Abstract
The pelagic stingray (Pteroplatytrygon violacea), perhaps the only stingray to inhabit open ocean waters, is highly interactive with longline and purse seine fisheries. The threat to P. violacea posed by high bycatch mortality has received widespread attention. To date, the environmental preference of P. violacea, which is important in designing conservation and management measures, has not been well studied. Based on data collected during a 2016–2019 survey in the Pacific Ocean by national observers of tuna longline fisheries, the relationship between the presence of P. violacea and spatiotemporal and environmental variables was first analyzed using the Generalized Additive Model. The results showed that geographic location (latitude and longitude) was the most influential variable. Monthly, P. violacea is frequently present in the Pacific high sea from December to May. The El Niño–Southern Oscillation had a significant impact on the presence of P. violacea in the Pacific high sea, with both the cold (Ocean Nino Index <−0.5) and warm (Ocean Nino Index >1) phases leading to a decrease in its presence. Regarding the environmental factors, we found that high presence was associated with low salinity (33.0~34.5 psu), a relatively high concentration of chlorophyll (0.2–0.35 mg/m3), and warm water (>20 °C). P. violacea was most likely observed in the waters offshore, closer to seamounts, and with water depths between 4000 and 5000 m. Four areas, including those east of the Solomon Islands and east of Kiribati, areas west of the Galapagos Islands, and areas near the coastal upwelling of northern Peru, related to upwelling systems or seamounts, were identified as the potential key habitats of P. violacea. Predicted distribution maps showed a significant seasonal variation in the presence of P. violacea. Moreover, the yearly change in the presence of P. violacea in the Pacific high sea indicated a possible decreasing trend in recent years. The information first provided here is essential for developing conservation and management measures for P. violacea to prevent the unavoidable ecological consequences of bycatch or other anthropogenic factors.
1. Introduction
Incidental catches in tuna longline fisheries have become the main threat to the diversity of the pelagic ocean ecosystem [,]. The Food and Agriculture Organization (FAO) developed technical guidelines for the conservation and management of bycatch, including sharks [], seabirds [], and marine turtles []. Great progress has been made in developing effective and commercially viable bycatch mitigation measures, which include finning controls, catch controls, operational and gear controls, as well as the modification of fishing gear and fishing behaviors. However, recent studies show that the effectiveness of these approaches remains to be evaluated []. Given that, area-based management tools (ABMTs), such as time–area closures, selective area-based fishery/gear closures, marine protected areas (MPAs), and adaptive/real-time management in blue-water ecosystems, have become a leading topic in fishery management []. ABMTs, such as time–area closures, have been used for a long time in tuna regional fishery management organizations to reduce the interactions of fisheries with dolphins, juvenile tunas, and swordfish []. However, ABMTs have seldom been applied to reduce the bycatch of other bycatch species, such as sharks, rays, and marine turtles, especially in the high seas, as the knowledge of habitat preference for these species is limited [].
Stingrays are a group of sea rays, which are elasmobranchs related to sharks. They are common in coastal tropical and subtropical marine waters throughout the world. Most stingrays are found in warm, temperate oceans and fresh waters, but some are pelagic. P. violacea, perhaps the only stingray to inhabit open ocean waters, is widespread throughout the tropical and subtropical areas of the Pacific, Atlantic, and Indian Oceans []. It is listed as “Least Concern” on the IUCN list []. This species is a regular bycatch in both purse seine and longline gears and is also caught by coastal fisheries for local subsistence. In the Pacific Ocean [], P. violacea is one of the most frequently caught elasmobranchs in tuna longline fisheries [,]. Although a previous study found that this species increased in central Pacific longline fisheries between the 1950s and 1990s [], a report based on Hawaii-based longline fishery catch and effort data showed a 5.4% annual decline in the catch rate of P. violacea []. More recently, ecological risk assessments for both tuna fisheries in the western and central Pacific Oceans and the eastern Pacific Ocean showed that P. violacea has been at medium risk due to its large removal from pelagic waters [,].
The key to minimizing the interactions of fisheries with bycatch species and identifying areas of high bycatch rates is to understand the relationship between species distributions and spatial–temporal and environmental factors []. Observer programs, designed to monitor catch and bycatch and collect fishery-relevant information [], are an important source of ecological data that underpin the application of ecological-based fishery management []. Data from these programs have been widely used in building species distribution models (SDMs) to explore the relationship between species’ presence or abundance and environmental characteristics and predict the potential habitat of species with high ecological risks [,,,,]. Several abiotic factors, such as temperature, salinity, and dissolved oxygen, and biotic factors, such as plankton aggregations, have been demonstrated to influence the movement of sharks and rays either indirectly or directly []. Climate changes are also important factors affecting the migration and distribution of sharks and rays []. In the case of P. violacea, a study has shown that the catch rate of P. violacea in the Spanish longline fleet in the Mediterranean during 2000–2013 was correlated with the climatic variability driven by the North Atlantic Oscillation. The study revealed that the high catch rate along the coast of the Iberian Peninsula might be induced by the negative North Atlantic Oscillation phase, which increases the contribution of land-based nutrients to the sea [].
A few pieces of research have been performed to better understand its biological and fishery characteristics [,,]. However, studies on the environmental characteristics associated with the presence of P. violacea are few in number, especially in the Pacific high sea. Thus, this paper aims to use observer data from Chinese tuna longline observer programs to (1) explore the environmental characteristics associated with the presence of P. violacea in the high seas of the Pacific Ocean and (2) find its potential key habitat and dynamics for future conservation issues.
2. Materials and Methods
2.1. Study Areas
The Pacific Ocean is the world’s largest tuna fishing ground, providing more than 55% of the global tuna catch []. The study area covers most of the tuna longline fishing grounds (30° S–25° N and 150° E–100° W), as shown in Figure 1, including both tropical and temperate waters.
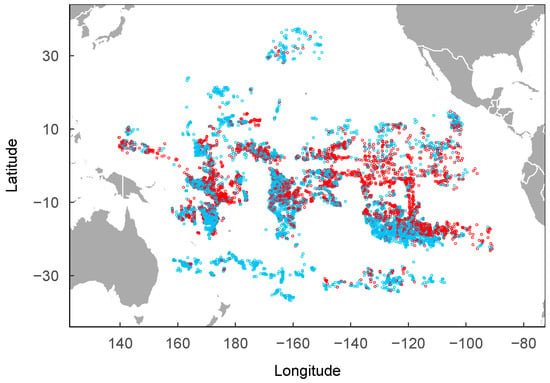
Figure 1.
Observed sets of tuna longline fishery from 2016 to 2019. The red dots represent the observed presence of P. violacea, and the blue dots represent the observed absence of P. violacea.
2.2. P. violacea Data
According to the Conservation and Management Measures for the Regional Observer Programs in the Western and Central Pacific Fisheries Commission (WCPFC), a total of 10,291 fishing data sets were collected by observers onboard Chinese tuna longline vessels in the Pacific Ocean from 2016 to 2019 []. The scientific observers were first rigorously trained to collect fishery data on tunas and other pelagic fish stocks. Next, the observers were randomly allocated to longline vessels on the fishing grounds. During their trips, the observers were required to record basic information about the fishing operations (which included the fishing/hauling time, positions, hooks between floats, length of float and branch lines, setting speed of the mainline and vessels, and target species) and catch information (which included all species captured by the sets) at the species level. More details are available in the China annual report to the WCPFC [].
2.3. Environmental Data
The details of the environmental variables are shown in Table 1. The values of the dynamic oceanographic variables for each fishing set were derived from the Copernicus Marine Service (CMEMS) (https://marine.copernicus.eu/) (accesed on 8 April 2022). The Ocean Nino Index (ONI), derived from the Climate Prediction Center, National Oceanic and Atmospheric Administration (https://origin.cpc.ncep.noaa.gov) (accesed on on 8 April 2022), was used to explore the impact of climate change (the El Niño–Southern Oscillation, ENSO) on the presence of P. violacea. The values of bathymetry and distance to the nearest land were obtained from Global Marine Environment Datasets (https://gmed.auckland.ac.nz/index.html) (accesed on on 8 April 2022). The distance of each longline set to the closest seamount was estimated using the simple spherical law of cosines and a Pacific seamount dataset [,]. Only seamounts with summits shallower than 400 m, which had a significant aggregation effect on pelagic species, such as tuna, sharks, billfishes, marine mammals, and sea turtles [], were used in the distance calculation.

Table 1.
Summary of the environmental variables.
2.4. Statistical Analysis
The Generalized Additive Model (GAM) [] was used to quantify the statistical relationship between the presence/absence and the environmental, spatial (latitude and longitude), and temporal (year and month) variables. The general structure of the GAM is as follows:
where g is the link function, Y is the expected response variable, α is the intercept, are smooth functions, are the covariates, and is the residual error.
The degree of freedom of the smooth functions was restricted for each explanatory variable to avoid additional overfitting. The number of the basis function (k) was set to k = 20 for the interaction effects, k = 4 for the year effects, and k = 6 for the rest effects [,].
To avoid correlations, one variable from the variable pairs with a high correlation (r > 0.7/r < −0.7) was removed based on the Pearson’s rank correlation []. To deal with multicollinearity problems, a variance inflation factor analysis (VIF) was conducted with a cut-off value of 5 []. Based on these tests, the variables SSH, O2, Phy, and Ni were removed owing to correlation/collinearity with more ecologically important variables [,]. All other covariates available for the model selection had low cross-correlation and cross-collinearity scores (Figure S1 and Table S1).
The best-fit model was selected using the forward stepwise variable selection procedure (Table S2). The model with the lowest Akaike’s Information Criterion (AIC) was chosen as the final model []. The percentage of the deviance explained was used to measure the model’s goodness-of-fit.
A 5-fold cross-validation was applied to assess the model’s performance [,]. This approach divides the data into two groups, where 80% of the data are used to establish relationships between the presence–absence data and the variables and 20% of the data are used to evaluate the quality of the model’s predictions. The area under the receiver operating curve (AUC) was used to evaluate the model’s performance [,]. It measures the ability of a model to correctly predict the presence or absence of a species. The AUC value ranges from 0 to 1. The closer the AUC value is to 1, the better the model’s prediction [].
The spatial (1° × 1°) and seasonal distributions of P. violacea were predicted using the best-fit models. The seasons were defined as follows: winter, Jan–Mar; spring, Apr–Jun; summer, Jul–Sep; autumn, Oct–Dec.
The functions and packages used in the analyses are shown in Table 2. All analyses were carried out in the R-4.1.1 software [].

Table 2.
Summary of the functions and packages used in the analyses.
3. Results
Out of the 10,291 sets, 4349 were found to have the presence of P. violacea during the study period between 2016 and 2019. Most of the fishing sets (75.6%) and the sets with the presence of P. violacea (76.1%) were observed from August to January. The highest number of fishing sets was observed during October (1508), followed by September (1451) and November (1356) (Figure 2a). The number of sets with the presence of P. violacea was the highest in September (662), followed by October (658) and December (615) (Figure 2b).
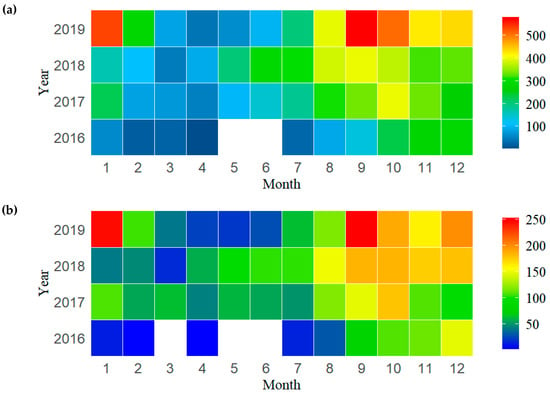
Figure 2.
(a) Monthly distribution of the observed number of fishing sets in Chinese tuna longline fisheries. (b) Monthly distribution of the observed number of fishing sets with the presence of P. violacea.
Based on the lowest AIC, the variables included in the best GAM model were as follows: the interaction of longitude and latitude, month, SAL, ONI, Chl, SST, year, MLT, Land_dis, Seamounts_dis, and depth (Table 3).

Table 3.
Summary analysis of the deviance of the generalized additive model.
The model achieved a 15.65% deviance explained with an adjusted R2 of 0.187. The individual contributions of the variables are shown in Table 3. The most important covariates to explain the presence of P. violacea are the interaction latitude–longitude (10.84%), followed by month (1.06%), ONI (0.91%), and SAL (0.89%) (Table 3).
The relationships between the covariates and the response variables are shown in Figure 3. The model suggests that the presence probability of P. violacea was higher in the eastern tropical Pacific (0°–20° S, 100°–140° W) from December to May. The significant annual variation indicates that the presence of P. violacea has decreased in recent years in the Pacific high sea. The presence of P. violacea during the climate periods with −0.5 < ONI < 1 was higher than that during the cold (ONI < −0.5) and warm (ONI > 1) phases. Regarding the environmental variables, P. violacea was more likely to be present in waters with low SAL (<34.5 psu), relatively high concentrations of chlorophyll (0.20–0.35 mg/m3), warm waters (>20 °C), and deep MLT (>80 m). P. violacea was more concentrated in open waters far offshore, closer to seamounts, and at seafloor depths of 4000–5000 m.
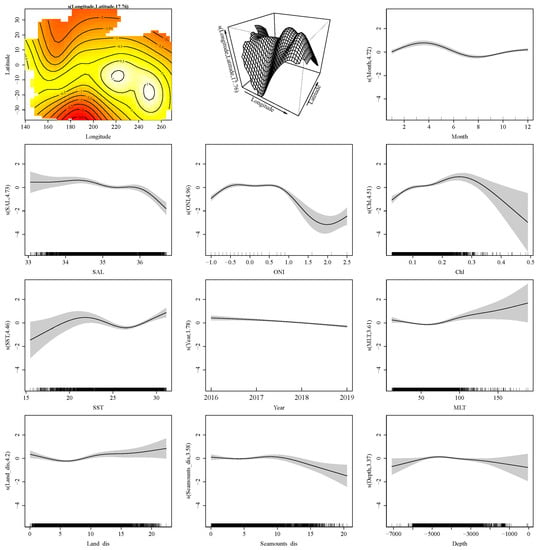
Figure 3.
Effects of the spatial–temporal and environmental variables on the presence of P. violacea in the Pacific Ocean.
The average AUC (0.76, 0.74, 0.74, 0.75, and 0.75) for the model was 0.75, which revealed that the ability of the model to predict the presence of P. violacea was moderate.
Based on the predicted spatial distribution of the presence probability of P. violacea from the best model, the following four areas with a concentrated distribution of grids (1° × 1°) with a high probability of presence (≥0.7) were identified as potential habitats: A1 (5°–16° S, 164°–180° E), A2 (1° N–8° S, 143°–158° W), A3 (3° N–9° S, 120°–140° W), and A4 (14°–24° S, 91°–110° W), as shown in Figure 4. Most of the grids with a probability of ≥0.7 were concentrated in the A3 area. The predicted seasonal spatial distribution suggests that P. violacea’s presence might change seasonally. For example, the high presence probability occurred in each season in A3, but only in the summer and autumn in A4 and in the spring in A2. The area with a high probability of P. violacea’s presence was more extensive in the spring and winter than in the summer and autumn in A1 (Figure 5).
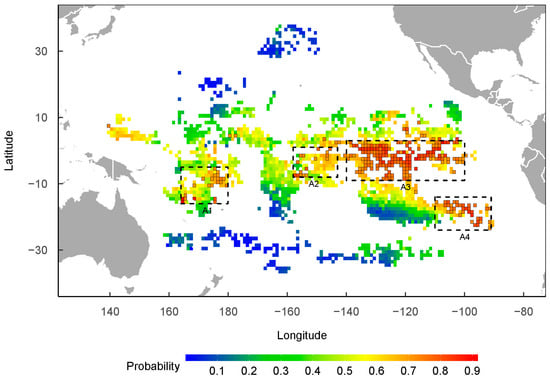
Figure 4.
The spatial distribution of P. violacea’s presence probability, predicted from the GAM model. The grids (1° × 1°) with a presence probability of ≥0.70 are bordered in black. The areas within the dashed border are considered the potential key habitat of P. violacea, as most of the high-probability grids are distributed in these areas.
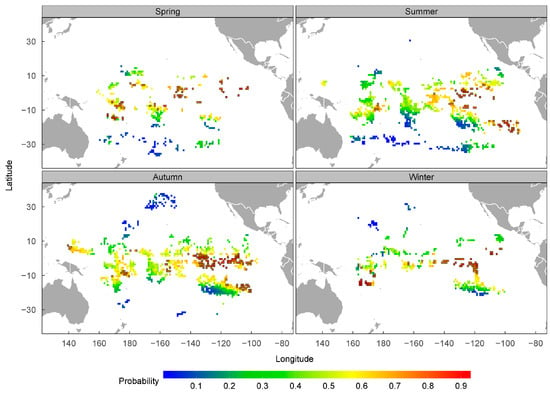
Figure 5.
The seasonal spatial distribution of P. violacea’s presence probability, predicted from the GAM model. The grids (1° × 1°) with a presence probability of ≥0.70 are bordered in black.
4. Discussion
Researchers have paid the most attention to the biological characteristics of P. violacea, but research on the species’ ecological habits and environmental preferences is lacking. This study examined the presence probability of P. violacea in the Pacific high sea using bycatch data from Chinese tuna longline fisheries. The key environmental variables that may affect P. violacea’s presence in this region were identified by using GAMs. The results revealed that the presence of P. violacea in the Pacific high sea was strongly associated with environmental variables (SAL, Chl, and SST) and was significantly impacted by the ENSO. The habitat hotspots for P. violacea generally occurred in waters associated with upwelling systems and showed significant annual and seasonal variations. This study will be fundamental to developing effective spatial fishery management regulations.
4.1. Environmental Preference of P. violacea
By understanding the environmental preference of the species, we could know if there is a higher probability of finding the species in other studied areas. Salinity and temperature are well-known to have a strong influence on the physiology of fish and are key drivers of fish migration [,]. Whereas many animals can tolerate a wide range of salinities, most sharks and rays strictly occupy a narrow range of salinities []. The normal range of ocean salinity ranges between 33 and 37 psu. Near the equator, the tropics receive the most consistent rain. As a result, the freshwater falling into the ocean helps to decrease the salinity of the surface water in that region []. Although P. violacea is nearly universal in subtropical and tropical seas and also occurs at temperate latitudes [], our findings show that it is more likely present in waters with relatively low salinities, such as those near the equator, and it is rarely observed in temperate high seas, especially in areas where the salinity is higher than 35 psu. Regarding the SST, the previous study showed that P. violacea prefers water temperatures above 19 °C and will die if the temperature drops to 15 °C []. Our study further demonstrated that P. violacea prefers warm water habitats with temperatures ranging between 20 and 25 °C, which is also a suitable temperature for many pelagic elasmobranchs, such as the scalloped hammerhead (Sphyrna lewini), the smooth hammerhead (Sphyrna zygaena), and the silky shark (Carcharhinus falciformis) [].
In the open ocean, areas with high chlorophyll concentrations are generally associated with upwelling systems, indicating an abundance of primary productivity. Unlike the spinetail devil ray (Mobula mobular), which prefers habitats in coastal waters with relatively high concentrations of chlorophyll (0.5–1 mg/m3) [], we found that P. violacea was more likely to be found in areas with chlorophyll concentrations between 0.20 and 0.35 mg/m3. Such environmental conditions, in combination with other oceanographic characteristics, have also attracted multiple pelagic species to form a species diversity hotspot in the eastern and central tropical Pacific [,].
4.2. Impact of ENSO on the Presence of P. violacea in the Pacific High Sea
For marine fisheries, the volume and dominant species of fish catches can change dramatically depending on the type of ENSO []. ENSOs are often simplified to reflect the following two main phases: El Niño, an anomalous warming phase, characterized by cooler ocean temperatures in the central and eastern tropical Pacific Ocean due to increased upwelling, and an opposite cooling phase called La Niña, characterized by an anomalous increase in SST in the eastern Pacific Ocean as upwelling of cold water occurs less or not at all in offshore northwestern South America. Our study first showed that the presence probability of P. violacea in the Pacific high sea was lower both during the cold (ONI < −0.5) and warm (ONI > 1) phases than in the neutral phase. Sharks and rays are known to exploit the thermal heterogeneity in their environment by selecting different temperatures throughout the day, a behavior known as thermotaxis [,]. For example, studies have shown that sharks and rays prefer to forage in warm waters, which maximizes their flexibility, and rest in cold waters to reduce their daily energy costs [,]. Thus, elasmobranchs may not change their latitudinal range as the climate warms but rather select deeper, colder water areas to enhance their physiological processes []. Based on this, one possible explanation for the relationship between P. violacea’s presence in the Pacific high sea and ENSO is that the El Nino events might drive P. violacea into deeper waters or away from the equator, and P. violacea might migrate towards warmer waters in the central and/or western Pacific under La Nina events. However, given the limited amount of data available, these effects are difficult to characterize with certainty.
4.3. Spatial–Temporal Distribution of P. violacea in the Pacific High Sea
The typical habitats or biodiversity hotspots of pelagic fishes are mostly located in areas characterized by upwelling systems, seamounts [,], or ocean fronts []. Our results showed that the hotspots of P. violacea’s presence in the Pacific high sea were mainly in four regions. The area east of the Solomon Islands, near the zone of dense seamount distribution [], is characterized by increased turbulence, mixing, and mesoscale eddies that can enhance local production by transporting nutrients to the poleward zone, where the high primary productivity and abundant bait attract predators to congregate in the area [,,]. The area east of Kiribati and west of the Galapagos Islands, which is rich in nutrients due to equatorial upwelling, is an important habitat for elasmobranchs [,]. The waters off the Peruvian coast, which are also influenced by upwelling systems, have a large accumulation of nutrients that affect the abundance and distribution of marine organisms []. These areas are either rich in fishery resources, such as the coastal waters of Peru, or have high biodiversity as critical habitats for many pelagic species, such as the area east of Kiribati and the west of the Galapagos Islands, and would be the priority areas for attention and management.
P. violacea spends the winter in oceanic waters near the equator and moves into higher latitudes and toward the coast in the spring []. The seasonal changes in P. violacea’s presence in the Pacific high sea might reflect its migrations between the coast and the open ocean. Furthermore, the significance of the year in the model indicated that there had been a decreasing trend concerning the presence of P. violacea in the Pacific high sea over the years. In the long run, the observed catch rate for P. violacea in longline and purse seine sets within the western and central Pacific Oceans tends to exhibit large annual fluctuations. The catch rate declined in both gears and all set types from the mid-1990s to the mid-2000s and then seemed to increase again after 2010 []. Researchers speculated that this increase may be related to the decline in mesopredator release as a result of declines in sharks and billfishes []. The short-term interannual variability in our study might also indicate a change in its abundance, or it may be related to the interannual fluctuation of climate change affecting its presence in the Pacific high sea. Whatever the underlying causes, the results suggested, to some extent, that anthropogenic factors have directly or indirectly influenced the biomass or distribution of P. violacea. Further consideration should be given to whether this impact is negative or neutral to prevent irreversible effects on the population.
4.4. Conservation Consideration
Stingray species are progressively becoming threatened or vulnerable to extinction, particularly as a consequence of unregulated fishing. The Food and Agriculture Organization of the United Nations published the International Plan of Action for Conservation and Management of Sharks, which includes non-shark species of chondrichthyan fishes, such as skates, rays, and chimaeras, in 1999 []. WCPFC was the first tuna regional fishery management organization to establish a formal shark research plan covering stock assessment, research coordination, and fishery statistic improvements []. Currently, more than ten species have been designated as key sharks, which have priorities for conservation and management []. P. violacea made up a high proportion of the non-key shark bycatch in longline fisheries []. To be listed as a WCPFC key shark species, the management of P. violacea could be improved by enhanced reporting by vessels. However, the scientific commission did not recommend P. violacea as a WCPFC key shark species because of their medium vulnerability to most fishing gear used in the WCPFC, as well as their medium productivity [,]. In this sense, there are no targeted bycatch mitigation measures or data collection and research plans for P. violacea. However, several bycatch mitigation measures, such as adjusting the gear depth, reducing the use of J-hooks, and encouraging the use of line cutters or de-hookers to release, have also been effective in reducing the bycatch rates and post-release mortality of P. violacea []. The continued removal of large numbers of these organisms from the pelagic realm will have unavoidable ecological consequences.
Data based on fisheries, especially those with long time series and large spatial coverage, provide us with information on the spatial and temporal distributions of bycatch species [,]. SDMs, based on fishery observer programs, are widely used to explore the relationship between environmental variables and bycatch species distribution, as well as to identify and predict habitat hotspots [,,]. The predictive maps of P. violacea allowed us to identify the seasonal–spatial dynamics of P. violacea’s distribution []. As data become more abundant for P. violacea and other vulnerable species, the dynamic changes in key habitats could be further predicted monthly, weekly, and even daily []. Such studies serve as a basis for dynamic adjustments of fishing locations to prevent vulnerable species from encountering fishing gear [,,,].
5. Conclusions
This study improves our understanding of P. violacea’s environmental preferences and spatial and temporal distributions in the Pacific high sea. Seawater salinity, the concentration of chlorophyll, and sea surface temperatures were the key environmental variables that shaped the distribution patterns of P. violacea in the study area. Both the warm and cold phases of ENSO could reduce the presence of P. violacea in the Pacific high sea. The key habitats of P. violacea were generally found in areas close to the seamounts in the western Pacific or the upwelling systems in the eastern Pacific. Moreover, the prediction output of the model revealed the seasonal variation and the interannual fluctuation of P. violacea’s presence in the Pacific high sea. This study provides important information for managers on how to shape their bycatch mitigation strategies to reduce mortality by understanding the ecological dynamics of bycatch species.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/fishes8010046/s1, Figure S1: Pearson’s correlation coefficient between the variables; Table S1: Collinearity between the variables by calculating the Variance Inflation Factor (VIF); Table S2: Model selected by stepwise analysis.
Author Contributions
Conceptualization, J.W. and C.G.; methodology, J.W.; formal analysis, J.W. and L.D.; data curation, F.W.; writing—original draft preparation, J.W.; writing—review and editing, Q.M. and S.T.; funding acquisition, S.T. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the National Key Research and Development Program of China (2019YFD0901404).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available on reasonable request from the corresponding author.
Acknowledgments
We would like to thank the teachers and students from the laboratory of Shanghai Ocean University for their work and help in sample collection and data analysis, as well as their valuable comments on the revision of the paper.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Hall, M.; Alverson, D.; Metuzals, K. By-Catch: Problems and Solutions. Mar. Pollut. Bull. 2000, 41, 204–219. [Google Scholar] [CrossRef]
- Wang, J.; Gao, X.; Xu, L.; Dai, L.; Chen, J.; Tian, S.; Chen, Y. Biodiversity in the Bycatch Community of Chinese Tuna Longline Fisheries in the Pacific Ocean. Glob. Ecol. Conserv. 2020, 24, e01276. [Google Scholar] [CrossRef]
- FAO. International Plan of Action for the Conservation and Management of Sharks; FAO: Rome, Italy, 1999. [Google Scholar]
- FAO. International Plan of Action for Reducing Incidental Catch of Seabirds in Longline Fisheries; FAO: Rome, Italy, 1999. [Google Scholar]
- FAO. Guidelines to Reduce Sea Turtle Mortality in Fishing Operations; FAO: Rome, Italy, 2009. [Google Scholar]
- Clarke, S.; Sato, M.; Small, C.; Sullivan, B.; Inoue, Y.; Ochi, D. Bycatch in Longline Fisheries for Tuna and Tuna-like Species: A Global Review of Status and Mitigation Measures. FAO Fish. Aquac. Tech. Pap. 2014, 588, 1–199. [Google Scholar]
- Hilborn, R.; Agostini, V.N.; Chaloupka, M.; Garcia, S.M.; Gerber, L.R.; Gilman, E.; Hanich, Q.; Himes-Cornell, A.; Hobday, A.J.; Itano, D.; et al. Area-Based Management of Blue Water Fisheries: Current Knowledge and Research Needs. Fish Fish. 2021, 1–27. [Google Scholar] [CrossRef]
- Schlaff, A.M.; Heupel, M.R.; Simpfendorfer, C.A. Influence of Environmental Factors on Shark and Ray Movement, Behaviour and Habitat Use: A Review. Rev. Fish Biol. Fish. 2014, 24, 1089–1103. [Google Scholar] [CrossRef]
- Neer, J.A. The Biology and Ecology of the Pelagic Stingray, Pteroplatytrygon Violacea (Bonaparte, 1832). Sharks Open Ocean Biol. Fish. Conserv. 2009, 152–159. [Google Scholar] [CrossRef]
- Báez, J.C.; Crespo, G.O.; García-Barcelona, S.; De Urbina, J.M.O.; Macías, D. Understanding Pelagic Stingray (Pteroplatytrygon Violacea) by-Catch by Spanish Longliners in the Mediterranean Sea. J. Mar. Biol. Assoc. U. K. 2016, 96, 1387–1394. [Google Scholar] [CrossRef]
- Hare, S.R.; Williams, P.G.; Castillo Jordán, C.D.; Hamer, P.A.; Scott, R.D.; Pilling, G. The Western and Central Pacific Tuna Fishery: 2020 Overview and Status of Stocks; Pacific Community: Noumea, NC, USA, 2021; ISBN 9789820014206. [Google Scholar]
- Wang, J.; Gao, C.; Wu, F.; Gao, X.; Chen, J.; Dai, X.; Tian, S.; Chen, Y. The Discards and Bycatch of Chinese Tuna Longline Fleets in the Pacific Ocean from 2010 to 2018. Biol. Conserv. 2021, 255, 109011. [Google Scholar] [CrossRef]
- Ward, P.; Myers, R.A. Shifts in Open-ocean Fish Communities Coinciding with the Commencement of Commercial Fishing. Ecology 2005, 86, 835–847. [Google Scholar] [CrossRef]
- Polovina, J.J.; Woodworth-Jefcoats, P.A. Fishery-Induced Changes in the Subtropical Pacific Pelagic Ecosystem Size Structure: Observations and Theory. PLoS ONE 2013, 8, e62341. [Google Scholar] [CrossRef]
- Kirby, D.S.; Hobday, A. Ecological Risk Assessment for the Effects of Fishing in the Western and Central Pacific Ocean: Productivity-Susceptibility Analysis. In Proceedings of the WCPFC-SC3-EB-SWG/WP-1.Third Scientific Committee Meeting of the Western and Central Pacific Fisheries Commission, Honolulu, HI, USA, 13–24 August 2007. [Google Scholar]
- Lin, Q.; Chen, Y.; Zhu, J. A Comparative Analysis of the Ecological Impacts of Chinese Tuna Longline Fishery on the Eastern Pacific Ocean. Ecol. Indic. 2022, 143, 109284. [Google Scholar] [CrossRef]
- Kindong, R.; Sarr, O.; Wang, J.; Xia, M.; Wu, F.; Dai, L.; Tian, S.; Dai, X. Size Distribution Patterns of Silky Shark Carcharhinus Falciformis Shaped by Environmental Factors in the Pacific Ocean. Sci. Total Environ. 2022, 850, 157927. [Google Scholar] [CrossRef] [PubMed]
- Gilman, E.; Passfield, K.; Nakamura, K. Performance of Regional Fisheries Management Organizations: Ecosystem-Based Governance of Bycatch and Discards. Fish Fish. 2014, 15, 327–351. [Google Scholar] [CrossRef]
- Gilman, E.; Weijerman, M.; Suuronen, P. Ecological Data from Observer Programmes Underpin Ecosystem-Based Fisheries Management. ICES J. Mar. Sci. 2017, 74, 1481–1495. [Google Scholar] [CrossRef]
- Diaz-Delgado, E.; Crespo-Neto, O.; Martínez-Rincón, R.O. Environmental Preferences of Sharks Bycaught by the Tuna Purse-Seine Fishery in the Eastern Pacific Ocean. Fish. Res. 2021, 243, 106076. [Google Scholar] [CrossRef]
- Lopetegui-Eguren, L.; Poos, J.J.; Arrizabalaga, H.; Guirhem, G.L.; Murua, H.; Lezama-Ochoa, N.; Griffiths, S.P.; Gondra, J.R.; Sabarros, P.S.; Báez, J.C.; et al. Spatio-Temporal Distribution of Juvenile Oceanic Whitetip Shark Incidental Catch in the Western Indian Ocean. Front. Mar. Sci. 2022, 9, 1–19. [Google Scholar] [CrossRef]
- Lezama-Ochoa, N.; Hall, M.A.; Pennino, M.G.; Stewart, J.D.; López, J.; Murua, H. Environmental Characteristics Associated with the Presence of the Spinetail Devil Ray (Mobula Mobular) in the Eastern Tropical Pacific. PLoS ONE 2019, 14, 854. [Google Scholar] [CrossRef]
- Lopez, J.; Alvarez-Berastegui, D.; Soto, M.; Murua, H. Using Fisheries Data to Model the Oceanic Habitats of Juvenile Silky Shark (Carcharhinus Falciformis) in the Tropical Eastern Atlantic Ocean. Biodivers. Conserv. 2020, 29, 2377–2397. [Google Scholar] [CrossRef]
- Vilmar, M.; Di Santo, V. Swimming Performance of Sharks and Rays under Climate Change. Rev. Fish Biol. Fish. 2022, 32, 765–781. [Google Scholar] [CrossRef]
- Mollet, H.F. Distribution of the Pelagic Stingray, Dasyatis Violacea (Bonaparte, 1832), off California, Central America, and Worldwide. Mar. Freshw. Res. 2002, 53, 525–530. [Google Scholar] [CrossRef]
- Mollet, H.F.; Ezcurra, J.M.; O’Sullivan, J.B. Captive Biology of the Pelagic Stingray, Dasyatis Violacea (Bonaparte, 1832). Mar. Freshw. Res. 2002, 53, 531–541. [Google Scholar] [CrossRef]
- Williams, P.; Ruaia, T. Overview of Tuna Fisheries in the Western and Central Pacific Ocean, Including Economic Conditions-2019. In Proceedings of the WCPFC-SC16-2020/GN-IP-1. Sixteenth Regular Session of the WCPFC Scientific Committee, Online Meeting, 11–22 August 2020. [Google Scholar]
- Dai, X.; Wu, F.; Wang, X. Annual Report to the Commission Part 1: Information on Fisheries, Research and Statistics. In Proceedings of the WCPFC-SC15-AR/CCM–03, Fifteenth Regular Session of the WCPFC Scientific Committee, Pohnpei, Micronesia, 12–20 August 2019. [Google Scholar]
- Morato, T.; Hoyle, S.D.; Allain, V.; Nicol, S.J. Seamounts Are Hotspots of Pelagic Biodiversity in the Open Ocean. Proc. Natl. Acad. Sci. USA 2010, 107, 9707–9711. [Google Scholar] [CrossRef] [PubMed]
- Yesson, C.; Clark, M.R.; Taylor, M.L.; Rogers, A.D. The Global Distribution of Seamounts Based on 30 Arc Seconds Bathymetry Data. Deep Sea Res. Part I Oceanogr. Res. Pap. 2011, 58, 442–453. [Google Scholar] [CrossRef]
- Morato, T.; Varkey, D.A.; Damaso, C.; Machete, M.; Santos, M.; Prieto, R.; Santos, R.S.; Pitcher, T.J. Evidence of a Seamount Effect on Aggregating Visitors. Mar. Ecol. Prog. Ser. 2008, 357, 23–32. [Google Scholar] [CrossRef]
- Guisan, A.; Edwards Jr, T.C.; Hastie, T. Generalized Linear and Generalized Additive Models in Studies of Species Distributions: Setting the Scene. Ecol. Modell. 2002, 157, 89–100. [Google Scholar] [CrossRef]
- Wood, S.N. Generalized Additive Models: An Introduction with R; Chapman and Hall/CRC: London, UK, 2006; ISBN 0429093152. [Google Scholar]
- Akaike, H. A New Look at the Statistical Model Identification. IEEE Trans. Autom. Control 1974, 19, 716–723. [Google Scholar] [CrossRef]
- Fielding, A.H.; Bell, J.F. A Review of Methods for the Assessment of Prediction Errors in Conservation Presence/Absence Models. Environ. Conserv. 1997, 24, 38–49. [Google Scholar] [CrossRef]
- R Core Team. R Development Core Team. R A Lang. Environ. Stat. Comput. 2013, 55, 275–286. [Google Scholar]
- Wood, S.; Wood, M.S. Package ‘Mgcv’. R Packag. Version 2015, 1, 729. [Google Scholar]
- Team, R.C.; Team, M.R.C.; Suggests, M.; Matrix, S. Package Stats. R Stats Packag. 2018, 1–489. [Google Scholar]
- Wei, T.; Simko, V.; Levy, M.; Xie, Y.; Jin, Y.; Zemla, J. Package ‘Corrplot’. Statistician 2017, 56, e24. [Google Scholar]
- Fox, J.; Weisberg, S.; Adler, D.; Bates, D.; Baud-Bovy, G.; Ellison, S.; Firth, D.; Friendly, M.; Gorjanc, G.; Graves, S. Package ‘Car’. Vienna R Found. Stat. Comput. 2012, 16, 1–158. [Google Scholar]
- Freeman, E.A.; Moisen, G. PresenceAbsence: An R Package for Presence Absence Analysis. J. Stat. Softw. 2008, 23, 1–31. [Google Scholar] [CrossRef]
- Kuhn, M. Building Predictive Models in R Using the Caret Package. J. Stat. Softw. 2008, 28, 1–26. [Google Scholar] [CrossRef]
- Bernal, D.; Carlson, J.K.; Goldman, K.J.; Lowe, C.G. Energetics, Metabolism, and Endothermy in Sharks and Rays. Biol. Sharks Relat. 2012, 211, 237. [Google Scholar]
- Talley, L.D. Salinity Patterns in the Ocean. Earth Syst. Phys. Chem. Dimens. Glob. Environ. Chang. 2002, 1, 629–640. [Google Scholar]
- Musyl, M.K.; Brill, R.W.; Curran, D.S.; Fragoso, N.M.; McNaughton, L.M.; Nielsen, A.; Kikkawa, B.S.; Moyes, C.D. Postrelease Survival, Vertical and Horizontal Movements, and Thermal Habitats of Five Species of Pelagic Sharks in the Central Pacific Ocean. Fish. Bull. 2011, 109, 341–368. [Google Scholar]
- Lezama-Ochoa, N.; Murua, H.; Hall, M.; Román, M.; Ruiz, J.; Vogel, N.; Caballero, A.; Sancristobal, I. Biodiversity and Habitat Characteristics of the Bycatch Assemblages in Fish Aggregating Devices (FADs) and School Sets in the Eastern Pacific Ocean. Front. Mar. Sci. 2017, 4. [Google Scholar] [CrossRef]
- Bertrand, A.; Lengaigne, M.; Takahashi, K.; Avadi, A.; Poulain, F.; Harrod, C. El Niño Southern Oscillation (ENSO) Effects on Fisheries and Aquaculture; Food & Agriculture Org.: Rome, Italy, 2020; Volume 660, ISBN 9251323275. [Google Scholar]
- DiGirolamo, A.L.; Gruber, S.H.; Pomory, C.; Bennett, W.A. Diel Temperature Patterns of Juvenile Lemon Sharks Negaprion Brevirostris, in a Shallow-water Nursery. J. Fish Biol. 2012, 80, 1436–1448. [Google Scholar] [CrossRef]
- Speed, C.W.; Meekan, M.G.; Field, I.C.; McMahon, C.R.; Bradshaw, C.J.A. Heat-Seeking Sharks: Support for Behavioural Thermoregulation in Reef Sharks. Mar. Ecol. Prog. Ser. 2012, 463, 231–244. [Google Scholar] [CrossRef]
- Sims, D.W.; Wearmouth, V.J.; Southall, E.J.; Hill, J.M.; Moore, P.; Rawlinson, K.; Hutchinson, N.; Budd, G.C.; Righton, D.; Metcalfe, J.D. Hunt Warm, Rest Cool: Bioenergetic Strategy Underlying Diel Vertical Migration of a Benthic Shark. J. Anim. Ecol. 2006, 75, 176–190. [Google Scholar] [CrossRef] [PubMed]
- Worm, B.; Sandow, M.; Oschlies, A.; Lotze, H.K.; Myers, R.A. Ecology: Global Patterns of Predator Diversity in the Open Oceans. Science 2005, 309, 1365–1369. [Google Scholar] [CrossRef] [PubMed]
- Worm, B.; Lotze, H.K.; Myers, R.A. Predator Diversity Hotspots in the Blue Ocean. Proc. Natl. Acad. Sci. USA 2003, 100, 9884–9888. [Google Scholar] [CrossRef] [PubMed]
- Morato, T.; Hoyle, S.D.; Allain, V.; Nicol, S.J. Tuna Longline Fishing around West and Central Pacific Seamounts. PLoS ONE 2010, 5, 453. [Google Scholar] [CrossRef] [PubMed]
- Lezama-Ochoa, N.; Hall, M.; Román, M.; Vogel, N. Spatial and Temporal Distribution of Mobulid Ray Species in the Eastern Pacific Ocean Ascertained from Observer Data from the Tropical Tuna Purse-Seine Fishery. Environ. Biol. Fishes 2019, 102, 1–17. [Google Scholar] [CrossRef]
- Tremblay-Boyer, L.; Brouwer, S. Review of Available Information on Non-Key Shark Species Including Mobulids and Fisheries Interactions. In Proceedings of the EB-WP-08 Twelfth Regular Session of the Scientific Committee to the Western Central Pacific Fisheries Commission, Bali, Indonesia, 3–11 August 2016. [Google Scholar]
- Clarke, S.C.; Harley, S.J. A Proposal for a Research Plan to Determine the Status of the Key Shark Species. 2010,WCPFC-SC6-2010/EB-WP01. Available online: https://meetings.wcpfc.int/node/7033 (accessed on 19 October 2022).
- Rice, J.; Tremblay-Boyer, L.; Scott, R.; Hare, S.; Tidd, A. Analysis of Stock Status and Related Indicators for Key Shark Species of the Western Central Pacific Fisheries Commission. In Proceedings of the WCPFC-SC11-2015/EB-WP-04-Rev 1, Eleventh Regular Session of the WCPFC Scientific Committee, Pohnpei, Micronesia, 5–13 August 2015. [Google Scholar]
- Dunn, D.C.; Maxwell, S.M.; Boustany, A.M.; Halpin, P.N. Dynamic Ocean Management Increases the Efficiency and Efficacy of Fisheries Management. Proc. Natl. Acad. Sci. USA 2016, 113, 668–673. [Google Scholar] [CrossRef]
- Hazen, E.L.; Scales, K.L.; Maxwell, S.M.; Briscoe, D.K.; Welch, H.; Bograd, S.J.; Bailey, H.; Benson, S.R.; Eguchi, T.; Dewar, H.; et al. A Dynamic Ocean Management Tool to Reduce Bycatch and Support Sustainable Fisheries. Sci. Adv. 2018, 4, 1–8. [Google Scholar] [CrossRef]
- Maxwell, S.M.; Hazen, E.L.; Lewison, R.L.; Dunn, D.C.; Bailey, H.; Bograd, S.J.; Briscoe, D.K.; Fossette, S.; Hobday, A.J.; Bennett, M.; et al. Dynamic Ocean Management: Defining and Conceptualizing Real-Time Management of the Ocean. Mar. Policy 2015, 58, 42–50. [Google Scholar] [CrossRef]
- Pons, M.; Watson, J.T.; Ovando, D.; Andraka, S.; Brodie, S.; Domingo, A.; Fitchett, M.; Forselledo, R.; Hall, M.; Hazen, E.L.; et al. Trade-Offs between Bycatch and Target Catches in Static versus Dynamic Fishery Closures. Proc. Natl. Acad. Sci. USA 2022, 119, e2114508119. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).