Differences in Intestinal Microbial Composition between Red Claw Crayfish (Cherax quadricarinatus) and Red Swamp Crayfish (Procambarus clarkii) Cultured in Pond
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Collection
2.2. Genomic DNA (gDNA) Extraction, Polymerase Chain Reaction (PCR) Amplification, and Sequencing
2.3. Data Analysis
2.4. Phylogenic Distance and Community Distribution
2.5. Statistical Analysis
3. Results
3.1. OTU Analysis and Alpha Diversity
3.2. Beta Diversity
3.3. Taxonomic Composition
3.4. Variance Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Acknowledgments
Conflicts of Interest
References
- Crandall, K.A.; Buhay, J.E. Global diversity of crayfish (Astacidae, Cambaridae, and Parastacidae—Decapoda) in freshwater. In Freshwater Animal Diversity Assessment; Springer: Dordrecht, The Netherlands, 2007; pp. 295–301. [Google Scholar]
- Mu, F.; Cheng, Y.; Wu, X. Distribution and industrial development of crayfish in the world. J. Shanghai Ocean Univ. 2007, 16, 64–72. (In Chinese) [Google Scholar]
- Bao, J.; Xing, Y.; Feng, C.; Kou, S.; Jiang, H.; Li, X. Acute and sub-chronic effects of copper on survival, respiratory metabolism, and metal accumulation in Cambaroides dauricus. Sci. Rep. 2020, 10, 16700. [Google Scholar] [CrossRef]
- Holdich, D.M. A review of astaciculture: Freshwater crayfish farming. Aquat. Living Resour. 1993, 6, 307–317. [Google Scholar] [CrossRef]
- King, C.R. Growth and survival of redclaw crayfish hatchlings (Cherax quadricarinatus von Martens) in relation to temperature, with comments on the relative suitabitity of Cherax quadricarinatus and Cherax destructor for culture in Queensland. Aquaculture 1994, 122, 75–80. [Google Scholar] [CrossRef]
- Holt, C.C.; Bass, D.; Stentiford, G.D.; Giezen, M. Understanding the role of the shrimp gut microbiome in health and disease. J. Invertebr. Pathol. 2021, 186, 107387. [Google Scholar] [CrossRef]
- Li, T.; Li, H.; Gatesoupe, F.J.; She, R.; Lin, Q.; Yan, X.; Li, J.; Li, X. Bacterial signatures of “Red-Operculum” disease in the gut of crucian carp (Carassius auratus). Microb. Ecol. 2017, 74, 510–521. [Google Scholar] [CrossRef]
- Mora-Sánchez, B.; Balcázar, J.L.; Pérez-Sánchez, T. Effect of a novel postbiotic containing lactic acid bacteria on the intestinal microbiota and disease resistance of rainbow trout (Oncorhynchus mykiss). Biotechnol. Lett. 2020, 42, 1957–1962. [Google Scholar] [CrossRef]
- Zhu, C.Z.; Li, D.; Chen, W.J.; Ban, S.N.; Liu, T.; Wen, H.; Jiang, M. Effects of dietary host-associated Lactococcus lactis on growth performance, disease resistance, intestinal morphology and intestinal microbiota of mandarin fish (Siniperca chuatsi). Aquaculture 2021, 540, 736702. [Google Scholar] [CrossRef]
- You, C.; Chen, B.; Wang, M.; Wang, S.; Zhang, M.; Sun, Z.; Juventus, A.J.; Ma, H.; Li, Y. Effects of dietary lipid sources on the intestinal microbiome and health of golden pompano (Trachinotus ovatus). Fish Shellfish Immunol. 2019, 89, 187–197. [Google Scholar] [CrossRef]
- Foysal, M.J.; Fotedar, R.; Tay, A.C.Y.; Gupta, S.K. Effects of long-term starvation on health indices, gut microbiota and innate immune response of fresh water crayfish, marron (Cherax cainii, Austin 2002). Aquaculture 2020, 514, 734444. [Google Scholar] [CrossRef]
- Mu, D.; Meng, J.; Bo, X.; Wu, M.; Xiao, H.; Wang, H. The effect of cadmium exposure on diversity of intestinal microbial community of Rana chensinensis tadpoles. Ecotoxicol. Environ. Saf. 2018, 154, 6–12. [Google Scholar] [CrossRef]
- Li, E.; Xu, C.; Wang, X.; Wang, S.; Zhao, Q.; Zhang, M.; Qin, J.G.; Chen, L. Gut microbiota and its modulation for healthy farming of Pacific white shrimp Litopenaeus vannamei. Rev. Fish. Sci. Aquac. 2018, 26, 381–399. [Google Scholar] [CrossRef]
- Dragičević, P.; Bielen, A.; Petrić, I.; Vuk, M.; Žučko, J.; Hudina, S. Microbiome of the successful freshwater invader, the signal crayfish, and its changes along the invasion range. Microbiol. Spectr. 2021, 9, e00389-21. [Google Scholar] [CrossRef]
- Skelton, J.; Geyer, K.M.; Lennon, J.T.; Creed, R.P.; Brown, B.L. Multi-scale ecological filters shape the crayfish microbiome. Symbiosis 2017, 72, 159–170. [Google Scholar] [CrossRef]
- Magoč, T.; Salzberg, S.L. FLASH: Fast length adjustment of short reads to improve genome assemblies. Bioinformatics 2011, 27, 2957–2963. [Google Scholar] [CrossRef]
- McDonald, D.; Price, M.N.; Goodrich, J.; Nawrocki, E.P.; DeSantis, T.Z.; Probst, A.; Andersen, G.L.; Knight, R.; Hugenholtz, P. An improved Greengenes taxonomy with explicit ranks for ecological and evolutionary analyses of bacteria and archaea. ISME J. 2012, 6, 610–618. [Google Scholar] [CrossRef]
- Liu, Q.; Long, Y.N.; Li, B.; Zhao, L.; Luo, J.; Xu, L.; Luo, W.; Du, Z.; Zhou, J.; Yang, S. Rice-shrimp culture: A better intestinal microbiota, immune enzymatic activities, and muscle relish of crayfish (Procambarus clarkii) in Sichuan Province. Appl. Microbiol. Biotechnol. 2020, 104, 9413–9420. [Google Scholar] [CrossRef]
- Guo, K.; Ruan, G.; Fan, W.; Fang, L.; Wang, Q.; Luo, M.; Yi, T. The effect of nitrite and sulfide on the antioxidant capacity and microbial composition of the intestines of red swamp crayfish, Procambarus clarkii. Fish Shellfish Immunol. 2020, 96, 290–296. [Google Scholar] [CrossRef]
- Zhang, Z.; Liu, J.; Jin, X.; Liu, C.; Fan, C.; Guo, L.; Liang, Y.; Zheng, J.; Peng, N. Developmental, dietary, and geographical impacts on gut microbiota of red swamp crayfish (Procambarus clarkii). Microorganisms 2020, 8, 1376. [Google Scholar] [CrossRef]
- Chen, H.; Wang, Y.; Zhang, J.; Bao, J. Intestinal microbiota in white spot syndrome virus infected red swamp crayfish (Procambarus clarkii) at different health statuses. Aquaculture 2021, 542, 736826. [Google Scholar] [CrossRef]
- Chen, X.; Fan, L.M.; Qiu, L.P.; Dong, X.X.; Wang, Q.; Hu, G.D.; Meng, S.L.; Li, D.D.; Chen, J.Z. Metagenomics Analysis Reveals Compositional and Functional Differences in the Gut Microbiota of Red Swamp Crayfish, Procambarus clarkii, Grown on Two Different Culture Environments. Front. Microbiol. 2021, 12, 735190. [Google Scholar] [CrossRef]
- Feng, Y.; Li, M.H.; Duan, H.M.; Li, L.Y.; Ouyang, P.; Chen, D.F.; Geng, Y.; Huang, X.L.; Yang, S.Y.; Yin, L.Z.; et al. Microbial analysis reveals the potential colonization of pathogens in the intestine of crayfish (Procambarus clarkii) in traditional aquaculture environments. Ecotoxicol. Environ. Saf. 2021, 224, 112705. [Google Scholar] [CrossRef]
- Liu, S.B.; Qi, C.L.; Jia, Y.Y.; Gu, Z.M.; Li, E.C. Growth and intestinal health of the red claw crayfish, Cherax quadricarinatus, reared under different salinities. Aquaculture 2020, 524, 735256. [Google Scholar] [CrossRef]
- Pérez-Sánchez, T.; Ruiz-Zarzuela, I.; de Blas, I.; Balcázar, J.L. Probiotics in aquaculture: A current assessment. Rev. Aquac. 2014, 6, 133–146. [Google Scholar] [CrossRef]
- De Schryver, P.; Vadstein, O. Ecological theory as a foundation to control pathogenic invasion in aquaculture. ISME J. 2014, 8, 2360–2368. [Google Scholar] [CrossRef]
- Zhu, J.; Dai, W.; Qiu, Q.; Dong, C.; Zhang, J.; Xiong, J. Contrasting ecological processes between intestinal bacterial community in healthy and diseased shrimp. Microb. Ecol. 2016, 72, 975–985. [Google Scholar] [CrossRef]
- Matozzo, V.; Marin, M.G. First evidence of gender-related differences in immune parameters of the clam Ruditapes philippinarum (Mollusca, Bivalvia). Mar. Biol. 2010, 157, 1181–1189. [Google Scholar] [CrossRef]
- Yurkovetskiy, L.; Burrows, M.; Khan, A.A.; Graham, L.; Volchkov, P.; Becker, L.; Antonopoulos, D.; Umesaki, Y.; Chervonsky, A.V. Gender bias in autoimmunity is influenced by microbiota. Immunity 2013, 39, 400–412. [Google Scholar] [CrossRef]
- Rungrassamee, W.; Klanchui, A.; Maibunkaew, S.; Chaiyapechara, S.; Jiravanichpaisal, P.; Karoonuthaisiri, N. Characterization of intestinal bacteria in wild and domesticated adult black tiger shrimp (Penaeus monodon). PLoS ONE 2014, 9, e91853. [Google Scholar] [CrossRef]
- Sugita, H.; Ito, Y. Identification of intestinal bacteria from Japanese flounder (Paralichthys olivaceus) and their ability to digest chitin. Lett. Appl. Microbiol. 2006, 43, 336–342. [Google Scholar] [CrossRef]
- Prachumwat, A.; Wechprasit, P.; Srisala, J.; Kriangsaksri, R.; Flegel, T.W.; Thitamadee, S.; Sritunyalucksana, K. Shewanella khirikhana sp. nov.–a shrimp pathogen isolated from a cultivation pond exhibiting early mortality syndrome. Microb. Biotechnol. 2020, 13, 781–795. [Google Scholar] [CrossRef]
- Radhakrishnan, E.V.; Kizhakudan, J.K. Health Management in Lobster Aquaculture. In Lobsters: Biology, Fisheries and Aquaculture; Springer: Singapore, 2019; pp. 571–601. [Google Scholar]
- Muthukrishnan, S.; Defoirdt, T.; Ina-Salwany, M.Y.; Yusoff, F.M.; Shariff, M.; Ismail, S.I.; Natrah, I. Vibrio parahaemolyticus and Vibrio harveyi causing Acute Hepatopancreatic Necrosis Disease (AHPND) in Penaeus vannamei (Boone, 1931) isolated from Malaysian shrimp ponds. Aquaculture 2019, 511, 734227. [Google Scholar] [CrossRef]
- Kurtz, J.; Wiesner, A.; Götz, P.; Sauer, K.P. Gender differences and individual variation in the immune system of the scorpionfly Panorpa vulgaris (Insecta: Mecoptera). Dev. Comp. Immunol. 2000, 24, 1–12. [Google Scholar] [CrossRef]
- Ben-Haim, Y.; Thompson, F.L.; Thompson, C.C.; Cnockaert, M.C.; Hoste, B.; Swings, J.; Rosenberg, E. Vibrio coralliilyticus sp. nov., a temperature-dependent pathogen of the coral Pocillopora damicornis. Int. J. Microbiol. 2003, 53, 309–315. [Google Scholar] [CrossRef]
- Kimes, N.E.; Grim, C.J.; Johnson, W.R.; Hasan, N.A.; Tall, B.D.; Kothary, M.H.; Kiss, H.; Munk, A.C.; Tapia, R.; Green, L.; et al. Temperature regulation of virulence factors in the pathogen Vibrio coralliilyticus. ISME J. 2012, 6, 835–846. [Google Scholar] [CrossRef]
- Kesarcodi-Watson, A.; Miner, P.; Nicolas, J.L.; Robert, R. Protective effect of four potential probiotics against pathogen-challenge of the larvae of three bivalves: Pacific oyster (Crassostrea gigas), flat oyster (Ostrea edulis) and scallop (Pecten maximus). Aquaculture 2012, 344, 29–34. [Google Scholar] [CrossRef]
- Richards, G.P.; Watson, M.A.; Needleman, D.S.; Church, K.M.; Häse, C.C. Mortalities of Eastern and Pacific oyster larvae caused by the pathogens Vibrio coralliilyticus and Vibrio tubiashii. Appl. Environ. Microbiol. 2015, 81, 292–297. [Google Scholar] [CrossRef]
- Torres, M.; Reina, J.C.; Fuentes-Monteverde, J.C.; Fernández, G.; Rodríguez, J.; Jiménez, C.; Llamas, I. AHL-lactonase expression in three marine emerging pathogenic Vibrio spp. reduces virulence and mortality in brine shrimp (Artemia salina) and Manila clam (Venerupis philippinarum). PLoS ONE 2018, 13, e0195176. [Google Scholar] [CrossRef]
- Austin, B.; Austin, D.; Sutherland, R.; Thompson, F.; Swings, J. Pathogenicity of vibrios to rainbow trout (Oncorhynchus mykiss, Walbaum) and Artemia nauplii. Environ. Microbiol. 2005, 7, 1488–1495. [Google Scholar] [CrossRef]
- Albuquerque, C.R.; Cristina, S.G.; Lima, A.R.; Edirsana, M.R.; Regine, H.S. Microbiota of Vibrio sp. in the hepatopancreas of cultured white pacific shrimp (Litopenaeus vannamei). Rev. MVZ Cordoba 2013, 18, 3439–3443. [Google Scholar] [CrossRef][Green Version]
- Dos Santos Rocha, R.; De Sousa, O.V.; Dos Fernandes Vieira, R.H.S. Multidrug-resistant Vibrio associated with an estuary affected by shrimp farming in Northeastern Brazil. Mar. Pollut. Bull. 2016, 105, 337–340. [Google Scholar] [CrossRef] [PubMed]
- Thomas, F.; Hehemann, J.H.; Rebuffet, E.; Czjzek, M.; Michel, G. Environmental and gut bacteroidetes: The food connection. Front. Microbiol. 2011, 2, 93. [Google Scholar] [CrossRef] [PubMed]
- Mazmanian, S.K.; Round, J.L.; Kasper, D.L. A microbial symbiosis factor prevents intestinal inflammatory disease. Nature 2008, 453, 620–625. [Google Scholar] [CrossRef] [PubMed]
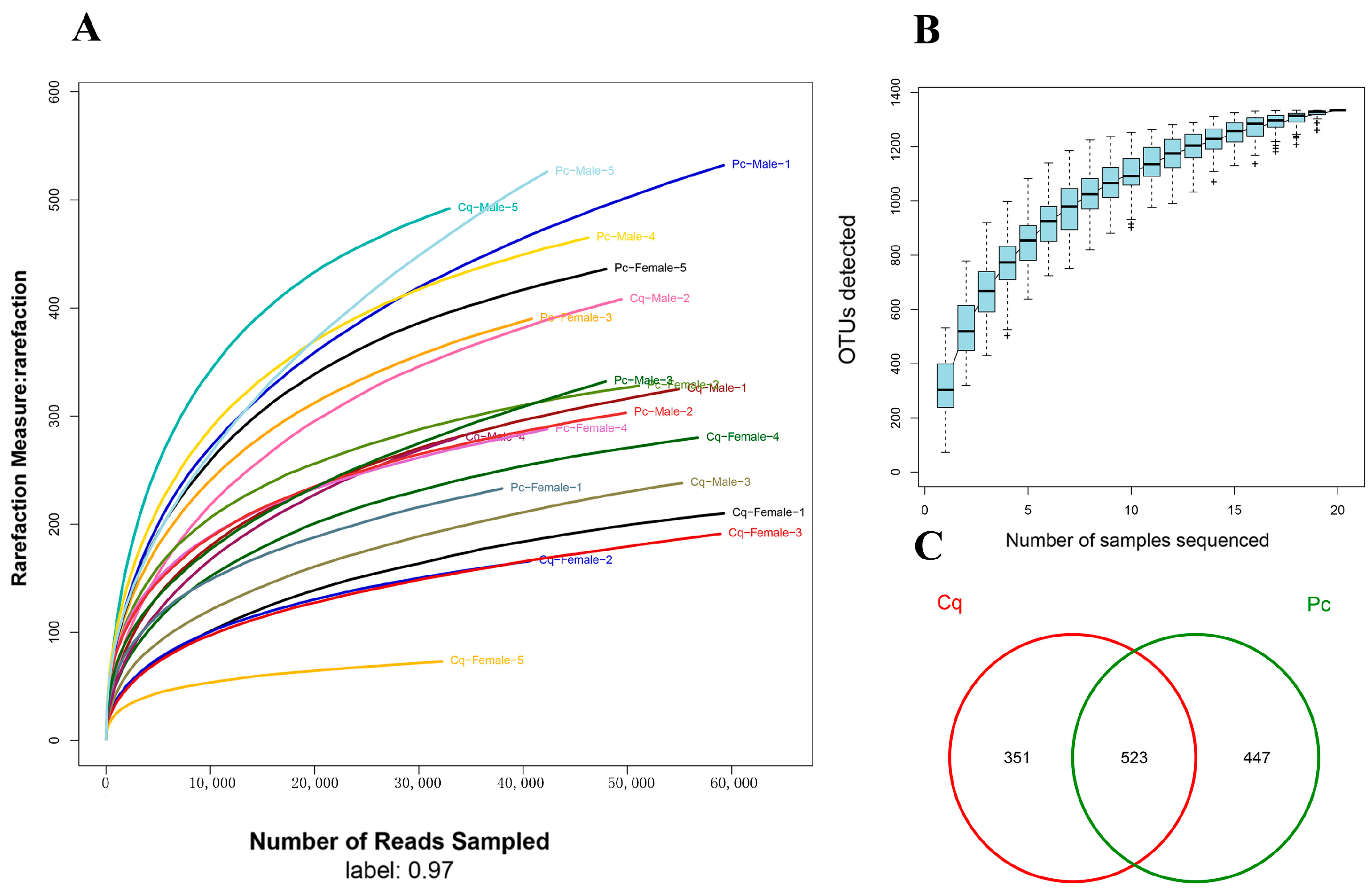
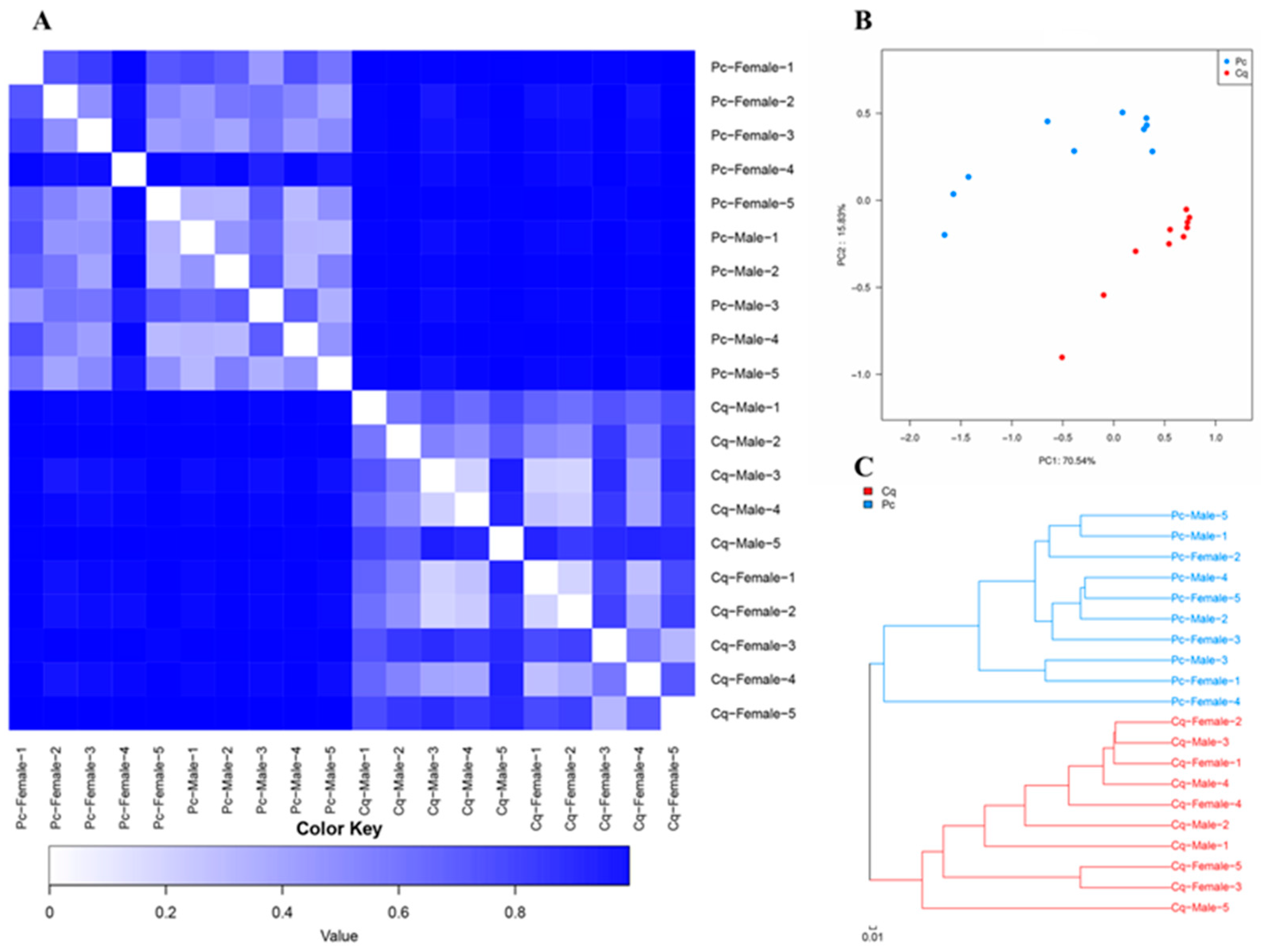
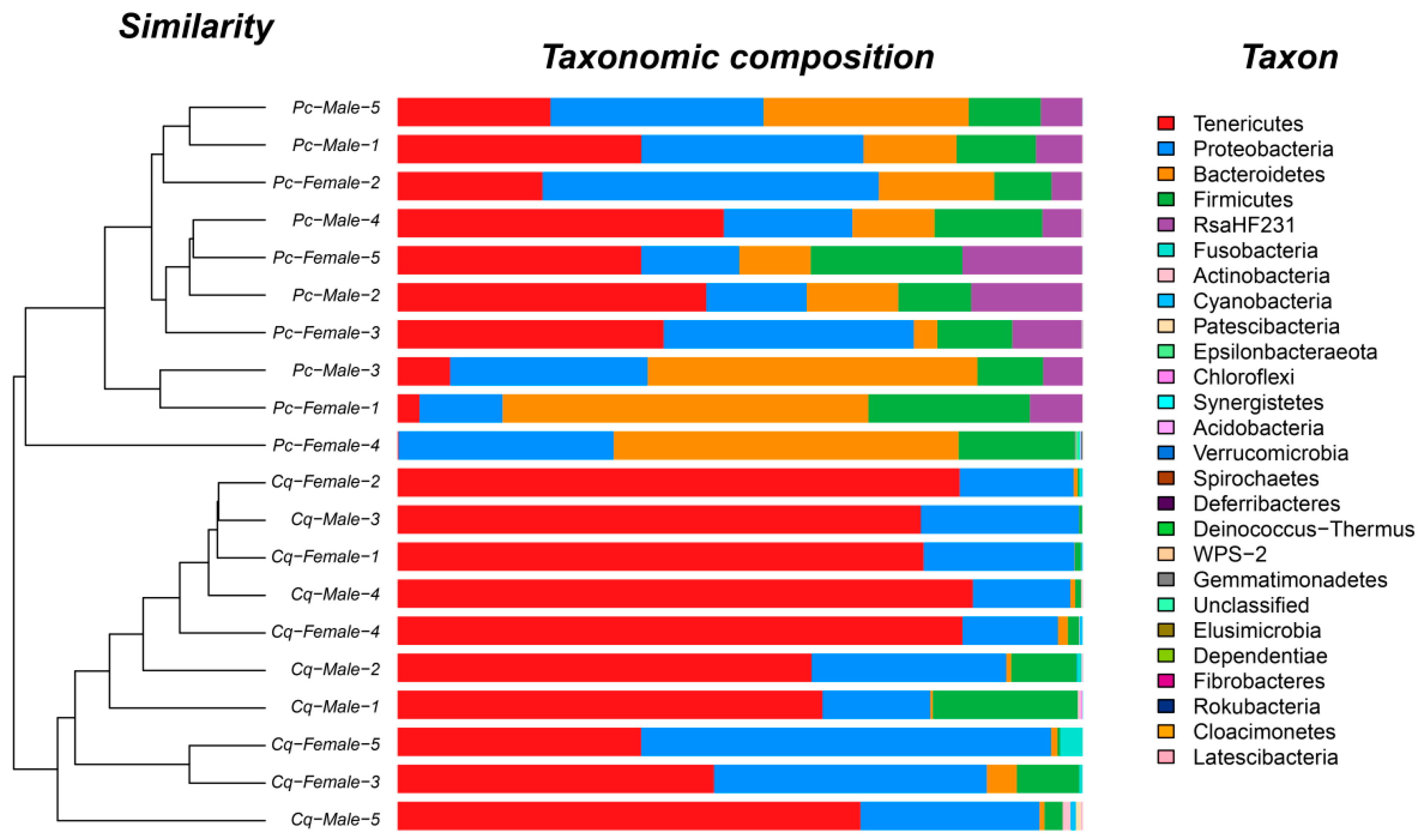
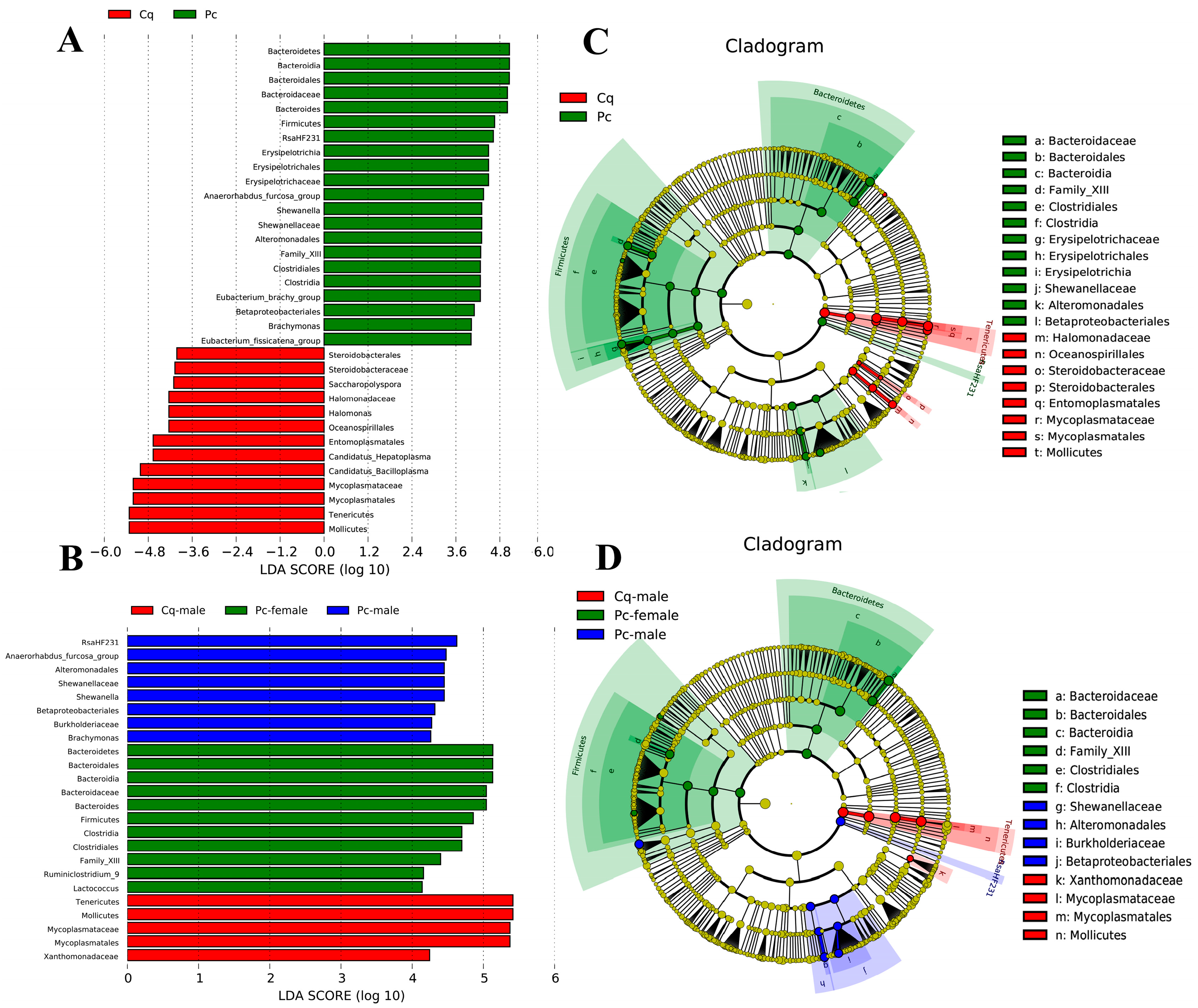
| Pc | Pc Female | Pc Male | Cq | Cq Female | Cq Male | |
|---|---|---|---|---|---|---|
| Chao | 538.24 ± 210.95 | 426.19 ± 73.83 | 650.29 ± 251.57 | 351.28 ± 137.62 | 254.01 ± 93.12 | 448.56 ± 101.42 |
| Shannon | 4.46 ± 0.30 | 4.33 ± 0.28 | 4.59 ± 0.28 | 2.76 ± 0.49 | 2.53 ± 0.29 | 2.98 ± 0.58 |
| Simpson | 0.11 ± 0.02 | 0.12 ± 0.02 | 0.10 ± 0.03 | 0.32 ± 0.10 | 0.33 ± 0.07 | 0.31 ± 0.13 |
| ACE | 526.76 ± 192.91 | 421.48 ± 80.33 | 632.04 ± 222.64 | 354.00 ± 139.59 | 249.87 ± 94.37 | 458.13 ± 88.47 |
| Good’s coverage | 0.9982 ± 0.001 | 0.9980 ± 0.000 | 0.9969 ± 0.002 | 0.9975 ± 0.001 | 0.9989 ± 0.000 | 0.9976 ± 0.001 |
| OTU (97%) | 346.9 ± 87.2 | 311.40 ± 71.80 | 382.40 ± 93.83 | 236.5 ± 118.5 | 158.60 ± 58.65 | 314.40 ± 113.86 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, H.; Liu, F.; Ouyang, M.; Zhou, H.; Lou, B. Differences in Intestinal Microbial Composition between Red Claw Crayfish (Cherax quadricarinatus) and Red Swamp Crayfish (Procambarus clarkii) Cultured in Pond. Fishes 2022, 7, 241. https://doi.org/10.3390/fishes7050241
Chen H, Liu F, Ouyang M, Zhou H, Lou B. Differences in Intestinal Microbial Composition between Red Claw Crayfish (Cherax quadricarinatus) and Red Swamp Crayfish (Procambarus clarkii) Cultured in Pond. Fishes. 2022; 7(5):241. https://doi.org/10.3390/fishes7050241
Chicago/Turabian StyleChen, Honglin, Fangfang Liu, Miaofeng Ouyang, Huan Zhou, and Bao Lou. 2022. "Differences in Intestinal Microbial Composition between Red Claw Crayfish (Cherax quadricarinatus) and Red Swamp Crayfish (Procambarus clarkii) Cultured in Pond" Fishes 7, no. 5: 241. https://doi.org/10.3390/fishes7050241
APA StyleChen, H., Liu, F., Ouyang, M., Zhou, H., & Lou, B. (2022). Differences in Intestinal Microbial Composition between Red Claw Crayfish (Cherax quadricarinatus) and Red Swamp Crayfish (Procambarus clarkii) Cultured in Pond. Fishes, 7(5), 241. https://doi.org/10.3390/fishes7050241





