Comparing Body Density of Lumpfish (Cyclopterus lumpus) to Different Operational Welfare Indicators
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Fish and Location
2.2. Sample Collection
2.3. Measuring Body Density of Lumpfish
2.4. Hepatosomatic Index (HSI)
2.5. Condition Factor
2.6. Operational Welfare Indicators
2.7. Statistical Analyses
3. Results
3.1. Disease at Location D
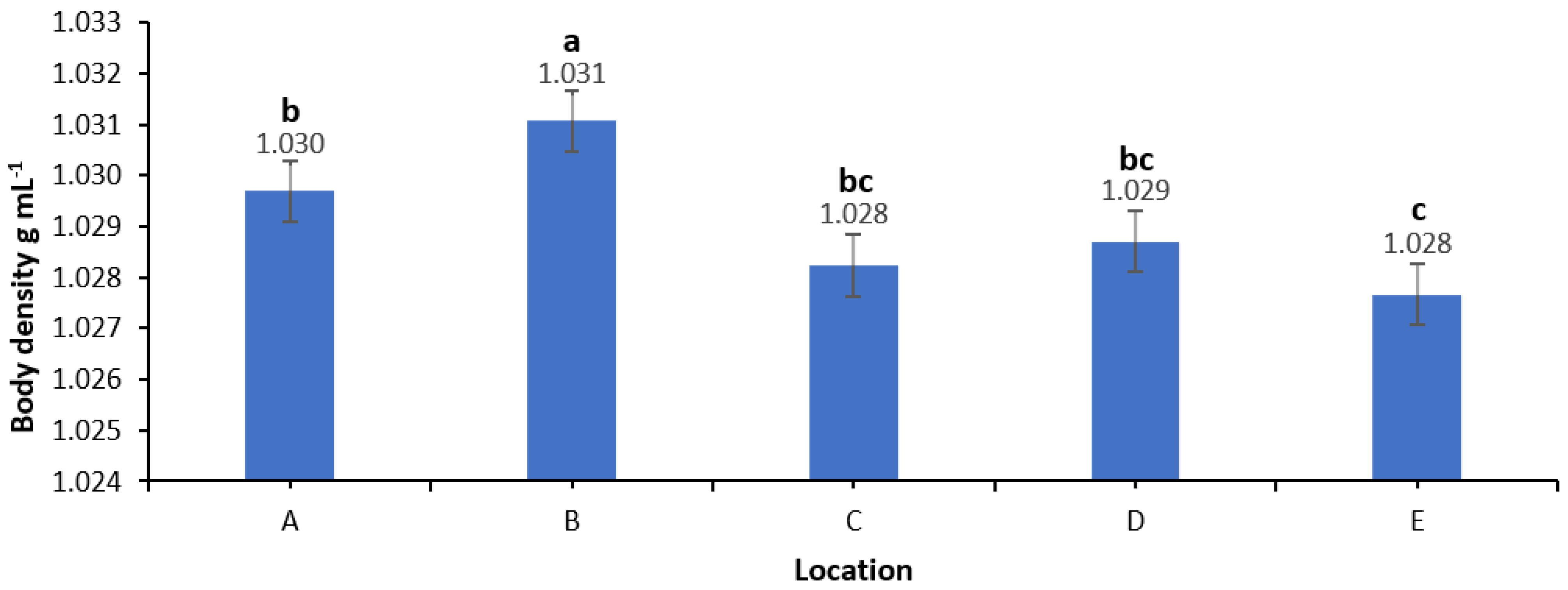
3.2. Body Density
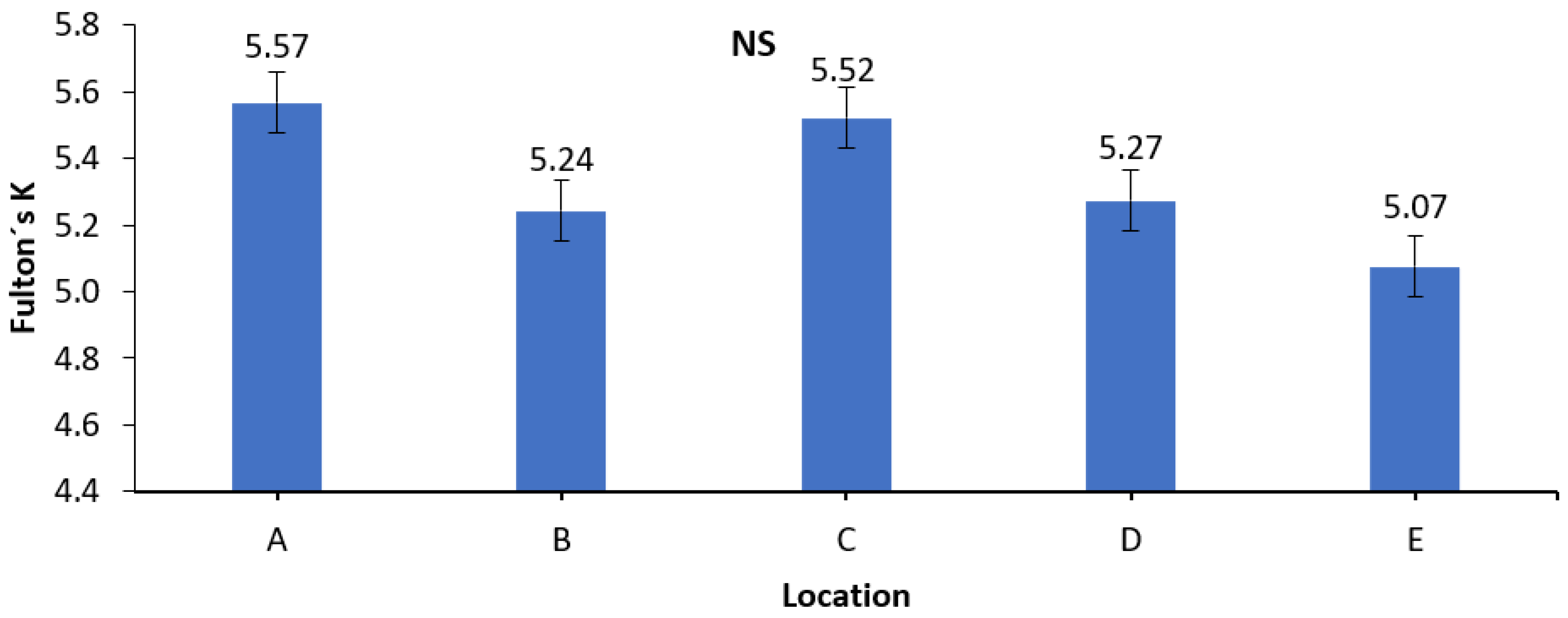
3.3. Fulton’s K
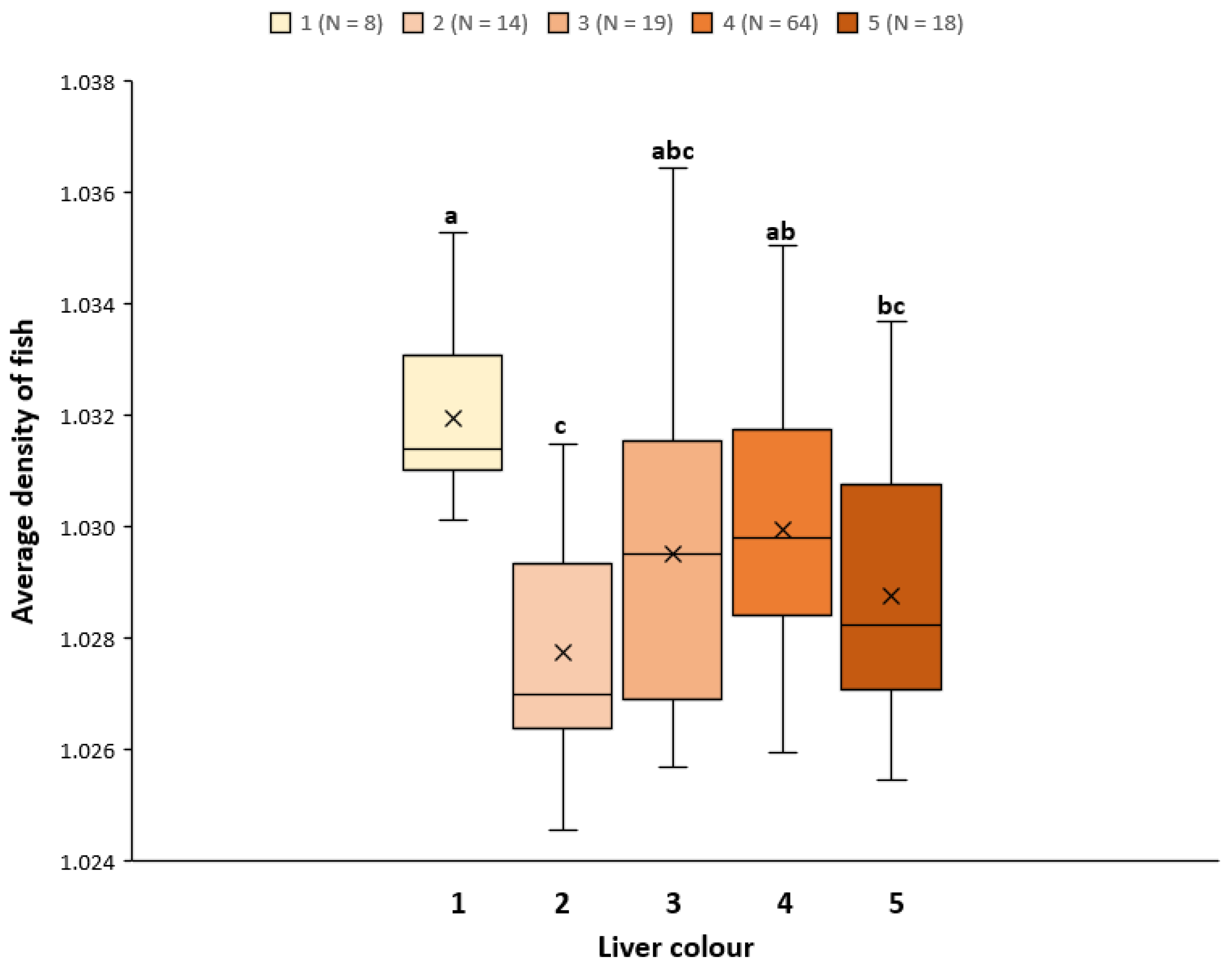
3.4. Welfare Status
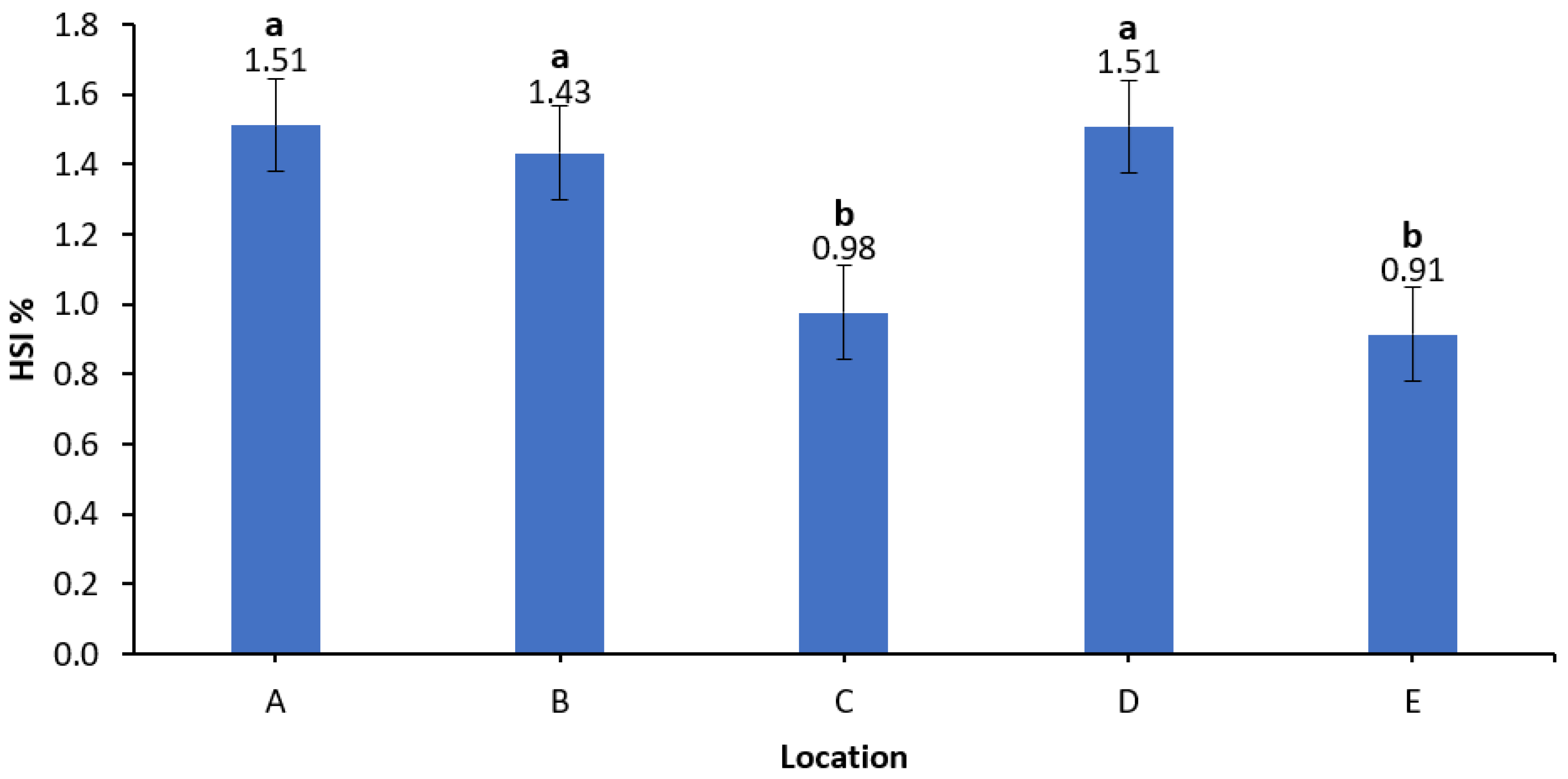
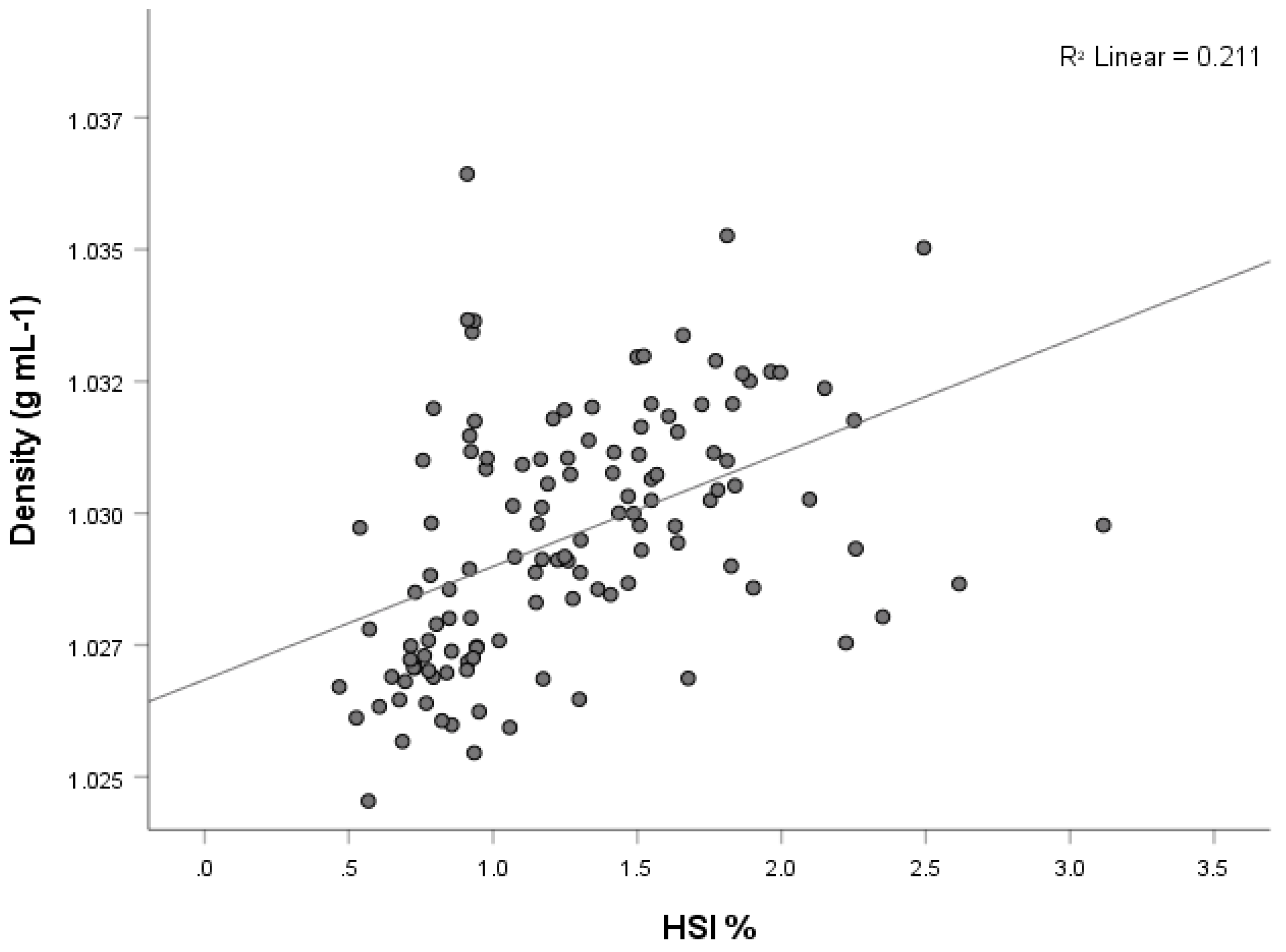
3.5. Hepatosomatic Index (HSI)
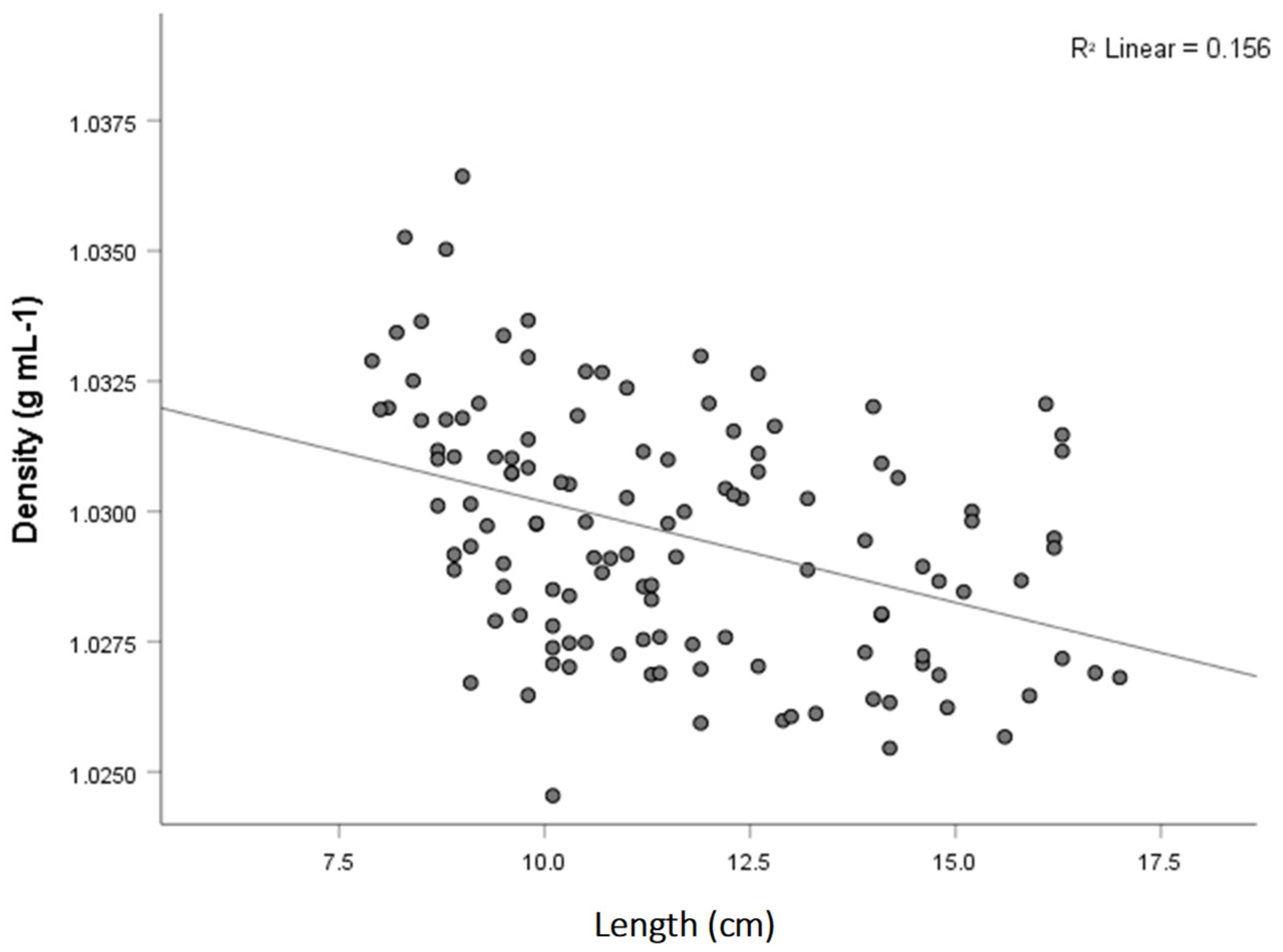
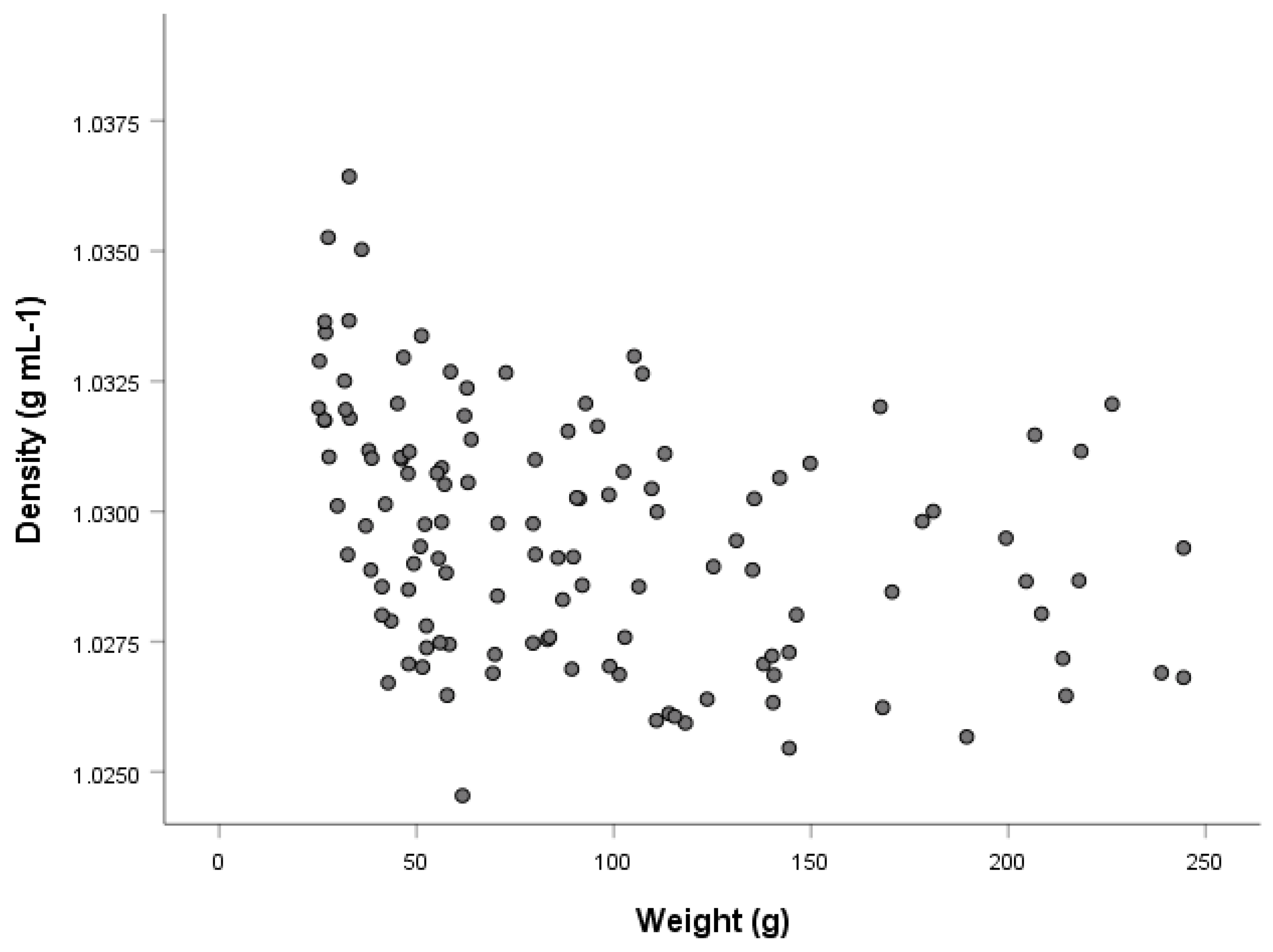
3.6. Length and Weight
4. Discussion
4.1. Lumpfish Body Density and Welfare Status
4.2. Lumpfish Body Density and Buoyancy Compared to Other Species
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Haugland, G.T.; Imsland, A.K.D.; Reynolds, P.; Treasurer, J. Application of biological control; use of cleaner fish. In Aquaculture Health Management: Design and Operational Approaches; Kibenge, F.S.B., Powell, M.D., Eds.; Elsevier: London, UK, 2020; pp. 319–369. [Google Scholar]
- Imsland, A.K.D.; Reynolds, P.; Lorentzen, M.; Eilertsen, R.A.; Micallef, G.; Tvenning, R. Improving survival and health of lumpfish (Cyclopterus lumpus L.) by the use of feed blocks and operational welfare indicators (OWIs) in commercial Atlantic salmon cages. Aquaculture 2020, 527, 735476. [Google Scholar] [CrossRef]
- Powell, A.; Treasurer, J.W.; Pooley, C.L.; Keay, A.J.; Lloyd, R.; Imsland, A.K.; Garcia de Leaniz, C. Cleaner fish for sea-lice control in salmon farming: Challenges and opportunities using lumpfish. Rev. Aquac. 2018, 10, 683–702. [Google Scholar] [CrossRef]
- Council for Animal Ethics. Er det Mulig å Bruke Rensefisk på en Etisk og Velferdsmessig Forsvarlig Måte i Norske Oppdrettsanlegg? In Norwegian. 2020. Available online: https://www.radetfordyreetikk.no/er-det-mulig-a-bruke-rensefisk-pa-en-etisk-og-velferdsmessig-forsvarlig-mate-i-norske-oppdrettsanlegg/?fbclid=IwAR0gLvlhTbeWtdpgGE6_8QKOnTRhfk-32x_XvyM986G7FRJ6sl3y1SxfKVg (accessed on 1 June 2021).
- Davenport, J.; Kjørsvik, E. Buoyancy in the lumpsucker Cyclopterus lumpus. J. Mar. Biol. Assoc. UK 1986, 66, 159–174. [Google Scholar] [CrossRef]
- Phleger, C.F. Buoyancy in marine fishes: Direct and indirect role of lipids. Am. Zool. 1998, 38, 321–330. [Google Scholar] [CrossRef]
- Sargent, J.R.; Tocher, D.R.; Bell, J.G. The lipids. In Fish Nutrition, 3rd ed.; Halver, J.E., Hardy, R.W., Eds.; Elsevier Academic Press: San Diego, CA, USA, 2003; pp. 181–257. [Google Scholar]
- Eliasen, K.; Patursson, E.J.; McAdam, B.J.; Pino, E.; Morro, B.; Betancor, M.; Baily, J.; Rey, S. Liver colour scoring index, carotenoids and lipid content assessment as a proxy for lumpfish (Cyclopterus lumpus L.) health and welfare condition. Sci. Rep. 2020, 10, 8927. [Google Scholar] [CrossRef]
- Gutierrez Rabadan, C.; Spreadbury, C.; Consuegra, S.; Garcia de Leaniz, C. Development, validation and testing of an Operational Welfare Score Index for farmed lumpfish Cyclopterus lumpus L. Aquaculture 2021, 531, 735777. [Google Scholar] [CrossRef]
- Madaro, A.; Olsen, R.E.; Kristiansen, T.S.; Ebbesson, L.O.; Nilsen, T.O.; Flik, G.; Gorissen, M. Stress in Atlantic salmon: Response to unpredictable chronic stress. J. Exp. Biol. 2015, 218, 2538–2550. [Google Scholar] [CrossRef] [PubMed]
- Niclasen, B.A.; Simonsen, K. High Resolution Wave Climate of the Faroe Islands; University of the Faroe Islands: Tórshavn, Faroe Islands, 2012. [Google Scholar]
- Millero, F.J.; Poisson, A. International one-atmosphere equation of state of seawater. Deep Sea Res. Part A Oceanog. Res. Pap. 1981, 28, 625–629. [Google Scholar] [CrossRef]
- Willora, F.P.; Grønevik, B.; Liu, C.; Palihawadana, A.; Sørensen, M.; Hagen, Ø. Total replacement of marine oil by rapeseed oil in plant protein rich diets of juvenile lumpfish (Cyclopterus lumpus): Effects on growth performance, chemical and fatty acid composition. Aquac. Rep. 2021, 19, 100560. [Google Scholar] [CrossRef]
- Zar, J.H. Biostatistical Analysis, 2nd ed.; Prentice-Hall, Inc.: Englewood Cliffs, NJ, USA, 1984; 718p. [Google Scholar]
- Skoge, R.H.; Brattespe, J.; Økland, A.L.; Plarre, H.; Nylund, A. New virus of the family Flaviviridae detected in lumpfish (Cyclopterus lumpus). Arc. Virol. 2018, 163, 679–685. [Google Scholar] [CrossRef] [PubMed]
- Arrington, D.A.; Winemiller, K.O.; Loftus, W.F.; Akin, S. How Often Do Fishes “Run on Empty”? Ecology 2002, 83, 2145–2151. [Google Scholar]
- Eliasen, K.; Danielsen, E.; Johannesen, Á.; Joensen, L.L.; Patursson, E.J. The cleaning efficacy of lumpfish (Cyclopterus lumpus L.) in Faroese salmon (Salmo salar L.) farming pens in relation to lumpfish size and seasonality. Aquaculture 2018, 488, 61–65. [Google Scholar] [CrossRef]
- Fernández, D.A.; Ceballos, S.G.; Malanga, G.; Boy, C.C.; Vanella, F.A. Buoyancy of sub-Antarctic notothenioids including the sister lineage of all other notothenioids (Bovichtidae). Polar Biol. 2012, 35, 99–106. [Google Scholar] [CrossRef]
- Eastman, J.T. The evolution of neutrally buoyant notothenioid fishes: Their specializations and potential interactions in the Antarctic marine food web. In Antarctic Nutrient Cycles and Food Webs; Siegfried, W.R., Condy, P.R., Laws, R.M., Eds.; Springer: Berlin/Heidelberg, Germany, 1985; pp. 430–436. [Google Scholar]

| Location | A | B | C | D | E |
|---|---|---|---|---|---|
| Start of sampling | 16 September 2020 | 8 September 2020 | 14 September 2020 | 15 October 2020 | 19 October 2020 |
| End of sampling | 28 October 2020 | 04 November 2020 | 12 October 2020 | 15 October 2020 | 19 October 2020 |
| Hm0_50 m | 1–2 m | 1–2 m | 2–4 m | 6 m | 1–2 m |
| Average current 10 m | 6 cm/s | 6 cm/s | 5 cm/s | 50 cm/s (Measured) | 110 cm/s (Measured) |
| Ring Circumference and dept | 120 × 25 m | 120 × 25 m | 128 × 15 m | 160 × 12 m | 160 × 9 m |
| Salmon stock (#) at first sampling | 138.875 | 67.016 | 54.875 | 77.416 | 58.722 |
| Average salmon weight (g) | 891 | 2.406 | 5050 | 6.200 | 6.100 |
| Salmon Feed | Athena 600 | Athena 1200 | Biomar 2.2 mm | Skretting express 2500 50A | Skretting express 2500 50A |
| Lumpfish stock (#) at first sampling | 22.968 | 7.484 | 13.313 | 10.274 | 5.843 |
| Lumpfish feed | Unknown | Unknown | 9 mm margæti | 3 mm margæti | 3 mm margæti |
| Average lumpfish weight (g) at first measurement | 146 | 32 | 85 | 143 | 157 |
| Parameters | All Fish (D Excluded) | Lowest 30 Density Fish | Highest 30 Density Fish | Location D (n = 15) |
|---|---|---|---|---|
| Density ± SD | 1.030 ± 0.002 | 1.027 ± 0.001 | 1.033 ± 0.001 | 1.029 ± 0.002 |
| Fin | 1.52 | 1.70 | 1.56 | 1.73 |
| Skin | 1.32 | 1.50 | 1.20 | 2.07 |
| Stomach | 2.28 | 2.27 | 2.17 | 2.53 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Imsland, A.K.D.; Berg, M.S.; Haugland, G.T.; Eliasen, K. Comparing Body Density of Lumpfish (Cyclopterus lumpus) to Different Operational Welfare Indicators. Fishes 2022, 7, 284. https://doi.org/10.3390/fishes7050284
Imsland AKD, Berg MS, Haugland GT, Eliasen K. Comparing Body Density of Lumpfish (Cyclopterus lumpus) to Different Operational Welfare Indicators. Fishes. 2022; 7(5):284. https://doi.org/10.3390/fishes7050284
Chicago/Turabian StyleImsland, Albert Kjartan Dagbjartarson, Magnus Sunason Berg, Gyri Teien Haugland, and Kirstin Eliasen. 2022. "Comparing Body Density of Lumpfish (Cyclopterus lumpus) to Different Operational Welfare Indicators" Fishes 7, no. 5: 284. https://doi.org/10.3390/fishes7050284
APA StyleImsland, A. K. D., Berg, M. S., Haugland, G. T., & Eliasen, K. (2022). Comparing Body Density of Lumpfish (Cyclopterus lumpus) to Different Operational Welfare Indicators. Fishes, 7(5), 284. https://doi.org/10.3390/fishes7050284







