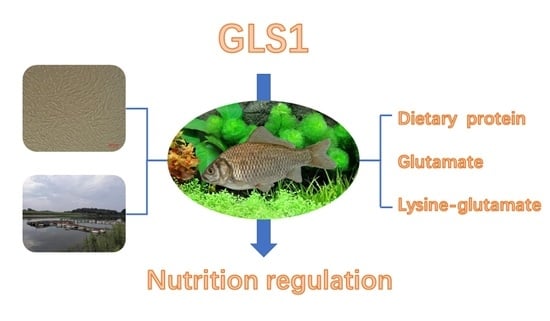
Molecular Characterization and Dietary Regulation of Glutaminase 1 (gls1) in Triploid Crucian Carp (Carassius auratus)
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals and Tissue Preparation
2.2. gls1 Expression in Various Tissues and Diurnal Variation
2.3. Muscle Cell Culture and Treatment with Glutamate
2.4. Dietary Protein Level and Protein Source Regulation
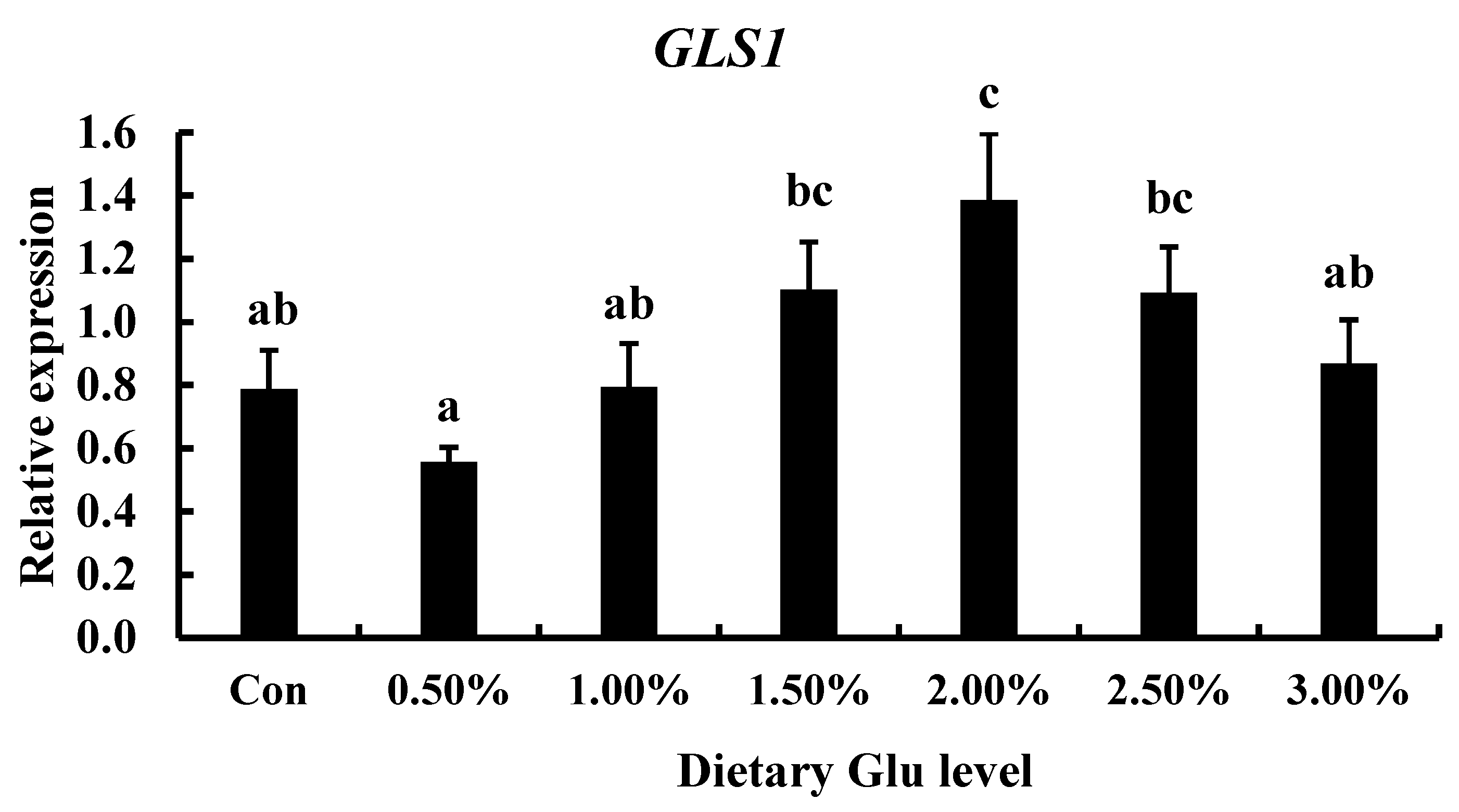
2.5. Dietary Glutamate Regulations
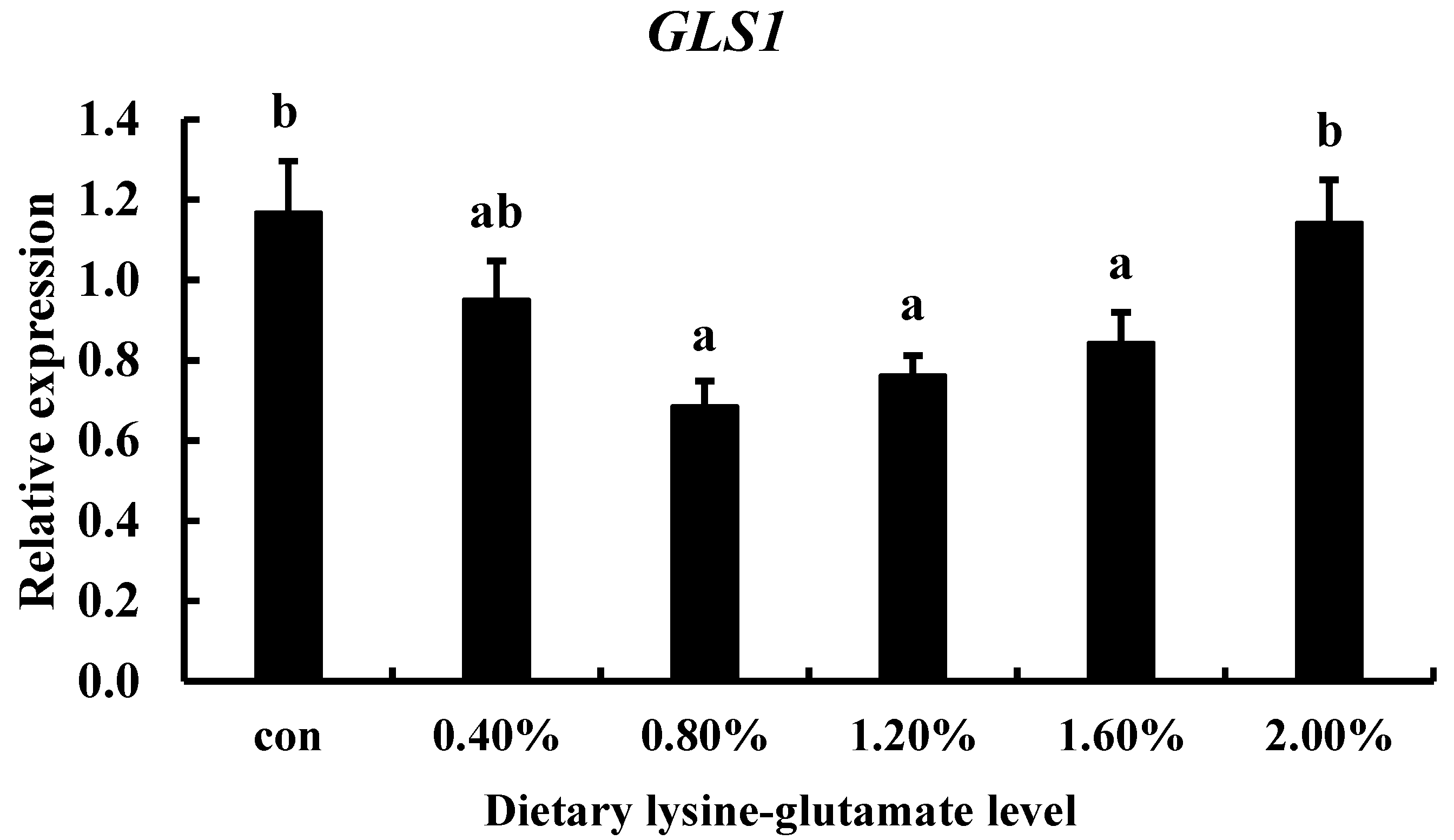
2.6. Dietary Lysine-Glutamate Dipeptide Regulation
2.7. RNA Isolation and cDNA Synthesis
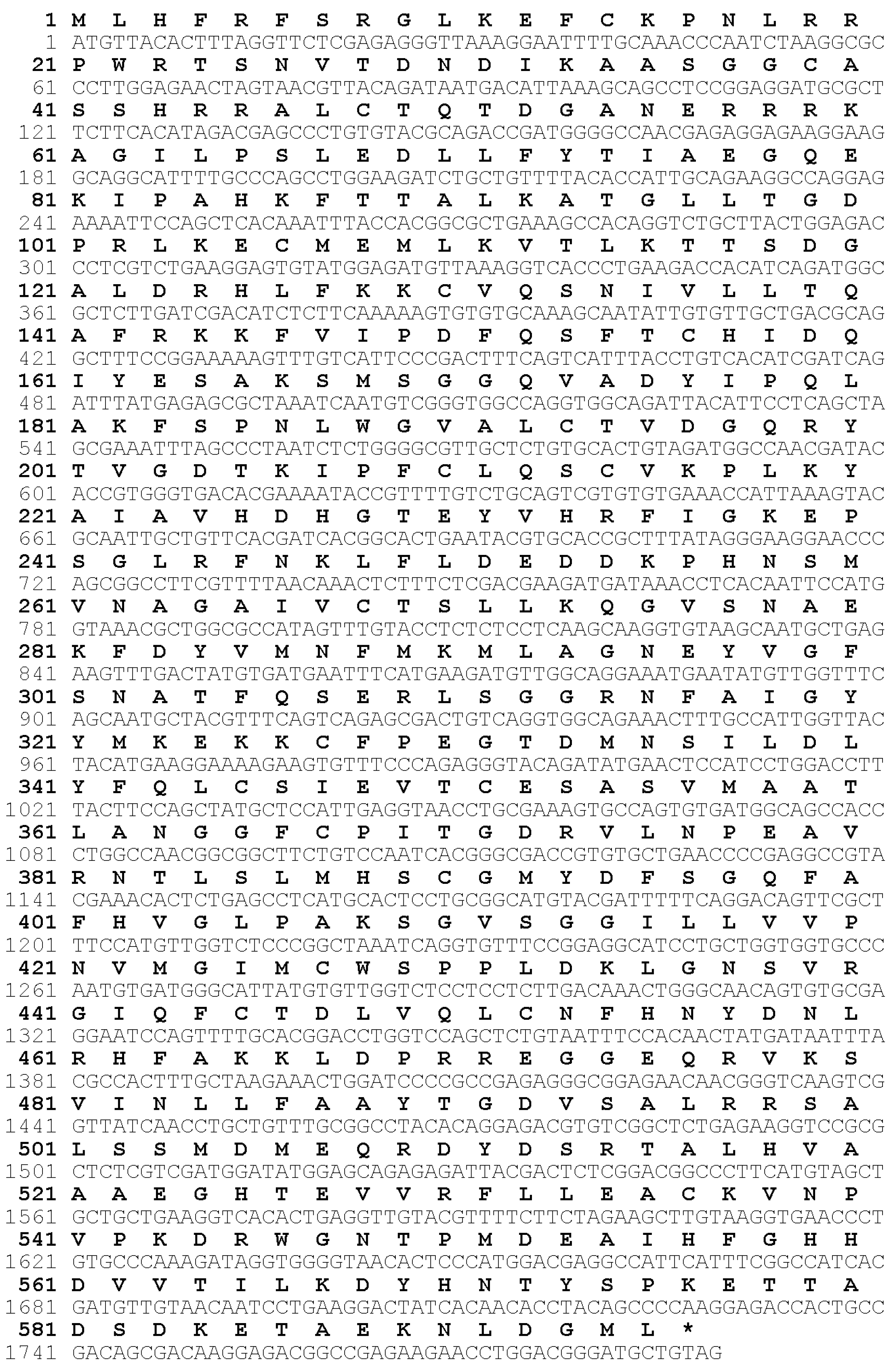
2.8. Cloning of gls1 cDNA
2.9. Quantitative Real-Time PCR
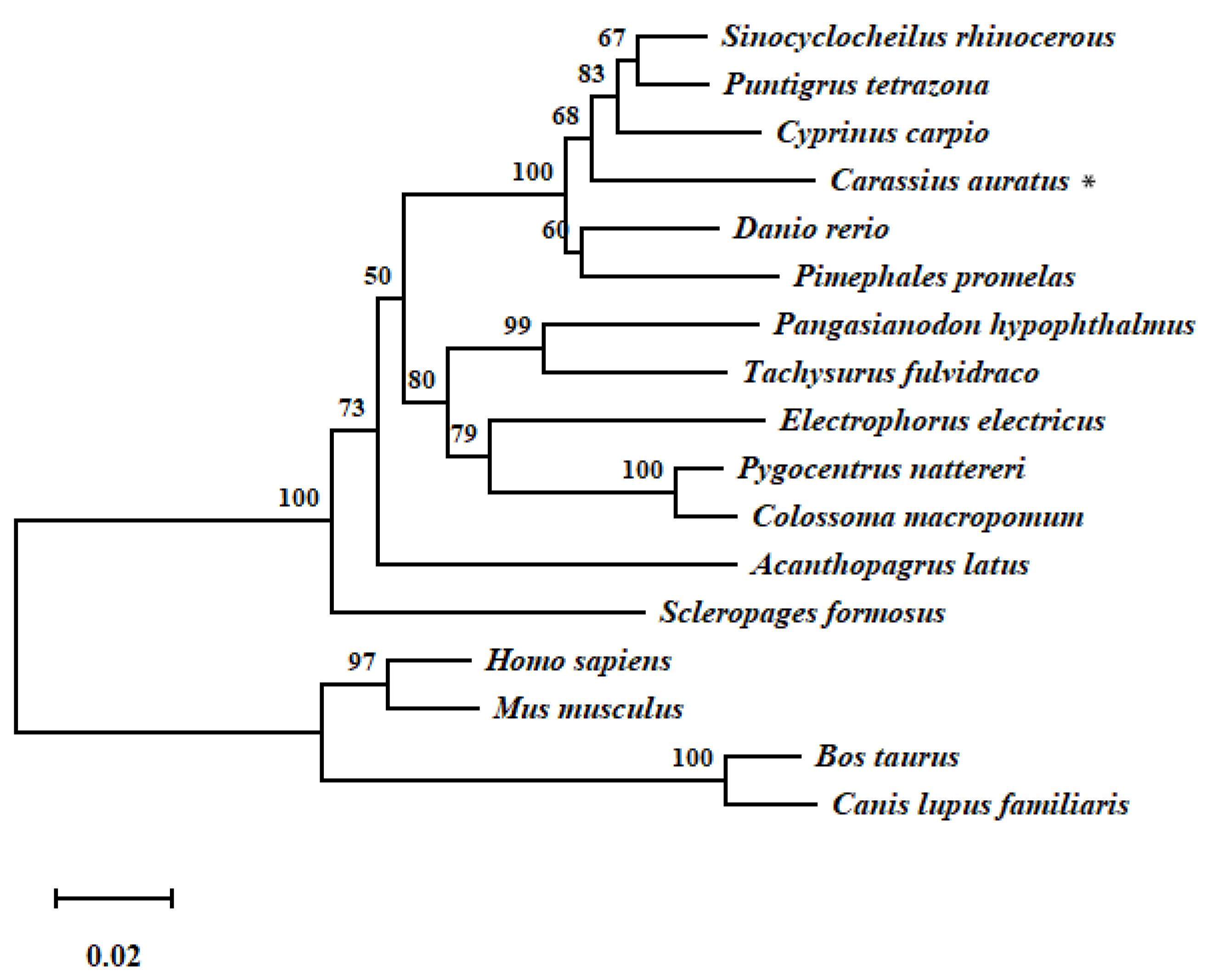
2.10. Phylogenetic Analysis of gls1
2.11. Statistical Analysis
3. Results
3.1. Cloning and Sequence Analysis of gls1 cDNA from TCC
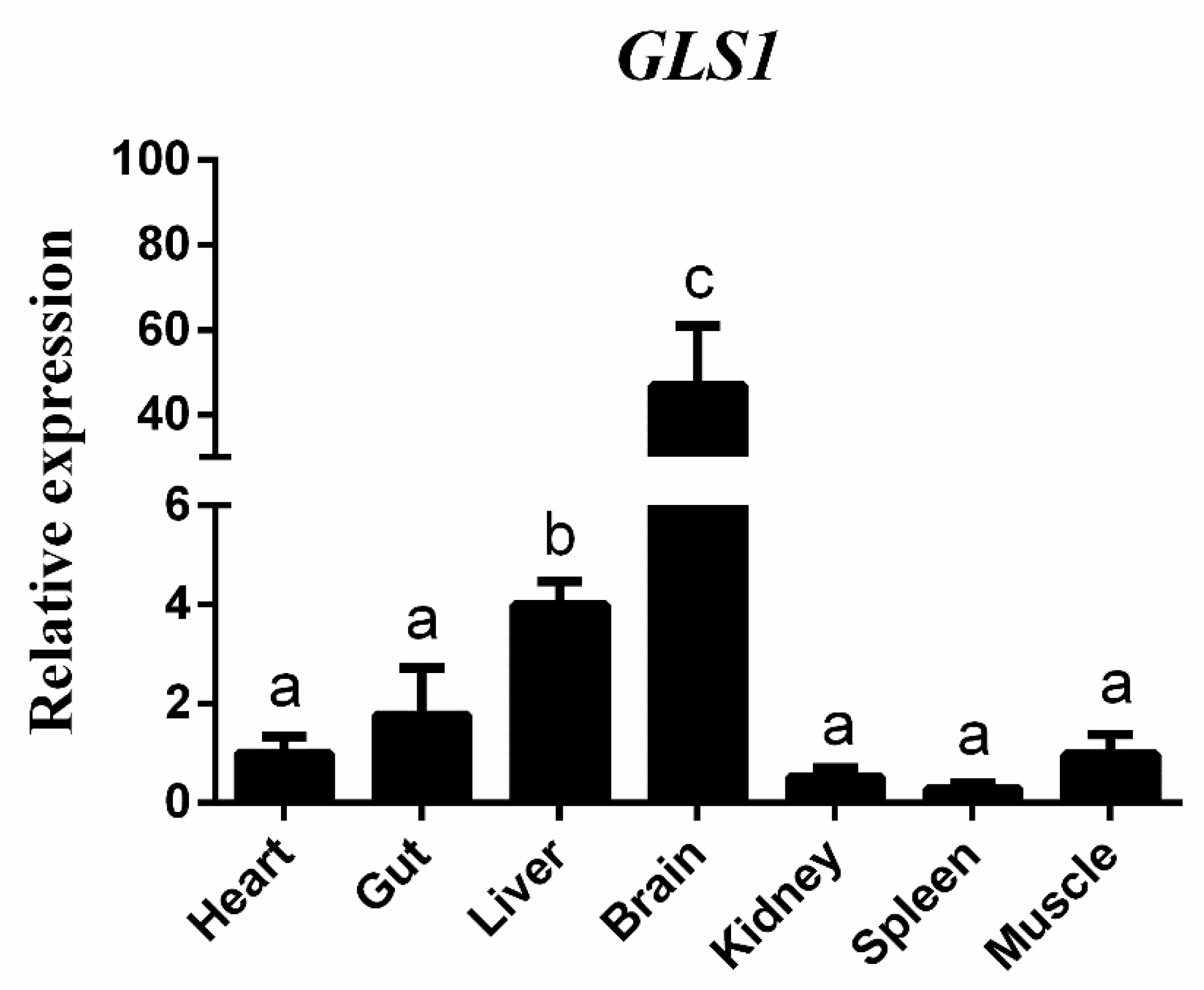
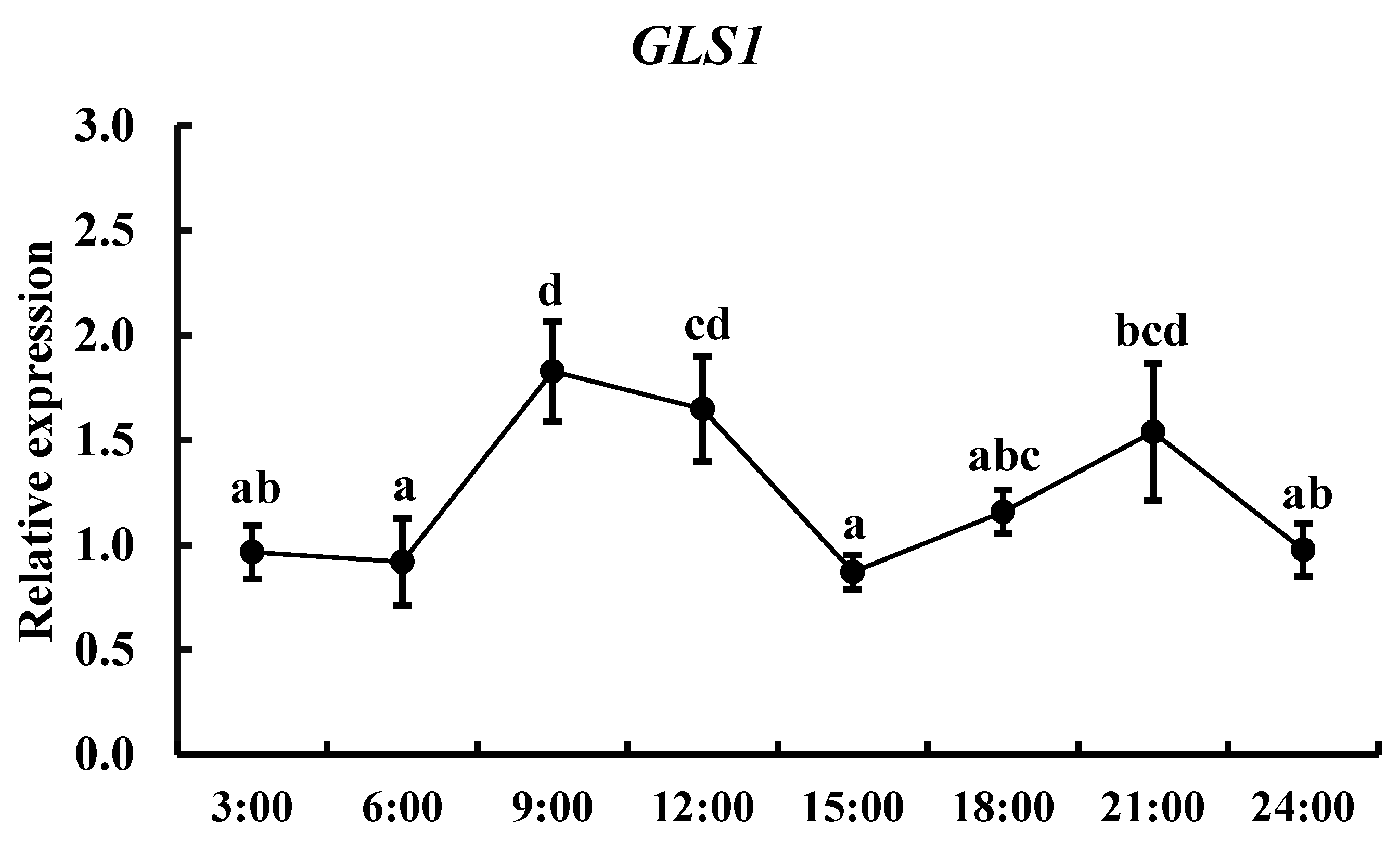
3.2. Tissue Expression Patterns and Circadian Expression of gls1 mRNAs in TCC
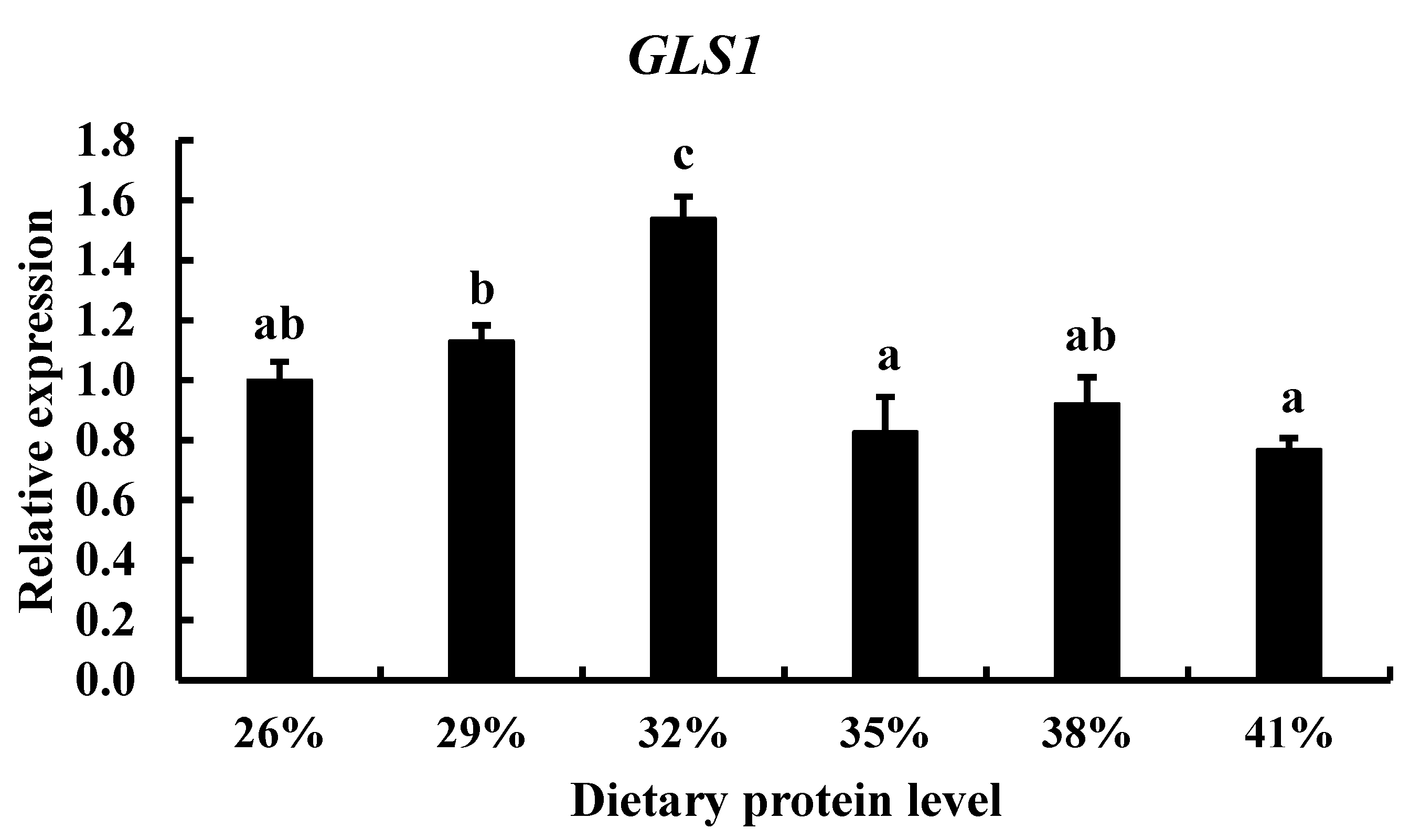
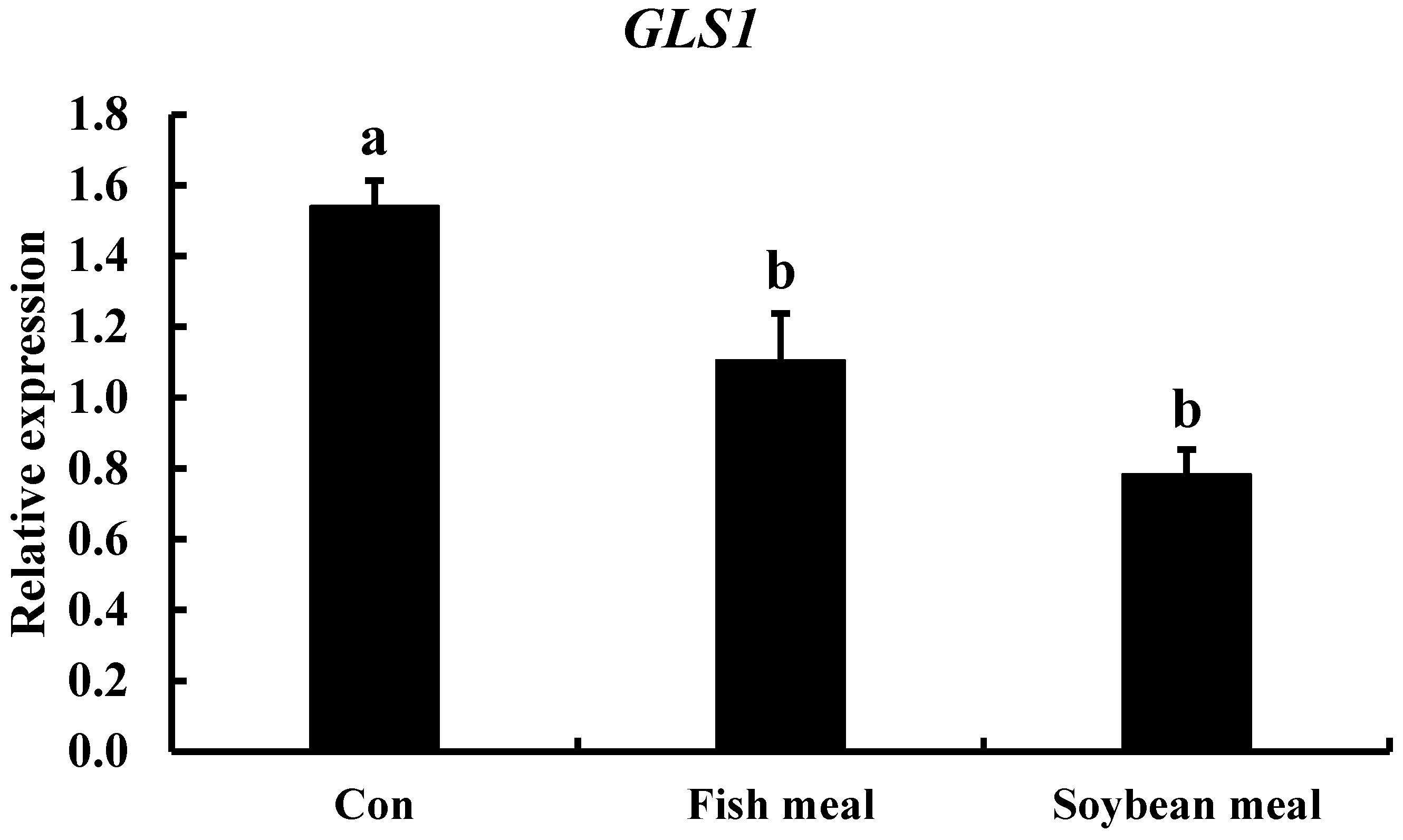
3.3. Effect of Diets with Different Protein Levels and Protein Sources on TCC gls1 Gene Expression
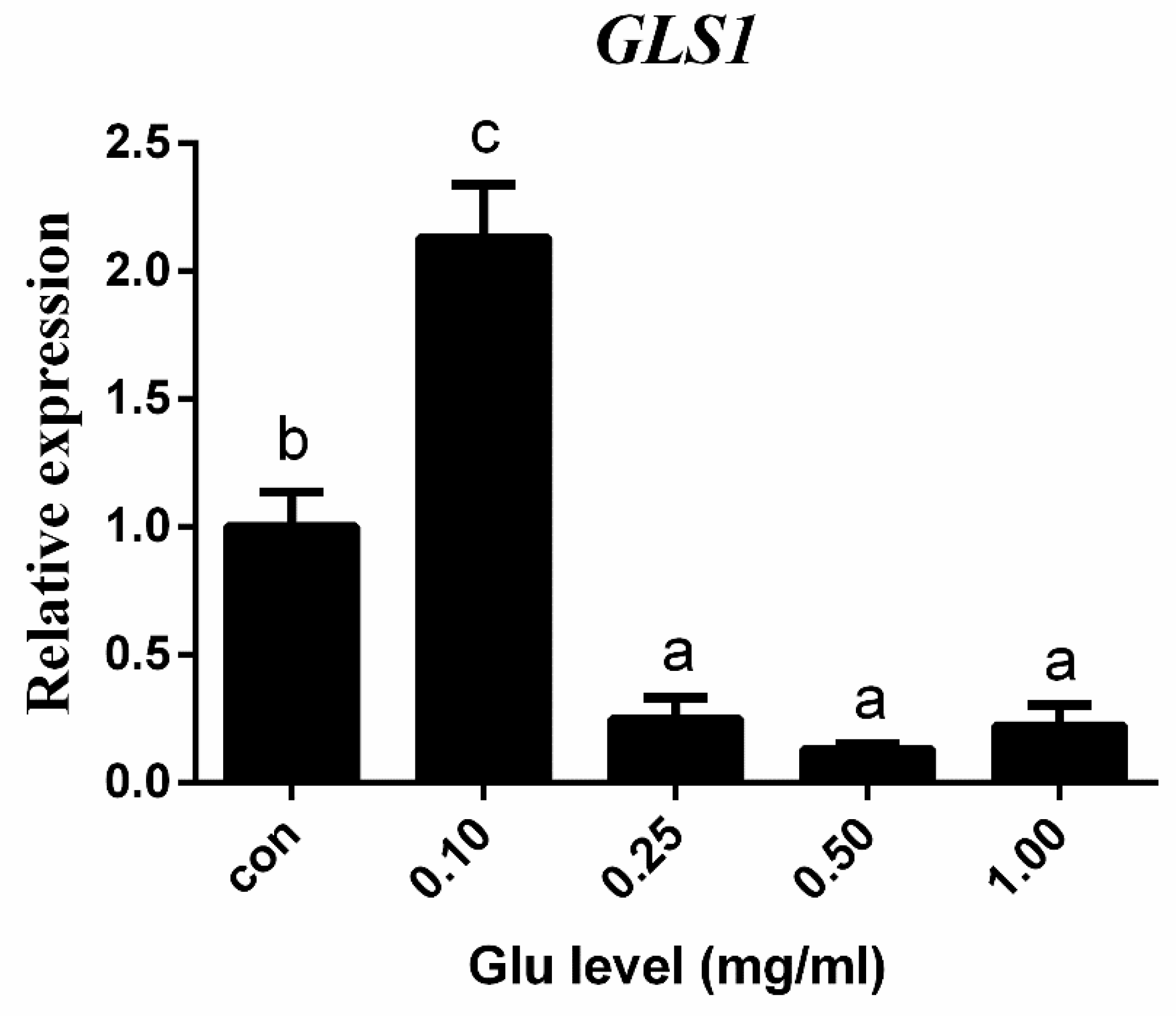
3.4. The Effects of Glutamate In Vitro and In Vivo on TCC gls1 Gene Expression
3.5. The Effect of Diets with Lysine-Glutamate on TCC gls1 Gene Expression
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Grigorakis, K.; Alexi, N.; Vasilaki, A.; Giogios, I.; Fountoulaki, E. Chemical quality and sensory profile of the Mediterranean farmed fish shi drum (Umbrina cirrosa) as affected by its dietary protein/fat levels. Ital. J. Anim. Sci. 2016, 15, 681–688. [Google Scholar] [CrossRef]
- Yang, G.; Jiang, W.; Chen, Y.; Hu, Y.; Kumar, V. Effect of oil source on growth performance, antioxidant capacity, fatty acid composition and fillet quality of juvenile grass carp (Ctenopharyngodon idella). Aquac. Nutr. 2020, 26, 1186–1197. [Google Scholar] [CrossRef]
- Hoffmann, K. What is quality? Definitions, measurement and evaluation of meat quality. Meat Focus Int. 1994, 3, 73–82. [Google Scholar]
- Becker, T. Consumer perception of fresh meat quality: A framework for analysis. Br. Food J. 2000, 102, 158–176. [Google Scholar] [CrossRef]
- Watanabe, G.; Kobayashi, H.; Shibata, M.; Kubota, M.; Kadowaki, M.; Fujimura, S. Reduction in dietary lysine increases muscle free amino acids through changes in protein metabolism in chickens. Poult. Sci. 2020, 99, 3102–3110. [Google Scholar] [CrossRef] [PubMed]
- Bigiani, A. Glutamate Receptors in Taste Receptor Cells. In Glutamate Receptors in Peripheral Tissue: Excitatory Transmission Outside the CNS; Springer: Boston, MA, USA, 2005. [Google Scholar]
- Halpern, B.P. Glutamate and the Flavor of Foods. J. Nutr. 2000, 130, 910S–914S. [Google Scholar] [CrossRef]
- Ballantyne, J.S. Amino acid metabolism. Fish Physiol. 2001, 20, 77–107. [Google Scholar]
- Blanco, E.; Campos-Sandoval, J.; Palomino, A.; Luque-Rojas, M.J.; Bilbao, A.; Suárez, J.; Márquez, J.; Fonseca, F. Cocaine modulates both glutaminase gene expression and glutaminase activity in the brain of cocaine-sensitized mice. Psychopharmacology 2012, 219, 933–944. [Google Scholar] [CrossRef]
- Curthoys, N.P.; Watford, M. Regulation of glutaminase activity and glutamine metabolism. Annu. Rev. Nutr. 1995, 15, 133–159. [Google Scholar] [CrossRef]
- Gómez-Fabre, P.; Aledo, J.C.; Castillo-Olivares, A.D.; Alonso, F.J.; Ignacio, N.; Campos, J.A.; Márquez, J. Molecular cloning, sequencing and expression studies of the human breast cancer cell glutaminase. Biochem. J. 2000, 345, 365. [Google Scholar] [CrossRef]
- Rosa, V.; Campos-Sandoval, J.A.; Martin-Rufian, M.; Cardona, C.; Mates, J.M.; Segura, J.A.; Alonso, F.J.; Marquez, J. A novel glutaminase isoform in mammalian tissues. Neurochem. Int. 2009, 55, 76–84. [Google Scholar] [CrossRef]
- Elgadi, K.M.; Meguid, R.A.; Qian, M.; Souba, W.W.; Abcouwer, S.F. Cloning and analysis of unique human glutaminase isoforms generated by tissue-specific alternative splicing. Physiol. Genom. 1999, 1, 51–62. [Google Scholar] [CrossRef]
- Pérez-Gómez, C.; Matés, J.; Gómez-Fabre, P.; Castillo-Olivares, A.D.; Alonso, F.J.; Márquez, J. Genomic organization and transcriptional analysis of the human l-glutaminase gene. Biochem. J. 2003, 370, 771. [Google Scholar] [CrossRef]
- Jiang, J.; Feng, L.; Liu, Y.; Jiang, W.D.; Hu, K.; Li, S.H.; Zhou, X.Q. Molecular cloning and expression of kidney-type glutaminase from common carp (Cyprinus carpio) and its up-regulation by glutamine in primary culture enterocyte. Aquac. Nutr. 2014, 20, 731–740. [Google Scholar] [CrossRef]
- Liu, S.; Li, N.; Lin, Q.; Liu, L.; Niu, Y.; Liang, H.; Huang, Z.; Fu, X. Glutaminase 1 in mandarin fish Siniperca chuatsi: Molecular characterization, expression pattern and function involving in virus replication. Aquaculture 2020, 519, 734924. [Google Scholar] [CrossRef]
- Cambier, S.; Gonzalez, P.; Mesmer-Dudons, N.; Brethes, D.; Fujimura, M.; Bourdineaud, J.P. Effects of dietary methylmercury on the zebrafish brain: Histological, mitochondrial, and gene transcription analyses. Biometals 2012, 25, 165–180. [Google Scholar] [CrossRef]
- Aledo, J.C.; Gomez-Fabre, P.M.; Olalla, L.; Marquez, J. Identification of two human glutaminase loci and tissue-specific expression of the two related genes. Mamm. Genome 2000, 11, 1107–1110. [Google Scholar] [CrossRef]
- Castell, L.; Vance, C.; Abbott, R.; Marquez, J.; Eggleton, P. Granule localization of glutaminase in human neutrophils and the consequence of glutamine utilization for neutrophil activity. J. Biol. Chem. 2004, 279, 13305–13310. [Google Scholar] [CrossRef]
- Masson, J.; Darmon, M.; Conjard, A.; Chuhma, N.; Ropert, N.; Thoby-Brisson, M.; Foutz, A.S.; Parrot, S.; Miller, G.M.; Jorisch, R. Mice lacking brain/kidney phosphate-activated glutaminase have impaired glutamatergic synaptic transmission, altered breathing, disorganized goal-directed behavior and die shortly after birth. Knock-Out and Glutamate Transmission. J. Neurosci. 2006, 26, 4660–4671. [Google Scholar] [CrossRef]
- Le, A.; Lane, A.; Hamaker, M.; Bose, S.; Gouw, A.; Barbi, J.; Tsukamoto, T.; Rojas, C.; Slusher, B.; Zhang, H. Glucose-independent glutamine metabolism via TCA cycling for proliferation and survival in B cells. Cell Metab. 2012, 15, 110–121. [Google Scholar] [CrossRef]
- Huang, F.; Zhang, Q.; Hong, M.; Lv, Q.; Tao, Z. Expression of glutaminase is upregulated in colorectal cancer and of clinical significance. Int. J. Clin. Exp. Pathol. 2014, 7, 1093. [Google Scholar] [PubMed]
- Mousumi, D.; Dayanand, A. Production and antioxidant attribute of L-glutaminase from Streptomyces enissocaesilis DMQ-24. Int. J. Latest Res. Sci. Technol. 2013, 2, 1–9. [Google Scholar]
- Kong, S.E.; Hall, J.C.; Cooper, D.; Mccauley, R.D. Glutamine-enriched parenteral nutrition regulates the activity and expression of intestinal glutaminase. Biochim. Biophys. Acta BBA-Gen. Subj. 2000, 1475, 67–75. [Google Scholar] [CrossRef] [PubMed]
- Marquez, J.; Tosina, M.; de la Rosa, V.; Segura, J.A.; Alonso, F.J.; Mates, J.M.; Campos-Sandoval, J.A. New insights into brain glutaminases: Beyond their role on glutamatergic transmission. Neurochem. Int. 2009, 55, 64–70. [Google Scholar] [CrossRef] [PubMed]
- Fontaine, K.A.; Camarda, R.; Lagunoff, M. Vaccinia Virus Requires Glutamine but Not Glucose for Efficient Replication. J. Virol. 2014, 88, 4366. [Google Scholar] [CrossRef]
- Amobonye, A.; Singh, S.; Pillai, S. Recent advances in microbial glutaminase production and applications—A concise review. Crit. Rev. Biotechnol. 2019, 39, 944–963. [Google Scholar] [CrossRef]
- Chen, S.; Wang, J.; Liu, S.J.; Qin, Q.B.; Xiao, J.; Duan, W.; Luo, K.K.; Liu, J.H.; Liu, Y. Biological characteristics of an improved triploid crucian carp. Sci. China 2009, 52, 733–738. [Google Scholar] [CrossRef]
- Fu, Y.; Liang, X.; Li, D.; Gao, H.; Hu, F. Effect of Dietary Tryptophan on Growth, Intestinal Microbiota, and Intestinal Gene Expression in an Improved Triploid Crucian Carp. Front. Nutr. 2021, 8, 322. [Google Scholar] [CrossRef]
- Luo, W.; Qu, F.; Song, P.; Xiong, D.; Yin, Y.; Li, J.; Liu, Z. Molecular characterization and taurine regulation of two novel CDOs (CDO1 and CDO2) from Carassius auratus. Comp. Biochem. Physiol. B Biochem. Mol. Biol. 2019, 235, 54–61. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of Relative Gene Expression Data using Real-Time Quantitative PCR. Methods 2002, 25, 402–408. [Google Scholar] [CrossRef]
- Kong, S.E.; Hall, J.C.; Cooper, D.; McCauley, R.D. Starvation alters the activity and mRNA level of glutaminase and glutamine synthetase in the rat intestine. J. Nutr. Biochem. 2000, 11, 393–400. [Google Scholar] [CrossRef]
- Kuttykrishnan, C.; Santhanam, K.; Rao, R.; Vijayaraghavan, P.K. Effect of dietary protein quality on rat kidney glutaminase activity. Ann. Nutr. Metab. 1981, 25, 151–157. [Google Scholar] [CrossRef]
- Viana, M.T.; D’Abramo, L.R.; Gonzalez, M.A.; García-Suárez, J.V.; Shimada, A.; Vásquez-Peláez, C. Energy and nutrient utilization of juvenile green abalone (Haliotis fulgens) during starvation. Aquaculture 2007, 264, 323–329. [Google Scholar] [CrossRef]
- Shiau, C.Y.; Pong, Y.J.; Chiou, T.K.; Tin, Y.Y. Effect of starvation on free histidine and amino acids in white muscle of milkfish Chanos chanos. Comp. Biochem. Physiol. Part B Biochem. Mol. Biol. 2001, 128, 501–506. [Google Scholar] [CrossRef]
- Pérez-Gómez, C.; Matés, J.; Gómez-Fabre, P.; Castillo-Olivares, A.D.; Alonso, F.J.; Márquez, J. Co-expression of glutaminase K and L isoenzymes in human tumour cells. Biochem. J. 2005, 386, 535–542. [Google Scholar] [CrossRef]
- FAO. The State of World Fisheries and Aquaculture 2020; FAO: Rome, Italy, 2020. [Google Scholar]
- Kobayashi, H.; Eguchi, A.; Takano, W.; Shibata, M.; Kadowaki, M.; Fujimura, S. Regulation of muscular glutamate metabolism by high-protein diet in broiler chicks. Anim. Sci. J. 2011, 82, 86–92. [Google Scholar] [CrossRef]
- Deng, D.F.; Dominy, W.; Ju, Z.Y.; Koshio, S.; Murashige, R.; Wilson, R.P. Dietary lysine requirement of juvenile Pacific threadfin (Polydactylus sexfilis). Aquaculture 2010, 308, 44–48. [Google Scholar] [CrossRef]
- Daniel, H. Molecular and integrative physiology of intestinal peptide transport. Annu. Rev. Physiol. 2004, 66, 361–384. [Google Scholar] [CrossRef]
- Gilbert, E.R.; Wong, E.A.; Webb, K.E. Board-invited review: Peptide absorption and utilization: Implications for animal nutrition and health. J. Anim. Sci. 2008, 86, 2135–2155. [Google Scholar] [CrossRef]
- Mai, K.; Lu, Z.; Ai, Q.; Duan, Q.; Zhang, C.; Li, H.; Wan, J.; Liufu, Z. Dietary lysine requirement of juvenile Japanese seabass, Lateolabrax japonicus. Aquaculture 2006, 258, 535–542. [Google Scholar] [CrossRef]
| Dietary Protein Levels | ||||||
|---|---|---|---|---|---|---|
| 26% | 29% | 32% | 35% | 38% | 41% | |
| Fishmeal 1 | 12.00 | 12.00 | 12.00 | 12.00 | 12.00 | 12.00 |
| Soybean meal 1 | 20.00 | 20.00 | 20.00 | 20.00 | 20.00 | 20.00 |
| Rapeseed meal 1 | 15.00 | 15.00 | 15.00 | 15.00 | 15.00 | 15.00 |
| Casein 1 | 0.00 | 3.20 | 6.50 | 9.80 | 13.10 | 16.40 |
| Fish oil | 3.00 | 3.00 | 3.00 | 3.00 | 3.00 | 3.00 |
| Soybean oi | 3.00 | 3.00 | 3.00 | 3.00 | 3.00 | 3.00 |
| Corn starch | 25.00 | 21.00 | 16.80 | 12.60 | 8.40 | 4.20 |
| Wheat flour | 10.00 | 10.00 | 10.00 | 10.00 | 10.00 | 10.00 |
| Choline | 0.50 | 0.50 | 0.50 | 0.50 | 0.50 | 0.50 |
| Premix 2 | 3.00 | 3.00 | 3.00 | 3.00 | 3.00 | 3.00 |
| CMC | 3.00 | 3.00 | 3.00 | 3.00 | 3.00 | 3.00 |
| Cellulose | 5.50 | 6.30 | 7.20 | 8.10 | 9.00 | 9.90 |
| Total | 100.00 | 100.00 | 100.00 | 100.00 | 100.00 | 100.00 |
| Proximate composition | ||||||
| Crude protein | 26.08 | 29.00 | 32.01 | 35.03 | 38.04 | 41.05 |
| Crude lipid | 8.07 | 8.07 | 8.07 | 8.07 | 8.07 | 8.07 |
| Gross energy (MJ/kg) | 18.05 | 18.06 | 18.07 | 18.08 | 18.08 | 18.09 |
| Moisture | 9.12 | 6.01 | 10.05 | 8.19 | 5.75 | 7.26 |
| Ash | 6.15 | 6.12 | 6.78 | 6.13 | 6.34 | 6.75 |
| Ingredients | Control Group | Fishmeal Group | Soybean Meal Group |
|---|---|---|---|
| Fishmeal 1 | 12.00 | 44.40 | 0.00 |
| Soybean meal 1 | 20.00 | 0.00 | 37.10 |
| Rapeseed meal 1 | 15.00 | 0.00 | 15.00 |
| Casein 1 | 6.50 | 0.00 | 6.50 |
| Fish oil | 3.00 | 1.63 | 3.50 |
| Soybean oil | 3.00 | 1.63 | 3.50 |
| Cornstarch | 16.80 | 31.00 | 10.00 |
| Wheat flour | 10.00 | 10.00 | 10.00 |
| Choline | 0.50 | 0.50 | 0.50 |
| Premix 2 | 3.00 | 3.00 | 3.00 |
| CMC | 3.00 | 3.00 | 3.00 |
| Cellulose | 7.20 | 4.84 | 7.90 |
| Total | 100.00 | 100.00 | 100.00 |
| Proximate composition | |||
| Crude protein | 32.01 | 32.05 | 32.03 |
| Crude lipid | 8.07 | 8.05 | 8.06 |
| Gross energy (MJ/kg) | 18.07 | 18.06 | 18.00 |
| Moisture | 10.05 | 9.73 | 9.86 |
| Ash | 6.78 | 9.11 | 5.65 |
| Dietary Glutamate Levels (%) | |||||||
|---|---|---|---|---|---|---|---|
| 0.0 | 0.5 | 1.0 | 1.5 | 2.0 | 2.5 | 3.0 | |
| Glutamate 1 | 0.00 | 0.50 | 1.00 | 1.50 | 2.00 | 2.50 | 3.00 |
| Fishmeal 2 | 2.00 | 2.00 | 2.00 | 2.00 | 2.00 | 2.00 | 2.00 |
| Soybean meal 2 | 34.00 | 34.00 | 34.00 | 34.00 | 34.00 | 34.00 | 34.00 |
| Rapeseed meal 2 | 23.60 | 23.60 | 23.60 | 23.60 | 23.60 | 23.60 | 23.60 |
| Wheat flour 2 | 16.00 | 16.00 | 16.00 | 16.00 | 16.00 | 16.00 | 16.00 |
| Fish oil | 2.00 | 2.00 | 2.00 | 2.00 | 2.00 | 2.00 | 2.00 |
| Soybean oil | 2.50 | 2.50 | 2.50 | 2.50 | 2.50 | 2.50 | 2.50 |
| Cornstarch | 6.00 | 6.00 | 6.00 | 6.00 | 6.00 | 6.00 | 6.00 |
| Choline | 0.11 | 0.11 | 0.11 | 0.11 | 0.11 | 0.11 | 0.11 |
| Premix 3 | 1.50 | 1.50 | 1.50 | 1.50 | 1.50 | 1.50 | 1.50 |
| Methionine | 0.50 | 0.50 | 0.50 | 0.50 | 0.50 | 0.50 | 0.50 |
| CMC | 3.00 | 3.00 | 3.00 | 3.00 | 3.00 | 3.00 | 3.00 |
| Cellulose | 8.79 | 8.29 | 7.79 | 7.29 | 6.79 | 6.29 | 5.79 |
| Total | 100.00 | 100.00 | 100.00 | 100.00 | 100.00 | 100.00 | 100.00 |
| Proximate composition | |||||||
| Crude protein | 31.02 | 31.52 | 32.02 | 32.52 | 33.02 | 33.52 | 34.02 |
| Crude lipid | 6.03 | 6.03 | 6.03 | 6.03 | 6.03 | 6.03 | 6.03 |
| Moisture | 6.63 | 5.75 | 6.53 | 6.84 | 6.58 | 6.21 | 6.32 |
| Ash | 5.66 | 5.79 | 5.63 | 5.70 | 5.30 | 5.55 | 5.62 |
| Dietary Lysine-Glutamate Levels (%) | ||||||
|---|---|---|---|---|---|---|
| 0.0 | 0.4 | 0.8 | 1.2 | 1.6 | 2.0 | |
| Fishmeal 1 | 12.00 | 12.00 | 12.00 | 12.00 | 12.00 | 12.00 |
| Soybean meal 1 | 20.00 | 20.00 | 20.00 | 20.00 | 20.00 | 20.00 |
| Rapeseed meal 1 | 15.00 | 15.00 | 15.00 | 15.00 | 15.00 | 15.00 |
| Casein 1 | 6.50 | 6.50 | 6.50 | 6.50 | 6.50 | 6.50 |
| Fish oil | 3.00 | 3.00 | 3.00 | 3.00 | 3.00 | 3.00 |
| Soybean oil | 3.00 | 3.00 | 3.00 | 3.00 | 3.00 | 3.00 |
| Cornstarch | 16.80 | 16.80 | 16.80 | 16.80 | 16.80 | 16.80 |
| Wheat flour | 10.00 | 10.00 | 10.00 | 10.00 | 10.00 | 10.00 |
| Choline | 0.50 | 0.50 | 0.50 | 0.50 | 0.50 | 0.50 |
| Premix 2 | 3.00 | 3.00 | 3.00 | 3.00 | 3.00 | 3.00 |
| CMC | 3.00 | 3.00 | 3.00 | 3.00 | 3.00 | 3.00 |
| Cellulose | 7.20 | 6.80 | 6.40 | 6.00 | 5.60 | 5.20 |
| Lysine-glutamate 3 | 0.00 | 0.40 | 0.80 | 1.20 | 1.60 | 2.00 |
| Total | 100.00 | 100.00 | 100.00 | 100.00 | 100.00 | 100.00 |
| Proximate composition | ||||||
| Crude protein | 32.01 | 32.41 | 32.81 | 33.21 | 33.61 | 34.01 |
| Crude lipid | 8.07 | 8.07 | 8.07 | 8.07 | 8.07 | 8.07 |
| Moisture | 10.05 | 12.31 | 9.80 | 9.70 | 11.18 | 0.93 |
| Ash | 6.78 | 6.48 | 6.97 | 6.54 | 6.77 | 7.15 |
| Primer | Primer Sequence | Purpose |
|---|---|---|
| GLS-F | 5′-GACTGTCTAAAAAGCGGATT-3′ | CDS |
| GLS-R | 5′-ATGAGGCTACATTCTCCCGA-3′ | CDS |
| GLS-RT-F | 5′-TCTGCTTACTGGAGACCCTCG-3′ | Real-time PCR |
| GLS-RT-R | 5′-TTGCTTTGCACACACTTTTTG-3′ | Real-time PCR |
| β-actin-F | 5′-GAAACTGGAAAGGGAGGTAGC-3′ | Real-time PCR |
| β-actin-R | 5′-CTGTGAGGGCAGAGTGGTAGA-3′ | Real-time PCR |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xiao, Y.; Huang, R.; Cao, S.; Zhao, D.; Mao, Z.; Xiao, C.; Xu, Z.; Zhou, X.; Zhang, X.; Zhang, Y.; et al. Molecular Characterization and Dietary Regulation of Glutaminase 1 (gls1) in Triploid Crucian Carp (Carassius auratus). Fishes 2022, 7, 377. https://doi.org/10.3390/fishes7060377
Xiao Y, Huang R, Cao S, Zhao D, Mao Z, Xiao C, Xu Z, Zhou X, Zhang X, Zhang Y, et al. Molecular Characterization and Dietary Regulation of Glutaminase 1 (gls1) in Triploid Crucian Carp (Carassius auratus). Fishes. 2022; 7(6):377. https://doi.org/10.3390/fishes7060377
Chicago/Turabian StyleXiao, Yangbo, Rong Huang, Shenping Cao, Dafang Zhao, Zhuangwen Mao, Chuchu Xiao, Zhehua Xu, Xiaomei Zhou, Xinran Zhang, Yu Zhang, and et al. 2022. "Molecular Characterization and Dietary Regulation of Glutaminase 1 (gls1) in Triploid Crucian Carp (Carassius auratus)" Fishes 7, no. 6: 377. https://doi.org/10.3390/fishes7060377
APA StyleXiao, Y., Huang, R., Cao, S., Zhao, D., Mao, Z., Xiao, C., Xu, Z., Zhou, X., Zhang, X., Zhang, Y., Tang, J., Jin, J., Li, Y., Zou, J., & Liu, Z. (2022). Molecular Characterization and Dietary Regulation of Glutaminase 1 (gls1) in Triploid Crucian Carp (Carassius auratus). Fishes, 7(6), 377. https://doi.org/10.3390/fishes7060377