Comparison of Artificial Feed and Natural Food by the Growth and Blood Biochemistry in Chinese Sturgeon Acipenser sinensis
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Fish
2.2. Feeding Management
2.3. Experimental Diet
2.4. Blood Sample Collection and Pre-Treatment
2.5. Serum Biochemical Index Test
2.6. Growth Performance
2.7. Statistical Analysis
3. Results
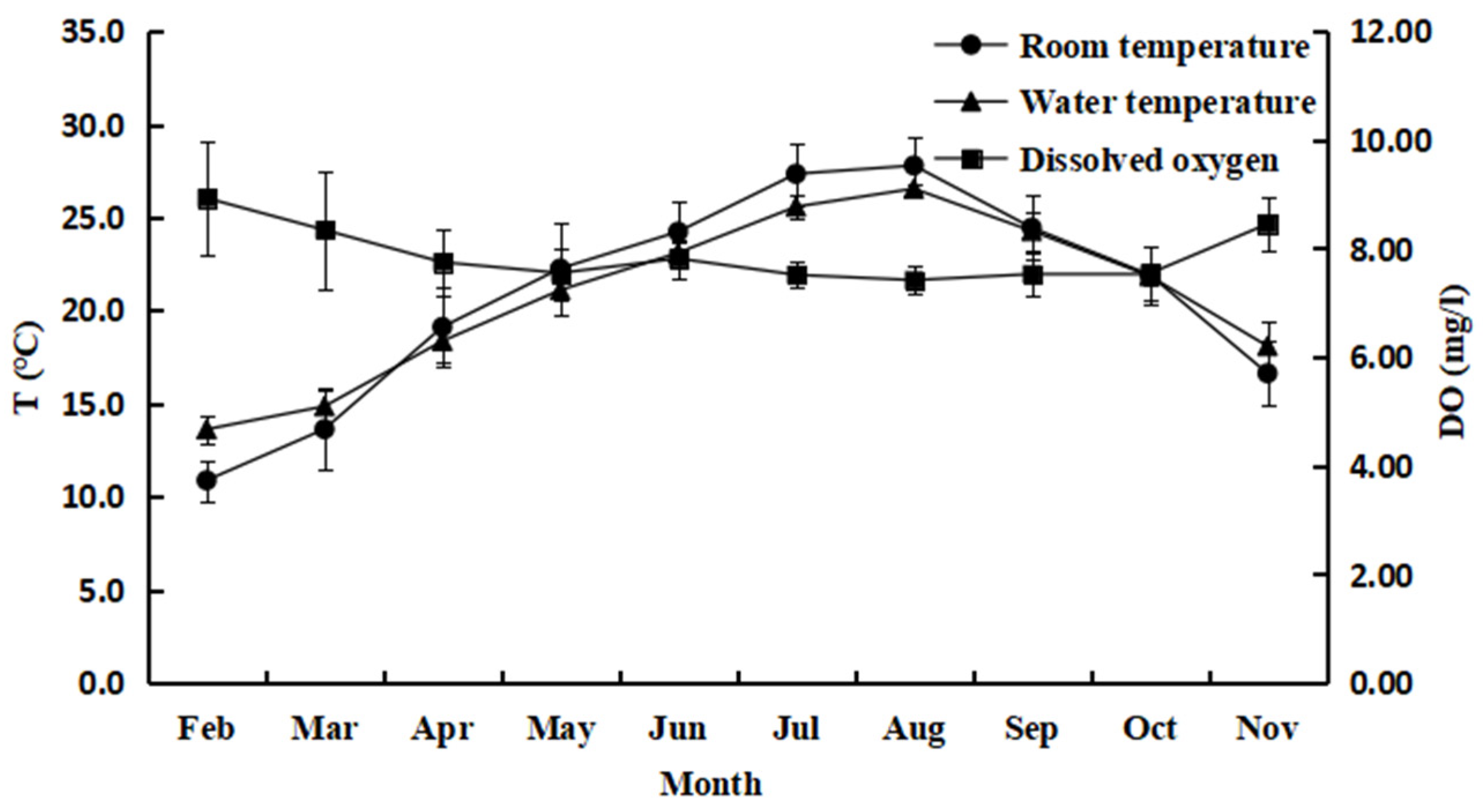
3.1. Changes in Water Quality
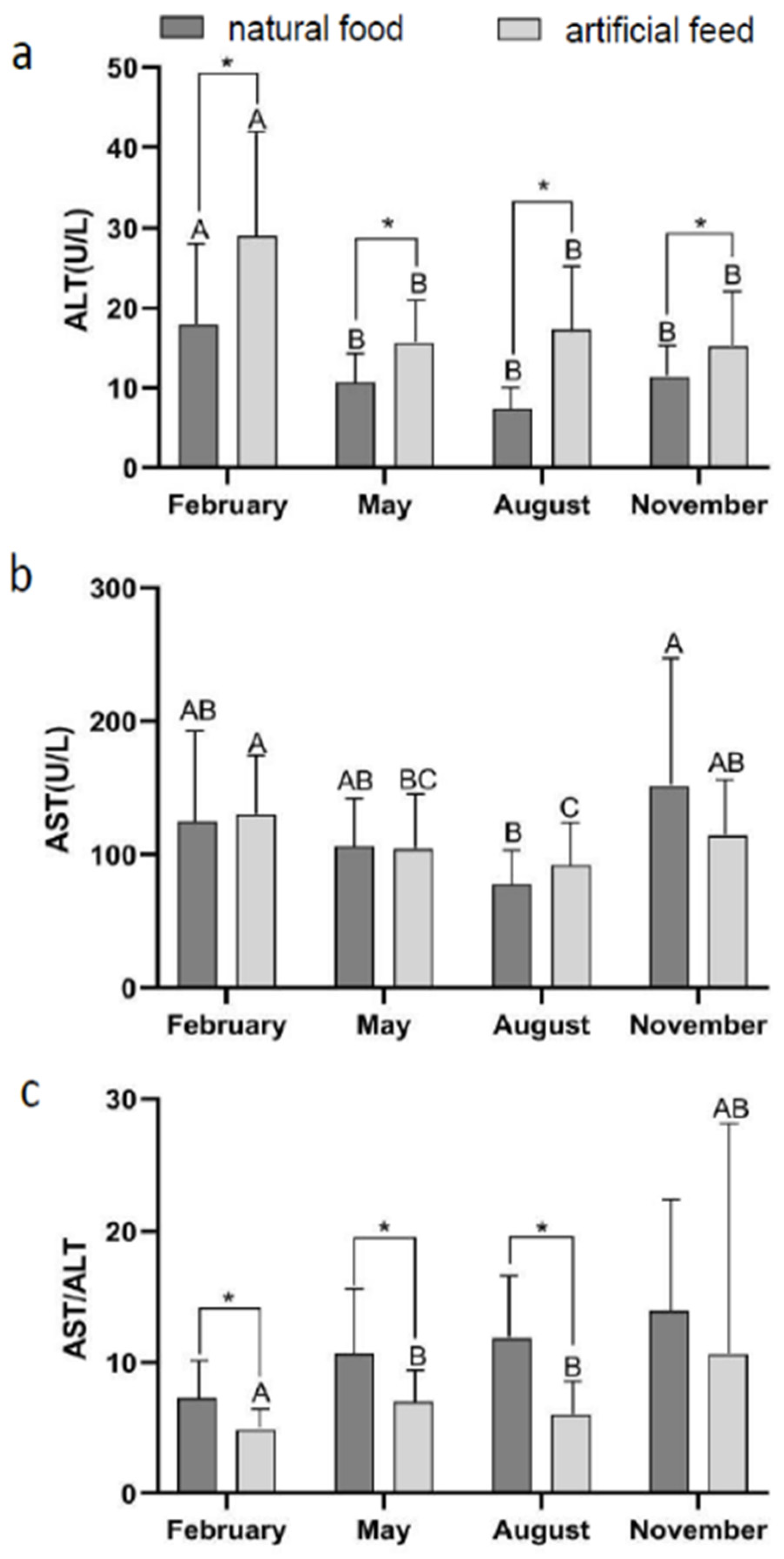
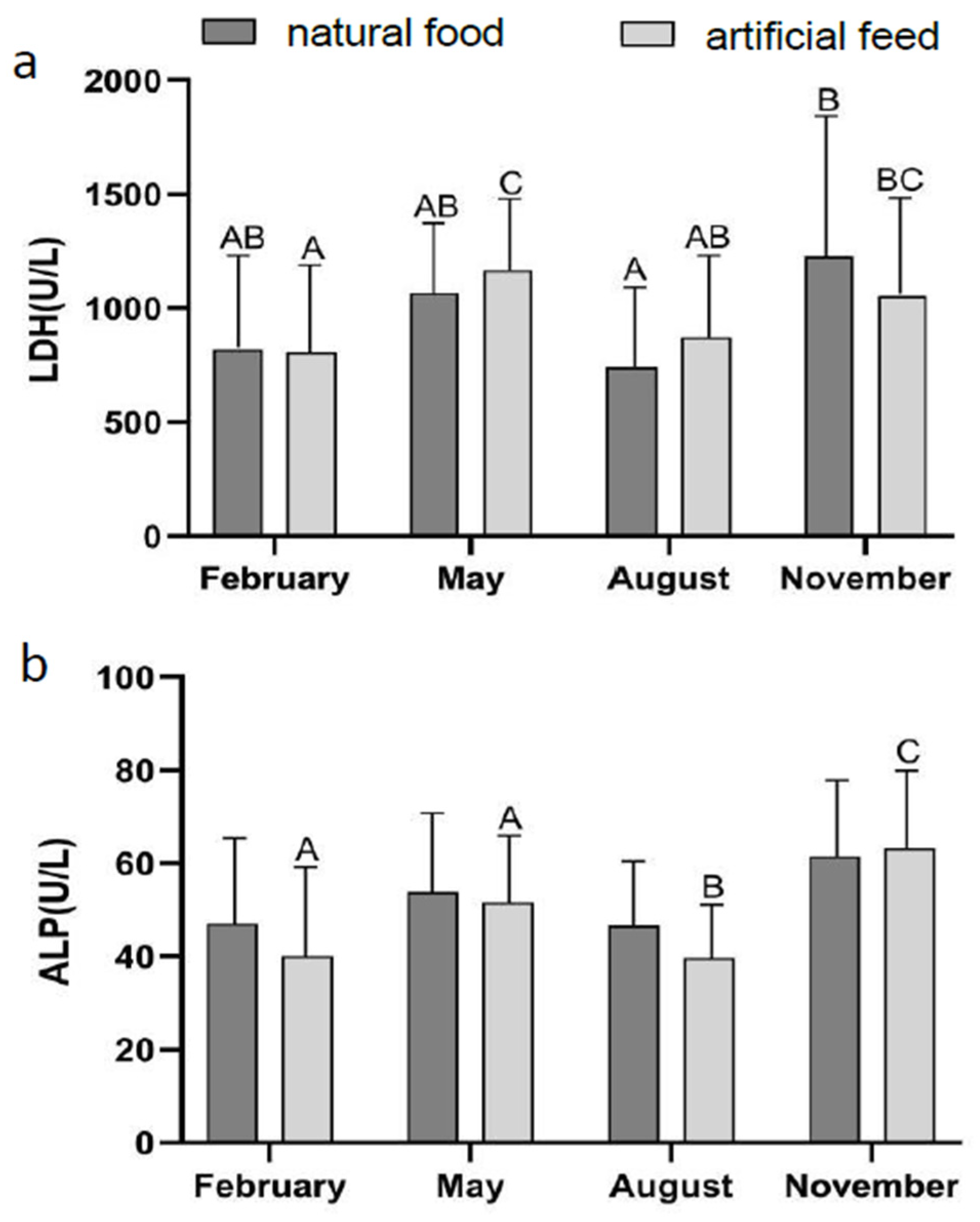
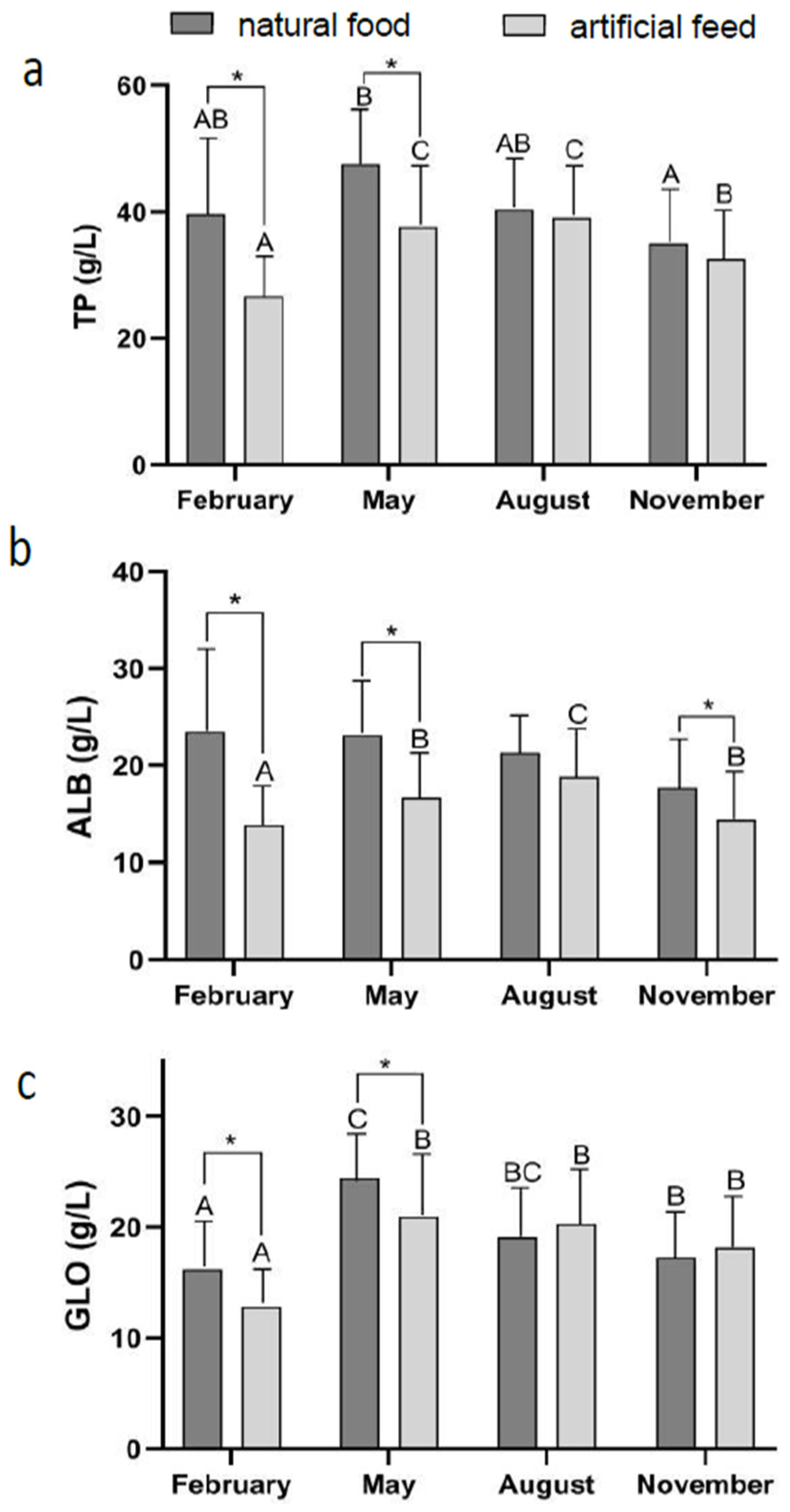
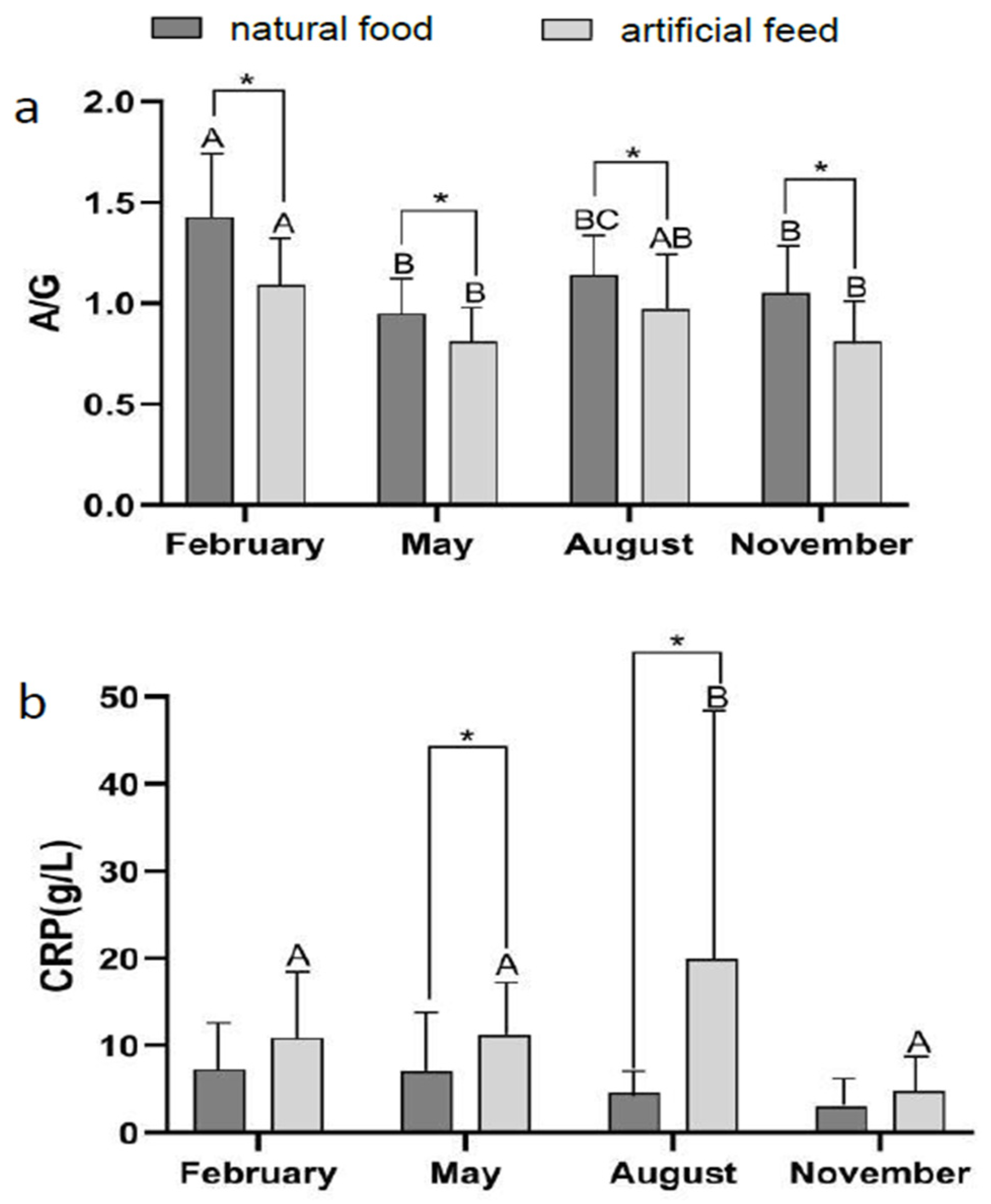
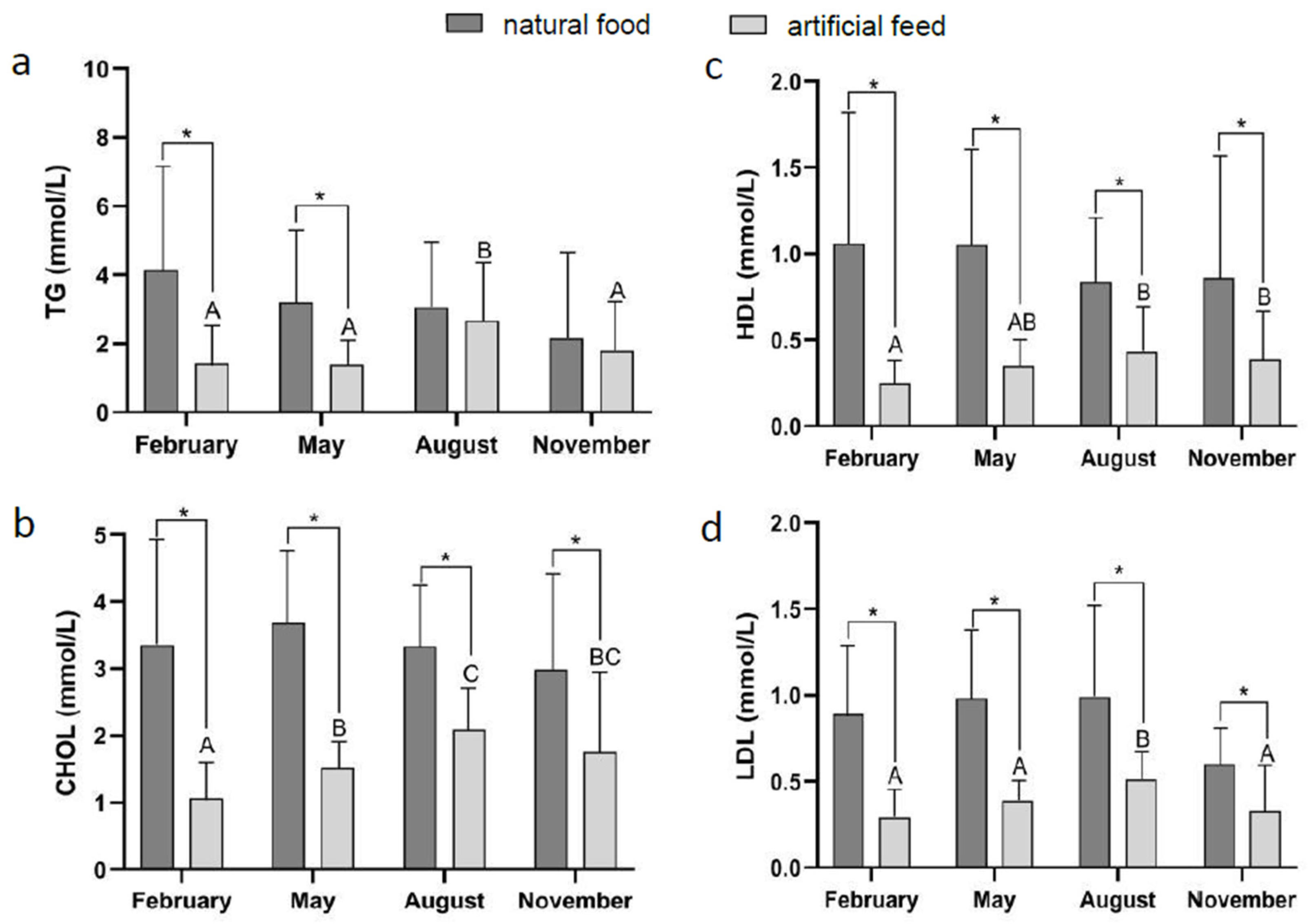
3.2. Effect of Diet Type and Seasons on Blood Physiological Indicators
3.3. Effect of Diet Type on Growth
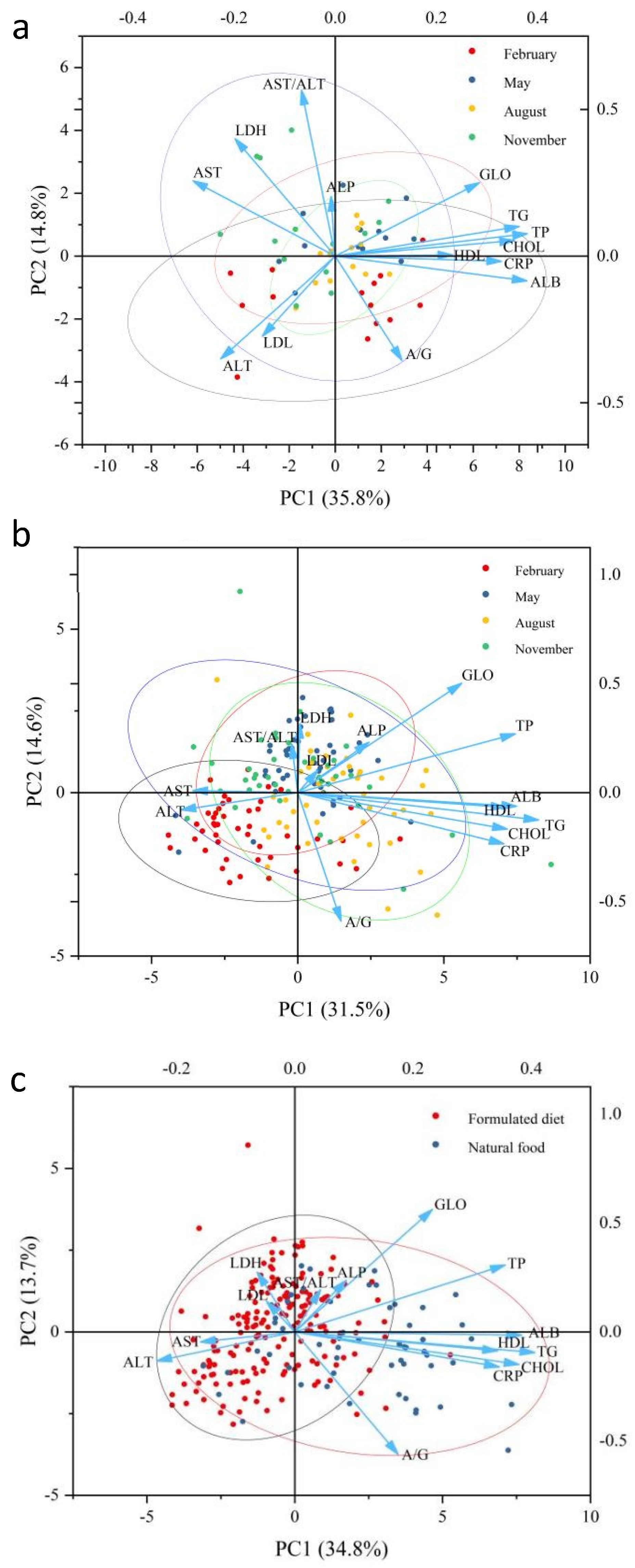
3.4. Principal Component Analysis and Correlation Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Huang, Z.L.; Wang, L.H. Yangtze Dams Increasingly Threaten the Survival of the Chinese Sturgeon. Curr. Biol. 2018, 28, 3640–3647. [Google Scholar] [CrossRef]
- Chen, Y.S.; Qu, X.; Xiong, F.Y.; Lu, Y.; Wang, L.Z.; Hughes, R.M. Challenges to saving China’s freshwater biodiversity: Fishery exploitation and landscape pressures. Ambio 2020, 49, 926–938. [Google Scholar] [CrossRef] [PubMed]
- Xie, P. Can we save the disappearing sturgeons in the Yangtze River. J. Lake. Sci. 2020, 32, 899–914. (In Chinese) [Google Scholar]
- Zhou, X.F.; Chen, L.; Yang, J.; Wu, H.Q. Chinese sturgeon needs urgent rescue. Science 2020, 370, 1175. [Google Scholar] [CrossRef]
- Wei, Q.W.; Li, L.X.; Du, H.; Zhang, X.Y.; Xiong, W.; Zhang, H.; Shen, L.; Wu, J.M.; Zhang, S.H.; Wang, C.Y.; et al. Research on technology for controlled propagation of cultured Chinese sturgeon (Acipenser sinensis). J. Fish. Sci. China 2013, 20, 1–11. (In Chinese) [Google Scholar]
- Sun, L.T.; Zhao, F.; Wang, S.K.; Wang, Y.; Yang, G.; Zhuang, P. Growth and feeding ecology of juvenile Chinese sturgeon, Acipenser sinensis, in the Yangtze Estuary. J. Appl. Ichthyol. 2019, 35, 47–53. [Google Scholar] [CrossRef]
- Xiao, H.; Cui, Y.B.; Hung, S.S.O.; Zhu, X.M.; Zou, Z.J.; Xie, S.Q. Growth of juvenile Chinese sturgeon Acipenser sinensis fed live and formulated diets. N. Am. J. Aquac. 1999, 61, 184–188. [Google Scholar] [CrossRef]
- Hung, S.S.O. Recent advances in sturgeon nutrition. Anim. Nutr. 2017, 3, 191–204. [Google Scholar] [CrossRef]
- Wu, J.P.; Yang, D.Q.; Du, H.; Yu, T.; Luo, J.; Xu, Q.Q.; Zhu, J.Q.; Wei, Q.W. The influence of dietary arachidonic acid on growth, fatty acid profile and sex steroid hormones of F2 generation Chinese sturgeon (Acipenser sinensis). Aquac. Rep. 2021, 21, e100818. [Google Scholar] [CrossRef]
- Kals, J.; Blonk, R.J.W.; Palstra, A.P.; Sobotta, T.K.; Mongile, F.; Schneider, O.; Planas, J.V.; Schrama, J.W.; Verreth, J.A.J. Feeding ragworm (Nereis virens Sars) to common sole (Solea solea L.) alleviates nutritional anaemia and stimulates growth. Aquac. Res. 2017, 48, 752–759. [Google Scholar] [CrossRef]
- Roh, H.; Park, J.; Kim, A.; Kim, N.; Lee, Y.; Kim, B.S.; Vijayan, J.; Lee, M.K.; Park, C.I.; Kim, D. Overfeeding-Induced Obesity Could Cause Potential Immuno-Physiological Disorders in Rainbow Trout (Oncorhynchus mykiss). Animals 2020, 10, e1499. [Google Scholar] [CrossRef]
- Wu, N.; Xu, X.; Wang, B.; Li, X.M.; Cheng, Y.Y.; Li, M.; Xia, X.Q.; Zhang, Y.A. Anti-foodborne enteritis effect of galantamine potentially via acetylcholine anti-inflammatory pathway in fish. Fish Shellfish. Immunol. 2020, 97, 204–215. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.H.; Xia, J.G.; Zhang, X.; He, X.G.; Li, L.; Tang, R.; Chi, W.; Li, D.P. Diet Affects Muscle Quality and Growth Traits of Grass Carp (Ctenopharyngodon idellus): A Comparison Between Grass and Artificial Feed. Front. Physiol. 2018, 9, 283. [Google Scholar] [CrossRef] [PubMed]
- Meyer, H.A.; Chipps, S.R.; Graeb, B.D.; Klumb, R.A. Growth, food consumption, and energy status of juvenile pallid sturgeon fed natural or artificial diets. J. Fish Wildl. Manag. 2016, 7, 388–396. [Google Scholar] [CrossRef]
- Lee, L.Y.; Normaiyudin, N.A.; Wong, S.C.; Shu-Chien, A.C.; Rahmah, S.; Jaya-Ram, A. First description of mantis shrimp, Miyakella nepa (Latreille, 1828), feeding preference behaviour in captive conditions. Aquac. Rep. 2022, 22, e100969. [Google Scholar] [CrossRef]
- Deng, X.; Cui, Y.; Hung, S.S.O. Initial trials with feeding of Chinese sturgeon (Acipenser sinensis) larvae on artificial diet. Acta Hydrobiol. Sin. 1998, 22, 189–191. [Google Scholar]
- Sontakke, R.; Chaturvedi, C.S.; Saharan, N.; Tiwari, V.K.; Haridas, H.; Rani, A.M.B. Growth response, digestive enzyme activity and stress enzyme status in early stages of an endangered fish, Notopterus chitala (Hamilton, 1822) fed with live feed and formulated diet. Aquaculture 2019, 510, 182–190. [Google Scholar] [CrossRef]
- Ramesh, M.; Thilagavathi, T.; Rathika, R.; Poopal, R.K. Antioxidant status, biochemical, and hematological responses in a cultivable fish Cirrhinus mrigala exposed to an aquaculture antibiotic Sulfamethazine. Aquaculture 2018, 491, 10–19. [Google Scholar] [CrossRef]
- Seibel, H.; Bassmann, B.; Rebl, A. Blood Will Tell: What Hematological Analyses Can Reveal About Fish Welfare. Front. Vet. Sci. 2021, 8, e616955. [Google Scholar] [CrossRef]
- Fazio, F. Fish hematology analysis as an important tool of aquaculture: A review. Aquaculture 2019, 500, 237–242. [Google Scholar] [CrossRef]
- Lulijwa, R.; Young, T.; Symonds, J.E.; Walker, S.P.; Delorme, N.J.; Alfaro, A.C. Uncoupling Thermotolerance and Growth Performance in Chinook Salmon: Blood Biochemistry and Immune Capacity. Metabolites 2021, 11, e547. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Li, J.Z.; Lu, J.T.; Li, Z.; Shi, S.C.; Liu, Z.J. Effects of live and artificial feeds on the growth, digestion, immunity and intestinal microflora of mandarin fish hybrid (Siniperca chuatsi× Siniperca scherzeri). Aquac. Res. 2017, 48, 4479–4485. [Google Scholar] [CrossRef]
- Zheng, Y.P.; Zhang, Y.; Xie, Z.; Shin, P.K.S.; Xu, J.N.; Fan, H.Y.; Zhuang, P.; Hu, M.H.; Wang, Y.J. Seasonal Changes of Growth, Immune Parameters and Liver Function in Wild Chinese Sturgeons Under Indoor Conditions: Implication for Artificial Rearing. Front. Physiol. 2022, 13, 894729. [Google Scholar] [CrossRef]
- Chen, M.H.; Sun, Y.Y.; Kong, C.M.; Tang, H.J.; Gan, L. Effect of dietary phosphorus levels on growth and body composition of crucian carp, Carassius auratus under indoor and outdoor experiments. Aquac. Nutr. 2017, 23, 702–709. [Google Scholar] [CrossRef]
- Harimana, Y.; Tang, X.; Xu, P.; Xu, G.C.; Karangwa, E.; Zhang, K.; Sun, Y.J.; Li, Y.R.; Ma, S.H.; Uriho, A.; et al. Effect of long-term moderate exercise on muscle cellularity and texture, antioxidant activities, tissue composition, freshness indicators and flavor characteristics in largemouth bass (Micropterus salmoides). Aquaculture 2019, 510, 100–108. [Google Scholar] [CrossRef]
- Chen, X.H. Biology and Resources of Acipenseriformes Fishes; China Ocean Press: Beijing, China, 2007. (In Chinese) [Google Scholar]
- Roo, J.; Hernandez-Cruz, C.M.; Mesa-Rodriguez, A.; Fernandez-Palacios, H.; Izquierdo, M.S. Effect of increasing n-3 HUFA content in enriched Artemia on growth, survival and skeleton anomalies occurrence of greater amberjack Seriola dumerili larvae. Aquaculture 2019, 500, 651–659. [Google Scholar] [CrossRef]
- Kokou, F.; Henry, M.; Nikoloudaki, C.; Kounna, C.; Vasilaki, A.; Fountoulaki, E. Optimum protein-to-lipid ratio requirement of the juvenile shi drum (Umbrina cirrosa) as estimated by nutritional and histological parameters. Aquac. Nutr. 2019, 25, 444–455. [Google Scholar] [CrossRef]
- Liu, H.; Yang, J.J.; Dong, X.H.; Tan, B.P.; Zhang, S.; Chi, S.Y.; Yang, Q.H.; Liu, H.Y.; Yang, Y.Z. Effects of different dietary carbohydrate-to-lipid ratios on growth, plasma biochemical indexes, digestive and immune enzymes activities of juvenile orange-spotted grouper Epinephelus coioides. Aquac. Res. 2020, 51, 4152–4164. [Google Scholar] [CrossRef]
- Azimi, A.; Shekarabi, S.P.H.; Paknejad, H.; Harsij, M.; Khorshidi, Z.; Zolfaghari, M.; Hatami, A.S.; Dawood, M.A.O.; Mazloumi, N.; Zakariaee, H. Various carbon/nitrogen ratios in a biofloc-based rearing system of common carp (Cyprinus carpio) fingerlings: Effect on growth performance, immune response, and serum biochemistry. Aquaculture 2022, 548, e737622. [Google Scholar] [CrossRef]
- Sokooti, R.; Chelemal Dezfoulnejad, M.; Javaheri Baboli, M. Effects of olive leaf extract (Olea europaea Leecino) on growth, haematological parameters, immune system and carcass composition in common carp (Cyprinus carpio). Aquac. Res. 2021, 52, 2415–2423. [Google Scholar] [CrossRef]
- Reshma, K.J.; Sumithra, T.G.; Vishnu, B.; Jyothi, R.; Kumar, R.R.; Pootholathil, S.; Sanil, N.K. Indexing serum biochemical attributes of Lutjanus argentimaculatus (Forsskal, 1775) to instrument in health assessment. Aquac. Res. 2020, 51, 2590–2602. [Google Scholar] [CrossRef]
- Kim, S.Y.; Park, C.; Kim, M.Y.; Ji, S.Y.; Hwangbo, H.; Lee, H.; Hong, S.H.; Han, M.H.; Jeong, J.W.; Kim, G.Y.; et al. ROS-Mediated Anti-Tumor Effect of Coptidis Rhizoma against Human Hepatocellular Carcinoma Hep3B Cells and Xenografts. Int. J. Mol. Sci. 2021, 22, e4797. [Google Scholar] [CrossRef]
- Meton, I.; Mediavilla, D.; Caseras, A.; Canto, E.; Fernandez, F.; Baanante, I.V. Effect of diet composition and ration size on key enzyme activities of glycolysis-gluconeogenesis, the pentose phosphate pathway and amino acid metabolism in liver of gilthead sea bream (Sparus aurata). Br. J. Nutr. 1999, 82, 223–232. [Google Scholar] [CrossRef]
- Silva, A.L.N.; Rodrigues, R.A.; Siqueira, M.S.; Farias, K.N.N.; Kuibida, K.V.; Franco-Belussi, L.; Fernandes, C.E. Transaminase profile and hepatic histopathological traits in Piaractus mesopotamicus exposed to insecticide Diflubenzuron. Environ. Sci. Pollut. Res. 2021, 28, 22002–22010. [Google Scholar] [CrossRef]
- Ozer, J.; Ratner, M.; Shaw, M.; Bailey, W.; Schomaker, S. The current state of serum biomarkers of hepatotoxicity. Toxicology 2008, 245, 194–205. [Google Scholar] [CrossRef] [PubMed]
- Gao, X.Q.; Fei, F.; Huo, H.H.; Huang, B.; Meng, X.S.; Zhang, T.; Liu, B.L. Impact of nitrite exposure on plasma biochemical parameters and immune-related responses in Takifugu rubripes. Aquat. Toxicol. 2020, 218, e105362. [Google Scholar] [CrossRef]
- Roychowdhury, P.; Aftabuddin, M.; Pati, M.K. Thermal stress altered growth performance and metabolism and induced anaemia and liver disorder in Labeo rohita. Aquac. Res. 2020, 51, 1406–1414. [Google Scholar] [CrossRef]
- Ferri, J.; Matic-Skoko, S.; Coz-Rakovac, R.; Strunjak-Perovic, I.; Ljubic, B.B.; Popovic, N.T. Assessment of Fish Health: Seasonal Variations in Blood Parameters of the Widely Spread Mediterranean Scorpaenid Species, Scorpaena porcus. Appl. Sci. 2022, 12, e4106. [Google Scholar] [CrossRef]
- Nyblom, H.; Bjornsson, E.; Simren, M.; Aldenborg, F.; Almer, S.; Olsson, R. The AST/ALT ratio as an indicator of cirrhosis in patients with PBC. Liver Int. 2006, 26, 840–845. [Google Scholar] [CrossRef]
- Bonifacio, A.F.; Cazenave, J.; Bacchetta, C.; Ballesteros, M.L.; Bistoni, M.D.; Ame, M.V.; Bertrand, L.; Hued, A.C. Alterations in the general condition, biochemical parameters and locomotor activity in Cnesterodon decemmaculatus exposed to commercial formulations of chlorpyrifos, glyphosate and their mixtures. Ecol. Indic. 2016, 67, 88–97. [Google Scholar] [CrossRef]
- Zhu, T.; Corraze, G.; Plagnes-Juan, E.; Quillet, E.; Dupont-Nivet, M.; Skiba-Cassy, S. Regulation of genes related to cholesterol metabolism in rainbow trout (Oncorhynchus mykiss) fed a plant-based diet. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2018, 314, R58–R70. [Google Scholar] [CrossRef]
- Chen, Y.F.; Miura, Y.; Sakurai, T.; Chen, Z.; Shrestha, R.; Kato, S.; Okada, E.; Ukawa, S.; Nakagawa, T.; Nakamura, K.; et al. Comparison of dimension reduction methods on fatty acids food source study. Sci. Rep. 2021, 11, e18748. [Google Scholar] [CrossRef]
- Alves-Bezerra, M.; Cohen, D.E. Triglyceride Metabolism in the Liver. Compr. Physiol. 2018, 8, 1–22. [Google Scholar] [CrossRef]
- Yun, B.A.; Mai, K.S.; Zhang, W.B.; Xu, W. Effects of dietary cholesterol on growth performance, feed intake and cholesterol metabolism in juvenile turbot (Scophthalmus maximus L.) fed high plant protein diets. Aquaculture 2011, 319, 105–110. [Google Scholar] [CrossRef]
- Lewis, G.F.; Rader, D.J. New insights into the regulation of HDL metabolism and reverse cholesterol transport. Circ. Res. 2005, 96, 1221–1232. [Google Scholar] [CrossRef]
- Kudinov, V.A.; Alekseeva, O.Y.; Torkhovskaya, T.I.; Baskaev, K.K.; Artyushev, R.I.; Saburina, I.N.; Markin, S.S. High-Density Lipoproteins as Homeostatic Nanoparticles of Blood Plasma. Int. J. Mol. Sci. 2020, 21, e8737. [Google Scholar] [CrossRef]
- Wallaert, C.; Babin, P.J. Age-related, sex-related, and seasonal changes of plasma lipoprotein concentrations in trout. J. Lipid Res. 1994, 35, 1619–1633. [Google Scholar] [CrossRef]
- AtallahBenson, L.; Merly, L.; Cray, C.; Hammerschlag, N. Serum Protein Analysis of Nurse Sharks. J. Aquat. Anim. Health. 2020, 32, 77–82. [Google Scholar] [CrossRef]
- Kari, Z.A.; Kabir, M.A.; Mat, K.; Rusli, N.D.; Razab, M.; Ariff, N.; Edinur, H.A.; Rahim, M.Z.A.; Pati, S.; Dawood, M.A.O.; et al. The possibility of replacing fish meal with fermented soy pulp on the growth performance, blood biochemistry, liver, and intestinal morphology of African catfish (Clarias gariepinus). Aquac. Rep. 2021, 21, e100815. [Google Scholar] [CrossRef]
- Rehulka, J.; Minarik, B. Blood parameters in brook trout Salvelinus fontinalis (Mitchill, 1815), affected by columnaris disease. Aquac. Res. 2007, 38, 1182–1197. [Google Scholar] [CrossRef]
- Adeshina, I.; Abdel-Tawwab, M. Dietary taurine incorporation to high plant protein-based diets improved growth, biochemical, immunity, and antioxidants biomarkers of African catfish, Clarias gariepinus (B.). Fish Physiol. Biochem. 2020, 46, 1323–1335. [Google Scholar] [CrossRef]
- Yan, X.B.; Yang, J.J.; Dong, X.H.; Tan, B.P.; Zhang, S.; Chi, S.Y.; Yang, Q.H.; Liu, H.Y.; Yang, Y.Z. The optimal dietary protein level of large-size grouper Epinephelus coioides. Aquac. Nutr. 2020, 26, 705–714. [Google Scholar] [CrossRef]
- Ihut, A.; Raducu, C.; Cocan, D.; Munteanu, C.; Luca, I.T.; Uiuiu, P.; Latiu, C.; Rus, V.; Miresan, V. Seasonal variation of blood biomarkers in huchen, Hucho hucho (Actinopterygii: Salmoniformes: Salmonidae) reared in captivity. Acta Ichthyol. Piscat. 2020, 50, 381–390. [Google Scholar] [CrossRef]
- Asadi, F.; Hallajian, A.; Asadian, P.; Shahriari, A.; Pourkabir, M. Serum lipid, free fatty acid, and proteins in juvenile sturgeons: Acipenser persicus and Acipenser stellatus. Comp. Clin. Path. 2009, 18, 287–289. [Google Scholar] [CrossRef]
- Lopez, L.M.; Flores-Ibarra, M.; Banuelos-Vargas, I.; Galaviz, M.A.; True, C.D. Effect of fishmeal replacement by soy protein concentrate with taurine supplementation on growth performance, hematological and biochemical status, and liver histology of totoaba juveniles (Totoaba macdonaldi). Fish Physiol. Biochem. 2015, 41, 921–936. [Google Scholar] [CrossRef]
- Shekarabi, S.P.H.; Javarsiani, L.; Mehrgan, M.S.; Dawood, M.A.O.; Adel, M. Growth performance, blood biochemistry profile, and immune response of rainbow trout (Oncorhynchus mykiss) fed dietary Persian shallot (Allium stipitatum) powder. Aquaculture 2022, 548, e737627. [Google Scholar] [CrossRef]
- Roy, S.; Kumar, V.; Kumar, V.; Behera, B.K. Acute Phase Proteins and their Potential Role as an Indicator for Fish Health and in Diagnosis of Fish Diseases. Protein Pept. Lett. 2017, 24, 78–89. [Google Scholar] [CrossRef]
- Lee, P.T.; Bird, S.; Zou, J.; Martin, S.A.M. Phylogeny and expression analysis of C-reactive protein (CRP) and serum amyloid-P (SAP) like genes reveal two distinct groups in fish. Fish Shellfish. Immunol. 2017, 65, 42–51. [Google Scholar] [CrossRef]
- Aversa-Marnai, M.; Castellano, M.; Quartiani, I.; Conijesky, D.; Perretta, A.; Villarino, A.; Silva-Alvarez, V.; Ferreira, A.M. Different response of Acipenser gueldenstaedtii CRP/SAP and SAA to bacterial challenge and chronic thermal stress sheds light on the innate immune system of sturgeons. Fish Shellfish. Immunol. 2022, 121, 404–417. [Google Scholar] [CrossRef]
- Hiroshi, K.; Yuji, M.; Yoshiaki, T.; Youchang, L.; Tadashi, I.; Shinobu, W. Changes of C-reactive protein levels in rainbow trout (Oncorhynchus mykiss) sera after exposure to anti-ectoparasitic chemicals used in aquaculture. Fish Shellfish. Immunol. 2004, 16, 589–597. [Google Scholar] [CrossRef]
| Ingredient | Diet Treatment | ||
|---|---|---|---|
| Crucian Carp Diet | Largemouth Bass Diet | Formulated Diet | |
| Crude protein | 15.03 ± 0.16 | 18.19 ± 1.51 | ≥40 |
| Crude lipid | 10.32 ± 0.26 | 6.32 ± 0.83 | ≥12 |
| Ash | 3.57 ± 0.03 | 1.01 ± 0.02 | ≤18 |
| Moisture | 67.00 ± 0.37 | 84.59 ± 0.21 | ≤12 |
| Groups | Natural Food | |||
|---|---|---|---|---|
| February | May | August | November | |
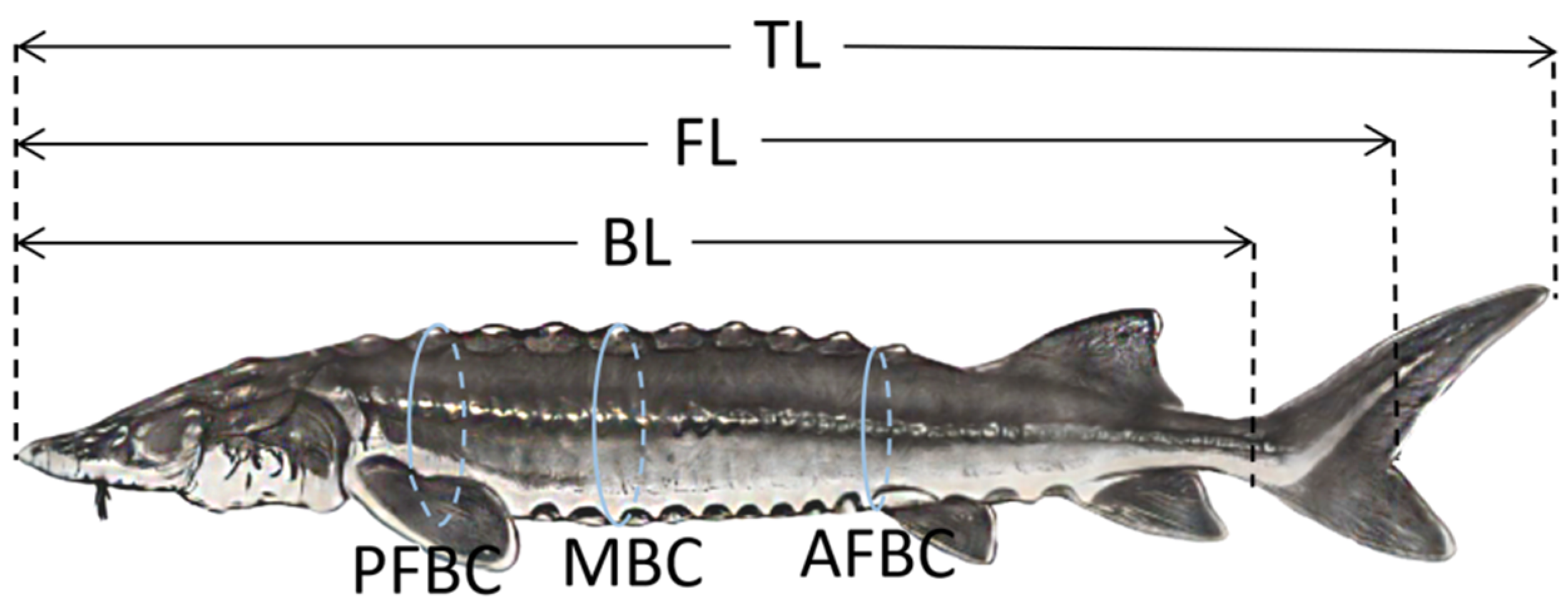
| TL (cm) | 248.8 ± 18.4 A | 244.1 ± 19.0 A | 245.3 ± 17.5 A | 240.3 ± 18.9 A |
| FL (cm) | 224.3 ± 17.1 A | 219.3 ± 17.3 A | 222.8 ± 16.0 A | 219.8 ± 16.0 A |
| BL (cm) | 201.6 ± 15.2 A | 199.3 ± 15.3 A | 200.3 ± 15.0 A | 199.3 ± 14.2 A |
| PFBC (cm) | 107.8 ± 9.2 A | 98.6 ± 6.9 B | 99.4 ± 8.3 B | 98.8 ± 9.7 B |
| MBC (cm) | 116.1 ± 10.7 A | 109.8 ± 9.8 A | 107.4 ± 10.6 A | 106.4 ± 10.8 A |
| AFBC (cm) | 98 ± 11.2 B | 91.8 ± 9.5 AB | 89.5 ± 9.1 A | 86.3 ± 9.7 A |
| Body weight (kg) | 115.2 ± 28.9 A | 112.4 ± 28.4 A | 110.4 ± 26.7 A | 110.4 ± 27.4 A |
| Groups | Formulated Diet | |||
|---|---|---|---|---|
| February | May | August | November | |
| TL (cm) | 214 ± 16.0 A | 212.4 ± 15.9 B | 211.7 ± 15.3 A | 210.8 ± 16.4 A |
| FL (cm) | 194 ± 14.8 A | 185.9 ± 14.2 B | 191.7 ± 14.2 AB | 193.2 ± 14.3 AB |
| BL (cm) | 174.2 ± 13.6 A | 168.6 ± 12.9 A | 171.6 ± 12.8 A | 171.9 ± 18.5 A |
| PFBC (cm) | 88.6 ± 6.1 C | 82.0 ± 6.6 A | 83.8 ± 6.4 AB | 85.8 ± 7.4 BC |
| MBC (cm) | 92.7 ± 7.2 B | 86.4 ± 6.9 A | 88.8 ± 7.0 A | 90.0 ± 7.9 AB |
| AFBC (cm) | 75.7 ± 5.3 A | 70.4 ± 6.0 B | 72.4 ± 5.7 BC | 73.8 ± 6.8 AC |
| Body weight (kg) | 60.1 ± 12.1 A | 61.2 ± 13.0 A | 63.5 ± 13.4 A | 65.9 ± 17.8 A |
| Diet | Natural Food | Formulated Diet |
|---|---|---|
| Initial weight (kg) | 115.2 ± 28.9 | 60.1 ± 12.1 |
| Final weight (kg) | 110.4 ± 27.4 | 65.9 ± 17.8 |
| Initial TL (cm) | 248.8 ± 18.4 | 214 ± 16.0 |
| Final TL (cm) | 240.3 ± 18.9 | 210.8 ± 16.39 |
| SGR(%) | −1.8 | 4.2 |
| SGRL(%) | −1.47 | −0.63 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zheng, Y.; Liu, J.; Xu, J.; Fan, H.; Wang, Y.; Zhuang, P.; Hu, M. Comparison of Artificial Feed and Natural Food by the Growth and Blood Biochemistry in Chinese Sturgeon Acipenser sinensis. Fishes 2023, 8, 45. https://doi.org/10.3390/fishes8010045
Zheng Y, Liu J, Xu J, Fan H, Wang Y, Zhuang P, Hu M. Comparison of Artificial Feed and Natural Food by the Growth and Blood Biochemistry in Chinese Sturgeon Acipenser sinensis. Fishes. 2023; 8(1):45. https://doi.org/10.3390/fishes8010045
Chicago/Turabian StyleZheng, Yueping, Jiehao Liu, Jianan Xu, Houyong Fan, Youji Wang, Ping Zhuang, and Menghong Hu. 2023. "Comparison of Artificial Feed and Natural Food by the Growth and Blood Biochemistry in Chinese Sturgeon Acipenser sinensis" Fishes 8, no. 1: 45. https://doi.org/10.3390/fishes8010045
APA StyleZheng, Y., Liu, J., Xu, J., Fan, H., Wang, Y., Zhuang, P., & Hu, M. (2023). Comparison of Artificial Feed and Natural Food by the Growth and Blood Biochemistry in Chinese Sturgeon Acipenser sinensis. Fishes, 8(1), 45. https://doi.org/10.3390/fishes8010045











