Demographic and Life History Characteristics of Black Bullheads Ameiurus melas in a North Temperate USA Lake
Abstract
:1. Introduction
2. Materials and Methods
2.1. Black Bullhead Population Characteristics
2.2. Spawning, Maturity, and Fecundity
2.3. Seasonal Diet Analysis
3. Results
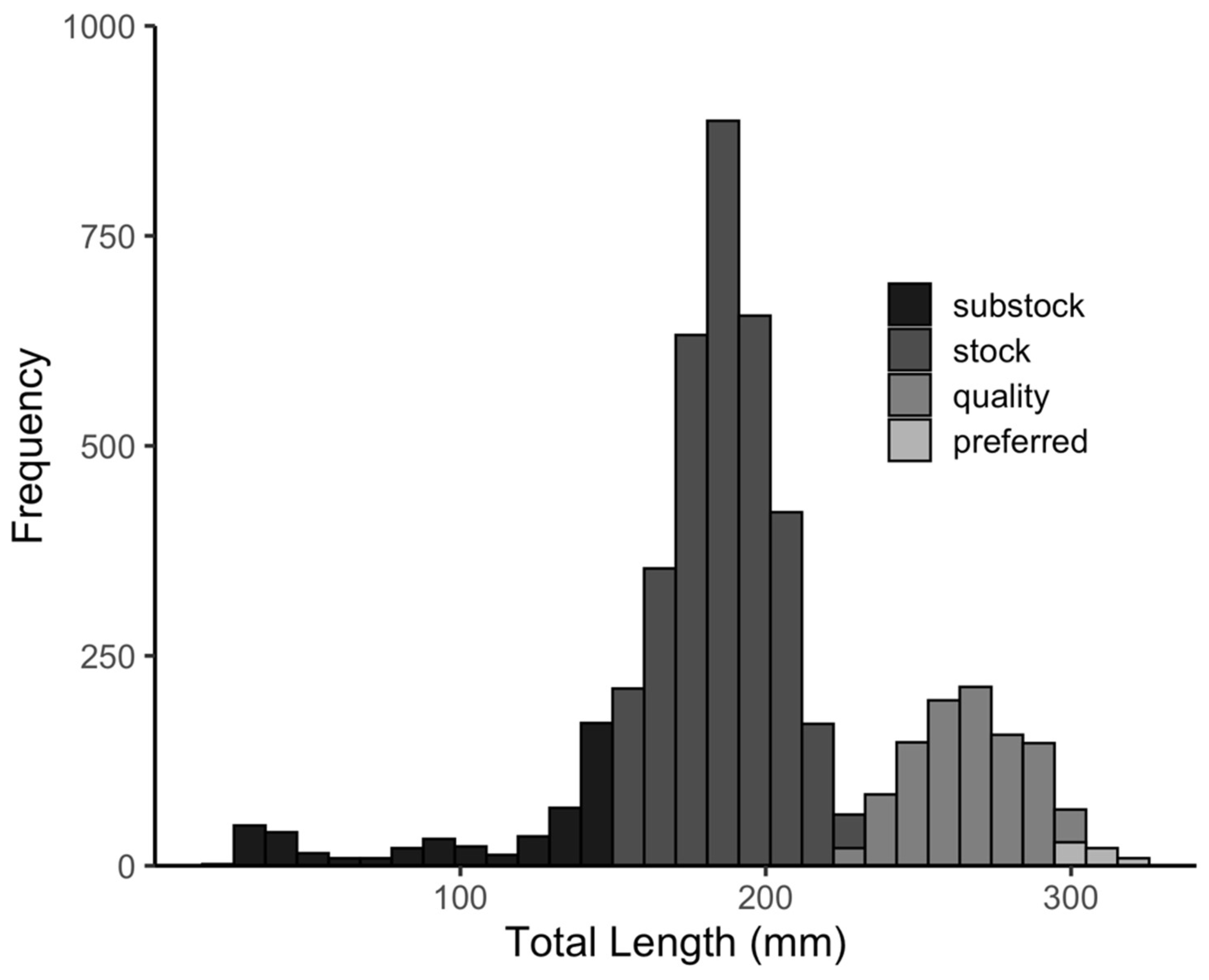
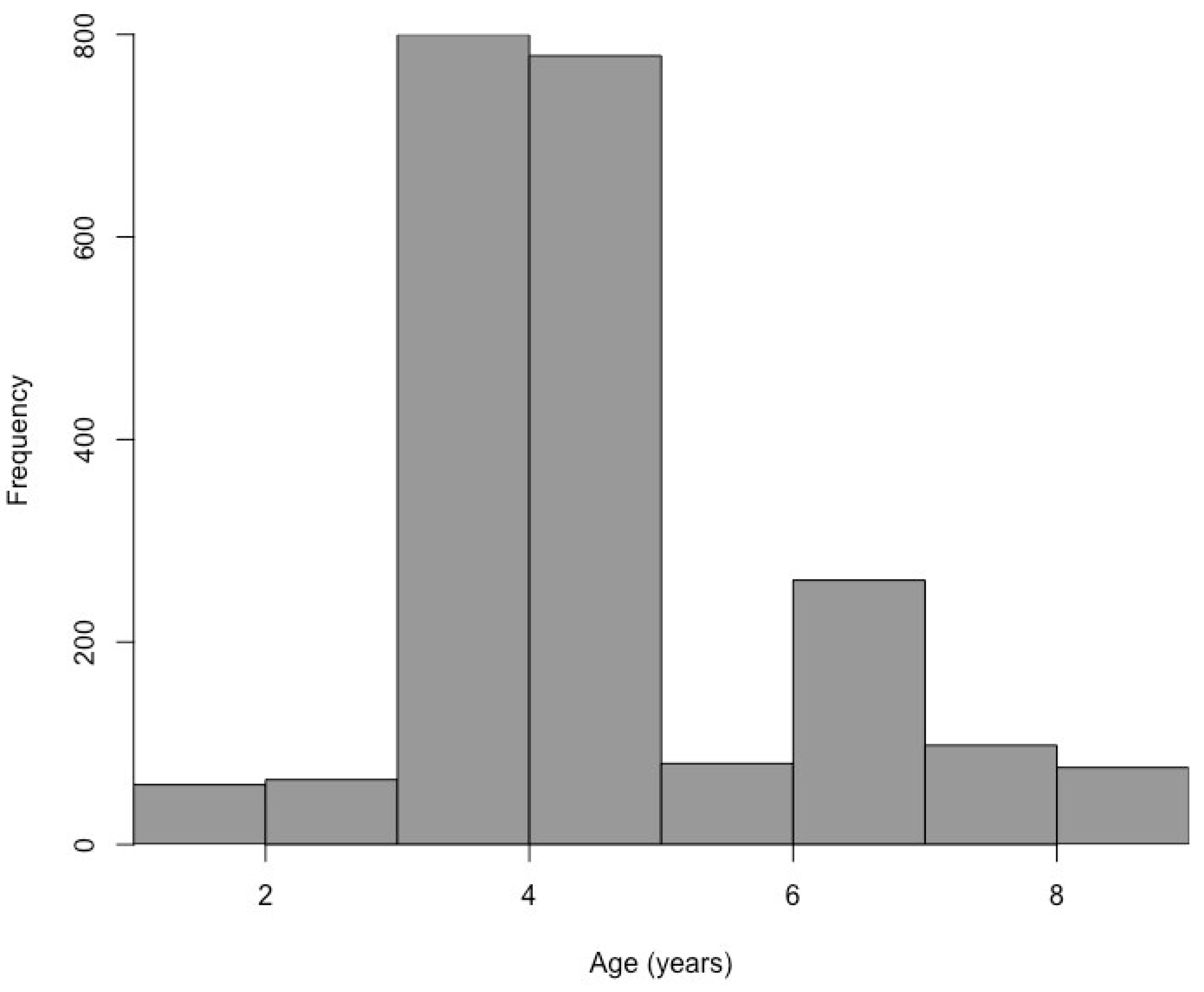
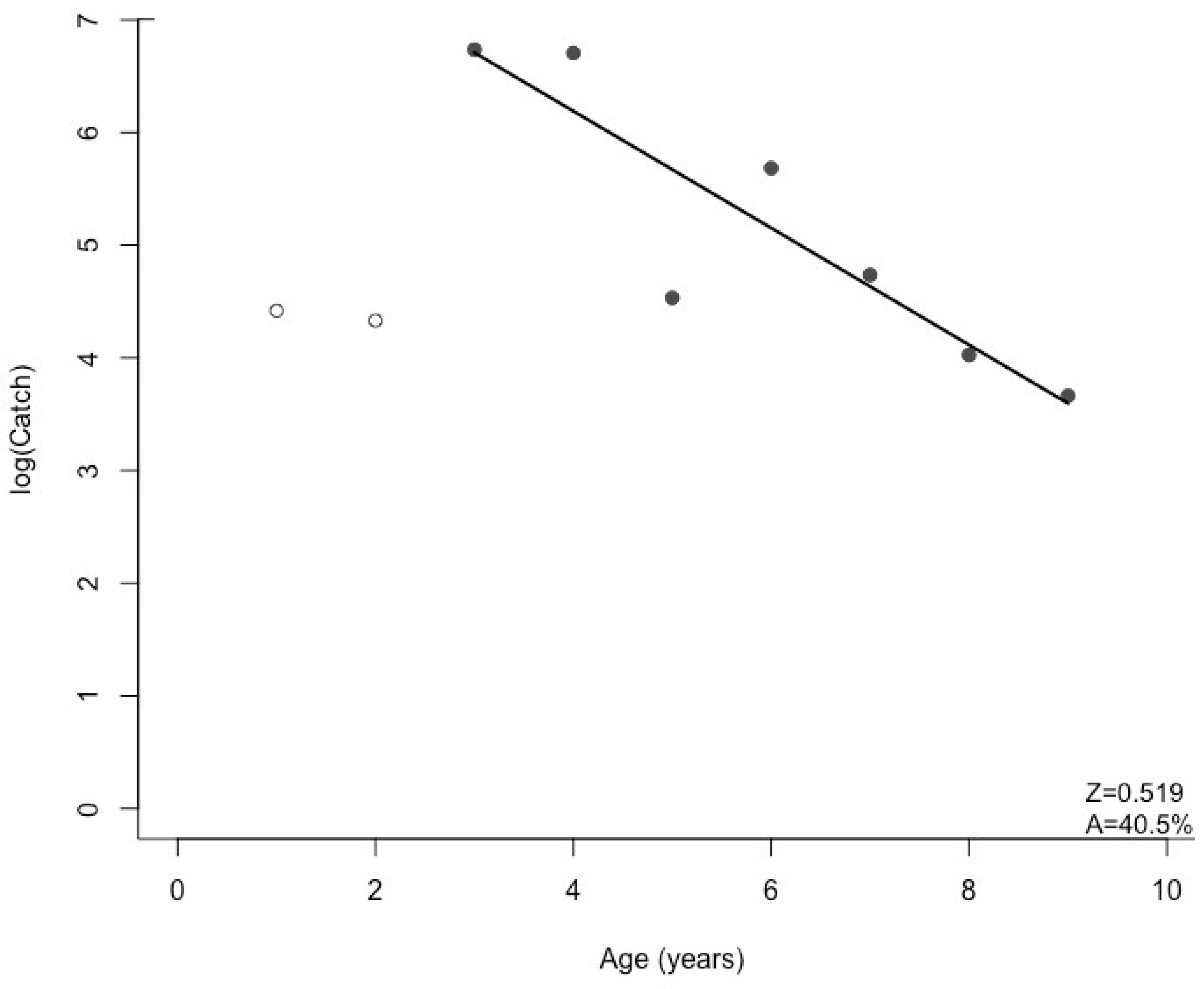
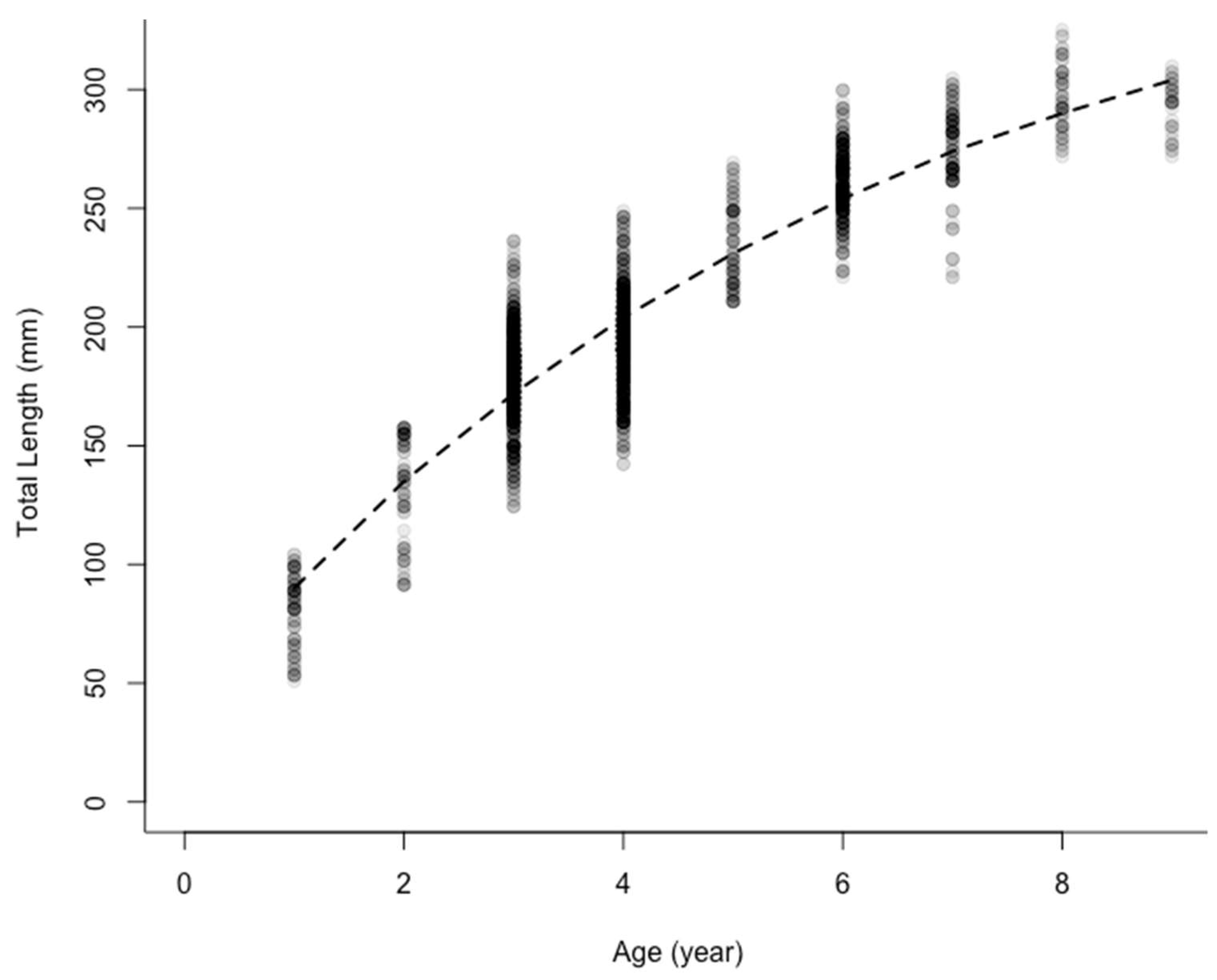
3.1. Black Bullhead Population Characteristics
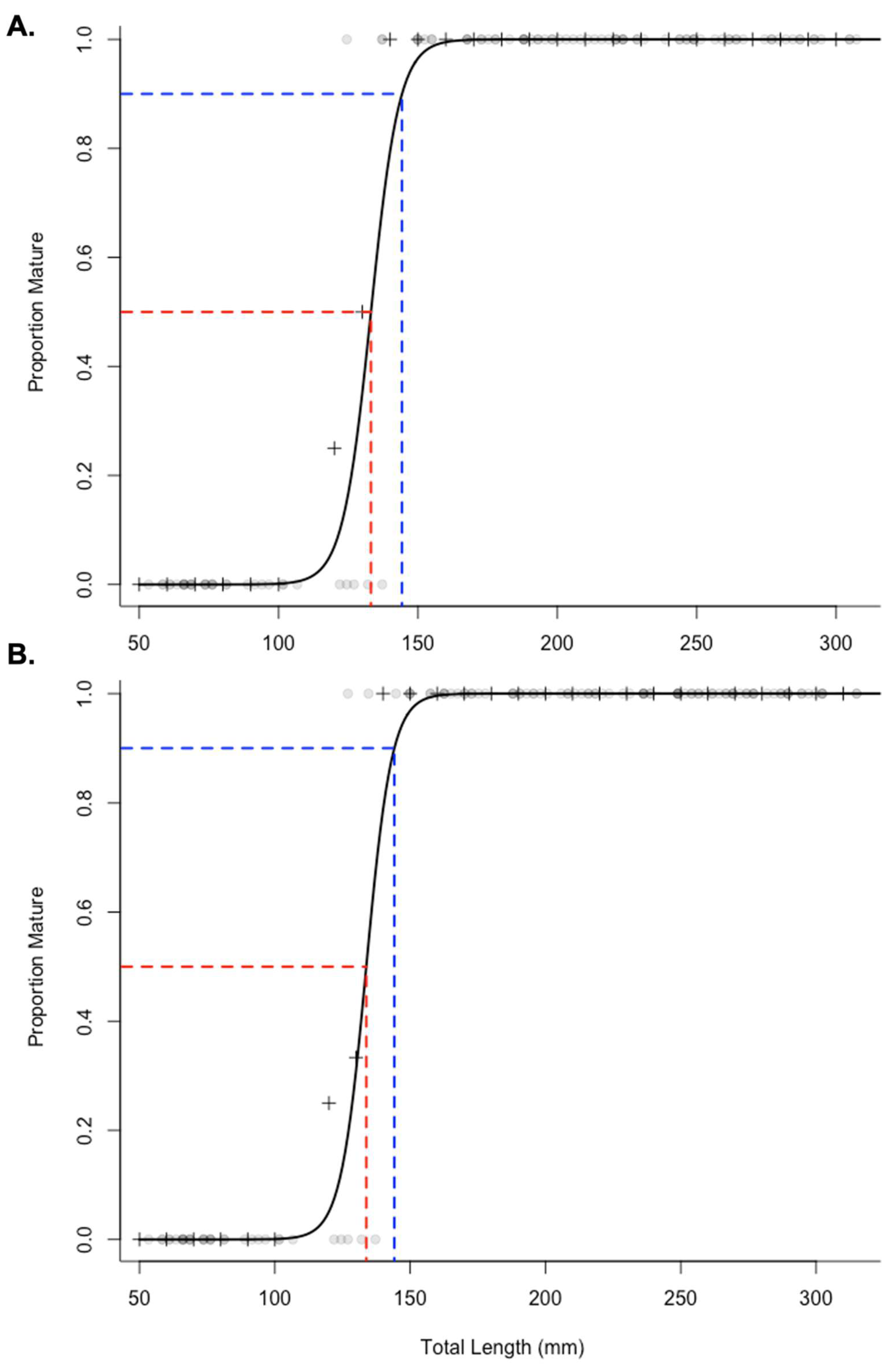
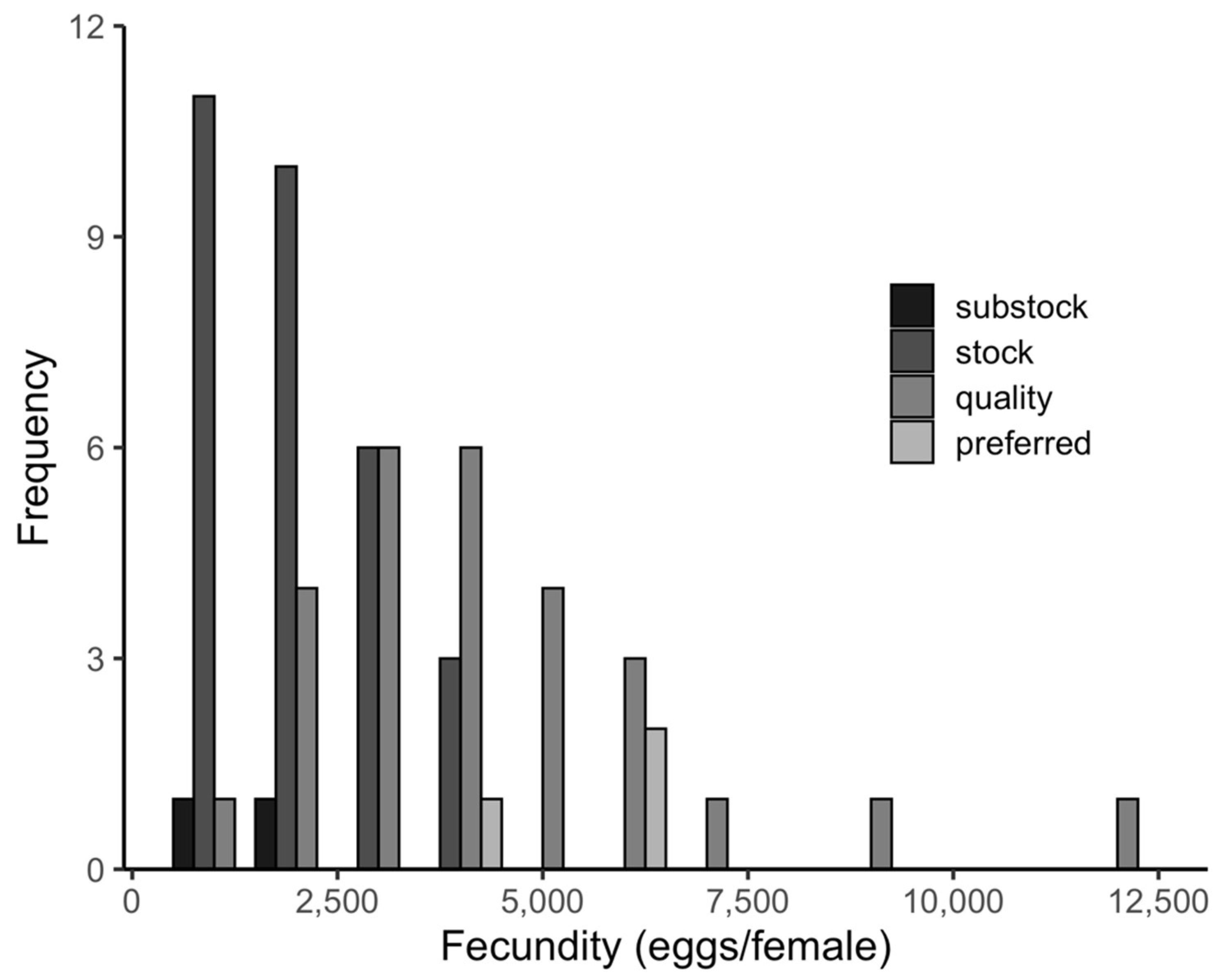
3.2. Spawning, Maturity, and Fecundity
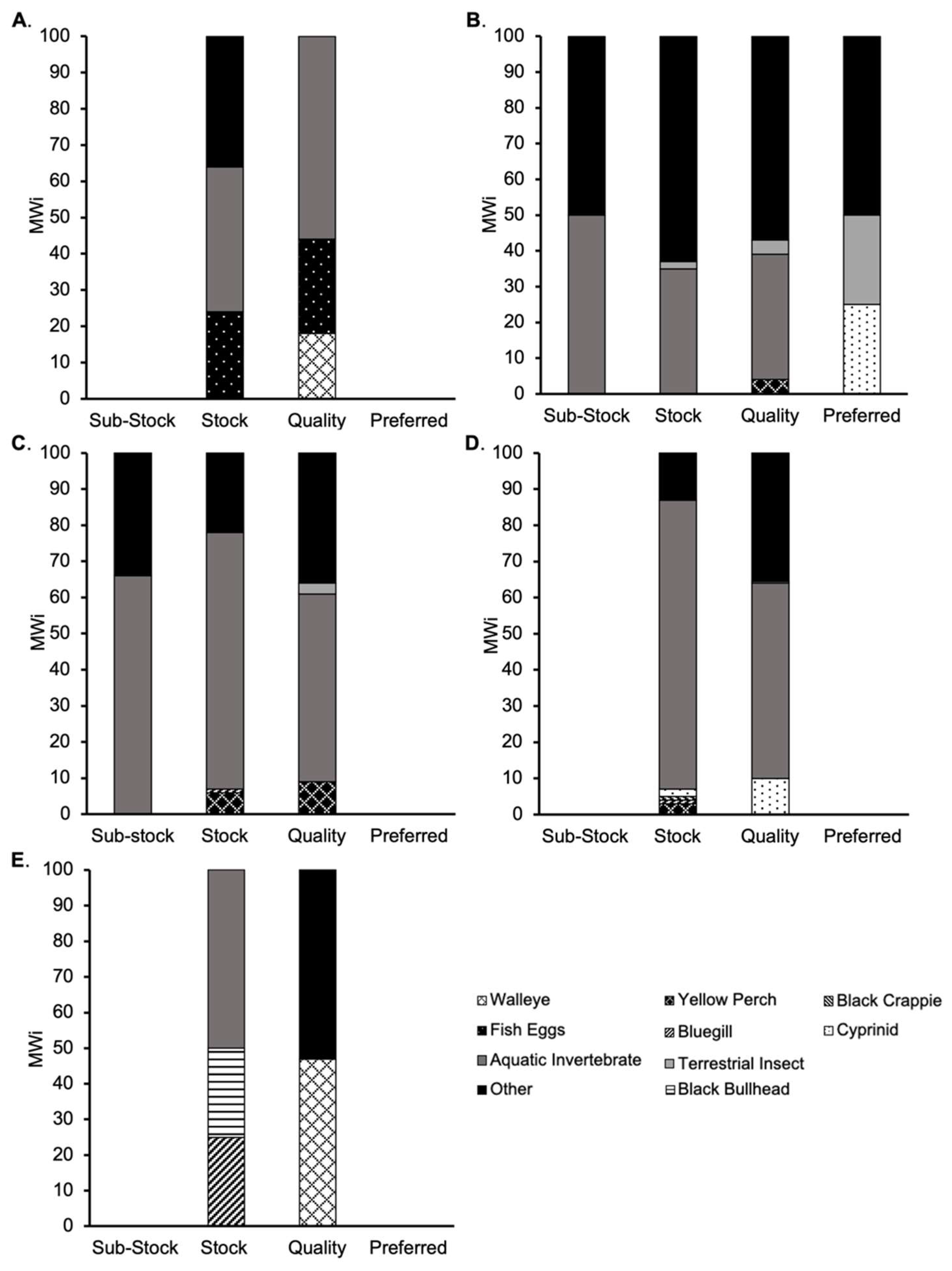
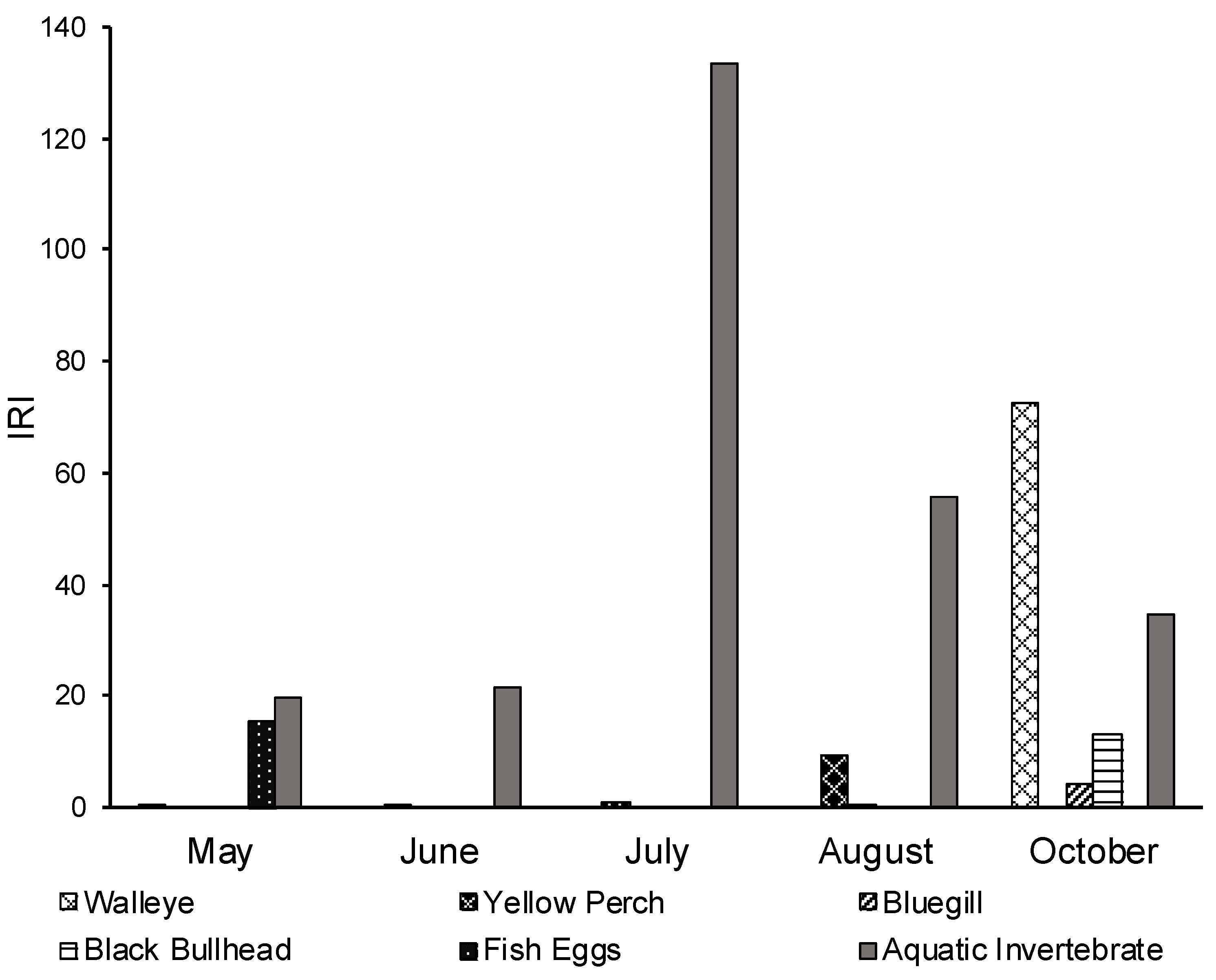
3.3. Seasonal Diet Analysis
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wheeler, A.C. Ictalurus melas (Rafinesque, 1820) and I. nebulosus (Lesueur, 1819): The North American catfishes in Europe. J. Fish. Biol. 1978, 12, 435–439. [Google Scholar] [CrossRef]
- Page, L.M.; Burr, B.M. A Field Guide to Freshwater Fishes: North America North of Mexico; Houghton Mifflin: Boston, MA, USA, 1991. [Google Scholar]
- Copp, G.H.; Bianco, P.G.; Bogutskaya, N.; Erős, T.; Falka, I.; Ferreira, M.T.; Fox, M.G.; Freyhof, J.; Gozlan, R.E.; Grabowska, J.; et al. To be, or not to be, a non-native freshwater fish? J. Appl. Ichthyol. 2005, 21, 242–262. [Google Scholar] [CrossRef]
- Copp, G.H.; Tarkan, A.S.; Masson, G.; Godard, M.J.; Koščo, J.; Novomeská, A.; Miranda, R.; Cucherousset, J.; Pedicillo, G.; Blackwell, B. A review of growth and life-history traits of native and non-native European populations of black bullhead Ameiurus melas. Rev. Fish Biol. Fish. 2016, 26, 441–469. [Google Scholar] [CrossRef] [Green Version]
- Vooren, C. Ecological aspects of the introduction of fish species into natural habitats in Europe, with special reference to the Netherlands. J. Fish. Biol. 1971, 4, 565–583. [Google Scholar] [CrossRef]
- Cucherousset, J.; Paillisson, J.M.; Carpentier, A.; Eybert, M.C.; Olden, J.D. Habitat use of an artificial wetland by the invasive catfish Ameiurus melas. Ecol. Freshw. Fish. 2006, 15, 589–596. [Google Scholar] [CrossRef]
- Copp, G.H. The habitat diversity and fish reproductive function of floodplain ecosystems. Environ. Biol. Fish. 1989, 26, 1–26. [Google Scholar] [CrossRef]
- Copp, G.H. The upper River Rhône revisited: An empirical model of microhabitat use by 0+ juvenile fishes. Folia. Zool. 1993, 42, 329–340. [Google Scholar]
- Gozlan, R.E.; Mastrorillo, S.; Dauba, F.; Tourenq, J.N.; Copp, G.H. Multi-scale analysis of habitat use by 0+ fishes during late summer in the River Garonne (France). Aquat. Sci. 1998, 60, 99–117. [Google Scholar] [CrossRef]
- Cucherousset, J.; Paillisson, J.M.; Carpentier, A. Is mass removal an efficient measure to regulate the North American catfish Ameiurus melas outside of its native range? J. Freshw. Ecol. 2008, 21, 699–704. [Google Scholar] [CrossRef]
- Sikora, L.W.; VanDeHey, J.A.; Sass, G.G.; Matzke, G.; Preul, M. Fish Community Changes Associated with Bullhead Removals in Four Northern Wisconsin Lakes. N. Am. J. Fish. Manag. 2021, 41, S71–S81. [Google Scholar] [CrossRef]
- Braig, E.C.; Johnson, D.L. Impact of black bullhead (Ameiurus melas) on turbidity in a diked wetland. Hydrobiologia 2003, 490, 11–21. [Google Scholar] [CrossRef]
- Scott, W.B.; Crossman, E.G. Freshwater Fishes of Canada; Fisheries Resource Board Canada: Ottawa, ON, Canada, 1973; Volume 184, p. 966. [Google Scholar]
- Stuber, R.J. Habitat Suitability Index Models: Black Bullhead; United States Department of Interior, Fish and Wildlife Service: Fairfax, VA, USA, 1982.
- Ribeiro, F.; Elvira, B.; Collares-Pereira, M.J.; Moyle, P.B. Life-history traits of non-native fishes in Iberian watersheds across several invasion stages: A first approach. Biol. Invasions 2008, 10, 89–102. [Google Scholar] [CrossRef]
- Carlander, K.D. Handbook of Freshwater Fishery Biology; Iowa State University Press: Ames, IA, USA, 1996; Volume 1. [Google Scholar]
- Bister, T.J.; Willis, D.W.; Brown, M.L.; Jordan, S.M.; Neumann, R.M.; Quist, M.C.; Guy, C.S. Proposed standard weight (Ws) equations and standard length categories for 18 warmwater nongame and riverine fish species. N. Am. J. Fish. Manag. 2000, 20, 570–574. [Google Scholar] [CrossRef]
- Sharma, S.; Jackson, D.A.; Minns, C.K.; Shuter, B.J. Will northern fish populations be in hot water because of climate change? Glob. Chang. Biol. 2007, 13, 2052–2064. [Google Scholar] [CrossRef]
- Hansen, G.J.A.; Gaeta, J.W.; Hansen, J.F.; Carpenter, S.R. Learning to manage and managing to learn: Sustaining freshwater recreational fisheries in a changing environment. Fisheries 2015, 40, 56–64. [Google Scholar] [CrossRef]
- Lynch, A.J.; Myers, B.J.; Chu, C.; Eby, L.A.; Falke, J.A.; Kovach, R.P.; Krabbenhoft, T.J.; Kwak, T.J.; Lyons, J.; Paukert, C.P.; et al. Climate change effects on North American inland fish populations and assemblages. Fisheries 2016, 41, 346–361. [Google Scholar] [CrossRef]
- Hansen, G.J.A.; Read, J.S.; Hansen, J.F.; Winslow, L.A. Projected shifts in fish species dominance in Wisconsin lakes under climate change. Glob. Chang. Biol. 2017, 23, 1463–1476. [Google Scholar] [CrossRef] [PubMed]
- Sass, G.G.; Rypel, A.L.; Stafford, J.D. Fisheries habitat management: Lessons learned from wildlife ecology and a proposal for change. Fisheries 2017, 42, 197–209. [Google Scholar] [CrossRef]
- Mrnak, J.T.; Shaw, S.L.; Eslinger, L.D.; Cichosz, T.A.; Sass, G.G. Characterizing the joint tribal spearing and angling walleye fisheries in the Ceded Territory of Wisconsin. N. Am. J. Fish. Manag. 2018, 38, 1381–1393. [Google Scholar] [CrossRef]
- Embke, H.S.; Rypel, A.L.; Carpenter, S.R.; Sass, G.G.; Ogle, D.; Cichosz, T.; Hennessy, J.; Essington, T.E.; Zanden, M.J.V. Production dynamics reveal hidden overharvest of inland recreational fisheries. Proc. Natl. Acad. Sci. USA 2019, 116, 24676–24681. [Google Scholar] [CrossRef]
- Sass, G.G.; Shaw, S.L. Walleye population responses to experimental exploitation in a northern Wisconsin lake. Trans. Am. Fish. Soc. 2018, 147, 869–878. [Google Scholar] [CrossRef]
- Sass, G.G.; Shaw, S.L. Catch-and-release influences on inland recreational fisheries. Rev. Fish. Sci. Aquacult. 2020, 28, 211–227. [Google Scholar] [CrossRef]
- Carpenter, S.R.; Cole, J.J.; Pace, M.L.; Batt, R.; Brock, W.A.; Cline, T.; Coloso, J.; Hodgson, J.R.; Kitchell, J.F.; Seekell, D.A.; et al. Early warnings of regime shifts: A whole-ecosystem experiment. Science 2011, 332, 1079–1082. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Carle, F.L.; Strub, M.R. A new method for estimating population size from removal data. Biometrics 1978, 34, 621–630. [Google Scholar] [CrossRef]
- Bettoli, P.W.; Miranda, L.E. A cautionary note about estimating mean length at age with sub- sampled data. N. Am. J. Fish. Manag. 2001, 21, 425–428. [Google Scholar] [CrossRef]
- Stewart, D.R.; Long, J.M. Verification of otolith identity used by fisheries scientists for aging Channel Catfish. Trans. Am. Fish. Soc. 2010, 139, 1775–1779. [Google Scholar] [CrossRef]
- Buckmeier, D.L.; Howells, R.G. Validation of otoliths for estimating ages of Largemouth Bass to 16 years. N. Am. J. Fish. Manag. 2003, 23, 590–593. [Google Scholar] [CrossRef]
- Crim, L.W.; Glebe, B.D. Reproduction. Methods for Fish Biology; Schreck, C.B., Moyle, P.B., Eds.; American Fisheries Society: Bethesda, MA, USA, 1990; pp. 529–547. [Google Scholar]
- Quist, M.C.; Pegg, M.A.; De Vries, D.R. Age and Growth. Fisheries Techniques, 3rd ed.; Alexander, A.V., Parrish, D.L., Sutton, T.M., Eds.; American Fisheries Society: Bethesda, MA, USA, 2012; pp. 677–731. [Google Scholar]
- Buckmeier, D.L.; Irwin, E.R.; Betsill, R.K.; Prentice, J.A. Validity of otoliths and pectoral spines for estimating ages of Channel Catfish. N. Am. J. Fish. Manag. 2002, 22, 934–942. [Google Scholar] [CrossRef]
- Ricker, W.E. Computation and Interpretation of Biological Statistics of Fish Populations; Fisheries Research Board of Canada Bulletin: Ottawa, ON, Canada, 1975; p. 191. [Google Scholar]
- Von Bertalanffy, L. A quantitative theory of organic growth (Inquiries on growth laws. II). Hum. Biol. 1938, 10, 181–213. [Google Scholar]
- Gabelhouse, D.W., Jr. A length-categorization system to assess fish stocks. N. Am. J. Fish. Manag. 1984, 4, 273–285. [Google Scholar] [CrossRef]
- Maceina, M.J.; Bettoli, P.W. Variation in largemouth bass recruitment in four mainstream impoundments of the Tennessee River. N. Am. J. Fish. Manag. 1998, 18, 998–1003. [Google Scholar] [CrossRef] [Green Version]
- Bowen, S.H. Quantitative Description of the Diet; Fisheries Techniques; Nielsen, L.A., Johnson, D.L., Eds.; American Fisheries Society: Bethesda, MA, USA, 1983; pp. 325–336. [Google Scholar]
- Kline, J.L.; Wood, B.M. Food Habits and Diet Selectivity of the Brown Bullhead. J. Freshw. Ecol. 1996, 11, 145–151. [Google Scholar] [CrossRef]
- Pope, K.L.; Brown, M.L.; Duffy, W.G.; Michaletz, P.H. A caloric-based evaluation of diet indices for largemouth bass. Environ. Biol. Fish. 2001, 61, 329–339. [Google Scholar] [CrossRef]
- Chipps, S.R.; Garvey, J.E. Assessment of Diets and Feeding Patterns. Analysis and Interpretation of Freshwater Fisheries Data; Guy, C.S., Brown, M.L., Eds.; American Fisheries Society: Bethesda, MA, USA, 2007; pp. 473–514. [Google Scholar]
- Hanchin, P.A.; Willis, D.W.; Hubers, M.J. Black Bullhead Growth in South Dakota Waters: Limnological and Community Influences. J. Freshw. Ecol. 2002, 17, 65–73. [Google Scholar] [CrossRef]
- Becker, G. Fishes of Wisconsin; University of Wisconsin Press: Madison, WI, USA, 1983; pp. 693–732. [Google Scholar]
- Jenkins, R.E.; Burkhead, N.M. Freshwater Fishes of Virginia; American Fisheries Society: Bethesda, MA, USA, 1993. [Google Scholar]
- Barnickol, P.G.; Starrett, W.C. Commercial and sport fishery of the Mississippi River between Caruthersville, Missouri, and Dubuque, Iowa. Bull. Ill. Nat. Hist. Surv. 1951, 23, 267–350. [Google Scholar] [CrossRef]
- Forney, J.L. Life history of the Black Bullhead, Ameiurus melas (Rafinesque), of Clear Lake, Iowa. Iowa State Coll. J. Sci. 1955, 30, 145–162. [Google Scholar]
- Carlander, K.D.; Sprugel, G. Project 42. Bullhead management. Quart. Rep. Iowa Coop. Wildl. Res. Units 1951, 15, 44–45. [Google Scholar]
- Dennison, S.G.; Bulkley, R.V. Reproductive potential of the Black Bullhead, Ictalurus melas, in Clear Lake, Iowa. Trans. Am. Fish. Soc. 1972, 101, 483–486. [Google Scholar] [CrossRef]
- Houde, E.D. Mortality. In Fishery Science. The Unique Contributions of Early Life Stages; Fuiman, L.A., Werner, R.G., Eds.; Blackwell Publishing: Oxford, UK, 2002; pp. 64–87. [Google Scholar]
- Harrison, H.M. The foods used by some common fish of the Des Moines River drainage. Iowa Conserv. Comm. 1950, 1, 31–44. [Google Scholar]
- Welker, B.D. Summer food habits of Yellow Bass and Black Bullheads in Clear Lake. Proc. Iowa Acad. Sci. 1962, 69, 286–295. [Google Scholar]
- Bauer, R.J. Digestion rate of the Clear Lake Black Bullhead. Proc. Iowa Acad. Sci. 1970, 77, 112–121. [Google Scholar]
- Williams, W.E. Summer food of juvenile Black Bullheads (Ictalurus melas) of Mitchell Lake, Wexford Country, Michigan. Trans. Am. Fish. Soc. 1970, 99, 597–599. [Google Scholar] [CrossRef]
- Snow, R.A.; Porta, M.J.; Robison, A.L. Seasonal Diet Composition of Black Bullhead (Ameiurus melas) in Lake Carl Etling, Oklahoma. Proc. Okla. Acad. Sci. 2018, 97, 54–60. [Google Scholar]
- Clady, M.D. Food Habits of Yellow Perch, Smallmouth Bass and Largemouth Bass in Two Unproductive Lakes in Northern Michigan. Am. Midl. Nat. 1974, 91, 453–459. [Google Scholar] [CrossRef]
- Lyons, J.; Magnuson, J.J. Effects of Walleye Predation on the Population Dynamics of Small Littoral-Zone Fishes in a Northern Wisconsin Lake. Trans. Am. Fish. Soc. 1987, 116, 29–39. [Google Scholar] [CrossRef]
- Margenau, T.L.; Rasmussen, P.W.; Kampa, J.M. Factors Affecting Growth of Northern Pike in Small Northern Wisconsin Lakes. N. Am. J. Fish. Manag. 1998, 18, 625–639. [Google Scholar] [CrossRef] [Green Version]
| Age (year) | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Length (mm) | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 |
| 50 | 1.00 (3) | ||||||||
| 60 | 1.00 (10) | ||||||||
| 70 | 1.00 (6) | ||||||||
| 80 | 1.00 (3) | ||||||||
| 90 | 0.67 (2) | 0.33 (1) | |||||||
| 100 | 0.33 (1) | 0.67 (2) | |||||||
| 120 | 0.60 (3) | 0.40 (2) | |||||||
| 130 | 0.40 (2) | 0.60 (3) | |||||||
| 140 | 0.14 (1) | 0.71 (5) | 0.14 (1) | ||||||
| 150 | 0.40 (2) | 0.40 (2) | 0.20 (1) | ||||||
| 160 | 0.62 (5) | 0.38 (3) | |||||||
| 170 | 0.73 (8) | 0.27 (3) | |||||||
| 180 | 0.75 (6) | 0.25 (2) | |||||||
| 190 | 0.33 (4) | 0.67 (8) | |||||||
| 200 | 0.25 (2) | 0.75 (6) | |||||||
| 210 | 0.10 (1) | 0.70 (7) | 0.20 (2) | ||||||
| 220 | 0.18 (2) | 0.36 (4) | 0.27 (3) | 0.09 (1) | 0.09 (1) | ||||
| 230 | 0.11 (1) | 0.33 (3) | 0.22 (2) | 0.33 (3) | |||||
| 240 | 0.17 (2) | 0.25 (3) | 0.50 (6) | 0.80 (1) | |||||
| 250 | 0.11 (1) | 0.89 (8) | |||||||
| 260 | 0.08 (1) | 0.62 (8) | 0.31 (4) | ||||||
| 270 | 0.60 (6) | 0.20 (2) | 0.10 (1) | 0.10 (1) | |||||
| 280 | 0.18 (2) | 0.55 (6) | 0.18 (2) | 0.09 (1) | |||||
| 290 | 0.14 (1) | 0.29 (2) | 0.29 (2) | 0.29 (2) | |||||
| 300 | 0.17 (1) | 0.50 (3) | 0.33 (2) | ||||||
| 310 | 1.00 (2) | ||||||||
| Age (year) | N | Mean Length (mm) | Standard Deviation (SD) | Predicted Length (mm) | Lower 95% CI | Upper 95% CI |
|---|---|---|---|---|---|---|
| 1 | 84 | 80 | 15.0 | 90 | 87 | 93 |
| 2 | 76 | 132 | 22.0 | 135 | 133 | 136 |
| 3 | 842 | 180 | 18.3 | 172 | 171 | 173 |
| 4 | 816 | 196 | 18.0 | 204 | 203 | 205 |
| 5 | 92 | 235 | 17.3 | 231 | 230 | 233 |
| 6 | 295 | 260 | 14.6 | 254 | 253 | 256 |
| 7 | 117 | 274 | 18.5 | 274 | 272 | 276 |
| 8 | 57 | 297 | 14.0 | 290 | 288 | 283 |
| 9 | 35 | 292 | 11.1 | 304 | 301 | 307 |
| Diet Item | May | June | July | August | October |
|---|---|---|---|---|---|
| Oi | Oi | Oi | Oi | Oi | |
| Fish | |||||
| Bluegill (Lepomis macrochirus) | 0.7 (1) | 1.5 (2) | 16.7 (1) | ||
| Yellow Perch (Perca flavescens) | 1.0 (1) | 8.3 (12) | 3.8 (5) | ||
| Black Crappie (Pomoxis nigromaculatus) | 0.8 (1) | ||||
| Black Bullhead (Ameiurus melas) | 16.7 (1) | ||||
| Walleye (Sander vitreus) | 7.1 (1) | 16.7 (1) | |||
| Common Shiner (Luxilus cornutus) | 1.0 (1) | 1.5 (2) | |||
| Bluntnose Minnow (Pimephales notatus) | 2.3 (3) | ||||
| Eggs | 14.3 (2) | ||||
| Aquatic Invertebrates | |||||
| Gastropod (snails) | 35.7 (5) | 18.5 (19) | 67.6 (98) | 63.9 (85) | 50 (3) |
| Arthropoda (crayfish) | 3.9 (4) | 1.4 (2) | 1.5 (2) | 16.7 (1) | |
| Ephemeroptera (larvae) | 7.1 (1) | 4.9 (5) | 0.8 (1) | ||
| Odonota (larvae) | 21.4 (3) | 4.9 (5) | 0.7 (1) | 1.5 (2) | |
| Dytiscidae (beetle) | 1.9 (2) | ||||
| Diptera (larvae and pupae) | 1 (1) | 2.6 (3) | 12.8 (17) | ||
| Trichoptera (larvae) | 1.5 (2) | ||||
| Hirundea (leeches) | 28.6 (4) | 2.9 (3) | 1.5 (2) | ||
| Terrestrial Insects | |||||
| Odonota (adult) | 1.9 (2) | 0.7 (1) | |||
| Tipulidae (adult) | 1.9 (2) | 0.8 (1) | |||
| Lepidoptera (adult) | 1.0 (1) | ||||
| Other | |||||
| Aquatic Vegetation | 17.5 (18) | 17.2 (25) | 14.3 (19) | ||
| Detritus | 21.4 (3) | 38.8 (40) | 8.3 (12) | 3.8 (5) | |
| Unknown | 1.0 (1) |
| Diet Item | May | June | July | August | October |
|---|---|---|---|---|---|
| IRI | IRI | IRI | IRI | IRI | |
| Fish | |||||
| Bluegill (Lepomis macrochirus) | 0.01 | 0.03 | 5.57 | ||
| Yellow Perch (Perca flavescens) | 0.02 | 1.13 | 0.25 | ||
| Black Crappie (Pomoxis nigromaculatus) | 0.01 | ||||
| Black Bullhead (Ameiurus melas) | 5.57 | ||||
| Walleye (Sander vitreus) | 0.64 | 4.01 | |||
| Common Shiner (Luxilus cornutus) | 0.02 | 0.03 | |||
| Bluntnose Minnow (Pimephales notatus) | 0.07 | ||||
| Eggs | 15.55 | ||||
| Aquatic Invertebrates | |||||
| Gastropod (snails) | 10.67 | 6.68 | 89.49 | 78.28 | 37.98 |
| Arthropoda (crayfish) | 0.22 | 0.03 | 0.04 | 5.57 | |
| Ephemeroptera (larvae) | 0.02 | 0.46 | 0.00 | ||
| Odonota (larvae) | 4.10 | 0.48 | 0.01 | 0.00 | |
| Dytiscidae (beetle) | 0.07 | ||||
| Diptera (larvae and pupae) | 0.01 | 0.10 | 3.19 | ||
| Trichoptera (larvae) | 0.01 | ||||
| Hirundea (leeches) | 4.85 | 0.15 | 0.02 | ||
| Terrestrial Insects | |||||
| Odonota (adult) | 0.05 | 0.00 | |||
| Tipulidae (adult) | 0.04 | 0.00 | |||
| Lepidoptera (adult) | 0.02 | ||||
| Other | |||||
| Aquatic Vegetation | 3.21 | 2.77 | 1.76 | ||
| Detritus | 3.42 | 15.95 | 0.65 | 0.13 | |
| Unknown | 0.02 |
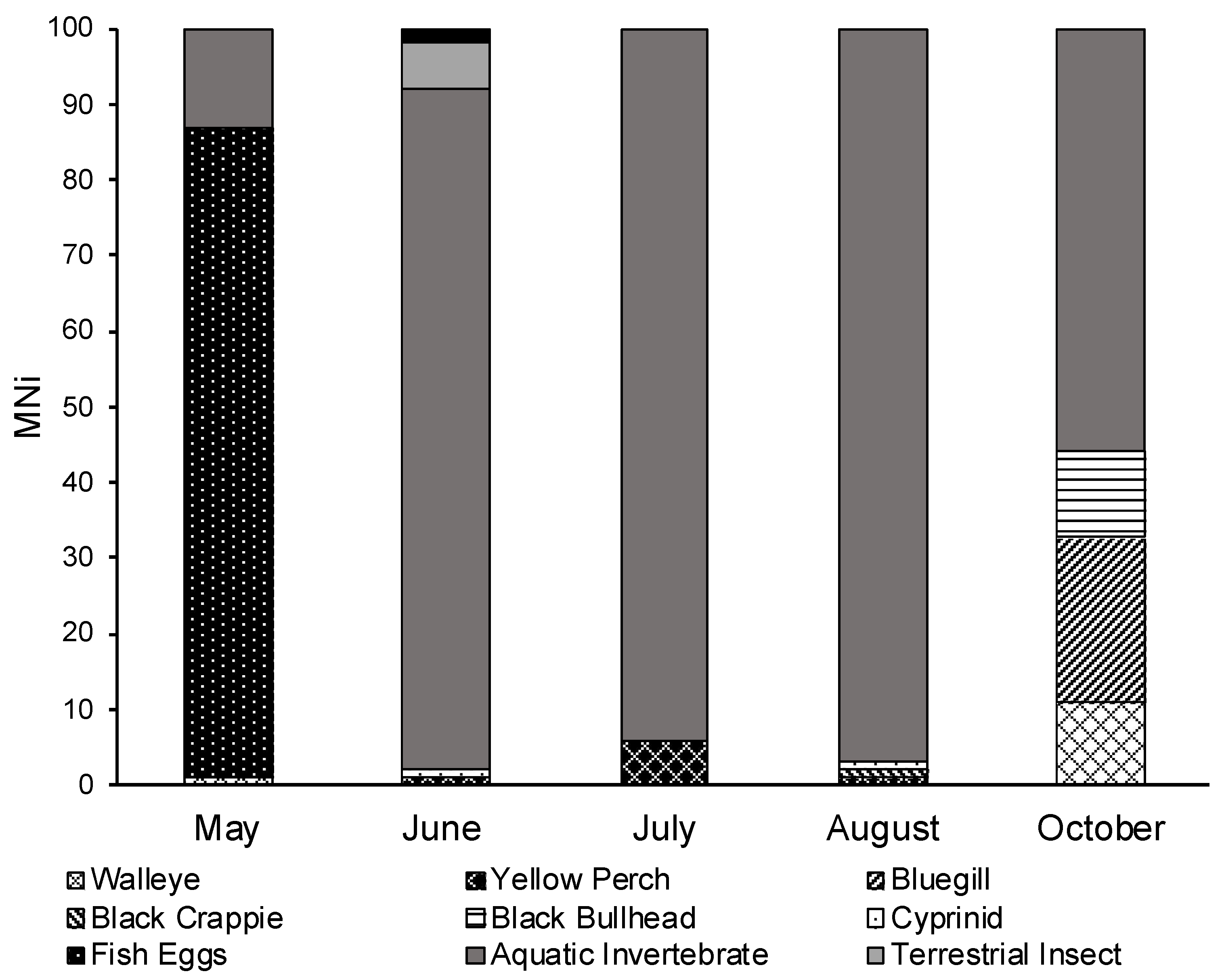
| Diet Item | May | June | July | August | October |
|---|---|---|---|---|---|
| Mni | Mni | Mni | Mni | Mni | |
| Fish | |||||
| Bluegill (Lepomis macrochirus) | 0.69 | 1.13 | 16.67 | ||
| Yellow Perch (Perca flavescens) | 0.97 | 7.47 | 3.38 | ||
| Black Crappie (Pomoxis nigromaculatus) | 0.38 | ||||
| Black Bullhead (Ameiurus melas) | 16.67 | ||||
| Walleye (Sander vitreus) | 0.25 | 8.33 | |||
| Common Shiner (Luxilus cornutus) | 0.97 | 0.75 | |||
| Bluntnose Minnow (Pimephales notatus) | 1.45 | ||||
| Eggs | 85.86 | ||||
| Aquatic Invertebrates | |||||
| Gastropod (snails) | 7.00 | 18.01 | 66.55 | 61.92 | 41.67 |
| Arthropoda (crayfish) | 3.11 | 0.92 | 1.13 | 16.67 | |
| Ephemeroptera (larvae) | 0.25 | 4.94 | 0.30 | ||
| Odonota (larvae) | 4.8 | 4.85 | 0.69 | 1.13 | |
| Dytiscidae (beetle) | 1.94 | ||||
| Diptera (larvae and pupae) | 0.97 | 2.3 | 12.71 | ||
| Trichoptera (larvae) | 0.45 | ||||
| Hirundea (leeches) | 1.77 | 2.91 | 0.83 | ||
| Terrestrial Insects | |||||
| Odonota (adult) | 1.46 | 0.23 | |||
| Tipulidae (adult) | 1.46 | 0.08 | |||
| Lepidoptera (adult) | 0.97 | ||||
| Other | |||||
| Aquatic Vegetation | |||||
| Detritus | |||||
| Unknown | 1.13 |
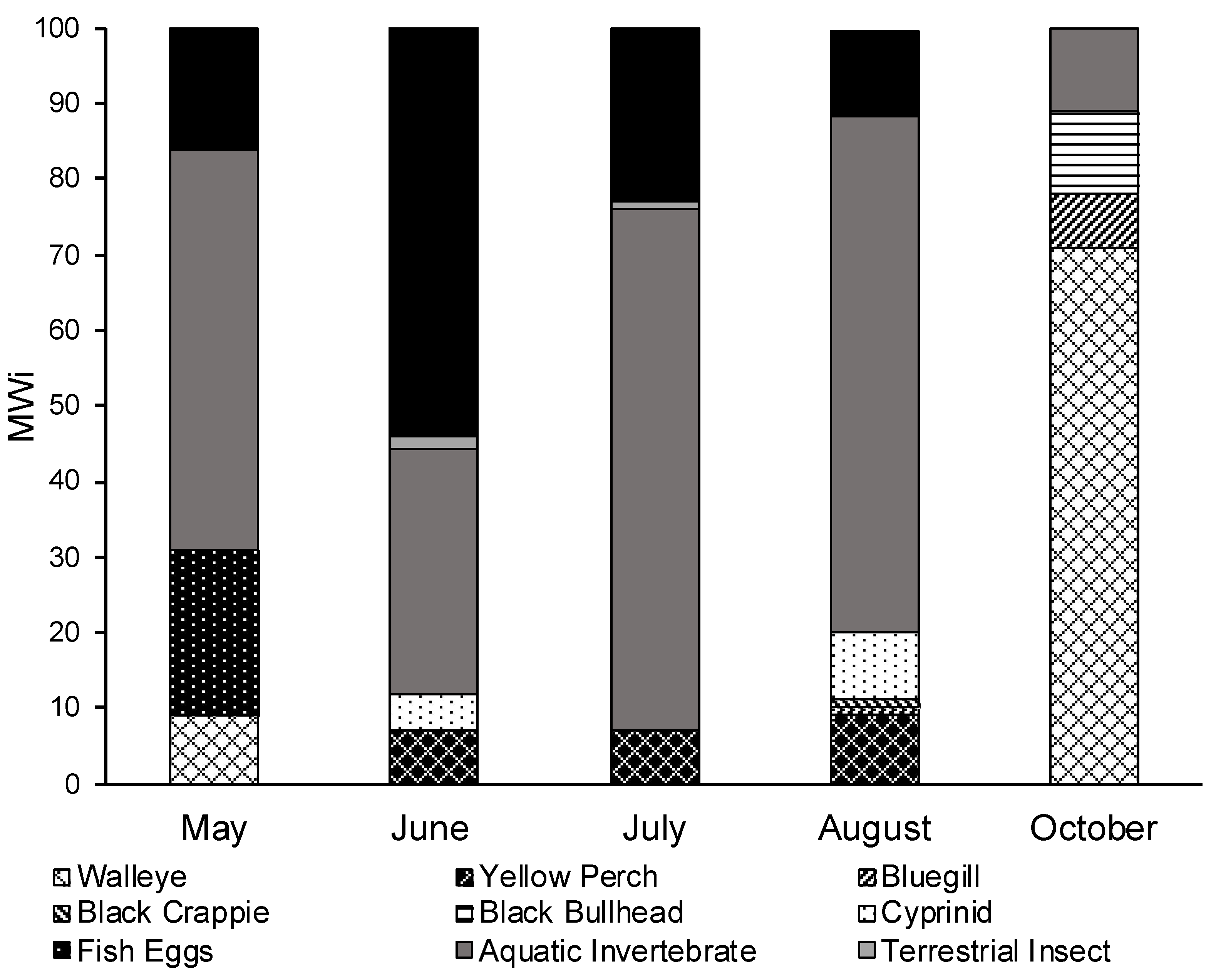
| Diet Item | May | June | July | August | October |
|---|---|---|---|---|---|
| Mwi | Mwi | Mwi | Mwi | Mwi | |
| Fish | |||||
| Bluegill (Lepomis macrochirus) | 0.69 | 1.09 | 16.67 | ||
| Yellow Perch (Perca flavescens) | 0.97 | 6.10 | 3.12 | ||
| Black Crappie (Pomoxis nigromaculatus) | 0.41 | ||||
| Black Bullhead (Ameiurus melas) | 16.67 | ||||
| Walleye (Sander vitreus) | 8.72 | 15.70 | |||
| Common Shiner (Luxilus cornutus) | 0.97 | 1.33 | |||
| Bluntnose Minnow (Pimephales notatus) | 1.47 | ||||
| Eggs | 22.87 | ||||
| Aquatic Invertebrates | |||||
| Gastropod (snails) | 22.90 | 18.07 | 65.8 | 60.58 | 34.30 |
| Arthropoda (crayfish) | 2.57 | 1.16 | 1.23 | 16.67 | |
| Ephemeroptera (larvae) | 0.05 | 4.46 | 0.30 | ||
| Odonota (larvae) | 14.36 | 4.85 | 0.69 | 1.20 | |
| Dytiscidae (beetle) | 1.94 | ||||
| Diptera (larvae and pupae) | 0.32 | 1.45 | 12.22 | ||
| Trichoptera (larvae) | 0.12 | ||||
| Hirundea (leeches) | 15.20 | 2.14 | 0.77 | ||
| Terrestrial Insects | |||||
| Odonota (adult) | 1.25 | 0.20 | |||
| Tipulidae (adult) | 0.90 | 0.02 | |||
| Lepidoptera (adult) | 0.97 | ||||
| Other | |||||
| Aquatic Vegetation | 18.32 | 16.10 | 12.30 | ||
| Detritus | 16.00 | 41.10 | 7.80 | 3.42 | |
| Unknown | 1.15 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sikora, L.W.; Mrnak, J.T.; Henningsen, R.; VanDeHey, J.A.; Sass, G.G. Demographic and Life History Characteristics of Black Bullheads Ameiurus melas in a North Temperate USA Lake. Fishes 2022, 7, 21. https://doi.org/10.3390/fishes7010021
Sikora LW, Mrnak JT, Henningsen R, VanDeHey JA, Sass GG. Demographic and Life History Characteristics of Black Bullheads Ameiurus melas in a North Temperate USA Lake. Fishes. 2022; 7(1):21. https://doi.org/10.3390/fishes7010021
Chicago/Turabian StyleSikora, Logan W., Joseph T. Mrnak, Rebecca Henningsen, Justin A. VanDeHey, and Greg G. Sass. 2022. "Demographic and Life History Characteristics of Black Bullheads Ameiurus melas in a North Temperate USA Lake" Fishes 7, no. 1: 21. https://doi.org/10.3390/fishes7010021
APA StyleSikora, L. W., Mrnak, J. T., Henningsen, R., VanDeHey, J. A., & Sass, G. G. (2022). Demographic and Life History Characteristics of Black Bullheads Ameiurus melas in a North Temperate USA Lake. Fishes, 7(1), 21. https://doi.org/10.3390/fishes7010021







