Different Animal Metabolism Markers for Artemia Nauplii in Crude Protein Digestibility Assay for Lophiosilurus alexandri Larvae
Abstract
1. Introduction
2. Materials and Methods
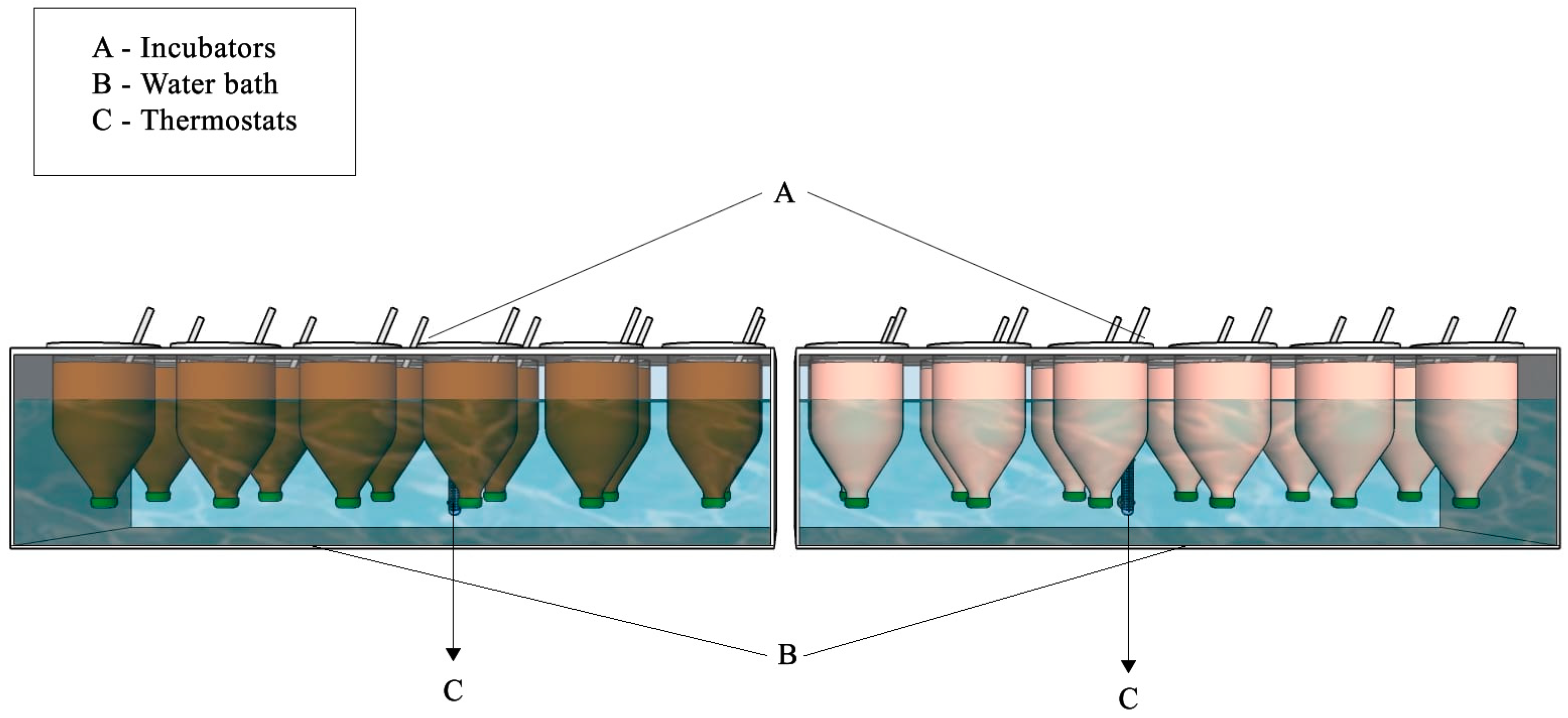
2.1. Phase I—Ingestion and Passage Time of Different Makers in Artemia
2.1.1. Obtainment of Colloidal Particles of the Markers Chromic Oxide and Titanium Dioxide
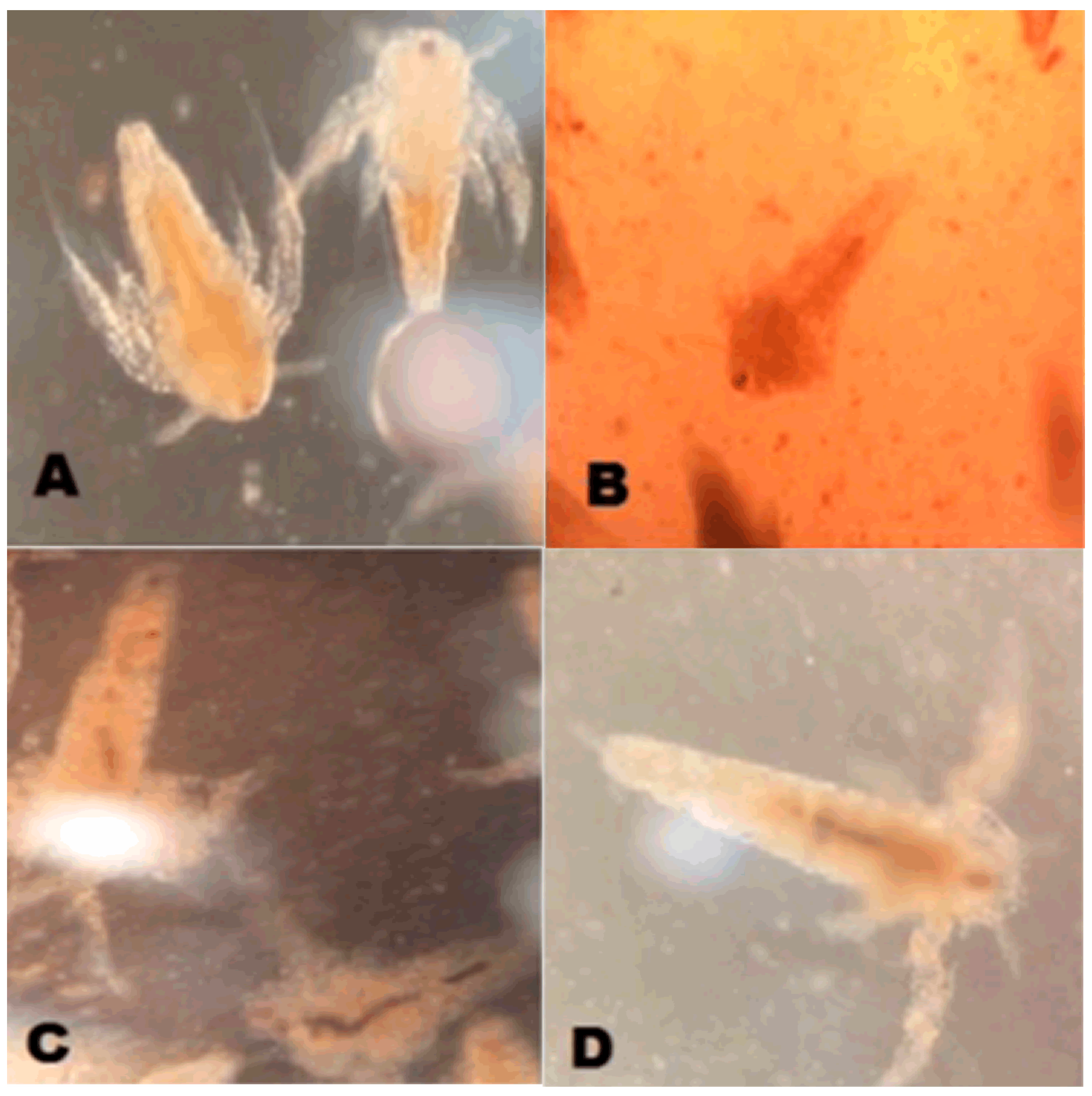
2.1.2. Marking Artemia
2.1.3. Chemical Analyses of Markers
2.1.4. Mathematical Model for Passage Time in Artemia: age-dependent
2.2. Phase II—Apparent Digestibility of Artemia Nauplii for Lophiosilurus alexandri Larvae of Different Ages
2.2.1. Animals and Conditions
2.2.2. Marking Artemia
2.2.3. Digestibility Test
2.2.4. Analyses
2.3. Data Analysis
3. Results
3.1. Phase I—Ingestion and Passage Time of Different Markers in Artemia
3.2. Phase II—Apparent Digestibility of Artemia nauplii for Lophiosilurus alexandri Larvae of Different Ages
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Santos, J.C.E.; Luz, R.K. Effect of Salinity and Prey Concentrations on Pseudoplatystoma corruscans, Prochilodus costatus and Lophiosilurus alexandri Larviculture. Aquaculture 2009, 287, 324–328. [Google Scholar] [CrossRef]
- Podrabsky, J.E.; Hand, S.C. Physiological Strategies during Animal Diapause: Lessons from Brine Shrimp and Annual Killifish. J. Exp. Biol. 2015, 218, 1897–1906. [Google Scholar] [CrossRef]
- Takeuchi, T. Progress on Larval and Juvenile Nutrition to Improve the Quality and Health of Seawater Fish: A Review. Fish. Sci. 2014, 80, 389–403. [Google Scholar] [CrossRef]
- Talens-perales, D.; Marín-Navarro, J.; Garrido, D.; Almansa, E.; Polaina, J. Fixation of Bioactive Compounds to the Cuticle of Artemia. Aquaculture 2017, 474, 95–100. [Google Scholar] [CrossRef]
- Kolkovski, S.; Curnow, J.; King, J. Intensive Rearing System for Fish Larvae Research II Artemia Hatching and Enriching System. Aquac. Eng. 2004, 31, 309–317. [Google Scholar] [CrossRef]
- Luz, R.K.; Zaniboni, E.F. Utilização de Diferentes Dietas Na Primeira Alimentação Do Mandi-Amarelo (Pimelodus maculatus, Lacépéde). Acta Sci. Biol. Sci. 2001, 23, 483–489. [Google Scholar]
- Reynalte-tataje, D.; Luz, R.K.; Meurer, S.; Zaniboni-filho, E.; Pires, A.; Nuñer, D.O. Influência Do Fotoperíodo No Crescimento e Sobrevivência de Pós—Larvas de Piracanjuba Brycon orbignyanus (Valenciennes, 1849). Acta Sci. Biol. Sci. 2002, 24, 439–443. [Google Scholar]
- Luz, R.K. Resistência Ao Estresse e Crescimento de Larvas de Peixes Neotropicais Alimentadas Com Diferentes Dietas. Pesqui. Agropecuária Bras. 2007, 42, 65–72. [Google Scholar] [CrossRef]
- Luz, R.K.; Dos Santos, J.C.E. Densidade de Estocagem e Salinidade Da Água Na Larvicultura Do Pacamã. Pesqui. Agropecuária Bras. 2008, 43, 903–909. [Google Scholar] [CrossRef]
- Jomori, R.K.; Luz, R.K.; Takata, R.; Perez Fabregat, T.E.H.; Portella, M.C. Água Levemente Salinizada Aumenta a Eficiência Da Larvicultura De Peixes Neotropicais. Pesqui. Agropecuária Bras. 2013, 48, 809–815. [Google Scholar] [CrossRef]
- Luz, R.K.; Portella, M.C. Effect of Prey Concentrations and Feed Training on Production of Hoplias lacerdae Juvenile. An. Da Acad. Bras. De Ciências 2015, 87, 1125–1132. [Google Scholar] [CrossRef]
- Santos, F.A.C.; Gustavo, S.; Julio, C.; Luz, R.K. Stocking Density in Colossoma macropomum Larviculture, a Freshwater Fish, in Recirculating Aquaculture System. Aquac. Res. 2020, 52, 1185–1191. [Google Scholar] [CrossRef]
- Santos, F.A.C.; Julio, G.S.C.; Batista, F.S.; Miranda, N.L.; Pedras, P.P.C.; Luz, R.K. High Stocking Densities in the Larviculture of Colossoma macropomum in a Recirculating Aquaculture System: Performance, Survival and Economic Viability. Aquaculture 2022, 552, 738016. [Google Scholar] [CrossRef]
- Léger, P.; Bengtson, D.A.; Simpson, K.L.; Sorgeloos, P. The use and nutritional value of Artemia as a food source. Oceanogr. Mar. Biol. Annu. Rev. 1986, 24, 521–623. [Google Scholar]
- Mesarič, T.; Gambardella, C.; Milivojević, T.; Faimali, M.; Drobne, D.; Falugi, C.; Sepčić, K. High Surface Adsorption Properties of Carbon-Based Nanomaterials Are Responsible for Mortality, Swimming Inhibition, and Biochemical Responses in Artemia Salina Larvae. Aquat. Toxicol. 2015, 163, 121–129. [Google Scholar] [CrossRef]
- Hamzah, M.; Shaik, M.I.; Sarbon, N.M. Effect of Fish Protein Hydrolysate on Physicochemical Properties and Oxidative Stability of Shortfin Scad (Decapterus macrosoma) Emulsion Sausage. Food Res. 2021, 5, 225–235. [Google Scholar] [CrossRef]
- Siddik, M.A.B.; Pham, H.D.; Francis, D.S.; Vo, B. Van Dietary Supplementation of Fish Protein Hydrolysate in High Plant Protein Diets Modulates Growth, Liver and Kidney Health, and Immunity of Barramundi (Lates calcarifer). Aquac. Nutr. 2021, 27, 86–98. [Google Scholar] [CrossRef]
- Miranda, A.; Altiery, D.S.; Daniela, S.; Bacconi, F.; Fernando, J.; Melo, B.; Thiago, A.; Luiz, S.; Oliveira, V. Corn Substitution by Mesquite Bean Flour (Prosopis juliflora) Maintains Growth and Improves Protein Metabolism of Nile Tilapia Juveniles (Oreochromis niloticus). Trop. Anim. Health Prod. 2021, 53, 1–15. [Google Scholar] [CrossRef]
- Mohseni, M.; Najjar, S.; Younes, L.; Sarameh, S.P. Effects of Different Dietary Canola and Fish Oil Levels on Overall Performance, Fatty Acid Profile, Haemato—Biochemical Responses, and Digestibility of Macronutrients of Caspian Brown Trout (Salmo trutta caspius Kessler) Fingerling. J. Appl. Ichthyol. 2022, 38, 212–222. [Google Scholar] [CrossRef]
- Zaretabar, A.; Ouraji, H.; Abedian, A.; Yeganeh, S.; Esmaeili, M.; Keramat, A. One Step toward Aquaculture Sustainability of a Carnivorous Species: Fish Meal Replacement with Barley Protein Concentrate plus Wheat Gluten Meal in Caspian Brown Trout (Salmo trutta caspius). Aquac. Rep. 2021, 20, 100714. [Google Scholar] [CrossRef]
- Takakuwa, F.; Sato, H.; Mineyama, N.; Yamada, S.; Biswas, A.; Tanaka, H. Bioavailability of Porcine Blood Meal as a Fish Meal Substitute in the Diet for Red Sea Bream (Pagrus major, Temminck & Schlegel) Fingerling. Aquac. Res. 2022, 53, 4616–4626. [Google Scholar] [CrossRef]
- Abedi, S.Z. The Influence of Probiotic (Isolated Based on Phytase Activity) on Growth Performance, Body Composition, and Digestibility of Rainbow Trout, Oncorhynchus mykiss. J. World Aquac. Soc. 2022, 53, 1006–1030. [Google Scholar] [CrossRef]
- Marcos, A.; Ramos, J.; Machado, D.; Eduardo, L.; De Freitas, L.; Rodrigues, V.; Paula, A.; Rodrigues, O. Optimizing Methodological Aspects of Stool Collection by Sedimentation in Digestibility Studies with Pirarucu (Arapaima gigas). Aquac. Res. 2022, 53, 1456–1467. [Google Scholar] [CrossRef]
- Hamilton, L.; Yadav, R.; Kumar, N.; Ved, C.; Saini, P.; Banerjee, P.; Manohar, S.; Ojha, L.; Jayant, M.; Raj, N. Substitution of De-Oiled Rice Bran with Khejri Pod Meal and Groundnut Oilcake with Khejri Seed Meal in the Diet Of. Aquac. Res. 2022, 53, 642–656. [Google Scholar] [CrossRef]
- Major, P.; Shadrack, R.S.; Manabu, I.; Koshio, S.; Yokoyama, S.; Zhang, Y.; Mzengereza, K.; Fouad, M.; Basuini, E.; Dawood, M.A.O. Effects of Single and Mixture Probiotic Supplements on Growth, Digestive Activity, Antioxidative Status, Immune and Growth-Related Genes, and Stress Response of Juvenile Red Sea Bream. Aquac. Nutr. 2022, 2022, 1–17. [Google Scholar]
- Mzengereza, K.; Ishikawa, M.; Koshio, S.; Yokoyama, S.; Yukun, Z.; Shadrack, R.S.; Seo, S.; Kotani, T.; Dossou, S.; El Basuini, M.F.; et al. Growth Performance, Growth-Related Genes, Digestibility, Digestive Enzyme Activity, Immune and Stress Responses of de novo Camelina Meal in Diets of Red Seabream (Pagrus major). Animals 2021, 11, 3118. [Google Scholar] [CrossRef]
- Bowen, S.H. Chromic Acid in Assimilation Studies—A Caution. Trans. Am. Fish. Soc. 1978, 107, 755–756. [Google Scholar] [CrossRef]
- Vandenberg, G.W.; Nou, J.D.E.L.A. Apparent Digestibility Comparison in Rainbow Trout (Oncorhynchus mykiss) Assessed Using Three Methods of Faeces Collection and Three Digestibility Markers. Aquac. Nutr. 2021, 7, 237–245. [Google Scholar] [CrossRef]
- Dumas, V.; Labbé, L.; Pelissier, P. Rainbow trout (Oncorhynchus mykiss) freshwater recirculating aquaculture system: Nutrient flow management with aquaponics. In Proceedings of the World Aquaculture 2018, #We R Aquaculture, Montpellier, France, 25–29 August 2018; 848p. [Google Scholar]
- Mayumeoshiro, F.; Fraga, T.; Honorato, C. Tempo de trânsito gastrointestinal do pintado (Pseudoplatystoma sp.). J. Agron. Sci. 2012, 1, 128–138. [Google Scholar]
- Langeland, M.; Vidakovic, A.; Vielma, J.; Lindberg, J.E.; Kiessling, A.; Lundh, T. Digestibility of microbial and mussel meal for Arctic charr (Salvelinus alpinus) and Eurasian perch (Perca fluviatilis). Aquac. Nutr. 2016, 22, 485–495. [Google Scholar] [CrossRef]
- Heinitz, M.C.; Lemme, A.; Schulz, C. Measurement of Digestibility in Agastric Fish Based on Stripping Method—Apparent Nutrient, Energy and Amino Acid Digestibilities of Common Feed Ingredients for Carp Diets (Cyprinus carpio). Aquac. Nutr. 2016, 22, 1065–1078. [Google Scholar] [CrossRef]
- Myers, W.D.; Ludden, P.A.; V Nayigihugu, V.; Hess, B.W. Technical Note: A procedure for the preparation and quantitative analysis of samples for titanium dioxide. J. Anim. Sci. 2004, 82, 179–183. [Google Scholar] [CrossRef] [PubMed]
- Evans, O.; Jones, E.; Teixeira, A.E. Validation of the external marker Nanolipe as an indicator of apparent nutrient and energy digestible in juvenile Nile Tilápia (Oreochromos niloticus). J. Anim. Sci. 2012, 90, 558–559. [Google Scholar]
- Rosenfeld, I.; Austbø, D.; Volden, H. Models for Estimating Digesta Passage Kinetics in the Gastrointestinal Tract of the Horse. J. Anim. Sci. 2006, 84, 3321–3328. [Google Scholar] [CrossRef]
- Sciences, A.; Stensig, T.; Martin, R. Evaluation of Different Methods for the Determination of Digestion and Passage Rates of Fibre in the Rumen of Dairy Cows. Acta Agric. Scand. Part A 1998, 48, 141–154. [Google Scholar]
- Firkins, J.L. Effects of Feeding Nonforage Fiber Sources on Site of Fiber Digestion 1. J. Dairy Sci. 1997, 80, 1426–1437. [Google Scholar] [CrossRef]
- Lascano, C.; Quiroz, R. Metodologia para estimar la dinámica de la digestión en rumiantes. In Nutrición de Rumiantes: Guía Metodológica de Investigación; Ruiz, M.E., Ruiz, A., Eds.; IICA: San Jose, Costa Rica, 1990; pp. 89–104. [Google Scholar]
- Luz, R.K.; dos Santos, J.C.E. Evaluation of the Tolerance of “Pacamã” Lophiosilurus alexandri Steindachner, 1877 (Pisces: Siluriformes) Larvae to Different Salinities. Acta Scientiarum. Biol. Sci. 2008, 30, 345–350. [Google Scholar] [CrossRef]
- Cordeiro, B.N.I.S.; Costa, D.C.; Silva, W.D.S.; Takata, R.; Luz, R.K. High Stocking Density during Larviculture and Effect of Size and Diet on Production of Juvenile Lophiosilurus alexandri Steindachner, 1876 (Siluriformes: Pseudopimelodidae). J. Appl. Ichthyol. 2016, 32, 61–66. [Google Scholar] [CrossRef]
- Santos, J.C.E.; Correia, E.; Luz, R.K. Effect of Daily Artemia Nauplii Concentrations during Juvenile Production of Lophiosilurus alexandri. Bol. do Inst. de Pesca 2015, 41, 771–776. [Google Scholar] [CrossRef]
- Takata, R.; Silva, W.; Costa, D.C.; Filho, R.M.; Luz, R.K. Effect of Water Temperature and Prey Concentrations on Initial Development of Lophiosilurus alexandri Steindachner, 1876 (Siluriformes: Pseudopimelodidae), a Freshwater Fish. Neotrop. Ichthyol. 2014, 12, 853–860. [Google Scholar] [CrossRef]
- Claudio, J.; Dos, E.; Pedreira, M.M.; Luz, R.K. Feeding Frequency in pacamã larviculture. Revista Caatinga 2016, 2125, 512–518. [Google Scholar]
- Luz, R.K.; Santos, J.C.E.; Pedreira, M.M.; Teixeira, E.A. Effect of Water Flow Rate and Feed Training on “Pacamã” (Siluriforme: Pseudopimelodidae) Juvenile Production. Arq. Bras. De Med. Vet. E Zootec. 2011, 63, 973–979. [Google Scholar] [CrossRef]
- Melillo Filho, R.; Takata, R.; Santos, E.H.; De Souza, W.; Luz, R.K.; Ana, L. Draining System and Feeding Rate during the Initial Development of Lophiosilurus alexandri (Steindachner, 1877), a Carnivorous Freshwater Fish. Aquac. Res. 2014, 45, 1913–1920. [Google Scholar] [CrossRef]
- Pedreira, M.M.; Luz, R.K.; Cláudio, J. Biofiltração Da Água e Tipos de Substrato Na Larvicultura Do Pacamã. Pesqui. Agropecuária Bras. 2009, 44, 511–518. [Google Scholar] [CrossRef]
- Torres, I.F.A.; Júlio, G.S.C.; Figueiredo, L.G.; De Lima, N.L.C.; Soares, A.P.N.; Luz, R.K. Larviculture of a Carnivorous Freshwater Catfish, Lophiosilurus alexandri, Screened by Personality Type. Behav. Process. 2017, 145, 44–47. [Google Scholar] [CrossRef]
- Cook, M.A.; Johnson, R.B.; Nicklason, P.; Barnett, H.; Rust, M.B. Marking Live Feeds with Inert Metal Oxides for Fish Larvae Feeding and Nutrition Studies. Aquac. Res. 2008, 39, 347–353. [Google Scholar] [CrossRef]
- Silva, J.D.; Queiroz, A.C. Análise de Alimentos—Métodos Químicos e Biológicos, 3rd ed.; UFV: Viçosa, Brazil, 2009; p. 235. [Google Scholar]
- Detmann, E.; Souza, M.A.; Valadares Filho, S.C.; Rocha, G.C.; Palma, M.N.N.; Rodrigues, J.P.P. Métodos Para análise de Alimentos; Suprema: Visconde do Rio Branco, MG, Brazil, 2012; p. 214. [Google Scholar]
- Saliba, E.; Moreira, G.R.; Federal, U.; De Pernambuco, R.; Borges, I.; Costa, A.L. Use of Infrared Spectroscopy to Estimate Fecal Output with Marker Lipe®. Int. J. Food Sci. Nutr. Diet. 2015, 4, 1–10. [Google Scholar] [CrossRef]
- Ellis, W.C.; Matis, J.H.; Hill, T.M.; Murphy, M.R. Methodology for estimation digestion and passage kinetics of forages. In Forage Quality, Evaluation, and Utilization; Fahey, G.C., Jr., Collins, M., Mertens, D.R., Moser, L.E., Eds.; American Society of Agronomy: Madison, WI, USA, 1994; pp. 682–756. [Google Scholar]
- Quiroz, R.A.; Pond, K.R.; Tolley, E.A.; Johnson, W.L. Selection among nonlinear models for rate of passage studies in ruminants. J. Anim. Sci. 1988, 66, 2977–2986. [Google Scholar] [CrossRef]
- Pond, K.R.; Ellis, W.; Matis, C. Compartment models for estimating attributes of digesta flow in cattle. Br. J. Nutr. 1988, 60, 571–595. [Google Scholar] [CrossRef]
- Faichney, G.J. The use of markers to partition digestion within the gastrointestinal tract. In Digestion and Metabolism in the Ruminant; Macdonald, I.W., Warner, A.A.I., Eds.; University of New England Publishing Unit: Armidale, NSW, Australia, 1975; pp. 277–291. [Google Scholar]
- Beauchemin, K.A.; Buchanan-Smith, J.G. Evaluation of markers, sampling sites and models for estimating rates of passage of silage or hay in dairy cows. Anim. Feed Sci. Technol. 1989, 27, 59–75. [Google Scholar] [CrossRef]
- Uden, P. The influence of leaf and stem particle size in vitro and sample size in sacco on neutral detergent fiber fermentation kinetics. Anim. Feed Sci. Technol. 1992, 37, 85. [Google Scholar] [CrossRef]
- Lavens, P.; Sorgeloos, P.; FAO. Manual on the Production and Use of Live Food for Aquaculture; Fisheries Technical Paper; FAO: Rome, Italy, 1996; p. 295. [Google Scholar]
- Mattioli, C.C.; Takata, R.; De Oliveira, F.; Leme, P.; Cristina, D.; Melillo, R.; de Souza, W.; Kennedy, R. The Effects of Acute and Chronic Exposure to Water Salinity on Juveniles of the Carnivorous Freshwater Catfish Lophiosilurus alexandri. Aquaculture 2017, 481, 255–266. [Google Scholar] [CrossRef]
- Burns, J.C.; Pond, K.R.; FIisher, D.S. Measurement of forage intake. In Forage Quality, Evoluatin, and Utilization; Fahey, J.R., Ed.; American Society of Agronomy: Madison, WI, USA, 1994; pp. 494–532. [Google Scholar]
- Di Rienzo, J.A.; Casanoves, F.; Balzarini, M.G.; Gonzalez, L.A.; Tablada, M.; Robledo, C.W. InfoStat Version. Grupo InfoStat, FCA, Universidad Nacional de Cordoba, Argentina. 2015. Available online: http://www.infostat.com.ar (accessed on 1 December 2022).
- Teshima, T.; Ishikawa, M.; Koshio, S. Nutritional assessment and feed intake of microparticulate diets in crustaceans and fish. Aquat. Res. 2000, 31, 691–702. [Google Scholar]
- Arndt, C.; Sommer, U.; Ueberschär, B. A comparative in-vitro-test on the digestibility of live prey for fish larvae under specific consideration of trypsin. Aquaculture 2015, 446, 12–16. [Google Scholar] [CrossRef]
- Hansen, J.M.; Lazo, J.P.; Kling, L.J. A method to determine protein digestibility of microdiets for larval and early juvenile fish. Aquac. Nutr. 2009, 15, 615–626. [Google Scholar] [CrossRef]
- Gamboa-delgado, J.; Cañavate, J.P.; Zerolo, R.; Le Vay, L. Natural carbon stable isotope ratios as indicators of the relative contribution of live and inert diets to growth in larval Senegalese sole (Solea senegalensis). Aquaculture 2008, 280, 190–197. [Google Scholar] [CrossRef]
- Gonçalves, N.C. Validação Do Nanolipe Como Indicador Para Estimativa Do Consumo Em Bovinos Leiteiros; UFMG: Belo Horizonte, MG, Brazil, 2012; p. 40. [Google Scholar]
- Hafez, S.; Junge, W.; Kalm, E. Schatzung der verdaulichkeit mit einer indikatormethode bei milchku¨hen im vergleich zum hohenheimer-futterwert-test. Arch. Für Tierernaehrung 1988, 38, 929–945. [Google Scholar] [CrossRef]
- Titgemeyer, E.C.; Armendariz, C.K.; Bindel, D.J.; Greenwood, R.H.; Löest, C.A. Evaluation of titanium dioxide as a digestibility marker for cattle. J. Anim. Sci. 2001, 79, 1059–1063. [Google Scholar] [CrossRef]
- Rodriguez, N.M.; Saliba, E.O.S.; Guimarães-Junior, R. Uso de indicadores para estimar consumo y digestibilidad de pasto. LIPE, lignina purificada y enriquecida. Rev. Colomb. De Cienc. Pecu. 2007, 20, 518–525. [Google Scholar]
- Coutteau, P.; Segner, H.; Huisman, E.A.; Sorgeloos, P. Biochemical and Enzymatic Characterization of Decapsulated Cysts and Nauplii of the Brine Shrimp Artemia at Different Developmental Stages. Aquaculture 1998, 161, 501–514. [Google Scholar]
- Conceicão, L.E.C.; Teresa, M.; Rønnestad, I. A Method for Radiolabeling Artemia with Applications in Studies of Food Intake, Digestibility, Protein and Amino Acid Metabolism in Larval Fish. Aquaculture 2004, 231, 469–487. [Google Scholar] [CrossRef]
- Engrola, S.; Dinis, M.T.; Conceição, L.E.C. Senegalese sole larvae growth and protein utilization is depressed when co-fed high levels of inert diet and Artemia since first feeding. Aquac. Nutr. 2010, 16, 457–465. [Google Scholar] [CrossRef]
- Johnson, R.B.; Cook, A.M.; Nicklason, P.M.; Rust, M.B. Determination of apparent protein digestibility of live Artemia and a microparticulate diet in 8- week-old Atlantic cod Gadus morhua larvae. Aquaculture 2009, 288, 290–298. [Google Scholar] [CrossRef]
- Melo, K.D.M.; Oliveira, G.R.; Brito, T.S.; Soares, D.R.P.; Tessitore, A.J.D.A.; Alvarenga, É.R.D.; Teixeira, E.D.A. Digestibilidade de ingredientes em dietas para juvenis de pacamã (Lophiosilurus alexandri). Pesqui. Agropecuária Bras. 2016, 51, 785–788. [Google Scholar] [CrossRef]
- Owens, F.N.; Hanson, C.F. External and internal markers for appraising site and extent of digestion in ruminants. J. Dairy Sci. 1992, 75, 2605–2617. [Google Scholar] [CrossRef]
| Composition | Value |
|---|---|
| Total dry matter (%) | 57.65 |
| Dry matter 105 °C (%) | 82.98 |
| Crude protein (%) | 16.37 |
| Ethereal extract (%) | 2.42 |
| Crude fiber (%) | 6.67 |
| Calcium (%) | 8.90 |
| Phosphor (%) | 2.15 |
| Gross energy (cal/g) | 4615.15 |
| ANOVA | F (Value) |
|---|---|
| Marker (M) | <0.0001 * |
| Duration (D) | 0.0286 * |
| M × D | 0.4066 ns |
| Factors | Concentration (ppm) |
| Marker | |
| T2 | 9.53 ± 0.74 c |
| T3 | 67.79 ± 6.59 a |
| T4 | 25.08 ± 0.62 b |
| Duration (minutes) | |
| 30 | 29.82 ± 6.01 b |
| 60 | 33.74 ± 6.86 ab |
| 90 | 38.85 ± 8.75 a |
| Time (min) | ||||
|---|---|---|---|---|
| Marker | 0 | 10 | 20 | 30 |
| T2 | 16.7 c | 10.6 c | 5.34 c | 0 |
| T3 | 75.4 a | 42.03 a | 20.75 a | 0.06 |
| T4 | 43.8 b | 24.6 b | 12.05 b | 0 |
| Standard error | 0.8 | 0.8 | 0.8 | 0.8 |
| p-value | <0.05 | <0.05 | <0.05 | 0.9983 |
| MRT | Tx %/min | TT | |
|---|---|---|---|
| T2 | 6.5 | 15.34 | 19.5 |
| T3 | 6.1 | 16.53 | 15.5 |
| T4 | 6.1 | 16.53 | 15.5 |
| Treatments | |||||
|---|---|---|---|---|---|
| T1 | T2 | T3 | T4 | p-Value | |
| L. alexandri larvae after 3 days of exogenous feeding | |||||
| Digestibility % * | 94.38 ± 0.94 ab | 93.53 ± 1.09 b | SR | 95.50 ± 0.97 a | 0.0152 |
| Ti (min.) ** | 38.83 ± 1.32 b | 40.17 ± 2.71 b | 56.83 ± 3.76 a | 40.17 ± 1.72 b | 0.0015 |
| Tf (min.) ** | 29.83 ± 0.75 | 30.33 ± 0.51 | 29.17 ± 1.47 | 30.6 7 ± 0.81 | 0.1229 |
| L. alexandri larvae after 15 days of exogenous feeding | |||||
| Digestibility % * | 96.65 ± 1.00 a | 96.53 ± 0.90 a | 94.75 ± 1.34 b | 96.62 ± 0.81 a | 0.0245 |
| Ti (min.) ** | 39.50 ± 1.04 b | 40.00 ± 0.89 b | 59.83 ± 2.04 a | 40.50 ± 0.54 b | 0.0015 |
| Tf (min.) ** | 29.83 ± 1.16 | 31.17 ± 0.75 | 30.17 ± 1.60 | 30.50 ± 0.83 | 0.2341 |
| L. alexandri larvae after 25 days of exogenous feeding | |||||
| Digestibility % * | 97.22 ± 0.44 a | 96.27 ± 0.61 bc | 95.43 ± 0.68 c | 96.62 ± 0.52 ab | 0.0391 |
| Ti (min.) ** | 40.16 ± 1.47 b | 40.17 ± 1.32 b | 59.83 ± 0.75 a | 40.50 ± 1.22 b | 0.0034 |
| Tf (min.) ** | 29.67 ± 0.81 | 30.50 ± 0.83 | 30.33 ± 0.81 | 29.50 ± 1.04 | 0.1724 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Saliba, J.S.; Santos, F.A.C.d.; Saliba, E.d.O.S.; Luz, R.K. Different Animal Metabolism Markers for Artemia Nauplii in Crude Protein Digestibility Assay for Lophiosilurus alexandri Larvae. Fishes 2023, 8, 110. https://doi.org/10.3390/fishes8020110
Saliba JS, Santos FACd, Saliba EdOS, Luz RK. Different Animal Metabolism Markers for Artemia Nauplii in Crude Protein Digestibility Assay for Lophiosilurus alexandri Larvae. Fishes. 2023; 8(2):110. https://doi.org/10.3390/fishes8020110
Chicago/Turabian StyleSaliba, Jaqueline Simões, Fabio Aremil Costa dos Santos, Eloísa de Oliveira Simões Saliba, and Ronald Kennedy Luz. 2023. "Different Animal Metabolism Markers for Artemia Nauplii in Crude Protein Digestibility Assay for Lophiosilurus alexandri Larvae" Fishes 8, no. 2: 110. https://doi.org/10.3390/fishes8020110
APA StyleSaliba, J. S., Santos, F. A. C. d., Saliba, E. d. O. S., & Luz, R. K. (2023). Different Animal Metabolism Markers for Artemia Nauplii in Crude Protein Digestibility Assay for Lophiosilurus alexandri Larvae. Fishes, 8(2), 110. https://doi.org/10.3390/fishes8020110









