Swimbladder Function in the European Eel Anguilla anguilla
Abstract
1. Introduction
2. Opening of the Swimbladder in Glass Eel
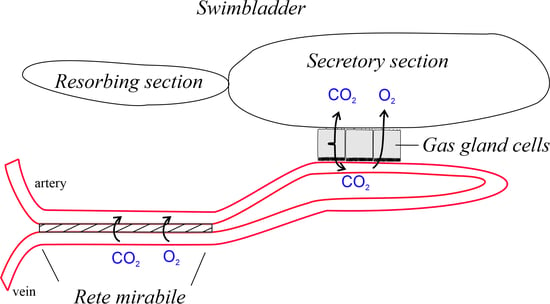
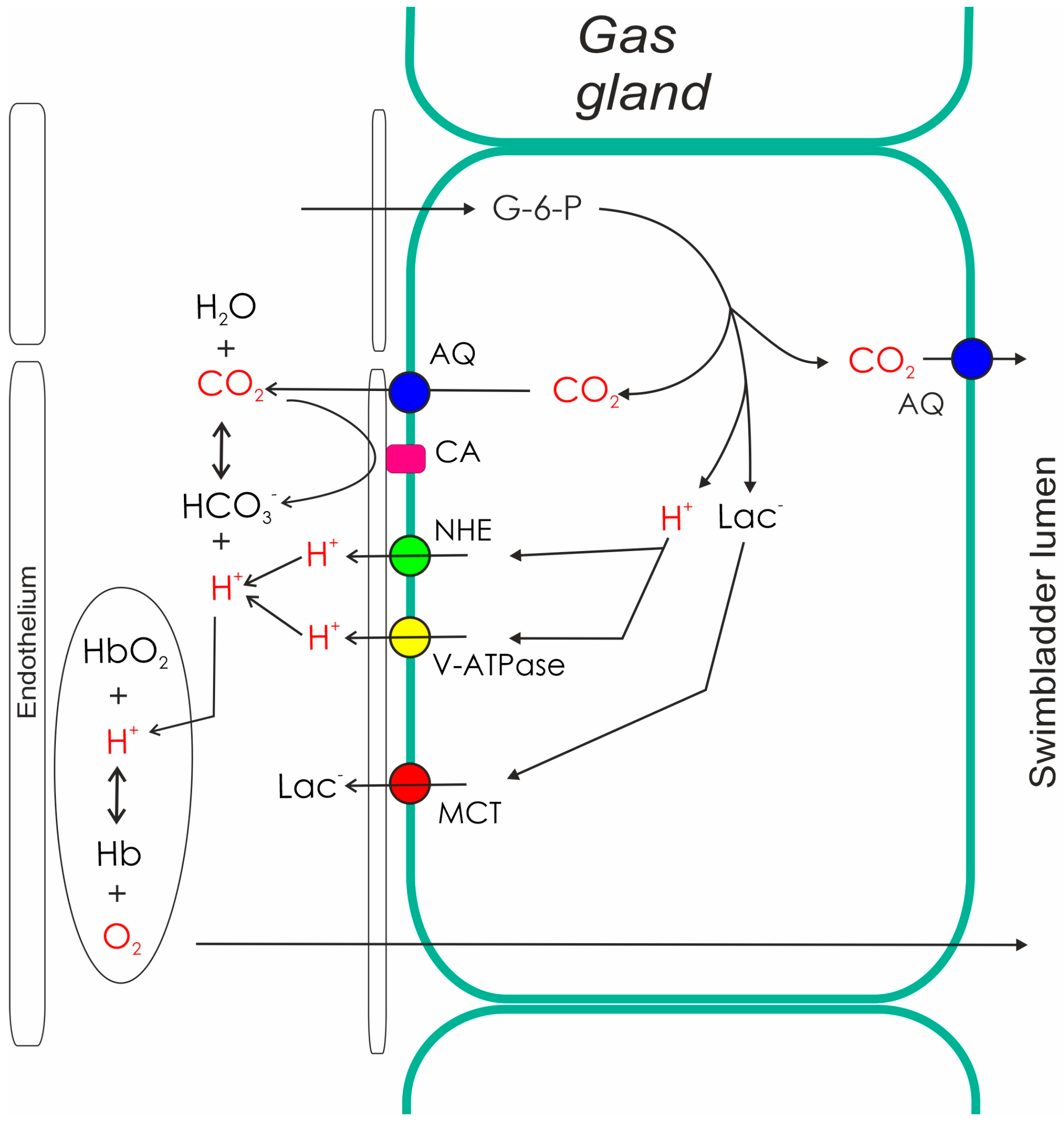
3. Swimbladder Function in Yellow Eels
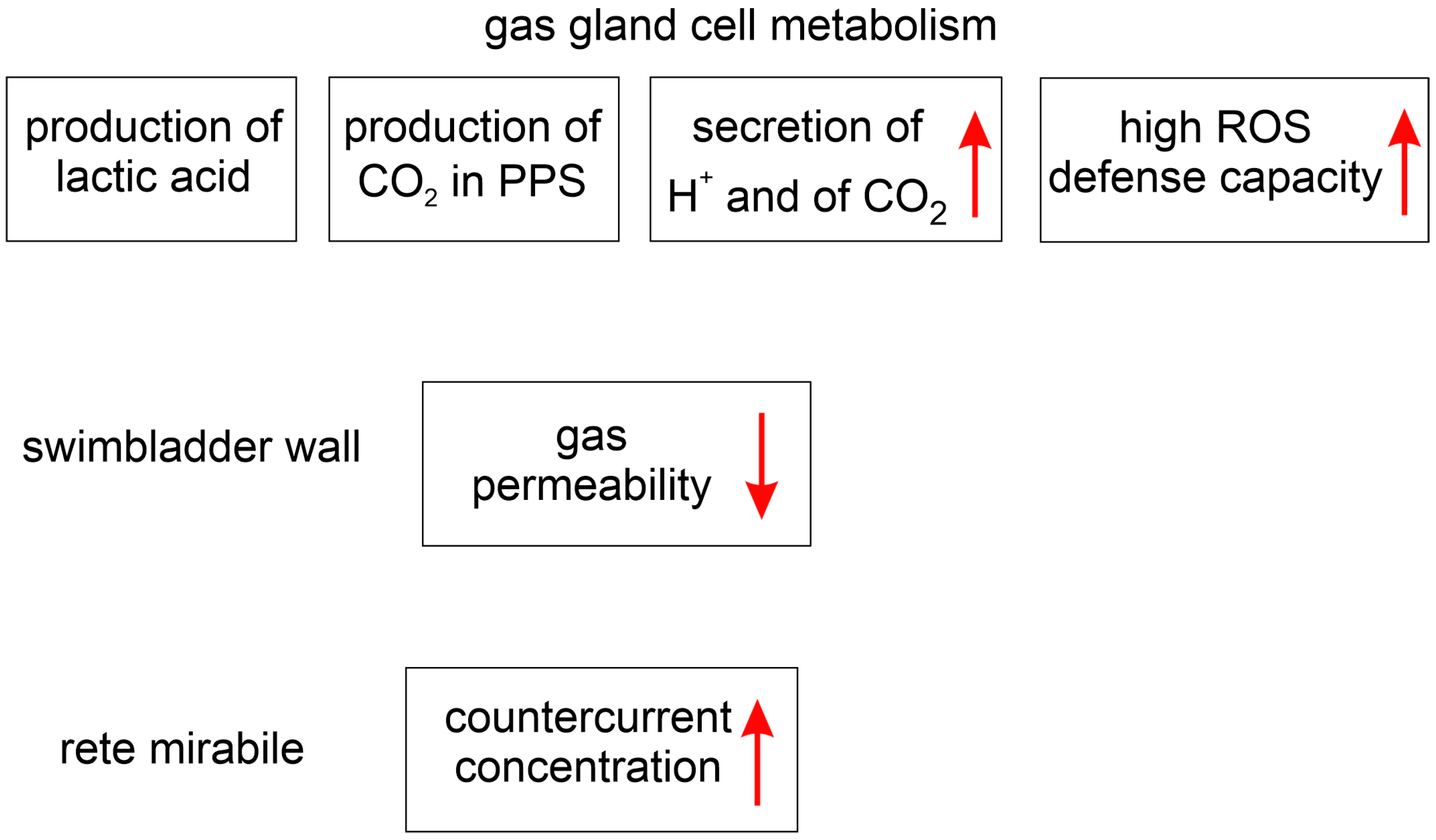
4. The Effect of Silvering
5. Swimbladder Function during the Spawning Migration
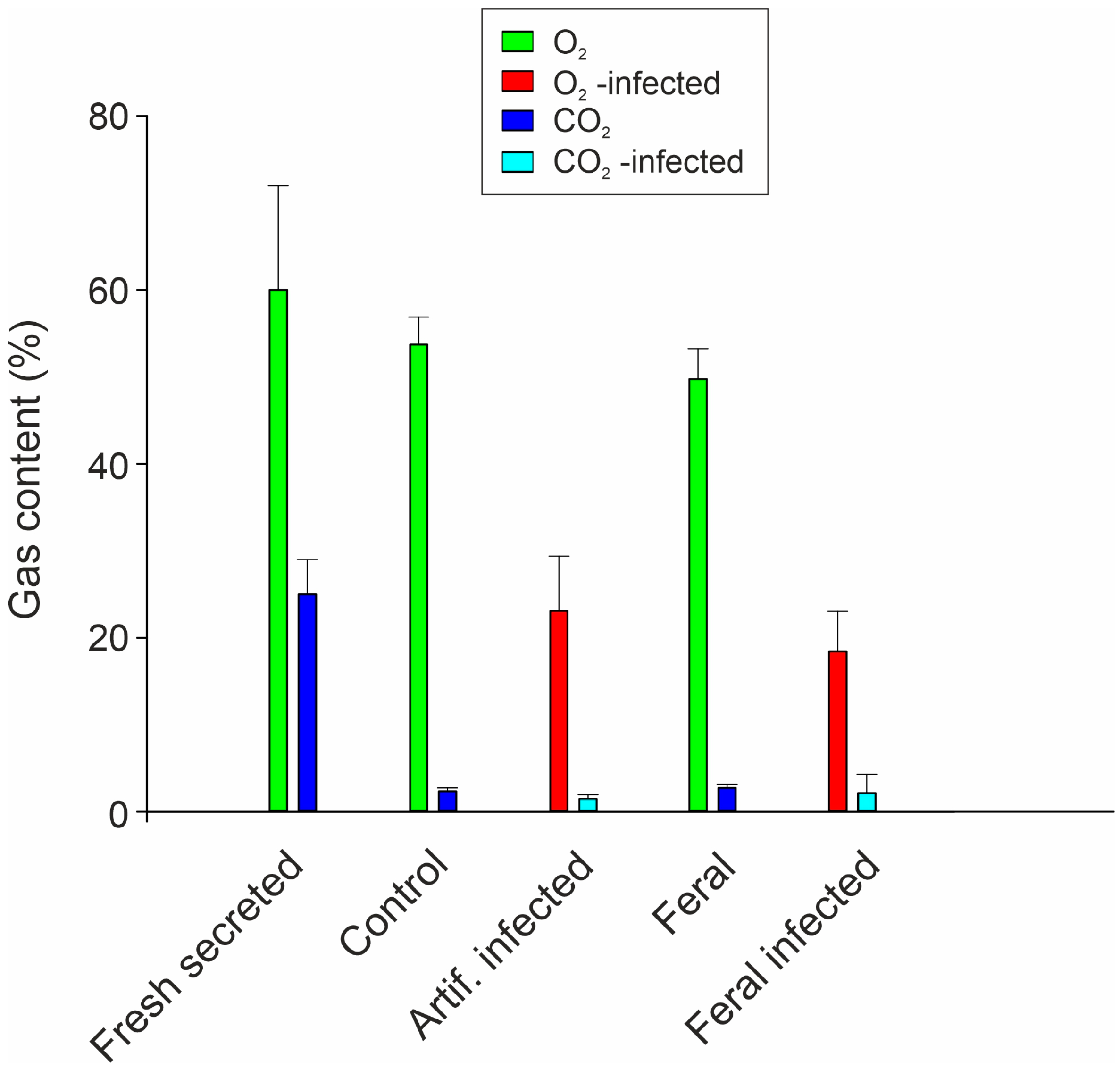
6. The Nematode Anguillicola crassus Impairs Swimbladder Function
7. Future Directions
Funding
Conflicts of Interest
References
- Tzeng, W.N.; Wang, C.H.; Wickström, H.; Reizenstein, M. Occurrence of the semi-catadromous European eel Anguilla anguilla in the Baltic Sea. Marine Biol. 2000, 137, 93–98. [Google Scholar] [CrossRef]
- Wright, R.; Piper, A.; Aarestrup, K.; Azevedo, J.; Cowan, G.; Don, A.; Gollock, M.; Rodriguez Ramallo, S.; Velterop, R.; Walker, A.; et al. First direct evidence of adult European eels migrating to their breeding place in the Sargasso Sea. Sci. Rep. 2022, 12, 15362. [Google Scholar] [CrossRef]
- Righton, D.; Aarestrup, K.; Jellyman, D.; Sebert, P.; Van den Thillart, G.; Tsukamoto, K. The Anguilla spp. migration problem: 40 million years of evolution and two millennia of speculation. J. Fish Biol. 2012, 81, 365–386. [Google Scholar] [CrossRef]
- Righton, D.; Westerberg, H.; Feunteun, E.; Okland, F.; Gargan, P.; Amilhart, E.; Metcalfe, J.; Lobon-Cervia, J.; Sjöberg, J.; Acou, A.; et al. Empirical observations of the spawning migration of European eels: The long and dangerous road to the Sargasso Sea. Sci. Adv. 2016, 2, e1501694. [Google Scholar] [CrossRef]
- Wysujack, K.; Westerberg, H.; Aarestrup, K.; Trautner, J.; Kurwie, T.; Nagel, F.; Hanel, R. The migration behaviour of European silver eels (Anguilla anguilla) released in open ocean conditions. Mar. Freshw. Res. 2015, 66, 145–157. [Google Scholar] [CrossRef]
- Aarestrup, K.; Okland, F.; Hansen, M.; Righton, D.; Gargan, P.; Castonguay, M.; Bernatchez, L.; Howey, P.; Sparholt, H.; Pedersen, M.; et al. Oceanic spawning migration of the european eel (Anguilla anguilla). Science 2009, 325, 1660. [Google Scholar] [CrossRef] [PubMed]
- Kaup, J.J. Catalogue of Apodal Fish, in the Collection of the British Museum; British Museum: London, UK, 1856. [Google Scholar]
- Grassi, G.B.; Calandruccio, S. Fortpflanzung und Metamorphose des Aales. Allg. Fisch Zeitung. 1897, 22, 402–408. [Google Scholar]
- Tesch, F.-W. Der Aal; Paul Parey Verlag: Singhofen, Germany, 1999. [Google Scholar]
- Bonhommeau, S.; Castonguay, M.; Rivot, E.; Sabatié, R.; Le Pape, O. The duration of migration of Atlantic Anguilla larvae. Fish Fish. 2010, 11, 289–306. [Google Scholar] [CrossRef]
- Tsukamoto, K.; Yamada, Y.; Okamura, A.; Kaneko, T.; Tanaka, H.; Miller, M.; Horie, N.; Mikawa, N.; Utoh, T.; Tanaka, S. Positive buoyancy in eel leptocephali: An adaptation for life in the ocean surface layer. Mar. Biol. 2009, 156, 835–846. [Google Scholar] [CrossRef]
- Durif, C.M.F.; van Ginneken, V.; Dufour, S.; Müller, T.; Elie, P. Seasonal Evolution and Individual Differences in Silvering Eels from Different Locations. In Spawning Migration of the European Eel; Springer: Dordrecht, The Netherlands, 2009; pp. 13–38. [Google Scholar]
- Rousseau, K.; Aroua, S.; Schmitz, M.; Elie, P.; Dufour, S. Silvering: Metamorphosis or Puberty? In Spawning Migration of the European Eel; Springer: Dordrecht, The Netherlands, 2009; pp. 39–63. [Google Scholar]
- van Ginneken, A.C.G.; Maes, G.E. The European eel (Anguilla anguilla, Linnaeus), its lifecycle, evolution and reproduction: A literature review. Rev. Fish Biol. Fish. 2005, 15, 367–398. [Google Scholar] [CrossRef]
- van Ginneken, V.; Durif, C.; Balm, S.; Boot, R.; Verstegen, M.; Antonissen, E.; van den Thillart, G. Silvering of European eel (Anguilla anguilla L.): Seasonal changes of morphological and metabolic parameters. Anim. Biol. 2007, 57, 63–77. [Google Scholar] [CrossRef]
- Kleckner, R.C.; Krueger, W.H. Changes in swimbladder retial morphology in Anguilla rostrata during premigration metamorphosis. J. Fish Biol. 1981, 18, 569–577. [Google Scholar] [CrossRef]
- Yamada, Y.; Zhang, H.; Okamura, A.; Tanaka, S.; Horie, N.; Mikawa, N.; Utoh, T.; Oka, H. Morphological and histological changes in the swim bladder during maturation of the Japanese eel. J. Fish Biol. 2001, 58, 804–814. [Google Scholar] [CrossRef]
- Kleckner, R.C. Swim bladder volume maintenance related to migratory depth in silver phase Anguilla rostrata. Science 1980, 208, 1481–1482. [Google Scholar] [CrossRef] [PubMed]
- Kleckner, R.C. Swimbladder wall guanine enhancement related to migratory depth in silver phase Anguilla rostrata. Comp. Biochem. Physiol. Part A 1980, 65, 351–354. [Google Scholar] [CrossRef]
- Zwerger, P.; Nimeth, K.; Würtz, J.; Salvenmoser, W.; Pelster, B. Development of the swimbladder in the European eel (Anguilla anguilla). Cell Tissue Res. 2002, 307, 155–164. [Google Scholar] [CrossRef] [PubMed]
- Prem, C.; Salvenmoser, W.; Würtz, J.; Pelster, B. Swimbladder gas gland cells produce surfactant: In vivo and in culture. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2000, 279, 2336–2343. [Google Scholar] [CrossRef] [PubMed]
- Dorn, E. Über den Feinbau der Schwimmblase von Anguilla vulgaris L. Licht- und Elektronenmikroskopische Untersuchungen. Zeitschrift für Zellforsch. 1961, 55, 849–912. [Google Scholar] [CrossRef]
- Alexander, R.M. Physical aspects of swimbladder function. Biol. Rev. 1966, 41, 141–176. [Google Scholar] [CrossRef]
- Pelster, B. Buoyancy control in aquatic vertebrates. In Cardio-Respiratory Control in Vertebrates; Glass, M.L., Wood, S.C., Eds.; Springer: Berlin/Heidelberg, Germany, 2009; pp. 65–98. [Google Scholar]
- Wagner, R.C.; Froehlich, R.; Hossler, F.E.; Andrews, S.B. Ultrastructure of capillaries in the red body (rete mirabile) of the eel swim bladder. Microvasc. Res. 1987, 34, 349–362. [Google Scholar] [CrossRef]
- Pelster, B. The swimbladder. In Eel Physiology; Trischitta, F., Takei, Y., Sebert, P., Eds.; CRC Press: Enfield, FL, USA, 2013; pp. 44–67. [Google Scholar]
- Fänge, R. The mechanisms of gas transport in the euphysoclist swimbladder. Acta Physiol. Scand. Suppl. 1953, 30, 1–133. [Google Scholar] [PubMed]
- Jones, F.R.H.; Marshall, N.B. The structure and functions of the teleostean swimbladder. Biol. Bull. 1953, 28, 16–83. [Google Scholar]
- Steen, J.B. The physiology of the swimbladder in the eel Anguilla vulgaris. III. The mechanism of gas secretion. Acta Physiol. Scand. 1963, 59, 221–241. [Google Scholar] [CrossRef] [PubMed]
- Pelster, B.; Scheid, P. Glucose Metabolism of the Swimbladder Tissue of the European Eel Anguilla anguilla. J. Exp. Biol. 1993, 185, 169–178. [Google Scholar] [CrossRef]
- Pelster, B. Metabolism of the swimbladder tissue. Biochem. Mol. Biol. Fishes 1995, 4, 101–118. [Google Scholar]
- Drechsel, V.; Schneebauer, G.; Sandbichler, A.M.; Fiechtner, B.; Pelster, B. Oxygen consumption and acid secretion in isolated gas gland cells of the European eel Anguilla anguilla. J. Comp. Physiol. B 2022, 192, 447–457. [Google Scholar] [CrossRef] [PubMed]
- Walsh, P.J.; Milligan, C.L. Roles of buffering capacity and pentose phosphate pathway activity in the gas gland of the gulf toadfish Opsanus beta. J. Exp. Biol. 1993, 176, 311–316. [Google Scholar] [CrossRef]
- Pelster, B.; Hicks, J.; Driedzic, W.R. Contribution of the Pentose Phosphate Shunt to the formation of CO2 in swimbladder tissue of the eel. J. Exp. Biol. 1994, 197, 119–128. [Google Scholar] [CrossRef]
- Pelster, B. Mechanisms of acid release in isolated gas gland cells of the European eel Anguilla anguilla. Am. J. Physiol. 1995, 269, R793–R799. [Google Scholar] [CrossRef]
- Sialana, F.J.; Schneebauer, G.; Paunkov, A.; Pelster, B.; Lubec, G. Proteomic Studies on the Swim Bladder of the European Eel (Anguilla anguilla). Proteomics 2018, 18, 1700445. [Google Scholar] [CrossRef]
- Pelster, B.; Schneebauer, G.; Dirks, R.P. Anguillicola crassus infection significantly affects the silvering related modifications in steady state mRNA levels in gas gland tissue of the European eel. Front. Physiol. 2016, 7, 175. [Google Scholar] [CrossRef]
- Schneebauer, G.; Dirks, R.P.; Pelster, B. Anguillicola crassus infection affects mRNA expression levels in gas gland tissue of European yellow and silver eel. PLoS ONE 2017, 12, e0183128. [Google Scholar] [CrossRef] [PubMed]
- Niederstätter, H.; Pelster, B. Expression of two vacuolar-type ATPase B subunit isoforms in swimbladder gas gland cells of the European eel: Nucleotide sequences and deduced amino acid sequences. Bba. Gene Struct. Express 2000, 1491, 133–142. [Google Scholar] [CrossRef]
- Pelster, B. pH regulation and swimbladder function in fish. Respir. Physiol. Neurobiol. 2004, 144, 179–790. [Google Scholar] [CrossRef]
- Würtz, J.; Taraschewski, H.; Pelster, B. Changes in gas composition in the swimbladder of the European eel (Anguilla anguilla) infected with Anguillicola crassus (Nematoda). Parasitology 1996, 112, 233–238. [Google Scholar] [CrossRef] [PubMed]
- Drechsel, V.; Schneebauer, G.; Fiechtner, B.; Cutler, C.P.; Pelster, B. Aquaporin expression and cholesterol content in eel swimbladder tissue. J. Fish Biol. 2022, 100, 609–618. [Google Scholar] [CrossRef]
- Chen, L.M.; Zhao, J.; Musa-Aziz, R.; Pelletier, M.F.; Drummond, I.A.; Boron, W.F. Cloning and characterization of a zebrafish homologue of human AQP1: A bifunctional water and gas channel. AJP-Regul. Integr. Comp. Physiol. 2010, 299, R1163–R1174. [Google Scholar] [CrossRef]
- Perry, S.F.; Braun, M.H.; Noland, M.; Dawdy, J.; Walsh, P.J. Do zebrafish Rh proteins act as dual ammonia-CO2 channels? J. Exp. Zool. Part A Ecol. Genet. Physiol. 2010, 313, 618–621. [Google Scholar] [CrossRef]
- Talbot, K.; Kwong, R.W.M.; Gilmour, K.M.; Perry, S.F. The water channel aquaporin-1a1 facilitates movement of CO2 and ammonia in zebrafish (Danio rerio) larvae. J. Exp. Biol. 2015, 218, 3931–3940. [Google Scholar] [CrossRef] [PubMed]
- Pelster, B. The Generation of Hyperbaric Oxygen Tensions in Fish. Physiology 2001, 16, 287–291. [Google Scholar] [CrossRef]
- Root, R.W. The respiratory function of the blood of marine fishes. Biol. Bull. 1931, 61, 427–456. [Google Scholar] [CrossRef]
- Berenbrink, M.; Koldkjaer, P.; Kepp, O.; Cossins, A.R. Evolution of Oxygen Secretion in Fishes and the Emergence of a Complex Physiological System. Science 2005, 307, 1752–1757. [Google Scholar] [CrossRef]
- Berenbrink, M.; Koldkaer, P.; Hannah Wright, E.; Kepp, O.; Jose da Silva, A. Magnitude of the Root effect in red blood cells and haemoglobin solutions of fishes: A tribute to August Krogh. Acta Physiol. 2011, 202, 583–592. [Google Scholar] [CrossRef] [PubMed]
- Kuhn, W.; Ramel, A.; Kuhn, H.J.; Marti, E. The filling mechanism of the swimbladder. Generation of high gas pressures through hairpin countercurrent multiplication. Experientia 1963, 19, 497–511. [Google Scholar] [CrossRef]
- Kobayashi, H.; Pelster, B.; Scheid, P. CO2 back-diffusion in the rete aids O2 secretion in the swimbladder of the eel. Respir. Physiol. 1990, 79, 231–242. [Google Scholar] [CrossRef]
- Kobayashi, H.; Pelster, B.; Scheid, P. Water and lactate movement in the swimbladder of the eel, Anguilla anguilla. Respir. Physiol. 1989, 78, 45–57. [Google Scholar] [CrossRef] [PubMed]
- Schneebauer, G.; Drechsel, V.; Dirks, R.; Faserl, K.; Sarg, B.; Pelster, B. Expression of transport proteins in the rete mirabile of european silver and yellow eel. BMC Genom. 2021, 22, 866. [Google Scholar] [CrossRef]
- Kelley, J.L.; Taylor, I.; Hart, N.S.; Partridge, J.C. Aquatic prey use countershading camouflage to match the visual background. Behav. Ecol. 2017, 28, 1314–1322. [Google Scholar] [CrossRef]
- Pankhurst, N.W. Relation of visual changes to the onset of sexual maturation in the European eel Anguilla anguilla (L.). J. Fish Biol. 1982, 21, 127–140. [Google Scholar] [CrossRef]
- Schneebauer, G.; Hanel, R.; Pelster, B. Anguillicola crassus impairs the silvering-related enhancements of the ROS defense capacity in swimbladder tissue of the European eel (Anguilla anguilla). J. Comp. Physiol. B 2016, 186, 867–877. [Google Scholar] [CrossRef]
- Westerberg, H.; Lagenfelt, I.; Svedang, H. Silver eel migration behaviour in the Baltic. ICES J. Mar. Sci. 2007, 64, 1457–1462. [Google Scholar] [CrossRef]
- Wahlberg, M.; Westerberg, H.; Aarestrup, K.; Feunteun, E.; Gargan, P.; Righton, D. Evidence of marine mammal predation of the European eel (Anguilla anguilla L.) on its marine migration. Deep Sea Res. Part I Oceanogr. Res. Pap. 2014, 86, 32–38. [Google Scholar] [CrossRef]
- Palstra, A.; van Ginneken, V.; van den Thillart, G. Cost of transport and optimal swimming speed in farmed and wild European silver eels (Anguilla anguilla). Comp. Biochem. Physiol. Part A Mol. Integr. Physiol. 2008, 151, 37–44. [Google Scholar] [CrossRef]
- Trancart, T.; Tudorache, C.; van den Thillart, G.; Acou, A.; Carpentier, A.; Boinet, C.; Gouchet, G.; Feunteun, E. The effect of thermal shock during diel vertical migration on the energy required for oceanic migration of the European silver eel. J. Exp. Mar. Bio. Ecol. 2015, 463, 168–172. [Google Scholar] [CrossRef]
- Palstra, A.P.; van den Thillart, G. Swimming physiology of European silver eels (Anguilla anguilla L.): Energetic costs and effects on sexual maturation and reproduction. Fish Physiol. Biochem. 2010, 36, 297–322. [Google Scholar] [CrossRef]
- van den Thillart, G.; van Ginneken, V.; Korner, F.; Heijmans, R.; van der Linden, R.; Gluvers, A. Endurance swimming of European eel. J. Fish Biol. 2004, 65, 312–318. [Google Scholar] [CrossRef]
- van Ginneken, V.; Antonissen, E.; Müller, U.K.; Booms, R.; Eding, E.; Verreth, J.; van den Thillart, G. Eel migration to the Sargasso: Remarkably high swimming efficiency and low energy costs. J. Exp. Biol. 2005, 208, 1329–1335. [Google Scholar] [CrossRef] [PubMed]
- Tudorache, C.; Burgerhout, E.; Brittijn, S.; van den Thillart, G. Comparison of swimming capacity and energetics of migratory European eel (Anguilla anguilla) and New Zealand short-finned eel (A. australis). Front. Physiol. 2015, 6, 256. [Google Scholar] [CrossRef] [PubMed]
- Sébert, P.; Scaion, D.; Belhomme, M. High hydrostatic pressure improves the swimming efficiency of European migrating silver eel. Respir. Physiol. Neurobiol. 2009, 165, 112–114. [Google Scholar] [CrossRef]
- Pelster, B. Swimbladder function and the spawning migration of the European eel Anguilla anguilla. Front. Physiol. 2015, 5, 486. [Google Scholar] [CrossRef]
- Pelster, B.; Scheid, P. The influence of gas gland metabolism and blood flow on gas deposition into the swimbladder of the European eel Anguilla anguilla. J. Exp. Biol. 1992, 173, 205–216. [Google Scholar] [CrossRef]
- Pelster, B. Buoyancy at Depth. In Fish Physiology; Randall, C.D.J., Farrel, A.P., Eds.; Academic Press: San Diego, CA, USA, 1997; Volume 15, pp. 195–237. [Google Scholar]
- Haenen, O.L.M.; van Banning, P. Experimental transmission of Anguillicola crassus (Nematoda, Dracunculoidea) larvae from infected prey fish to the eel Anguilla anguilla. Aquaculture 1991, 92, 115–119. [Google Scholar] [CrossRef]
- Lefebvre, F.; Wielgoss, S.; Nagasawa, K.; Moravec, F. On the origin of Anguillicoloides crassus, the invasive nematode of anguillid eels. Aquat. Invasions 2012, 7, 443–453. [Google Scholar] [CrossRef]
- Schabuss, M.; Kennedy, C.R.; Konecny, R.; Grillitsch, B.; Reckendorfer, W.; Schiemer, F.; Herzig, A. Dynamics and predicted decline of Anguillicola crassus infection in European eels, Anguilla anguilla, in Neusiedler See, Austria. J. Helminthol. 2005, 79, 159–167. [Google Scholar] [CrossRef] [PubMed]
- Würtz, J.; Knopf, K.; Taraschewski, H. Distribution and prevalence of Anguillicola crassus (Nematoda) in eels Anguilla anguilla of the rivers Rhine and Naab, Germany. Dis. Aquat. Organ. 1998, 32, 137–143. [Google Scholar] [CrossRef] [PubMed]
- Knopf, K. The swimbladder nematode Anguillicola crassus in the European eel Anguilla anguilla and the Japanese eel Anguilla japonica: Differences in susceptibility and immunity between a recently colonized host and the original host. J. Helminthol. 2006, 80, 129–136. [Google Scholar] [CrossRef] [PubMed]
- Kirk, R.S. The impact of Anguillicola crassus on European eels. Fish. Manag. Ecol. 2003, 10, 385–394. [Google Scholar] [CrossRef]
- Lefebvre, F.; Fazio, G.; Mounaix, B.; Crivelli, A.J. Is the continental life of the European eel Anguilla anguilla affected by the parasitic invader Anguillicoloides crassus? Proc. R. Soc. B Biol. Sci. 2013, 280, 20122916. [Google Scholar] [CrossRef]
- Moravec, F.; Skoríkková, B. Amphibians and larvae of aquatic insects as new paratenic hosts of Anguillicola crassus (Nematoda: Dracunculoidea), a swimbladder parasite of eels. Dis. Aquat. Organ. 1998, 34, 217–222. [Google Scholar] [CrossRef]
- Nimeth, K.; Zwerger, P.; Würtz, J.; Salvenmoser, W.; Pelster, B. Infection of the glass-eel swimbladder with the nematode Anguillicola crassus. Parasitology 2000, 121, 75–83. [Google Scholar] [CrossRef]
- Polzer, M.; Taraschewski, H. Identification and characterization of the proteolytic enzymes in the developmental stages of the eel-pathogenic nematode Anguillicola crassus. Parasitol. Res. 1993, 79, 24–27. [Google Scholar] [CrossRef]
- Boon, J.H.; Cannaerts, V.; Augustijn, H.; Machiels, M.; De Charleroy, D.; Ollevier, F. The effect of different infection levels with infective larvae of Anguillicola crassus on haematological parameters of European eel (Anguilla anguilla). Aquaculture 1990, 87, 243–253. [Google Scholar] [CrossRef]
- Würtz, J.; Taraschewski, H. Histopathological changes in the swimbladder wall of the European eel Anguilla anguilla due to infections with Anguillicola crassus. Dis. Aquat.Org. 2000, 39, 121–134. [Google Scholar] [CrossRef] [PubMed]
- Molnár, K.; Szakolczai, J.; Vetési, F. Histological changes in the swimbladder wall of eels due to abnormal location of adults and second stage larvae of Anguillicola crassus. Acta Vet. Hung. 1995, 43, 125–137. [Google Scholar]
- Barry, J.; McLeish, J.; Dodd, J.A.; Turnbull, J.F.; Boylan, P.; Adams, C.E. Introduced parasite Anguillicola crassus infection significantly impedes swim bladder function in the European eel Anguilla anguilla (L.). J. Fish Dis. 2014, 37, 921–924. [Google Scholar] [CrossRef] [PubMed]
- van Banning, P.; and Haenen, O. Effects of the swimbladder nematode Anguillicola crassus in wild and farmed eel, Anguilla anguilla. In Pathology in Marine Science; Elsevier: Amsterdam, The Netherlands, 1990; pp. 317–330. [Google Scholar]
- Knopf, K.; Madriles Helm, A.; Lucius, R.; Bleiss, W.; Taraschewski, H. Migratory response of European eel (Anguilla anguilla) phagocytes to the eel swimbladder nematode Anguillicola crassus. Parasitol. Res. 2008, 102, 1311–1316. [Google Scholar] [CrossRef]
- Beregi, A.; Molnár, K.; Békési, L.; Székely, C. Radiodiagnostic method for studying swimbladder inflammation caused by Anguillicola crassus (Nematoda:Dracunculoidea). Dis. Aquat. Organ. 1998, 34, 155–160. [Google Scholar] [CrossRef] [PubMed]
- Burgerhout, E.; Minegishi, Y.; Brittijn, S.; de Wijze, D.; Henkel, C.; Jansen, H.; Spaink, H.; Dirks, R.; van den Thillart, G. Changes in ovarian gene expression profiles and plasma hormone levels in maturing European eel (Anguilla anguilla); Biomarkers for broodstock selection. Gen. Comp. Endocrinol. 2016, 225, 185–196. [Google Scholar] [CrossRef] [PubMed]
- Dufour, S.; Burzawa-Gerard, E.; Le Belle, N.; Sbaihi, M.; Vidal, B. Reproductive Endocrinology of the European Eel, Anguilla anguilla. In Eel Biology; Springer: Tokyo, Japan, 2003; pp. 373–383. [Google Scholar]
- Schneebauer, G.; Lindemann, C.; Drechsel, V.; Marohn, L.; Wysujack, K.; Santidrian, E.; Dirks, R.; Hanel, R.; Pelster, B. Swimming under elevated hydrostatic pressure increases glycolytic activity in gas gland cells of the European eel. PLoS ONE 2020, 15, e0239627. [Google Scholar] [CrossRef]
- Palstra, A.P.; Heppener, D.; van Ginneken, V.; Székely, C.; van den Thillart, G. Swimming performance of silver eels is severely impaired by the swim-bladder parasite Anguillicola crassus. J. Exp. Mar. Bio. Ecol. 2007, 352, 244–256. [Google Scholar] [CrossRef]
- Sprengel, G.; Luchtenberg, H. Infection by endoparasites reduces maximum swimming speed of European smelt Osmerus eperlanus and European eel Anguilla anguilla. Dis. Aquat. Organ. 1991, 11, 31–35. [Google Scholar] [CrossRef]
- Newbold, L.R.; Hockley, F.A.; Williams, C.; Cable, J.; Reading, A.; Auchterlonie, N.; Kemp, P. Relationship between European eel Anguilla anguilla infection with non-native parasites and swimming behaviour on encountering accelerating flow. J. Fish Biol. 2015, 86, 1519–1533. [Google Scholar] [CrossRef] [PubMed]
- Simon, J.; Westerberg, H.; Righton, D.; Sjöberg, N.; Dorow, M. Diving activity of migrating silver eel with and without Anguillicola crassus infection. J. Appl. Ichthyol. 2018, 34, 659–668. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pelster, B. Swimbladder Function in the European Eel Anguilla anguilla. Fishes 2023, 8, 125. https://doi.org/10.3390/fishes8030125
Pelster B. Swimbladder Function in the European Eel Anguilla anguilla. Fishes. 2023; 8(3):125. https://doi.org/10.3390/fishes8030125
Chicago/Turabian StylePelster, Bernd. 2023. "Swimbladder Function in the European Eel Anguilla anguilla" Fishes 8, no. 3: 125. https://doi.org/10.3390/fishes8030125
APA StylePelster, B. (2023). Swimbladder Function in the European Eel Anguilla anguilla. Fishes, 8(3), 125. https://doi.org/10.3390/fishes8030125