Abstract
The blood clam (Anadara granosa) is an economic bivalve that is relatively tolerant to hypoxia, but its molecular mechanism of hypoxia tolerance is unclear. We found that a significant decrease in extracellular Ca2+ concentration and a marked increase in intracellular Ca2+ concentration was observed in the blood clam through the fluorescence probe method, under hypoxic conditions at 0.5 mg/L. Concomitantly, there was a downward trend in the expression level of CaV2 mRNA, whereas NFAT (nuclear factor of activated T cells) expression increased by qRT-PCR. These findings suggest that the elevated intracellular Ca2+ concentration may activate negative transcription factors of NFAT, which subsequently suppresses the transcription of CaV2, leading to its decreased expression. Then, the NFAT RNA interference experiments supported this hypothesis. Sequence analysis and 3D structure prediction revealed conserved and mutated residue sites in blood clam compared to other bivalves. Hypoxia-induced changes in intracellular and extracellular Ca2+ concentrations, activating transcription factor NFAT and suppressing CaV2 expression. This study highlights the key roles of CaV2 and NFAT in hypoxia adaptation, paving the way for further exploration of hypoxia tolerance mechanisms in mollusca.
Key Contribution:
(1) Under hypoxic conditions, we observed altered Ca2+ homeostasis in the blood clam (Anadara granosa), which triggered an upregulation of NFAT and subsequent downregulation of CaV2 mRNA expression, revealing a novel molecular mechanism for hypoxia tolerance. (2) The sequence and structural analyses identified conserved and unique features of CaV2 in blood clams, emphasizing the significance in molluscan hypoxia adaptation.
1. Introduction
Anadara granosa, commonly known as the blood clam, is an economically important mollusca species that is highly valued for its delicious taste and nutritional benefits [1,2,3]. As a benthic mollusca, the blood clam grows in an anoxic environment all year round—the dissolved oxygen rate in the environment is lower than 3.5 mg/L [4]. Our earlier study results have also demonstrated that the blood clam exhibits significantly greater hypoxia tolerance capabilities compared to other bivalves [2,5]. However, the molecular regulatory mechanisms involved in blood clam response to hypoxia stress have not been reported so far.
Calcium (Ca2+) and calcium channel (CaV) homeostasis are critical for normal cellular physiology and, if hypoxia occurs, lead to calcium overload and cell death [6]. In vitro evidence indicates that the hypoxic conditions result in excessive intracellular calcium ions generation [7]. Intracellular calcium overload can be taken as a measure of impaired cellular health and has been a known symptom of an early marker of cell apoptosis phenomena [8].
In primary cardiomyocytes’ epithelial cell cultures, cells tend to exhibit higher Ca2+ and accelerated cell death under hypoxia [8,9] because excessive input of calcium ions signaling in the intracellular will lead to excessive downstream reactions of organisms and consume too much energy; this is unfavorable to the organism and accelerates the death of cells and even the organism [10]. Hypoxia can negatively affect the immune process and increase the apoptosis rate of hemocytes in Mytilus coruscus [11], in which metabolic physicochemical substances such as ROS and lysosome are regulated by calcium ion signals and the calcium channel [12,13]. However, to the best of our knowledge, there is no investigation of the time course of Ca2+ overload and calcium signal of invertebrate species under hypoxic stress. We hypothesize that the time-dependent increase in Ca2+ channel expression may induce Ca2+ overloading, and that calcium channel proteins are the direct regulatory factors controlling the influx of calcium ions from the extracellular space into the cytoplasm. When rats are exposed to hypoxia, calcium channel protein CaV was significantly up-regulated [14]; it was also found that hypoxia causes an increase in CaV protein expression in PC12 cells [15]. In particular, previous studies with hypoxia indicated that NFAT (nuclear factor of activated T cells) may regulate the expression of voltage-dependent Ca2+ channel (CaV) [16]. Hypoxia stress activated transcription factor NFAT generation in ex vivo cellular cultures [17].
Among invertebrates, the blood clam is a special animal with red blood [18,19], and blood is closely related to respiration and oxygen transport. Therefore, this study takes the blood as the representative for in-depth research. To further delve into the mechanisms of hypoxia tolerance in blood clams, this study employed the fluorescence probe method to verify alterations in calcium signaling in blood clam hemocytes under hypoxic stress. Additionally, bioinformatics techniques were utilized to analyze the structural characteristics of the calcium channel protein CaV2 in blood clams and common mollusca. RNA interference technology was then implemented to validate the transcriptional regulation of CaV2 by the NFAT transcription factor. With these approaches, we monitor the calcium signaling in blood clam cells as CaV2 expression varies. The findings of this research enhance our understanding of the molecular mechanisms underlying hypoxia tolerance in blood clams, particularly focusing on calcium signaling.
2. Materials and Methods
2.1. Prediction of the Three-Dimensional Conformation of the CaV2 Domain
The SIB Swiss-model program was run to select the sequences of blood clam and Sinonvacta constricta to predict the three-dimensional conformation of the domain according to the results in 2.5 [20]. A representative sequence from each classification group was selected to predict the tertiary conformation for comparative analysis, and the differences between the tertiary conformation of blood clam and other bivalve mollusca at specific sites were compared [21]. The corresponding sequences of the key conformation differences were selected for comparative analysis. PyMOL software (version 2.3.2) was used to edit, adjust and enhance the above work [22].
2.2. Phylogenetic Analysis and Motif Analysis of CaV2 Characteristic Sequences of Bivalves
According to the sequence alignment results, the MEGA Maximum Likelihood (ML) substitution model program was run to evaluate the optimal tree construction model, and then the MEGA ML program was run to construct the ML tree according to the optimal model [23]. The sequences of calcium ion transport domains of Biomphalaria glabrata (B. gl) and Haliotis rubra (H. ru) were set as outgroups. The bootstrap test was used, and the bootstrap value was set to 500. After the final establishment of the tree, the step value was greater than 60, which was regarded as having high homology and clustering into a group. The group classification of CaV2 feature sequences of bivalves was determined according to the large group determined by the bootstrap value. After that, the TBtools MEME program was executed to conduct motif analysis on the feature sequences of CaV2 of the bivalve [24,25]. The minimum length of searching motif blocks was set to 6 and the longest length was set to 50, and 10 different motif blocks were searched.
2.3. Prediction of Transcription Factor Regulation of CaV2
Combined with the literature, the transcription factor NFAT that may regulate CaV2 transcription was selected [26]. The TBTools program was used to pick the sequence promoter (location: −2000 → +100) of the transcription promoter region of blood clam CaV2, and then the JASPAR program was run to search whether there is a binding site of NFAT in the promoter region with “NFAT binding site” as the search object.
2.4. Hypoxia Experiment
One hundred grains were selected from the breeding pond in Ningbo City, Zhejiang Province. The shell was not damaged, and the axe foot was flexible and active. They were randomly divided equally into experimental and control groups. Each group included 3 replicates and about 17 grains, which were all kept in a container (80 cm × 68 cm × 60 cm, L × W × H), at temperature 25 °C. We changed the water once a day, and fed them once with Chlorella vulgaris (1 mL/tank, 3.5 × 109 cells/mL).
The nitrogen oxygen control method was used to control the dissolved oxygen in the water. Glass-reinforced plastic tanks were filled with seawater, and nitrogen gas was bubbled into them. Real-time monitoring of DO was conducted using a portable DO monitor (HQ30D, HACH, Loveland, CO, USA). The DO of the experimental group was adjusted to 0.5 mg/L and the concentration of dissolved oxygen was measured and adjusted every 2 h. The control group was routinely inflated, and the salinity of the aquaculture water was controlled at about 22 psu. We changed the water once a day, and fed them once with Chlorella vulgaris (1 mL/tank, 3.5 × 109 cells/mL). After 24 h and 48 h of hypoxia stress, a blood sample was taken from each experimental group, and hemocytes and serum were taken from each blood sample after centrifugation. Samples were repeated for 5 times in each group.
2.5. Quantitative Real-Time PCR
RNA from hemocytes was extracted after a 48 h hypoxia experiment (Trizol method). RNA quality and concentration were assessed using electrophoresis gels and Nanodrop spectrophotometers (Thermo Fisher Scientific, Waltham, MA, USA), respectively. Subsequently, total RNA was reverse transcribed into cDNA utilizing the HiScript III RT Super Mix kit designed for quantitative real-time PCR (qRT-PCR) (Vazyme, Nanjing, China). qRT-PCR was performed according to the method by Peng et al. [5], which was conducted in triplicate using the Kubo Quanta gene q225 RT-PCR system (Kubo Technology, Beijing, China) with the ChamQ Universal SYBR qPCR master mix (Vazyme, Nanjing, China). We examined the expression of CaV2 and NFAT, with 18S rRNA as a constitutive gene. Sangon Biotech (Shanghai, China) synthesized the primers, listed in Table 1. The 2−ΔΔCT method was used to analyze the relative levels of gene expression.

Table 1.
Primers for quantitative fluorescence PCR.
2.6. Experiment for Detecting Changes in Intracellular and Extracellular Calcium Ion Signaling
2.6.1. Extracellular (Plasma) Calcium Ion Signal Change Detection
The determination of calcium ion (Ca2⁺) concentration in plasma samples collected during hypoxia experiments at two time points (24 h and 48 h) was conducted using the Methyl Thymol Blue (MTB) method.
According to the specified protocol, the quantification of Ca2⁺ can be represented by the formula: c (Ca2+) ∝ [A determination (sample) − A hole (blank)], where “A” represents the absorbance measurement recorded for a particular well in the enzyme-linked immunosorbent assay (ELISA). Therefore, we utilize the expression “[A (determination hole) − A (measured blank holes)]” to represent the relative content of Ca2+. The relative content of Ca2+ at 610 nm for each sample was determined by ELISA, then normality and variance homogeneity tests and a two-tailed t test were subsequently performed to evaluate whether there were statistically significant differences between the experimental group and the control group. Statistical significance was set at a p < 0.05.
2.6.2. Intracellular Calcium Ion Signal Change Detection
In the hypoxia experiment, hemocyte samples were collected at two specifics distinct time points (24 h and 48 h), and the concentration of calcium ions in hemocytes was measured determined using the Fluo-4 fluorescence probe method. Drawing from pertinent literature sources [27,28], we adopted the parameter denoted as “A (determination hole)” to represent the relative intracellular Ca2+ content. Subsequently, fluorescence microscopy was then utilized used to capture images and evaluate and quantify the fluorescence intensity of intracellular Ca2+ at each designated time point. Following the imaging procedure, the relative Ca2+ levels content in each sample were quantified using a fluorescence microplate reader, with an excitation and emission wavelength of 488 nm and an emission wavelength of 516 nm, respectively. Normality and variance homogeneity tests and two-tailed t-tests were subsequently performed to determine whether there were statistically significant differences between the experimental and control groups, with a p < 0.05 considered statistically significant.
2.7. RNA Interference (RNAi) Experiments Targeting NFAT
The RNAi sequence targeting NFAT was carefully designed to trigger interference in blood clam specimens exposed to hypoxia (0.5 mg/L). Simultaneously, a control group underwent the same conditions without the RNAi treatment. The interference process lasted 48 h. Once the interference period concluded, hemocyte samples were collected to assess the initial efficacy of the RNAi. Following this, an analysis was performed to determine any modifications in the expression of CaV2, and to detect changes in both intracellular and extracellular calcium ion signal of blood clam’s hemocytes.
3. Results
3.1. Explore the Domain of CaV2 Protein Sequence
A total of five domains were matched (Figure S1, Table S1), among which the ion transport domain (pfam00520) had a better matching degree. The best matching range is 669–927.
A total of 27 sequences were obtained through multiple sequences comparison, with other bivalve mollusks sharing similarity with this domain under investigation. Alignment analysis showed that this domain had a high degree of conservation across various bivalve mollusks, albeit with some unique amino acid mutations observed in the conserved region specifically in Arcidae Mollusca. These mutations include a Y→F substitution at position 314 (Figure S2b), a 290-bit an L→A mutation at position 290 (Figure S2b), an A→M mutation at position 198 (Figure S2a) and a T→I mutation at position 10 (Figure S2a). Comparison results from the UniProt program indicated that the voltage-dependent 1-type calcium channel subunit of Opisthorchis viverrini demonstrated the greatest sequence similarity. This finding corroborates the protein species examined in this study, suggesting a high degree of accuracy in the matching results. Upon closer inspection of the sequence, it was found that the domain is located on the cellular membrane and performs the essential function of calcium ion binding. Furthermore, the domain contains multiple transmembrane regions.
3.2. Phylogenetic and Motif Analysis of Calcium Ion Transport Domain Sequences in Bivalves
The results of ML tree construction are presented on the left side of Figure S3. The sequence of this domain in Scapharca broughtonii exhibits the highest degree of similarity with the blood clam, evidenced by a bootstrap value of 99%. Additionally, the calcium transport domain in S. broughtonii exhibits homology in the blood clam. Furthermore, the sequences of this domain in Argopecten purpuratus and Modiolus philippinarum share some degree of homology with the Arcidae family, although the connection is weak. Regarding the rate of evolution, it varies considerably for this domain—particularly in Arcidae mollusca, where the branch length is relatively short. Based on the grouping criteria established in this study, the calcium ion transport domain across all bivalves is broadly categorized into five groups, labeled as groups 1–5 in Figure S3 on the left side; they are roughly classified by family, based on the taxonomic information of each species. The results of the motif analysis of protein sequences reveal a relatively conserved motif arrangement of calcium ion transport domains in bivalves as illustrated on the right side of Figure S3. Notably, there are significant similarities in the motif patterns among Group 1 to 5. Upon careful closer inspection, however, distinct motif differences can be observed within each group. For instance, the presence of a unique short red motif in Group 3 and a short pink motif exclusive to Group 3 and 4 suggest potential unique structural and functional characteristics of the proteins. These specific motif blocks likely reflect distinct features of the proteins’ structure and function.
3.3. Prediction of the Three-Dimensional Conformation of the Domain
A comparative analysis of the calcium ion transport domains across representative species from five distinct groups (as illustrated in Figure S3a–f) revealed a general consistency in the spatial conformation of the bivalves. However, within the Pos23→37 segment (highlighted as yellow circle), other bivalves exhibited irregular coiling patterns, whereas the blood clam showed a distinct folded structure in its conformation. This observation underscores the unique tertiary conformation of the CaV2 in the blood clam. Upon comparing the calcium ion transport domain of blood clam and S. constricta, and superimposing their spatial conformations, it became evident that within the Pos23→37 interval, the conformation of S. constricta notably diverged from that of the blood clam. Specifically, S. constricta exhibited a lengthy and irregular coiled structure, whereas the blood clam displayed a comparatively less irregular coiled and more compact structure in this region (upper right of Figure 1).
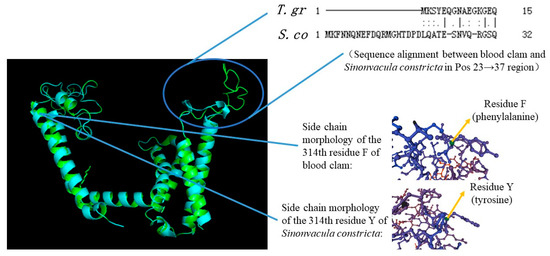
Figure 1.
Conformational difference and sequence alignment between blood clam (T. gr) and S. constricta (S. co) in Pos23→37 and residue 314.
3.4. The Validation of CaV2 and NFAT Gene Expression Trends in the Transcriptome
The qRT-PCR detection conducted during the hypoxia experiment revealed a consistent pattern with the transcriptome detection results (please refer to the transcriptome previously determined by our research group, GSA: CRA007322) [29], as illustrated in Figure 2. This finding verifies that, upon exposure to hypoxia stress, NFAT levels in blood clam hemocytes increase, whereas CaV2 levels decrease.
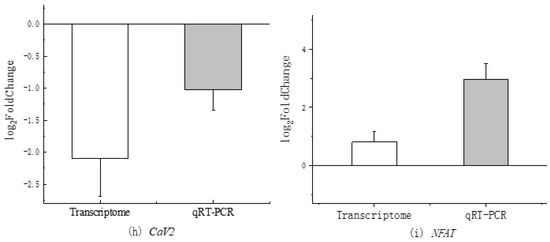
Figure 2.
Comparison of CaV2 and NFAT expression between transcriptome and qRT-PCR.
3.5. Results of Intracellular and Extracellular Calcium Signal Changes after Hypoxia Stress
3.5.1. Evaluate Results of Extracellular (Plasma) Calcium Ion Signal Changes of Hemocytes
After 24 h of stress, a downward trend was observed in the plasma calcium ion concentration (Figure 3a). Additionally, following 48 h of stress, there was a statistically significant decrease in the plasma calcium ion concentration (p < 0.05) (Figure 3b).
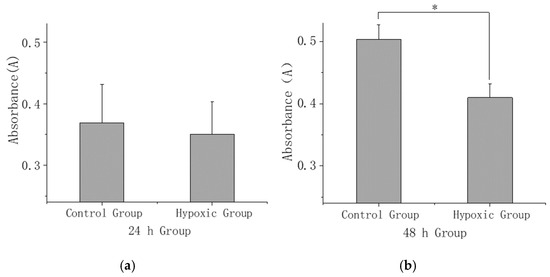
Figure 3.
(a) Comparison of extracellular calcium ion concentration at 24 h under hypoxia stress. (b) Comparison of extracellular calcium ion concentration at 48 h under hypoxia stress. The asterisk (*) in the bar chart presented signifies a statistically significant difference (p < 0.05) between the control group and hypoxic group.
3.5.2. Detection Results of Alterations in Intracellular Calcium Ion Signals of Hemocytes
The results for detecting changes in intracellular calcium ion signals revealed a significant increase in concentration at both 24 and 48 h (p < 0.05) (Figure 4). Upon observation under a fluorescence microscope, the experimental group showed a markedly stronger fluorescence intensity in comparison to the control group after 48 h of stress (Figure 5a,b).
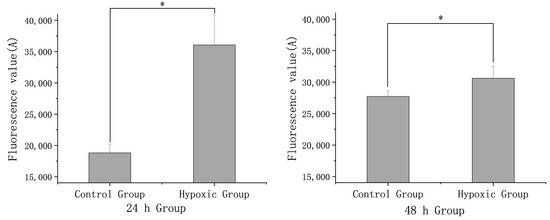
Figure 4.
Comparison of intracellular calcium ion concentration signals between the experimental group and the control group after 24 h and 48 h stress. The asterisk (*) in the bar chart presented signifies a statistically significant difference (p < 0.05) between the control group and hypoxic group.
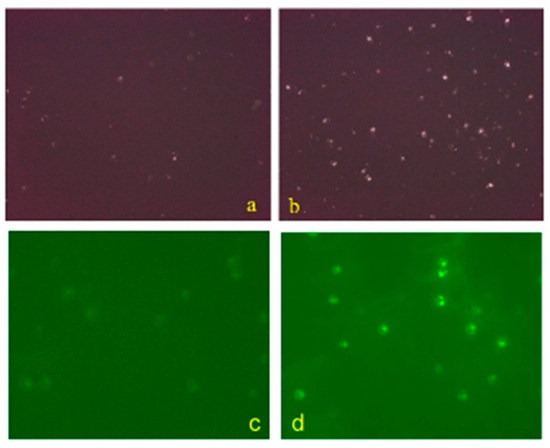
Figure 5.
Comparison of intracellular calcium ion fluorescence intensity of hemocytes between control group (a) and experimental group (b) after 48 h of hypoxia stress. The fluorescence intensity of the experimental group was stronger than that of the control group. Comparison of intracellular calcium ion fluorescence intensity of hemocytes between control group (c) and interference group (d). The fluorescence intensity of the interference group was stronger than that of the control group.
3.6. Transcriptional Regulation of CaV2
The results revealed that NFAT had a significant downregulation in the interference group (as shown in Figure 6a), thus confirming the successful execution of the interference.
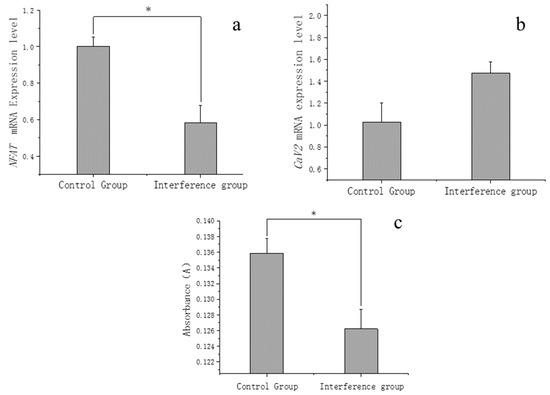
Figure 6.
(a) Expression changes of NFAT after RNAi. (b) Expression changes of CaV2 after NFAT interference. (c) Comparison of extracellular calcium ion concentration signal between the interference group and the control group. The asterisk (*) in the bar chart presented signifies a statistically significant difference (p < 0.05) between the control group and interference group.
Subsequently, the change in CaV2 expression within the interference group was quantified. The results indicated an upregulation of blood clam CaV2 expression following the reduction in NFAT due to the interference (as shown in Figure 6b); concurrently, a significant decrease in extracellular calcium ions signaling was observed (Figure 6c). Additionally, the fluorescence signal of intracellular calcium ions demonstrated a marked intensification (as depicted Figure 5c,d).
4. Discussion
The CaV2 channel belongs to the P/Q type alpha-1A subtype of voltage-dependent calcium channels, and its regulatory influence spans key pathways such as the MAPK signaling pathway, calcium signaling pathway, synaptic vesicle cycle, and glutamatergic synapse [30,31,32].
In the blood clam, CaV2 expresses three calcium ion transport domains (PF00520), which are integral components for facilitating the transport of the calcium ions. The ion transport domain family (PF00520) encompasses sodium, potassium, and calcium ion channels, distinguished by their six transmembrane helices. Notably, the last two helices encircle a ring that determines ion selectivity. Although certain subfamilies, like sodium channels, display a fourfold repetition of domain, the calcium channel family assembles as a tetramer within the membrane [33]. To ascertain the transmembrane properties of the domain, predictions were made using the UniProt program. The results indicated that the calcium ion transport domain in the blood clam demonstrates positive transmembrane traits, suggesting a direct interaction with calcium ions, as proposed by the Consortium [34].
Previous studies have identified both the blood clam and S. broughtonii as mollusks demonstrating remarkable hypoxia tolerance [35,36,37]. Upon comparing the calcium ion transport protein domain sequences, a high degree of similarity was observed between these two species, with a strong bootstrap value of 99%. It is noteworthy that variations were exclusively found at four specific amino acid residue sites in the blood clam and S. broughtonii, while these sites remained conserved in other bivalves. This phenomenon is hypothesized to contribute significantly to the adaptive advantages exhibited by the blood clam and S. broughtonii in hypoxic environments.
The modifications observed in calcium ion transporters within these species are believed to enhance their adaptability to environmental changes and provide enhanced cellular protection. It is noteworthy that even a single amino acid residue modification can exert a profound effect on the internal hydrogen bonding pattern, free energy, and various physicochemical properties of the polypeptide chain and protein, as elucidated in previous studies [38,39,40,41]. Such alterations can lead to significant changes in the stability and characteristics of the molecule. To further explore this discovery, a comprehensive analysis of the changes in residue sites has been conducted, as detailed in Table 2.

Table 2.
Changes in residues at specific sites in the calcium ion transport domain of Arcidae mollusca compared with other bivalves.
According to this table, a remarkable feature emerges regarding the distinct residue sites. Specifically, the sites corresponding sites to Arcidae mollusca are characterized by the presence of non-polar amino acids, presenting a sharp contrast to the polar amino acid sites found in other bivalve mollusca. Furthermore, the table reveals that the isoelectric point of residues at these corresponding sites in Arcidae mollusca is relatively low compared to other bivalve mollusca. This difference indicates that the residues located at these specific sites in Arcidae mollusca possess a higher level of acidity, accompanied by an increased negative charge.
We hypothesize that modifications in residue sites have the potential to significantly impact physicochemical properties and functions of CaV2 [42]. Specifically, these changes could negatively impact the transport of calcium ions within CaV2. For instance, using the NetPhos-3.1 program of EMBL to predict phosphorylation sites, we discovered that threonine (T) at position 10 served as a binding site for protein kinases GSK3, CaM-II, CK-I, and CK-II in the calcium ion transporter domain sequence of other bivalve mollusca.
Additionally, tyrosine (Y) at position 314 was identified as a binding site for protein kinases SRC, INSR, EGFR, and UNSP [43]. When these protein kinases bind to the matrix, the serine/threonine/tyrosine site on the protein undergoes phosphorylation, exerting a positive regulatory effect on the protein [44]. However, due to the mutations at these critical sites in the blood clam, resulting in non-phosphorylated sites (I and F), these essential active sites are lost. This loss could potentially have adverse effects on the functional activity of CaV2 in the blood clam.
Based on the results of transcriptome analyses and qRT-PCR experiments, a notable observation emerged: the transcription expression of CaV2 significantly decreased under conditions of hypoxia stress. This reduction in transcription activity may be attributed to a decreased diminished cellular demand for CaV2 protein in cell membranes, subsequently suppressing the activity of positive transcription factors linked to CaV2. Consequently, this regulatory cascade influences the transcriptional level of CaV2 mRNA, leading to a decrease in CaV2 mRNA transcription. Alternatively, it could be postulated that during hypoxia stress, there is an excessive influx of calcium ions in blood clam cells, activating specific negative regulatory transcription factors such as NFAT. These activated transcription factors then hinder the transcription of CaV2 [16,45]. Our transcriptome data revealed a responsive and up-regulated trend in the expression of NFAT in blood clam under hypoxia stress (FC = 1.75), which was further confirmed by qRT-PCR results from the hypoxia experiment. Additionally, using the JASPAR program, we identified multiple regulatory sites in the promoter region of the CaV2 gene in the blood clam where NFAT can bind [46]. To further validate the negative regulatory effect of NFAT on CaV2, we designed a hypoxia interference experiment targeting NFAT. Successfully reducing NFAT expression through interference led to the up-regulation of CaV2 expression, supporting the hypothesis that CaV2 is negatively regulated after hypoxia. By monitoring the intracellular and extracellular calcium signals following the upregulation of CaV2, we obtained initial evidence indicating that elevated CaV2 expression facilitates the influx of extracellular calcium ions into the cell. The upstream of NFAT is regulated by calcineurin. It is possible that it acts in a dephosphorylated manner and then negatively regulates CaV2 expression by recruiting corepressors or cooperating with known transcriptional repressors on DNA [47,48,49]. The details of how to regulate in blood clam are our next step.
Based on our experimental findings, a significant increase in intracellular calcium concentration was observed in blood clam hemocytes following exposure to hypoxia stress. This response is consistent with observations made in various cell types, including rat hippocampal neurons and cardiac muscle cells [7,50]. The possible mechanism is that low oxygen leads to the production of more ROS in cell mitochondria [51,52], and then ROS can induce the increase in expression of some calcium ion channel proteins in cells [53,54], thereby accelerating the inflow of calcium ions into cells and causing an increase in calcium ion content in cells.
By evaluating the expression level of the calcium channel protein gene CaV2, it is speculated that the blood clam might regulate the influx of calcium ions by adjusting the abundance of CaV2 in the membrane. Upon comparing the time difference at 24 h and 48 h of stress relative to the control group, it was noticed that from 24 h to 48 h of stress, the time difference in calcium ion concentration within the blood clam shows a decreasing trend. This reduction could be linked to the decreased presence of CaV2 in the cell membrane, leading to a slowdown in the accumulation of intracellular calcium ions in the blood clam.
Blood clam had a notable decrease in extracellular calcium concentration, which was detected after hypoxia stress, similar to changes seen in neonates with hypoxic–ischemic encephalopathy (HIE) [55]. The literature suggests that, following hypoxia stress, calcium ions outside blood clam cells migrate into the cells, a process primarily controlled by calcium ion channel proteins located on the cell membrane. However, an excessive buildup of intracellular calcium ions may intensify cellular damage induced by hypoxia [56,57,58].
Despite this, the blood clam might possess protective mechanisms to prevent intracellular calcium ion overload triggered by hypoxia. By evaluating the expression level of the calcium channel protein gene CaV2, it is speculated that the blood clam might regulate the influx of calcium ions by adjusting the abundance of CaV2 in the membrane.
5. Conclusions
In this study, we have accurately mapped changes in intracellular and extracellular Ca2+ concentrations, as well as variations in CaV2 mRNA expression levels. We hypothesize that modifications in residue sites and spatial structure have the potential to significantly impact the physicochemical properties and functions of CaV2. Specifically, these changes could negatively impact the transport of calcium ions within CaV2. Furthermore, it could be postulated that, during hypoxia stress, there is an influx of calcium ions in blood clam cells, activating specific negative regulatory transcription factors, such as NFAT. These activated transcription factors then hinder the transcription of CaV2. Our research, focused on elucidating the functional characteristics and regulatory mechanisms of CaV2 in hypoxia, promises to enhance our understanding of the adaptive strategies adopted by this economically important mollusca species. In the next phase of research, we plan to investigate protein-level expression of genes related to the calcium signaling pathway in the blood clam, to further validate the conclusions drawn from this study.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/fishes9100409/s1, Figure S1: Positions of matched domains on CaV2 sequence of blood clam; Table S1: domain matching results; Figure S2: Full-length comparison of the sequences of 27 calcium ion transport domains of bivalves; Figure S3. Phylogenetic tree of calcium ion transport domain in bivalves and Spatial configuration of calcium ion transport domains of M. ga (a), S. br (b), T. gr (c), O. ed (d), M. ye (e), M. me (f).
Author Contributions
Conceptualization, Y.B., Z.P., H.L. and Y.Z.; methodology, Y.Z.; software, Y.Z.; validation, Z.P. and Y.Z.; formal analysis, Z.P. and Y.Z.; investigation, Z.P. and Y.Z.; resources, Y.B. and Z.P.; data curation, Y.Z.; writing—original draft preparation, Z.P. and Y.Z.; writing—review and editing, Y.B., Z.P. and Y.Z.; visualization, Y.Z.; supervision, Y.B. and Z.P.; project administration, Y.B., Z.P. and H.L.; funding acquisition, Y.B. and Z.P. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by Zhejiang Provincial Top Discipline of Biological Engineering Level A (ZS2024002); Zhejiang Province Public Welfare Technology Application Research Project (LGN21C190012); Zhejiang Major Program of Science and Technology Special Project (2021C02069-7); Ningbo Public Benefit Research Key Project (2021S014); Open Foundation from Marine Sciences in the First-Class Subjects of Zhejiang (OFMS007).
Institutional Review Board Statement
The animal study was reviewed and approved by the Institutional Animal Care and Use Committee (IACUC) of Zhejiang Wanli University, China (Approval code: 20221003001).
Informed Consent Statement
Not applicable.
Data Availability Statement
The datasets generated for this study can be found in the CNCB GSA accession no. CRA007322.
Acknowledgments
The authors thank Cheng Haoxiang, Wu Zongming, Fu Lulu, Kong Xianghui and Wang Shasha of Zhejiang Wanli University for their guidance and assistance in the relevant experiments.
Conflicts of Interest
The authors declare no competing or financial interest.
References
- Bao, Y.; Wang, J.; Li, C.; Li, P.; Wang, S.; Lin, Z. A preliminary study on the antibacterial mechanism of Tegillarca granosa hemoglobin by derived peptides and peroxidase activity. Fish Shellfish Immunol. 2016, 51, 9–16. [Google Scholar] [CrossRef]
- Zhan, Y.; Zha, S.; Peng, Z.; Lin, Z.; Bao, Y. Hypoxia-mediated immunotoxicity in the blood clam Tegillarca granosa. Mar. Environ. Res. 2022, 177, 105632. [Google Scholar] [CrossRef]
- Liu, Y.; Liu, Y. Rational utilization of monomonas alga during high density cultivation of Tegillarca granosa larvae. Aquaculture 2018, 39, 30–32. (In Chinese) [Google Scholar]
- Zang, Y.; Sun, Y.; Yang, L.; Li, Y.; Luo, T.; Mu, F. Spatiotemporal distribution pattern of meiofauna and its influencing factors in the jinshatan beach, Dalian. Mar. Sci. 2020, 44, 76–89. [Google Scholar]
- Peng, Z.; Liu, X.; Jin, M.; Zhan, Y.; Zhang, X.; Bao, Y.; Liu, M. Hypoxia activates HIF-1α and affects gene expression and transcriptional regulation of phd in Tegillarca granosa. Fishes 2023, 8, 359. [Google Scholar] [CrossRef]
- Xiao, D.; Liu, Y.; Qiao, S. Study on damage of cultured cardiomyocytes induced by low calcium in external environment. Chin. J. Endem. Dis. Control 2000, 6, 341–342. (In Chinese) [Google Scholar]
- Kou, T.; Zhang, Y. Effects of salidroside on calcium ion content, calcium activated neutral protease and calcium channel protein expression in hippocampal neurons cultured in vitro by physical hypoxia. J. Xinxiang Med. Coll. 2012, 29, 260–264. (In Chinese) [Google Scholar]
- Kwong, J.C.; Schwartz, K.L.; Campitelli, M.A.; Chung, H.; Crowcroft, N.S.; Karnauchow, T.; Katz, K.; Ko, D.T.; Mcgeer, A.J.; Mcnally, D.; et al. Acute myocardial infarction after laboratory-confirmed influenza infection. N. Engl. J. Med. 2018, 378, 345–353. [Google Scholar] [CrossRef]
- Arnould, T.; Michiels, C.; Alexandre, I.; Remacle, J. Effect of hypoxia upon intracellular calcium concentration of human endothelial cells. J. Cell. Physiol. 1992, 152, 215–221. [Google Scholar] [CrossRef] [PubMed]
- Orrenius, S.; Zhivotovsky, B.; Nicotera, P. Regulation of cell death: The calcium–apoptosis link. Nat. Rev. Mol. Cell Biol. 2003, 4, 552–565. [Google Scholar] [CrossRef] [PubMed]
- Sui, Y.; Kong, H.; Shang, Y.; Huang, X.; Wu, F.; Hu, M.; Lin, D.; Lu, W.; Wang, Y. Effects of short-term hypoxia and seawater acidification on hemocyte responses of the mussel Mytilus coruscus. Mar. Pollut. Bull. 2016, 108, 46–52. [Google Scholar] [CrossRef] [PubMed]
- Brookes, P.; Yoon, Y.; Robotham, J.; Anders, M.; Sheu, S. Calcium, ATP, and ROS: A mitochondrial love-hate triangle. Am. J. Physiol. Cell Physiol. 2004, 287, C817–C833. [Google Scholar] [CrossRef] [PubMed]
- Jaconi, M. Cytosolic free calcium elevation mediates the phagosome-lysosome fusion during phagocytosis in human neutrophils. J. Cell Biol. 1990, 110, 1555–1564. [Google Scholar] [CrossRef] [PubMed]
- Sellak, H.; Liu, B.; Zhou, C.; Chen, H.; Wu, S. Mechanism of hypoxia-induced α1h (cav3.2) gene expression: Examining the transcriptional regulation. FASEB J. 2008, 22, 920–960. [Google Scholar] [CrossRef]
- Green, K.N.; Boyle, J.P.; Peers, C. Hypoxia potentiates exocytosis and Ca2+ channels in pc12 cells via increased amyloid beta peptide formation and reactive oxygen species generation. J. Physiol. Lond. 2002, 541, 1013–1023. [Google Scholar] [CrossRef]
- Kuklina, E.M.; Shirshev, S.V. Role of transcription factor NFAT in the immune response. Biochem. Mosc. 2001, 66, 467–475. [Google Scholar] [CrossRef]
- Chen, C.Y.; Del Gatto-Konczak, F.; Wu, Z.; Karin, M. Stabilization of interleukin-2 mRNA by the c-jun NH2-terminal kinase pathway. Science 1998, 280, 1945–1949. [Google Scholar] [CrossRef]
- An, H.Y.; Park, J.Y. Ten new highly polymorphic microsatellite loci in the blood clam Scapharca broughtonii. Mol. Ecol. Notes 2005, 5, 896–898. [Google Scholar] [CrossRef]
- Nishida, K.; Ishimura, T.; Suzuki, A.; Sasaki, T. Seasonal changes in the shell microstructure of the bloody clam, Scapharca broughtonii (mollusca: Bivalvia: Arcidae). Palaeogeogr. Palaeoclimatol. Palaeoecol. 2012, 363, 99–108. [Google Scholar]
- Waterhouse, A.; Bertoni, M.; Bienert, S.; Studer, G.; Tauriello, G.; Gumienny, R.; Heer, F.T.; de Beer, T.; Rempfer, C.; Bordoli, L.; et al. Swiss-model: Homology modelling of protein structures and complexes. Nucleic Acids Res. 2018, 46, W296–W303. [Google Scholar] [CrossRef]
- Schoch, C.L.; Ciufo, S.; Domrachev, M.; Hotton, C.L.; Kannan, S.; Khovanskaya, R.; Leipe, D.; Mcveigh, R.; O’Neill, K.; Robbertse, B.; et al. NCBI taxonomy: A comprehensive update on curation, resources and tools. Database 2020, 2020, baaa062. [Google Scholar] [CrossRef] [PubMed]
- Schrodinger, L. The PYMOL Molecular Graphics System, version 1.8; Science and Education Publisher: Newark, De, USA, 2015. [Google Scholar]
- Kumar, S.; Stecher, G.; Li, M.; Knyaz, C.; Tamura, K. Mega x: Molecular evolutionary genetics analysis across computing platforms. Mol. Biol. Evol. 2018, 35, 1547–1549. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.; Li, Y.; Yu, H.; Zhang, L.; Hu, J.; Bao, Z.; Wang, S. Molluscdb: An integrated functional and evolutionary genomics database for the hyper-diverse animal phylum mollusca. Nucleic Acids Res. 2021, 49, D988–D997. [Google Scholar] [CrossRef] [PubMed]
- Bailey, T.L.; Elkan, C. Fitting a mixture model by expectation maximization to discover motifs in biopolymers. Proc. Int. Conf. Intell. Syst. Mol. Biol. 1994, 2, 28–36. [Google Scholar] [PubMed]
- Rao, A.; Luo, C.; Hogan, P.G. Transcription factors of the NFAT family: Regulation and function. Annu. Rev. Immunol. 1997, 15, 707–747. [Google Scholar] [CrossRef]
- Evans, A.M.; Mustard, K.J.; Wyatt, C.N.; Peers, C.; Dipp, M.; Kumar, P.; Kinnear, N.P.; Hardie, D.G. Does amp-activated protein kinase couple inhibition of mitochondrial oxidative phosphorylation by hypoxia to calcium signaling in O2-sensing cells? J. Biol. Chem. 2005, 280, 41504–41511. [Google Scholar] [CrossRef]
- Liu, Z.; Chen, J.; Tan, Y.; Qiu, J.; Chen, D.; Liu, J. Effects of 8 hz 90 db infrasonic on the expression of calcium ion and endoplasmic reticulum calcium channel protein ryrs in rat hippocampus cells. J. Fourth Mil. Med. Univ. 2005, 2, 185–188. (In Chinese) [Google Scholar]
- CNCB-NGDC, M.A.P. Database resources of the national genomics data center, China national center for bioinformation in 2024. Nucleic Acids Res. 2024, 52, D18–D32. [Google Scholar] [CrossRef]
- Few, A.P.; Lautermilch, N.J.; Westenbroek, R.E.; Scheuer, T.; Catterall, W.A. Differential regulation of cav2.1 channels by calcium-binding protein 1 and visinin-like protein-2 requires N-terminal myristoylation. J. Neurosci. 2005, 25, 7071–7080. [Google Scholar] [CrossRef]
- Hans, M.; Urrutia, A.; Deal, C.; Brust, P.F.; Stauderman, K.; Ellis, S.B.; Harpold, M.M.; Johnson, E.C.; Williams, M.E. Structural elements in domain iv that influence biophysical and pharmacological properties of human alpha1a-containing high-voltage-activated calcium channels. Biophys. J. 1999, 76, 1384–1400. [Google Scholar] [CrossRef][Green Version]
- Kanehisa, M.; Araki, M.; Goto, S.; Hattori, M.; Hirakawa, M.; Itoh, M.; Katayama, T.; Kawashima, S.; Okuda, S.; Tokimatsu, T.; et al. KEGG for linking genomes to life and the environment. Nucleic Acids Res. 2008, 36, D480–D484. [Google Scholar] [CrossRef] [PubMed]
- Necci, M.; Piovesan, D.; Dosztányi, Z.; Tosatto, S.C.E. Mobidb-lite: Fast and highly specific consensus prediction of intrinsic disorder in proteins. Bioinformatics 2017, 33, 1402–1404. [Google Scholar] [CrossRef] [PubMed]
- Consortium, T.U. Uniprot: The universal protein knowledgebase in 2023. Nucleic Acids Res. 2022, 51, D523–D531. [Google Scholar] [CrossRef] [PubMed]
- Zhan, Y. Immune Response to Hypoxia Stress and the Mechanism of hif-1α Regulation in Blood Clam Tegillarca granosa; Shanghai Ocean University: Shanghai, China, 2022. [Google Scholar]
- Zhang, G.W. Effects of Hypoxia Stress and Molecular Response in Scapharca broughtonii; Shanghai Ocean University: Shanghai, China, 2019. [Google Scholar]
- Zhao, Q. Molecular Cloning and Functional Analysis of Hemoglobin Genes from Ark Shell Scapharca broughtonii; Shanghai Ocean University: Shanghai, China, 2018. [Google Scholar]
- Chan, H.S.; Dill, K.A. Origins of structure in globular proteins. Proc. Natl. Acad. Sci. USA 1990, 87, 6388–6392. [Google Scholar] [CrossRef] [PubMed]
- Gadkari, R.A.; Varughese, D.; Srinivasan, N. Recognition of interaction interface residues in low-resolution structures of protein assemblies solely from the positions of c (alpha) atoms. PLoS ONE 2009, 4, e4476. [Google Scholar] [CrossRef]
- Liu, X.; Taylor, R.D.; Griffin, L.; Coker, S.F.; Adams, R.; Ceska, T.; Shi, J.; Lawson, A.D.; Baker, T. Computational design of an epitope-specific keap1 binding antibody using hotspot residues grafting and cdr loop swapping. Sci. Rep. 2017, 7, 41306. [Google Scholar] [CrossRef]
- Wieczorek, R.; Dannenberg, J.J. Hydrogen-bond cooperativity, vibrational coupling, and dependence of helix stability on changes in amino acid sequence in small 310-helical peptides. A density functional theory study. J. Am. Chem. Soc. 2003, 125, 14065–14071. [Google Scholar] [CrossRef]
- Hsieh, A.H.; Kuo, C.F.; Chou, I.J.; Tseng, W.Y.; Chen, Y.F.; Yu, K.H.; Luo, S.F. Human cytomegalovirus pp65 peptide-induced autoantibodies cross-reacts with taf9 protein and induces lupus-like autoimmunity in BALB/c mice. Sci. Rep. 2020, 10, 9662. [Google Scholar] [CrossRef]
- Blom, N.; Sicheritz-Pontén, T.; Gupta, R.; Gammeltoft, S.; Brunak, S. Prediction of post-translational glycosylation and phosphorylation of proteins from the amino acid sequence. Proteomics 2004, 4, 1633–1649. [Google Scholar] [CrossRef]
- Koch, C.A.; Anderson, D.; Moran, M.F.; Ellis, C.; Pawson, T. SH2 and SH3 domains: Elements that control interactions of cytoplasmic signaling proteins. Science 1991, 252, 668–674. [Google Scholar] [CrossRef]
- Loh, C.; Shaw, K.T.Y.; Carew, J.; Viola, J.P.B.; Luo, C.; Perrino, B.A.; Rao, A. Calcineurin binds the transcription factor NFAT1 and reversibly regulates its activity (*). J. Biol. Chem. 1996, 271, 10884–10891. [Google Scholar] [CrossRef] [PubMed]
- Castro-Mondragon, J.A.; Riudavets-Puig, R.; Rauluseviciute, I.; Berhanu Lemma, R.; Turchi, L.; Blanc-Mathieu, R.; Lucas, J.; Boddie, P.; Khan, A.; Manosalva Pérez, N.; et al. JASPAR 2022: The 9th release of the open-access database of transcription factor binding profiles. Nucleic Acids Res. 2021, 50, D165–D173. [Google Scholar] [CrossRef] [PubMed]
- Hogan, P.; Chen, L.; Nardone, J.; Rao, A. Transcriptional regulation by calcium, calcineurin, and nfat. Genes dev 17: 2205–2232. Genes Dev. 2003, 17, 2205–2232. [Google Scholar] [CrossRef] [PubMed]
- Okamura, H.; Aramburu, J.; García-Rodríguez, C.; Viola, J.P.B.; Raghavan, A.; Tahiliani, M.; Zhang, X.; Qin, J.; Hogan, P.G.; Rao, A. Concerted dephosphorylation of the transcription factor nfat1 induces a conformational switch that regulates transcriptional activity. Mol. Cell 2000, 6, 539–550. [Google Scholar] [CrossRef] [PubMed]
- Baksh, S.; Widlund, H.R.; Frazer-Abel, A.A.; Du, J.; Fosmire, S.; Fisher, D.E.; Decaprio, J.A.; Modiano, J.F.; Burakoff, S.J. NFATc2-mediated repression of cyclin-dependent kinase 4 expression. Mol. Cell 2002, 10, 1071–1081. [Google Scholar] [CrossRef]
- Kondo, R.P.; Apstein, C.S.; Eberli, F.R.; Tillotson, D.L.; Suter, T.M. Increased calcium loading and inotropy without greater cell death in hypoxic rat cardiomyocytes. Am. J. Physiol. 1998, 275, H2272–H2282. [Google Scholar] [CrossRef]
- Chandel, N.; Schumacker, P. Cellular oxygen sensing by mitochondria: Old questions, new insight. J. Appl. Physiol. 2000, 88, 1880–1889. [Google Scholar] [CrossRef]
- Chandel, N.S.; Maltepe, E.; Goldwasser, E.; Mathieu, C.E.; Simon, M.C.; Schumacker, P.T. Mitochondrial reactive oxygen species trigger hypoxia-induced transcription. Proc. Natl. Acad. Sci. USA 1998, 95, 11715–11720. [Google Scholar] [CrossRef]
- Green, K.; Peers, C. Amyloid β peptides mediate hypoxic augmentation of Ca2+ channels. J. Neurochem. 2001, 77, 953–956. [Google Scholar] [CrossRef]
- Taylor, S.; Batten, T.; Peers, C. Hypoxic enhancement of quantal catecholamine secretion. Evidence for the involvement of amyloid β-peptides. J. Biol. Chem. 1999, 274, 31217–31222. [Google Scholar] [CrossRef]
- Prempunpong, C.; Efanov, I.; Sant’Anna, G. Serum calcium concentrations and incidence of hypocalcemia in infants with moderate or severe hypoxic-ischemic encephalopathy: Effect of therapeutic hypothermia. Early Hum. Dev. 2015, 91, 535–540. [Google Scholar] [CrossRef] [PubMed]
- Rimessi, A.; Bonora, M.; Marchi, S.; Patergnani, S.; Marobbio, C.M.; Lasorsa, F.M.; Pinton, P. Perturbed mitochondrial Ca2+ signals as causes or consequences of mitophagy induction. Autophagy 2013, 9, 1677–1686. [Google Scholar] [CrossRef] [PubMed]
- Yu, S.; Zheng, S.; Leng, J.; Wang, S.; Zhao, T.; Liu, J. Inhibition of mitochondrial calcium uniporter protects neurocytes from ischemia/reperfusion injury via the inhibition of excessive mitophagy. Neurosci. Lett. 2016, 628, 24–29. [Google Scholar] [CrossRef] [PubMed]
- Yamada, H.; Chen, Y.; Aihara, M.; Araie, M. Neuroprotective effect of calcium channel blocker against retinal ganglion cell damage under hypoxia. Brain Res. 2006, 1071, 75–80. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).