The Effects of Different LED Lights on the Main Nutritional Compositions of Isochrysis zhanjiangensis
Abstract
1. Introduction
2. Materials and Methods
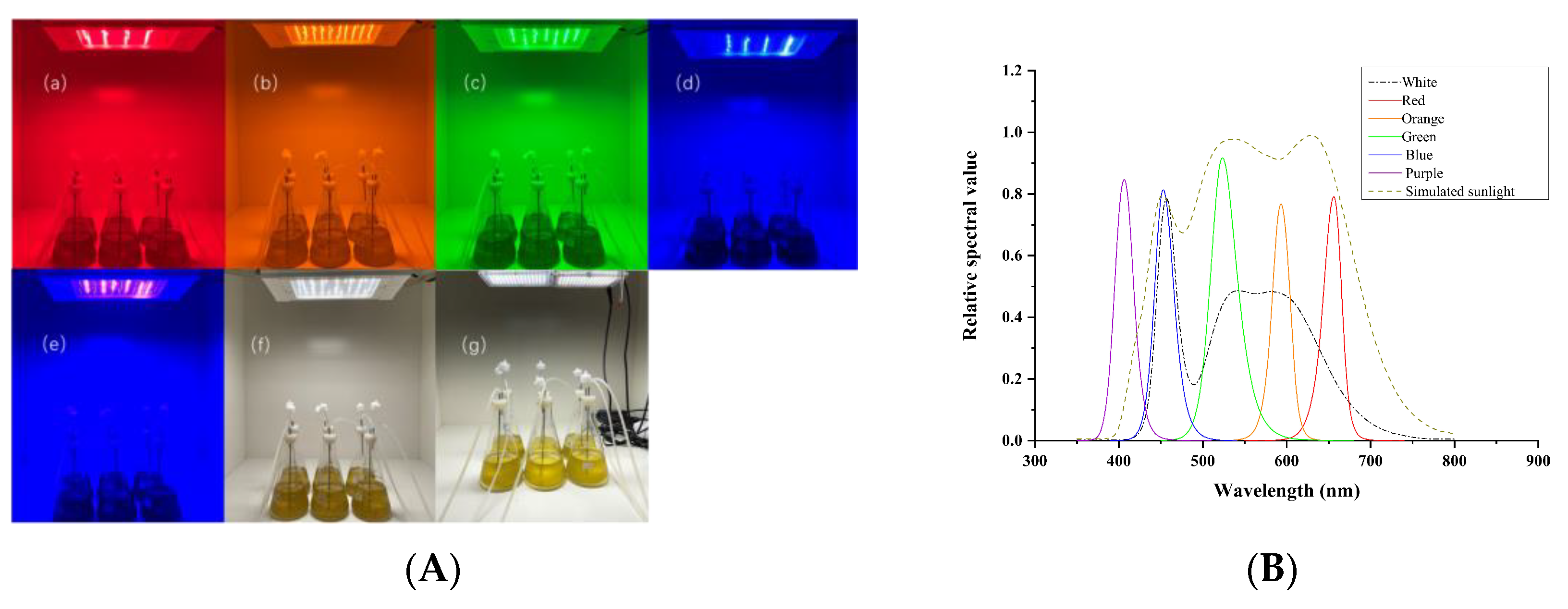
2.1. Instruments and Equipment
2.2. Microalgae Cultivation and Harvesting
2.3. Determination of Soluble Protein Content
2.4. Determination of Soluble Carbohydrate Content
2.5. Fatty Acid Analysis
2.6. Statistical Analysis
3. Results
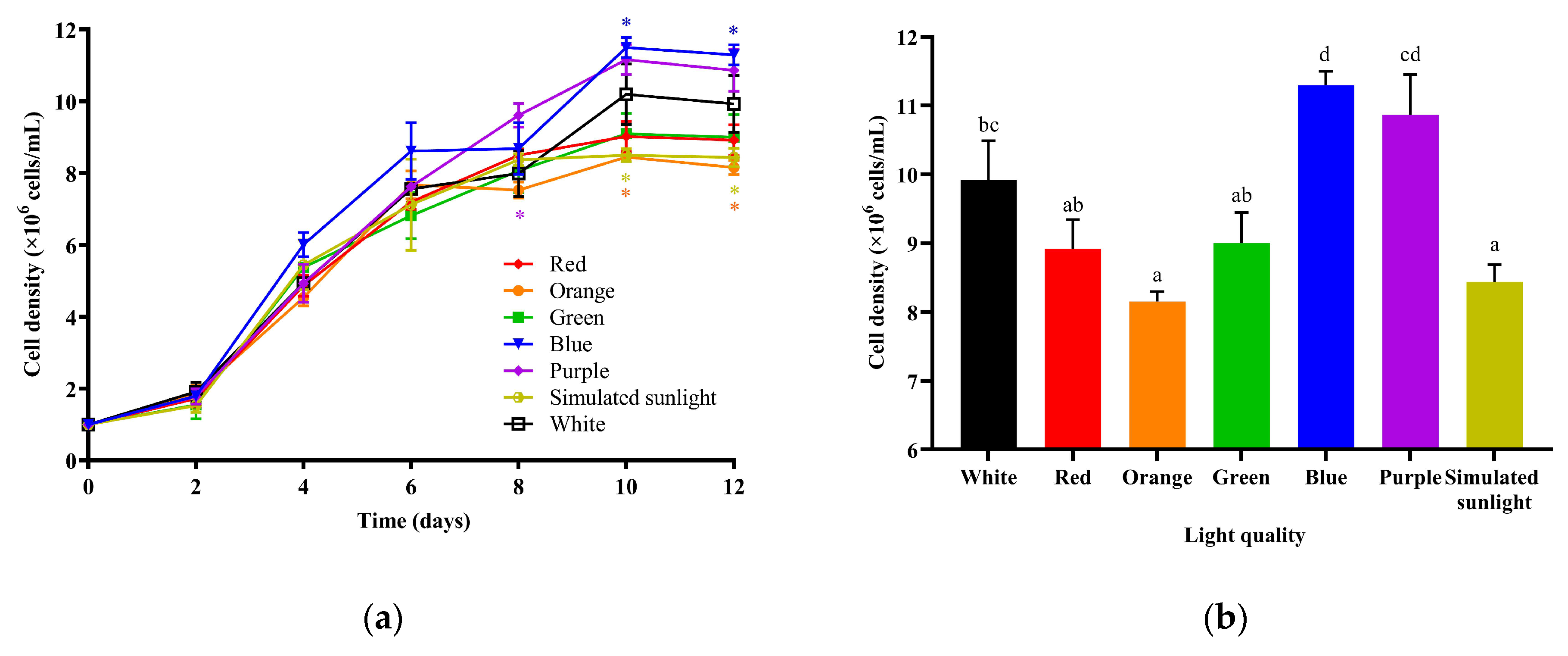
3.1. The Effects of Different LED Lights on the Growth of I. zhanjiangensis
3.2. The Effects of Light Source on the Fatty Acid Composition of I. zhanjiangensis
3.3. The effects of Light Source on Soluble Protein and Soluble Carbohydrate in I. zhanjiangensis
4. Discussion
4.1. The Effects of Light Source on the Growth of I. zhanjiangensis
4.2. The Effects of Different Lights on the Fatty Acids of I. zhanjiangensis
4.3. The Effects of Different Lights on Soluble Protein and Soluble Carbohydrate of I. zhanjiangensis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Little, D.C.; Newton, R.W.; Beveridge, M.C.M. Aquaculture: A rapidly growing and significant source of sustainable food? Status, transitions and potential. Proc. Nutr. Soc. 2016, 75, 274–286. [Google Scholar] [CrossRef]
- Wijsman, J.W.M.; Troost, K.; Fang, J.; Roncarati, A. Global Production of Marine Bivalves. Trends and Challenges. In Goods and Services of Marine Bivalves; Springer: Berlin, Germany, 2019; pp. 7–26. [Google Scholar]
- Mishra, B.; Tiwari, A.; Mahmoud, A.E.D. Microalgal potential for sustainable aquaculture applications: Bioremediation, biocontrol, aquafeed. Clean Technol. Environ. Policy 2022, 25, 675–687. [Google Scholar] [CrossRef]
- Dineshbabu, G.; Goswami, G.; Kumar, R.; Ankan, S.; Debasish, D. Microalgae–nutritious, sustainable aqua-and animal feed source. J. Funct. Foods 2019, 62, 103545. [Google Scholar] [CrossRef]
- Hemaiswarya, S.; Raja, R.; Ravi Kumar, R.; Ganesan, V.; Anbazhagan, C. Microalgae: A sustainable feed source for aquaculture. World J. Microbiol. Biotechnol. 2011, 27, 1737–1746. [Google Scholar] [CrossRef]
- Da Fontoura Prates, D.; Duarte, J.H.; Vendruscolo, R.G.; Wagner, R.; Ballus, C.A.; da Silva Oliveira, W.; Godoy, H.T.; Barcia, M.T.; Morais, M.G.; Radmann, E.M.; et al. Role of light emitting diode (LED) wavelengths on increase of protein productivity and free amino acid profile of Spirulina sp. cultures. Bioresour. Technol. 2020, 306, 123184. [Google Scholar] [CrossRef]
- Pereira, S.; Ptero, A. Haematococcus pluvialis bioprocess optimization: Effect of light quality, temperature and irradiance on growth, pigment content and photosynthetic response. Algal Res. 2020, 51, 102027. [Google Scholar] [CrossRef]
- Chen, H.B.; Wu, J.Y.; Wang, C.F.; Fu, C.C.; Shieh, C.J.; Chen, C.I.; Wang, C.Y.; Liu, Y.C. Modeling on chlorophyll a and phycocyanin production by Spirulina platensis under various light-emitting diodes. Biochem. Eng. J. 2010, 53, 52–56. [Google Scholar] [CrossRef]
- Duarte, J.H.; Costa, J.A.V. Blue light emitting diodes (LEDs) as an energy source in Chlorella fusca and Synechococcus nidulans cultures. Bioresour. Technol. 2017, 247, 1242–1245. [Google Scholar] [CrossRef]
- Zhang, H.; Chen, A.; Huang, L.; Zhang, C.; Gao, B. Transcriptomic Analysis Unravels the Modulating Mechanisms of the Biomass and Value-Added Bioproducts Accumulation by Light Spectrum in Eustigmatos cf. polyphem (Eustigmatophyceae). Bioresour. Technol. 2021, 338, 125523. [Google Scholar] [CrossRef]
- Li, Y.X.; Cai, X.; Gu, W.; Wang, G. Transcriptome analysis of carotenoid biosynthesis in Dunaliella salina under red and blue light. J. Oceanol. Limnol. 2020, 38, 179–187. [Google Scholar] [CrossRef]
- Pagels, F.; Lopes, G.; Vasconcelos, V.; Guedesa, A.C. White and red LEDs as two-phase batch for cyanobacterial pigments production. Bioresour. Technol. 2020, 307, 123105. [Google Scholar] [CrossRef]
- Ra, C.H.; Kang, C.H.; Jung, J.H.; Jeong, G.T.; Kim, S.K. Effects of light-emitting diodes (LEDs) on the accumulation of lipid content using a two-phase culture process with three microalgae. Bioresour. Technol. 2016, 212, 254–261. [Google Scholar] [CrossRef]
- Jung, J.H.; Sirisuk, P.; Ra, C.H.; Kim, J.M.; Jeong, G.T.; Kim, S.K. Effects of green LED light and three stresses on biomass and lipid accumulation with two-phase culture of microalgae. Process Biochem. 2019, 77, 93–99. [Google Scholar] [CrossRef]
- Kim, S.H.; Sunwoo, I.Y.; Hong, H.J.; Awah, C.C.; Jeong, G.T.; Kim, S.K. Lipid and unsaturated fatty acid productions from three microalgae using nitrate and light-emitting diodes with complementary LED wavelength in a two-phase culture system. Bioprocess Biosyst. Eng. 2019, 42, 1517–1526. [Google Scholar] [CrossRef]
- Suhaimi, N.B.; Kwan, P.P.; Banerje, S.; Shariff, M. Influence of blue and yellow light-emitting diodes on the lipid and carbohydrate composition of Isochrysis galbana. Aquac. Res. 2021, 52, 3226–3232. [Google Scholar] [CrossRef]
- Chen, K.; Wang, Y.Y.; Yang, Y.; Cao, J.Y.; Cao, X.P.; Xu, J.L. Analysis of relationship between bacterial community and high temperature tolerance of Isochrysis zhangjiangensis. J. Nucl. Agric. Sci. 2021, 35, 807–814. [Google Scholar]
- Soudant, P.; Chu, F.L.E.; Samain, J.F. Lipids requirements in some economically important marine bivalves. J. Shellfish. Res. 2000, 19, 605. [Google Scholar]
- Lv, B.; Liu, Z.; Chen, Y.; Lan, S.; Mao, J.; Gu, Z.; Wang, A.; Yu, F.; Zheng, X.; Vasquez, H.E. Effect of Different Colored LED Lighting on the Growth and Pigment Content of Isochrysis zhanjiangensis under Laboratory Conditions. J. Mar. Sci. Eng. 2022, 10, 1752. [Google Scholar] [CrossRef]
- Li, S.; Chen, X.; Wong, M.H.; Chen, H.; Tao, L.; Liufu, G.; Cheng, J.J.; Yang, X. Mechanism study on the regulation of metabolite flux for producing promising bioactive substances in microalgae Desmodesmus sp.YT through salinity stress. Algal Res. 2022, 64, 102721. [Google Scholar] [CrossRef]
- Wang, Y.Y.; Xu, S.M.; Cao, J.Y.; Wu, M.N.; Lin, J.H.; Zhou, C.-X.; Zhang, L.; Zhou, H.-B.; Li, Y.-R.; Xu, J.-L.; et al. Co-cultivation of Isochrysis galbana and Marinobacter sp. can enhance algal growth and docosahexaenoic acid production. Aquaculture 2022, 556, 738248. [Google Scholar] [CrossRef]
- Liu, X.; Hong, Y.; Liu, Y. Cultivation of Chlorella sp. HQ in inland saline-alkaline water under different light qualities. Front. Environ. Sci. Eng. 2022, 16, 1–10. [Google Scholar] [CrossRef]
- Sirisuk, P.; Ra, C.H.; Jeong, G.T.; Kim, S.K. Effects of wavelength mixing ratio and photoperiod on microalgal biomass and lipid production in a two-phase culture system using LED illumination. Bioresour. Technol. 2018, 253, 175–181. [Google Scholar] [CrossRef]
- Peraman, M.; Nachimuthu, S. Identification and quantification of fucoxanthin in selected carotenoid-producing marine microalgae and evaluation for their chemotherapeutic potential. Pharmacogn. Mag. 2019, 15, 243–249. [Google Scholar] [CrossRef]
- Ra, C.H.; Sirisuk, P.; Jung, J.H.; Jeong, G.T.; Kim, S.K. Effects of light-emitting diode (LED) with a mixture of wavelengths on the growth and lipid content of microalgae. Bioprocess Biosyst. Eng. 2018, 41, 457–465. [Google Scholar] [CrossRef]
- Huang, J.J.; Lin, S.L.; Xu, W.W.; Cheung, P.C.K. Enhancement of the production of bioactive microalgal metabolites by ultraviolet radiation (uva 365 nm). J. Agric. Food Chem. 2018, 66, 10215–10224. [Google Scholar] [CrossRef]
- Ran, Z.; Kong, F.; Xu, J.; Liao, K.; Xu, X.; Shi, P.; Chen, K.; Zhou, C.; Yan, X. Fad and Elovl expressions, fatty acid compositions, and feed effects of three representative microalgae in Sinonovacula constricta (Lamarck 1818) at early developmental stages. Aquaculture 2020, 521, 735101. [Google Scholar] [CrossRef]
- Ma, R.; Thomas-Hall, S.R.; Chua, E.T.; Eltanahy, E.; Netzel, M.E.; Netzel, Z.; Lu, Y.; Schenk, P.M. LED power efficiency of biomass, fatty acid, and carotenoid production in Nannochloropsis microalgae. Bioresour. Technol. 2018, 252, 118–126. [Google Scholar] [CrossRef]
- Amaro, H.M.; Pagels, F.; Azevedo, I.C.; Azevedo, J.; Pinto, I.S.; Malcata, F.X.; Guedes, A.C. Light-emitting diodes—A plus on microalgae biomass and high-value metabolite production. J. Appl. Phycol. 2020, 32, 3605–3618. [Google Scholar] [CrossRef]
- Pagels, F.; Bonomi-Barufi, J.; Vega, J.; Abdala-Díaz, R.; Vasconcelos, V.; Guedes, A.C.; Figueroa, F.L. Light quality triggers biochemical modulation of Cyanobium sp.—Photobiology as tool for biotechnological optimization. J. Appl. Phycol. 2020, 32, 2851–2861. [Google Scholar] [CrossRef]
- Chai, Y.; Wu, Y.; Zhao, H.H.; Yu, D. Effect of light qualities on growth and fatty acid composition of Isochrysis zhanjiangensis Hu & Liu. Plant Physiol. Commun. 2009, 45, 571–574. [Google Scholar]
- Ratledge, C. Fatty acid biosynthesis in microorganisms being used for single cell oil production. Biochimie 2004, 86, 807–815. [Google Scholar] [CrossRef]
- Bišová, K.; Zachleder, V. Cell-cycle regulation in green algae dividing by multiple fission. J. Exp. Bot. 2014, 65, 2585–2602. [Google Scholar] [CrossRef]
- Li, X.P.; Manuel, J.; Slavens, S.; Crunkleton, D.W.; Johannes, T.W. Interactive effects of light quality and culturing temperature on algal cell size, biomass doubling time, protein content, and carbohydrate content. Appl. Microbiol. Biotechnol. 2021, 105, 587–597. [Google Scholar] [CrossRef]
- Kaur, M.; Saini, K.C.; Ojah, H.; Sahoo, R.; Gupta, K.; Kumar, A.; Bast, F. Abiotic stress in algae: Response, signaling and transgenic approaches. J. Appl. Phycol. 2022, 34, 1843–1869. [Google Scholar] [CrossRef]
- Yaakob, M.A.; Mohamed, R.M.S.R.; Al-Gheethi, A.; Gokare, R.A.; Ambati, R.R. Influence of nitrogen and phosphorus on microalgal growth, biomass, lipid, and fatty acid production: An overview. Cells 2021, 10, 393. [Google Scholar] [CrossRef]
- Mirizadeh, S.; Nosrati, M.; Shojaosadati, S.A. Synergistic effect of nutrient and salt stress on lipid productivity of Chlorella vulgaris through two-stage cultivation. BioEnergy Res. 2020, 13, 507–517. [Google Scholar] [CrossRef]
| Fatty Acid | White | Red | Orange | Green | Blue | Purple | Simulated Sunlight |
|---|---|---|---|---|---|---|---|
| C14:0 | 17.23 ± 0.11 c | 16.65 ± 0.85 bc | 16.70 ± 0.56 c | 16.85 ± 0.44 c | 14.13 ± 0.47 a | 16.14 ± 0.44 bc | 15.68 ± 0.33 b |
| C15:0 | 0.69 ± 0.03 | 0.64 ± 0.14 | 0.66 ± 0.04 | 0.69 ± 0.03 | 0.63 ± 0.02 | 0.75 ± 0.02 | 0.61 ± 0.01 |
| C16:0 | 13.17 ± 0.10 a | 13.23 ± 0.39 ab | 13.49 ± 0.56 abc | 13.21 ± 0.23 ab | 13.21 ± 0.56 ab | 14.15 ± 0.14 bc | 14.24 ± 0.15 c |
| C17:0 | 0.27 ± 0.03 | 0.24 ± 0.02 | ND | 0.27 ± 0.01 | 0.22 ± 0.01 | 0.25 ± 0.00 | 0.23 ± 0.01 |
| C18:0 | 1.30 ± 0.28 a | 1.28 ± 0.17 a | 1.02 ± 0.19 a | 1.34 ± 0.07 a | 1.77 ± 0.22 b | 1.20 ± 0.06 a | 1.93 ± 0.15 b |
| C20:0 | 0.52 ± 0.18 b | 0.50 ± 0.08 b | 0.29 ± 0.00 ab | 0.37 ± 0.18 ab | 0.23 ± 0.12 a | 0.44 ± 0.04 ab | 0.45 ± 0.44 ab |
| C22:0 | 1.19 ± 0.09 ab | 1.14 ± 0.02 a | 0.98 ± 0.10 a | 1.22 ± 0.07 ab | 1.42 ± 0.19 bc | 1.28 ± 0.05 abc | 1.51 ± 0.00 c |
| SFA | 34.37 ± 0.67 b | 33.68 ± 1.84 ab | 33.15 ± 2.02 ab | 33.95 ± 0.88 b | 31.62 ± 1.17 a | 34.21 ± 1.03 b | 34.66 ± 0.47 b |
| C14:1n-5 | 0.20 ± 0.03 | 0.22 ± 0.03 | 0.19 ± 0.00 | 0.20 ± 0.01 | 0.12 ± 0.00 | 0.17 ± 0.01 | 0.17 ± 0.01 |
| C16:1n-7 | 3.82 ± 0.26 ab | 3.78 ± 0.68 ab | 4.05 ± 0.58 b | 3.90 ± 0.06 ab | 3.50 ± 0.26 ab | 4.00 ± 0.09 b | 3.24 ± 0.28 a |
| C17:1n-7 | 0.44 ± 0.05 | 0.43 ± 0.09 | 0.37 ± 0.01 | 0.42 ± 0.04 | 0.37 ± 0.02 | 0.42 ± 0.03 | 0.36 ± 0.06 |
| C18:1n-9t | 20.19 ± 0.62 ab | 19.29 ± 1.71 ab | 18.32 ± 1.27 a | 19.55 ± 0.47 ab | 20.60 ± 1.28 b | 19.75 ± 0.51 ab | 21.25 ± 0.50 b |
| C18:1n-9c | 3.45 ± 0.50 | 3.82 ± 0.78 | 3.33 ± 0.58 | 3.39 ± 0.14 | 4.10 ± 0.46 | 3.76 ± 0.36 | 3.55 ± 0.10 |
| C22:1n-9 | 0.46 ± 0.03 b | 0.38 ± 0.00 ab | 0.34 ± 0.08 ab | 0.43 ± 0.06 ab | 0.32 ± 0.03 a | 0.46 ± 0.01 b | 0.33 ± 0.05 a |
| MUFA | 28.57 ± 0.46 | 27.91 ± 1.81 | 26.59 ± 1.07 | 27.88 ± 0.45 | 29.00 ± 0.95 | 28.55 ± 0.81 | 28.91 ± 0.21 |
| C16:2n-6 | 0.65 ± 0.11 b | 0.51 ± 0.06 ab | 0.64 ± 0.09 b | 0.62 ± 0.02 b | 0.49 ± 0.10 ab | 0.60 ± 0.05 b | 0.40 ± 0.05 a |
| C18:2n-6 | 3.39 ± 0.07 bc | 3.63 ± 0.19 bc | 2.75 ± 0.16 a | 3.27 ± 0.12 bc | 4.85 ± 0.17 c | 3.10 ± 0.03 ab | 5.17 ± 0.21 c |
| C18:3n-3 | 6.04 ± 1.05 | 8.08 ± 2.46 | 6.06 ± 0.65 | 6.93 ± 1.21 | 7.20 ± 0.67 | 6.10 ± 0.50 | 6.05 ± 0.32 |
| C18:4n-3 | 15.37 ± 1.03 abc | 14.55 ± 1.66 abc | 16.51 ± 1.52 c | 15.08 ± 0.49 abc | 16.17 ± 1.03 bc | 14.18 ± 0.38 ab | 14.00 ± 0.24 a |
| C20:2n-6 | 0.51 ± 0.06 a | 0.54 ± 0.07 a | 0.38 ± 0.02 ab | 0.49 ± 0.07 a | 0.32 ± 0.11 b | 0.42 ± 0.13 ab | 0.31 ± 0.02 b |
| C20:5n-3 | 0.24 ± 0.02 ab | 0.10 ± 0.05 a | 0.18 ± 0.18 ab | 0.36 ± 0.03 b | 0.18 ± 0.05 ab | 0.32 ± 0.04 b | 0.20 ± 0.07 ab |
| C22:5n-6 | 1.42 ± 0.10 ab | 1.55 ± 0.11 ab | 1.59 ± 0.16 b | 1.49 ± 0.03 ab | 1.38 ± 0.06 a | 1.58 ± 0.06 ab | 1.41 ± 0.01 ab |
| C22:6n-3 | 8.56 ± 0.64 bc | 7.55 ± 0.71 ab | 9.66 ± 0.78 d | 8.67 ± 0.16 bcd | 7.45 ± 0.22 ab | 9.56 ± 0.35 cd | 6.67 ± 0.45 a |
| PUFA | 36.18 ± 1.03 ab | 36.51 ± 3.68 ab | 39.56 ± 1.95 b | 36.91 ± 1.61 ab | 38.56 ± 2.15 ab | 35.86 ± 0.81 ab | 35.21 ± 1.02 a |
| TUFA | 64.59 ± 0.64 a | 64.42 ± 2.05 a | 65.92 ± 0.99 ab | 64.79 ± 0.79 a | 67.04 ± 1.32 b | 64.41 ± 0.79 a | 63.12 ± 0.67 a |
| Light | Maximum Soluble Protein Content (mg/106 cell) | Maximum Soluble Carbohydrate Content (mg/106 cell) |
|---|---|---|
| White | 0.91 ± 0.16 a | 0.99 ± 0.21 a |
| Red | 0.85 ± 0.03 a | 1.09 ± 0.10 a |
| Orange | 1.04 ± 0.13 a | 0.95 ± 0.08 a |
| Green | 0.94 ± 0.04 a | 1.10 ± 0.13 a |
| Blue | 0.83 ± 0.00 a | 0.99 ± 0.66 a |
| Purple | 0.82 ± 0.03 a | 0.94 ± 0.11 a |
| Simulated sunlight | 1.37 ± 0.07 b | 1.26 ± 0.09 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sun, Y.; Zhang, J.; Xu, J.; Cao, J.; Li, Y. The Effects of Different LED Lights on the Main Nutritional Compositions of Isochrysis zhanjiangensis. Fishes 2023, 8, 124. https://doi.org/10.3390/fishes8030124
Sun Y, Zhang J, Xu J, Cao J, Li Y. The Effects of Different LED Lights on the Main Nutritional Compositions of Isochrysis zhanjiangensis. Fishes. 2023; 8(3):124. https://doi.org/10.3390/fishes8030124
Chicago/Turabian StyleSun, Yanbin, Jiaxing Zhang, Jilin Xu, Jiayi Cao, and Yanrong Li. 2023. "The Effects of Different LED Lights on the Main Nutritional Compositions of Isochrysis zhanjiangensis" Fishes 8, no. 3: 124. https://doi.org/10.3390/fishes8030124
APA StyleSun, Y., Zhang, J., Xu, J., Cao, J., & Li, Y. (2023). The Effects of Different LED Lights on the Main Nutritional Compositions of Isochrysis zhanjiangensis. Fishes, 8(3), 124. https://doi.org/10.3390/fishes8030124







