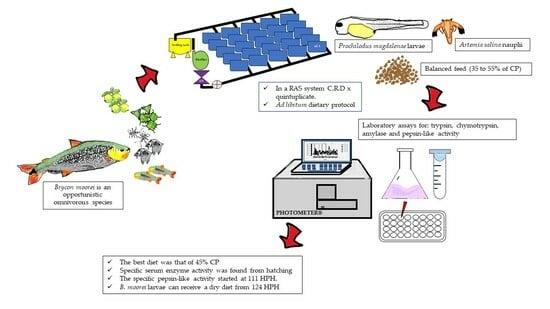
Zootechnical Parameters and Enzyme Activity in the Species Brycon moorei (Steindachner 1878)
Abstract
1. Introduction
2. Materials and Methods
2.1. Location
2.2. Environmental Parameters and Larval Collection
2.3. Feeding Protocol Design
2.4. Zootechnical Parameters
2.5. Enzyme Extraction and Total Soluble Protein Dosing
2.6. Enzyme Activity Assays
2.7. Statistical Analysis
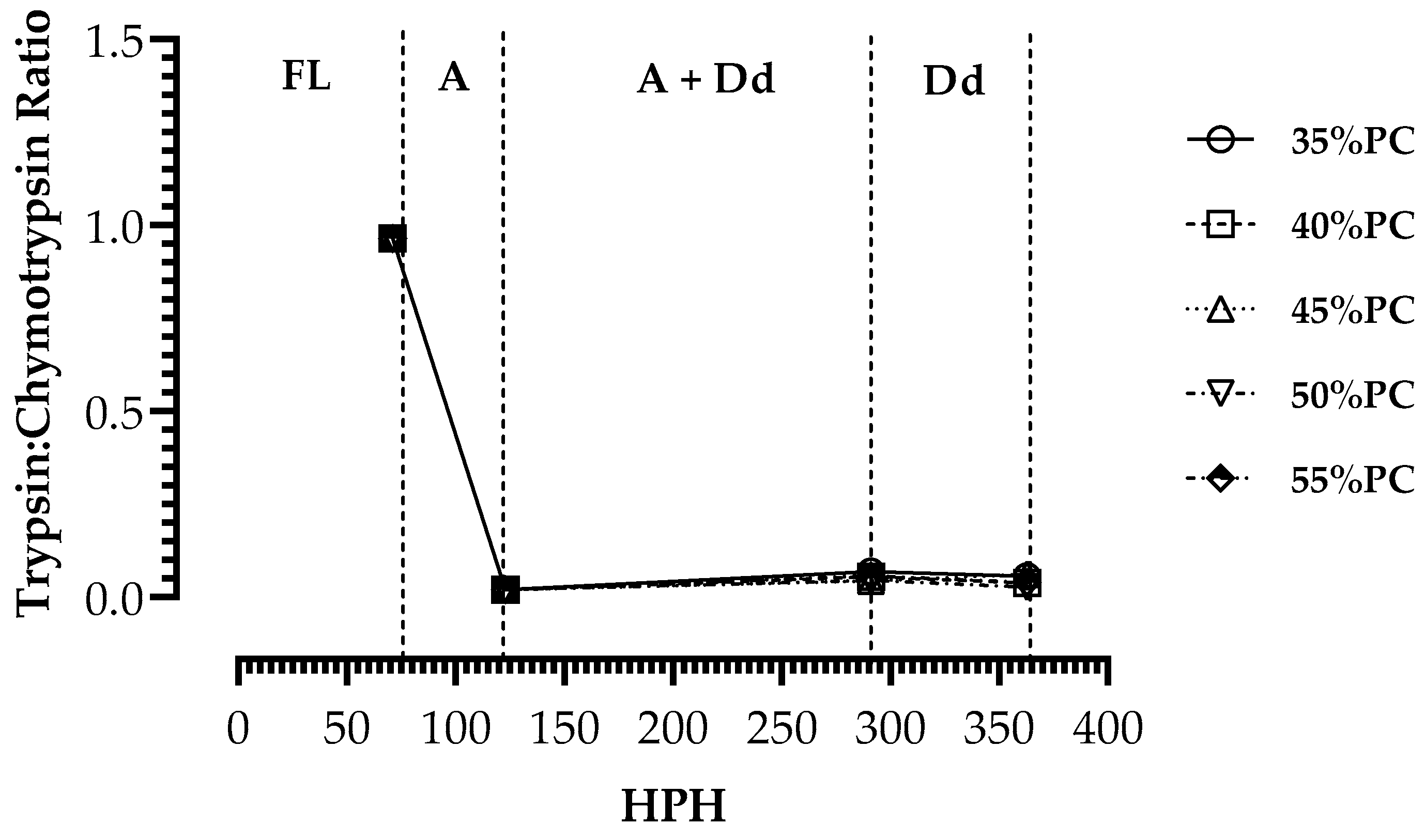
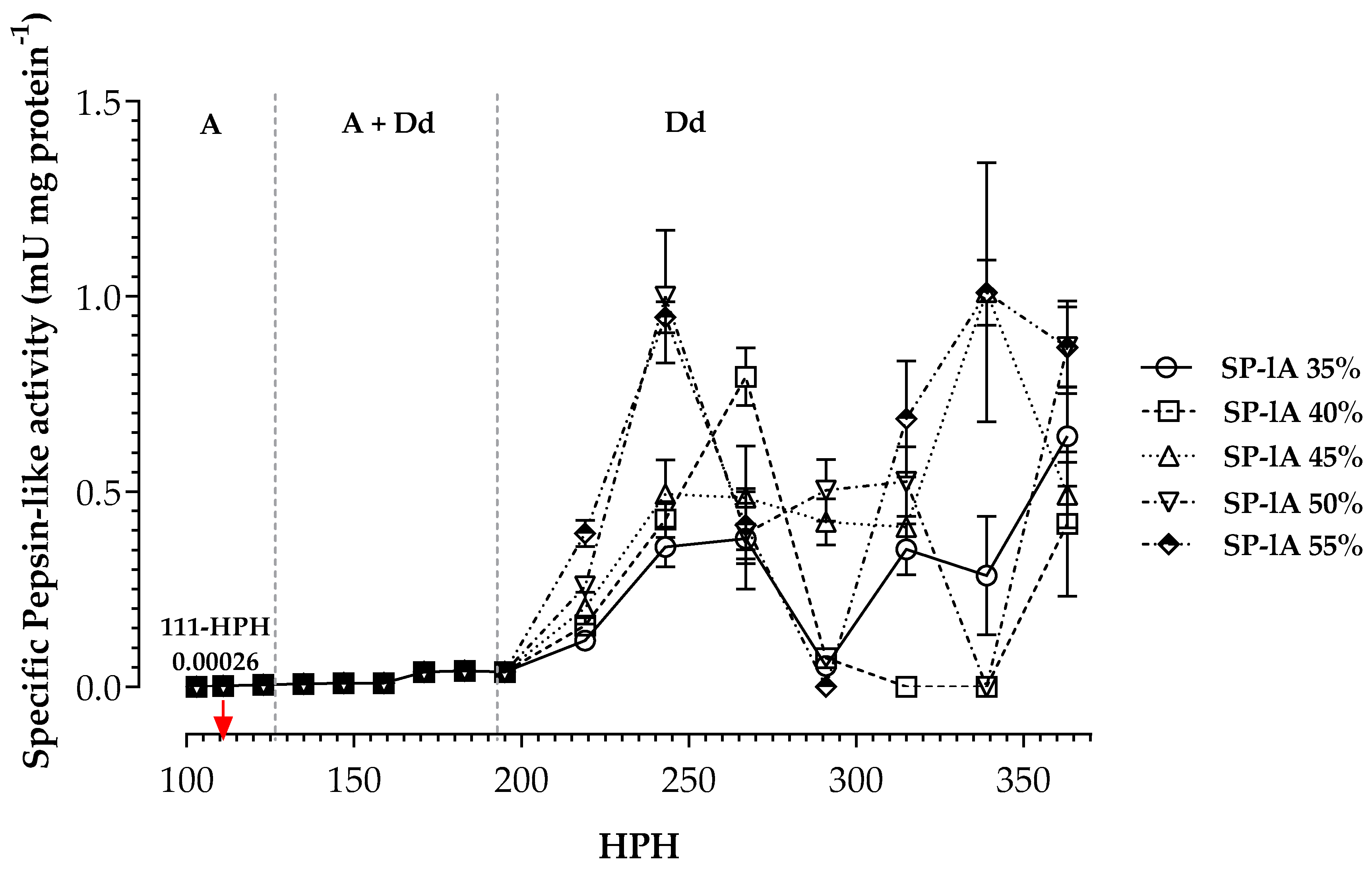
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Atencio-García, V.; Zaniboni-Filho, E. El canibalismo en la larvicultura de peces. Rev. MVZ Córdoba 2006, 11, 9–19. [Google Scholar] [CrossRef]
- David-Ruales, C.A.; Fracalossi, D.; Vásquez-Torres, W. Desarrollo temprano en larvas de peces, clave para el inicio de la alimentación exógena. Rev. Lasallista Investig. 2018, 15, 180–194. [Google Scholar] [CrossRef]
- Mai, M.G.; Zaniboni-Filho, E. Efeito da idade de estocagem em tanques externos no desempenho da larvicultura do dourado Salminus brasiliensis. Acta Sci. 2005, 27, 287–296. [Google Scholar]
- Jomori, R.K.; Carneiro, D.J.; Malheiros, E.B.; Portella, M.C. Growth and survival of pacu Piaractus mesopotamicus (Holmberg, 1887) juveniles reared in ponds or at different initial larviculture periods indoors. Aquaculture 2003, 221, 277–287. [Google Scholar] [CrossRef]
- Senhorini, J.A.; Gaspar, L.A.; Fransozo, A. Crescimento, sobrevivência e preferência alimentar de larvas de matrinxã (Brycon cephalus) e de piracanjuba (Brycon orbignianus) em viveiros. Bol. Téc. CEPTA Pirassununga 2002, 15, 9–21. [Google Scholar]
- Balon, E.K. Saltatory Processes and Altricial to Precocial Forms in the Ontogeny of Fishes. Am. Zool. 1981, 21, 573–596. [Google Scholar] [CrossRef]
- Baras, E.; Ndao, M.; Maxi, M.Y.J.; Jeandrain, D.; Thomé, J.P.; Vandewalle, P.; Mélard, C. Sibling cannibalism in dorada under experimental conditions. I. Ontogeny, dynamics, bioenergetics of cannibalism and prey size selectivity. J. Fish. Biol. 2000, 57, 1001–1020. [Google Scholar] [CrossRef]
- Baras, E.; Maxi, M.Y.J.; Ndao, M.; Mélard, C. Sibling cannibalism in dorada under experimental conditions. II. Effect of initial size heterogeneity, diet and light regime on early cannibalism. J. Fish. Biol. 2000, 57, 1021–1036. [Google Scholar] [CrossRef]
- David-Ruales, C.A.; Castañeda-Álvarez, G.D. Manual de Producción de Mueluda o Dorada el Magdalena (Brycon moorei), 1st ed.; Editorial Lasallista: Caldas, Colombia, 2018. [Google Scholar]
- David-Ruales, C.A.; Castañeda-Álvarez, G.D. Larvicultura de Peces Comerciales en Sistemas de Recirculación, 1st ed.; En: Perspectivas y Avances en Investigación; Editorial Artes y Letras SAS: Itagüí, Colombia, 2010; pp. 199–215. [Google Scholar]
- David-Ruales, C.A.; Castañeda-Álvarez, G.D. Recirculation system for larviculture stage of Brycon moorei Evaluation of a recirculation system for Dorada (Brycon moorei-Steindachner 1878) larvae in the Magdalena River. Rev. CES Med. Vet. Zootec. 2014, 9, 179–189. [Google Scholar]
- Rønnestad, I.; Yúfera, M.; Ueberschär, B.; Ribeiro, L.; Sæle, Ø.; Boglione, C. Feeding behaviour and digestive physiology in larval fish: Current knowledge, and gaps and bottlenecks in research. Rev. Aquac. 2013, 5, S59–S98. [Google Scholar] [CrossRef]
- David-Ruales, C.A.; Machado-Fracalossi, D.; Betancur-Gonzalez, E.M.; Rodríguez-Franco, N.; Castañeda-Álvarez, G.D.; Florez-Restrepo, C.; Vásquez-Torres, W. Relaciones alométricas en estadios tempranos de la especie Brycon moorei Steindachner (Characidae), en condiciones controladas. Actual. Biol. 2020, 42, 1–21. [Google Scholar] [CrossRef]
- Zambonino-Infante, J.L.; Gisbert, E.; Sarasquete, C.; Navarro, I.; Gutierrez, J.; Cahu, C.L. Ontogeny and Physiology of the digestive system of marine fish larvae. In Feeding and Digestive Functions in Fishes; Cyrino, J.E.P., Bureau, D.P., Kapoor, B.G., Eds.; Science Publishers: Enfield, NH, USA, 2008; pp. 281–348. [Google Scholar]
- Portella, M.C.; Leitão, N.J.; Takata, R.; Lopes, T.S. Alimentação e nutrição de larvas. In NUTRIAQUA—Nutrição e Alimentação de Espécies de Interesse para a Aquicultura Brasileira, 1st ed.; Fracalossi, D.M., Cyrino, J.E.P., Eds.; Sociedade Brasileira de Aquicultura e Biologia Aquática: Florianópolis, Brazil, 2012; pp. 185–216. [Google Scholar]
- Gisbert, E.; Morais, S.; Moyano, F. Feeding and Digestion. In Larval Fish Aquaculture; Qin, J.G., Ed.; Nova Science Publishers, Inc.: New York, NY, USA, 2013; pp. 73–124. [Google Scholar]
- Kolkovski, S.; Curnow, J.; King, J. Intensive rearing system for fish larvae research: I. Marine fish larval rearing system. Aquac. Eng. 2004, 31, 295–308. [Google Scholar] [CrossRef]
- German, D.P.; Horn, M.H.; Gawlicka, A. Digestive Enzyme Activities in Herbivorous and Carnivorous Prickleback Fishes (Teleostei: Stichaeidae): Ontogenetic, Dietary, and Phylogenetic Effects. Physiol. Biochem. Zool. 2004, 77, 789–804. [Google Scholar] [CrossRef] [PubMed]
- Lazo, J.P. Conocimiento actual y nuevas perspectivas en el desarrollo de dietas para larvas de peces marinos. In Proceedings of the Nutrición Acuícola V Memorias del V Simposium Internacional de Nutrición Acuícola, Mérida, Mexico, 19–22 November 2000; pp. 300–312. [Google Scholar]
- AOAC Int. Official Methods of Analysis of AOAC International, 20th ed.; AOAC International: Gaithersburg, MD, USA, 2016. [Google Scholar]
- Faustino, F. Desenvolvimento Embrionário e Larval de Brycon gouldingi (Teleostei, Characidae). Master’s Thesis, Universidad Estadual Paulista, Bauru, Brazil, 2010. [Google Scholar]
- Neumann, E. Desenvolvimento Inicial de Jatuarana, Brycon amazonicus (Teleostei, Characidae). Ph.D. Thesis, Centro de Aqüicultura da UNESP, Universidade Estadual Paulista, Bauru, Brazil, 2008. [Google Scholar]
- Fracalossi-Machado, D.; Rodrigues-Oeda, A.P.; ESilva-De Castro, T.; Cyrino-Possebon, J.E. Técnicas experimentais em Nutrição de peixes. In NUTRIAQUA—Nutrição e Alimentação de Espécies de Interesse para a Aquicultura Brasileira; Fracalossi-Machado, D., Cyrino-Possebon, J.E., Eds.; Sociedade Brasileira de Aquicultura e Biologia Aquática: Florianópolis, Brazil, 2012; pp. 37–63. [Google Scholar]
- Ricker, W.E. Growth rates and models. In Bioenergetics and Growth; Fish Physiology Book Series; Hoar, W., Randall, D., Brett, J., Eds.; Academy Press: New York, NY, USA, 1979; pp. 678–685. [Google Scholar]
- Le Cren, E.D. The Length-Weight Relationship and Seasonal Cycle in Gonad Weight and Condition in the Perch (Perca fluviatilis). J. Anim. Ecol. 1951, 20, 201–219. [Google Scholar] [CrossRef]
- Vega-Orellana, O.M.; Fracalossi, D.M.; Sugai, J.K. Dourado (Salminus brasiliensis) larviculture: Weaning and ontogenetic development of digestive proteinases. Aquaculture 2006, 252, 484–493. [Google Scholar] [CrossRef]
- Lowry, O.H.; Rosebrough, N.J.; Farr, A.L.; Randall, R.J. Protein measurement with the folin phenol reagent. J. Biol. Chem. 1951, 193, 265–275. [Google Scholar] [CrossRef]
- Erlanger, B.F.; Kokowsky, N.; Cohen, W. The preparation and properties of two new chromogenic substrates of trypsin. Arch. Biochem. Biophys. 1961, 95, 271–278. [Google Scholar] [CrossRef]
- DelMar, E.G.; Largman, C.; Brodrick, J.W.; Geokas, M.C. A sensitive new substrate for chymotrypsin. Anal. Biochem. 1979, 99, 316–320. [Google Scholar] [CrossRef]
- Noelting, G.; Bernfeld, P. Sur les enzymes amylolytiques III. La β-amylase: Dosage d’activité et contrôle de l’absence d’α-amylase. Helv. Chim. Acta 1948, 31, 286–290. [Google Scholar] [CrossRef]
- Anson, M.L. The estimation of pepsin, trypsin, papain and cathepsin with hemoglobin. J. Gen. Physiol. 1938, 22, 79–89. [Google Scholar] [CrossRef]
- Montes-Girao, P.J.; Fracalossi, D.M. Dietary Lysine Requirement as Basis to Estimate the Essential Dietary Amino Acid Profile for Jundiá, Rhamdia quelen. J. World Aquac. Soc. 2006, 37, 388–396. [Google Scholar] [CrossRef]
- Fuiman, L.A.; Higgs, D.M. Ontogeny, growth, and the recruitment process. In Early Life History and Recruitment in Fish Populations; Chambers, R.C., Trippel, E.A., Eds.; Chapman and Hall: London, UK, 1997; pp. 225–249. [Google Scholar]
- Osse, J.W.M.; van den Boogaart, J.G.M.; van Snik, G.M.J.; van der Sluys, L. Priorities during early growth of fish larvae. Aquaculture 1997, 155, 249–258. [Google Scholar] [CrossRef]
- Osse, J.W.M.; van den Boogaart, J.G.M. Fish larvae, development, allometric growth and the aquatic environment. ICES Mar. Sci. Symp. 1995, 201, 21–34. [Google Scholar] [CrossRef]
- Restrepo-Santamaria, D.; Herrera-Pérez, J.; Muñoz-Duque, S.; Ospina-Pabón, J.G.; Londoño, J.P.; Loaiza-Santana, C.A.; Álvarez-Bustamante, J.; Valencia-Rodríguez, D.; Jiménez-Segura, L. Inventarios de peces en la cuenca del río Magdalena (Colombia) como herramienta para la gestión de su conservación [Fish inventories in the Magdalena River basin (Colombia) as a tool for their conservation management]. Caldasia 2022, 44, 356–367. [Google Scholar] [CrossRef]
- Atencio-Garcia, V.; Pertuz-Buelvas, V.M.; Pérez-Espitia, F.; Ortiz-Mestra, R.; Pardo-Carrasco, S.C. Manejo de la primera alimentación de dorada Brycon sinuensis ofreciendo larvas de bocachico Prochilodus magdalenae. Rev. Colomb. Cienc. Pecu. 2010, 23, 317–324. [Google Scholar]
- Baras, E.; Jobling, M. Dynamics of intracohort cannibalism in cultured fish. Aquac. Res. 2002, 33, 461–479. [Google Scholar] [CrossRef]
- Sales, J. First feeding of freshwater fish larvae with live feed versus compound diets: A meta-analysis. Aquac. Int. 2011, 19, 1217–1228. [Google Scholar] [CrossRef]
- Baras, E. Minimización del canibalismo en especies de peces con larvas piscívoras: Estrategias y éxitos con el carácido Brycon moorei. In Biología de las Poblaciones de Peces de la Amazonía y Acuicultura; Renno, J.F., García, C., Duponchelle, F., Nuñez, J., Eds.; Red de Investigación sobre la Ictiofauna Amazónica: Iquitos, Perú, 2005; pp. 227–233. [Google Scholar]
- Kolkovski, S. Digestive enzymes in fish larvae and juveniles—Implications and applications to formulated diets. Aquaculture 2001, 200, 181–201. [Google Scholar] [CrossRef]
- Baras, E.; Lucas, M.C. Individual growth trajectories of sibling Brycon moorei raised in isolation since egg stage, and their relationship with aggressive behaviour. J. Fish Biol. 2010, 77, 985–997. [Google Scholar] [CrossRef]
- Atencio Garcia, V.; Zaniboni-Filho, E.; Pardo Carrasco, S.; Arias-Castellanos, A. Influência da primeira alimentação na larvicultura e alevinagem do yamú Brycon siebenthalae (Characidae). Acta Sci. 2003, 25, 61–72. [Google Scholar] [CrossRef]
- Gonçalves, L.U.; França, L.A.; Epifânio, C.M.; da Fonseca, F.A.L.; de Alcântara, A.M.; do Nascimento, R.G.; Silva, E.N.S.; da Conceição, L.E.C. Ostracoda impairs growth and survival of Arapaima gigas larvae. Aquaculture 2019, 505, 344–350. [Google Scholar] [CrossRef]
- Silveira, J.; Silva, C.P.; Cargnin-Ferreira, E.; Alexandre, D.; Elias, M.A.; Fracalossi, D.M. Freshwater catfish jundiá (Rhamdia quelen) larvae are prepared to digest inert feed at the exogenous feeding onset: Physiological and histological assessments. Fish Physiol. Biochem. 2013, 39, 1581–1590. [Google Scholar] [CrossRef] [PubMed]
- Castro-Ruiz, D.; Mozanzadeh, M.T.; Fernández-Méndez, C.; Andree, K.B.; García-Dávila, C.; Cahu, C.; Gisbert, E.; Darias, M.J. Ontogeny of the digestive enzyme activity of the Amazonian pimelodid catfish Pseudoplatystoma punctifer (Castelnau, 1855). Aquaculture 2019, 504, 210–218. [Google Scholar] [CrossRef]
- Fernández-Méndez, C.; David, F.; Darias, M.J.; Castro-Ruiz, D.; Núñez-Rodríguez, J. Rearing of the Amazon catfish Pseudoplatystoma punctifer (Castelnau, 1855): Weaning with dry and moist diets. J. Appl. Ichthyol. 2015, 31, 83–87. [Google Scholar] [CrossRef]
- Jomori, R.K.; Carneiro, D.J.; Martins, M.I.E.G.; Portella, M.C. Economic evaluation of Piaractus mesopotamicus juvenile production in different rearing systems. Aquaculture 2005, 243, 175–183. [Google Scholar] [CrossRef]
- Beux, L.F.; Zaniboni-Filho, E. Artemia sp. Proportions and Effects on Survival and Growth of Pintado, Pseudoplatystoma corruscans Larvae. J. Appl. Aquac. 2008, 20, 184–199. [Google Scholar] [CrossRef]
- David-Ruales, C.A.; Zapata-Berruecos, B.; Vásquez-Torres, W. Efectos de la primera alimentación en larvas de Rhamdia sobre la ganancia de peso y longitud. Rev. Lasallista Investig. 2009, 6, 7–13. [Google Scholar]
- David-Ruales, C.; Lenis-Sucerquia, G.; Castañeda-Alvarez, G.; Lopera, A.; Restrepo, L. Initial diet composition affects weight gain and total length of Pacu (Piaractus brachypomus) larvae. Rev. Colomb. Ciencias Pecu. 2011, 24, 48–53. [Google Scholar]
- Tesser, M.B.; Carneiro, D.J.; Portella, M.C. Co-Feeding of Pacu, Piaractus mesopotamicus Holmberg (1887), Larvae with Artemia Nauplii and a Microencapsulated Diet. J. Appl. Aquac. 2005, 17, 47–59. [Google Scholar] [CrossRef]
- Cestarolli, M.; Portella, M.; Rojas, N. Efeito do nível de alimentação e do tipo de alimento na sobrevivência e no desempenho inicial de larvas de Curimbatá Prochilodus scrofa (Steindachner, 1881). Bol. Inst. Pesca 1997, 24, 119–129. [Google Scholar]
- Pradhan, P.K.; Jena, J.; Mitra, G.; Sood, N.; Gisbert, E. Effects of different weaning strategies on survival, growth and digestive system development in butter catfish Ompok bimaculatus (Bloch) larvae. Aquaculture 2014, 424–425, 120–130. [Google Scholar] [CrossRef]
- Hamre, K.; Yúfera, M.; Rønnestad, I.; Boglione, C.; Conceição, L.E.C.; Izquierdo, M. Fish larval nutrition and feed formulation: Knowledge gaps and bottlenecks for advances in larval rearing. Rev. Aquac. 2013, 5, S26–S58. [Google Scholar] [CrossRef]
- Portella, M.C.; Jomori, R.K.; Leitão, N.J.; Menossi, O.C.C.; Freitas, T.M.; Kojima, J.T.; Lopes, T.S.; Clavijo-Ayala, J.A.; Carneiro, D.J. Larval development of indigenous South American freshwater fish species, with particular reference to pacu (Piaractus mesopotamicus): A review. Aquaculture 2014, 432, 402–417. [Google Scholar] [CrossRef]
- Conceição, L.; Aragão, C.; Rønnestad, I. Proteins. In Larval Fish Nutrition, 1st ed.; Holt, J.G., Ed.; John Wiley & Sons, Inc.: Ames, IA, USA, 2011; pp. 83–116. [Google Scholar]
- Jomori, R.K.; Ducatti, C.; Carneiro, D.J.; Portella, M.C. Stable carbon (δ 13 C) and nitrogen (δ 15 N) isotopes as natural indicators of live and dry food in Piaractus mesopotamicus (Holmberg, 1887) larval tissue. Aquac. Res. 2008, 39, 370–381. [Google Scholar] [CrossRef]
- Solovyev, M.; Kashinskaya, E.; Gisbert, E. A meta-analysis for assessing the contributions of trypsin and chymotrypsin as the two major endoproteases in protein hydrolysis in fish intestine. Comp. Biochem. Physiol. Part A Mol. Integr. Physiol. 2023, 278, 111372. [Google Scholar] [CrossRef]
- Frías-Quintana, C.A.; Álvarez-González, C.A.; Guerrero-Zárate, R.; Valverde-Chavarría, S.; Ulloa-Rojas, J.B. Changes in digestive enzymes activities during the initial ontogeny of wolf cichlid, Parachromis dovii (Perciformes: Cichlidae). Neotrop. Ichthyol. 2019, 17, e180161. [Google Scholar] [CrossRef]
- Jimenez-Martinez, L.D.; Alvarez-González, C.A.; Tovar-Ramírez, D.; Gaxiola, G.; Sanchez-Zamora, A.; Moyano, F.J.; Alarcón, F.J.; Márquez-Couturier, G.; Gisbert, E.; Contreras-Sánchez, W.M.; et al. Digestive enzyme activities during early ontogeny in Common snook (Centropomus undecimalis). Fish. Physiol. Biochem. 2012, 38, 441–454. [Google Scholar] [CrossRef]
- Yanes-Roca, C.; Toledo-Cuevas, M.E.; Sánchez, L.J.; Born-Torrijos, A.; Rhody, N.; Main, K.L. Digestive Enzyme Activity during Larval Development of Black Snook, Centropomus nigrescens. J. World Aquac. Soc. 2018, 49, 612–624. [Google Scholar] [CrossRef]
- Hernández-López, I.A.; Ibarra-Castro, L.; Álvarez-González, C.A.; Martínez-Brown, J.M.; Maytorena-Verdugo, C.I.; Peña-Marín, E.S. Characterization of digestive enzymes during early ontogeny of white Snook (Centropomus viridis). Aquaculture 2021, 535, 736399. [Google Scholar] [CrossRef]
- Castañeda-Alvarez, G.D. Desarrollo de la actividad de la tripsina, quimotripsina, proteasa ácida, lipasa y amilasa en la etapa larval de Pseudoplatystoma fasciatum alimentadas con Artemia salina. Master’s Thesis, Instituto de Acuicultura de los Llanos, Universidad de los Llanos, Villavicencio, Colombia, 2009; 86p. [Google Scholar]
- Zambonino Infante, J.; Cahu, C. Ontogeny of the gastrointestinal tract of marine fish larvae. Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2001, 130, 477–487. [Google Scholar] [CrossRef]
- Pérez-Jiménez, A.; Cardenete, G.; Morales, A.E.; García-Alcázar, A.; Abellán, E.; Hidalgo, M.C. Digestive enzymatic profile of Dentex dentex and response to different dietary formulations. Comp. Biochem. Physiol. Part A Mol. Integr. Physiol. 2009, 154, 157–164. [Google Scholar] [CrossRef] [PubMed]
- Chakrabarti, R.; Rathore, R.M. Ontogenic changes in the digestive enzyme patterns and characterization of proteases in Indian major carp Cirrhinus mrigala. Aquac. Nutr. 2010, 16, 569–581. [Google Scholar] [CrossRef]
- Gilannejad, N.; Moyano, F.J.; Martínez-Rodríguez, G.; Yúfera, M. Feeding Protocol Modulates the Digestive Process in Senegalese Sole (Solea senegalensis) Juveniles. Front. Mar. Sci. 2021, 8, 698403. [Google Scholar] [CrossRef]
- Tong, X.H.; Xu, S.H.; Liu, Q.H.; Li, J.; Xiao, Z.Z.; Ma, D.Y. Digestive enzyme activities of turbot (Scophthalmus maximus L.) during early developmental stages under culture condition. Fish. Physiol. Biochem. 2012, 38, 715–724. [Google Scholar] [CrossRef]
- Fernández, I.; Moyano, F.; Díaz, M.; Martínez, T. Characterization of α-amylase activity in five species of Mediterranean sparid fishes (Sparidae, Teleostei). J. Exp. Mar. Bio Ecol. 2001, 262, 1–12. [Google Scholar] [CrossRef]
- López-Vásquez, K.; Castro-Pérez, C.A.; Val, A.L. Digestive enzymes of eight amazonian teleosts with different feeding habits. J. Fish. Biol. 2009, 74, 1620–1628. [Google Scholar] [CrossRef]
- Dabrowski, K.; Portella, M.C. Feeding Plasticity and Nutritional Physiology in Tropical Fishes. Fish Physiol. 2005, 21, 155–224. [Google Scholar] [CrossRef]
- Cara, B.; Moyano, F.J.; Zambonino, J.L.; Fauvel, C. Trypsin and chymotrypsin as indicators of nutritional status of post-weaned sea bass larvae. J. Fish. Biol. 2007, 70, 1798–1808. [Google Scholar] [CrossRef]
- Gisbert, E.; Giménez, G.; Fernández, I.; Kotzamanis, Y.; Estévez, A. Development of digestive enzymes in common dentex Dentex dentex during early ontogeny. Aquaculture 2009, 287, 381–387. [Google Scholar] [CrossRef]
- Sveinsdóttir, H.; Thorarensen, H.; Gudmundsdóttir, Á. Involvement of trypsin and chymotrypsin activities in Atlantic cod (Gadus morhua) embryogenesis. Aquaculture 2006, 260, 307–314. [Google Scholar] [CrossRef]
- Gisbert, E.; Doroshov, S.I. Allometric growth in green sturgeon larvae. J. Appl. Ichthyol. 2006, 22, 202–207. [Google Scholar] [CrossRef]
- Solovyev, M.M.; Kashinskaya, E.N.; Izvekova, G.I.; Gisbert, E.; Glupov, V.V. Feeding habits and ontogenic changes in digestive enzyme patterns in five freshwater teleosts. J. Fish. Biol. 2014, 85, 1395–1412. [Google Scholar] [CrossRef] [PubMed]
- Rungruangsak-Torrissen, K.; Moss, R.; Andresen, L.H.; Berg, A.W.R. Different expressions of trypsin and chymotrypsin in relation to growth in Atlantic salmon (Salmo salar L.). Fish. Physiol. Biochem. 2006, 32, 7–23. [Google Scholar] [CrossRef]
- Martínez-Llorens, S.; Peruzzi, S.; Falk-Petersen, I.B.; Godoy-Olmos, S.; Ulleberg, L.; Tomás-Vidal, A.; Puvanendran, V.; Odei, D.K.; Hagen, Ø.; Fernandes, J.M.O.; et al. Digestive tract morphology and enzyme activities of juvenile diploid and triploid Atlantic salmon (Salmo salar) fed fishmeal-based diets with or without fish protein hydrolysates. PLoS ONE 2021, 16, e0245216. [Google Scholar] [CrossRef]
- Mai, M.G. Estudos da Ontogenia e da Alimentação Inicial de Larvas de Peixes, com Ênfase em Dourado Salminus brasiliensis (Characiformes, Characidae). Ph.D. Thesis, Universidade Federal de São Carlos, São Carlos, Brazil, 2009. [Google Scholar]
- Santos, A.E.; Pedreira, M.M.; Santos, T.G.; Moura, G.d.S.; dos Santos, J.C.E.; Silva, R.C. Desenvolvimento do sistema digestório em larvas do peixe neotropical Prochilodus argenteus (Characiformes, prochilodontidae). Acta Sci. Anim. Sci. 2016, 38, 9–16. [Google Scholar] [CrossRef][Green Version]
- Gisbert, E.; Moreira, C.; Castro-Ruiz, D.; Öztürk, S.; Fernández, C.; Gilles, S.; Nuñez, J.; Duponchelle, F.; Tello, S.; Renno, J.; et al. Histological development of the digestive system of the Amazonian pimelodid catfish Pseudoplatystoma punctifer. Animal 2014, 8, 1765–1776. [Google Scholar] [CrossRef] [PubMed]
- Santos, J.E.; dos Godinho, H.P. Ontogenic events and swimming behavior of larvae of the characid fish Salminus brasiliensis (Cuvier) (Characiformes, Characidae) under laboratory conditions. Rev. Bras. Zool. 2002, 19, 163–171. [Google Scholar] [CrossRef]
- Tesser, M.B. Desenvolvimento do Trato Digestório e Crescimento de Larvas de Pacu, Piaractus mesopotamicus (Holmberg, 1887) em Sistemas de Co-Alimentação com Náuplios de Artemia e Dieta Microencapsulada. Master’s Thesis, Aquaculture Center, Sao Paulo State University, Jaboticabal, Brazil, 2002; 59p. [Google Scholar]
- David-Ruales, C.A. Desarrollo Temprano de la Especie Brycon moorei—Steindachner, 1878 (Dorada del Magdalena): Aspectos Embrionarios, Morfométricos, Histológicos e Histoquímicos, Parámetros Productivos y Actividad de las Enzimas Digestivas en Función de la Dieta. Ph.D. Thesis, Instituto de Acuicultura de los Llanos, Universidad de los Llanos, Villavicencio, Colombia, 2019. [Google Scholar]
- Ribeiro, L.; Zambonino-Infante, J.L.; Cahu, C.; Dinis, M.T. Development of digestive enzymes in larvae of Solea senegalensis, Kaup 1858. Aquaculture 1999, 179, 465–473. [Google Scholar] [CrossRef]
- Rønnestad, I.; Dominguez, R.P.; Tanaka, M. Ontogeny of digestive tract functionality in Japanese flounder, Paralichthys olivaceus studied by in vivo microinjection: pH and assimilation of free amino acids. Fish. Physiol. Biochem. 2000, 22, 225–235. [Google Scholar] [CrossRef]
- Darias, M.J.; Murray, H.M.; Gallant, J.W.; Douglas, S.E.; Yúfera, M.; Martínez-Rodríguez, G. Ontogeny of pepsinogen and gastric proton pump expression in red porgy (Pagrus pagrus): Determination of stomach functionality. Aquaculture 2007, 270, 369–378. [Google Scholar] [CrossRef]


| Parameter | Comfort Index |
|---|---|
| DO (mg L−1) | 5.5 ± 0.5 |
| pH | 6.5 ± 0.8 |
| T (°C) | 26 ± 0.4 |
| Alk/Hard (mg L−1 CaCO3) | 20.8 ± 1.7 |
| NH3 (mg L−1) | 0.002 ± 0.00001 |
| DPH | Feeding Timing | ||||
|---|---|---|---|---|---|
| (Hours) | |||||
| 7 | 9 | 11 | 13 | 15 | |
| 1 | FL | FL | FL | FL | FL |
| 2 | FL | FL | FL | FL | FL |
| 3 | FL | FL | FL | FL | FL |
| 4 | A | A | A | A | A |
| 5 | A | A | A | A | A |
| 6 | A | A | A + Dd | A | A |
| 7 | A | A | A + Dd | A | A |
| 8 | A | A | A + Dd | A | A |
| 9 | A + Dd | A | A | Dd | A + Dd |
| 10 | A + Dd | Dd | Dd | A + Dd | Dd |
| 11 | Dd | Dd | A + Dd | Dd | Dd |
| 12 | A + Dd | Dd | Dd | Dd | Dd |
| 13 | Dd | Dd | Dd | Dd | Dd |
| 14 | Dd | Dd | Dd | Dd | Dd |
| 15 | Dd | Dd | Dd | Dd | Dd |
| Ingredient | Protein Level% | ||||
|---|---|---|---|---|---|
| 55 | 50 | 45 | 40 | 35 | |
| Fish meal a | 65.0 | 56.4 | 49.0 | 41.0 | 31.0 |
| Earthworm meal b | 11.7 | 11.7 | 11.7 | 11.7 | 12.7 |
| Whole egg flour c | 4.3 | 4.3 | 4.3 | 3.0 | 4.3 |
| Pregelatinized starch d | 0.3 | 8.9 | 20.8 | 34.3 | 37.0 |
| Alpha-cellulose e | 0.0 | 0.0 | 0.0 | 0.0 | 5.8 |
| Fish oil f | 6.5 | 6.5 | 3.0 | 0.5 | 0.1 |
| Canola oil g | 3.2 | 3.2 | 2.2 | 0.5 | 0.1 |
| Soy lecithin h | 1.1 | 1.1 | 1.1 | 1.1 | 1.1 |
| Choline chloride e | 3.2 | 3.2 | 3.2 | 3.2 | 3.2 |
| Dicalcium phosphate e | 0.5 | 0.5 | 0.5 | 0.5 | 0.5 |
| Premiz Vit/Min i | 4.1 | 4.1 | 4.1 | 4.1 | 4.1 |
| Vit C j | 0.2 | 0.2 | 0.2 | 0.2 | 0.2 |
| Proximate analysis (dry basis) | |||||
| Dry matter | 92.5 | 91.6 | 92.8 | 91.8 | 92.5 |
| Crude protein | 54.4 | 48.9 | 44.3 | 38.7 | 34.5 |
| Crude fat | 18.3 | 17.3 | 16.3 | 14.7 | 12.1 |
| Ashes | 7.6 | 7.5 | 7.1 | 7.0 | 6.9 |
| Free nitrogen | 11.1 | 10.1 | 9.1 | 7.9 | 5.5 |
| Crude fiber | 1.1 | 7.8 | 16.0 | 23.5 | 33.5 |
| Energy kcal/kg | 5022.0 | 4904.0 | 4546.0 | 4180.0 | 3805.0 |
| Variables | Protein Levels (%) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 35 | 40 | 45 | 50 | 55 | ||||||
| WG (mg) | 62.8 ± 31.6 | bc | 61.5 ± 20.4 | c | 176.2 ± 61.3 | a | 69.3 ± 27.7 | bc | 114.0 ± 50.0 | bc |
| DWG (mg) | 4.1 ± 2.1 | bc | 4.09 ± 1.3 | c | 11.7 ± 4.1 | a | 4.6 ± 1.8 | bc | 7.6 ± 3.3 | bc |
| LG (mm) | 0.4 ± 0.2 | b | 0.5 ± 0.2 | b | 0.7 ± 0.2 | a | 0.5 ± 0.1 | ab | 0.3 ± 0.1 | b |
| DLG (mm) | 0.03 ± 0.01 | b | 0.03 ± 0.01 | b | 0.05 ± 0.01 | a | 0.03 ± 0.01 | ab | 0.02 ± 0.01 | b |
| FC | 0.9 ± 0.06 | b | 0.9 ± 0.04 | b | 0.6 ± 0.03 | a | 1.2 ± 0.06 | c | 0.5 ± 0.07 | a |
| C (mg/day/larva) | 5.6 ± 0.4 | c | 5.4 ± 0.2 | c | 10.2 ± 0.5 | a | 8.3 ± 0.4 | bc | 5.7 ± 1.6 | c |
| SGR (%) | 7.4 ± 3.2 | b | 6.6 ± 2.3 | b | 13.0 ± 1.7 | a | 7.4 ± 1.9 | b | 9.8 ± 3.9 | ab |
| K | 1.08 ± 0.2 | 1.09 ± 0.4 | 1.1 ± 0.06 | 1.1 ± 0.23 | 1.1 ± 0.2 | |||||
| S% | 43.7 | 42.8 | 45.6 | 39.9 | 43.5 | |||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
David-Ruales, C.; Machado Fracalossi, D.; Collazos-Lasso, F. Zootechnical Parameters and Enzyme Activity in the Species Brycon moorei (Steindachner 1878). Fishes 2023, 8, 592. https://doi.org/10.3390/fishes8120592
David-Ruales C, Machado Fracalossi D, Collazos-Lasso F. Zootechnical Parameters and Enzyme Activity in the Species Brycon moorei (Steindachner 1878). Fishes. 2023; 8(12):592. https://doi.org/10.3390/fishes8120592
Chicago/Turabian StyleDavid-Ruales, Carlos, Débora Machado Fracalossi, and Felipe Collazos-Lasso. 2023. "Zootechnical Parameters and Enzyme Activity in the Species Brycon moorei (Steindachner 1878)" Fishes 8, no. 12: 592. https://doi.org/10.3390/fishes8120592
APA StyleDavid-Ruales, C., Machado Fracalossi, D., & Collazos-Lasso, F. (2023). Zootechnical Parameters and Enzyme Activity in the Species Brycon moorei (Steindachner 1878). Fishes, 8(12), 592. https://doi.org/10.3390/fishes8120592