1. Introduction
Lipid, as one of the most essential nutrients for fish, plays a crucial role in their growth, development, and metabolism [
1]. Lipid not only provides energy for fish but also serves as an important component of their bodies [
2]. However, both excessive and insufficient levels of dietary lipid supplementation can have inhibitory effects on growth, development, and disease resistance [
3]. The serum physiological and biochemical indicators of fish are closely related to their nutritional status, metabolism, and diseases [
4,
5]. Therefore, studying the influence of dietary lipid supplementation on the serum biochemical indicators of fish is an important approach for evaluating the nutritional value of feed.
Fish possess natural antioxidant and non-specific immune systems [
6]. Superoxide dismutase (SOD), glutathione peroxidase (GSH-Px), and catalase (CAT) are the main antioxidant enzymes involved in the elimination of free radicals in the fish, while lysozyme (LZM), alkaline phosphatase (ALP), and the complement system are important components of the non-specific immune system [
7,
8,
9]. Previous studies have shown that lipids play a crucial role in regulating the antioxidant and immune responses, and the level of dietary lipid supplementation is closely related to the antioxidant capacity and immune function of fish [
10,
11,
12]. The inflammatory factors include tumor necrosis factor α (TNF-α), interleukin 1β (IL-1β), and interleukin 6 (IL-6). These factors are important inflammatory mediators in animals and play a significant role in immune regulation. Their expression levels are often used as indicators of the inflammatory response in the body [
13]. The expression levels of inflammatory-related genes are influenced by the composition of the diet [
14,
15], but the mechanisms by which dietary lipid levels affect the immune response in juvenile tilapia are still not clear and require further research.
The mechanistic target of rapamycin (mTOR) is one of the major pathways involved in protein metabolism in animals. It has been found in various studies that the mTOR signaling pathway is positively regulated by substances such as hormones, nutrients, and growth factors [
16]. Activation of the mTOR signaling pathway leads to increased expression of ribosomal protein S6 kinase Beta-1 (S6K1) and Akt protein kinase B (Akt) [
17]. Wacyk et al. found that the mTOR signaling pathway in rainbow trout (
Oncorhynchus mykiss) was influenced by dietary protein, and altering the protein content in the diet activated the mTOR signaling pathway [
18]. Wang et al. found that the addition of glutamine to the diet activated the mTOR signaling pathway in the malabar grouper (
Pinephelus malabricus), resulting in improved growth performance [
19]. However, there is currently limited research on the effect of dietary lipid levels on the mTOR signaling pathway in juvenile tilapia. Investigating the impact of dietary lipid levels on the TOR signaling pathway in juvenile tilapia can provide insights into the mechanisms by which dietary lipids regulate lipid synthesis and protein synthesis in juvenile tilapia.
Genetic improvement-farmed tilapia (GIFT,
Oreochromis niloticus) is an important commercially cultured species worldwide and known for its strong disease resistance, fast growth rate, high nutritional value, and low-cost feed ingredients [
2]. Considering the omnivorous nature and economic significance of GIFT tilapia, this study selected GIFT tilapia as the experimental fish. This study primarily investigates the effect of different dietary lipid levels on the serum biochemicals, antioxidant capacity, non-specific immunity, inflammatory response, and the expression of mTOR signaling pathway-related genes in juvenile GIFT tilapia. This study aims to enrich and enhance the research on fish feed nutrition and fish physiology and provide references for the nutritional standards and commercial aquaculture production of juvenile GIFT tilapia in terms of their lipid requirements in the formulated feed.
4. Discussion
The levels of total protein (TP), albumin (ALB), and globulin (GLB) in serum represent the metabolic status and amino acid or peptide absorption of the organism and can also reflect the non-specific immune function of the fish [
23]. The results of this study showed that, compared to the control group, the addition of lipids in the feed significantly increased the levels of TP, ALB, and GLB in the serum, indicating that increasing the lipid level in the feed could promote metabolic processes and amino acid or peptide absorption in the fish, as well as enhance the non-specific immune function of the fish. The reason might be that the higher lipid content in the feed accelerated the rate of protein synthesis, resulting in an increased secretion of proteins into the serum [
24]. However, when the lipid content is excessively high, it could lead to liver cell damage. After the digestion and absorption of lipids into the bloodstream, they participate in the metabolism of blood substances or generate feedback mechanisms to regulate blood osmotic pressure and fish immune function by synthesizing albumin and affecting the transport of globulins [
25]. A similar study had shown that the addition of lipids in the feed (80–160 g/kg) significantly increased the levels of TP and ALB in the serum of tilapia [
26]. In this study, the GLU content in the serum increased with the increasing lipid content in the feed, indicating that increasing the lipid content in the feed could enhance glucose metabolism in fish and promote an increase in serum glucose levels. The main sources of glucose (GLU) in fish serum are primarily obtained through three aspects: the absorption of carbohydrates in the food, the breakdown of liver glycogen, and the gluconeogenesis of proteins and lipids [
27]. A similar result had also been observed in triploid rainbow trout, where the GLU content in the serum increased with increasing lipid levels [
28]. The levels of total cholesterol (T-CHO) and triglycerides (TG) in serum are important indicators of lipid metabolism and the nutritional status of fish [
29]. In this study, the levels of T-CHO and TG in the serum increased with the increasing lipid levels in the feed, indicating that the lipid content in the feed could affect the levels of T-CHO and TG in the serum. Serum T-CHO is mainly synthesized in the liver and is a major component of blood lipids. Changes in its levels could reflect the chemical reactions of liver fat [
30,
31]. The increase in the levels of T-CHO and TG in the serum might be due to the higher lipid levels in the feed, which increased the lipid metabolism pressure in the fish, leading to increased activity of endogenous lipid transport in the body, resulting in liver cell damage and elevated levels of T-CHO and TG in the serum [
32]. A similar study showed that in the case of turbot (
Scophthalmus maximus L.), the levels of T-CHO and TG gradually increased with increasing dietary lipid levels [
33]. Low-density lipoprotein (LDL) is a lipoprotein particle that transports cholesterol into peripheral tissue cells, while high-density lipoprotein (HDL) transports cholesterol from peripheral tissues to the liver for metabolic clearance [
34]. In this study, the levels of HDL and LDL increased with the increasing lipid levels, which could be attributed to the increased cholesterol content in the blood under high-fat feeding conditions. The fish synthesized more HDL to transport cholesterol back to the liver for metabolism. As cholesterol continued to be transported to the liver, the metabolic pressure on the liver increased, leading to liver cell damage. Consequently, LDL levels increased in the serum, facilitating the removal of accumulated cholesterol from the liver [
35].
In this study, compared to the control group, the addition of lipids to the feed significantly increased the activities of superoxide dismutase (SOD), total antioxidant capacity (T-AOC), glutathione peroxidase (GSH-Px), and catalase (CAT) in the liver and serum of juvenile tilapia, indicating that lipids could enhance the antioxidant capacity of juvenile tilapia. This might be attributed to several factors: First, lipids were not only important nutrients for fish but also played crucial physiological roles in growth, development, and reproduction, as well as influencing the antioxidant and immune functions of fish through various physiological mechanisms [
7]. For example, feeding a juvenile yellow croaker (
Larmichthys crocea) with fish oil-enriched feed could promote the activities of CAT, SOD, and T-AOC in the liver, thereby enhancing the antioxidant capacity of the yellow croaker [
36]. Second, polyunsaturated fatty acids (PUFAs) present in lipids possess the ability to combat or eliminate free radicals, especially long-chain PUFAs. They not only had a strong free radical scavenging ability, reducing intracellular reactive oxygen species (ROS) and cell death, but they also effectively eliminated H
2O
2 in the body, thereby enhancing the antioxidant capacity, reducing cell damage, and improving the overall antioxidant function [
37]. Third, lipids could form stable hydroperoxides through their peroxidation reactions and react with ROS, effectively clearing free radicals. They could also regulate the level of ROS in the body by inhibiting the activity of reductive coenzyme oxidases, thus participating in the modulation of the antioxidant capacity [
38].
However, in this study, as the lipid levels in the feed increased, the activities of SOD, GSH-Px, CAT, and T-AOC in the liver and serum of juvenile tilapia showed an initial increase followed by a decrease. This indicated that high levels of dietary lipids inhibited the antioxidant capacity of juvenile tilapia. This might be because the high lipid levels exceeded the tolerance of juvenile tilapia, resulting in lipid peroxidation and oxidative damage [
39]. Malondialdehyde (MDA) is the product of lipid peroxidation in the body, which can cause cell membrane damage and functional impairment, thus affecting other physiological functions of the organism. The MDA content in the body is an important indicator reflecting the degree of oxidative damage [
33]. In this study, the MDA content in the liver and serum increased with the increasing lipid levels in the feed, indicating that high levels of dietary lipid could lead to increased lipid peroxidation in the body. The characteristics of lipids, especially highly unsaturated fatty acids, being easily oxidized, coupled with the consumption of feed with excessive fat content, triggered lipid peroxidation reactions in the fish, resulting in elevated levels of the lipid peroxidation product MDA in the tissues [
40].
The results of this study showed that, compared to the control group, the addition of lipids to the feed significantly increased the activities of LZM and ALP in the serum of juvenile tilapia, as well as the levels of C3 and IgM, indicating that lipids could enhance the non-specific immune response of juvenile tilapia. The level of lipids in the feed could affect the non-specific immune function of fish for several reasons: First, the cell membrane was an important barrier to the non-specific immune system in organisms, and lipids were essential components of cell membranes. The addition of lipids to the feed could significantly increase the levels of IgM and LZM enzyme activity in the serum of fish, possibly because fatty acids are important constituents of fish tissue cell membranes. They played a crucial role in maintaining membrane fluidity, promoting lymphocyte proliferation and differentiation, and regulating immune function [
41]. Second, lipids could regulate the immune response of fish and enhance the bactericidal ability of macrophages in vitro. Unsaturated fatty acids in lipids could prevent damage to immune cells caused by free radicals produced in the fish body, ensuring the integrity of immune cell structure and function, thereby maintaining immune cell activity and function [
42]. Third, lipids could stimulate the development of immune organs in fish, to some extent enhancing the organism’s immune capacity. In finless eel (
Monopterus albus), the addition of lipids to the feed significantly increased the activity of IgM and LZM in the serum. This might be attributed to the essential fatty acids in lipids, which not only enhanced the humoral and cellular immune functions in animals’ specific immunity but also enhanced the production of cytokines in immune responses and stimulated the proliferation and differentiation of immune cells [
43].
However, in this study, the activities of LZM and ALP in the serum, as well as the levels of C3 and IgM, showed a trend of initial increase followed by a decrease with increasing levels of dietary lipids. This suggested that excessive levels of dietary lipids could inhibit the non-specific immune performance of juvenile tilapia. This might be attributed to the following reasons: First, excessive lipids reduced the expression of pathogen recognition receptors and their adaptor proteins on the surface of fish immune organs [
44]. Second, excessive unsaturated fatty acids in the fish body were prone to lipid peroxidation, and superoxide anions could attack immune cell membranes, thereby reducing the immune performance of the organism [
45]. Studies have indicated that LZM in non-specific immunity was highly sensitive to stress, with increased activity under acute stimulation, while chronic long-term stress could inhibit LZM activity [
46]. Lipids in the feed acted as immune stimulants for fish, and appropriate levels of lipids could enhance the activity of fish immune enzymes. However, high levels of lipids could induce metabolic stress and increase the metabolic rate of the liver and pancreas, thereby impairing the function of these organs and reducing the activity of fish immune enzymes, ultimately inhibiting the immune system of the organism [
47].
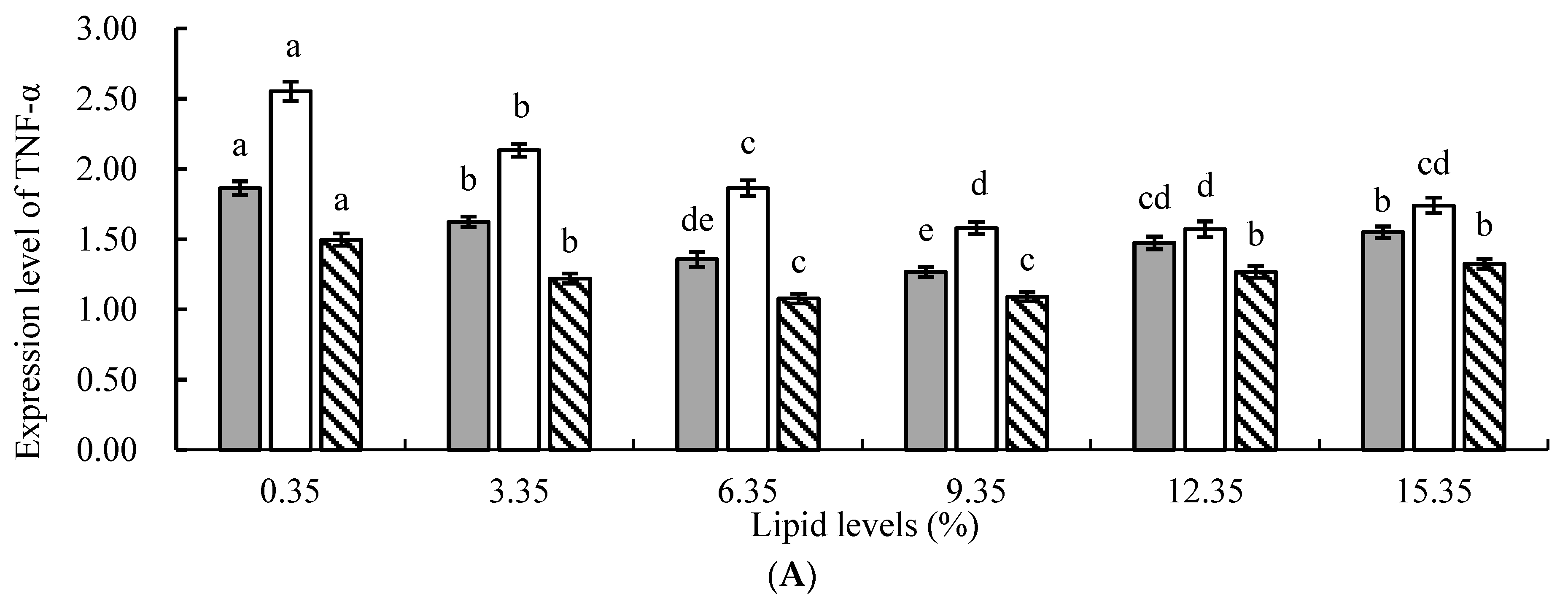
In animals, ROS generated by cellular oxidative stress is considered one of the main factors leading to chronic inflammation [
48]. The results of this study revealed that, compared to the control group, the addition of lipids to the feed significantly suppressed the relative expression levels of tumor necrosis factor α (TNF-α), interleukin 1β (IL-1β), and interleukin 6 (IL-6) genes in juvenile tilapia, indicating that lipids could significantly reduce the occurrence of inflammatory responses in fish. This might be attributed to the following reasons: First, the protective effect of n−3 PUFAs in lipids against inflammation was mediated by upregulating the expression of heme oxygenase-1 (HO-1) through nuclear factor erythroid 2-related factor 2 (Nrf2), thereby inhibiting the expression of the nuclear transcription factor kB (NF-kB) signaling pathway and subsequently suppressing the inflammatory response in the organism [
49]. For example, in the study on tunny (
Puntius gonionotous), an increase in dietary lipid levels resulted in higher levels of n – 3 PUFAs and n−3-LC-PUFAs, which upregulated the expression of anti-inflammatory genes and downregulated the expression of pro-inflammatory genes (such as TNF-α, IL-1β, and IL-6), thereby enhancing the immune capacity of the fish [
50]. In mice supplemented with n − 3 PUFA-rich oils, the secretion of TNF-α, IL-1β, and IL-6 by peritoneal macrophages decreased [
51]. Feeding with a high ratio of n − 3/n − 6 PUFAs-rich diet could reduce the expression of TNF-α, IL-1β, and IL-6 mRNA, indicating that different ratios of n − 3/n − 6 PUFAs could affect the immune function of the organism by altering the expression of TNF-α, IL-1β, IL-6 genes, and the composition of fatty acids in immune cell membranes [
52,
53]. Second, TNF-α, IL-1β, and IL-6, as important immune-regulatory cytokines in fish, played a crucial role in the non-specific immune response of the organism. Under normal conditions without pathogen infection, the expression levels of TNF-α, IL-1β, and IL-6 genes in fish were maintained at a low level. However, when fish are subjected to external stress or pathogen invasion, an upregulation of their expression levels occurs [
54]. In this study, the relative expression levels of TNF-α, IL-1β, and IL-6 genes in the experimental groups were significantly lower than those in the control group, indicating that the addition of lipids to the feed effectively alleviated the stress response in fish, enhanced their disease resistance, and inhibited the inflammatory response.
However, this study found that when the level of lipid in the feed was too high, there was an increasing trend in the relative expression levels of TNF-α, IL-1β, and IL-6 genes. This may be because PUFAs in lipids could metabolize and generate a series of bioactive substances, such as eicosanoids, which play an important regulatory role in inflammation, T lymphocyte, and B lymphocyte function [
44]. The level of prostaglandin E2 (PGE2), a derivative of arachidonic acid (AA), was negatively correlated with the strength of animal immune function. Concentrations within the appropriate physiological range were beneficial for maintaining normal immune function in the body. However, when the level of lipids was too high and the PGE2 content exceeded the normal level, it could lead to the occurrence of inflammatory reactions or diseases such as cancer and could also promote the secretion of cytokines such as TNF-α, IL-1β, and IL-6, thereby affecting the function of the immune system [
55]. Generally, a high level of n-6 PUFAs in the feed could increase the levels of eicosanoids derived from AA before the inflammatory response, thus enhancing the inflammatory response in the body [
56]. In addition, a certain concentration of saturated fatty acids and arachidonic acid in lipids could stimulate the TLR4/NF-kB signaling pathway, triggering an inflammatory response in the body [
57].
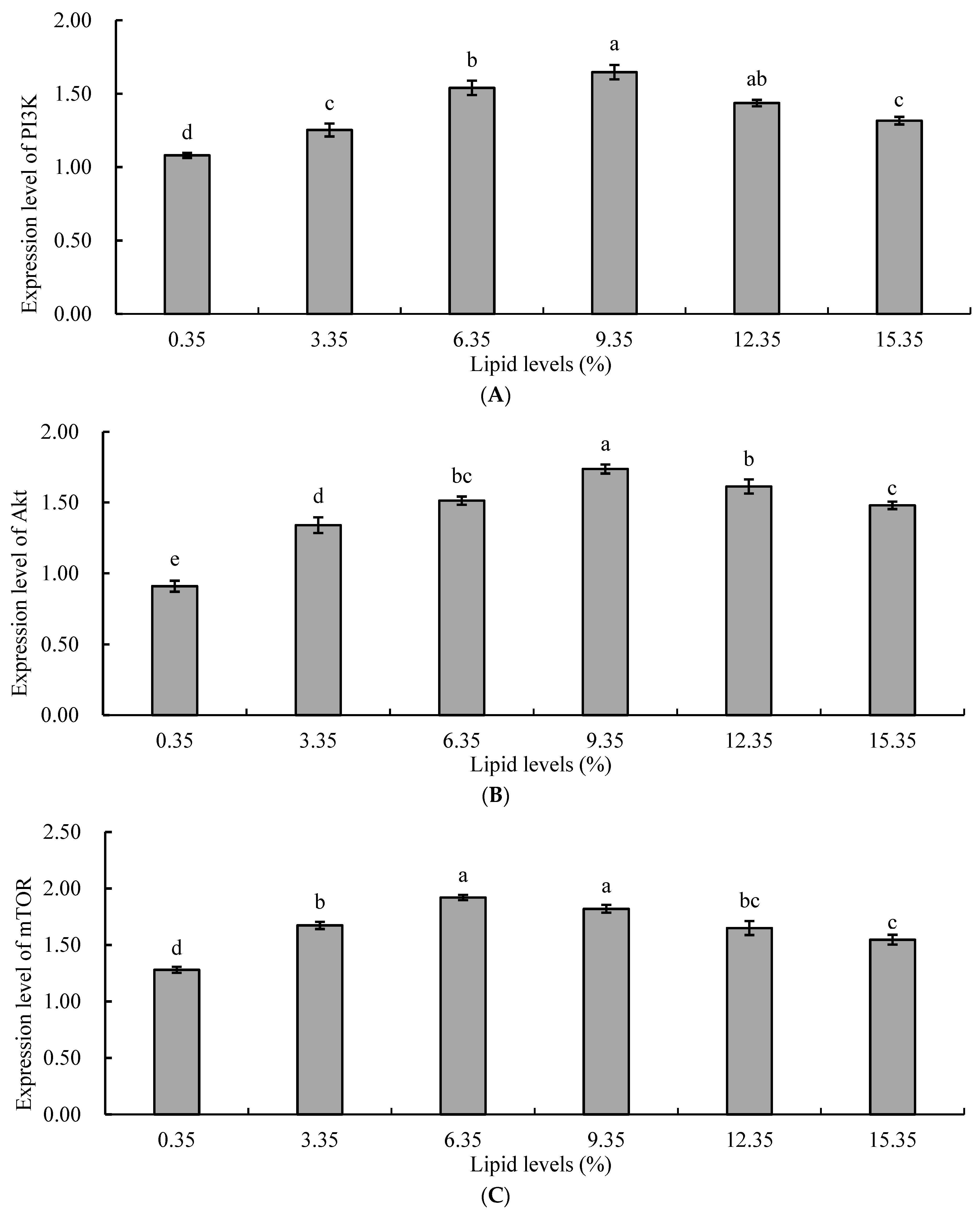
Protein synthesis in aquatic animals is regulated by the rapamycin target protein signaling pathway (PI3K/Akt/mTOR). The PI3K/Akt/mTOR signaling pathway plays a crucial role in protein synthesis metabolism by regulating the activity of ribosomal protein S6 kinases (S6Ks) and eukaryotic translation initiation factor 4E-binding protein (4E-BP), thereby controlling protein synthesis and lipid synthesis [
58]. In this study, the expression levels of mTOR, PI3K, and Akt genes in the liver of juvenile tilapia in the lipid supplementation groups were significantly higher than those in the control group. With increasing levels of lipids, the relative expression levels of mTOR, PI3K, and Akt genes in the liver of juvenile tilapia in the experimental groups initially increased and then decreased. It could be inferred that an appropriate concentration of lipids could increase the rate of protein metabolism, enhance the utilization and absorption of protein in juvenile tilapia, and promote the expression of protein metabolism-related genes. The reason for this might be that an increase in lipid intake increases the metabolic energy of the organism, resulting in a decreased proportion of protein in the feed being used for energy, and more protein being used for the synthesis of fish protein, promoting growth, development, and reproduction in fish [
59]. Additionally, the PI3K/Akt/mTOR pathway was also an important signaling pathway involved in regulating lipid metabolism, mainly by regulating the expression of lipid synthesis-related factors, and enzymes and played a role in regulating lipid metabolism [
60]. Therefore, an increase in the level of dietary lipids also upregulated the expression of genes related to the PI3K/Akt/mTOR pathway. When the level of dietary lipid was too high, a large amount of lipid accumulated in liver cells, inducing oxidative damage to liver tissue, disrupting lipid metabolism in the body, and subsequently feedback inhibiting the expression of genes related to the PI3K/Akt/mTOR pathway [
61]. In Li’s study, high-lipid diets were found to inhibit the expression of genes related to the PI3K/Akt/mTOR pathway in Chinese soft-shelled turtles (
Pelodiscus sinensis) [
62]. In Xin’s study on cows, high concentrations of fatty acid diets were found to downregulate the phosphorylation levels of mTOR and S6K1 [
63]. In Yu’s study on coho salmon (
Oncorhynchus kisutch), as the level of dietary lipids increased, the expression of Akt, PI3K, and mTOR genes initially increased and then decreased [
64]. In a study on Senegalese sole (
Solea senegalensis), feeding a high concentration of lipids led to the downregulation of genes related to the PI3K/AKT/mTOR pathway in the muscle [
65].
 ) liver. All the above data are mean ± SE (n = 3 tanks per condition). Different superscript letters in the figure indicate significant differences among the data (p < 0.05). The F-value of TNF-α in the spleen, head kidney, and liver are 23.647, 47.424, and 17.965, respectively. The F-value of IL-1β in the spleen, head kidney, and liver are 63.120, 39.355, and 17.616, respectively. The F-values of IL-6 in the spleen, head kidney, and liver are 73.451, 21.784, and 28.580, respectively. The p-values of TNF-α, IL-1β, and IL-6 in the spleen, head kidney, and liver are all 0.000.
) liver. All the above data are mean ± SE (n = 3 tanks per condition). Different superscript letters in the figure indicate significant differences among the data (p < 0.05). The F-value of TNF-α in the spleen, head kidney, and liver are 23.647, 47.424, and 17.965, respectively. The F-value of IL-1β in the spleen, head kidney, and liver are 63.120, 39.355, and 17.616, respectively. The F-values of IL-6 in the spleen, head kidney, and liver are 73.451, 21.784, and 28.580, respectively. The p-values of TNF-α, IL-1β, and IL-6 in the spleen, head kidney, and liver are all 0.000.
 ) liver. All the above data are mean ± SE (n = 3 tanks per condition). Different superscript letters in the figure indicate significant differences among the data (p < 0.05). The F-value of TNF-α in the spleen, head kidney, and liver are 23.647, 47.424, and 17.965, respectively. The F-value of IL-1β in the spleen, head kidney, and liver are 63.120, 39.355, and 17.616, respectively. The F-values of IL-6 in the spleen, head kidney, and liver are 73.451, 21.784, and 28.580, respectively. The p-values of TNF-α, IL-1β, and IL-6 in the spleen, head kidney, and liver are all 0.000.
) liver. All the above data are mean ± SE (n = 3 tanks per condition). Different superscript letters in the figure indicate significant differences among the data (p < 0.05). The F-value of TNF-α in the spleen, head kidney, and liver are 23.647, 47.424, and 17.965, respectively. The F-value of IL-1β in the spleen, head kidney, and liver are 63.120, 39.355, and 17.616, respectively. The F-values of IL-6 in the spleen, head kidney, and liver are 73.451, 21.784, and 28.580, respectively. The p-values of TNF-α, IL-1β, and IL-6 in the spleen, head kidney, and liver are all 0.000.