Effects of Snail Bellamya purificata Farming at Different Stocking Densities on the Algal and Fungal Communities in Sediment
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experiment Design
2.2. Sample Collection
2.3. PCR Amplification and Sequencing
2.4. Data Processing
2.5. Statistical Analysis
3. Results
3.1. Overview of Fungal and Algal Communities in Sediment
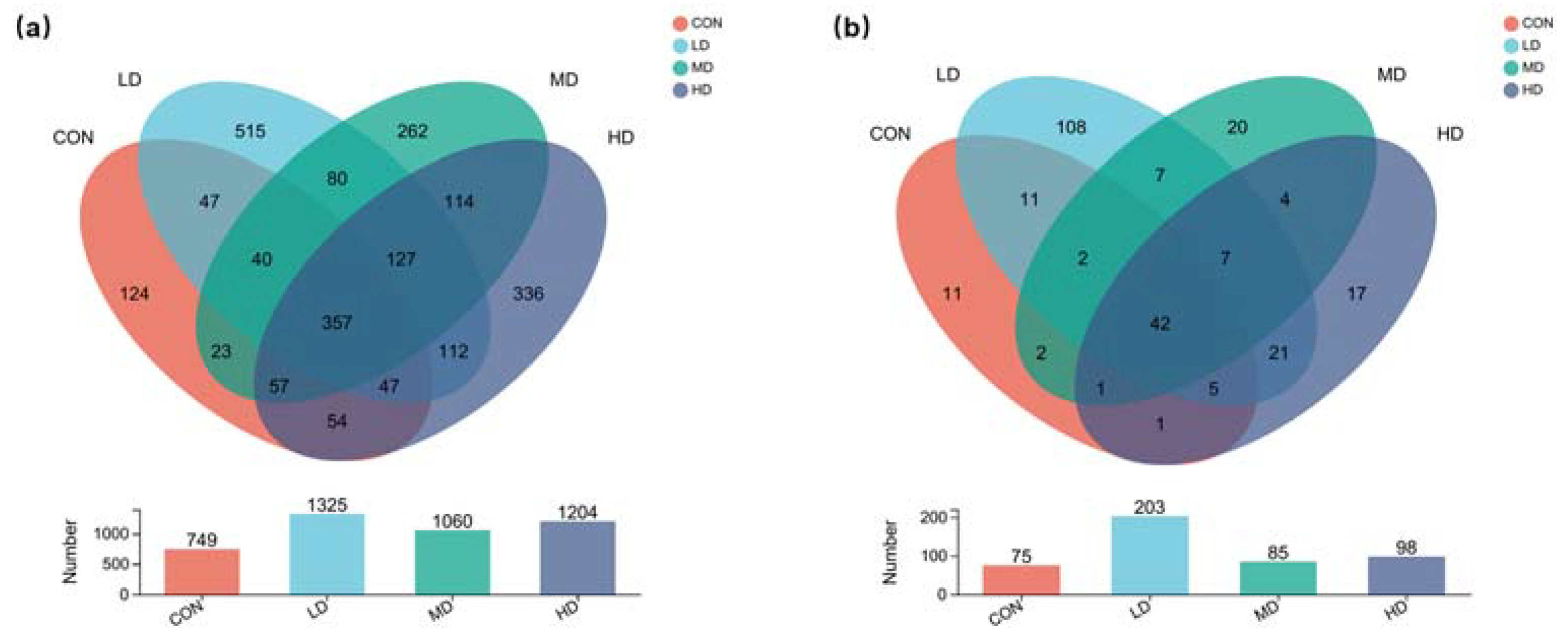
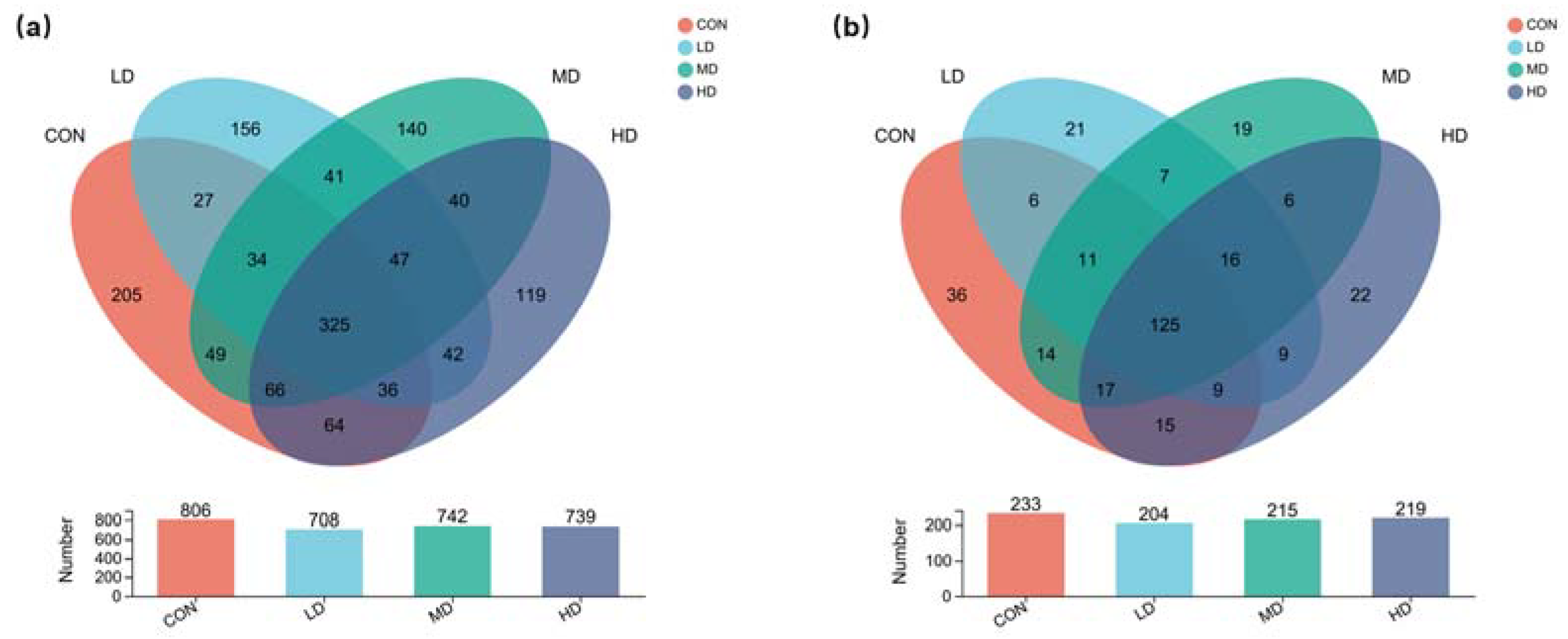
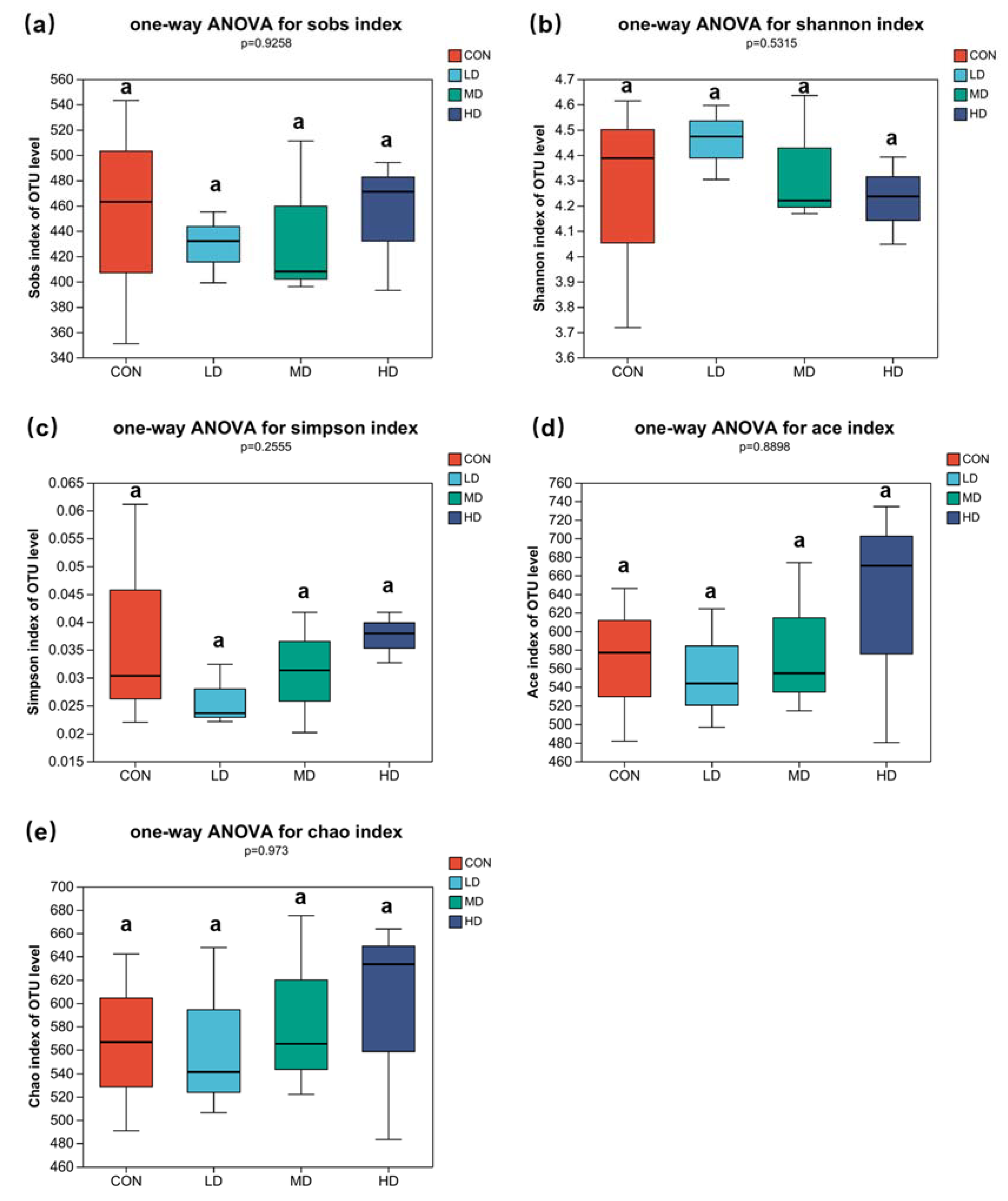
3.2. Alpha and Beta Diversities of Fungal Community in Sediment
3.3. Alpha and Beta Diversities of Algal Community in Sediment
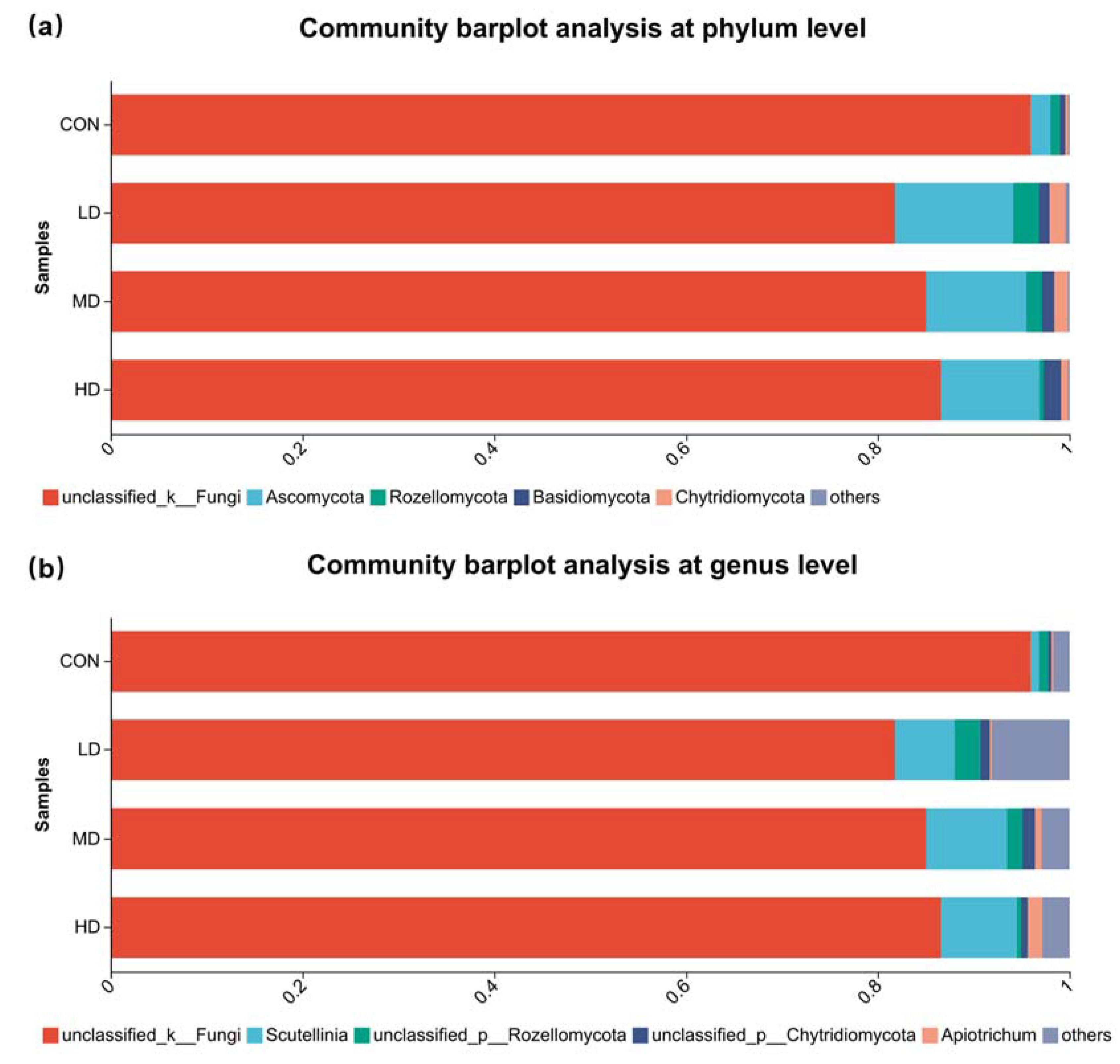
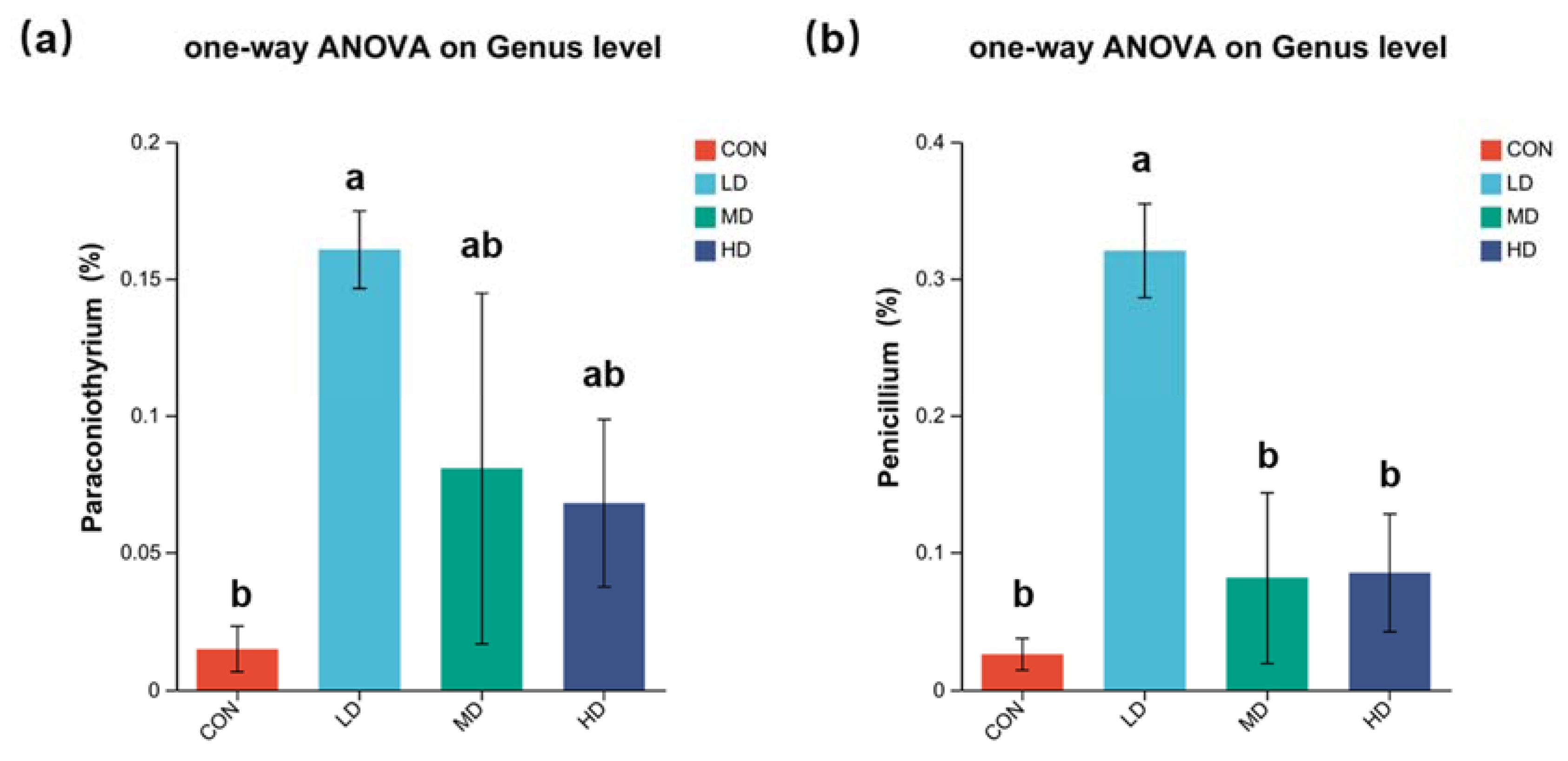
3.4. Composition of Fungal Community in Sediment
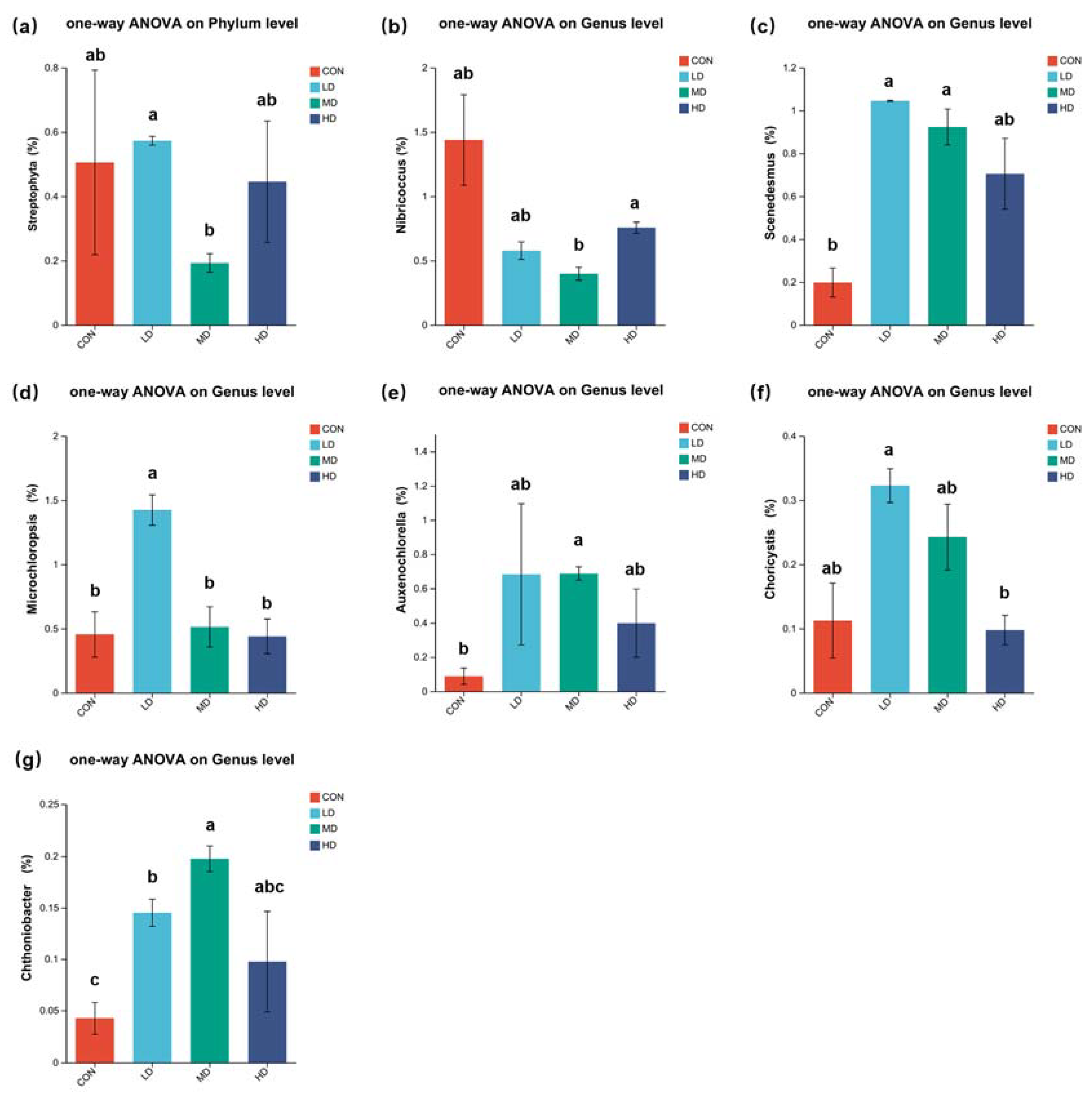
3.5. Composition of Algal Community in Sediment
4. Discussion
4.1. Fungal Community in Sediment Affect by B. purificata Farming Activities
4.2. Algal Community in Sediment Affect by B. purificata Farming Activities
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Food and Agriculture Organization of the United Nations. The State of World Fisheries and Aquaculture 2022; Food and Agriculture Organization of the United Nations: Rome, Italy, 2022. [Google Scholar]
- Costello, C.; Cao, L.; Gelcich, S.; Cisneros-Mata, M.A.; Free, C.M.; Froehlich, H.E.; Golden, C.D.; Ishimura, G.; Maier, J.; Macadam-Somer, I.; et al. The future of food from the sea. Nature 2020, 588, 95–100. [Google Scholar] [CrossRef]
- Cao, L.; Wang, W. Wastewater management in freshwater pond aquaculture in China. In Sustainability in Food and Water. Alliance for Global Sustainability Bookseries (Science and Technology: Tools for Sustainable Development); Sumi, A., Fukushi, K., Honda, R.K.H., Eds.; Springer: Dordrecht, The Netherlands, 2010; Volume 18. [Google Scholar]
- Deng, L.F.; Jiang, X.L. The application and research progress of bioremediation in pond aquaculture. Oceanol. Limnol. Sin. 2013, 44, 1270–1275. [Google Scholar]
- Liu, H.L.; Cao, X.Y.; Song, C.L.; Zhou, Y.Y. Environmental effects of organic matter enrichment and restoration strategy in sediment of aquaculture ponds. J. Hydroecol. 2011, 32, 130–134. [Google Scholar]
- Liu, X.G. Study on the Pond Aquaculture Pollution and Ecological Engineering Regulation Techniques; Nanjing Agricultural University: Nanjing, China, 2011. [Google Scholar]
- Ji, X.; Lin, X.; Xu, Z.; Lin, Y. Machenism of mariculture self-pollution and its effects on environment. Mar. Environ. Sci. 2000, 19, 66–71. [Google Scholar]
- Zhou, H.; Jiang, C.; Zhu, L.; Wang, X.; Hu, X.; Cheng, J.; Xie, M. Impact of pond and fence aquaculture on reservoir environment. Water Sci. Eng. 2011, 4, 92–100. [Google Scholar]
- Knowler, D.; Chopin, T.; Martinez-Espineira, R.; Neori, A.; Nobre, A.; Noce, A.; Reid, G. The economics of Integrated Multi-Trophic Aquaculture: Where are we now and where do we need to go? Rev. Aquac. 2020, 12, 1579–1594. [Google Scholar] [CrossRef]
- Neori, A.; Chopin, T.; Troell, M.; Buschmann, A.H.; Kraemer, G.P.; Halling, C.; Shpigel, M.; Yarish, C. Integrated aquaculture: Rationale, evolution and state of the art emphasizing seaweed biofiltration in modem mariculture. Aquaculture 2004, 231, 361–391. [Google Scholar] [CrossRef]
- Shi, H.H.; Zheng, W.; Zhang, X.L.; Zhu, M.Y.; Ding, D.W. Ecological-economic assessment of monoculture and integrated multi-trophic aquaculture in Sanggou Bay of China. Aquaculture 2013, 410, 172–178. [Google Scholar] [CrossRef]
- Troell, M.; Joyce, A.; Chopin, T.; Neori, A.; Buschmann, A.H.; Fang, J.G. Ecological engineering in aquaculture—Potential for integrated multi-trophic aquaculture (IMTA) in marine offshore systems. Aquaculture 2009, 297, 1–9. [Google Scholar] [CrossRef]
- Yu, L.Q.J.; Mu, Y.; Zhao, Z.; Lamc, V.W.Y.; Sumaila, U.R. Economic challenges to the generalization of integrated multi-trophic aquaculture: An empirical comparative study on kelp monoculture and kelp-mollusk polyculture in Weihai, China. Aquaculture 2017, 471, 130–139. [Google Scholar] [CrossRef]
- Chen, J.; Song, G.; Wang, X.; She, L.; Wu, S.; Wu, M.; Ding, F. The efficiency of water purification by different densities of Bellamya aeruginosa and Bellamya purificata. J. Anhui Agric. Sci. 2012, 40, 11708–11709. [Google Scholar]
- Ma, B.; Wang, H.; Jin, W.; Huang, B.; Li, Y.; Li, Z.; Wang, M.; Li, C. Study on the water purification effect of the algae Elodea nuttallii and snail Bellamya purificata. Jiangxi Fish. Sci. Technol. 2022, 2, 3–6. [Google Scholar]
- Duan, X.; Xie, C.; Lv, Y.; Zhang, N.; ZHao, F.; Li, R. Feeding habits of Bellamya purificata and its function in water purification system of ecological ditch. Fish. Mod. 2013, 40, 17–21. [Google Scholar]
- Cao, Z.; Jiang, X. The influence of environmental factors on Bellamya purificate. J. Shanghai Fish. Univ. 1998, 7, 200–205. [Google Scholar]
- Yan, Y.; Jin, W.; Wen, H.; Ma, X.; Xue, T.; Sun, C.; He, Y.; Bing, X. Estimation of genetic parameters for growth traits of Bellamya purificata in 60 days. Freshw. Fish. 2018, 48, 108–111. [Google Scholar]
- Hou, Y.R.; Li, B.; Luo, J.W.; Zhang, C.F.; He, J.; Zhu, J. Effect of Bellamya purificata on organic matter degradation in surface sediment as revealed by amino acids. Aquac. Environ. Interact. 2021, 13, 1–12. [Google Scholar] [CrossRef]
- Zhao, F.; Xie, C.; Zhang, N.; Lv, Y.; Li, R. Efficiency of water purification and the nitrogen and phosphorous release of the sediment by different densities of Bellamya purificata. J. Hydroecol. 2014, 35, 32–38. [Google Scholar]
- Zhou, M.; Hou, Y.; Jia, R.; Li, B.; Zhu, J. Effects of Bellamya purificata Cultivation at Different Stocking Densities on the Dynamics and Assembly of Bacterial Communities in Sediment. Biomolecules 2023, 13, 254. [Google Scholar] [CrossRef]
- Liu, L.; Xu, M. Microbial biomass in sediments affects greenhouse gas effluxes in Poyang Lake in China. J. Freshw. Ecol. 2016, 31, 109–121. [Google Scholar] [CrossRef]
- Sabater, S.; Buchaca, T.; Cambra, J.; Catalan, J.; Guasch, H.; Ivorra, N.; Muñoz, I.; Navarro, E.; Real, M.; Romaní, A. Structure and function of benthic algal communities in an extremely acid river. J. Phycol. 2003, 39, 481–489. [Google Scholar] [CrossRef]
- Marino, J.A.; Denef, V.J.; Dick, G.J.; Duhaime, M.B.; James, T.Y. Fungal community dynamics associated with harmful cyanobacterial blooms in two Great Lakes. J. Great Lakes Res. 2022, 48, 1021–1031. [Google Scholar] [CrossRef]
- Xu, S. Diversity of Microbial Communities in Mixed Crab Breeding Rice Plantations; Tianjing University: Tianjing, China, 2021. [Google Scholar]
- Zhang, H.; Jia, J.; Chen, S.; Huang, T.; Wang, Y.; Zhao, Z.; Feng, J.; Hao, H.; Li, S.; Ma, X. Dynamics of Bacterial and Fungal Communities during the Outbreak and Decline of an Algal Bloom in a Drinking Water Reservoir. Int. J. Environ. Res. Public Health 2018, 15, 361. [Google Scholar] [CrossRef]
- Zhang, Z.; Deng, Q.; Cao, X.; Zhou, Y.; Song, C. Patterns of sediment fungal community dependent on farming practices in aquaculture ponds. Front. Microbiol. 2021, 12, 542064. [Google Scholar] [CrossRef] [PubMed]
- Hou, Y.; Li, B.; Feng, G.; Zhang, C.; He, J.; Li, H.; Zhu, J. Responses of bacterial communities and organic matter degradation in surface sediment to Macrobrachium nipponense bioturbation. Sci. Total Environ. 2021, 759, 143534. [Google Scholar] [CrossRef]
- Nie, X.; Wu, S.; Wu, F.; Sun, S.; Jiang, C.; Tan, W.; Wan, J. Effects of the bioturbation activity of Tubifex tubifex on nitrogen release from sediments. Acta Sci. Circumstantiae 2011, 31, 107–113. [Google Scholar]
- Wang, Y.; Song, J.; Weiwei, J.; Cheng, D.; Xue, J.; Yang, X.; Zhang, B.; Zhang, J. Effect of bioturbation of Chironomid larvae and Limno drilus hoffmeiteri on the release of nitrogen, oxygen and phosphate in the sediments from a river. Acta Sci. Circumstantiae 2015, 35, 2504–2511. [Google Scholar]
- Yoon, T.-H.; Kang, H.-E.; Kang, C.-K.; Lee, S.H.; Ahn, D.-H.; Park, H.; Kim, H.-W. Development of a cost-effective metabarcoding strategy for analysis of the marine phytoplankton community. PeerJ 2016, 4, e2115. [Google Scholar] [CrossRef]
- Chen, S.F.; Zhou, Y.Q.; Chen, Y.R.; Gu, J. fastp: An ultra-fast all-in-one FASTQ preprocessor. Bioinformatics 2018, 34, 884–890. [Google Scholar] [CrossRef]
- Magoc, T.; Salzberg, S.L. FLASH: Fast length adjustment of short reads to improve genome assemblies. Bioinformatics 2011, 27, 2957–2963. [Google Scholar] [CrossRef]
- Edgar, R.C. UPARSE: Highly accurate OTU sequences from microbial amplicon reads. Nat. Methods 2013, 10, 996–998. [Google Scholar] [CrossRef]
- Nilsson, R.H.; Larsson, K.-H.; Taylor, A.F.S.; Bengtsson-Palme, J.; Jeppesen, T.S.; Schigel, D.; Kennedy, P.; Picard, K.; Gloeckner, F.O.; Tedersoo, L.; et al. The UNITE database for molecular identification of fungi: Handling dark taxa and parallel taxonomic classifications. Nucleic Acids Res. 2019, 47, D259–D264. [Google Scholar] [CrossRef] [PubMed]
- Schloss, P.D.; Westcott, S.L.; Ryabin, T.; Hall, J.R.; Hartmann, M.; Hollister, E.B.; Lesniewski, R.A.; Oakley, B.B.; Parks, D.H.; Robinson, C.J.; et al. Introducing mothur: Open-Source, Platform-Independent, Community-Supported Software for Describing and Comparing Microbial Communities. Appl. Environ. Microbiol. 2009, 75, 7537–7541. [Google Scholar] [CrossRef] [PubMed]
- Xu, F.; Zhu, L.; Wang, J.; Xue, Y.; Liu, K.; Zhang, F.; Zhang, T. Nonpoint Source Pollution (NPSP) Induces Structural and Functional Variation in the Fungal Community of Sediments in the Jialing River, China. Microb. Ecol. 2023, 85, 1308–1322. [Google Scholar] [CrossRef] [PubMed]
- Jiang, G.; Yao, R.; Lin, Y. A survey of study on secondary metabolite s from marine imperfect fungi. J. Microbiol. 2001, 21, 41–44. [Google Scholar]
- Fan, L. Microbial Community in Tilapia (Oreochromis niloticus) Cultural Ponds; Nanjing Agricultural University: Nanjing, China, 2015. [Google Scholar]
- Zhang, Y.; Guo, L. Progress of fungal DNA barcode. Mycosystema 2012, 31, 809–820. [Google Scholar]
- Wang, P.; Xiao, H.; Yuan, R.; Zhao, J.; Yu, X. Fungal community in the estuarine sediment of Poyang Lake. Acta Sci. Circumstantiae 2018, 38, 1949–1956. [Google Scholar]
- Zhao, Y.; Zheng, S.; Fang, Z.; Yu, Z. Vertical distribution of fungal communities in Honghu Lake sediment. Mycosystema 2011, 30, 721–728. [Google Scholar]
- Wang, J.F.; Shao, S.C.; Liu, C.S.; Song, Z.Q.; Liu, S.S.; Wu, S.H. The genus Paraconiothyrium: Species concepts, biological functions, and secondary metabolites. Crit. Rev. Microbiol. 2021, 47, 781–810. [Google Scholar] [CrossRef]
- Du, L.; Li, D.H.; Zhu, T.J.; Cai, S.X.; Wang, F.P.; Xiao, X.; Gu, Q.Q. New alkaloids and diterpenes from a deep ocean sediment derived fungus Penicillium sp. Tetrahedron 2009, 65, 1033–1039. [Google Scholar] [CrossRef]
- Xu, T.; Wu, Y.; Jia, Y.; Liu, B.; Ye, B. A Penicillium sp. XGH2321 isolated from the rhizospheric soil of Rhizophora stylosa griff and its antibacterial activity. Microbiol. China 2009, 36, 1682–1687. [Google Scholar]
- Corte, A.M.; Liotta, M.; Venturi, C.B.; Calegari, L. Antibacterial activity of Penicillium spp. strains isolated in extreme environments. Polar Biol. 2000, 23, 294–297. [Google Scholar] [CrossRef]
- Bringmann, G.; Lang, G.; Gulder, T.A.M.; Tsuruta, H.; Muhlbacher, J.; Maksimenka, K.; Steffens, S.; Schaumann, K.; Stohr, R.; Wiese, J.; et al. The first sorbicillinoid alkaloids, the antileukemic sorbicillactones A and B, from a sponge-derived Penicillium chrysogenum strain. Tetrahedron 2005, 61, 7252–7265. [Google Scholar] [CrossRef]
- Petit, K.E.; Mondeguer, F.; Roquebert, M.F.; Biard, J.F.; Pouchus, Y.F. Detection of griseofulvin in a marine strain of Penicillium waksmanii by ion trap mass spectrometry. J. Microbiol. Methods 2004, 58, 59–65. [Google Scholar] [CrossRef] [PubMed]
- Silva, M.G.; Aracari, N.; Furtado, J.C.; Pupo, M.T.; Fonseca, M.J.V.; Said, S.; da Silva, A.A.; Bastos, J.K. Antibacterial activity from Penicillium corylophilum Dierckx. Microbiol. Res. 2004, 159, 317–322. [Google Scholar] [CrossRef]
- Xu, M.J.; Gessner, G.; Groth, I.; Lange, C.; Christner, A.; Bruhn, T.; Deng, Z.W.; Li, X.; Heinemann, S.H.; Grabley, S.; et al. Shearinines D-K, new indole triterpenoids from an endophytic Penicillium sp (strain HKI0459) with blocking activity on large-conductance calcium-activated potassium channels. Tetrahedron 2007, 63, 435–444. [Google Scholar] [CrossRef]
- De Schryver, P.; Vadstein, O. Ecological theory as a foundation to control pathogenic invasion in aquaculture. Isme J. 2014, 8, 2360–2368. [Google Scholar] [CrossRef]
- Francoeur, S.N.; Pinowska, A.; Clason, T.A.; Makosky, S.; Lowe, R.L. Unionid bivalve influence on benthic algal community composition in a Michigan Lake. J. Freshw. Ecol. 2002, 17, 489–500. [Google Scholar] [CrossRef]
- James, M.R.; Hawes, I.; Weatherhead, M.; Stanger, C.; Gibbs, M. Carbon flow in the littoral food web of an oligotrophic lake. Hydrobiologia 2000, 441, 93–106. [Google Scholar] [CrossRef]
- Xu, Z.; Chen, Y.; Meng, X.; Wang, F.; Zheng, Z. Phytoplankton community diversity is influenced by environmental factors in the coastal East China Sea. Eur. J. Phycol. 2016, 51, 107–118. [Google Scholar] [CrossRef]
- Liang, Y.; Liu, C.; Tian, C.; Sun, M. Effects of nutrients sources and input mode on the community succession of six marine microalgal species. Trans. Oceanol. Limnol. 2014, 2, 23–30. [Google Scholar] [CrossRef]
- Zhao, B.; Hu, W.; Li, C.; Zhou, H.; Zhao, H.; Sun, Y.; Ju, W.; Han, S. Community structure of phytoplankton and benthophyte in Apostichopus japonicus aquaculture ponds and water quality characteristics. J. Guangxi Acad. Sci. 2020, 36, 399–405. [Google Scholar]
- Dal Bo, D.; Magneschi, L.; Bedhomme, M.; Billey, E.; Deragon, E.; Storti, M.; Menneteau, M.; Richard, C.; Rak, C.; Lapeyre, M.; et al. Consequences of Mixotrophy on Cell Energetic Metabolism in Microchloropsis gaditana Revealed by Genetic Engineering and Metabolic Approaches. Front. Plant Sci. 2021, 12, 628684. [Google Scholar] [CrossRef]
- El-Sheekh, M.M.; Mansour, H.M.; Bedaiwy, M.Y.; El-shenody, R.A. Influence of nutrient supplementation and stress conditions on the biomass and lipid production of Microchloropsis salina for biodiesel production. Biomass Convers. Biorefin. 2022. Available online: https://link.springer.com/article/10.1007/s13399-022-03434-9#article-info (accessed on 1 December 2022). [CrossRef]
- Fierro, S.; Sanchez-Saavedra, M.D.P.; Copalcua, C. Nitrate and phosphate removal by chitosan immobilized Scenedesmus. Bioresour. Technol. 2008, 99, 1274–1279. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.K.; Yu, Z.Y.; Wei, D. High-yield production of biomass, protein and pigments by mixotrophic Chlorella pyrenoidosa through the bioconversion of high ammonium in wastewater. Bioresour. Technol. 2020, 313, 123499. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.C.; Tan, X.B.; Yang, L.B.; Liao, J.Y.; Li, X.Y. Cultivation of Chlorella pyrenoidosa in anaerobic wastewater: The coupled effects of ammonium, temperature and pH conditions on lipids compositions. Bioresour. Technol. 2019, 284, 90–97. [Google Scholar] [CrossRef] [PubMed]
- Talib, A.; Recknagel, F.; Cao, H.; van der Molen, D.T. Forecasting and explanation of algal dynamics in two shallow lakes by recurrent artificial neural network and hybrid evolutionary algorithm. Math. Comput. Simul. 2008, 78, 424–434. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hou, Y.; Zhou, M.; Jia, R.; Sun, W.; Yang, Y.; Huang, X.; Li, B.; Zhu, J. Effects of Snail Bellamya purificata Farming at Different Stocking Densities on the Algal and Fungal Communities in Sediment. Fishes 2023, 8, 488. https://doi.org/10.3390/fishes8100488
Hou Y, Zhou M, Jia R, Sun W, Yang Y, Huang X, Li B, Zhu J. Effects of Snail Bellamya purificata Farming at Different Stocking Densities on the Algal and Fungal Communities in Sediment. Fishes. 2023; 8(10):488. https://doi.org/10.3390/fishes8100488
Chicago/Turabian StyleHou, Yiran, Mengmeng Zhou, Rui Jia, Wei Sun, Yanhong Yang, Xiongjian Huang, Bing Li, and Jian Zhu. 2023. "Effects of Snail Bellamya purificata Farming at Different Stocking Densities on the Algal and Fungal Communities in Sediment" Fishes 8, no. 10: 488. https://doi.org/10.3390/fishes8100488
APA StyleHou, Y., Zhou, M., Jia, R., Sun, W., Yang, Y., Huang, X., Li, B., & Zhu, J. (2023). Effects of Snail Bellamya purificata Farming at Different Stocking Densities on the Algal and Fungal Communities in Sediment. Fishes, 8(10), 488. https://doi.org/10.3390/fishes8100488







