Abstract
Alterations to the functions and connectivity of mangrove–seagrass–patch reef (MSP) seascapes have the potential to impact the survival, foraging activities, and movement of reef-dependent invertebrates (e.g., crabs and shrimp) and fishes. In the current study, we examined carbon flow in the Guánica Biosphere Reserve in southwestern Puerto Rico using pigment analysis of particulate organic matter and stable isotope analysis of carbon (δ13C) and nitrogen (δ15N) in flora and fauna. Several lines of evidence pointed to N2 fixers (cyanobacteria) being important for fueling primary productivity in this oligotrophic ecosystem including low (<0.7 µg L−1) chlorophyll, prevalence of cyanobacteria based on pigment signatures, and the isotope signatures of seagrass and red mangrove leaf tissue (enriched δ15N values) and consumers (depleted δ15N values). Food web mixing models based on stable isotopes (δ13C and δ15N) revealed that multiple producers (phytoplankton, benthic microalgae, seagrasses, etc.) contributed organic matter to the consumers (zooplankton, invertebrates, and fishes) in the MSP seascape at the center of the reserve. Contribution estimates for common benthic invertebrates (crabs and shrimp) were taxon-specific, and the highest input was generally linked to particulate organic matter (POM) and benthic microalgae (BMA)/seagrass producer categories, although meaningful mangrove contribution was observed for some taxa. Similarly, contribution estimates for fishes were highest for POM and BMA/seagrass, with the latter producer category being more important for species known to migrate from mangroves or patch reefs to seagrass beds at night (bluestriped grunt, French grunt, and white grunt). Although all fish investigated were observed in mangrove prop-root habitats, input of organic matter from mangroves to these consumers was typically limited for most of the species examined. Understanding these complex seascapes contributes to our understanding of the ecology of these vital ecosystems.
1. Introduction
Coral reefs and adjacent back-reef habitats vary in their health, assemblage composition, and geomorphology. These shallow-water seascapes are increasingly threatened by coastal development, particularly those in which humans have altered adjacent landscapes [1,2,3,4,5,6,7,8]. Globally, mangroves found in back-reef seascapes are declining rapidly as these areas are cleared for development, aquaculture, and logged for timber and/or fuel production [9]. Habitat destruction and exploitation have led to declines in mangroves and seagrasses, which alter seascape functions and connectivity, and in turn have a negative impact on fisheries [3,10]. While mangroves do not appear to be an important food source for reef-dependent invertebrates and fishes, mangrove–seagrass–patch reef (MSP) seascapes serve as the principal nursery for these diverse assemblages, providing shelter for the survival of newly settled invertebrates and fishes [4,11,12,13,14,15,16].
Most coral reefs are nearshore in the tropics and thus corals are typically associated with both seagrass and mangrove habitats even if the coral reef is not in a back-reef system [10,17,18]. Given that many marine fishes require or utilize these habitats during early life stages, the alteration, decline, and/or loss of any component of a seascape can profoundly influence foraging success, predation risk, and early life survival [18,19,20]. An improved understanding of trophic connectivity within tropical seascapes is therefore critical not only for effective marine spatial management but also efforts to evaluate ecosystem services provided by coral reef habitats [8,21].
Phytoplankton are used as indicators of ecological functioning in coastal waters and carbon flow through MSP reef seascapes [7,22] with species composition, biomass, and abundance thought to reflect water quality [23]. Photosynthetic pigments of phytoplankton (i.e., chlorophyll (chl) a, chl b, chl c, lutein, zeaxanthin, fucoxanthin, peridinin, and others) can be used as biomarkers for taxonomic groups [24,25,26,27]. Information on phytoplankton biomass and community composition can be used to determine important drivers of ecosystem function. In addition, it is known that N2 fixation is an important source of new nitrogen in oligotrophic waters [28,29]. Similarly, the composition and distribution of seagrasses provides details of the water column light environment [30] while the density of macroalgae often reflects perturbations due to excessive nutrients [1].
In order to identify important relationships between primary producers and consumers, dietary tracers (stable isotopes) are a widely applied tool in MSP seascapes [8,12,18,31]. In comparison to other methods, this approach is more cost effective for studying habitat relationships than electronic tagging studies, and the data generate a more holistic dietary picture than stomach content analysis alone. The ratio of carbon isotopes in animals (13C/12C, δ13C) can be used to distinguish among broad taxonomic groups of primary producers and then, evaluate the ultimate food source of consumers as changes in δ13C are mirrored as carbon moves through food webs [32,33,34,35,36]. In contrast, the ratio of stable isotopes of nitrogen (15N/14N, δ15N) is influenced by trophic transfer dynamics, consumer physiology, and changes in the nitrogen isotope ratios of primary producers at the base of the food web.
The objective of this study was to identify key sources of organic matter supporting consumers in an MSP seascape located at the center of the Guánica Biosphere Reserve in southwestern Puerto Rico using stable isotopes of carbon (δ13C) and nitrogen (δ15N) (Figure 1). This was accomplished by sampling the primary sources of organic matter supporting higher trophic levels and a suite of consumers common to MSP seascapes in the Caribbean. This included water column (phytoplankton) and benthic primary producers (benthic microalgae, seagrasses, macroalgae, and mangroves) as well as a wide range of consumers from zooplankton to fishes. We used (1) pigment biomarkers to examine the phytoplankton community (biomass and distribution) and (2) Mix Stable Isotope (δ13C, δ15N) Analysis in R (MixSIAR) to identify the primary source(s) of organic matter supporting numerically dominant consumers in this MSP seascape. Given that nursery habitats in the tropics support highly diverse communities, often mixed species assemblages where several fish taxa school together or overlap in space and time (e.g., [18]), a better understanding of how resources are partitioned and how back-reef food webs function to support these diverse assemblages is needed.
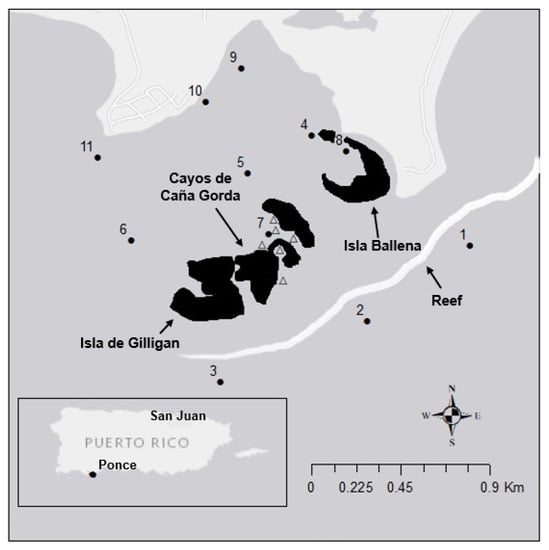
Figure 1.
Map of study stations in the Guánica Biosphere Reserve located off the southwestern coast of Puerto Rico. POM and zooplankton were collected from the top portion of the water column (circles) at all 11 stations in the reserve. The MSP reef seascape (station 7) was sampled extensively for both primary producers and consumers. In addition, BMA (triangles) were collected from six stations within the MSP.
2. Materials and Methods
2.1. Study Area
The study was conducted in the Guánica Biosphere Reserve (17°56′ N, 66°52′ W) (Figure 1). This UNESCO recognized site has minimal coastal development, and the majority of land area consists of natural vegetation (https://en.unesco.org/ (accessed on 01 December 2021)). The MSP seascape is located in and around station 7 adjacent to the two small mangrove keys (Gilligan’s Island and Isla Ballena) within the Cayos de Caña Gorda that are vegetated with red mangrove (Rhizophora mangle), seagrass, macroalgae, and patch reefs, typical of MSP seascapes in the Caribbean. The mangrove keys were separated by a deeper channel with a sand bottom and mixed stands of turtle grass (Thalassia testudinum), manatee grass (Syringodium filiforme) and shoal grass (Halodule wrightii). Large stands of turtle grass were also present on the leeward side of each mangrove key in the northwestern region of the seascape. The southeastern region of the seascape contained several small patch reefs, comprised primarily of mustard hill coral (Porites astreoides), hump coral or finger coral (P. porites), shallow-water starlet coral (Siderastrea radians) and the round starlet coral (S. sidereal). In addition, a variety of macroalgae (Table S1) were present throughout the MSP seascape; given they represent a small fraction of the total primary producer biomass they were not included in the MixSIAR. Across the study area, salinities (36–38 psu) and temperatures (26 ± 2 °C) were similar during the sampling campaign (data not shown).
2.2. Sample Collection
Particulate organic matter (POM) associated with primary producers and zooplankton in were collected from surface waters (top 1 m) at 11 stations (~depths 1–3 m, Figure 1) in the reserve to account for the spatial heterogeneity associated with these populations. All samples were collected in April and May 2014 in triplicate 4 L acid washed cubitainers for high-performance liquid chromatography (HPLC) pigment biomarkers (including chl a) and stable isotope analysis of POM. Samples were filtered (<130 kPa) onto separate precombusted 0.7 μm Whatman GF/F filters and frozen at −80 °C and −20 ˚C, respectively. POM pigment signatures may also contain pigment signatures from nearby plants (see more below). Samples were prepared and run as described in Pinckney et al. [26], with specific details in [37]. Briefly, filters for HPLC analysis were freeze-dried for 24 h and then the pigments were extracted in a −20 °C cryo-cooler using 100% acetone. To the pigment extract, 200 µL of carotenal was added as an internal standard. Blank samples were prepared concurrently with acetone and internal standard. Pigment data were processed using CHEMTAX V1.95 to determine the contributions of different phytoplankton classes to the total community [38]. The phytoplankton targeted in the pigment analysis included diatoms, dinoflagellates, cyanobacteria, chlorophytes, cryptophytes, and haptophytes, using the Schlüter matrix [27].
Zooplankton (chaetognaths, copepods, and other crustaceans) were collected in the mornings at the 11 stations by towing a 333 μm mesh plankton net multiple times to collect replicates, and then isolated using forceps, and placed onto filters which were rinsed with filtered (0.2 μm) seawater three times, and frozen [28]. As with the phytoplankton, the 11 stations were chosen to account for the spatial heterogeneity associated with zooplankton populations in the reserve.
Benthic microalgae (BMA), invertebrates and fishes were only collected in the MSP seascape (station 7). The vertical migration technique of Wells et al. [39] was used to separate the BMA collected at the six sites (Figure 1) with a grab sampler from the surrounding sediment material before they were filtered and frozen (−20 °C). Plant material from macrophytes (seagrass, macroalgae, and mangrove) was collected by hand and stored in Ziploc bags in a cooler until returning to the laboratory. This material was then scraped to remove sediments, encrusting organisms and epiphytic algae, and then rinsed extensively with filtered seawater and frozen (−20 °C). All epiphytes were taken from the red mangrove prop roots and seagrasses in the MSP seascape (Figure 1). Red mangrove epiphyte biomass was scraped by hand and the biomass filtered onto precombusted Whatman GF/F filters and frozen at −20 °C. To remove epiphytes from live seagrasses, leaves were gently scraped with a razor blade and the biomass transferred into a scintillation vial. This slurry was mixed and then filtered as described above. Although we initially planned to examine epiphytes from macroalgal thalli, the complex blade structures did not allow complete epiphyte removal, therefore, these associated epiphytes were ultimately included with the tissue samples.
Consumers (Table 1 and Table S1) were collected from the MSP seascape (Figure 1) using a variety of gear types. Gears included a 30 m length x 1 m height seine net, pole spears, and manually collecting benthic invertebrates by hand. Muscle tissue from consumers was dissected and frozen at −20 °C as described in [18]. Based on size and other characteristics (color and markings), all fishes selected for MixSIAR were assumed to be juveniles or sub-adults.

Table 1.
Stable isotope values (means ± standard deviations) and C:N (mol:mol) ratios measured in the flora and fauna in the Guánica Biosphere Reserve. POM and zooplankton were collected from all around the reserve while all other samples including the BMA were taken from only within the MSP reef.
2.3. Stable Isotope Analysis
Samples were thawed and then dried at 60 °C for 24–48 h prior to stable isotope analyses. Dried filters for POM, BMA and zooplankton were packed into tin capsules. Tissue from invertebrates and fishes was dried and then powdered using a mortar and pestle; 0.5–1.5 mg was packaged in tin capsules. All samples were analyzed for stable isotopes of carbon (δ13C) and nitrogen (δ15N) at the Stable Isotope Facility at the University of California at Davis, CA USA. Results of the stable isotope ratios are reported relative to the Vienna Pee Dee Belemnite standard for carbon and atmospheric N2 for nitrogen. Stable isotope values are expressed using the standard δ notation, as part per thousand (‰) deviation from a standard. Carbon and nitrogen contents of each sample were determined separately using a CHN analyzer (Fisons NA1500). All samples were combusted at 500 °C for 3 h to remove organic carbon and reanalyzed. Analytical error for C was 0.2‰ and N is 0.3‰.
Given the observed effects of HCl treatment on δ13C in marine primary producers [40,41,42], we elected not to acid-wash our filtered POM and BMA samples to avoid introducing unnecessary (and unpredictable) error to our values. As with other dual-stable-isotope food-web studies where correction for lipids was considered unlikely to result in ecologically meaningful adjustments to δ13C (e.g., [4]), we did not perform a lipid extraction or a mathematical adjustment on samples used in food web models.
2.4. Data Analysis
All means are presented with standard deviations. While POM, pigment samples and zooplankton were collected from all 11 stations in the reserve, all other flora and fauna examined were only collected from station 7, the location of the MSP seascape. One-way analysis of variance (ANOVA) was performed to test for the differences in pigment concentration among stations. ANOVAs were used to test for differences between δ13C and δ15N among all the different producers and consumers separately. A posteriori differences among means were analyzed with Tukey’s honestly significant difference (HSD) test. The potential carbon contribution of several primary producers to invertebrate and fish consumers was estimated with Bayesian mixing models using MixSIAR [43,44]. Only primary producers, invertebrates, and fishes with an n ≥ 2 were included in the MixSIAR models (Table 1). Primary producers included POM (proxy for water column/pelagic production), BMA/seagrass (proxy for benthic production), and red mangrove (representative mangrove). A total of 13 consumers were used in the MixSIAR including four benthic invertebrates and nine fish species from the MSP seascape (station 7): snapping shrimp (Synalpheus sp.) (n = 7), arrow crab (Stenorhynchus sp.) (n = 3), rubble crab (Paractaea sp.) (n = 4), and clinging crab (Mithraculus sp.) (n = 6), white grunt (Haemulon plumierii) (n = 8), schoolmaster (Lutjanus apodus) (n = 7), great barracuda (Sphyraena barracuda) (n = 4), squirrelfish (Holocentrus adscensionis) (n = 3), red lionfish (Pterois volitans) (n = 4), lane snapper (Lutjanus synagris) (n = 3), graysby (Cephalopholis cruentata) (n = 2), French grunt (Haemulon flavolineatum) (n = 17), and bluestriped grunt (Haemulon sciurus) (n = 9). As categorized in [18], BMA and seagrass δ13C and δ15N values were combined into a single producer category (BMA/Seagrass). For the mixing model, a carbon trophic enrichment factor of +1.0‰ (standard deviation; SD ± 0.3‰) was used [45,46]. δ15N enrichment values range from +2.5 to +3.5‰ (SD ± 0.6‰) in aquatic systems [47,48], therefore a nitrogen enrichment value of 3.0‰ was used per trophic position [46,49]. Model inputs for MixSIAR included no concentration dependence, 500,000 iterations, and 50,000 burnins or number of initial iterations to discard. Gelman–Rubin diagnostics were used to verify model convergence [50].
3. Results
3.1. Primary Producers
In order to understand the community composition of the dominant primary producers in the water column as well as their spatial heterogeneity, pigment biomarkers from 10 additional stations around the MSP seascape were assessed. Chl a, a proxy for phytoplankton biomass, was low at all stations except 8 (Figure 2a). The values ranged from 0.11 to 0.24 µg L−1 at stations 1–3 along the forereef (i.e., outer reef) to 0.21 to 0.69 µg L−1 at stations 9–11 closest to the coastline. Significantly higher (p < 0.01) chl a concentrations (3.04 µg L−1 ± 0.66 µg L−1) were measured adjacent to the inner mangrove channel at station 8 (Figure 1 and Figure 2). The HPLC analysis detected high concentrations of lutein, β-carotene, chlorophyll b and other pigments collectively associated with green plants (Figure 2b; green bars). The likely source of this pigment is the detrital materials rather than microscopic green algae in the water column targeted by the pigment analysis. It is not possible to tease out the exact source because all the plants in the study area have similar pigment composition. Removing this “green plant” signal from the analysis (Figure 2c), we found that surface water samples were dominated by cyanobacteria and diatoms (blue and orange bars, respectively), and to a lesser extent, dinoflagellates, haptophytes and cryptophytes. At all stations, cyanobacteria and diatoms accounted for 50–75% of the pigment composition (Figure 2c). At station 8, however, they only accounted for 18% of the signal, while dinoflagellates were 53% of the community. Haptophytes were only detected at the offshore stations (1–3), accounting for 2–15% of the signal while the cryptophytes were measured at all stations, varying from 12 to 34% of the community (Figure 2c). The phytoplankton in the MSP seascape had a similar pigment composition to all the surrounding stations except station 8 (Figure 2).
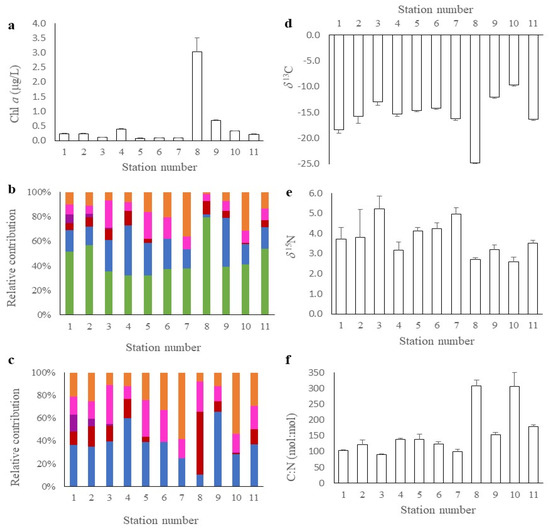
Figure 2.
At each of the 11 sampling stations, replicate samples were collected to measure (a) Chlorophyll (chl) a (µg L−1); (b) HPLC pigment analysis of the phytoplankton community with chlorophytes (green), cyanobacteria (blue), dinoflagellates (red), cryptophytes (pink), haptophytes (purple) and diatoms (orange); and (c) HPLC pigment analysis minus the chlorophytes. Simultaneously, we sampled replicates to determine the (d) δ13C; (e) δ15N; and (f) C:N (mol:mol) of phytoplankton at each of the stations.
δ13C of POM ranged from −25.8‰ (inner mangrove channel) to −9.9‰ at the nearshore locations, respectively (Figure 2d). The average δ13C of POM across all 11 stations was −15.5‰ (±−1.2‰) (Figure 3). An examination of C:N ratios revealed a similar profile (Figure 2f) with mean values of 160 mol:mol (±23.2 mol:mol). The highest values were measured at stations 8 and 10 (~308 mol:mol), while at the other stations, values were lower and more variable. The mean δ13C for BMA was −8.9‰ (±−0.7‰) (Figure 3; Table 1), which is enriched relative to the POM. C:N ratios of BMA were 184 mol:mol (±4.47 mol:mol) and similar to the average C:N ratios for POM. The δ13C of the three dominant seagrasses in the MSP reef varied from −7.4‰ (±−1.1‰) for shoal grass, −6.6‰ (±−0.4‰) for turtle grass and −3.1‰ (±−0.7‰) for manatee grass (Table 1). C:N ratios were 823 mol:mol (±191 mol:mol), 1347 mol:mol (±706 mol:mol) and 2085 mol:mol (±949 mol:mol) for these three seagrasses (Table 1). Epiphytes found on seagrasses had a δ13C of −4.7‰ (±−0.78‰) and C:N of 159 mol:mol (±36 mol:mol) (Table 1). The δ13C and δ15N values for seagrasses were significantly different to that of the BMA (Figure 3; p < 0.0001 and p < 0.01, respectively), and enriched relative to that of the POM. Turtle grass was the dominant species throughout the area, with shoal grass and manatee grass nearer the red mangrove. Of the nine macroalgae examined, δ13C values ranged from −19.8‰ for Chaetomorpha sp. to −8.1‰ for Avrainvillea sp. (Table S1). The green and yellow leaves of red mangrove did not have different δ13C values (−28.3 ± −1.5‰), but we did measure significantly (p < 0.001) lower C:N ratios in the green leaves (734 mol:mol ± 121 mol:mol) compared with the yellow leaves (2247 mol:mol ± 918 mol:mol) reflecting the reallocation of nutrients with senescence (Table 1). The epiphytic community on red mangroves was different to that found on seagrasses, with a δ13C of −13.6‰ (±−2.4‰) and C:N ratios of 151 mol:mol (±46 mol:mol), respectively. δ13C values for red mangrove were significantly (p < 0.001) different from all other autotrophs in the MSP (Figure 3) but this was not the case for δ15N values which were similar amongst red mangrove and macroalgae.
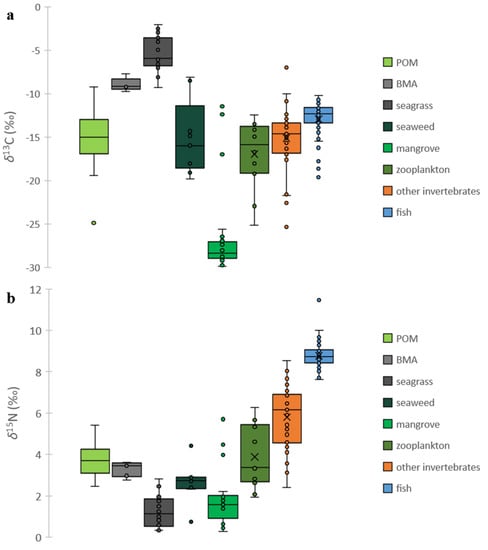
Figure 3.
Box and whisker plots of (a) δ13C and (b) δ15N values showing the median, 25% and 75% quartiles, with the whiskers showing the minimum and maximum. If the mean is significantly different from the median, an X is used to mark the mean. If present, outliers are shown (circles).
Across the reserve, there were similar δ15N values for POM, from 2.6 to 5.2‰ (Figure 2e), with a mean δ15N of 3.8‰ (±0.3‰). The mean δ15N value of BMA was 3.4‰ (±0.3‰), and not significantly different from POM (p > 0.05) (Figure 3). The shoal grass, turtle grass and manatee grass δ15N values were 1.4‰ (±0.3‰), 1.0‰ (±0.5‰) and 0.7‰ (±0.6‰), respectively, while their epiphytes had an average δ15N of 2.4‰ (±0.3‰) (Table 1). δ15N values of the macroalgae were less variable than their corresponding δ13C values, with an average of 2.7‰ (±0.9‰) (Figure 3; Table S1). Green leaves on red mangrove had significantly higher (p < 0.001) δ15N (1.7 ± 0.3‰) compared to that found in yellow leaves (0.6 ± 0.2‰) and both these values were different from that measured in red-mangrove epiphytes = 4.3 ± 0.7‰ (Table 1). δ15N values for red mangrove were similar to those measured in seagrasses, but not the other autotrophs (Figure 3).
3.2. Consumers
δ13C and δ15N values of zooplankton averaged −17.2 ± −1.0‰ and 3.9 ± 0.4‰, respectively (Table 1; Figure 3). Within the Cayos de Caña Gorda, the two small mangrove keys were home to a variety of shrimp and crabs (Table 1; Table S1), predominately of the genera of snapping shrimp, arrow crab, rubble crab, and clinging crab. δ13C and δ15N values for these invertebrates were similar, ranging from −14.8 ± 0.4‰ to −13.2 ± 1.3‰ and 4.7 ± 0.3‰ and 7.5 ± 0.4‰, respectively (Table 1, Figure 3). Other invertebrate consumers (e.g., mollusk, cnidarian, sea cucumber, and brittlestar) showed a considerably larger range in δ13C and δ15N values (Table S1).
Nine species of fish were collected in the MSP seascape (station 7), and the mean δ13C values were lowest for squirrelfish (−16.9 ± −1.81‰) and schoolmaster (−15.9 ± −3.1‰). In contrast, the mean δ13C values were highest for species known to inhabit seagrass meadows at night: French grunt (−12.1 ± −1.2‰), white grunt (−12.1 ± −0.5‰), bluestriped grunt (−11.4 ± −0.8‰), and lane snapper (−11.2 ± −0.4‰) (Table 1; Figure 3). Intermediate δ13C values were measured for the remaining species examined: graysby (−13.9 ± −0.5‰), great barracuda (−13.7 ± −0.7‰), and red lionfish (−13.1 ± −0.9‰). The mean δ15N values were relatively similar among the fishes examined with eight species within a 1.0‰ range: squirrelfish (8.3 ± 0.1‰), French grunt (8.5 ± 0.9‰), lane snapper (8.5 ± 0.3‰), bluestriped grunt (8.6 ± 0.3‰), red lionfish (8.8 ± 0.4‰), graysby (9.0 ± 0.3‰), white grunt (9.0 ± 0.4‰), and schoolmaster (9.2 ± 0.4‰). The highest δ15N value was observed for great barracuda (9.6 ± 0.1‰) (Table 1).
Stable isotope biplots displaying individual δ13C and δ15N values for the POM, BMA/seagrass and red mangrove (circles) summarized the major sources of primary producers supporting invertebrate and fish consumers (squares) in the MSP seascape (Figure 4a). Mean (±SD) δ13C and δ15N values for zooplankton and other invertebrates (see MixSIAR analysis) show that these communities rely on POM and BMA/seagrasses as a food source to invertebrates and fishes (squares), but for the most part little from the red mangrove. Similarly. an isotope biplot displaying individual δ13C and δ15N values for the four benthic invertebrates and nine fish species (circles) used in the MixSIAR analysis (Figure 4b) reveals which members of the community are utilizing the POM, BMA/seagrass and red mangrove (mean (±SD) δ13C and δ15N values; squares).
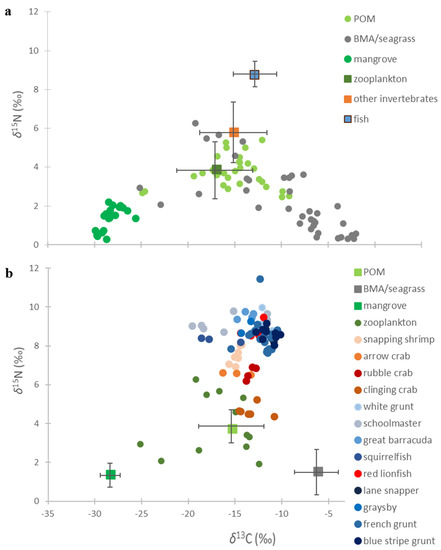
Figure 4.
(a) Isotope biplot displaying individual δ13C and δ15N values for the POM, BMA/seagrass and red mangrove (circles) and the mean (±SD) δ13C and δ15N values for zooplankton, other invertebrates and fish (squares), and (b) Isotope biplot displaying individual δ13C and δ15N values for the benthic invertebrates and fish species (circles) and the mean (±SD) δ13C and δ15N values for POM, BMA/seagrass and red mangrove (squares).
3.3. Mixing Models
Contribution estimates for benthic invertebrates collected in the MSP seascape were species-specific, and the mean contribution of POM ranged from a high of 30% for rubble crab to a low of 6% for clinging crab (Figure 5). The contribution of POM to snapping shrimp and arrow crab averaged 32% and 23%, respectively. BMA/seagrass contribution estimates showed the highest contributions to clinging crab at 62% and moderate contributions to the other species: 39% for snapping shrimp, 44% for arrow crab, and 47% for rubble crab (Figure 5). Red mangrove contribution estimates ranged from a high of 33% for arrow crab to a low of 23% for rubble crab, 29% for snapping shrimp, and 32% for clinging crab (Figure 5).
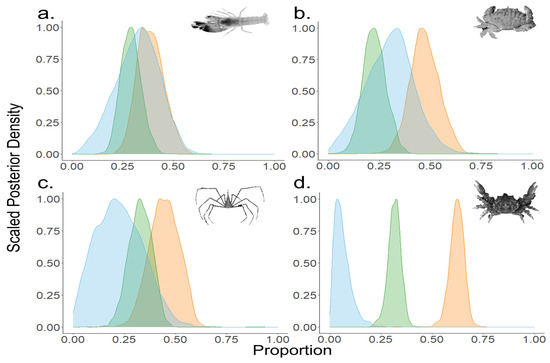
Figure 5.
Relative contribution estimates of the three dominant primary producers [POM (blue), BMA/seagrass (orange), mangrove (green)] for the four most prevalent invertebrate species. Plots represent outputs from MixSIAR models. Invertebrate species are (a) snapping shrimp, (b) rubble crab, (c) arrow crab, and (d) clinging crab.
Overall contribution estimates of fishes collected in the MSP seascape were highest by POM (mean 40%) and BMA/seagrass (mean 39%) and had typically less input from red mangrove (Figure 6). Contributions by POM ranged from a high of 61% for great barracuda to a low of 26% for squirrelfish. BMA/seagrass contributions exceeded 50% for three species (lane snapper, French grunt, and bluestriped grunt) but more commonly, around 20–40% for most consumers. Contributions by POM and BMA/seagrass were relatively equal at 43% and 42% for white grunt, while in schoolmaster, red lionfish, lane snapper, French grunt and bluestriped grunt, they were similar, on average 37% and 44%, respectively (Figure 6). Unlike other fish species, squirrelfish contributions by POM (26%) and BMA/seagrass (29%) were lower than red mangrove (45%). Contributions by POM (45%) and BMA/seagrass (35%) were similar in graysby with a moderate contribution by red mangrove (21%).
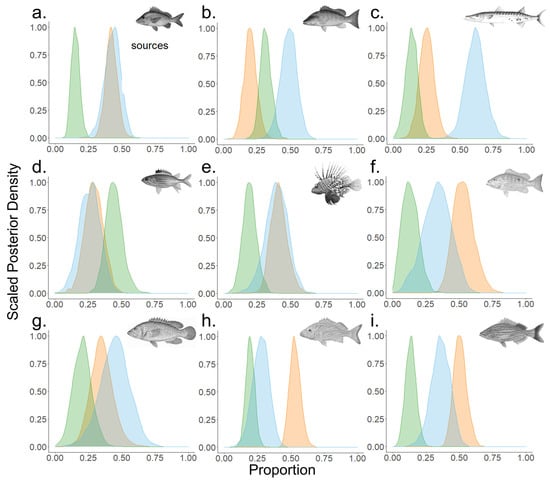
Figure 6.
Relative contribution estimates of three dominant primary producers (POM (blue), BMA/seagrass (orange), mangrove (green)) for nine fish species. Plots outputs from MixSIAR models. Fish species are (a) white grunt, (b) schoolmaster snapper, (c) great barracuda, (d) squirrelfish, (e) red lionfish, (f) lane snapper, (g) graysby, (h) French grunt, and (i) bluestriped grunt.
4. Discussion
Having a better understanding of the role of organic matter contributions in supporting consumers in any ecosystem is useful for evaluating changes due to climate shifts, anthropogenic inputs, and/or human alterations to the environment (e.g., dredging, fishing, and deforestation). MSP seascapes are particularly vulnerable given their coastal locations (see recent review [8]). We found the Guánica Biosphere Reserve in southwestern Puerto Rico to be oligotrophic; this is an important constraint on primary producers. Several lines of evidence pointed to this important finding. First, we measured low chlorophyll (<0.7 µg L−1) in the reserve, consistent with findings in other coral reef systems (e.g., Princess Charlotte Bay, Great Barrier Reef, Australia) [22,24,51]. Low chlorophyll values are indicative of areas experiencing low human disturbance and/or an absence of eutrophication [7]. Second, the δ15N values of seagrass leaf tissues is known to reflect the nature (e.g., oligotrophic versus eutrophic) of the water bodies in which they are collected [5,30,52,53]; Table S1). Our δ15N values for seagrasses collected in the MSP seascape were relatively low (1.0 ± 0.5‰), similar to those of Loneragan et al. [54]; 0.32–1.88‰), and thereby indicative of oligotrophy and limited anthropogenic influences. Third, our δ15N values for red mangrove (1.39 ± 0.57‰; Table 1) are indicative of nitrogen derived from N2 fixation in planktonic marine systems as was observed in other studies conducted in oligotrophic areas (e.g., [55,56]). Fourth, depleted δ15N values in consumers which suggest N2 fixation is indeed important [28,57]. N2 fixation was also reported to supply the majority of combined nitrogen for the ecosystems at Twin Cays, Florida [56]. The pigment analysis performed revealed a dominance of cyanobacteria, as well as diatoms, and other eukaryotic groups associated with tropical environments [22,24,51]. Cyanobacteria are the most important water column N2 fixers, providing nitrogen for other primary producers [29,58]. Indeed, N2 fixation has been shown to be an important source of new nitrogen in oligotrophic oceans [28,29]. This finding is critical given that consumer assemblages are influenced by nutrient supply in reef-level communities [4].
A variety of factors are known to impact primary producers. δ13C values of seagrasses are known to vary in response to light intensity experienced during their growth cycles. Given that the seagrasses reported here were found in the open regions of the MSP seascape, our findings are consistent with those examining seagrasses grown in the open (Table 1; e.g., [30]). Zostera capricorni, Halophila spinulosa, Syringodium isoetifolium, Cymodocea serrulata and Halodule uninervis collected from Moreton Bay (Australia) had leaf δ13C values that were 3 to 4‰ less negative and higher C:N ratios when grown in full sunlight compared to those found in more shaded regions of the ecosystem [30]. The red mangroves in this location do not appear to be experiencing physiological stress (e.g., due to elevated salinity) that would lead to enrichment in δ13C [56]. δ13C values were also similar to those for Laguncularia racemosa (white mangrove) from Florida and Belize [56] with an average value ~–27‰ for these C3 plants (see also Table S1). We did not find a significant difference in the spatial distributions in phytoplankton composition and biomass (Figure 2); this was likely due to the similar salinities and temperatures throughout the reserve during the study period [59]. The pigment data associated with pelagic primary productivity, however, provided valuable insights into the sources of POM in the MSP seascape as well as in the Guánica Biosphere Reserve. The high levels of chlorophytes (green pigments; Figure 2) reflect a combination of contributions from not only phytoplankton, but also detritus, macroalgae, and seagrass fragments being mobilized around the reef by currents. While diatoms were found in the water column, BMA is composed almost entirely of diatoms; both sources are an important food source for grazers, invertebrates, and some fishes [60,61].
Collectively, our findings reveal that consumers in the MSP seascape are strongly supported by new water column production (POM), benthic primary production (BMA), and seagrasses but less so by the epiphytic material on seagrasses or reef-associated detritus, macroalgae or the red mangrove themselves (Figs. 4, 5 and 6). BMA/seagrass was the most enriched (−6.14 ± −2.35‰), followed by POM at −15.5‰ (±−1.17‰), and the most depleted values were measured in red mangrove leaves (−28.3‰ (±−1.07‰) (Table 1; Figure 3 and Figure 4). The difference in δ13C between POM and BMA was 6.6‰ which is consistent with the findings of France [62] who reported that the global average difference in δ13C is ~7‰ for these primary producers. While a suite of producers within the MSP seascape contributed organic matter to consumers, a single primary source often dominated dietary carbon assimilation. For example, the source of organic matter supporting schoolmaster and great barracuda was 49% and 61% POM, respectively (Figure 6), while clinging crabs favored BMA/seagrasses (Figure 5) and squirrelfish had a relatively high contribution of organic matter from mangroves (Figure 6). Similarly, McMahon et al. [10] found that while a variety of source endmembers (BMA, detritus) contributed to the production of coral reef fishes, a single source often dominated, even for mobile predators. Since graysby, schoolmaster and great barracuda are reef-associated without a strong mangrove or seagrass connection, we may be missing an important component in the MixSIAR analysis. Future studies should consider their epiphytes as well as possibly those found on macroalgae in the reef as these have been found to be important contributors to the POM pool in some locations [8].
Shrimp are omnivores and consume small crustaceans and BMA. Mixing models presented here suggest that BMA/seagrass (39%) was the primary source contributing to snapping shrimp diet with an additional contribution from POM (32%) (Figure 4). Given that both autotrophs had similar δ13C and δ15N signatures, the two other shrimp found (Brachycarpus sp., commensal shrimp and Lysmata sp., peppermint shrimp) may have had similar source(s) contributing organic matter to their diet (Table S1). However, this is difficult to assert as they are also likely consuming a variety of zooplankton (Table 1; Figure 3 and Figure 4). Studies in a small mangrove-lined lagoon nearby yielded shrimp with a δ13C of −16‰, similar to the present study for POM and shrimp [54]. There appears to be little evidence for the assimilation of mangrove detritus by shrimp [63,64], but consumption of red mangrove epiphytes that have a similar δ13C of −13‰ may be occurring. In the MSP seascape, we found that all three crabs relied on organic matter from BMA/seagrasses (~51%) and less so on red mangroves (~29%) and POM (~20%) (Figure 4 and Figure 5). Ocypodid (fiddler) crabs from a nearby lagoon (Laguna Joyuda) in Puerto Rico were found to have preferentially selected BMA over plant detritus [52], but these authors did not measure POM, seagrasses or mangroves, thereby preventing a more in-depth comparison.
We also identified the primary source(s) of organic matter supporting a wide range of juvenile reef fishes. The majority of juvenile fishes in the MSP seascape were supported by organic matter derived primarily from water column (POM) and benthic (BMA/seagrass) sources as has been observed at other back-reef ecosystems in the Caribbean Sea [3,6,16]. The majority of juvenile fishes examined in this study sheltered in the red mangrove prop roots and, to a lesser extent, the adjacent seagrass meadows or patch reefs. Rooker et al. [18] found that two common constituents of back-reef ecosystems (schoolmaster and white grunt) relied on multiple habitats within this MSP seascape, occupying areas with more structure (i.e., mangroves and patch reef) during the day and areas with less structure (i.e., central channel and seagrasses) at night to minimize encounter rates with a potential predator. In this way, the use of multiple habitats within the MSP seascape offers favorable conditions for the survival of juveniles, including shelter, lower predator abundance, and a high supply of food [14,18,65,66]. The limited mix of producers in the MixSIAR performed may bias our interpretation for this ecosystem since it was not possible to include every potential source of carbon production. By incorporating all putative sources of primary production (i.e., including the epiphytes and macroalgae), the results may have changed slightly; however, these additional sources likely had little effect on our contribution estimates.
We found no convincing evidence for a trophic role of red mangrove or its detritus in supporting consumers within the MSP seascape, while production by pelagic and benthic algae was important, similar to reports in earlier studies [36,64,67]. Refuge appears to be the most important contribution of mangrove systems to benthic invertebrates and juvenile fishes in the Guánica Biosphere Reserve. Young fishes in particular use mangrove prop roots as a nursery habitat [18,68] while reduced predation has been shown to be critical for recruitment success and year–class strength [64]. In general terms, we did, however, find that there was a higher R. mangle contribution for taxa living/residing directly within the mangrove prop roots (e.g., crabs) compared to most fishes that reside near them (i.e., French grunt and bluestriped grunt) (Figure 5 and Figure 6).
This study contributes to our understanding of trophic connectivity in MSP seascapes located within tropical back-reef ecosystems. These areas represent important nurseries for a variety of reef-dependent invertebrates and fishes, but they are also disproportionately threatened by coastal development, anthropogenic disturbance, and climate change [1,2,3,6,7,8,9,66]. While the reserve is not a protected area in terms of restrictions on exploitation [5], it is a very popular tourist area and targeted by artisanal fishers. Future concerns for this region are alterations from the development of ports (and associated dredging) which will take place nearby in Guayanilla and Ponce. Understanding how primary producers and consumers thrive in these complex seascapes contributes not only to our knowledge of their ecology but also to the protection (value, functional role) of the components (seagrasses, mangroves, etc.) from on-going human disturbances [10,21]. Future studies may consider similar sampling strategies in altered seascapes to understand how eutrophication alters the dominant primary producers, and hence carbon flow, in these important systems.
5. Conclusions
Alterations to the functions and connectivity of MSP seascapes have the potential to negatively impact the survival, foraging activities, and movement of reef-dependent invertebrates and fishes. In the current study, we examined carbon flow in the Guánica Biosphere Reserve in southwestern Puerto Rico. Our findings show N2 fixers (cyanobacteria) being important for fueling water column primary productivity in this oligotrophic area while diatom-dominated benthic microalgae were important food sources for a variety of invertebrates. Food web mixing models based on stable isotopes revealed that while multiple producers contributed to consumers, a single source often accounted for the majority of the dietary carbon assimilation for a given species. Enriched δ15N values of seagrass and R. mangle leaf tissue and depleted values in consumers supported the assertion that N2 fixation is critical for primary productivity. Understanding these complex seascapes contributes not only to our knowledge of their ecology but also to their long-term conservation and management.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/fishes8010044/s1, Table S1: Summary of isotopic data from the literature from studies performed in Puerto Rico and other similar shallow reef systems.
Author Contributions
Conceptualization, A.Q., R.J.D.W. and J.R.R.; methodology, A.Q., R.J.D.W., J.R.R., R.L.H. and B.P.F.; formal analysis, all; investigation, all; data curation, A.Q.; writing—original draft preparation, A.Q., R.J.D.W. and J.R.R.; writing—review and editing, all; project administration, J.R.R.; funding acquisition, A.Q. and J.R.R. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Texas Institute of Oceanography (TIO), the McDaniel Charitable Foundation, and the Brazilian Research Council (CNPq- PVE 401594/2014-9) to B.P.F and J.R.R. NOAA Coral Reef Conservation Program for RLH.
Institutional Review Board Statement
The animal study protocol was approved by the Institutional Review Board of Puerto Rico University for studies involving animals. For this study, an ethics review and collection permit were not needed due to the approval by the Puerto Rico Department of Environment and Natural Resources (DNER) for sample collections and processing.
Data Availability Statement
Data are contained within this article and the supplementary material, and are available upon request to the corresponding author.
Acknowledgments
We thank Alexandre Aschenbrenner (The Federal University of Pernambuco), Richard Appeldoorn (University of Puerto Rico) and Kevin Boswell (Florida International University) for their invaluable contributions to the study. Carlos Ruiz prepared samples which the Stable Isotope Facility at the University of California at Davis processed, Allyson Lucchese ran the HPLC, Jessica Hillhouse prepared the sampling map and Emily Meese prepared the contribution plots. We thank the three anonymous reviewers for their comments which improved the manuscript.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
References
- Bell, P.R.F. Eutrophication and coral reefs—Some examples in the Great Barrier Reef lagoon. Water Res. 1992, 26, 553–568. [Google Scholar] [CrossRef]
- Pandolfi, J.M.; Bradbury, R.H.; Sala, E.; Hughes, T.P.; Bjorndal, K.A.; Cooke, R.G.; McArdle, D.; McClenachan, L.; Newman, M.J.H.; Paredes, G.; et al. Global trajectories of the long-term decline of coral reef ecosystems. Science 2003, 301, 955–958. [Google Scholar] [CrossRef]
- Mumby, P.J.; Edwards, A.J.; Arias-González, J.E.; Lindeman, K.C.; Blackwell, P.G.; Gall, A.; Gorczynska, M.I.; Harborne, A.R.; Pescod, C.L.; Renken, H.; et al. Mangroves enhance the biomass of coral reef fish communities in the Caribbean. Nature 2004, 427, 533–536. [Google Scholar] [CrossRef]
- Wyatt, A.S.J.; Waite, A.M.; Humphries, S. Stable isotope analysis reveals community-level variation in fish trophodynamics across a fringing coral reef. Coral Reefs 2012, 31, 1029–1044. [Google Scholar] [CrossRef]
- Whithall, D.; Bauer, L.J.; Sherman, C.; Edwards, K.; Mason, A.; Pait, T.; Caldow, C. Baseline Assessment of Guánica Bay, Puerto Rico in Support of Watershed Restoration; NOAA Technical Memorandum NOS NCCOS 176; NOAA Institutional Repository: Silver Spring, MD, USA, 2013; p. 169. [Google Scholar]
- Nagelkerken, I.; Sheaves, M.; Baker, R.; Connolly, R.M. The seascape nursery: A novel spatial approach to identify and manage nurseries for coastal marine fauna. Fish Fish. 2015, 2015, 362–371. [Google Scholar] [CrossRef]
- Freeman, L.A.; Corbett, D.R.; Fitzgerald, A.; Lemley, D.A.; Quigg, A.; Steppe, C. Impacts of Urbanization on Estuarine Ecosystems and Water Quality. Estuar Coasts 2019, 42, 1821–1838. [Google Scholar] [CrossRef]
- Skinner, C.; Cobain, M.; Zhu, Y.; Wyatt, A.; Polunin, N. Progress and direction in the use of stable isotopes to understand complex coral reef ecosystems: A review. Oceanogr. Mar. Biol. 2022, 60, 375–434. [Google Scholar] [CrossRef]
- Polidoro, B.A.; Carpenter, K.E.; Collins, L.; Duke, N.C.; Ellison, A.M.; Ellison, J.C.; Farnsworth, E.J.; Fernando, E.S.; Kathiresan, K.; Koedam, N.E.; et al. The Loss of Species: Mangrove Extinction Risk and Geographic Areas of Global Concern. PLoS ONE 2010, 5, e10095. [Google Scholar] [CrossRef] [PubMed]
- McMahon, K.W.; Berumen, M.L.; Thorrold, S.R. Linking habitat mosaics and connectivity in a coral reef seascape. Proc. Nat. Acad. Sci. USA 2012, 109, 15372–15376. [Google Scholar] [CrossRef]
- Acosta, C.; Butler, M.H. Role of mangrove habitat as a nursery for juvenile spiny lobster, Panulirus argus, in Belize. Mar. Freshw. Res. 1997, 48, 721–727. [Google Scholar] [CrossRef]
- Nagelkerken, I.; van der Velde, G. Are Caribbean mangroves important feeding grounds for juvenile reef fish from adjacent seagrass beds? Mar. Ecol. Prog. Ser. 2004, 274, 143–151. [Google Scholar] [CrossRef]
- Adams, A.; Dahlgren, C.P.; Kellison, G.T.; Kendall, M.S.; Layman, C.A.; Ley, J.A.; Nagelkerken, I.; Serafy, J.E. Nursery function of tropical backreef systems. Mar. Ecol. Prog. Ser. 2006, 318, 287–301. [Google Scholar] [CrossRef]
- Verweij, M.C.; Nagelkerken, I.; de Graaf, D.; Peeters, M.; Bakker, E.J.; van der Velde, G. Structure, food and shade attract juvenile coral reef fish to mangrove and seagrass habitats: A field experiment. Mar. Ecol. Prog. Ser. 2006, 306, 257–268. [Google Scholar] [CrossRef]
- Nagelkerken, I.; Bothwell, J.; Nemeth, R.S.; Pitt, J.M.; van der Velde, G. Interlinkage between Caribbean coral reefs and seagrass beds through feeding migrations by grunts (Haemulidae) depends on habitat accessibility. Mar. Ecol. Prog. Ser. 2008, 368, 155–164. [Google Scholar] [CrossRef]
- Huijbers, C.M.; Grol, M.G.G.; Nagelkerken, I. Shallow patch reefs as alternative habitats for early juveniles of some mangrove/seagrass-associated fish species in Bermuda. Rev. Biol. Trop. 2008, 56, 161–169. [Google Scholar] [CrossRef]
- McMahon, K.W.; Thorrold, S.R.; Houghton, L.A.; Berumen, M.L. Tracing carbon flow through coral reef food webs using a compound specific stable isotope approach. Oecologia 2015, 180, 809–821. [Google Scholar] [CrossRef] [PubMed]
- Rooker, J.R.; Dance, M.A.; Wells, R.J.D.; Quigg, A.; Hill, R.L.; Appeldoorn, R.S.; Padovani Ferreira, B.; Boswell, K.M.; Sanchez, P.J.; Moulton, D.L.; et al. Seascape connectivity and the influence of predation risk on the movement of fishes inhabiting a back-reef ecosystem. Ecosphere 2018, 4, e02200. [Google Scholar] [CrossRef]
- Rooker, J.R.; Dennis, G.D. Diel, lunar and seasonal changes in a mangrove fish assemblage off southwestern Puerto Rico. Bull. Mar. Sci. 1991, 49, 684–698. [Google Scholar]
- Dahlgren, C.P.; Eggleston, D.B. Ecological processes underlying ontogenetic habitat shifts in a coral reef fish. Ecology 2000, 81, 2227–2240. [Google Scholar] [CrossRef]
- Sanchirico, J.N.; Mumby, P.J. Mapping ecosystem functions to the valuation of ecosystem services: Implications of species-habitat associations for coastal land-use decisions. Theor. Ecol. 2009, 2, 67–77. [Google Scholar] [CrossRef]
- Damar, A.; Colijn, F.; Hesse, K.-J.; Kurniawan, F. Coastal phytoplankton pigments composition in three tropical estuaries of Indonesia. J. Mar. Sci. Eng. 2020, 8, 311. [Google Scholar] [CrossRef]
- Cloern, J.E. Our evolving conceptual model of the coastal eutrophication problem. Mar. Ecol. Prog. Ser. 2001, 210, 223–253. [Google Scholar] [CrossRef]
- Jeffrey, S.W. Photosynthetic pigments of the phytoplankton of some coral reef waters. Limnol. Oceangr. 1968, 13, 350–355. [Google Scholar] [CrossRef]
- Pinckney, J.L.; Quigg, A.; Roelke, D.L. Interannual and seasonal patterns of estuarine phytoplankton diversity in Galveston Bay, Texas, USA. Estuaries Coasts 2017, 40, 310–316. [Google Scholar] [CrossRef]
- Pinckney, J.L.; Millie, D.F.; Howe, K.E.; Paerl, H.W.; Hurley, J.P. Flow scintillation counting of 14C-labeled microalgal photosynthetic pigments. J. Plankton Res. 1996, 18, 1867–1880. [Google Scholar] [CrossRef]
- Schlüter, L.; Møhlenberg, F.; Havskum, H.; Larsen, S. The use of phytoplankton pigments for identifying and quantifying phytoplankton groups in coastal areas: Testing the influence of light and nutrients on pigment/chlorophyll a ratios. Mar. Ecol. Prog. Ser. 2000, 192, 49–63. [Google Scholar] [CrossRef]
- Dorado, S.; Rooker, J.R.; Wissel, B.; Quigg, A. Isotope baseline shifts in pelagic food webs of the Gulf of Mexico. Mar. Ecol. Prog. Ser. 2012, 464, 37–49. [Google Scholar] [CrossRef]
- Quigg, A.; Al-Anasi, M.; Nour El Din, N.; Wei, C.-L.; Nunnally, C.C.; Al-Ansari, I.S.; Rowe, G.; Soliman, Y.; Al-Maslamani, I.; Mahmoud, I.; et al. Phytoplankton along the coastal shelf of an oligotrophic hypersaline environment in a semi-enclosed marginal sea: Qatar (Arabian Gulf). Cont. Shelf Res. 2013, 60, 1–16. [Google Scholar] [CrossRef]
- Grice, A.M.; Loneragan, N.R.; Dennison, W.C. Light intensity and the interactions between physiology, morphology and stable isotope ratios in five species of Seagrass. J. Exp. Mar. Biol. Ecol. 1996, 195, 91–110. [Google Scholar] [CrossRef]
- Kimirei, I.A.; Nagelkerken, I.; Mgaya, Y.D.; Huijbers, C.M. The mangrove nursery paradigm revisited: Otolith stable isotopes support nursery to reef movements by Indo-Pacific fishes. PLoS ONE 2013, 8, e66320. [Google Scholar] [CrossRef]
- Peterson, B.J.; Fry, B. Stable isotopes in ecosystem studies. Ann. Rev. Ecol. Syst. 1987, 18, 293–320. [Google Scholar] [CrossRef]
- Peterson, B.J.; Howarth, R.W.; Garritt, R.H. Multiple stable isotopes used to trace the flow of organic matter in estuarine food webs. Science 1985, 227, 1361–1363. [Google Scholar] [CrossRef]
- France, R.L.; Peters, R.H. Ecosystem differences in the trophic enrichment of 13C in aquatic food webs. Can. J. Fish. Aquat. Sci. 1997, 54, 1255–1258. [Google Scholar] [CrossRef]
- Post, D.M. Using stable isotopes to estimate trophic position: Models, methods, and assumptions. Ecology 2002, 83, 703–718. [Google Scholar] [CrossRef]
- Bouillon, S.; Connolly, R.M.; Gillikin, D.P. Use of stable isotopes to understand food webs and ecosystem functioning in estuaries. In Treatise on Estuarine and Coastal Science; Wolanski, E., McLusky, D.S., Eds.; Waltham Academic Press: Cambridge, MA, USA, 2011; Volume 7, pp. 143–173. [Google Scholar]
- Zhao, Y.; Quigg, A. Nutrient limitation in Northern Gulf of Mexico (NGOM): Phytoplankton communities and photosynthesis respond to nutrient pulse. PLoS ONE 2014, 9, e88732. [Google Scholar] [CrossRef] [PubMed]
- Latasa, M. Improving estimations of phytoplankton class abundances using CHEMTAX. Mar. Ecol. Prog. Ser. 2007, 329, 13–21. [Google Scholar] [CrossRef]
- Wells, R.J.D.; Cowan, J.H., Jr.; Fry, B. Feeding ecology of red snapper Lutjanus campechanus in the northern Gulf of Mexico. Mar. Ecol. Prog. Ser. 2008, 361, 213–225. [Google Scholar] [CrossRef]
- Bunn, S.E.; Loneragan, N.R.; Kempster, M.A. Effects of acid washing on stable isotope ratios of C and N in penaeid shrimp and seagrass: Implications for food-web studies using multiple stable isotopes. Limnol. Oceanogr. 1995, 40, 622–625. [Google Scholar] [CrossRef]
- Ng, J.S.; Wai, T.C.; Williams, G.A. The effects of acidification on the stable isotope signatures of marine algae and molluscs. Mar. Chem. 2007, 103, 97–102. [Google Scholar] [CrossRef]
- Serrano, O.; Serrano, L.; Mateo, M.A.; Colombini, I.; Chelazzi, L.; Gagnarli, E.; Fallaci, M. Acid washing effect on elemental and isotopic composition of whole beach arthropods: Implications for food web studies using stable isotopes. Acta Oecologica 2008, 34, 89–96. [Google Scholar] [CrossRef]
- Stock, B.C.; Semmens, B.X. MixSIAR GUI User Manual, Version 3.1; Scripps Institution of Oceanography: San Diego, CA, USA, 2013; Available online: https://github.com/brianstock/MixSIAR (accessed on 13 June 2021).
- Phillips, D.L.; Inger, R.; Bearhop, S.; Jackson, A.L.; Moore, J.W.; Parnell, A.C.; Semmens, B.X.; Ward, E.J. Best practices for use of stable isotope mixing models in food-web studies. Can. J. Zoo. 2014, 92, 823–835. [Google Scholar] [CrossRef]
- DeNiro, M.J.; Epstein, S. Influence of diet on the distribution of carbon isotopes in animals. Geochim. Cosmochim. Acta 1978, 42, 495–506. [Google Scholar] [CrossRef]
- Fry, B.; Sherr, E.B. δ13C measurements as indicators of carbon flow in marine and freshwater ecosystems. Contributions Mar. Sci. 1984, 27, 13–47. [Google Scholar]
- Vanderklift, M.A.; Ponsard, S. Sources of variation in consumer-diet δ15N enrichment: A meta-analysis. Oecologia 2003, 136, 169–182. [Google Scholar] [CrossRef] [PubMed]
- Vander Zanden, M.J.; Rasmussen, J.B. Variation in d15N and d13C trophic fractionation: Implications for aquatic food web studies. Limnol. Oceanogr. 2001, 46, 2061–2066. [Google Scholar] [CrossRef]
- Wells, R.J.D.; Rooker, J.R.; Quigg, A.; Wissel, B. Influence of mesoscale oceanographic features on pelagic food webs in the Gulf of Mexico. Mar. Biol. 2017, 164, 92–103. [Google Scholar] [CrossRef]
- Gelman, A.; Rubin, D.B. A single series from the Gibbs sampler provides a false sense of security. Bayesian Stat. 1992, 4, 625–631. [Google Scholar]
- Furnas, M.J.; Mitchell, A.W. Phytoplankton dynamics in the central Great Barrier Reef—I. Seasonal changes in biomass and community structure and their relation to intrusive activity. Cont. Shelf Res. 1986, 6, 363–384. [Google Scholar] [CrossRef]
- France, R.L. Estimating the assimilation of mangrove detritus by fiddler crabs in Laguna Joyuda, Puerto Rico, using dual stable isotopes. J. Trop. Ecol. 1988, 14, 413–425. [Google Scholar] [CrossRef]
- Briand, M.J.; Bonnet, X.; Goiran, C.; Guillou, G.; Letourneur, Y. Major sources of organic matter in a complex coral reef lagoon: Identification from isotopic signatures (δ13C and δ15N). PLoS ONE 2015, 10, e0131555. [Google Scholar] [CrossRef]
- Longeragan, N.R.; Bunn, S.E.; Kellaway, D.M. Are mangroves and seagrasses sources of organic carbon for penaeid prawns in a tropical Australian estuary? A multiple stable-isotope study. Mar. Biol. 1997, 130, 289–300. [Google Scholar] [CrossRef]
- Macko, S.A.; Ostrum, N.E. Pollution studies using stable isotopes. In Stable Isotopes in Ecology and Environmental Science; Lajtha, K., Michener, R., Eds.; Blackwell Scientific Publications: Oxford, UK, 1994; pp. 45–62. [Google Scholar]
- Wooller, M.; Smallwood, B.; Jacobson, M.; Fogel, M. Carbon and nitrogen stable isotopic variation in Laguncularia racemosa (L.) (White mangrove) from Florida and Belize: Implications for trophic level studies. Hydrobiologia 2003, 499, 13–23. [Google Scholar] [CrossRef]
- Wells, R.J.D.; Rooker, J.R. Feeding ecology of pelagic fish larvae and juveniles in slope waters of the Gulf of Mexico. J. Fish Biol. 2009, 75, 1719–1732. [Google Scholar] [CrossRef]
- Berman-Frank, I.; Falkowski, P. Nitrogen fixation and photosynthetic oxygen evolution in cyanobacteria. Res. Microbiol. 2003, 154, 157–164. [Google Scholar] [CrossRef]
- Van Duyl, F.C.; Gast, G.J.; Steinhoff, W.; Kloff, S.; Veldhuis, M.J.W.; Bak, R.P.M. Factors influencing the short-term variation in phytoplankton composition and biomass in coral reef waters. Coral Reefs 2002, 21, 293–306. [Google Scholar] [CrossRef]
- Gruber, N.; Sarmiento, J.L. Global patterns of marine nitrogen fixation and denitrification. Glob. Biogeochem Cycles 1997, 11, 235–266. [Google Scholar] [CrossRef]
- Goldberg, W.M. The Biology of Reefs and Reef Organisms; University of Chicago Press: Chicago, IL, USA, 2013. [Google Scholar]
- France, R.L. Carbon-13 enrichment in benthic compared to planktonic algae: Food web implications. Mar. Ecol. Prog. Ser. 1995, 124, 307–312. [Google Scholar] [CrossRef]
- Stoner, A.W.; Zimmerman, R.J. Food pathways associated with penaeid shrimps in a mangrove fringed estuary. Fish. Bull. 1988, 86, 543–551. [Google Scholar]
- Fry, B.; Ewel, K.C. Using stable isotopes in mangrove fisheries research—A review and outlook. Isot. Env. Health Stud. 2003, 3, 191–196. [Google Scholar] [CrossRef] [PubMed]
- Parrish, J.D. Fish communities of interacting shallow-water habitats in tropical oceanic regions. Mar. Ecol. Prog. Ser. 1989, 58, 143–160. [Google Scholar] [CrossRef]
- Blanar, C.A.; Hornbeck, J.R.; Kerstetter, D.W.; Hirons, A.C. Stable isotopes and community surveys reveal differential use of artificial and natural reefs by South Florida fishes. Heliyon 2021, 7, e07413. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez, C.; Stoner, A.W. The epiphyte community of mangrove roots in a tropical estuary: Distribution and biomass. Aquat. Bot. 1990, 36, 117–126. [Google Scholar] [CrossRef]
- Serafy, J.; Shideler, G.; Araújo, R.; Nagelkerken, I. Mangroves Enhance Reef Fish Abundance at the Caribbean Regional Scale. PLoS ONE 2015, 10, e0142022. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).