1. Introduction
Antioxidants in the immune system are no doubt run through the whole process of ontogenetic organisms, in which they are exposed to changes in environmental factors. It is well known that aquatic livestock in their natural habitats undergo the simultaneous impacts of various environmental variables [
1]. Among them, salinity and temperature are the most significant environmental factors affecting aquatic organisms, which can indirectly or directly regulate the rate of all bioprocesses, such as development, growth, survival, and reproduction [
1,
2,
3]. Salinity and temperature have thus been described as a dominant “ecological principal factor” for many marine species [
1]. However, most of the auto-biologic studies on bivalves have mainly concentrated on single effects rather than multiple environmental factors. For example, some studies by Japanese researchers were in the context of a single-factor influence of salinity or temperature on the pearl oyster [
4,
5,
6,
7,
8]. Wang et al. [
1] suggest that the interaction between single factors is critical, and single-factor experiments cannot provide information on interactive effects. Multiple factors may have posed for the mass mortalities of Japanese pearl oysters since the 1960s, and it has long been recognized that the influence of one factor can be modified by another factor [
9]. Accordingly, a study of the combined effects of multiple environmental factors is necessary.
The Box–Benhnken design (BBD) was created byBox [
10], and firstly applied byDavis [
11], who investigated ovum development, growth, and larval survival of hard clam
Mercenaria mercenaria and American oyster
Crassostrea virginia. Such a technique makes multi-factor synergy possible under broader environmental conditions rather than through laboratory experiments, and its first application was under the combined effect of salinity and temperature factors on bivalve larvae [
1]. Subsequently, the combined influences of the two factors have been investigated for numerous mollusks, such as European flat oyster
Ostrea edulis [
12], mussel
Mytilus edulis [
13], bivalve molluso
Adula californiensis (Pelecypoda: Mytilidae) [
14], brackish water clam
Rangia cuneata [
15], Chinese pearl oyster
Pinctada fucata martensii [
1], pearl oyster
Pinctada imbricata Röding [
9], coot clam
Mulinia lateralis [
16], Northern Bay scallop
Argopecten irradians irradians [
17], noble scallop
Chlamys nobilis [
18], Mediterranean mussel
Mytilus galloprovincialis, and Japanese oyster
Crassostrea gigas [
19].
The pearl oyster is the main shellfish species for cultivating seawater pearls worldwide [
1]. Since 1949, the pearl oyster has been cultivated in Guangdong, Guangxi, and Hainan Provinces of China until pearl production peaked in the 1990s. The yield of seawater pearl in China reached 6 tons in 2010, and the gross value was about USD 20 million. The pearl oyster has greatly promoted the development of the pearl industry in China and created considerable economic income for the nation [
20]. However, massive mortalities of the pearl oyster are frequently reported and have caused considerable economic losses in the past 10 years. A series of studies have been conducted to understand the causes of such mortalities. Evidence has indicated that temperature, salinity, dissolved oxygen, and pH can change the physiological indexes of the pearl oyster, including cellular immune level, oxygen consumption rate, osmotic pressure (OSM), and ammonia excretion rate, as well as inhibit growth, disturb the balance of basal metabolism, and even lead to death [
20,
21,
22,
23]. All of the evidence reveals that massive mortality of the pearl oyster is closely related to these environmental changes. To date, most studies have focused on one environmental factor at a time, while the existing multi-factor studies have focused on the immunological expression [
24], survival, growth [
25], energy budget [
26], fertilization, hatching [
1], transcriptome, and biomineralization [
27]. Therefore, it is necessary to explore in detail how salinity and temperature, in particular, jointly influence antioxidants in the immune system, and to determine the optimal combination of factors with practical significance. Undoubtedly, insight into such issues will be beneficial for maintaining the optimal living conditions of the pearl oyster, assuring their development, growth, and survival, thereby reducing economic losses and increasing production.
The objectives of this study were to (1) examine the synergistic effects of salinity and temperature on antioxidants in the immune system in the pearl oyster using response surface method and BBD, (2) model the relationship of antioxidants in the immune system with salinity and temperature, and (3) determine the optimal salinity–temperature combination using the statistical optimization technique. The results from the present study will provide a theoretical reference for shellfish culture with the variation of two environmental factors and the establishment of index models for P. pucata aquaculture.
4. Discussion
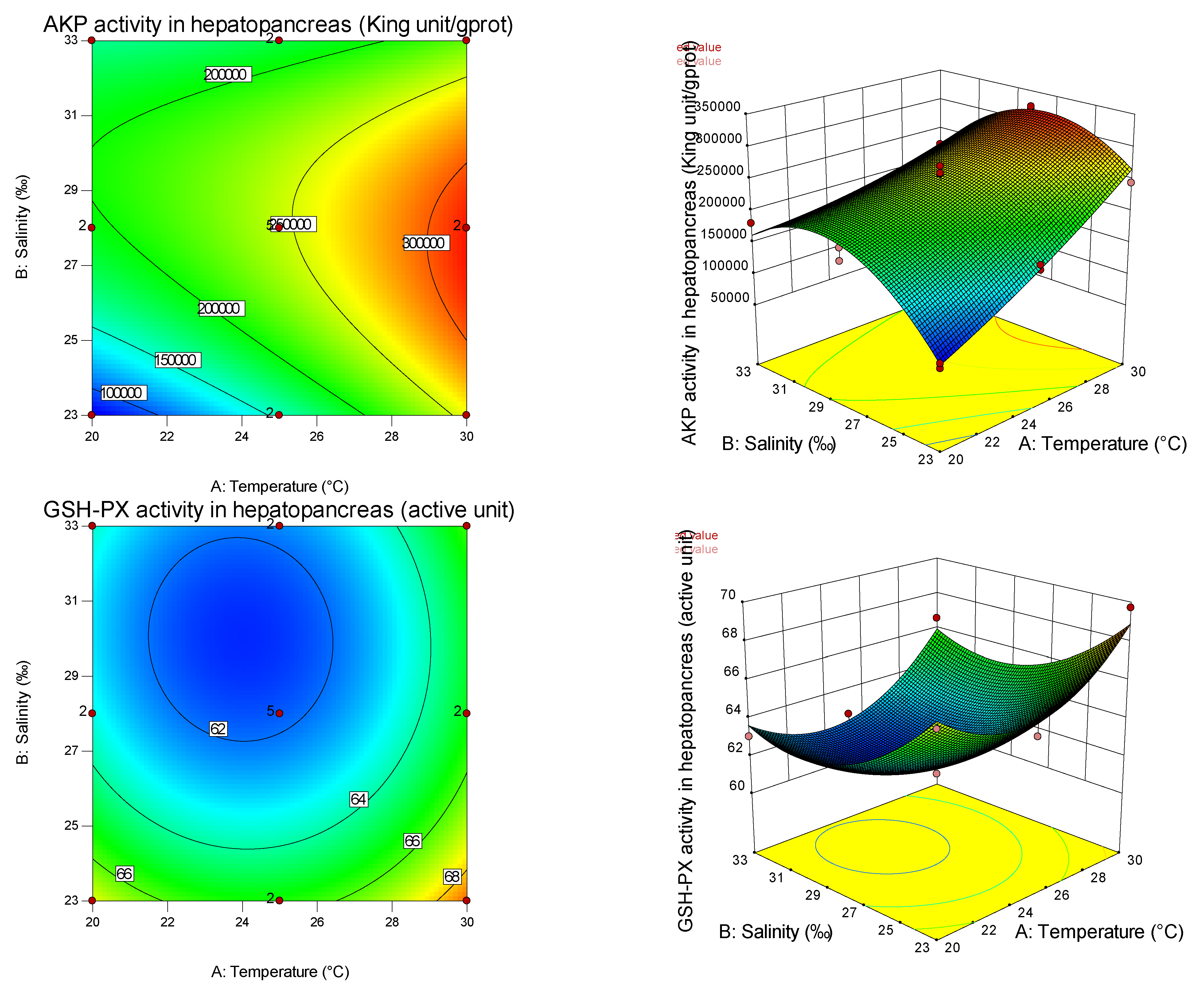
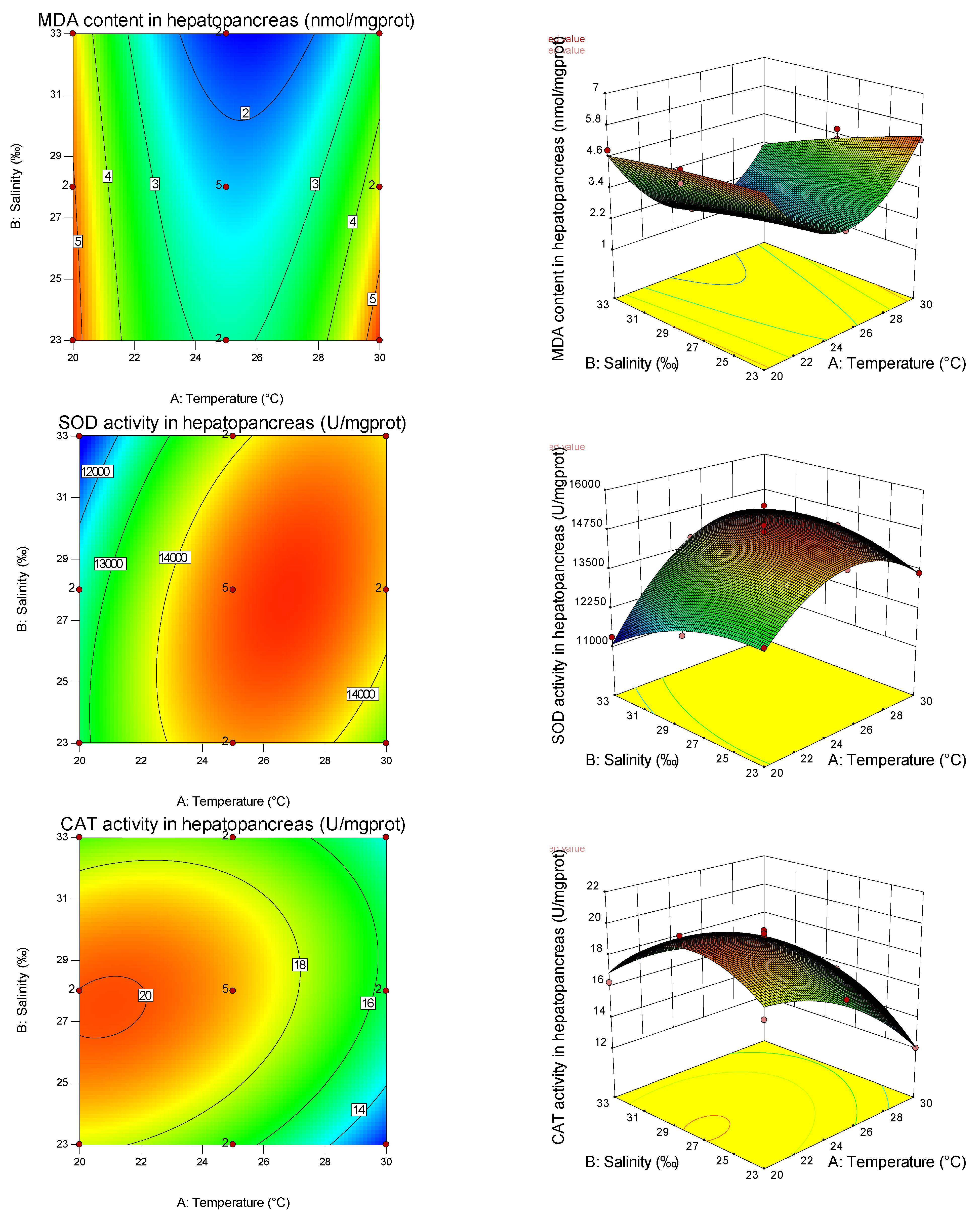
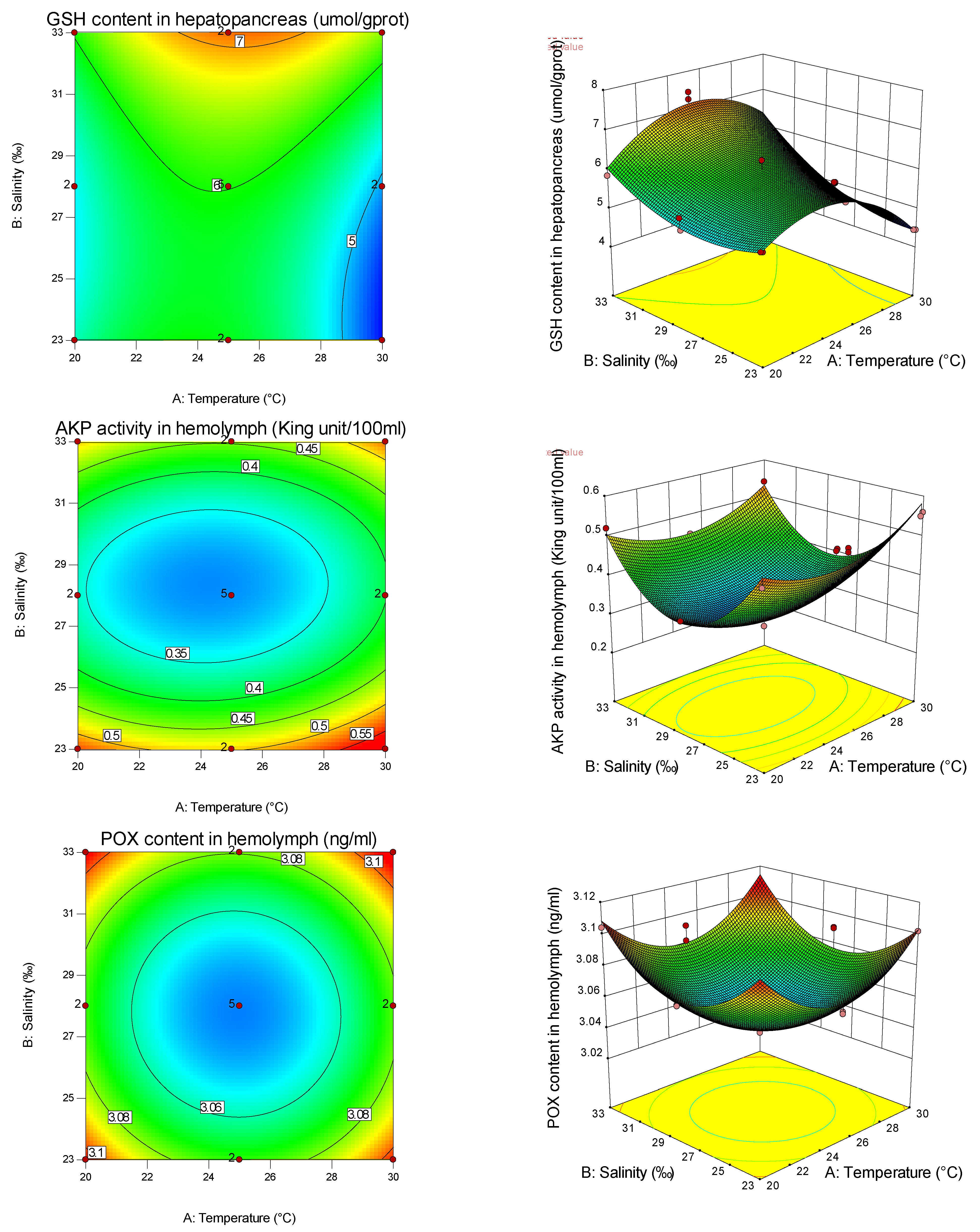
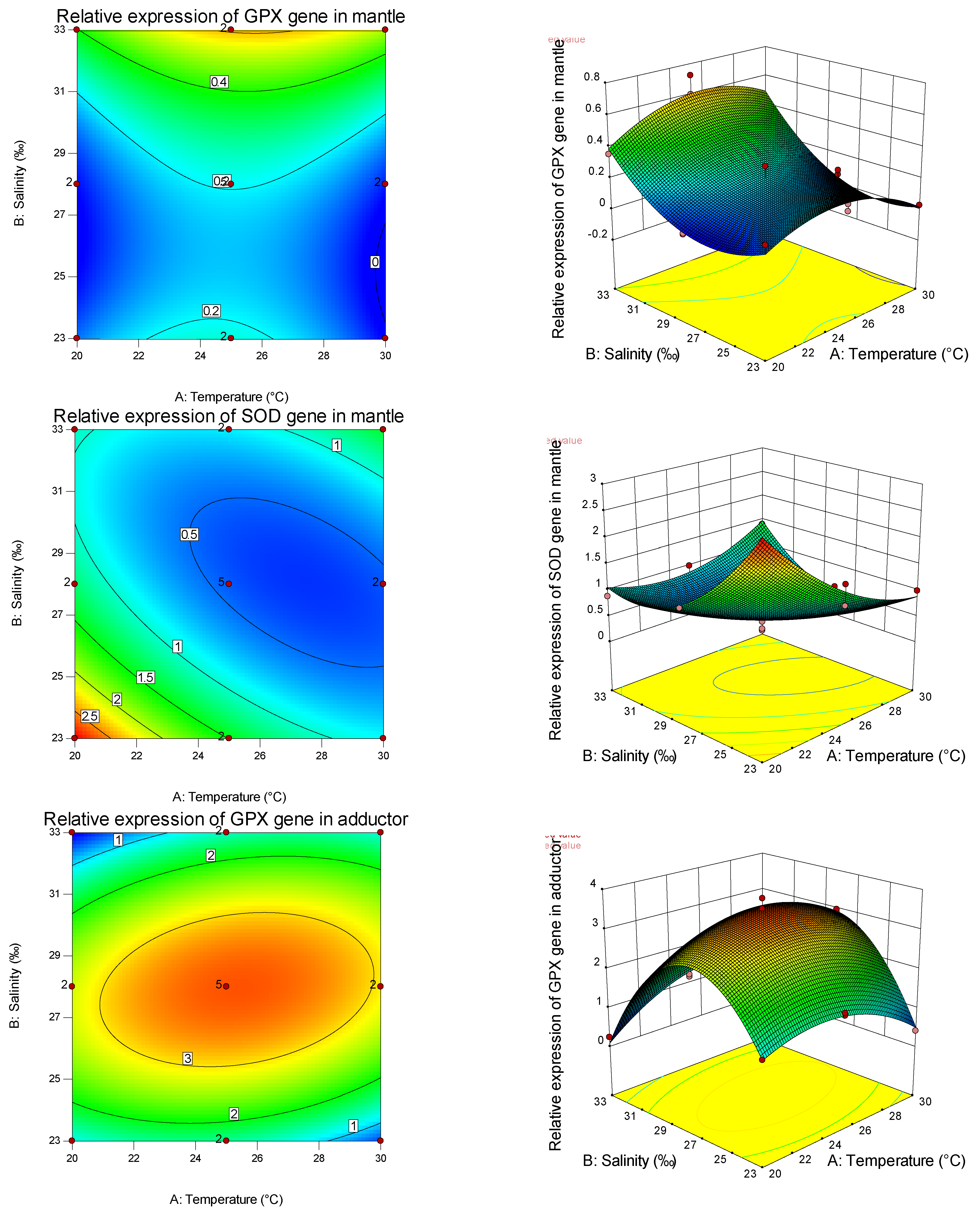
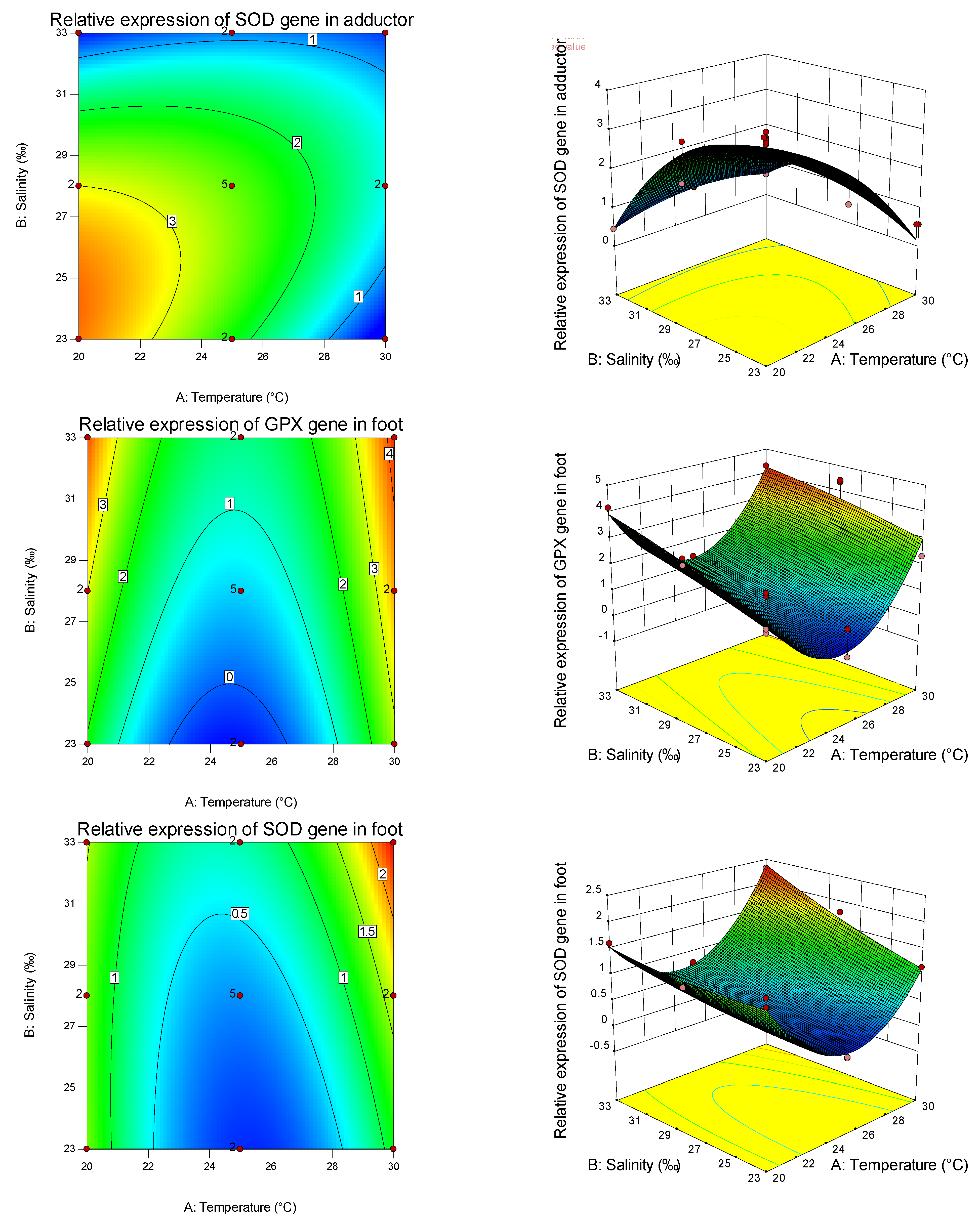
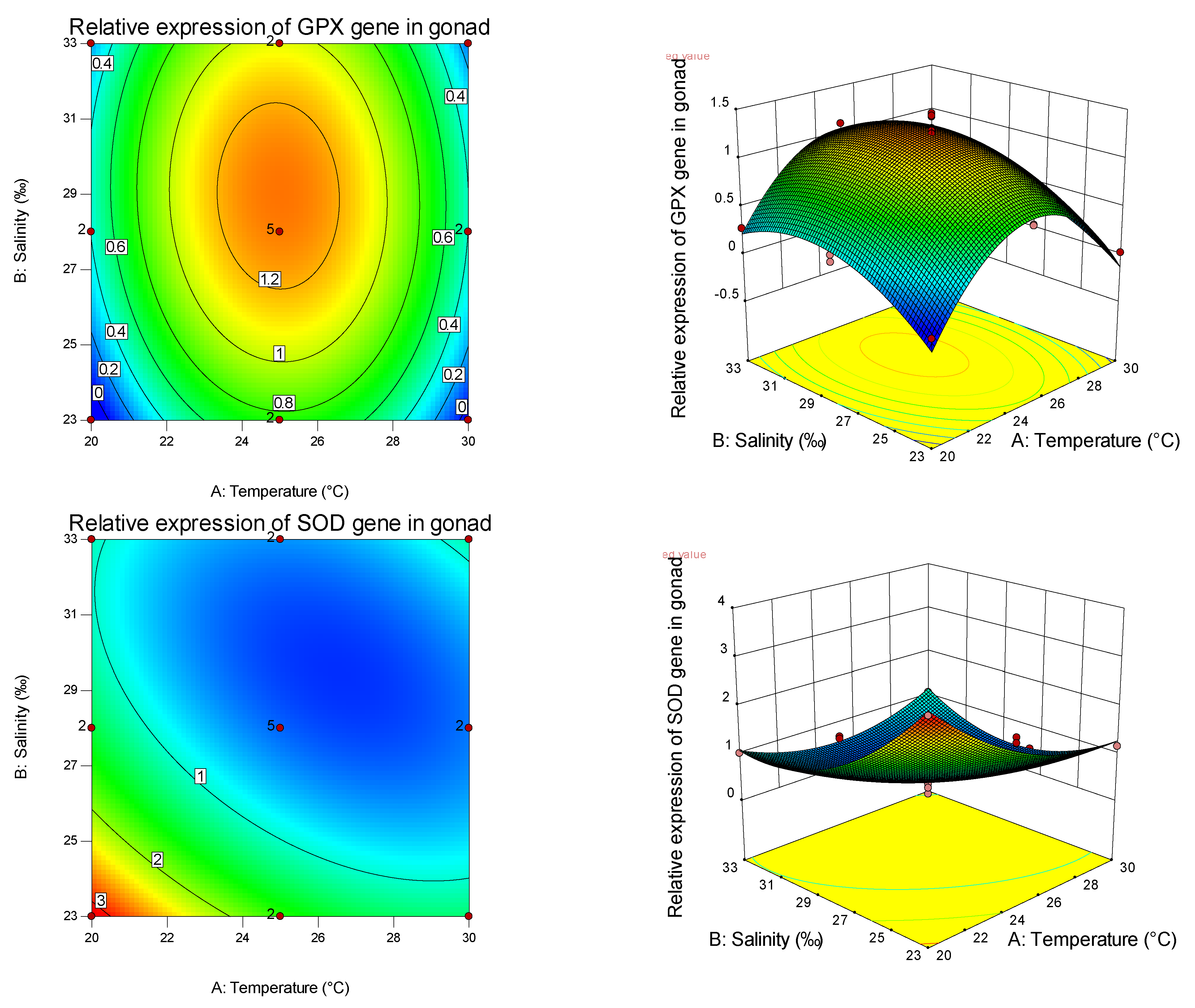
From the present study, it is clear that both temperature and salinity have significant impacts on the AKP, GSH-PX, MDA, SOD, CAT, GSH, POX, and GPX of the pearl oyster in a curvilinear fashion (
Figure 1,
Figure 2,
Table 7 and
Table 8). From the magnitude of the linear effect of two coded factors in
Table 7 and
Table 8, it can be seen that temperature is more important than salinity in affecting AKP, SOD, and CAT. Similar results have been found in other studies on fertilization and hatching in the pearl oyster [
1], embryonic development in the coot clam
Mulinia lateralis (Say) [
38], the brackish water clam
Rangia cuneata [
15], the northern bay scallop
Argopecten irradians irradians [
17], and the black-lip pearl oyster
Pinctada margaritifera (L.) [
39]. From the magnitude of the linear effect of two coded factors in
Table 7 and
Table 8, it can also be seen that temperature is more important than salinity in affecting the SOD gene, while salinity is more important than temperature in affecting the GPX gene. Thus, different antioxidants are affected by different empositions of temperature and salinity, and have tissue specificity. It can be found from the graphs that the curvature of the response surface is related to whether the first or second effects of temperature and salinity are significant, while the ellipse of the contour is related to the significance of the interaction between temperature and salinity.
The elimination and production of free radicals in aquatic animals are in a dynamic equilibrium state in daily production and life. As a critical (non-biological) factor affecting the physiology and biochemistry of aquatic animals, the salinity change destroys the dynamic balance of free radicals in the body, thereby affecting the body’s antioxidant defense system. In particular, as antioxidant enzymes, CAT and SOD, which can enhance cellular immunity and defense function, are regarded as the first line of defense against oxidative damage [
40,
41]. SOD plays a crucial role in the body’s immunity, mainly by scavenging free radicals to remove the harmful components left in the body during the oxidation process and reduce damage to the body [
42]. CAT is indispensable in living organisms and catalyzes the decomposition of hydrogen peroxide (produced by SOD, metabolized waste, damaging organisms) into oxygen and water (less toxic or harmless) [
43]. GSH-PX can catalyze the reduction of peroxides to hydroxyl compounds in aquatic cells, which is the second line of defense in the antioxidant defense system [
44,
45]. In addition to antioxidant enzymes, antioxidant defense systems include antioxidants, mainly fat-soluble carotenoids and VE, as well as water-soluble VC and GSH [
46,
47]. GSH is an important antioxidant and hydrogen donor of GSH-PX, which is used to catalyze organic peroxide. It is synthesized by cells and acts on the repair of injury in vivo and eliminating free radicals [
43]. In addition, MDA levels can be used to measure the degree of oxidative damage in the body, as it is a product of lipid peroxidation and can also indirectly reflect biological activity, free radical production, and antioxidant capacity [
48,
49]. In immune defense, AKP in hydrolases mainly exists in serum, which plays a role in removing foreign bodies [
50]. POX mainly exists in the hemolymph of invertebrates, as well as in multiple tissues of invertebrates, and plays different main functions in different tissues. POX is an indispensable enzyme in the immunity of mollusks, and is mainly involved in humoral immunity and cellular immunity in animals. Its activity can be directly used to measure the immune function of invertebrates [
20].
In this experiment, salinity had a significant effect on SOD and GSH-PX in the primary effect, but had no significant effect on CAT, indicating that SOD and GSH-PX were susceptible to salinity. In the study of the noble scallop [
51], it was found that the primary effect of salinity had a significant effect on CAT and SOD activities, but had no significant effect on GSH-PX. This may be because the antioxidant regulation mechanisms of different species on salinity are different, and the interaction effect shields the primary effect of CAT in the pearl oyster [
52,
53]. The secondary effects of salinity on CAT, SOD, and GSH-PX activities were significant, indicating that salinity had a nonlinear relationship with these antioxidant indexes, and CAT and SOD increased first and then decreased with the increase in salinity, which was consistent with the results of the noble scallop [
51]. This may be because (1) the oxygen consumption rate of two kinds of shellfish near the isotonic point was high, and the oxygen consumption rate under the salinity being lower or higher than the isotonic point showed a decreasing trend [
54], which inhibited the metabolism and redox reaction of the body, thus reducing the production of oxygen free radicals, and eventually leading to the decrease in antioxidant enzyme activity; (2) under non-isosmotic conditions, in order to adapt to the change of seawater salinity, shellfish need to consume the energy stored in the body, which will affect and change the activity of immune and digestive enzymes in the body; (3) when a large amount of reactive oxygen species far exceeds the scavenging capacity of the antioxidant system in the body, the cell structure will be oxidatively damaged, resulting in the decrease in the activity of antioxidant enzymes in the body [
55]. The enzyme activities of the ivory shell
Babylonia areolate [
55]; SOD activity of the mud crabs
Scylla serrata [
56]; SOD, CAT, and GSH-PX activity of the Manila clam
Ruditapes philippinarum [
57]; CAT activity of hepatopancreas of the Nile tilapia
Oreochromis niloticus [
58]; and SOD activity of the Suminoe oyster
Crassostrea rivularis [
59] were consistent with the trend of salinity. Therefore, in the process of artificial shellfish culture, the influence of seawater salinity on shellfish cannot be ignored. Salinity higher than 26–28‰ and 27–29‰ reduced the metabolism of the noble scallop [
51] and the pearl oyster, respectively, and increased the oxygen free radicals in the body, which could lead to the death of shellfish and cause economic losses.
In this study, the primary effect of salinity had a significant influence on MDA. In contrast, the secondary effect had no significant influence, indicating that salinity had a linear relationship with MDA, and showed a downward trend with the increase in salinity, indicating that low salinity had a greater degree of oxidative damage to shellfish. In this study, the effects of salinity on GSH-PX, GSH, and AKP (hemolymph) were significant, and the effects of secondary effects on POX were significant, indicating that salinity had a nonlinear relationship with these four indicators and its activity had a minimum value. The increase in antioxidant enzyme activity under high and low salinity may be the response of shellfish to maintain the dynamic balance of the antioxidant system under salinity stress. The AKP (hemolymph) of the pearl oyster increased significantly under high and low salinity conditions. Under suitable salinity conditions, the AKP (hemolymph) activity was low, which was the same as the results of Sun et al. [
23] on red-shell pearl oysters, Shi et al. [
59] on the oyster
Ostrea rivularis Gould, and Li et al. [
60] on the Zhikong scallop
Chlamys farreri and other aquatic organisms [
61,
62,
63]. The results were opposite or inconsistent with those of black-shell pearl oysters [
23], AKP in the digestive gland of the razor clam
Sinonovacula grandis [
64], and AKP in hepatopancreas in this study. This may be related to the differences in species, shell color, tissue site, and experimental conditions. The reason for the changes in AKP, GSH-PX, GSH, and POX may be that shellfish need much energy to regulate the osmotic pressure balance after severe changes in osmotic pressure caused by high and low salt, and the immune level is thus enhanced, so the activity of these four enzymes will increase [
60]. However, when the salinity was appropriate, the low activities of the four enzymes might be because shellfish immunity required less energy under suitable environmental conditions, and a large amount of energy was used for the growth of shellfish itself, so the enzyme activities related to antioxidants in the immune system were relatively low [
52].
With the change in salinity, although all kinds of immune and antioxidant (non) enzyme indexes of the pearl oyster were significantly affected, there was no consistent rule or obvious linear relationship, and the change rule was specific. It may be because of the disturbance effect of salinity stress on the immune and antioxidant system, but the degree of stress is still within the scope of the pearl oyster, which may also be related to the high adaptation and high resistance of the pearl oyster.
Temperature is an important environmental factor in abiotic factors, which is necessary for the survival of aquatic organisms. It affects the metabolism, biochemical reaction process, and activity of some biological macromolecules [
51]. Temperature changes often lead to decreased dissolved oxygen in water, and a large number of oxygen free radicals are generated by respiratory bursts, eventually leading to oxidative damage to shellfish. In this study, the primary and secondary effects of temperature had significant effects on GSH, GSH-PX, SOD, and CAT, indicating that the activities of these four antioxidant enzymes were significantly affected by temperature and had a nonlinear relationship with temperature. Among them, GSH, SOD, and CAT had maximum values. In the study of the Pacific abalone
Haliotis discus hannai Ino [
65], the Yesso scallop
Patinopecten yessoensis [
48], and the lwagaki oysters
Crassostrea nippona [
66], it was found that the changes in CAT and SOD activities in cooling and heating treatments were significantly different, which might be owing to the different regulatory mechanisms of cooling and heating on the antioxidant enzyme activities of different species. Other studies found that salinity’s primary and secondary effects had significant effects on the activities of CAT, GSH-PX, and SOD in the noble scallop [
51], and they all had maximum values. The changes in SOD activity in the hepatopancreas of the bivalve
Cyclina sinensis [
67] were the same, and the results of this study were consistent with it. These changes in SOD and CAT activities in the pearl oyster may be induced by a large amount of reactive oxygen species caused by accelerated metabolism with the increase in temperature under a period of external temperature stress. However, as the temperature exceeds the optimal range, the body’s vitality and metabolic ability will be reduced, affected by the changes in the body environment, eventually leading to the decrease in these two related antioxidant enzyme activities [
68,
69]. Excessive ambient temperature can make the body produce many oxygen free radicals, leading to death. Combined with the team’s previous experiments, under the corresponding temperature conditions, when the CAT and SOD activities of the pearl oyster were the highest, their activities, feeding, digestion, and metabolism were often better than those of other experimental groups. This indicated that the activities of some antioxidant enzymes in the pearl oyster were usually high under the optimal environmental conditions, which was conducive to reducing individual respiratory burst, scavenging oxygen free radicals in the body, and reducing mortality, which was consistent with the findings of the Pacific abalone [
70], the noble scallop [
51], the striped venus clam
Chamelea gallina [
71], the Chinese sturgeon
Acipenser sinensis Gray [
72], and the infaunal clam
Macoma balthica [
73]. Both the noble scallop [
51] and the pearl oyster belong to warm water shellfish, so their seawater temperatures should be controlled at 26.40 °C and 24.95 °C, respectively, under artificial indoor culture conditions to ensure that the two shellfishes have the strongest antioxidant capacity and the lowest individual mortality. For the sudden death of shellfish in the pool, the change in water temperature can be first checked to maintain it within the normal temperature range to prevent the death caused by respiratory burst, and then other possible factors such as bait or disease can be checked.
The primary effect of temperature significantly influenced AKP, while the secondary effect was not significant, indicating that temperature was linearly correlated with AKP and increased with the increase in temperature within a certain range. When shellfish are subjected to low-temperature stress, the metabolic rate and oxygen consumption are reduced, which inhibits the growth of microorganisms, the production of CO
2, ammonia and lactic acid, and the activity of shellfish to a certain extent, and reduces the risk of disease infection. This is also why a low temperature is suitable for in vivo transportation of shellfish [
74]. The reaction of AKP under low-temperature stress proves this. However, when shellfish are under high-temperature stress, the physiological disorder, metabolic rate, and oxygen consumption increase in the body under a stress state, which is susceptible to bacterial infection and requires much energy. The activities of nonspecific immune-related enzymes and antioxidant enzymes in shellfish will rise over time to resist changes in the external environment [
74]. In this study, the primary and secondary effects of temperature on GSH-PX and AKP in the hemolymph of the pearl oyster were significant, indicating that temperature had a nonlinear relationship with them, and the activity increased at a high temperature, which is consistent with the above rules. The primary effect of temperature and the interaction between temperature and salinity had no significant effect on POX, and the secondary effect was significant, indicating that temperature had a nonlinear relationship with POX and had high activity at a high temperature, consistent with the above rules. Li et al. [
60] and Chen et al. [
75]’s research on the Zhikong scallop, Liu et al. [
76]’ s research on the Zhikong scallop and the bay scallop
Argopectehs irradias, and Liu [
74]’ s research on the clam
Lutraria sieboldii Reeve found that AKP maintained a high activity level at a high temperature, which was consistent with the results of this study and conformed to the above laws. This may be related to the physical and chemical properties of AKP, and shellfish may be more sensitive to high-temperature stress. The change in AKP activity may also be due to the threat of high or low temperature to the normal survival of shellfish, and a high temperature leads to the emergence of many pathogens. Therefore, to resist adverse environments and ensure normal physiological activities, the pearl oyster must enhance its immunity [
60]. Hu et al. [
66] found that the AKP of the lwagaki oysters also increased significantly after 4 h and 8 h at 35 °C, but it was significantly lower than that of the control group, which was inconsistent with the results of this study, indicating that the lwagaki oysters had poor environmental adaptability, and the regulation of AKP was unbalanced under the temperature mutation of 35 °C. Under high-temperature stress, the AKP of the Manila clam [
77] showed a decreasing trend, and finally increased (returned) to a normal salt temperature level. The reason for these different results may be that high-temperature tolerance has species specificity. The primary and secondary effects of temperature had significant effects on MDA, indicating that there was a non-linear relationship between temperature and MDA, and a U-shaped distribution with the increase in temperature, indicating that both high and low temperatures caused oxidative damage to pearl oysters.
In this study, the combined effects of salinity and temperature on antioxidant and immune-related (non) enzyme indexes of the pearl oyster were analyzed by response surface methodology. The response surface method can obtain the model equation with a high fitting degree, optimize the experimental results, and obtain the optimal factor (factor) combination, which is its biggest advantage. However, given the influence of environmental factors on shellfish’s immune and antioxidant indexes, there is no reliable model established in domestic and foreign studies focusing on the analysis of some isolated levels under a single environmental condition. In this paper, based on the response surface analysis, we found that the response surface of SOD and CAT showed a convex curve shape, indicating that the two enzymes had a peak (maximum). By optimizing the established model, the theoretical conditions for SOD and CAT to reach the maximum activity were 24.95 °C/28.11‰, which are close to 26.4 °C and 27.7‰ of the noble scallop [
51], indicating that, under this condition, the external environment did not cause stress to shellfish, and the living environment was suitable for shellfish. The results showed that the interaction of salinity and temperature had significant effects on CAT and SOD of the pearl oyster, but had no significant effect on GSH-PX, which was completely consistent with the results of the noble scallop [
51]. This indicated that, for CAT and SOD, salinity and temperature had a mutual influence, and for GSH-PX, these two factors were independent without interference or superposition between additive effects. Studies on other aquatic organisms such as the cobia
Rachycentron canadum [
78] and juvenile GIFT Nile tilapia [
58] showed that the interaction between salinity and temperature on CAT and SOD activities were significant, which was consistent with the results of this study. However, there were few studies on the effect of the temperature–salt combination on GSH-PX. From the response surface diagram, the contour lines of AKP in hemolymph, GSH-PX, and POX under temperature and salinity stress were round, indicating that there was no interaction between temperature and salinity on AKP (hemolymph), GSH-PX, and POX. The interaction between salinity and temperature on AKP of the pearl oyster was obvious, which was contrary to the results of AKP (hemolymph) in this paper, and was consistent with the results of Li et al. [
60] on AKP, ACP, and GR in the hemolymph of the Zhikong scallop, and Zhu [
52] on AKP and ACP in juvenile pearl oysters. This may be because (1) the difference in AKP interaction may be related to the different combined effects of salinity and temperature on antioxidant indexes of different ages, different species, and different tissues; (2) AKP and POX in hemolymph were not affected by the interaction of temperature and salinity, either because the activity of antioxidant and immune enzyme in hemolymph was too low, the kit could not accurately determine its effective activity, or temperature and salinity did not interact with each other; (3) temperature changed the spatial conformation of AKP, and salinity provided the metal ions needed to activate AKP, so as to achieve the synergy between the two and jointly promote the regulation of AKP activity.
Many domestic and foreign experts and scholars have studied the interaction between marine invertebrate environmental factors and antioxidant enzymes. It is mainly reflected in the spatio-temporal and organizational differences in enzyme activity determination and expression level under various stress factors. So far, GPX, SOD, and GST are the most commonly studied antioxidant enzyme genes related to marine biological stress tolerance [
79]. However, there are few studies on the effects of temperature or salinity on the relative expression of SOD and GPX genes in shellfish.
Studies have shown that the shellfish antioxidant enzyme system has high tissue specificity [
80,
81]. In this study, for the GPX gene, the primary and secondary effects of salinity significantly affected its expression in the mantle, adductor muscle, and gonad, while the primary effect of salinity significantly affected its expression in the foot, and the secondary effect was not significant. This indicated that salinity had a significant influence on the expression of GPX in the mantle, adductor muscle, and gonad and had a non-linear relationship with it, and had a significant influence on the expression of GPX in the foot and had a linear relationship with it. Salinity had the maximum expression of GPX in the obturator muscle and gonad and the minimum expression of GPX in the mantle, while the expression of GPX in the foot increased with salinity. This indicates that salinity has tissue specificity for GPX gene expression. Similarly, the expression of the GPX gene by temperature and the expression of the SOD gene by salinity and temperature also have tissue specificity, and the relative expression of different genes in the same tissue is also different. In the study on salinity stress of the mud crabs [
82], with the increase in salinity, GPX activity in the abdominal muscle did not change, and SOD activity gradually decreased. GPX activity in the hepatopancreas decreased with increasing salinity, while SOD activity increased first and then decreased. GPX and SOD activities in gills decreased first and then increased with the increase in salinity, indicating the tissue specificity of salinity stress on the antioxidant defense and oxidative stress in aquatic animals. Through the analysis of the expression of antioxidant genes among different tissues under the effects of salinity and temperature on the primary, secondary, and interaction effects, the expression levels of GPX and SOD are consistent with and different from the trend of antioxidant and immune-related (non-) enzymes mentioned above, indicating that, in the pearl oyster, antioxidant and immune-related (non-) enzymes interacted with the expression of tissue immune genes to enhance the resistance and adaptability of shellfish to the environment.