Abstract
Lumpfish are widely used for removing sea lice in salmonid sea-based aquaculture. If these fish are to be harvested and used for human consumption, it is necessary to know how the physical strain associated with removing the lumpfish from the net-cages affects the fish in the short-term, and if live-storage in tanks, well-boats, or nets awaiting slaughter, will result in stress and mortalities. In this study, we investigated the effect of physical stress and mortality in a group of lumpfish recaptured from commercial net-cages, transported to holding tanks, and stored for one week. In addition to cortisol (primary stress response), we analyzed ions directly related to osmoregulation (Na+ and Cl−), osmotic stress (Ca2+), and blood plasma pH as an indicator of a secondary stress response. The aim of the study was to increase the basic physiological understanding of the physiological effects of handling procedures and transport in lumpfish. Only minor, and temporary, effects on primary stress response and secondary stress response were seen in lumpfish recaptured from net-cages and transported to holding facilities, indicating that lumpfish cope well with short transport (here 5 h). These findings are important in a context where lumpfish are harvested for reuse, e.g., human consumption or processing, following their lice-eating stage in net-cages.
1. Introduction
Biological control [1,2], using cleanerfish that pick the sea lice from salmonids [3,4,5,6] has been effective in reducing lice numbers and is being adopted widely by the salmon farming industry. The advantages of cleaner fish are environmental, with a reduction in medicine use [7], they are a natural form of control and provide continuous cleaning of lice [3,4,5,6,7]. As a cold-water cleanerfish alternative, the common lumpfish Cyclopterus lumpus L. has been demonstrated to work effectively in small-scale cage trials [4,8,9,10,11], and there is increasing evidence of efficacy in larger commercial pens [12,13,14,15].
Lumpfish and other cleaner fish are commonly used to control and reduce sea lice infestations in salmonid aquaculture, but lumpfish, especially, have a limited window as efficient grazers [14,15,16]. As the fish grow, the interest in eating sea lice falls, and after exceeding a size of approximately 300–400 g [14,15,16], there is little to gain from keeping lumpfish in salmon cages. Due to the risk of spreading diseases, smaller fish that still may be efficient grazers are prohibited from being reused in other locations if a net-cage is emptied and sent to slaughter, thus the lumpfish will be slaughtered as well. For salmon producers, this constitutes a cost, as lumpfish are not very well suited for silage and the fish are, therefore, often ending up as waste. The current production and use of lumpfish in Norway alone is more than 30 million individuals per year [14], meaning that a potential biomass of more than 12,000 tons (provided a slaughter weight of 0.4 kg) ends up as waste or silage. The ethical part of this practice is undoubtedly a concern, but both the economic (potential biomass for human consumption) and environmental (harvest of wild broodstock fish) aspects should also certainly be highlighted.
One of the major challenges hindering the high survival and further exploitation of lumpfish is that the fish are being treated as salmonids, and forced to undergo normal industrial processes developed for salmon, such as delousing, pumping, transport, and slaughter, all of which may have a negative impact on welfare and eventually increase mortality for the salmon [17,18] as well as for the lumpfish [19]. These procedures must be improved and adjusted to fit the lumpfish to secure its welfare and the quality of the fish to make it suitable for human consumption. It is necessary to know how the physical strain associated with removing the lumpfish from the net-cage affects the fish in the short-term, and if live-storage in tanks, well-boats, or nets awaiting slaughter, will result in stress and mortalities. For practical reasons, temporary live storage of lumpfish awaiting higher volumes before transport and slaughter (or slaughter on-site) is preferential, but if this compromises welfare, alternative methods must be sought.
There is great variation in the robustness and survival of lumpfish transferred to salmon cages to eat salmon lice [14,19,20], and some of the variations are thought to be related to the transport and handling of the fish before release. There is very little documented knowledge on lumpfish survival during and after transport, and observations of mortality in cages in general often have little focus. The short- and long-term effects of transport and the physiological state and robustness of the fish before release into cages have been documented to a very limited extent, and the basis for establishing protocols for transport, handling, and reception control is therefore not well founded. Mapping the stress response to characteristic environmental conditions during transport is therefore important and will provide a better understanding of the importance of good animal welfare, what kind of stress reaction one can expect from the individual handling methods, and environmental conditions during transport.
In this experiment, the effects on physical stress and mortality in a group of lumpfish re-captured from commercial net-cages, transported to holding tanks, and stored for one week, were investigated. The aim of the study was to increase the basic physiological understanding of transport on lumpfish. In addition to cortisol (primary stress response), we analyzed ions (Na+ and Cl−) directly related to osmoregulation and osmotic stress (Ca2+), as disturbances in the osmoregulatory capacity of the fish are typical secondary stress responses often observed in lumpfish as well as in other teleost species, whereas blood plasma pH is a good indicator of acid-base disturbances.
2. Materials and Methods
2.1. Experimental Fish
Juvenile lumpfish from a commercial producer in Norway (Senja Akvakultursenter, Troms, Norway) with an average weight of 22 g were transported by well-boat to a commercial net-cage facility for salmon outside Tromsø, Northern Norway on 16 June 2021. The lumpfish were stocked at a 10% density (lumpfish to salmon ratio, [12,14,15]), with a total of approximately 15,000 individuals in each net-cage.
2.2. Experimental Set-Up
At the start of the experiment on 7 September 2021, 15 fish (control group) were collected one by one from the net cage using a dip-net and immediately killed with a sharp blow to the head. Blood was thereafter collected from the heart using heparinized syringes, centrifuged at 8000 RPM, and the plasma was extracted and stored on ice until transferred to a −80 °C freezer. Following the collection of undisturbed fish, a large dip-net operated by a crane collected several fish in each attempt and transferred them to a sorting tank where small individuals were removed. 200 fish with an average weight of 230 g were thereafter placed in two boat transport tanks (1500 L) with continuous water renewal and a 5 h transport to the experimental facilities of Akvaplan-niva at Kvaløya (Troms, Norway) commenced. Blood was collected from 10 fish from each transport tank, following the same procedure as described above, halfway through the transport. Upon arrival, the fish were transferred to two 2000 L tanks with running seawater, and blood was collected from 10 fish in each tank at 1 h, 24 h, and 1 week post transfer.
2.3. Blood Plasma Analysis
Blood plasma was analyzed for pH, sodium-(Na+), calcium-(Ca2+) and chloride-(Cl−) concentrations using a Convergys® ISE comfort Electrolyte Analyzer (Convergent Technologies, Cölbe, Germany). Plasma cortisol was analyzed using a DetectX® Cortisol Enzyme Immuno Assay Kit (Ann Arbor, Michigan, USA) according to the manufacturer’s procedure. Samples were run in duplicates and read photometrically at 450 nm using Multiskan™ FC (Thermo Scientific™, Ullernchausseen, Norway). The assay detection limit is 0.05 ng mL−1.
2.4. Statistical Analyses
All statistical analyses were performed using STATISTICATM 14.0. To assess the normality of distributions, a Kolmogorov-Smirnov test [21] was used, and homogeneity of variances was tested using Levene’s F test [22]. Two-way nested ANOVA (Zar, 1984) was used to test for possible effect of transport on blood plasma cortisol, pH, Na+, Ca2+, and Cl−. Student–Newman–Keuls (SNK) multiple comparison post hoc test [21] was used to identify differences among treatments. A significance level (α) of 0.05 was used if not stated otherwise.
3. Results
3.1. Blood Plasma Cortisol
Blood plasma cortisol rose significantly from a base-line level of 3.7 ng mL−1 in the sea pen to 52.0 ng mL−1 under transport, and 76.3 ng mL−1 1 h post transport (p < 0.01, Figure 1). 24 h post transport, the cortisol level had sunk to 7.6 ng mL−1 and further to 6.3 ng mL−1 one week post transport.
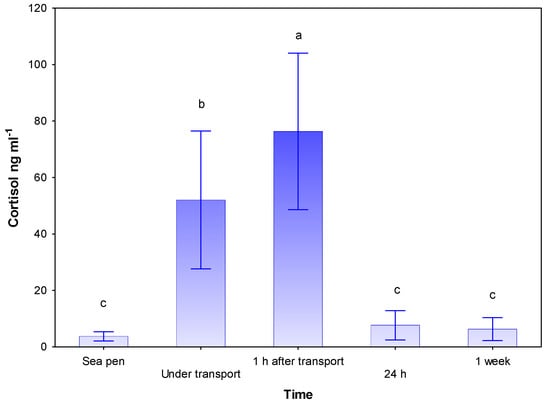
Figure 1.
Changes in blood plasma cortisol for lumpfish before and under transport, and 1 h, 24 h and one week after transport. Values are given as mean (SD). N = 20–30 for all groups. Different letters denote significant difference (Student–Newman–Keuls (SNK) test, p < 0.05) between treatments.
3.2. Blood Plasma pH, Ca2+, Cl−, Na+
The transport of lumpfish had no effect on blood plasma pH, Ca2+ or Na+ (p > 0.5, Figure 2A,B,D). Lower values (p < 0.05, Figure 2C) of Cl− were found under transport and after transport of lumpfish from sea pens to land-based tanks stabilizing at a lower level after 24 h post transport.
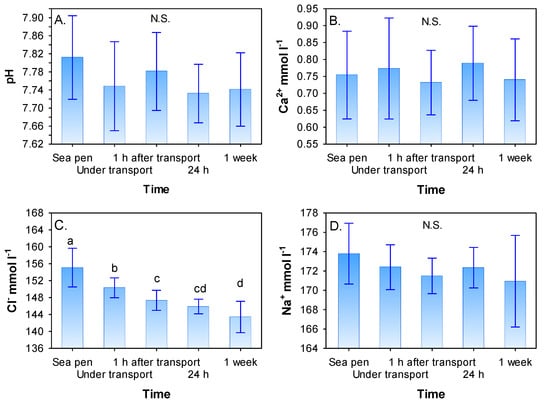
Figure 2.
Changes in blood plasma pH (A), Ca2+ (B), Cl− (C) and Na+ (D) for lumpfish before and under transport, 1 h, 24 h and one week after transport. Values are given as mean (SD). N = 20–30 for all groups. Different letters denote significant difference (Student–Newman–Keuls (SNK) test, p < 0.05) between treatments. N.S. = not significant.
4. Discussion
In this study changes in plasma cortisol were used as a quantitative measure of the fish’s primary stress response to various environmental changes and handling. The effect of the primary stress response includes mobilization of energy reserves, changes in immune function, and increased permeability of cell membranes that affect osmoregulation [23,24,25,26]. Baseline plasma cortisol was 3.7 and peaked at 76.3 ng mL−1 1 h post-transport (Figure 1). These levels of plasma cortisol under and immediately after transport are not high in comparison to experiments performed on Atlantic salmon [23] and ballan wrasse [24]. Similar resting levels of cortisol in lumpfish were found by [25,26,27]. Stress is known to build up when fish are exposed to repeated stressors within a limited period of time. Such an accumulation of stress can be avoided if the fish have time to recover from each stressor. Without recovery time, the next stressor that may not be fatal when acting as an individual case, can prove fatal [25,28,29,30]. There is a clear potential for this risk when lumpfish are transferred to cages where they again face new environmental changes that are likely to impose further stress. Whether the fish manage to respond to this additional stress and eventually restore physiological equilibrium (homeostasis) depends on whether the fish have the ability to respond and compensate. If it fails to do so, it will eventually die. In some cases, lumpfish mortality is reported after sea pen transfer [12], but it is complicated to follow up after it has been exposed to the sea as to whether this is linked to chronic stress built up during transport or if it has other causes. In this experiment, lumpfish display an immediate reaction to the physical strain of handling and transport with increased cortisol levels, but quickly return to pre-stress levels, indicating that the fish are able to fully compensate.
Blood plasma chloride levels fell during the experimental period (Figure 2C) and did not reach pre-transport levels one week post-transport, seemingly indicating a delayed effect of stress on plasma chloride. A similar reaction to a stressor on the hydromineral balance in lumpfish was found by [26] who suggested this is most likely a compensatory response on the cell level. Plasma chloride decrease may also be linked to active acid-base compensations [31]. However, acid-base regulation is linked to respiration, nitrogen secretion, and osmo- and ion-regulation. With the limited knowledge one currently has about these physiological processes in lumpfish, and how stress affects them, this must be seen as a loose hypothesis. It has been shown that fresh water can accumulate in the stomach/intestines of lumpfish [32] and that this species has tissues containing low levels of ions as one of several mechanisms for achieving near-neutral buoyancy [33,34]. There is, therefore, reason to believe that lumpfish regulation of its internal physiological equilibrium differs from other well-known farmed species studied in more detail.
The measured blood plasma concentrations, i.e., both mean values and variation in values in Figure 2 were in the range found for control groups in previously published results in studies on stress physiology in lumpfish (cortisol, [26,27]; pH, [35]; Cl−, [26,27,36]; Na+, [27]). At present, no published studies on blood plasma calcium in lumpfish exist for comparison.
In general changes in calcium ions (Ca2+) in blood plasma is a picture of osmotic stress influenced by increased permeability in the gill epithelial at increased levels of cortisol [37]. Compensation for this occurs by excretion of divalent ions over the kidneys. As no change in calcium ions and sodium in blood plasma was seen in the present study (Figure 2B,D) this may indicate low osmotic stress in relation to the transport procedure applied in this study. Overall, transport had no effect on blood plasma pH, calcium, and sodium. This may indicate a low-stress response in lumpfish, which is similar to the findings of [27] that suggested that this is related to the sedentary lifestyle of lumpfish where a strong stress response is not crucial for survival. Similar to the study of [27], the hematological effects in relation to stress presented here are generally similar to sturgeons [38,39,40,41], which are distantly related to lumpfish.
Fish densities under (15.3 kg m−3), and after transport, (11.5 kg m−3) were comparable to current transport practices with lumpfish in Norway [20]. Jonassen and Remen [20] found that increased fish density within the density restrictions practiced during primary transports (14–47 kg m−3) did not cause any increase in stress levels. Nor did an increase in transport time from 1 to 28 h affect the stress response measured at the end of transport. The follow-up of 15 commercial transports of lumpfish suggests that established practice for the transport of lumpfish of 20–60 g at densities from 30–50 kg m−3 of up to 20 h (and in some cases more) does not seem to adversely affect the physiological state of the fish. Current data are in line with these previous findings.
Overall, present findings indicate a limited stress response in recaptured lumpfish during and after transport. A reduced stress response can be beneficial for farmed fish, because production causes frequent disturbances e.g., daily maintenance, feeding, sorting, and transport [42]. Although the stress response is basically an adaptive response intended to help the animal re-establish its equilibrium in the face of a stressor [43], the frequent firing of a strong stress response will have a cost in aquaculture [44]. Stress can lead to reduced immune function, reduced ability to regulate ions, and reduced appetite [45,46]. It is too early to conclude that lumpfish are stress tolerant, simply because of their relatively low cortisol levels and minor changes in blood plasma pH, Ca2+, Cl−, and Na+ seen in the present study. There is currently no knowledge about cortisol metabolism (production and degradation) or cortisol receptor density in lumpfish, and there is currently limited knowledge about the relationship between measured cortisol levels in plasma and secondary/tertiary stress responses. Until more knowledge is available in this area, it is recommended that lumpfish are handled with as much gentleness as other farmed species during transport and handling.
5. Conclusions
In conclusion, only minor, and temporary, effects on the primary stress response (blood plasma cortisol) and secondary stress response directly related to osmoregulation (Na+ and Cl−), osmotic stress (Ca2+), and blood acid-base disturbances (pH) in lumpfish were seen in lumpfish recaptured from net-cages and transported to holding facilities, indicating that lumpfish cope well with short transport (here 5 h). These findings are important in a context where lumpfish are harvested for reuse, e.g., human consumption or processing, following their lice-eating stage in net-cages.
Author Contributions
A.F. conducted the experiment and analyzed the data, A.K.D.I. and A.F. wrote the article and reviewed the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
Financial support was given by the Research Council of Norway (Lumpfish4Food, 301494).
Institutional Review Board Statement
The present field trials were approved by the local responsible laboratory animal science specialist under the surveillance of the Norwegian Animal Research Authority (NARA) and registered by the Authority.
Data Availability Statement
Not applicable.
Acknowledgments
We would like to thank the staff at Kraknes Research Station for their assistance during the trials.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Grutter, A.S. Cleaner fish really do clean. Nature 1999, 398, 672–673. [Google Scholar] [CrossRef]
- Vaughan, D.B.; Grutter, A.S.; Costello, M.J.; Hutson, K.S. Cleaner fishes and shrimp diversity and re-evaluation of cleaning symbioses. Fish Fish. 2017, 18, 698–716. [Google Scholar] [CrossRef]
- Skiftesvik, A.B.; Bjelland, R.M.; Durif, C.M.F.; Johansen, I.S.; Browman, H.I. Delousing of Atlantic salmon (Salmo salar) by cultured vs. wild ballan wrasse (Labrus bergylta). Aquaculture 2013, 402–403, 113–118. [Google Scholar] [CrossRef]
- Imsland, A.K.; Reynolds, P.; Eliassen, G.; Hangstad, T.A.; Foss, A.; Vikingstad, E.; Elvegård, T.A. The use of lumpfish (Cyclopterus lumpus L.) to control sea lice (Lepeophtheirus salmonis Krøyer) infestations in intensively farmed Atlantic salmon (Salmo salar L.). Aquaculture 2014, 424–425, 18–23. [Google Scholar] [CrossRef]
- Powell, A.; Treasurer, J.W.; Pooley, C.L.; Keay, A.J.; Lloyd, R.; Imsland, A.K.; Garcia de Leaniz, C. Cleaner fish for sea-lice control in salmon farming: Challenges and opportunities using lumpfish. Rev. Aquac. 2018, 10, 683–702. [Google Scholar] [CrossRef]
- Brooker, A.J.; Papadopoulou, A.; Gutierrez, C.; Rey, S.; Davie, A.; Migaud, H. Sustainable production and use of cleaner fish for the biological control of sea lice: Recent advances and current challenges. Vet. Rec. 2018, 183, 383. [Google Scholar] [CrossRef]
- Treasurer, J.W. A review of potential pathogens of sea lice and the application of cleaner fish in biological control. Pest. Manag. Sci. 2002, 58, 546–558. [Google Scholar] [CrossRef] [PubMed]
- Imsland, A.K.; Reynolds, P.; Eliassen, G.; Hangstad, T.A.; Foss, A.; Vikingstad, E.; Elvegård, T.A. Notes on the behaviour of lumfish with and without Atlantic salmon present. J. Ethol. 2014, 32, 117–122. [Google Scholar] [CrossRef]
- Imsland, A.K.; Reynolds, P.; Eliassen, G.; Hangstad, T.A.; Nytrø, A.V.; Foss, A.; Vikingstad, E.; Elvegård, T.A. Assessment of growth and sea lice infection levels in Atlantic salmon stocked in small-scale cages with lumpfish. Aquaculture 2014, 433, 137–142. [Google Scholar] [CrossRef]
- Imsland, A.K.; Reynolds, P.; Eliassen, G.; Hangstad, T.A.; Nytrø, A.V.; Foss, A.; Vikingstad, E.; Elvegård, T.A. Feeding preferences of lumpfish (Cyclopterus lumpus L.) maintained in open net-pens with Atlantic salmon (Salmo salar L.). Aquaculture 2015, 436, 47–51. [Google Scholar] [CrossRef]
- Imsland, A.K.; Reynolds, P.; Eliassen, G.; Hangstad, T.A.; Nytrø, A.V.; Foss, A.; Vikingstad, E.; Elvegård, T.A. Assessment of suitable substrates for lumpfish in sea pens. Aquac. Int. 2015, 23, 639–645. [Google Scholar] [CrossRef]
- Imsland, A.K.; Hanssen, A.; Reynolds, P.; Nytrø, A.V.; Jonassen, T.M.; Hangstad, T.A.; Elvegård, T.A.; Urskog, T.C.; Mikalsen, B. It works! Lumpfish can significantly lower sea lice infections in large scale salmon farming. Biol. Open 2018, 7, bio036301. [Google Scholar] [CrossRef] [PubMed]
- Eliasen, K.; Danielsen, E.; Johannesen, Á.; Joensen, L.L.; Patursson, E.J. The cleaning efficacy of lumpfish (Cyclopterus lumpus L.) in Faroese salmon (Salmo salar L.) farming pens in relation to lumpfish size and season. Aquaculture 2018, 488, 61–65. [Google Scholar] [CrossRef]
- Boissonnot, L.; Kharlova, I.; Iversen, N.S.; Staven, F.R.; Austad, M. Characteristics of lumpfish (Cyclopterus lumpus) with high cleaning efficacy in commercial Atlantic salmon (Salmo salar) production. Aquaculture 2022, 560, 738544. [Google Scholar] [CrossRef]
- Imsland, A.K.D.; Reynolds, P. In lumpfish we trust? The efficacy of lumpfish to control Lepeophtheirus salmonis infestations on farmed Atlantic salmon: A review. Fishes 2022, 7, 220. [Google Scholar] [CrossRef]
- Imsland, A.K.; Reynolds, P.; Nytrø, A.V.; Eliassen, G.; Hangstad, T.A.; Jónsdóttir, Ó.D.B.; Emaus, P.A.; Elvegård, T.A.; Lemmens, S.C.A.; Rydland, R.; et al. Effects of lumpfish size on foraging behaviour and co-existence with sea lice infected Atlantic salmon in sea cages. Aquaculture 2016, 465, 19–27. [Google Scholar] [CrossRef]
- Roth, B. Avlusing av Laksefisk med Optilice: Effekt på Avlusing og Fiskevelferd. Nofima AS, Rapport 59/2016. 2016. In Norwegian with Abstract in English. Available online: https://nofima.no/publikasjon/1408716/ (accessed on 2 August 2022).
- Oliveira, V.H.S.; Dean, K.R.; Qviller, L.; Kirkeby, C.; Jensen, B.B. Factors associated with baseline mortality in Norwegian Atlantic salmon farming. Sci. Rep. 2021, 11, 14702. [Google Scholar] [CrossRef]
- Sommerset, I.; Walde, C.S.; Bang Jensen, B.; Bornø, B.; Haukaas, A.; Brun, E. The Health Situation in Norwegian Aquaculture 2019. Available online: https://www.vetinst.no/rapporter-og-publikasjoner/rapporter/2020/fiskehelserapporten-2019 (accessed on 2 August 2022).
- Jonassen, T.M.; Remen, M. Utvikling av Transport- og Mottaksprosedyrer for Rognkjeks Basert på Kartlegging av Miljø og Stress. Akvaplan-Niva Rapport nr. 7707-1. 2017. Available online: https://www.fhf.no/prosjekter/prosjektbasen/901158/ (accessed on 8 August 2022).
- Zar, J.H. Biostatistical Analysis, 2nd ed.; Prentice-Hall, Inc.: Englewood Cliffs, NJ, USA, 1984; 718p. [Google Scholar]
- Brown, M.B.; Forsythe, A.B. Robust tests for the equality of variances. J. Am. Stat. Assoc. 1974, 69, 364–367. [Google Scholar] [CrossRef]
- Iversen, M.H. Stress and Its Impact on Animal Welfare during Commercial Production of Atlantic Salmon (Salmo salar L.). Ph.D. Thesis, Nord University, Bodø, Norway, April 2013. [Google Scholar]
- Iversen, M.H.; Jakobsen, R.; Eliassen, R.; Ottesen, O. Sedasjon av berggylt og rognkjeks for å redusere stress og dødelighet. NFexpert 2015, 39, 42–46. [Google Scholar]
- Iversen, M.H.; Eliassen, R.A. The effect of allostatic load on hypothalamic-pituitary-interrenal (HPI) axis before and after secondary vaccination in Atlantic salmon postsmolts (Salmo salar L.). Fish Physiol. Biochem. 2014, 40, 527–538. [Google Scholar] [CrossRef]
- Hanssen, H.J. The Effect of Long-Term Stress on Basal Levels of Plasma Cortisol and Hypothalamic–Pituitary–Interrenal (hpi) Axis in Lumpsucker (Cyclopterus lumpus). Master’s Thesis, Nord University, Bodø, Norway, June 2016. [Google Scholar]
- Hvas, M.; Folkedal, O.; Imsland, A.K.; Oppedal, F. Metabolic rates, swimming capabilities, thermal niche and stress response of the lumpfish, Cyclopterus lumpus. Biol. Open 2018, 7, bio036079. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Finstad, B.; Iversen, M.; Sandodden, R. Stress reducing methods for releases of Atlantic salmon (Salmo salar) smolts in Norway. Aquaculture 2003, 222, 203–214. [Google Scholar] [CrossRef]
- Iversen, M.; Eliassen, R.A. The effect of AQUI-S® sedation on primary, secondary, and tertiary stress responses during salmon smolt, Salmo salar L., transport and transfer to sea. J. World Aquac. Soc. 2009, 40, 216–225. [Google Scholar] [CrossRef]
- Wendelaar Bonga, S.E. The stress response in fish. Physiol. Rev. 1997, 77, 591–625. [Google Scholar] [CrossRef]
- Evans, D.H.; Piermarini, P.M.; Choe, K.P. The multifunctional fish gill: Dominant site of gas exchange, osmoregulation, acid-base regulation, and excretion of nitrogenous waste. Physiol. Rev. 2005, 85, 97–177. [Google Scholar] [CrossRef]
- Frantzen, M.; Hansen, B.H.; Geraudie, P.; Palerud, J.; Falk-Petersen, I.B.; Olsen, G.H.; Camus, L. Acute and long-term biological effects of mechanically and chemically dispersed oil on lumpsucker (Cyclopterus lumpus). Mar. Environ. Res. 2015, 105, 8–19. [Google Scholar] [CrossRef]
- Davenport, J.; Kjørsvik, E. Buoyancy in the lumpsucker Cyclopterus lumpus. J. Mar. Biol. Assoc. U. K. 1986, 66, 159–174. [Google Scholar] [CrossRef]
- Berg, M. Comparing Density of Lumpfish (Cyclopterus lumpus) Used in Aquaculture to Different Welfare Indicators. Master’s Thesis, University of Bergen, Bergen, Norway, June 2021. [Google Scholar]
- Remen, M.; Nes, A.M.; Hangstad, T.A.; Geraudie, P.; Reynolds, P.; Urskog, T.C.; Hanssen, A.; Stefansson, S.O.; Imsland, A.K.D. Temperature and size-dependency of lumpfish (Cyclopterus lumpus) oxygen requirement and tolerance. Aquaculture 2022, 548, 737576. [Google Scholar] [CrossRef]
- Sällebrant, J.B. Chronic Allostatic Overload on the Hypothalamic-Pituitary-Interrenal Axis of Lumpfish (Cyclopterus lumpus). Effect of Size. Master’s Thesis, Nord University, Bodø, Norway, June 2018. [Google Scholar]
- Arends, R.J.; Mancera, J.M.; Munoz, J.L.; Wendelaar Bonga, S.E.; Flik, G. The stress response of the gilthead sea bream (Sparus aurata L.) to air exposure and confinement. J. Endocrionol. 1999, 163, 149–157. [Google Scholar] [CrossRef]
- Maxime, V.; Nonnotte, G.; Peyraud, C.; Williot, P.; Truchot, J.P. Circulatory and respiratory effects of an hypoxic stress in the Siberian sturgeon. Respir. Physiol. 1995, 100, 203–212. [Google Scholar] [CrossRef]
- Barton, B.A.; Bollig, H.; Hauskins, B.L.; Jansen, C.R. Juvenile pallid (Scaphirhynchus albus) and hybrid pallid x shovelnose (S. albus x platorynchus) sturgeons exhibit low physiological responses to acute handling and severe confinement. Comp. Biochem. Physiol. 2000, 126A, 125–134. [Google Scholar] [CrossRef]
- Belanger, J.M.; Son, J.H.; Laugero, K.D.; Moberg, G.P.; Doroshov, S.I.; Lankford, S.E.; Cech, J.J., Jr. Effects of short-term management stress and ACTH injections on plasma cortisol levels in cultured white sturgeon, Acipenser transmontanus. Aquaculture 2001, 203, 165–176. [Google Scholar] [CrossRef]
- Baker, D.W.; Wood, A.M.; Litvak, M.K.; Kieffer, J.D. Haematology of juvenile Acipenser oxyrinchus and Acipenser brevirostrum at rest and following forced activity. J. Fish Biol. 2005, 66, 208–221. [Google Scholar] [CrossRef]
- Øverli, Ø.; Sørensen, C.; Kiessling, A.; Pottinger, T.G.; Gjøen, H.M. Selection for improved stress tolerance in rainbow trout (Oncorhynchus mykiss) leads to reduced feed waste. Aquaculture 2006, 261, 776–781. [Google Scholar] [CrossRef]
- Barton, B.A. Stress in fishes: A diversity of responses with particular reference to changes in circulating corticosteroids. Int. Comp. Biol. 2002, 42, 517–525. [Google Scholar] [CrossRef]
- Barton, B.A.; Iwama, G.K. Physiological changes in fish from stress in aquaculture with emphasis on the response and effects of corticosteroids. Ann. Rev. Fish Dis. 1991, 1, 3–26. [Google Scholar] [CrossRef]
- Wendelaar Bonga, S.E. Hormonal Responses to Stress. In Encyclopaedia of Fish Physiology; Farrell, A., Ed.; Academic Press: San Diego, CA, USA, 2011; pp. 1515–1523. [Google Scholar]
- Remen, M.; Oppedal, F.; Torgersen, T.; Imsland, A.K.; Olsen, R.E. Effects of cyclic environmental hypoxia on physiology and feed intake of post-smolt Atlantic salmon: Initial responses and acclimation. Aquaculture 2012, 326, 148–155. [Google Scholar] [CrossRef] [Green Version]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).