Abstract
The growth of biofouling on aquaculture infrastructure is a universal challenge. Standard industry practices to remove biofouling in finfish aquaculture typically include in situ net cleaning via power washing. Since those cleaning practices can be potentially harmful to fish-gill health and expensive, development of other non-toxic biofouling controls is an industry priority. Deposit-feeding sea cucumbers are potentially well suited for biofouling control due to their feeding mechanism, but remain relatively untested in this capacity. We examined the use of California sea cucumbers (Apostichopus californicus) to control biofouling on cages containing adult Chinook salmon (Oncorhynchus tshawytscha) at a commercial farming operation. Four cage types were established: cages with salmon and sea cucumbers, cages with salmon only, cages with sea cucumbers only, and cages without either species. Results showed that the sea cucumbers actively fed on biofouling when salmon were absent (~16% cleaner on average) but preferred to consume uneaten feed/faeces at the bottom of the cages, neglecting the biofouling, when the salmon were present. It is hypothesized that biofouling control in cages with salmon may be possible with an increased density of sea cucumbers. This is the first study to examine the use of sea cucumbers as a direct net biofouling control agent with adult fish. Our results will be beneficial for industry to develop standard operating procedures for using California sea cucumbers as a biofouling control and could contribute to the development of a management framework for sea cucumber/salmon integrated multi-tropic aquaculture.
Key Contribution:
Results showed that the sea cucumbers actively fed on net biofouling when salmon were absent (~16% cleaner nets on average) but preferred to consume uneaten feed/faeces at the bottom of the cages, neglecting the biofouling, when the salmon were present. This is the first study to examine the use of sea cucumbers as a direct net biofouling control agent with adult fish.
1. Introduction
The growth of biofouling material on aquaculture infrastructure is an expensive and universal challenge faced by the industry. A recent study in Norway calculated that the cost of biofouling management per production cycle could be as much as USD 500,000 per eight standard production finfish cages [1]. The blockage of net openings by biofouling organisms can negatively impact fish health by reducing water flow through the nets [2] and affect local hydrodynamic conditions around farm sites [3,4], leading to poor flushing of waste and/or hypoxic conditions. Standard industry practice to remove biofouling typically includes in situ net cleaning via power washing on a monthly or even bi-weekly frequency when fouling levels are highest in the summer. However, that cleaning practice has recently been recognized as being potentially harmful to fish gills due to the plume of biofouling particulates, particularly stinging-capable hydroids, released into the water column as a result of the pressure-washing process [5,6,7,8]. The release of biofouling material through power washing can also promote the spread of invasive species to other cages in the vicinity and into the general marine environment [5].
A number of innovations have been explored by the finfish aquaculture industry in an attempt to improve the cost effectiveness and lower the environmental impacts of power washing, including novel antifouling coatings for nets [9,10], adding bubble streams around cages [11], in situ cleaning via net-crawling robots [12,13], and in situ cleaning with organisms such as the lumpfish (Cyclopterus lumpus [14,15]), ballan wrasse (Labrus bergylta [16]), West Indian spider crab (Maguimithrax spinosissimus [17]), and California sea cucumbers (Apostichopus californicus [18]). Economic and environmental impact analyses reveal that a combined approach of biofouling prevention and active control methods will likely need to be used together in order to achieve a sustainable and permanent solution for the aquaculture industry [1]. Therefore, development of a non-impactful toolkit of cost-effective and environmentally-sustainable options for the industry to rely on should continue to be a priority for the biofouling research community.
Deposit-feeding sea cucumbers are well known for their ability to ingest and assimilate organic material from sedimentary environments and have been shown to be efficient organic extractive species in integrated multi-trophic aquaculture (IMTA; [19,20,21,22]). They are also behaviourally passive, not prone to disease, and have well-established global markets [23], making them a safe and lucrative option for IMTA with finfish and shellfish. They are lesser known, however, for their potential biofouling mitigative properties. Nearly all studies involving sea cucumbers and biofouling control have focused on using bioactive compounds from their tissues and extracts as novel antifouling coatings [24,25,26]. However, many sea cucumber species have large peltate feeding tentacles that brush the surface of soft and hard substrates, picking up organic-rich material for ingestion. This feeding mechanism could potentially dislodge young/small biofouling organisms on nets used in finfish farming, providing active removal and control of biofouling. Indeed, one previous study in Alaska (USA) housed California sea cucumbers in cages with pink (Oncorhynchus gorbuscha) and chum (O. keta) salmon fry with results showing that nets were 58% cleaner when sea cucumbers were present than when they were absent [18]. Despite their potential as net cleaners, no other studies have tested the biofouling control capacity of California sea cucumbers (or any other sea cucumber species) in finfish cages, leading to questions about the effectiveness of the species with other stages of salmon (e.g., juveniles, adults) or with other commercial finfish species. Indeed, the Ahlgren (1998) [18] study is the only one to examine the active biofouling mitigative properties of any sea cucumber species globally.
The objective of the present study was to examine the biofouling control capacity of California sea cucumbers in open net cages with mature Chinook salmon (O. tshawytscha) broodstock. This study was the first of its kind for the Canadian aquaculture industry and the first study worldwide to assess deposit-feeding sea cucumbers for active biofouling control in cages with adult finfish. The results will be beneficial for the salmon aquaculture industry in order to develop standard operating procedures for using California sea cucumbers as net cleaners and will contribute to the development of a management framework for sea cucumber/salmon IMTA.
2. Materials and Methods
2.1. Study Area, Farm Parameters, and Source of Sea Cucumbers
The study was conducted in the summer of 2021 at two organic Chinook salmon farm sites in Clayoquot Sound, British Columbia (BC), Canada, which are Canada-owned and operated by Creative Salmon Co., Ltd. (Figure 1). One site, Dawley Pass (DP), was located in Fortune Channel (49°09′56.3” N, 125°46′10.7” W) and the other, Warne Island (WI), was situated off the southwest shore of Warne Island (W49°07′41.6” N, 125°44′58.6” W). Both farm sites used for this study had mature Chinook salmon broodstock housed within designated net cages (15 × 15 × 15-m Viking-style cages with 4-cm stretch nylon netting). Fish density was dependent on normal industry practice and stock availability, with densities in the 3–4 kg m−3 range in this study. Fish were fed daily, to satiation, using underwater cameras to ensure adequate amounts of food were made available. Four hundred wild California sea cucumbers (>15-cm contracted length, measured after 5 s of consistent handling in air) were hand collected by SCUBA in Clayoquot Sound at a distance >1 km from the farm sites used in the study (Figure 1).
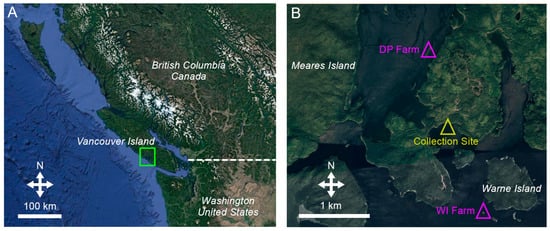
Figure 1.
(A) Map showing Western North America and Vancouver Island, British Columbia (BC), Canada, where the study took place. The dotted white line indicates the border division between Canada and the United States. (B) Chinook salmon farm locations used for the project in Clayoquot Sound, BC. Farm sites are indicated by purple triangles, DP = Dawley Pass, WI = Warne Island. Both farm sites are owned and operated by Creative Salmon Co., Ltd. (Tofino, BC, Canada). The sea cucumber collection site is shown by a yellow triangle.
2.2. Experimental Design
Four cage types were established at both farm sites in order to test the capacity of sea cucumbers to control biofouling on Chinook salmon net pens: (1) fish only without sea cucumbers (F), (2) sea cucumbers only without fish (S), (3) fish and sea cucumbers (F + S), and (4) no fish or sea cucumbers, designated as an “empty” cage control (E). Each cage with sea cucumbers had 100 individuals (density = 1 ind 11.25 m−2 of net surface area), and was chosen based on the density used by Ahlgren (1998) [18]. Four cages were randomly chosen from the cage array (4 × 4) at both farm sites, one for each cage type, with three replicate one-month trials being conducted at both sites beginning in July, August, and September 2021 (i.e., replication was conducted temporally in three “trials”). Once assigned, cages were used for all three replicate one-month trials. During each replicate trial, sea cucumbers were allowed to actively feed on biofouling material in S and F + S cages. All nets were cleaned after each trial via sun drying and power washing, and everything was reset for subsequent temporal replicates. Sea cucumbers were transferred by divers to floating cages in the center of each fish pen during net cleaning operations in order to prevent damage or stress to them while the nets were being manipulated (Figure 2). Sea cucumbers were reused among the trials.

Figure 2.
Large floating cage used to house sea cucumbers during regular net cleaning activities. Sea cucumbers were hand caught via SCUBA and transferred into the cage. It was left submerged and was attached in the middle of the cage before net cleaning began, preventing damage and stress to the sea cucumbers. This protocol and the cage design were developed by Creative Salmon Co., Ltd. personnel.
2.3. Analysis of Net Percent Cleanliness
Net percent cleanliness (PC) was defined as the percent area of net openings that was clear of biofouling growth. This was measured in each cage at the end of each one-month trial using image analysis of video footage collected by SCUBA at three different depth ranges: the top of the cage (~0–5 m), the middle of the cage (5–10 m), and the bottom of the cage (10–15 m). Three video transects per cage were taken at each sample date, with positions of the transects being chosen haphazardly. Image analysis was conducted by converting still frames from the video footage at each depth range into black/white threshold images in ImageJ software [27] and then calculating the percents of net openings that were clear (modified from [28]). A minimum of 25 net openings, chosen randomly, were analyzed at each net depth range per each of the three transects at each sampling point (~225 per cage per trial).
2.4. Stable Isotopes
Stable isotope ratios were used to explore feeding patterns of sea cucumbers housed with and without fish at the end of each 1-month replicate (i.e., August, September, October). Values for d13C and d 15N were obtained for sea cucumber muscle bands and body walls from S and F + S cages as well as wild-caught control individuals (from the original collection site, October only) using Elemental Analyzer Isotope Ratio Mass Spectrometry (EA-IRMS). Three sea cucumbers per cage type (F and F + S) were sampled at each time point. All samples were sub-sampled, run twice, and then averaged to account for sample heterogeneity. This work was completed by the Stable Isotopes Facility in the Faculty of Forestry at the University of British Columbia in Vancouver, BC.
2.5. Statistical Analysis
All statistical analyses were conducted using R statistical software [29] and graphics were produced using the ggplot2 package [30]. Significance was set at α = 0.05 for all tests. We first eliminated the possibility of seasonal variability and site variability on PC values using two-way Aligned Ranks Transformation (ART) ANOVA, since assumptions of normality were not met [31,32]. Since no significant seasonal or site effects were detected (Table 1), PC values were pooled from each one-month replicate trial and both farm sites for all subsequent analyses. Principle component analysis (PCA) was then used to compare patterns of PC values across net depths among experimental cage types (i.e., F, S, F + S, E). Follow-up two-way and one-way ART ANOVAs were performed to further explore the influence of net depth and cage type on PC values. Post-hoc pairwise comparisons were performed using an align-and-rank procedure with a Tukey correction of the p-values to correct for multiple pairwise comparisons.

Table 1.
Mean (±SD) percent cleanliness (%) by trial month and site and cage type and net depth. Statistical significance (p < 0.05) indicated by asterisks (two-factor aligned rank transform (ART) ANOVA) and pairwise groupings indicated by superscript letters (ART post-hoc multiple comparisons test).
A two-way ANOVA was then used to compare individual stable isotope ratios among factors (i.e., cage type, site) after each one-month replicate trial in order to assess the cumulative effects of sea cucumber exposure to biofouling at Chinook salmon farm sites. Parametric test assumptions of normality and homoscedasticity were confirmed using Shapiro tests and a plot of the residuals, respectively. Tukey post-hoc tests were used for pairwise comparisons of the treatments. No significant difference in stable isotope ratios between farm sites was detected.
3. Results
3.1. Net Percent Cleanliness
Over the course of the study, individual PC values exhibited a large range, from <10% to 100% (Figure 3). Average (±SD) PC values for the four cage types ranged from 62.1 ± 24.9% (F + S) to 79.4 ± 16.9% (S), but they did not differ significantly (Table 1). In addition, there was no significant cage-type × net-depth interaction (Table 1). There was, however, a significant net-depth effect, the top 5 m of the nets, with an average (±SD) PC value of 46.3 ± 33.5%, being significantly less clean at the end of each one-month trial than the middle (5−10 m) and bottom (10−15 m) regions of the nets, which had average PC values of 79.0 ± 18.1% and 77.5 ± 20.0%, respectively (Table 1).
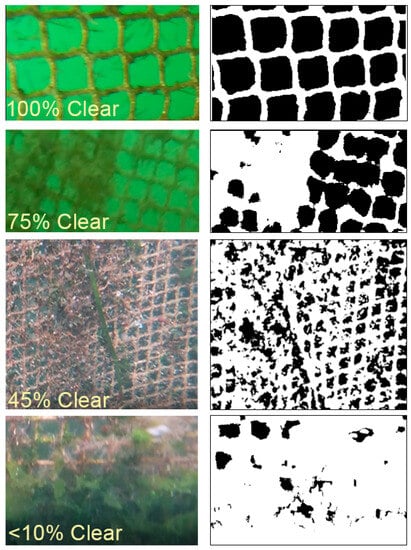
Figure 3.
Sample of various net percent cleanliness values from actual photographs (left) and edited images (right). Black in the edited images (right) indicates a clear space for water to pass through the net. ImageJ [27] was used for the analysis.
Principle component analysis indicated two potential groupings, one containing the S and E cages and another containing the F and F + S cages (Figure 4). It also revealed that patterns of net PC values among the cage types were primarily being driven by cleanliness at the bottom of the nets only (Figure 4). Closer analysis of these bottom PC values revealed significant differences among the four cage types: F (65.3 ± 25.8%), S (94.2 ± 6.8%), F + S (67.2 ± 19.5%), and E (83.2 ± 9.2%) (Table 2). Pairwise comparisons showed significantly higher PC values in the S cages relative to the F and F + S cages, with no other significant pairwise comparisons. There were no significant differences among the cage types in the top and middle regions (Table 2). Overall, the S cages had the cleanest nets of all cage types (16% cleaner on average).
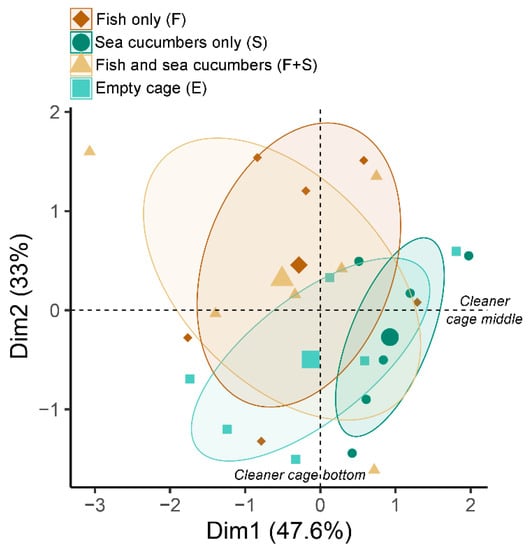
Figure 4.
Principle component analysis (PCA) comparing patterns of percent net cleanliness values among the four cage types: brown diamond = fish only (F), teal circle = sea cucumbers only (S), yellow triangle = fish and sea cucumbers (F + S), and light blue square = empty cage (E). Ellipses indicate 95% confidence intervals for each cage type. The center of each ellipse is indicated by a larger icon corresponding to each cage type. Dimension 1 (X-axis) was primarily explained by differences in middle-cage cleanliness while Dimension 2 (Y-axis) was primarily explained by differences in bottom-cage cleanliness.

Table 2.
Mean (± SD) percent cleanliness values (%) at each net depth across the four experimental cage types. Statistical significance (p < 0.05) indicated by asterisks (two-factor aligned rank transform (ART) ANOVA) and pairwise groupings indicated by superscript letters (ART post-hoc multiple comparisons test).
3.2. Stable Isotopes
Temporal patterns of d13C and d15N in the tissues of S and F + S sea cucumbers showed changes in diet between these two cage types after each sequential one-month replicate trial (PCA, Figure 5). These differences were significant after three months of sea cucumber integration into salmon culture for both d13C (two-way ANOVA, F1,15 = 7.60, p < 0.01) and d15N (F2,15 = 3.94, p = 0.04). At the three-month time point, F + S (+12.08‰) and S (+11.91‰) sea cucumbers were both enriched in 15N (Tukey Test, p = 0.04) relative to wild-caught reference (+11.14‰) sea cucumbers, and F + S (−18.07‰) individuals were depleted in 13C relative to S (−16.87‰) and wild-caught reference (−17.34‰) sea cucumbers (p < 0.01).
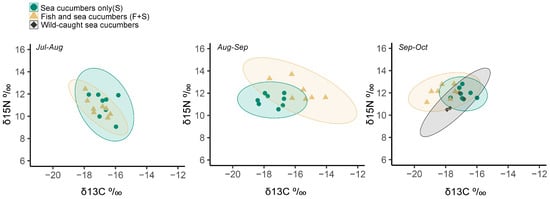
Figure 5.
Scatter plots of 15N versus 13C stable isotope ratios in tissues of California sea cucumbers at the end of each consecutive one-month replicate trial: teal circle = sea cucumbers only (S), yellow triangle = fish and sea cucumbers (F + S), and black diamond = wild-caught sea cucumbers (October only). Ellipses indicate 95% confidence intervals for each cage type.
4. Discussion
The present study tested the biofouling control capacity of California sea cucumbers in open net cages with mature Chinook salmon broodstock. Though it appeared that sea cucumbers were feeding on biofouling material in both cage types containing them, sea cucumbers at the present density could not remove enough biofouling to have a significant effect on net cleanliness when adult Chinook were present. This result was surprising given the high biofouling removal levels (58% cleaner with sea cucumbers than without them) reported by Ahlgren (1998) [18] in the only other study to conduct this type of work using deposit-feeding sea cucumber species to directly feed on and remove biofouling from finfish cages. However, there are two key differences between the studies that could explain why the sea cucumbers were less able to control biofouling in the present work. Firstly, Ahlgren (1998) [18] tested biofouling removal in cages with salmon fry whereas we tested it with mature adults. Salmon fry are very small and were likely fed a different diet and feed rate than adults would be, in addition to producing less faeces and less dissolved wastes. The likely difference in available nutrients at the farm sites used by Ahlgren (1998) [18] versus our study could have resulted in increased growth of biofouling when adult fish were present, making it more difficult for the same density of sea cucumbers to clean [33,34]. Additionally, Ahlgren (1998) [18] conducted their study in the late winter/early spring when baseline biofouling levels are typically lower, whereas we conducted our study in late summer, when biofouling levels are at their highest in the Northern Hemisphere [8,35,36]. Taken together, these differences suggest that our density of sea cucumbers (N = 100 per cage) was too low to control biofouling in cages with adult fish in the late summer and that this density should be increased for subsequent studies.
Interestingly, sea cucumbers housed alone (S) in the present study were more efficient at removing biofouling than those housed with fish (F + S) (S cages being on average 16% cleaner than the other cage types). The presence of fish can significantly increase levels of net biofouling [33,34], but sea cucumbers in the S and F + S cages actually exhibited different feeding behaviours that could also explain differences in their vertical net cleaning efficiencies. Individuals in the S and F + S cages had different depth preferences for feeding, with the former feeding at all net depths and the latter only feeding at the bottom of cages (Montgomery, pers. obs.; Figure 6). Sea cucumbers in the F + S cages seemed to feed primarily on waste fish food and faeces that sank to the bottom of the cages, rather than on net biofouling. We observed these partially digested fish pellets and faeces in the guts of F + S sea cucumbers that were not seen in the guts of S individuals. Sea cucumbers in the F + S cages, therefore, did not need to climb the nets in order to access quality food, which led to reduced net cleaning efficiency (Figure 6). However, as stocking densities of sea cucumbers are adjusted, intraspecific competition may encourage better net cleaning in F + S cages if individuals are forced to leave the bottom of the cages to access other food resources.
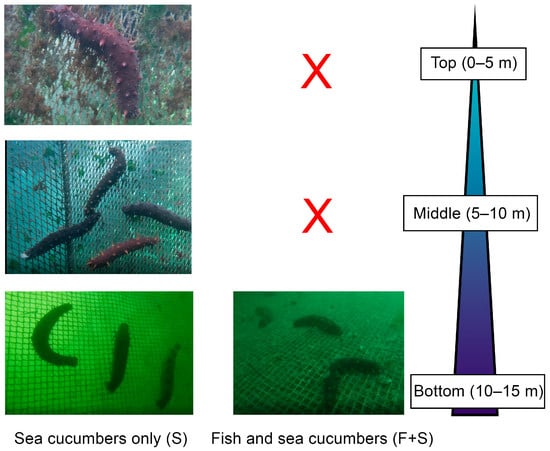
Figure 6.
Typical vertical position (0−15 m) of California sea cucumbers in sea cucumber only (S) and fish and sea cucumber (F + S) cages. Red crosses represent positions that sea cucumbers were not observed in.
The observed partitioned feeding behaviour of sea cucumbers in the F + S versus S cages was also supported by the stable isotopes results, which confirmed that sea cucumbers in those two cage types were eating different diets. Interestingly, the isotopic ratios of F + S versus S sea cucumbers shifted after each sequential one-month replicate (Figure 5). After three months of consecutive exposure to salmon culture, S sea cucumbers were more similar to wild-caught individuals, but F + S individuals were distinctly different, showing slight shifts in dietary carbon source and trophic position (higher d15N enrichment; Figure 5). These shifts would correspond with the observation of F + S sea cucumbers feeding on commercial salmon pellets containing organic wheat and fish extracts or their faeces. Previous studies of sea cucumbers cultured on the benthos near finfish cages have also reported shifts in d13C and d15N values corresponding to organic matter transfer from fish wastes to sea cucumber tissues, indicating a potential preference for these food sources by deposit-feeding sea cucumber species [37,38,39]. Though the sea cucumbers in the F + S cages were not efficient at cleaning net biofouling, their preference for salmon waste in the present study does support previous suggestions of using this species as a benthic bioremediatory in IMTA systems with finfish (see [19,40,41]).
Previous IMTA studies of sea cucumbers and finfish have reported additional compositional changes in sea cucumber tissues when they are exposed to fish wastes, including changes in fatty acids [39,42] and amino acids/proteins [38]. Compositional changes can positively or negatively influence taste and market favourability, since fatty acids/amino acids are often the building blocks for volatile compounds such as aldehydes, alcohols, and aromatics when sea cucumber tissues are exposed to various seasoning and processing methods [43]. It will therefore be critical to assess any compositional or nutritional differences in sea cucumbers integrated in salmon culture in order to assess the viability of industry selling such sea cucumbers as a secondary revenue stream.
5. Conclusions
The present study tested the biofouling control capacity of California sea cucumbers in open net cages with mature Chinook salmon broodstock. Results showed that the sea cucumbers actively fed on net biofouling when salmon were absent (~16% cleaner on average than all other net types) but preferred to consume uneaten feed/faeces at the bottom of the cages, neglecting the biofouling, when the fish were present. It is hypothesized that biofouling control in cages with salmon may be possible with an increased density of sea cucumbers. These results will be beneficial for industry to develop standard operating procedures for using California sea cucumbers (or other deposit-feeding sea cucumbers) as a net biofouling control, and they could contribute to the development of a management framework for sea cucumber/salmon integrated multi-tropic aquaculture.
Author Contributions
Conceptualization, E.M.M. and C.M.P.; methodology, E.M.M. and C.M.P.; software, E.M.M.; validation, E.M.M. and C.M.P.; formal analysis, E.M.M.; investigation, E.M.M.; resources, B.L.C. and C.M.P.; data curation, E.M.M. and C.M.P.; writing—original draft preparation, E.M.M.; writing—review and editing, E.M.M., B.L.C. and C.M.P.; visualization, E.M.M.; supervision, C.M.P.; project administration, E.M.M., B.L.C. and C.M.P.; funding acquisition, E.M.M. and C.M.P. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Aquaculture Collaborative Research and Development Program (ACRDP) of Fisheries and Oceans Canada (grant number ACRDP 20-P-05) and Creative Salmon Co., Ltd. (Tofino, British Columbia, Canada).
Institutional Review Board Statement
The study was conducted according to the guidelines of the Declaration of Helsinki and approved by the Institutional Review Board (Pacific Region Animal Care Committee) of Fisheries and Oceans Canada, Pacific Biological Station (AUP 21-005, approved 13 April 2021).
Data Availability Statement
The data are not publicly available.
Acknowledgments
The authors would like to thank the Aquaculture Collaborative Research and Development Program (ACRDP, Project: 20-P-05) of Fisheries and Oceans Canada and Creative Salmon Co., Ltd. (Tofino, British Columbia, Canada) for the funding and opportunity to conduct this study. In particular, E.M. would like to thank Rodrigo Leme, the field crews of Creative Salmon Co., Ltd., and the dive team at Mulder Marine for their enthusiasm and facilitation of the work at the farm sites. The authors would also like to thank Tim Green, Sarah Leduc, UB Diving, the Deep Bay Marine Field Station of Vancouver Island University for hosting us for preliminary laboratory tests on sea cucumber escapement, and the Stable Isotopes Lab at the University of British Columbia for helping conduct the stable isotopes work.
Conflicts of Interest
The sponsors had no role in the design, execution, or interpretation of the study. The sponsors did participate in a final review of the manuscript and provided resources during the study.
References
- Bloecher, N.; Floerl, O. Towards Cost-Effective Biofouling Management in Salmon Aquaculture: A Strategic Outlook. Rev. Aquac. 2021, 13, 783–795. [Google Scholar] [CrossRef]
- Cornejo, P.; Guerrero, N.M.; Montes, R.M.; Quiñones, R.A.; Sepúlveda, H.H. Hydrodynamic Effect of Biofouling in Fish Cage Aquaculture Netting. Aquaculture 2020, 526, 735367. [Google Scholar] [CrossRef]
- Chen, Q.-P.; Bi, C.-W.; Zhang, Z.-X.; Zhao, Y.-P. Hydrodynamic Effect of Different Biofouling Types on Aquaculture Netting. Ocean. Eng. 2023, 279, 114430. [Google Scholar] [CrossRef]
- Yu, S.; Qin, H.; Li, P.; Gong, F. Drag Force Coefficient and Flow Field Variations of Net with Different Levels of Biological Fouling under Large-Eddy Simulation. J. Mar. Sci. Technol. 2023, 28, 506–523. [Google Scholar] [CrossRef]
- Floerl, O.; Sunde, L.M.; Bloecher, N. Potential Environmental Risks Associated with Biofouling Management in Salmon Aquaculture. Aquac. Environ. Interact. 2016, 8, 407–417. [Google Scholar] [CrossRef]
- Åtland, Å.; Dale, T.; Bloecher, N. Environmental Considerations in Aquaculture Health Management. In Aquaculture Health Management; Elsevier: Amsterdam, The Netherlands, 2020; pp. 235–280. [Google Scholar]
- Østevik, L.; Stormoen, M.; Nødtvedt, A.; Alarcón, M.; Lie, K.-I.; Skagøy, A.; Rodger, H. Assessment of Acute Effects of in situ Net Cleaning on Gill Health of Farmed Atlantic Salmon (Salmo salar). Aquaculture 2021, 545, 737203. [Google Scholar] [CrossRef]
- Fletcher, L.M.; Davidson, I.C.; Bucknall, B.G.; Atalah, J. Salmon Farm Biofouling and Potential Health Impacts to Fish from Stinging Cnidarians. Aquaculture 2023, 568, 739315. [Google Scholar] [CrossRef]
- Bloecher, N.; Floerl, O. Efficacy Testing of Novel Antifouling Coatings for Pen Nets in Aquaculture: How Good Are Alternatives to Traditional Copper Coatings? Aquaculture 2020, 519, 734936. [Google Scholar] [CrossRef]
- Ashraf, P.M.; Lekshmi, N.M.; Chinnadurai, S.; Anjitha, S.; Archana, M.; Kumar, C.M.V.; Sandhya, K.M.; Gop, A.P. Impact Assessment of Biofouling Resistant Nano Copper Oxide–Polyaniline Coating on Aquaculture Cage Nets. Aquac. Fish 2023, 8, 538–543. [Google Scholar] [CrossRef]
- Haberlin, D.; McAllen, R.; Doyle, T.K. Field and Flume Tank Experiments Investigating the Efficacy of a Bubble Curtain to Keep Harmful Jellyfish out of Finfish Pens. Aquaculture 2021, 531, 735915. [Google Scholar] [CrossRef]
- Ohrem, S.J.; Kelasidi, E.; Bloecher, N. Analysis of a Novel Autonomous Underwater Robot for Biofouling Prevention and Inspection in Fish Farms. In Proceedings of the 2020 28th Mediterranean Conference on Control and Automation (MED), Saint-Raphaël, France, 15–18 September 2020; IEEE: Piscataway, NJ, USA, 2020; pp. 1002–1008. [Google Scholar]
- Haugaløkken, B.O.A.; Kelasidi, E.; Mulelid, M.; Bloecher, N. Docking Stations for Net-Crawling Underwater Vehicles in Aquaculture Net Pens. In Proceedings of the OCEANS 2021: San Diego–Porto, San Diego, CA, USA, 20–23 September 2021; IEEE: Piscataway, NJ, USA, 2021; pp. 1–10. [Google Scholar]
- Boissonnot, L.; Kharlova, I.; Iversen, N.S.; Staven, F.R.; Austad, M. Characteristics of Lumpfish (Cyclopterus lumpus) with High Cleaning Efficacy in Commercial Atlantic Salmon (Salmo salar) Production. Aquaculture 2022, 560, 738544. [Google Scholar] [CrossRef]
- Imsland, A.K.D.; Reynolds, P. In Lumpfish We Trust? The Efficacy of Lumpfish Cyclopterus lumpus to Control Lepeophtheirus salmonis Infestations on Farmed Atlantic Salmon: A Review. Fishes 2022, 7, 220. [Google Scholar] [CrossRef]
- Leclercq, E.; Zerafa, B.; Brooker, A.J.; Davie, A.; Migaud, H. Application of Passive-Acoustic Telemetry to Explore the Behaviour of Ballan Wrasse (Labrus bergylta) and Lumpfish (Cyclopterus lumpus) in Commercial Scottish Salmon Sea-Pens. Aquaculture 2018, 495, 1–12. [Google Scholar] [CrossRef]
- Zeinert, L.R.; Brooks, A.M.L.; Couturier, C.; McGaw, I.J. Potential Use of the Caribbean Spider Crab Maguimithrax spinosissimus for Biofouling Removal on Marine Aquaculture Cages. Aquaculture 2021, 545, 737202. [Google Scholar] [CrossRef]
- Ahlgren, M.O. Consumption and Assimilation of Salmon Net Pen Fouling Debris by the Red Sea Cucumber Parastichopus californicus: Implications for Polyculture. J. World Aquac. Soc. 1998, 29, 133–139. [Google Scholar] [CrossRef]
- Hannah, L.; Pearce, C.M.; Cross, S.F. Growth and Survival of California Sea Cucumbers (Parastichopus californicus) Cultivated with Sablefish (Anoplopoma fimbria) at an Integrated Multi-Trophic Aquaculture Site. Aquaculture 2013, 406–407, 34–42. [Google Scholar] [CrossRef]
- Tolon, M.T.; Emiroglu, D.; Gunay, D.; Ozgul, A. Sea Cucumber (Holothuria tubulosa Gmelin, 1790) Culture under Marine Fish Net Cages for Potential Use in Integrated Multi-Trophic Aquaculture (IMTA). Indian J. Geo-Mar. Sci. 2017, 46, 749–756. [Google Scholar]
- Zamora, L.N.; Yuan, X.; Carton, A.G.; Slater, M.J.; Marine, L. Role of Deposit-Feeding Sea Cucumbers in Integrated Multitrophic Aquaculture: Progress, Problems, Potential and Future Challenges. Rev. Aquac. 2018, 10, 57–74. [Google Scholar] [CrossRef]
- Cutajar, K.; Falconer, L.; Massa-Gallucci, A.; Cox, R.E.; Schenke, L.; Bardócz, T.; Sharman, A.; Deguara, S.; Telfer, T.C. Culturing the Sea Cucumber Holothuria poli in Open-Water Integrated Multi-Trophic Aquaculture at a Coastal Mediterranean Fish Farm. Aquaculture 2022, 550, 737881. [Google Scholar] [CrossRef]
- Purcell, S.W.; Mercier, A.; Conand, C.; Hamel, J.; Toral-Granda, M.V.; Lovatelli, A.; Uthicke, S. Sea Cucumber Fisheries: Global Analysis of Stocks, Management Measures and Drivers of Overfishing. Fish Fish. 2013, 14, 34–59. [Google Scholar] [CrossRef]
- Darya, M.; Sajjadi, M.; Yousefzadi, M.; Sourinejad, I.; Zarei, M. Bioactive Compounds of Sea Cucumber (Holothuria leucospilota) Added to Epoxy Resin as Environmental Friendly Antifouling Coats. Aquat. Physiol. Biotechnol. 2020, 8, 47–66. [Google Scholar]
- Darya, M.; Abdolrasouli, M.H.; Yousefzadi, M.; Sajjadi, M.M.; Sourinejad, I.; Zarei, M. Antifouling Coating Based on Biopolymers (PCL/PLA) and Bioactive Extract from the Sea Cucumber Stichopus herrmanni. AMB Express 2022, 12, 24. [Google Scholar] [CrossRef] [PubMed]
- Kamyab, E.; Goebeler, N.; Kellermann, M.Y.; Rohde, S.; Reverter, M.; Striebel, M.; Schupp, P.J. Anti-Fouling Effects of Saponin-Containing Crude Extracts from Tropical Indo-Pacific Sea Cucumbers. Mar. Drugs 2020, 18, 181. [Google Scholar] [CrossRef]
- Abràmoff, M.D.; Magalhães, P.J.; Ram, S.J. Image Processing with ImageJ. Biophotonics Int. 2004, 11, 36–41. [Google Scholar]
- Gansel, L.C.; Bloecher, N.; Floerl, O.; Guenther, J. Quantification of Biofouling on Nets: A Comparison of Wet Weight Measurements and Optical (Image Analysis) Methods. Aquac. Int. 2017, 25, 679–692. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2020. [Google Scholar]
- Wickham, H. Ggplot2; Use R! Springer International Publishing: Cham, Switzerland, 2016; ISBN 978-3-319-24275-0. [Google Scholar]
- Wobbrock, J.O.; Findlater, L.; Gergle, D.; Higgins, J.J. The Aligned Rank Transform for Nonparametric Factorial Analyses Using Only ANOVA Procedures. In Proceedings of the SIGCHI Conference on Human Factors in Computing Systems, Yokohama, Japan, 8–13 May 2011; pp. 143–146. [Google Scholar]
- Leys, C.; Schumann, S. A Nonparametric Method to Analyze Interactions: The Adjusted Rank Transform Test. J. Exp. Soc. Psychol. 2010, 46, 684–688. [Google Scholar] [CrossRef]
- Strain, P.M.; Hargrave, B.T. Salmon Aquaculture, Nutrient Fluxes and Ecosystem Processes in Southwestern New Brunswick. In Environmental Effects of Marine Finfish Aquaculture; Springer: Berlin/Heidelberg, Germany, 2005; pp. 29–57. [Google Scholar]
- Hodson, S.L.; Burke, C. Microfouling of Salmon-Cage Netting: A Preliminary Investigation. Biofouling 1994, 8, 93–105. [Google Scholar] [CrossRef]
- Bloecher, N.; Olsen, Y.; Guenther, J. Variability of Biofouling Communities on Fish Cage Nets: A 1-Year Field Study at a Norwegian Salmon Farm. Aquaculture 2013, 416–417, 302–309. [Google Scholar] [CrossRef]
- Bloecher, N.; Powell, M.; Hytterød, S.; Gjessing, M.; Wiik-Nielsen, J.; Mohammad, S.N.; Johansen, J.; Hansen, H.; Floerl, O.; Gjevre, A.G. Effects of Cnidarian Biofouling on Salmon Gill Health and Development of Amoebic Gill Disease. PLoS ONE 2018, 13, e0199842. [Google Scholar] [CrossRef]
- Park, H.J.; Han, E.; Lee, W.C.; Kwak, J.H.; Kim, H.C.; Park, M.S.; Kang, C.K. Trophic Structure in a Pilot System for the Integrated Multi-Trophic Aquaculture off the East Coast of Korean Peninsula as Determined by Stable Isotopes. Mar. Pollut. Bull. 2015, 95, 207–214. [Google Scholar] [CrossRef]
- Sadoul, B.; Caprioli, J.-P.; Barrier-Loiseau, C.; Cimiterra, N.; Laugier, T.; Lagarde, F.; Chary, K.; Callier, M.D.; Guillermard, M.-O.; D’Orbcastel, E.R. Is Holothuria tubulosa the Golden Goose of Ecological Aquaculture in the Mediterranean Sea? Aquaculture 2022, 554, 738149. [Google Scholar] [CrossRef]
- Cutajar, K.; Falconer, L.; Massa-Gallucci, A.; Cox, R.E.; Schenke, L.; Bardócz, T.; Andolina, C.; Signa, G.; Vizzini, S.; Sprague, M.; et al. Stable Isotope and Fatty Acid Analysis Reveal the Ability of Sea Cucumbers to Use Fish Farm Waste in Integrated Multi-Trophic Aquaculture. J. Environ. Manag. 2022, 318, 115511. [Google Scholar] [CrossRef] [PubMed]
- Orr, L.C. Co-Culture of Invertebrates with Sablefish (Anoplopoma fimbria) in IMTA in British Columbia: Use of Laboratory Feeding Trials to Assess the Organic Extractive Potential of Various Candidate Species. Master’s Thesis, University of Victoria, Victoria, BC, Canada, 2012. [Google Scholar]
- Hudson, B.; Hauser, L.; Vadopalas, B.; Hetrick, J.; Pride, A.; Hatchery, S.; Seward, H.; Carson, W. Development of Red Sea Cucumber (Parastichopus californicus) Poly-Aquaculture for Nutrient Uptake and Seafood Export; Final Report of the Saltonstall-Kennedy Program #NA15NMF4270322; 2019; Available online: https://www.pacshell.org/pdf/Final%20Report%20SK%20Cucumber%202019.pdf (accessed on 28 June 2023).
- Sun, J.; Hamel, J.-F.; Gianasi, B.L.; Graham, M.; Mercier, A. Growth, Health and Biochemical Composition of the Sea Cucumber Cucumaria frondosa after Multi-Year Holding in Effluent Waters of Land-Based Salmon Culture. Aquac. Environ. Interact. 2020, 12, 139–151. [Google Scholar] [CrossRef]
- Li, X.; Dong, Y.; Jiang, P.; Qi, L.; Lin, S. Identification of Changes in Volatile Compounds in Sea Cucumber Apostichopus japonicus during Seasonings Soaking Using HS-GC-IMS. LWT 2022, 154, 112695. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).