 0 | 100.00 | 100.00 |
| AQ + PC-AQ | 0 | 10 | 0 | 100.00 | ||
| TH | 0 | 0 | 5 | 100.00 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Song, C.; Yang, C.; Zhao, F.; Xie, J.; Tao, H.; Huang, X.; Zhuang, P. Using Muscle Element Fingerprint Analysis (EFA) to Trace and Determine the Source of Hypophthalmichthys nobilis in the Yangtze River Basin. Fishes 2024, 9, 316. https://doi.org/10.3390/fishes9080316
Song C, Yang C, Zhao F, Xie J, Tao H, Huang X, Zhuang P. Using Muscle Element Fingerprint Analysis (EFA) to Trace and Determine the Source of Hypophthalmichthys nobilis in the Yangtze River Basin. Fishes. 2024; 9(8):316. https://doi.org/10.3390/fishes9080316
Chicago/Turabian StyleSong, Chao, Chengyao Yang, Feng Zhao, Jilin Xie, Hong Tao, Xiaorong Huang, and Ping Zhuang. 2024. "Using Muscle Element Fingerprint Analysis (EFA) to Trace and Determine the Source of Hypophthalmichthys nobilis in the Yangtze River Basin" Fishes 9, no. 8: 316. https://doi.org/10.3390/fishes9080316
APA StyleSong, C., Yang, C., Zhao, F., Xie, J., Tao, H., Huang, X., & Zhuang, P. (2024). Using Muscle Element Fingerprint Analysis (EFA) to Trace and Determine the Source of Hypophthalmichthys nobilis in the Yangtze River Basin. Fishes, 9(8), 316. https://doi.org/10.3390/fishes9080316







